Abstract
Background
Diabetes in neonates usually has a monogenic etiology; however, the cause remains unknown in 20–30%. Heterozygous INS mutations represent one of the most common gene causes of neonatal diabetes mellitus.
Methods
Clinical and functional characterization of a novel homozygous intronic mutation (c.187+241G>A) in the insulin gene in a child identified through the Monogenic Diabetes Registry (http://monogenicdiabetes.uchicago.edu).
Results
The proband had insulin-requiring diabetes from birth. Ultrasonography revealed a structurally normal pancreas and C-peptide was undetectable despite readily detectable amylin, suggesting the presence of dysfunctional beta cells. Whole exome sequencing revealed the novel mutation. In silico analysis predicted a mutant mRNA product resulting from preferential recognition of a newly created splice site.
Wild-type and mutant human insulin gene constructs were derived and transiently expressed in INS-1 cells. We confirmed the predicted transcript and found an additional transcript created via an ectopic splice acceptor site.
Conclusion
Dominant INS mutations cause diabetes via a mutated translational product causing ER stress. We describe a novel mechanism of diabetes, without beta cell death, due to creation of two unstable mutant transcripts predicted to undergo nonsense and non-stop mediated decay respectively. Our discovery may have broader implications for those with insulin deficiency later in life.
Keywords: INS, Insulin gene, Neonatal Diabetes, Monogenic Diabetes
Introduction
Diabetes mellitus in neonates nearly always has a monogenic etiology, as autoimmune mediated type 1 diabetes is rarely seen before 6 months of age 1. Causal variations in the coding regions and splice sites of over 20 genes have been described. INS mutations are one of the most common causes of neonatal diabetes mellitus 2. Heterozygous dominant, compound heterozygous, and homozygous recessive mutations within the gene have been described leading to hyperglycemia, hyperinsulinemia or hyperproinsulinemia 3. Dominant mutations are thought to cause diabetes by the creation of a translated product that induces the unfolded protein response, leading to ER stress and ultimately beta cell apoptosis 4,5. Those few mutations associated with hyperinsulinemia or hyperproinsulinemia are typically not associated with dysglycemia 3.
The underlying genetic cause remains unknown in 20–30% of neonatal diabetes cases 6,7 and the University of Chicago Monogenic Diabetes Registry was established to identify novel etiological genetic mechanisms in families with suspected monogenic diabetes, in addition to following families with known forms of monogenic diabetes. Here we outline the identification of a deep intronic INS mutation causing diabetes through a novel mechanism.
Methods
Subjects
Subjects with diabetes diagnosed before one year of age, as well as family members, consented for participation through the University of Chicago Monogenic Diabetes Registry (http://monogenicdiabetes.uchicago.edu) 8. All subjects were consented for participation through protocols approved by the Institutional Review Board at the University of Chicago.
Purification of genomic DNA and sequencing analysis
Genomic DNA was extracted from peripheral leukocytes using standard methods. The proband had targeted Sanger sequencing of the exons, introns and promoter regions of candidate genes (INS, KCNJ11, ABCC8, EIF2AK3, PDX1) and was subsequently sequenced using a targeted-enrichment next generation sequencing (NGS) approach based on HaloPlex enrichment (Agilent Technologies, Santa Clara, CA, USA) followed by MiSeq Illumina NGS, and an in-house pipeline to call sequence variants 9. The VCHrome capture reagent (Roche NimbleGen, Madison, WI, USA; http://www.nimblegen.com) was used to target the exome that was then sequenced using a HiSeq 2000 instrument (Illumina, San Diego, CA, USA; http://www.illumina.com). Read mapping was performed using Burrows-Wheeler aligner 10, and variants were called using Atlas2 11. By design, at least 80% of the exome was covered 20x. Analysis was performed using Ingenuity® Variant Analysis™ software (QIAGEN, Redwood City, CA; www.qiagen.com/ingenuity). Variants with a minor allele frequency >3% in 1000 Genomes (phase 1 data released April 4, 2012 v3 NHLBI, ESP6500 data released June 2012) were removed. We further considered variants that were missense, nonsynonymous or annotated to a splice junction. To further prioritize variants, we restricted analyses to known monogenic diabetes genes and genes known to interact with the products of these genes, with exclusion of highly variable genes.
