Abstract
Field and laboratory investigations revealed phenotypic, target site and metabolic resistance to permethrin in an Anopheles gambiae s.s. (Diptera: Culicidae) population in Bungoma District, a region in western Kenya in which malaria is endemic and rates of ownership of insecticide-treated bednets are high. The sensitivity of individual An. gambiae s.l. females as indicated in assays using World Health Organization (WHO) test kits demonstrated reduced mortality in response to permethrin, deltamethrin and bendiocarb. Estimated time to knock-down of 50% (KDT50) of the test population in Centers for Disease Control (CDC) bottle bioassays was significantly lengthened for the three insecticides compared with that in a susceptible control strain. Anopheles arabiensis from all three sites showed higher mortality to all three insecticides in the WHO susceptibility assays compared with the CDC bottle assays, in which they showed less sensitivity and longer KDT50 than the reference strain for permethrin and deltamethrin. Microplate assays revealed elevated activity of β-esterases and oxidases, but not glutathione-S-transferase, in An. gambiae s.s. survivors exposed to permethrin in bottle bioassays compared with knocked down and unexposed individuals. No An. arabiensis showed elevated enzyme activity. The 1014S kdr allele was fixed in the Bungoma An. gambiae s.s. population and absent from An. arabiensis, whereas the 1014F kdr allele was absent from all samples of both species. Insecticide resistance could compromise vector control in Bungoma and could spread to other areas as coverage with longlasting insecticide-treated bednets increases.
Keywords: Anopheles arabiensis, Anopheles gambiae, insecticide resistance, metabolic detoxification, target site
Introduction
Malaria is one of the most important parasitic infections worldwide, placing at risk about half of the global population and killing approximately 655 000 people in 2010 [World Health Organization (WHO), 2011]. Between 2008 and 2010, nearly 300 million longlasting insecticide-treated bednets (LLINs) were distributed and over 78 million people were protected by indoor residual spraying (IRS) of insecticides in areas in which malaria is endemic (WHO, 2011). These achievements, along with improvements in malaria case management through the use of drugs, have resulted in fewer cases of malaria and fewer deaths caused by malaria in many regions of sub-Saharan Africa (O’Meara et al., 2010). However, the selection pressure imposed by exposure to the insecticides used in the vector control components of malaria control programmes is expected to result in resistance to insecticides, which could potentially lead to programme failure (Ranson et al., 2009).
Kenya has made significant progress in scaling up malaria control efforts, with targeted subsidies of ITNs beginning in 2003, a mass distribution of ITNs targeting children aged <5 years in 2006 (Okiro et al., 2010), and a universal coverage campaign in 2011 which provided one LLIN for every two people. Although recent surveys have indicated that 50% of children slept under an LLIN the previous night [Division of Malaria Control (DOMC), 2011], ownership and use rates reported by patients diagnosed at hospitals remained low (mean rate of 41% across eight sites) (Okiro et al., 2010). Malaria-related morbidity and mortality declined in western Kenya coincidentally with the instigation of these programmes and with a change in national policy towards the use of more efficacious drugs for malaria treatment (Fegan et al., 2007; O’Meara et al., 2008). However, recent site-specific trends suggest rebounds in malaria infection or malaria-attributed child mortality in western Kenya, the region of the country in which malaria is most endemic (Okiro et al., 2010; Hamel et al., 2011; Zhou et al., 2011). These observations raise the possibility that vector control programmes based upon a limited range of insecticides are faltering, possibly as a result of insecticide resistance as previously observed (Ranson et al., 2009; Mathias et al., 2011).
In western Kenya, reduced sensitivity to pyrethroid insecticides was first detected in Anopheles gambiae s.s. in a trial of permethrin-treated nets and curtains conducted in six villages in western Nyanza Province in the early 1990s (Vulule et al., 1994). However, this reported resistance was weak and unstable, and field populations regressed slightly after 1 year (Vulule et al., 1996). Additionally, reduced sensitivity was not observed in populations of An. gambiae s.s., Anopheles arabiensis and Anopheles funestus during and after a randomized control trial of conventional insecticide-treated bednets conducted in the same area from 1996 to 1999, or during subsequent follow-ups extending through 2007 during which time insecticide retreatment was provided free every 9 months (Gimnig et al., 2003; Bayoh et al., 2010). Vector populations were lower prior to the distribution of bednets as part of the trial (Lindblade et al., 2006) and the proportion of the relatively more zoophilic species An. arabiensis increased in comparison with that of the relatively more anthropophilic species An. gambiae s.s. (Bayoh et al., 2010), suggesting that vector control mediated by insecticide-treated bednets remained effective and had large-scale effects on vector population structure. More recently, An. arabiensis was reported to be less likely to be killed by LLINs than An. gambiae s.s., which may explain the increase in population proportions of An. arabiensis (Kitau et al., 2012). Several surveys in western Kenya have documented the emergence of genotypic, phenotypic and metabolic resistance to pyrethroid insecticides, DDT and bendiocarb (Chen et al., 2008; Kawada et al., 2011a, 2011b; Mathias et al., 2011). Of particular concern was the increase in frequency of the knock-down resistance allele 1014S at the voltage-gated sodium channel to near fixation in some populations of An. gambiae s.s., suggesting strong selection for target site resistance (Mathias et al., 2011).
Two main resistance mechanisms to pyrethroid insecticides have been observed in An. gambiae s.l. mosquitoes in Kenya: target site mutations (Ranson et al., 2000; Stump et al., 2004; Kamau et al., 2007), and detoxification by elevated enzymatic activity (Vulule et al., 1999). The high frequency of the 1014S kdr allele in the population of An. gambiae s.s. in western Kenya mentioned earlier did not, however, correlate well with phenotypic resistance (Mathias et al., 2011). Furthermore, there was regional variation in resistance, with An. gambiae s.s. populations in parts of Western Province showing relatively high phenotypic resistance and populations in Nyanza Province showing continued susceptibility (Mathias et al., 2011). Hence, the current study sought to make an in-depth evaluation of mechanisms of resistance to permethrin and other insecticides in a population of An. gambiae s.s. from Western Province previously determined by Mathias et al. (2011) to be phenotypically resistant, and to compare these with resistance in populations of An. arabiensis at that site and two other sites in Western and Nyanza Provinces.
