Abstract
Background
mTOR inhibitors are now approved by regulatory agencies for the treatment of a variety of malignancies. The risk of metabolic complications with these agents is not well characterized.
Methods
PubMed was searched for articles published from 2001 until 2011. Eligible studies included prospective randomized trials evaluating temsirolimus, everolimus, and ridaforolimus in patients with all solid tumor malignancies. Sixteen eligible phase II clinical trials and 8 randomized controlled clinical trials were included in a systematic review and meta-analysis and the number of metabolic related AEs (hyperglycemia, hypercholesterolemia, and hypertriglyceridemia) was extracted. Incidence rates and incident rate ratios were calculated.
Findings
Twenty-four trials, including 4261 patients, were included in the calculation of the incidence rate. The average incidence rate of all grade metabolic related events was 0.70 (95% CI, 0.47, 0.93). The average incidence rate of serious (grade 3 and 4) metabolic related adverse events was 0.11 (95% CI, 0.08, 0.15). The incidence rate ratio (IRR) of a metabolic adverse event with mTOR inhibitor therapy compared with control was 2.93 (95% CI, 2.33, 3.70) and of serious grade 3 and 4 metabolic adverse events was 4.58 (95% CI, 2.86, 7.34). The IRR of all grade hyperglycemia was 2.95 (95% CI, 2.14, 4.05) and of grade 3–4 hyperglycemia was 5.25 (95% CI, 3.07, 9.00). The IRR of all grade hypertriglyceridemia was 2.49 (95% CI, 1.76, 3.52) and of grade 3–4 hypertriglyceridemia was 2.01 (95% CI, 0.65, 6.27). The IRR of all grade hypercholesterolemia was 3.35 (95% CI, 2.17, 5.18) and of grade 3–4 hypercholesterolemia was 6.51 (95% CI, 1.48, 28.59). These findings suggest a statistically significant increase in the risk of hyperglycemia, hypercholesterolemia (all grades and grade 3 and 4), and all grade hypertriglyceridemia associated with mTOR therapy when compared with control.
Interpretation
The risk of all grade and grade 3–4, hyperglycemia, hypercholesterolemia, and hypertriglyceridemia, are increase in patients treated with mTOR inhibitors compared with control.
Keywords: Cancer, mTOR inhibitor, Metabolic, Temsirolimus, Everolimus, Ridaforolimus, Adverse events
Introduction
As our understanding of tumor biology has become more sophisticated, cancer therapeutics have shifted from traditional cytotoxic chemotherapy towards molecularly targeted agents. This shift has not only been accompanied by differences in mode of administration (e.g., frequently oral) and mechanisms of anticancer activity (e.g., frequently cytostatic) but also by a marked change in adverse event profiles. Though side effects of anticancer therapy were once dominated by transient myelosuppresion and nausea/vomiting, this new generation of therapies has generally been characterized by more chronic toxicities affecting a diverse range of organ systems. The team of specialists required to support patients being maintained on such therapies has expanded beyond oncologists, to include dermatologists, endocrinologists, pulmonologists, cardiologists, and others. An understanding of the likelihood, and severity, of particular side effects accompanying each new class of agent is therefore critical to optimizing patient management.
The mammalian target of rapamycin (mTOR) pathway is vital to cell processes such as proliferation, cell growth, metabolism, and angiogenesis.1,2 This critical role prompted the development and exploration of several pharmacologic inhibitors of the mTOR pathway for cancer therapy.3–23 Currently, two mTOR inhibitors have been approved for the treatment of cancer by the United States Food and Drug Administration (FDA). Temsirolimus has been approved for treatment of advanced renal cell carcinoma (RCC) while everolimus has been approved for RCC, pancreatic neuroendocrine tumors (PNET), and for subependymal giant cell astrocytoma associated with tuberous sclerosis. Ridaforolimus, although not yet FDA approved, is currently in phase III clinical trials.
The incidence of most cancers increases with age and risk factors for certain cancers (e.g., smoking) are also associated with an increased risk of other chronic illnesses. As a result, many patients with cancer have comorbidities including diabetes and cardiovascular disease. Metabolic toxicities have emerged as a common, and unique, side effect of mTOR inhibitors. Given the potential for these side effects to influence the comorbidities and general health of patients treated with mTOR inhibitors, we conducted a systematic review and meta-analysis of all published RCTs to characterize the incidence and risk of metabolic complications with mTOR inhibitors.