Human genomic DNA constructs
Using a 1,431 base pair fragment of genomic DNA cloned from heterozygous carrier of the INS mutation (c.187+241G>A), wild-type and mutant human insulin gene constructs were created containing the exon 1, 2 and 3 and both introns by PCR using targeting primers with additional restriction enzyme sites. After constructs (wild type and mutant) were confirmed by direct DNA sequencing they were subcloned into pcDNA3.1 vector.
Lymphoblastoid and INS-1 cell lines
We created Epstein-Barr virus immortalizes human B lymphocytes using published protocols 12. Rat insulinoma INS-1 cells (passages 70–74), were grown in 5% CO2 at 37°C in RPMI 1640 supplemented with 10 mM HEPES, 2 mM L-glutamine, 1 mM sodium pyruvate, 0.05 mM beta-mercaptoethanol, 10% FBS, 100 IU/ml penicillin and 100 μg/ml streptomycin. 1 × 106 INS-1 cells in 6 well plates were transfected with 4 μg of pcDNA3.1 wild-type (WT) or c.187+241G>A INS gene constructs using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). Total RNA was isolated from 24 hours post-transfection cells using Trizol (Invitrogen) and cDNA synthesis was performed using oligo dT-primer and Superscript® III First-Strand Synthesis System (Invitrogen).
Results
The proband was born at 36 weeks gestation after induction for concern about severe intrauterine growth retardation and was small for gestational age with a birth weight of 1740 g (<1st percentile; −2.6 SD) and length of 46 cm (5th percentile), despite maternal gestational diabetes requiring insulin therapy throughout the 3rd trimester. Severe hyperglycemia was noted from the first day of life and he has required subcutaneous insulin since. Ultrasonography revealed a structurally normal pancreas but C-peptide (which reflects endogenous insulin production) was undetectable. Further assessments of endogenous insulin production were repeatedly undetectable in the fasting or post-prandial states, as well as in response to an empiric trial of glyburide using a modified published protocol (maximum dose of 1 mg/kg/day) 13.
The proband was reassessed again at 3.4 years of age and despite his low birth weight, he had exhibited excellent post-natal catch-up growth, such that he had grown steadily between the 10th and 25th percentiles since approximately one year of age, with a weight of 13.9 kg (23rd percentile) and height of 93 cm (10th percentile). In order to stimulate any possible endogenous beta cell function, his insulin pump therapy was suspended and blood samples were drawn 30 minutes following a meal. Amylin is co-secreted with C-peptide and insulin by the human beta cell with a linear correlation between plasma levels of each of these beta cell products. Amylin levels are virtually absent in individuals with type 1 diabetes mellitus 14. Although his C-peptide was again undetectable (<0.033 pmol/L) despite an elevated glucose level of 368 mg/dL, his amylin level was readily detectable (10.06 pmol/L). This contrasted to C-peptide and amylin of 0.58 pmol/l and 5.52 pmol/l with a glucose of 110 mg/dl in his mother (heterozygous) and a C-peptide and amylin of 0.62 pmol/l and 11.85 pmol/l with a glucose of 92 mg/dl in a control subject. These data suggest that despite the absence of endogenous insulin and circulating C-peptide, viable beta cells were able to manufacture, package and release significant amounts of amylin. Antibodies associated with type 1 diabetes were undetectable (Anti-GAD, Anti-IA2, Anti-Insulin and Anti-ZnT8).