Materials and methods
Study site and sample collection
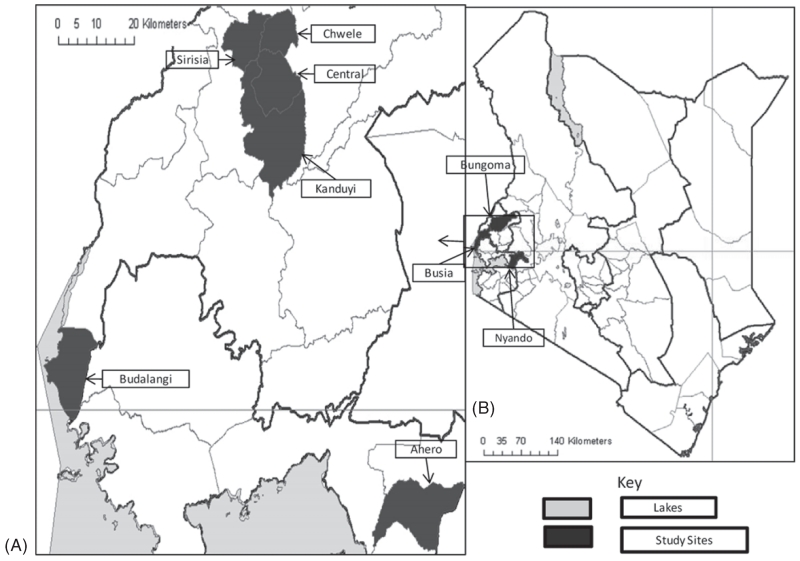
Mosquitoes were sampled from three districts (Fig. 1): (a) Bungoma District in Western Province, which includes Sirisia, Chwele, Central and Kanduyi Divisions (00°35′ N, 34°35′ E; 1386 m a.s.l.); (b) Budalangi Division (00°06′ N, 33°58′ E; 1147 m a.s.l.) in Busia District, Western Province, and (c) Ahero Division (00°11′ S, 34°55′ E; 1155 m a.s.l.) in Nyando District, Nyanza Province. Previous studies in Bungoma indicated a mixture of An. gambiae s.s. and An. arabiensis with the former exhibiting high levels of phenotypic resistance to pyrethroid insecticides. Anopheles arabiensis was the predominant species in Budalangi and Ahero, and was largely susceptible to pyrethroid insecticides at all three sites (Mathias et al., 2011). The rural economy in Bungoma District is mainly agricultural and depends on the intensive production of tobacco, sugar cane, grain cereals including maize, bananas, onions and other vegetables by large and small landholders (Jaetzold et al., 2005). In Ahero and Budalangi, rice production predominates as a result of local topography, annual flooding regimes and the development of rice field irrigation schemes (Mambala, 2007; Thairu, 2010).
Fig. 1.
(A) The study area showing the three districts in western Kenya in which sampling was conducted. (B) The locations within districts from which samples were obtained.
Bioassays
Mosquito dippers and hand pipettes were used to collect Anopheles larvae from various aquatic habitats. Samples were transported live to the laboratory, where they were placed in spring water in pans and reared on a mixture of fish food and brewer’s yeast provided daily. Upon pupation, individuals were transferred to cages and allowed to emerge as adults, and were provided with 10% sucrose in cotton pledgets.
Three-day-old adults that had emerged under these conditions were exposed to insecticides using WHO impregnated papers for 1 h at standard concentrations in the WHO tube bioassay (WHO, 1998). Mortality was scored at 24 h post-exposure. The insecticides used were permethrin (0.75%), deltamethrin (0.05%) and bendiocarb (0.1%). Centers for Disease Control (CDC) bioassays in glass bottles (Brogdon & McAllister, 1998) were conducted using the following recommended diagnostic doses: 21.5 μg/bottle permethrin; 12.5 μg/bottle deltamethrin, and 12.5 μg/bottle bendiocarb. Bottles were coated by adding the appropriate dose of insecticide in 1 mL of acetone. Mosquitoes were released into the bottles and knock-down recorded every 15 min for 1 h, after which the samples were removed from the bottle and knock-down status recorded. Samples were then placed in individual tubes and frozen for further experiments. A mosquito was considered to have been knocked down if it was unable to stand or fly in a coordinated way. These bottle bioassays were performed in addition to the WHO tube assays because the bottles allow easy visualization and measurement of time to knock-down, and knocked down or live individuals could be quickly retrieved from the bottles and immediately frozen for later biochemical assays. The control strain was a susceptible laboratory colony of An. gambiae s.s. called the Kisumu strain (Vulule et al., 1996, 1999).
Enzyme assays
Expression of detoxifying enzymes was measured using the microplate enzyme system as previously described in Brogdon et al. (1988). Mosquitoes were retrieved from the bottles and frozen as described above. Legs and wings were removed for molecular assays (see below). Carcasses (head, thorax and abdomen) were homogenized in 100 μL of 0.05 m potassium phosphate buffer, pH 7.2, and diluted to 1 mL with buffer. For measurement of esterase activity, the following protocol was conducted in 96-well microtitre plates. To each 100 μL of homogenate, a 100-μL aliquot of β-naphthyl acetate (56 mg/10 mL acetone with 90 mL buffer) was added and the preparation was incubated at room temperature (24 °C) for 10 min. A 100-μL aliquot of dianisidine (100 mg/100 mL water) was then added. Absorbances of samples were read with a plate reader at 540 nm.
For oxidase activity, 100 μL of mosquito homogenate was transferred into wells of microtitre plates and 200 μL of 3,3′ 5,5′ -tetramethyl-benzidine dihydrochloride hydrate (50 mg/25 mL methanol/75 mL 0.25 m Na acetate buffer) was added. A volume of 25 μL of 3% hydrogen peroxide was then added to each well and incubated for 5 min. Sample absorbance was read at 620 nm.