Methods
Data source
Study selection was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.24 An independent review of citations from PubMed published from January 1, 1997 until May 30, 2011 was conducted. Keywords included in the search were: temsirolimus, everolimus, and ridaforolimus. The search was limited to articles published in the English language. Abstracts and presentations containing the terms temsirolimus, everolimus, and ridaforolimus from the American Society of Clinical Oncology (www.ASCO.org) held between January 1997 and May 30, 2011 also were searched to identify relevant clinical trials; however, only trials published in peer-reviewed publications, in full manuscript form, or phase III trials with adequate adverse event reporting were included. Each publication was reviewed and in cases of duplicate publication only the most complete, recent, and updated report of the clinical trial was included in the meta-analysis.
Study selection
The primary objectives of this study were to evaluate the incidence of metabolic side effects (hyperglycemia, hypercholesterolemia, and hypertriglyceridemia) with mTOR inhibitors and the association between treatment with mTOR inhibitors and the development of such side effects. For incidence calculations, clinical trials that met the following criteria were included: (1) phase II and III trials of patients with solid tumors, (2) treatment with an mTOR inhibitor, (3) available data on metabolic side effects. For incidence rate ratio calculations, the selection criteria were the same but only trials that included a random assignment of participants to treatment with an mTOR inhibitor versus control (standard of care, placebo, or best supportive care) were included. Trials with combination therapy, which included an mTOR inhibitor as a component of the treatment regimen, were also included unless combined with a cytotoxic agent. For trials in which there were multiple arms, we pooled the adverse events for the arms that contained the mTOR inhibitor as long as the dosing schedule was the same.
Data extraction and clinical end point
We extracted data on study characteristics, treatment information, and follow-up. The primary end points of the analysis were all grade and severe hyperglycemia, all grade and severe hypercholesterolemia, all grade and severe hypertriglyceridemia, and total and severe “metabolic side effects” which was a composite of all three categories. Adverse events were defined as per the National Cancer Institute's Common Terminology Criteria for Adverse Events (CTCAE) criteria versions 2.0 and 3.0. Data extraction was performed independently by two authors (B.G., S.S.) who agreed on 99% of the observations. The sample size, number of all grade metabolic adverse events, adverse event type, and patient characteristics were recorded and most frequently the articles reported the worst grade per patient. Any discrepancies between reviewers were resolved by consensus. In cases where there was a crossover design, only data available from before the crossover was used.
Statistical analysis
Meta-analysis using a random effects model was performed as described25 to assess the incidence rate in mTOR inhibitor treatment group and incidence rate ratio between mTOR inhibitor treatment group and placebo treatment group. It was assumed that the event number X follows a Poisson distribution. The variance of the incidence rate X/N is X/N2, where N is patient number. Publication bias was assessed by Egger's regression test using sample size and standard error as predictors for incidence rate and incidence rate ratio respr sample size as the predictor respectively.26 All analysis was performed using R package metafor.27
Results
Search results
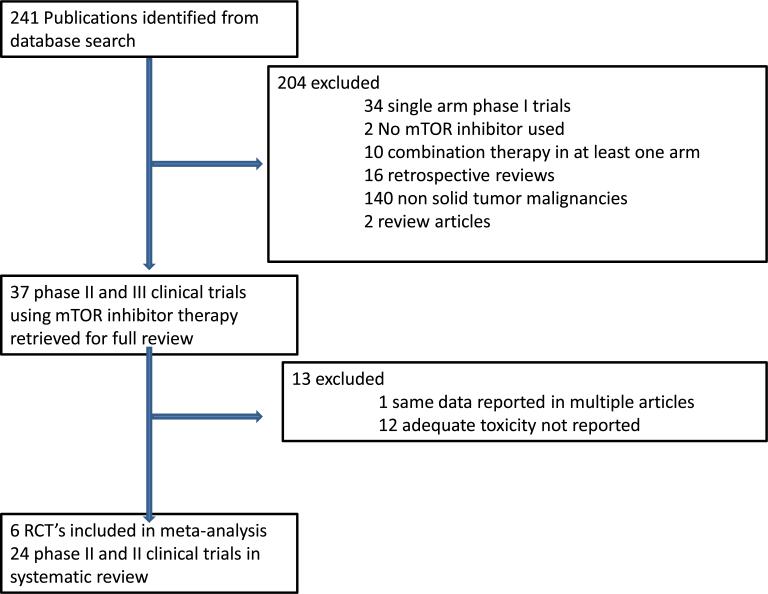
A literature search produced 243 potentially relevant human clinical studies evaluating temsirolimus, everolimus or ridaforolimus. Studies that were excluded from the final analysis, and the reasons for exclusion, are shown in Fig. 1. Twenty-four trials, including 4261 patients, were included in the systematic review for the incidence calculations.3–23,28–30 The characteristics of these 24 trials are listed in Table 1. Six trials, including 3125 patients, met the pre-specified criteria and were included in the meta-analysis for the incidence rate ratio calculations.16–20,30
Fig. 1.