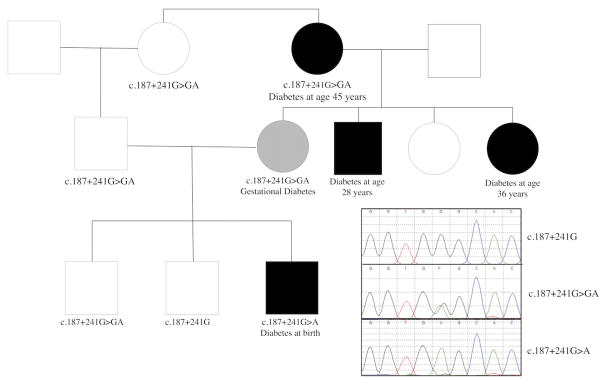
The proband and parents are of South East Asian origin. Any potential consanguinity was initially not reported but the parents subsequently acknowledged that they are first cousins. In addition to the mother’s gestational diabetes, others in the family also have a history of non-obese diabetes, all with BMIs less than 25 kg/m2 (Figure 1).
Figure 1. Pedigree of family and sequence traces of wild-type, heterozygous and homozygous carriers of the novel INS intron 2 mutation.
Closed and shaded symbols represent individuals with diabetes and gestational diabetes respectively.
After Sanger sequencing, performed soon after clinical diagnosis with diabetes, failed to reveal coding or splice site mutations within the most common known neonatal diabetes associated genes, the proband was further tested and found to be negative for mutations in any of 36 genes included in our NGS monogenic diabetes gene panel 9. Whole exome sequencing (WES) was performed and initial review did not reveal any potentially pathogenic variants. However, given the recent description of the heterozygous INS gene c.188-31G>A intronic variant leading to neonatal diabetes 15, we examined the low coverage intronic regions of genes associated with neonatal diabetes. A novel homozygous deep intronic INS variant was fortuitously found in the proband (c.187+241G>A). This region was not captured by WES in the parental samples but both were subsequently found to be heterozygous for the variant by targeted sequencing. The heterozygous mother required insulin to treat gestational diabetes during all three of her pregnancies. The proband’s maternal grandmother is also heterozygous for the variant and developed insulin requiring diabetes mellitus at 45 years of age. The proband has two healthy brothers, one of whom is heterozygous for the mutation and one who does not carry the mutation. Other family members were not available to provide DNA but a maternal aunt and uncle were diagnosed with insulin requiring diabetes mellitus at 28 and 36 years of age respectively.
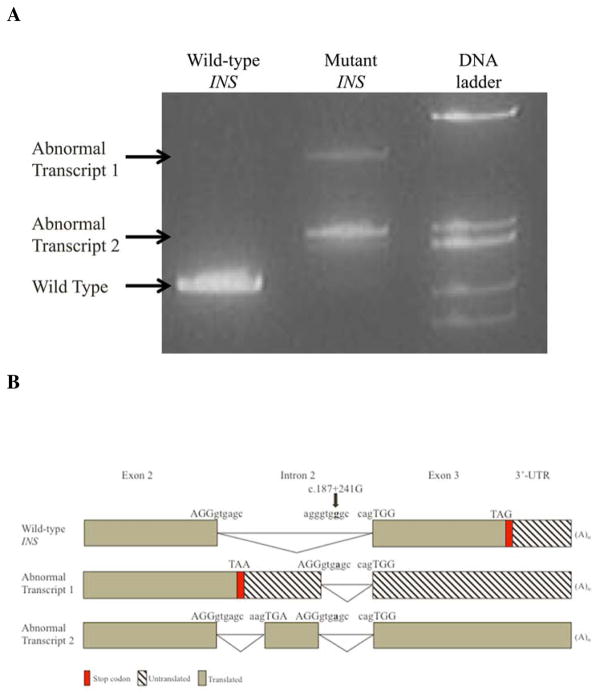
The novel c.187+241G>A mutation creates a donor 5′ splice site. In silico analysis to predict the mutant mRNA product suggests the splicing machinery will preferentially recognize the new splice site and read 237 bp of intron 2 as exonic sequence (Supplementary Figure 1A). This proposed truncated 482 bp mRNA product (Abnormal Transcript 1, Figure 2) is predicted to undergo nonsense-mediated mRNA decay triggered by a premature termination codon.