For quantification of glutathione-S-transferase (GST) activity, aliquots (100 μL) of the mosquito homogenate sample were transferred to wells of microtitre plates, and 100 μL of reduced glutathione (61 mg/100 mL KPO4 buffer) was added per well. Next, 100 μL of 1-chloro-2,4-dinitrobenzene (cDNB) (20 mg/10 mL acetone in 90 mL KPO4 buffer) was added and absorbance read immediately at 340 nm. Absorbance was recorded again at 5 min and the difference in absorbance between the two times per sample was determined.
To normalize enzyme activity readings, total protein of samples was determined as previously described in Brogdon et al. (1988). A 20-μL aliquot of mosquito homogenate was transferred into wells of microtitre plates and 80 μL KPO4 buffer added. A volume of 200 μL of protein dye reagent [20 mL Bio-Rad protein dye concentrate/80 mL distilled water (dH2O)] (Bio-Rad Laboratories, Inc., Hercules, CA, U.S.A.) was then added and optical densities read immediately at 620 nm. For all enzyme assays, each sample was tested in triplicate.
Genotypic analysis
DNA was extracted from the combined legs and wings of each specimen using ethanol precipitation (Collins et al., 1987). Conventional polymerase chain reaction (PCR) was used to distinguish between the two sibling species of the An. gambiae s.l. species complex native to western Kenya, An. gambiae s.s. and An. arabiensis (Scott et al., 1993). Real-time PCR (RT-PCR) was used to quantify the genotype at amino acid position 1014 of the voltage-gated sodium channel, following the methods of Bass et al. (2007) as modified by Mathias et al. (2011). Samples were genotyped for the wild-type (susceptible) allele using probe 5′ -CTTACGACTAAATTTC-3′, and for the 1014S kdr allele using probe 5′ -ACGACTGAATTTC-3′. A subset of samples was also genotyped for the 1014F kdr allele using probe 5′ -ACGACAAAATTTC-3′.
Data analysis
Results of the 24-h WHO bioassays were analysed using standard criteria (WHO, 1992, 1998). Results of bottle bioassays were expressed as the number knocked down out of the total per observation interval, and values for the time taken to achieve mortality of 50% [lethal time (LT50)] with 95% confidence limits were estimated using probit analysis. Resistance ratios (RR) for the LT50 and LT95 were determined by dividing the values of the respective LT50 and LT95 for each population by the values of the LT50 and LT95 of the controls. In order to determine significant differences in enzymatic expression in each population, absorbance values were compared between the knocked down and live individuals using analysis of variance (anova) with three groups, all from field samples: (a) individuals that survived exposure; (b) individuals that died during exposure, and (c) live, unexposed individuals. Similarly, absorbance values were compared between insecticide-exposed and unexposed, Kisumu strain An. gambiae s.s. females. Statistical analyses were performed using sas Version 9.1 (SAS, 2000).
Results
Site-specific species composition and sample size
A total of 3111 adult male and female An. gambiae s.l. were reared from larvae and subjected to PCR for identification of sibling species (Table 1). A total of 1986 female An. gambiae s.l. from these collections were used in the bioassays for phenotypic resistance properties. Of these, 734 were sourced from Bungoma, 473 from Budalangi and 779 from Ahero.
Table 1.
Percentage distribution of Anopheles gambiae s.l. vector species in the three study sites.
| Site | An. gambiae | An. arabiensis | Total, n |
|---|---|---|---|
| Bungoma | 77% | 23% | 1124 |
| Budalangi | 1% | 99% | 885 |
| Ahero | 0 | 100% | 1102 |
Phenotypic assays
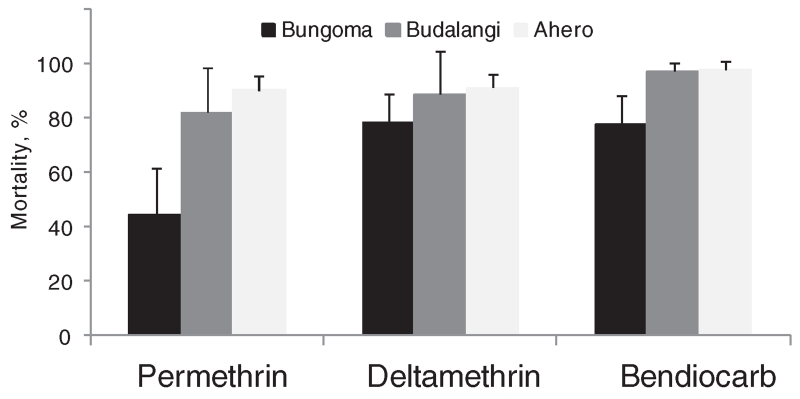
Results of bioassays in An. gambiae s.l. from the three study sites using WHO kits are shown in Fig. 2. In assays using WHO test kits, females of the Kisumu insecticide-susceptible strain showed 100% mortality to permethrin (n = 45), deltamethrin (n = 45) and bendiocarb (n = 25). By comparison, female An. gambiae s.s. from Bungoma showed low mortality to permethrin (44.5%), and intermediate mortality to deltamethrin (78.5%) and bendiocarb (78.0%). Female An. arabiensis from Budalangi showed intermediate mortality to permethrin (82.1%) and deltamethrin (88.9%), but relatively high mortality to bendiocarb (97.3%). Female An. arabiensis from Ahero exhibited relatively high mortality to all three insecticides (90.5% to permethrin, 91.2% to deltamethrin, 97.9% to bendiocarb).
Fig. 2.
Results of World Health Organization kit bioassays of Anopheles gambiae s.l. females from the three study sites (Bungoma, Budalangi, and Ahero) exposed to permethrin, deltamethrin and bendiocarb.
Results of CDC bottle bioassays showed 100% knockdown for females of the Kisumu strain at 30 min in response to bendiocarb and permethrin, and 45 min for deltamethrin. By contrast, time to knock-down was extended for wild-caught mosquitoes in exposures to permethrin, deltamethrin and, to a lesser extent, bendiocarb, compared with the Kisumu strain. In general, the Bungoma population was the least susceptible, the Budalangi population was intermediate, and the Ahero population was most susceptible in bottle bioassays. Estimated time to knock-down of 50% (KDT50) values (Table 2) indicated relatively short times to 50% knock-down for all populations when exposed to bendiocarb; however, time to knock-down was lengthened for deltamethrin and permethrin, compared with times in the Kisumu control strain. The highest resistance ratio observed, 4.3, referred to the Bungoma population for permethrin exposure.