Selection of phase 2 and 3 clinical trials included in the systematic review and selection of randomized controlled clinical trials (RCT's) for meta-analysis.
Table 1.
Trials included in the systematic review and meta-analysis.
| Source | Phase | # Participants treatment arm (mTOR) | # Participants control arm | Malignancy | MTOR inhibitor | Control arm | Jadad score |
|---|---|---|---|---|---|---|---|
| Okuno et al.3 | 2 | 40 | N/A | Advanced soft tissue sarcoma | Temsirolimus | None | N/A |
| Chawla et al.4 | 2 | 212 | N/A | Metastatic sarcoma | Ridaforolimus | None | N/A |
| Duran et al.5 | 2 | 36 | N/A | Advanced neuroendocrine carcinoma | Temsirolimus | None | N/A |
| Chang et al.6 | 2 | 43 | N/A | Recurrent glioblastoma multiforme | Temsirolimus | None | N/A |
| Galanis et al.7 | 2 | 65 | N/A | Recurrent glioblastoma multiforme | Temsirolimus | None | N/A |
| Atkins et al.23 | 2 | Arm 1:36 Arm 2: 38 Arm 3: 36 |
N/A | Advanced refractory renal cell carcinoma | Temsirolimus | Temsirolimus | N/A |
| Tarhini et al.8 | 2 | 40 | N/A | Relapsed small cell lung cancer | Everolimus | None | N/A |
| Slomovitz et al.9 | 2 | 35 | N/A | Recurrent endometrial carcinoma | Everolimus | None | N/A |
| Doi et al.10 | 2 | 53 | N/A | Metastatic gastric cancer | Everolimus | None | N/A |
| Yao et al.11 | 2 | 115 | N/A | Metastatic pancreatic neuroendocrine tumors | Everolimus | None | N/A |
| Ellard et al.12 | 2 | 33 | 16 | Metastatic breast cancer | Everolimus | Everolimus | N/A |
| Amato et al.13 | 2 | 39 | N/A | Renal cell cancer | Everolimus | None | N/A |
| Seront et al.14 | 2 | 37 | N/A | Locally advanced or metastatic bladder cancer | Everolimus | None | N/A |
| Wolpin et al.15 | 2 | 33 | N/A | Pancreatic cancer | Everolimus | None | N/A |
| Hudes et al.16 | 3 | 416 | 200 | Renal cell cancer | Temsiroliums | Interferon | 3 |
| Motzer et al.17 | 3 | 274 | 137 | Renal cell cancer | Everolimus | Placebo | 4 |
| Yao et al.18 | 3 | 204 | 203 | Pancreatic neuroendocrine | Everolimus | Placebo | 5 |
| Baselga et al.19 | 3 | 482 | 238 | Metastatic breast | Everolimus + exemstane | Placebo + exemestane | 5 |
| Baselga et al.20 | 2 | 137 | 132 | Metastatic breast | Everolimus + letrozole | Letrozole + placebo | 4 |
| Chan et al.21 | 2 | 55 | 51 | Locally advanced or metastatic breast cancer | Temsirolimus | Temsirolimus | N/A |
| Pandya et al.22 | 2 | 45 | 41 | Extensive-stage small-cell lung cancer | Temsirolimus | Temsirolimus | N/A |
| Chawla et al.30 | 3 | 343 | 359 | Metastastic sarcoma | Ridaforolimus | Placebo | Unable to assess |
| Javle et al.28 | 2 | 4 | N/A | Metastatic pancreatic cancer | Temsirolimus | None | N/A |
| Margolin et al.29 | 2 | 33 | N/A | Metastatic melanoma | Temsirolimus | None | N/A |
Study quality
The six RCT's included in the meta-analysis were assessed for study quality using the Jadad scoring system. Three trials had placebo control arms, 2 trials had a combined placebo and targeted therapy control arm, and one trial had interferon in the control arm. One trial was presented in abstract form and a Jadad score could not be assessed. All six trials utilized either CTCAE version two or three criteria. Jadad scores are listed in Table 1; the mean score was 4.2 with a range between 3 and 5 indicating that the overall study quality was good.31
Patients
The majority of patients had a baseline Eastern Cooperative Oncology Group status of 0–1. Most of the trials excluded patients who had previously been treated with mTOR inhibitors or who had a history of cardiovascular disease. In all trials, patients were assigned to either an mTOR inhibitor (temsirolimus, everolimus, ridaforolimus) or in the cases of randomized trials, a control (placebo, interferon, letrozole, exemestane, or no therapy).