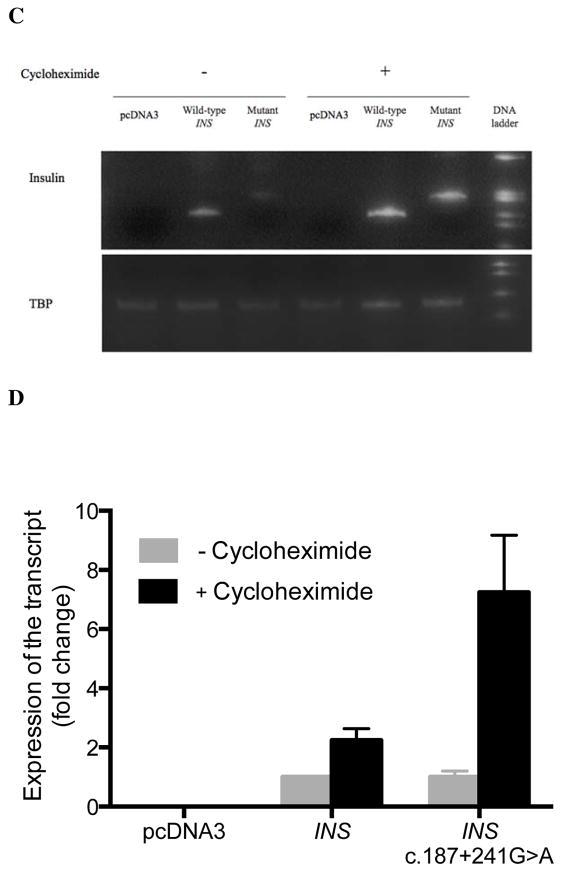
Figure 2. Wild-type and mutant insulin gene expression in INS-1 cells.
(A) RT-PCR of RNA isolated from INS-1 cells 24 hours post-transfection with wild-type and mutant INS gene constructs. Results shown are representative of three independent experiments. (B) A schematic diagram showing the pattern of alternative splicing that generates the aberrant transcripts from the mutant INS gene. (C) INS-1 cells were transfected with pcDNA3, wild-type INS and mutant INS (c.187+241G>A) gene constructs. RT-PCR was used to measure expression of human insulin with 30 cycles of PCR and TBP mRNA following treatment with 100 μM of cycloheximide for 3 hours that was started 24 hours post-transfection. (D) The level of mRNA of wild-type INS and mutant INS prior to cycloheximide treatment was normalized to a value of 1. The fold change in expression following treatment is shown. The mean ± SEM from three independent experiments is shown.
Abbreviations: ER, endoplasmic reticulum; NGS, next generation sequencing; RT-PCR, real-time polymerase chain reaction; SD, standard deviation; TBP, TATA-binding protein; WT, wild-type.
We sought to identify any transcriptional products resulting from this novel intronic mutation. No insulin RT-PCR product was detected from the proband’s lymphoblastoid cell line or blood. Wild type insulin was found in both the control and mother. An additional product (Abnormal Transcript 1), matching the size of in silico predicted mutant mRNA, was weakly detected in the maternal blood sample (Supplementary Figure 1B).
We assessed the expression of transcripts derived from wild-type and mutant human insulin gene constructs containing all three exons and both introns transfected into INS-1cells (Figure 2). RT-PCR of the mutant construct revealed two new transcripts without the wild-type transcript. Sequencing confirmed Abnormal Transcript 1 to be the predicted alternatively-spliced transcript. We also detected an additional transcript without a stop codon resulting from insertion of a 79 nucleotide pseudoexon following exon 2 through use of a native potential 3′ acceptor site (Abnormal Transcript 2, Figure 2). There is no evidence that this cryptic ectopic acceptor site is used in the control. We also examined insulin gene expression in cycloheximide treated INS-1 cells and found a 7.25±0.21 relative fold increase in Abnormal Transcript 2 while Abnormal Transcript 1 was not visible following cycloheximide treatment (Figure 2).