Table 2.
Estimated time to 50% mortality (KDT50) in three Anopheles gambiae s.l. populations sampled in western Kenya and exposed in bottle bioassays to three insecticides for 60 min.
| Insecticide | Population | KDT50, min | 95% CI | RR |
|---|---|---|---|---|
| Bendiocarb | Ahero | 12.3 | – | 1.0 |
| Budalangi | 18.9 | 15.9–21.7 | 1.5 | |
| Bungoma | 15.2 | 13.3–16.7 | 1.2 | |
| Kisumu (control) | 12.5 | – | – | |
| Deltamethrin | Ahero | 31.2 | 25.3–36.8 | 1.8 |
| Budalangi | 40.6 | 36.6–44.8 | 2.3 | |
| Bungoma | 56.6 | 50.3–70.1 | 3.3 | |
| Kisumu (control) | 17.3 | 13.2–20.7 | – | |
| Permethrin | Ahero | 32.0 | 23.2–39.4 | 2.3 |
| Budalangi | 48.8 | 41.6–59.8 | 3.5 | |
| Bungoma | 59.4 | 46.1–97.1 | 4.3 | |
| Kisumu (control) | 13.9 | 7.2–17.5 | – |
Estimates are from probit analysis with 95% confidence intervals (95% CI). Resistance ratios (RR) are calculated as the ratio of the KDT50 of the test population to that of the control population, represented here by the An. gambiae s.s. Kisumu laboratory strain, which is a standard insecticide-susceptible strain.
Because the populations of An. gambiae s.l. from Budalangi and Ahero proved to be almost entirely comprised of An. arabiensis, the knock-down data from the bottle bioassays refer to results for that species only. However, the population from Bungoma consisted of a mixture of An. gambiae s.s. and An. arabiensis; therefore we tallied knock-down of the two species separately in the bottle bioassays for all three insecticides for the purposes of comparison (Table 3). Results showed that both species were highly susceptible to bendiocarb and had equivalent but reduced susceptibility to deltamethrin, but that An. gambiae s.s. had relatively low rates of knock-down (11.1%) upon exposure to permethrin, whereas An. arabiensis had significantly higher knock-down (75.9%) at 30 min.
Table 3.
Percentage knock-down (KD) of Anopheles gambiae s.s. and Anopheles arabiensis females from Bungoma, western Kenya, after exposure of 60 min in glass bottle bioassays to three insecticides.
|
An. gambiae s.s.
|
An. arabiensis
|
||||
|---|---|---|---|---|---|
| Insecticide | % KD | n | % KD | n | χ2 test |
| Bendiocarb | 100 | 86 | 96.7 | 61 | Fisher’s exact test, P = 0.41 |
| Deltamethrin | 49.4 | 85 | 61.9 | 42 | χ2 = 1.3, P = 0.13 |
| Permethrin | 11.1 | 99 | 75.9 | 29 | χ2 = 45.82, P < 0.0001 |
Target site genotype
Table 4 summarizes kdr genotyping results in An. gambiae s.s. from Bungoma and Budalangi. There were no An. gambiae s.s. from Ahero. All of the An. arabiensis samples (160 from Bungoma, 117 from Budalangi, 300 from Ahero) that were assayed for the 1014S kdr allele, and 40 from Bungoma, 100 from Budalangi and 100 from Ahero assayed for the 1014F kdr allele were homozygous wild-type mosquitoes.
Table 4.
Frequencies of knock-down resistance (kdr) alleles in Anopheles gambiae s.s. populations in Bungoma and Budalangi.
|
kdr (L1014S) |
kdr (L1014F) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Site | RR | RS | SS | n | RR | RS | SS | n |
| Bungoma | 99.6% | 0.2% | 0.1% | 856 | 0 | 0 | 100% | 60 |
| Budalangi | 100% | 0 | 0 | 9 | 0 | 0 | 100% | 9 |
RR, homozygous resistant; RS, heterozygous resistant; SS, susceptible.
Enzyme assays
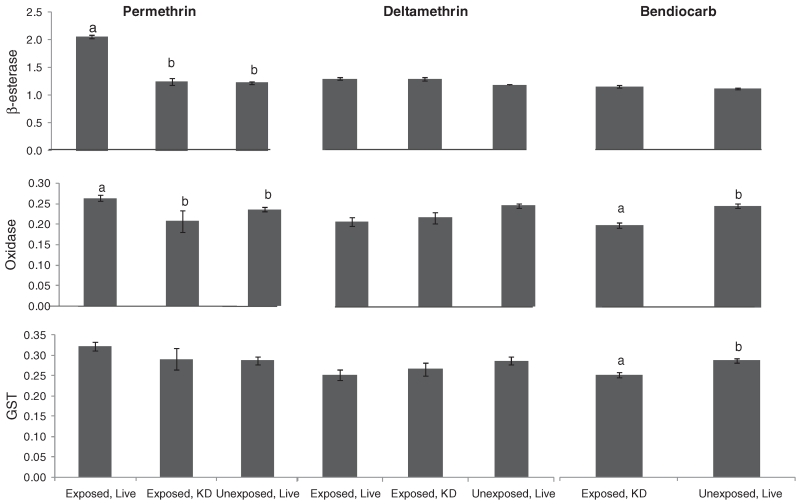
There were no significant enzyme elevations in the An. arabiensis samples from all three sites for all insecticide exposures (data not shown). Results of the enzyme assays for An. gambiae s.s. from Bungoma are shown in Fig. 3. There was a significant difference in β-esterase activity amongst the three test groups exposed to permethrin. Individuals that survived exposure had higher β-esterase activity levels compared with mosquitoes that were knocked down during exposure or mosquitoes that had not been exposed to permethrin (one-way anova, F2,173 = 326.3, P < 0.0001, Tukey’s mean separation test). There was a 1.7-fold elevation in β-esterase activity in live compared with knocked down permethrin-exposed mosquitoes. Esterase enzyme levels in the unexposed mosquitoes and in those that were knocked down in the bottle bioassay were not significantly different. Comparison of GST and oxidase enzyme levels in An. gambiae s.s. samples exposed to bendiocarb showed a decline in enzyme levels in comparison with those in the unexposed mosquitoes.
Fig. 3.
Bar graph of enzyme activity measured in biochemical assays in female Anopheles gambiae s.s. from Bungoma. Bars labelled with ‘a’ indicate findings that are significantly different from those labelled with ‘b’. Bars without letters were not significantly different. GST, glutathione-S-transferase; KD, knocked down.
Similarly, there were significant differences in oxidase activity amongst the three test groups exposed to permethrin. Mosquitoes that survived exposure to permethrin had higher oxidase activity levels compared with mosquitoes that were knocked down during exposure and with mosquitoes that had not been exposed (one-way anova, F2,162 = 7.49, P < 0.001, Tukey’s a posteriori test). There was a 1.4-fold elevation in oxidase enzyme activity in live compared with knocked down An. gambiae s.s. (Fig. 3). Oxidase enzyme levels in the unexposed mosquitoes and in those that were knocked down in the bottle bioassay were comparable.
There were no differences in GST activity among mosquitoes that were exposed to permethrin and there were no differences in β-esterase, oxidase or GST among mosquitoes exposed to deltamethrin. For bendiocarb, all mosquitoes were knocked down in the bottle bioassay. There were no differences in enzyme activity levels between mosquitoes that had been exposed to bendiocarb and those that had not (one-way anovas, P > 0.05).
Discussion
Ranson et al. (2009, 2011) reviewed the status of insecticide resistance of An. gambiae s.s. in Africa and predicted an increase in the frequency of resistant populations as vector control measures based upon pyrethroid insecticides expanded regionally. In Kenya, the Division of Malaria Control has emphasized wide-scale distribution of LLINs in which pyrethroid insecticides are incorporated into the fibres (DOMC, 2009; Noor et al., 2009). Household bednet ownership has increased in line with the heavily subsidized distribution through antenatal clinics beginning in 2003 and through a mass campaign conducted in 2006 (Bayoh et al., 2010; Mathias et al., 2011), although at the Bungoma study site insecticide-treated bednet ownership was reported to be low among patients diagnosed with malaria (36.0%) (Okiro et al., 2010). National surveys have shown relatively higher ownership of any type of bednet (untreated, conventionally treated or longlasting impregnated net), but modest ownership levels of the more effective longlasting, impregnated nets (DOMC, 2009, 2011). These pyrethroid-treated bednets are likely to represent a source of selection pressure for reduced sensitivity to pyrethroids in the An. gambiae s.s. population under study at Bungoma. Agricultural use of insecticides may also be a source of selection pressure (Lines, 1988; Fane et al., 2012) about which there is currently little information at our study sites. The rice irrigation schemes in Ahero and Budalangi represent a quite different agricultural landscape than the diverse cash crop economy of Bungoma, yet both types undoubtedly involve insecticide use for relevant insect pests. The relative contribution of agricultural insecticides and pyrethroids in bednets to the emergence of resistance in the An. gambiae s.s. population at Bungoma is therefore unknown. Resistance associated with insecticide use in agriculture has been observed in Uganda (Brogdon & Barber, 1990; Ramphul et al., 2009; Verhaeghen et al., 2010), Burkina Faso (Diabate et al., 2002) and Cameroon (Lines, 1988; Chouaibou et al., 2008; Müller et al., 2008a). Because the An. arabiensis populations studied here showed relatively greater sensitivity to pyrethroids, and larvae of this species would be just as likely as An. gambiae s.s. larvae to be exposed, it seems likely that bednets alone are responsible for most of the selection pressure in western Kenya.
Quantification of the intensity of resistance in populations of Anopheles vectors requires standardized assays for the purposes of comparison. We utilized two assays, the WHO standardized assay in kit form and the CDC bottle bioassay. Each provides different information about the responses of sampled populations to insecticide exposures and both provide inferences about resistance status. The WHO assay provides criteria for classifying populations into three categories on the basis of results as: (a) susceptible (>98% mortality); (b) having low-frequency resistance requiring additional confirmation (80–97% mortality), and (c) resistant (<80% mortality). These criteria were originally derived empirically based on the establishment of discriminating dosages of insecticides (WHO, 1998). According to these criteria, the Bungoma population, which was comprised primarily of An. gambiae s.s., was resistant to permethrin and deltamethrin, and has suspected resistance to bendiocarb. The populations at Budalangi and Ahero, by contrast, were comprised nearly entirely of An. arabiensis. The population from Budalangi was resistant to permethrin (just below the 80% mortality cut-off), had suspected resistance to deltamethrin and was susceptible to bendiocarb. The Ahero population had suspected resistance to permethrin and deltamethrin and low-frequency resistance to bendiocarb. The results of several recent surveys provide evidence for reduced sensitivity to pyrethroid insecticides in some, but not all, populations of An. gambiae s.s. and An. arabiensis sampled in western Kenya (Chen et al., 2008; Kawada et al., 2011a, 2011b; Mathias et al., 2011).
The categorical classifications of the status of the three populations under study in terms of their insecticide resistance are supported by the results of the bottle bioassays, which represent a more sensitive indicator of resistance than the WHO assay. In particular, all three field populations had reduced sensitivity to deltamethrin and permethrin, and the Budalangi An. arabiensis population showed a response to permethrin similar to that of the An. gambiae s.s. population from Bungoma. Growing resistance to pyrethroids observed in the study sites could be a challenge for IRS programmes implemented using pyrethroids. Apart from Bungoma, in which suspected resistance was observed in the population, bendiocarb may represent an alternative insecticide for IRS at these sites. The mild resistance to bendiocarb at some sites is likely to reflect carbamate use in agriculture in the area because the insecticide has not been used for malaria control.