These data supports the prediction that Abnormal Transcript 2 likely undergoes non-stop mediated decay rather than the rapid nonsense mediated decay predicted for Abnormal Transcript 1 16.
Discussion
Although rapid advancements over the last decade have led to the discovery of mutations in over 20 genes as causing neonatal forms of monogenic diabetes, the genetic etiology is not found in 20–30% of cases 9. Pathogenic intronic mutations are typically within or adjacent to conserved splice sites and are readily detected through Sanger sequencing of PCR amplicons targeting exons. Deeper intronic mutations may be missed through traditional techniques as demonstrated by the previously reported intronic point mutation in ABCC8 which also creates of a pseudo-exon 17. Our study highlights the possibility that mutations within even the deepest stretches of the non-coding regions may be the next frontier for explaining the molecular pathophysiology of heritable diseases.
Mutations in the INS gene are one of the most common causes of neonatal diabetes. Most other insulin gene mutations, including the only other known intronic mutation (heterozygous c.188-31G>A), lead to misfolded proteins that increase ER stress and beta cell death 5,15. Deletions and recessive mutations that lead to reduced insulin biosynthesis have also been described as rare causes of neonatal diabetes 18,19. We describe the first deep intronic homozygous mutation leading to altered splicing and creation of two unstable mutant transcripts. This unusual homozygous mutation causes failure of any translated INS product in otherwise viable beta cells, it provides an ideal system to study the role of endogenous insulin production in the development, function and maintenance of beta cells and other islet cell types. Utilization of this mutation represents an excellent model for examining the degradation of mRNAs with premature stop codons and those that lack a stop codon.
The prospect of advances in antisense gene therapy in combination with transplantation of insulin-producing pancreatic beta cells from stem cells in vitro offer hope for novel therapeutic interventions for individuals with INS mutations and other monogenic forms of diabetes 20. The frequency of deep intronic mutations in diabetes related genes remains unknown but this study and others demonstrate that intronic mutations may represent the etiology for neonatal diabetes cases without an established diagnosis 15,17.
Conclusion
We describe a novel mechanism for diabetes due to a deep intronic mutation leading to both a cryptic donor and acceptor site within the insulin gene, predicted to undergo nonsense and non-stop mediated decay respectively. Haplo-insufficiency of insulin has been suggested to contribute to the risk for diabetes in heterozygous individuals 19. Thus, our discovery extends the current understanding of how diabetes occurring in neonates may have broader implications for those with insulin deficiency leading to diabetes later in life.
Supplementary Material
Acknowledgments
Funding
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health [DK020595, UL1TR000430 and K23 DK094866]; the American Diabetes Association [1-11-CT-41]; and gifts from the Kovler Family Foundation.
This work was supported by the University of Chicago Diabetes Research and Training Center (DRTC), and our Clinical and Translational Research Award (The Institute for Translational Medicine) which along with Dr. Greeley’s K Award, was funded by grants from the National Institutes of Health (DK020595, UL1TR000430 and K23DK094866), as well as by a grant from the American Diabetes Association (1-11-CT-41), and gifts from the Kovler Family Foundation. We would like to acknowledge the support of Dr. Christine Billstrand with the development of viral transformation of lymphoblastoid cell lines. We are most grateful to the family for their continued participation in this study.
Footnotes
Conflict of Interest Statement
The authors report no conflicts of interest.