The frequency of the 1014S kdr allele at the voltage-gated sodium channel locus increased from <5% to nearly 100% in a population of An. gambiae s.s. located at the juncture of Bondo and Kisumu districts in Nyanza Province from 1996 to 2009, the period when pyrethroid-treated bednets became available (Mathias et al., 2011), yet at the same time the population of this species declined in proportion to An. arabiensis (Bayoh et al., 2010) and the latter species remained largely susceptible to permethrin and deltamethrin (Mathias et al., 2011). However, the protection conferred by this genotype is much less than that of the 1014F kdr genotype (Donnelly et al., 2009) and thus it is unclear what explanatory fraction of the phenotypic resistance we observed in the Bungoma population of An. gambiae s.s. would reflect the high frequency of the 1014S kdr allele vs. the relatively modest overexpression of β-esterase and oxidase enzymes. The two mechanisms co-occur in the same population, suggesting that both contribute to the phenotypic resistance to permethrin documented recently (Mathias et al., 2011) and confirmed in the current study. Further, these findings would support the hypothesis that An. gambiae s.s. populations in Bungoma persist in the presence of increased use of pyrethroid-treated bednets through combinations of mechanisms of resistance; such mechanisms may be lacking in other areas of Kenya where An. gambiae s.s. populations have steadily declined (Bayoh et al., 2010; Mathias et al., 2011). Thus far, only two resistance mechanisms (knock-down resistance and metabolic detoxification by oxidases and esterases) have been detected in these populations, but these findings do not rule out the possibility that other mechanisms may be active.
The results of enzyme expression analyses shown here with An. gambiae s.s. in Bungoma are similar to those of previous analyses (Vulule et al., 1994, 1996, 1999) carried out in the community of Gem in western Nyanza Province in western Kenya, in that both β-esterase and oxidase enzyme expression, but not GST enzymes, were elevated in the An. gambiae s.s. samples. Interestingly, although there was clear evidence for tolerance to deltamethrin phenotypically, there was no measurable elevated expression of these enzyme classes in surviving individuals that had been exposed to deltamethrin. Therefore, the mechanism of resistance to deltamethrin was undetected in the current study. It may involve merely target site resistance as a result of the presence of the kdr alleles, whereas resistance against permethrin may involve target site resistance as well as metabolic detoxification as a result of the elevated expression of these two classes of enzyme. Although the frequency of the knock-down resistance genotype in the Gem populations of An. gambiae s.s. under study previously (Vulule et al., 1994, 1996, 1999) was not known because the phenomenon had not yet been elucidated (Ranson et al., 2000), data from other studies (Stump et al., 2004; Mathias et al., 2011) would suggest that the frequency was low at that time. Therefore, the increase in frequency of the 1014S kdr allele in western Kenya populations reported in a previous study (Mathias et al., 2011) and confirmed in the current study must be a relatively recent event that has escalated over the decade beginning in 2001.
Our results on An. arabiensis in populations at the Budalangi and Ahero locations indicate reduced sensitivity to pyrethroids, albeit not to the extent of that of An. gambiae s.s. at Bungoma. The operational significance of these observations is uncertain. Furthermore, there was no evidence for any kdr alleles in the An. arabiensis populations sampled here, which is consistent with our previous findings in western Kenya (Mathias et al., 2011). This contrasts with the findings of Kawada et al. (2011a), who reported a high frequency and wide distribution of 1014S in An. arabiensis. Furthermore, we found no evidence of elevated oxidase enzymes in An. arabiensis populations from Ahero, which had been covered by an IRS programme employing pyrethroid insecticides since July 2010 and which is close to the location of collections in December 2010. These findings differ from those of Kawada et al. (2011b), who, by contrast, observed a restoration of permethrin susceptibility when mosquitoes were exposed to the synergist piperonyl butoxide, inferring cytochrome P450 oxidases as the main mechanism. The discrepancies in these findings require further investigation.
In conclusion, the results of the present study demonstrate marked phenotypic resistance to permethrin in a population of An. gambiae s.s. from Bungoma, western Kenya. Elevated expression of β-esterase and oxidase enzymes and the presence of kdr alleles at the voltage-gated sodium channel locus suggest that both metabolic and target site resistance mechanisms underlie phenotypic resistance. By contrast, although phenotypic resistance to deltamethrin was also present in the same population, but at more moderate levels, there was no elevated expression of detoxifying enzymes, suggesting that resistance could be attributed to target-site mechanisms solely. Although populations of An. arabiensis showed moderate phenotypic resistance to permethrin and deltamethrin based upon WHO and bottle bioassays, these populations lacked kdr alleles and had no elevated enzyme expression; thus mechanisms of resistance in these populations are unknown and require further investigation.
Acknowledgements
We thank Claudia Corredor-Medina for training in the enzyme assays, Richard Amito for mosquito rearing, Joseph Nduati for expert laboratory assistance, and staff of the Entomology Section, Centre for Global Health Research, Kenya Medical Research Institute, Kisumu, Kenya, for assistance with field collections. This study was funded by National Science Foundation grant EF-072377 and National Institutes of Health grant AI058542.