Contribution Statement
DC and SYP drafted the initial manuscript, coordinated and supervised data collection, analyzed data, performed the functional studies and interpreted results. HY, GAA, HMH, CLH performed the sequencing analyses. MEP, LHP, GIB and SAWG collected data, helped with study design and interpretation of results. All authors reviewed and revised the manuscript and approved the final manuscript as submitted. SAWG wrote the manuscript, conceptualized and designed the study and is the guarantor of this work
References
- 1.Greeley SAW, Naylor RN, Philipson LH, Bell GI. Neonatal diabetes: an expanding list of genes allows for improved diagnosis and treatment. Curr Diab Rep. 2011;11:519–32. doi: 10.1007/s11892-011-0234-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Støy J, Edghill EL, Flanagan SE, Ye H, Paz VP, Pluzhnikov A, Below JE, Hayes MG, Cox NJ, Lipkind GM, Lipton RB, Greeley SAW, Patch A-M, Ellard S, Steiner DF, Hattersley AT, Philipson LH, Bell GI Neonatal Diabetes International Collaborative Group. Insulin gene mutations as a cause of permanent neonatal diabetes. Proc Natl Acad Sci USA. 2007;104:15040–4. doi: 10.1073/pnas.0707291104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Støy J, Steiner DF, Park S-Y, Ye H, Philipson LH, Bell GI. Clinical and molecular genetics of neonatal diabetes due to mutations in the insulin gene. Rev Endocr Metab Disord. 2010;11:205–15. doi: 10.1007/s11154-010-9151-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colombo C, Porzio O, Liu M, Massa O, Vasta M, Salardi S, Beccaria L, Monciotti C, Toni S, Pedersen O, Hansen T, Federici L, Pesavento R, Cadario F, Federici G, Ghirri P, Arvan P, Iafusco D, Barbetti F Early Onset Diabetes Study Group of the Italian Society of Pediatric Endocrinology and Diabetes (SIEDP) Seven mutations in the human insulin gene linked to permanent neonatal/infancy-onset diabetes mellitus. J Clin Invest. 2008;118:2148–56. doi: 10.1172/JCI33777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park S-Y, Ye H, Steiner DF, Bell GI. Mutant proinsulin proteins associated with neonatal diabetes are retained in the endoplasmic reticulum and not efficiently secreted. Biochem Biophys Res Commun. 2010;391:1449–54. doi: 10.1016/j.bbrc.2009.12.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carmody D, Bell CD, Hwang JL, Dickens JT, Sima DI, Felipe DL, Zimmer CA, Davis AO, Kotlyarevska K, Naylor RN, Philipson LH, Greeley SAW. Sulfonylurea Treatment Before Genetic Testing in Neonatal Diabetes: Pros and Cons. J Clin Endocrinol Metab. 2014;99 doi: 10.1210/jc.2014-2494. jc20142494–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edghill EL, Flanagan SE, Ellard S. Permanent neonatal diabetes due to activating mutations in ABCC8 and KCNJ11. Rev Endocr Metab Disord. 2010;11:193–8. doi: 10.1007/s11154-010-9149-x. [DOI] [PubMed] [Google Scholar]
- 8.Greeley SAW, Naylor RN, Cook LS, Tucker SE, Lipton RB, Philipson LH. Creation of the Web-based University of Chicago Monogenic Diabetes Registry: using technology to facilitate longitudinal study of rare subtypes of diabetes. J Diabetes Sci Technol. 2011;5:879–86. doi: 10.1177/193229681100500409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alkorta-Aranburu G, Carmody D, Cheng YW, Nelakuditi V, Ma L, Dickens JT, Das S, Greeley SAW, del Gaudio D. Phenotypic heterogeneity in monogenic diabetes: The clinical and diagnostic utility of a gene panel-based next-generation sequencing approach. Mol Genet Metab. 2014;113:315–20. doi: 10.1016/j.ymgme.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–60. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Challis D, Yu J, Evani US, Jackson AR, Paithankar S, Coarfa C, Milosavljevic A, Gibbs RA, Yu F. An integrative variant analysis suite for whole exome next-generation sequencing data. BMC Bioinformatics. 2012;13:8. doi: 10.1186/1471-2105-13-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ling PD, Huls HM. Isolation and immortalization of lymphocytes. Curr Protoc Mol Biol. 2005;Chapter 28(Unit28.2) doi: 10.1002/0471142727.mb2802s70. [DOI] [PubMed] [Google Scholar]
- 13.Pearson ER, Flechtner I, Njølstad PR, Malecki MT, Flanagan SE, Larkin B, Ashcroft FM, Klimes I, Codner E, Iotova V, Slingerland AS, Shield J, Robert J-J, Holst JJ, Clark PM, Ellard S, Søvik O, Polak M, Hattersley AT Neonatal Diabetes International Collaborative Group. Switching from insulin to oral sulfonylureas in patients with diabetes due to Kir6.2 mutations. N Engl J Med. 2006;355:467–77. doi: 10.1056/NEJMoa061759. [DOI] [PubMed] [Google Scholar]
- 14.Kruger DF, Gloster MA. Pramlintide for the treatment of insulin-requiring diabetes mellitus: rationale and review of clinical data. Drugs. 2004;64:1419–32. doi: 10.2165/00003495-200464130-00003. [DOI] [PubMed] [Google Scholar]
- 15.Garin I, Pérez de Nanclares G, Gastaldo E, Harries LW, Rubio-Cabezas O, Castaño L. Permanent neonatal diabetes caused by creation of an ectopic splice site within the INS gene. PLoS ONE. 2012;7:e29205. doi: 10.1371/journal.pone.0029205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klauer AA, van Hoof A. Degradation of mRNAs that lack a stop codon: a decade of nonstop progress. Wiley Interdiscip Rev RNA. 2012;3:649–60. doi: 10.1002/wrna.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flanagan SE, Xie W, Caswell R, Damhuis A, Vianey-Saban C, Akcay T, Darendeliler F, Bas F, Guven A, Siklar Z, Ocal G, Berberoglu M, Murphy N, O’Sullivan M, Green A, Clayton PE, Banerjee I, Clayton PT, Hussain K, Weedon MN, Ellard S. Next-generation sequencing reveals deep intronic cryptic ABCC8 and HADH splicing founder mutations causing hyperinsulinism by pseudoexon activation. Am J Hum Genet. 2013;92:131–6. doi: 10.1016/j.ajhg.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garin I, Edghill EL, Akerman I, Rubio-Cabezas O, Rica I, Locke JM, Maestro MA, Alshaikh A, Bundak R, del Castillo G, Deeb A, Deiss D, Fernandez JM, Godbole K, Hussain K, O’Connell M, Klupa T, Kolouskova S, Mohsin F, Perlman K, Sumnik Z, Rial JM, Ugarte E, Vasanthi T, Johnstone K, Flanagan SE, Martínez R, Castaño C, Patch A-M, Fernández-Rebollo E, Raile K, Morgan N, Harries LW, Castaño L, Ellard S, Ferrer J, Pérez de Nanclares G, Hattersley AT Neonatal Diabetes International Group. Recessive mutations in the INS gene result in neonatal diabetes through reduced insulin biosynthesis. Proc Natl Acad Sci USA. 2010;107:3105–10. doi: 10.1073/pnas.0910533107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raile K, O’Connell M, Galler A, Werther G, Kühnen P, Krude H, Blankenstein O. Diabetes caused by insulin gene (INS) deletion: clinical characteristics of homozygous and heterozygous individuals. Eur J Endocrinol. 2011;165:255–60. doi: 10.1530/EJE-11-0208. [DOI] [PubMed] [Google Scholar]
- 20.Pagliuca FW, Millman JR, Gürtler M, Segel M, Van Dervort A, Ryu JH, Peterson QP, Greiner D, Melton DA. Generation of Functional Human Pancreatic _ Cells In Vitro. Cell. 2014;159:428–39. doi: 10.1016/j.cell.2014.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.