References
- Bass C, Nikou D, Donnelly MJ, et al. Detection of knockdown resistance (kdr) mutations in Anopheles gambiae: a comparison of two new high-throughput assays with existing methods. Malaria Journal. 2007;6:111. doi: 10.1186/1475-2875-6-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayoh MN, Mathias DK, Odiere MR, et al. Anopheles gambiae: historical population decline associated with regional distribution of insecticide-treated bednets in western Nyanza Province, Kenya. Malaria Journal. 2010;9:62. doi: 10.1186/1475-2875-9-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brogdon WG, Barber AM. Microplate assay of glutathione S-transferase activity for resistance detection in single-mosquito homogenates. Comparative Biochemical Physiology. 1990;96B:339–342. doi: 10.1016/0305-0491(90)90385-7. [DOI] [PubMed] [Google Scholar]
- Brogdon W, McAllister J. Insecticide resistance and vector control. Emerging Infectious Diseases. 1998;4:605–613. doi: 10.3201/eid0404.980410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brogdon WG, Beach RF, Stewart JM, Castanaza L. Microplate assay analysis of the distribution of organophosphate and carbamate resistance in Guatemalan Anopheles albimanus. Bulletin of the World Health Organization. 1988;66:339–346. [PMC free article] [PubMed] [Google Scholar]
- Chen H, Githeko AK, Githure JI, Mutunga J, Zhou G, Yan G. Monooxygenase levels and knock-down resistance (kdr) allele frequencies in Anopheles gambiae and Anopheles arabiensis in Kenya. Journal of Medical Entomology. 2008;45:242–250. doi: 10.1603/0022-2585(2008)45[242:mlakrk]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouaibou M, Etang J, Brevault T, Nwane P, Hinzoumbe CK, Mimpfoundi R, Simard F. Dynamics of insecticide resistance in the malaria vector Anopheles gambiae s.l. from an area of extensive cotton cultivation in northern Cameroon. Tropical Medicine and International Health. 2008;13:476–486. doi: 10.1111/j.1365-3156.2008.02025.x. [DOI] [PubMed] [Google Scholar]
- Collins FH, Mendez ME, Rasmussen MO, Mehaffey PC, Bensansky NJ, Finnerty V. A ribosomal RNA gene probe differentiates member species of the Anopheles gambiae complex. American Journal of Tropical Medicine and Hygiene. 1987;1:37–41. doi: 10.4269/ajtmh.1987.37.37. [DOI] [PubMed] [Google Scholar]
- Diabate A, Baldet T, Chandre F, et al. The role of agricultural use of insecticides in resistance to pyrethroids in Anopheles gambiae s.l. in Burkina Faso. American Journal of Tropical Medicine and Hygiene. 2002;67:617–622. doi: 10.4269/ajtmh.2002.67.617. [DOI] [PubMed] [Google Scholar]
- Division of Malaria Control . 2007 Kenya MALARIA Indicator Survey. Ministry of Public Health and Sanitation; Nairobi: 2009. [Google Scholar]
- Division of Malaria Control . 2010 Kenya MALARIA Indicator Survey. Ministry of Public Health and Sanitation; Nairobi: 2011. [Google Scholar]
- Donnelly MJ, Corbel V, Weetman D, Wilding CS, Williamson MS, Black WC., IV Does kdr genotype predict insecticide resistance phenotype in mosquitoes? Trends in Parasitology. 2009;25:213–219. doi: 10.1016/j.pt.2009.02.007. [DOI] [PubMed] [Google Scholar]
- Fane M, Cissé O, Sékou C, Traorea F, Sabatier P. Anopheles gambiae resistance to pyrethroid-treated nets in cotton versus rice areas in Mali. Acta Tropica. 2012;122:1–6. doi: 10.1016/j.actatropica.2011.11.013. [DOI] [PubMed] [Google Scholar]
- Fegan GW, Noor AM, Akhwale WS, Cousens S, Snow RW. Effect of expanded insecticide-treated bednet coverage on child survival in rural Kenya: a longitudinal study. Lancet. 2007;370:1035–1039. doi: 10.1016/S0140-6736(07)61477-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimnig J, Vulule J, Lo T, et al. Impact of permethrin-treated bednets on entomologic indices in an area of intense year around malaria transmission. American Journal of Tropical Medicine and Hygiene. 2003;68:16–22. [PubMed] [Google Scholar]
- Hamel MJ, Adazu K, Obor D, et al. A reversal in reductions of child mortality in western Kenya, 2003–2009. American Journal of Tropical Medicine and Hygiene. 2011;85:597–605. doi: 10.4269/ajtmh.2011.10-0678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaetzold R, Schmidt H, Hornetz B, Shisanya C. Farm Management Handbook of Kenya, Vol. II. Natural Conditions and Farm Management Information. Ministry of Agriculture, Kenya, in Cooperation with the German Agency for Technical Cooperation (GTZ); Nairobi: 2005. [Google Scholar]
- Kamau L, Agai D, Matoke D, Wachira L, Gikandi G, Vulule JM. Status of insecticide susceptibility in Anopheles gambiae sensu lato and Anopheles funestus mosquitoes from western Kenya. Journal of Insect Science. 2007;8:11. doi: 10.1673/031.008.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawada H, Dida GO, Ohashi K, et al. Multimodal pyrethroid resistance in malaria vectors, Anopheles gambiae s.s., Anopheles arabiensis, and Anopheles funestus s.s. in western Kenya. PLoS ONE. 2011a;6:e22574. doi: 10.1371/journal.pone.0022574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawada H, Futami K, Komagata O, et al. Distribution of a knock-down resistance mutation (L1014S) in Anopheles gambiaes. s. and Anopheles arabiensis in western and southern Kenya. PLoS ONE. 2011b;6:e24323. doi: 10.1371/journal.pone.0024323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitau J, Oxborough RM, Tungu PK, et al. Species shifts in the Anopheles gambiae complex: do LLINs successfully control Anopheles arabiensis? PLoS ONE. 2012;7:e31481. doi: 10.1371/journal.pone.0031481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindblade KA, Gimnig JE, Kamau L, et al. Impact of sustained use of insecticide-treated bednets on malaria vector species distribution and culicine mosquitoes. Journal of Medical Entomology. 2006;43:428–432. doi: 10.1603/0022-2585(2006)043[0428:iosuoi]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Lines JD. Do agricultural insecticides select for insecticide resistance in mosquitoes? A look at the evidence. Parasitology Today. 1988;4(Suppl.):17–20. doi: 10.1016/0169-4758(88)90083-x. [DOI] [PubMed] [Google Scholar]
- Mambala F. Bunyala Rice Irrigation Scheme, Kenya: A Case Study of the Munaka Outgrowers Community Based Organization. Institute for Sustainable Commodities (ISCOM); Bunyala, Kenya: [08 Feb 2012]. 2007. pp. 3–4. http://www.iscom.nl/publicaties/bunyalacasestudy.pdf. [Google Scholar]
- Mathias D, Ochomo E, Atieli F, et al. Spatial and temporal variation in the kdr allele L1014S in Anopheles gambiae s.s. and phenotypic variability in susceptibility to insecticides in western Kenya. Malaria Journal. 2011;10:10. doi: 10.1186/1475-2875-10-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller P, Chouaïbou M, Pignatelli P, Etang J, Walker ED, Donnelly MJ, Simard F. Pyrethroid tolerance is associated with elevated expression of antioxidants and agricultural practice in Anopheles arabiensis sampled from an area of cotton fields in northern Cameroon. Molecular Ecology. 2008a;17:1145–1155. doi: 10.1111/j.1365-294X.2007.03617.x. [DOI] [PubMed] [Google Scholar]
- Noor AM, Kirui VC, Brooker SJ, Snow RW. The use of insecticide-treated nets by age: implications for universal coverage in Africa. BMC Public Health. 2009;9:369. doi: 10.1186/1471-2458-9-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okiro E, Alegana V, Noor A, Snow R. Changing malaria intervention coverage, transmission and hospitalization in Kenya. Malaria Journal. 2010;9:285. doi: 10.1186/1475-2875-9-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Meara WP, Bejon P, Mwangi TW. Effect of a fall in malaria transmission on morbidity and mortality in Kilifi, Kenya. Lancet. 2008;372:1555–1562. doi: 10.1016/S0140-6736(08)61655-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Meara WP, Mangeni JN, Steketee R, Greenwood B. Changes in the burden of malaria in sub-Saharan Africa. Lancet Infectious Diseases. 2010;10:545–555. doi: 10.1016/S1473-3099(10)70096-7. [DOI] [PubMed] [Google Scholar]
- Ramphul U, Boase T, Bass C, Okedi LM, Donnelly MJ, Müller P. Insecticide resistance and its association with target-site mutations in natural populations of Anopheles gambiae from eastern Uganda. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2009;103:1121–1126. doi: 10.1016/j.trstmh.2009.02.014. [DOI] [PubMed] [Google Scholar]
- Ranson H, Jensen B, Vulule J, Wang X, Hemingway J, Collins F. Identification of a point mutation in the voltage-gated sodium channel gene of Kenyan Anopheles gambiae associated with resistance to DDT and pyrethroids. Insect Molecular Biology. 2000;9:491–497. doi: 10.1046/j.1365-2583.2000.00209.x. [DOI] [PubMed] [Google Scholar]
- Ranson H, Abdallah H, Badolo A, et al. Insecticide resistance in Anopheles gambiae: data from the first year of a multi-country study highlighting the extent of the problem. Malaria Journal. 2009;8:299. doi: 10.1186/1475-2875-8-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranson H, N’Guessan R, Lines JD, Moiroux N, Nkuni Z, Corbel V. Pyrethroid resistance in African anopheline mosquitoes: what are the implications for malaria control? Trends in Parasitology. 2011;27:2. doi: 10.1016/j.pt.2010.08.004. [DOI] [PubMed] [Google Scholar]
- SAS . SAS 9.00. Help and Documentation. SAS Institute, Inc.; Cary, NC: 2000. [Google Scholar]
- Scott JA, Brogdon WG, Collins FH. Identification of single specimens of the Anopheles gambiae complex by polymerase chain reaction. American Journal of Tropical Medicine and Hygiene. 1993;49:520–529. doi: 10.4269/ajtmh.1993.49.520. [DOI] [PubMed] [Google Scholar]
- Stump A, Atieli F, Vulule J, Besansky N. Dynamics of the pyrethroid knock-down resistance allele in western Kenyan populations of Anopheles gambiae in response to insecticide-treated bednet trials. American Journal of Tropical Medicine and Hygiene. 2004;70:591–596. [PubMed] [Google Scholar]
- Thairu NK. Graduate School of Development Studies, Masters of Arts in Development Studies, Specialization: Economics of Development (ECD) International Institute of Social Studies; The Hague: 2010. Agricultural production and irrigation management: the case of irrigated rice production in Kenya; pp. 20–28. [Google Scholar]
- Verhaeghen K, Bortel WV, Roelants P, Okello PE, Talisuna A, Coosemans M. Spatio-temporal patterns in kdr frequency in permethrin and DDT resistant Anopheles gambiae s.s. from Uganda. American Journal of Tropical Medicine and Hygiene. 2010;82:566–573. doi: 10.4269/ajtmh.2010.08-0668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vulule J, Beach R, Atieli F, Roberts J, Mount D, Mwangi R. Reduced susceptibility of Anopheles gambiae to permethrin associated with the use of permethrin-impregnated bednets and curtains in Kenya. Medical and Veterinary Entomology. 1994;8:71–75. doi: 10.1111/j.1365-2915.1994.tb00389.x. [DOI] [PubMed] [Google Scholar]
- Vulule JM, Beach RF, Atieli FK, Mount DL, Roberts JM, Mwangi RW. Longterm use of permethrin-impregnated nets does not increase Anopheles gambiae permethrin tolerance. Medical and Veterinary Entomology. 1996;10:71–79. doi: 10.1111/j.1365-2915.1996.tb00084.x. [DOI] [PubMed] [Google Scholar]
- Vulule JM, Beach RF, Atieli FK, McAllister JC, Brogdon WG, Roberts JM. Elevated oxidase and esterase levels associated with permethrin tolerance in Anopheles gambiae from Kenyan villages using permethrin-impregnated nets. Medical and Veterinary Entomology. 1999;13:239–244. doi: 10.1046/j.1365-2915.1999.00177.x. [DOI] [PubMed] [Google Scholar]
- World Health Organization . Vector Resistance to Insecticides, WHO Technical Report Series. WHO; Geneva: 1992. [Google Scholar]
- World Health Organization . Test Procedures for Insecticide Resistance Monitoring in Malaria Vectors: Bio-Efficacy and Persistence of Insecticides on Treated Surfaces. WHO; Geneva: 1998. [Google Scholar]
- World Health Organization . World Malaria Report. WHO; Geneva: 2011. [Google Scholar]
- Zhou G, Afrane YA, Vardo-Zalik AM, et al. Changing patterns of malaria epidemiology between 2002 and 2010 in western Kenya: the fall and rise of malaria. PLoS ONE. 2011;6:e20318. doi: 10.1371/journal.pone.0020318. [DOI] [PMC free article] [PubMed] [Google Scholar]