Systematic review of metabolic events
A total of 4261 patients receiving an mTOR inhibitor were available for analysis across 24 clinical trials. Several tumor types were represented including sarcoma (2 trials), pancreatic cancer (2 trials), glioblastoma (2 trials), melanoma (1 trial), endometrial cancer (1 trial), gastric cancer (1 trial), bladder cancer (1 trial), renal cell carcinoma (3 trials), breast cancer (3 trials), pancreatic neuroendocrine tumors (3 trials), and small cell lung cancer (2 trials). The average incidence rate across all 24 studies was 0.7 (95% CI, 0.47, 0.93). The average incidence rate of serious (grade 3 and 4) metabolic related adverse events was 0.11 (95% CI, 0.08, 0.15). The incidence rate of hyperglycemia of all grades was 0.25 (95% CI, 0.17, 0.33) and of grade 3–4 was 0.07 (95% CI, 0.05, 0.09). The incidence rate of hypertriglyceridemia of all grades was 0.35 (95% CI, 0.25, 0.45) and of grade 3–4 was 0.03 (95% CI, 0.01, 0.04). The incidence rate of hypercholesterolemia of all grades was 0.32 (95% CI, 0.20, 0.43) and of grade 3–4 was 0.03 (95% CI, 0.01, 0.04). No statistically significant publication bias was detected for the 24 trials included in the analysis of metabolic event incidence rate (p = 0.76).
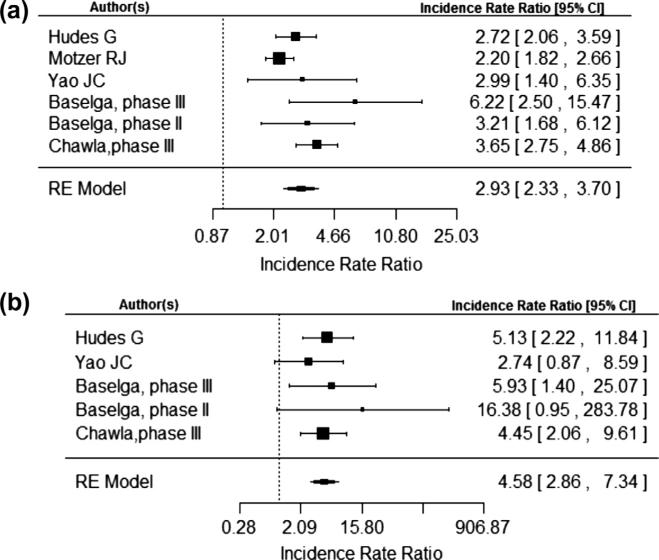
Incidence rate ratio of metabolic related adverse events
Analysis of the 3125 patients across 6 RCT's revealed that the incidence rate ratio (IRR) of a metabolic adverse events with mTOR inhibitor therapy compared with control was 2.93 (95% CI, 2.33, 3.70) using a random-effects model as seen in Fig. 2a. The risk of serious grade 3 and 4 metabolic adverse events was also increased as seen in Fig. 2a with an IRR of 4.58 (95% CI, 2.86, 7.34).
Fig. 2.
(a) and (b) are number of total metabolic event and total number of metabolic event grade 3 or 4 respectively.
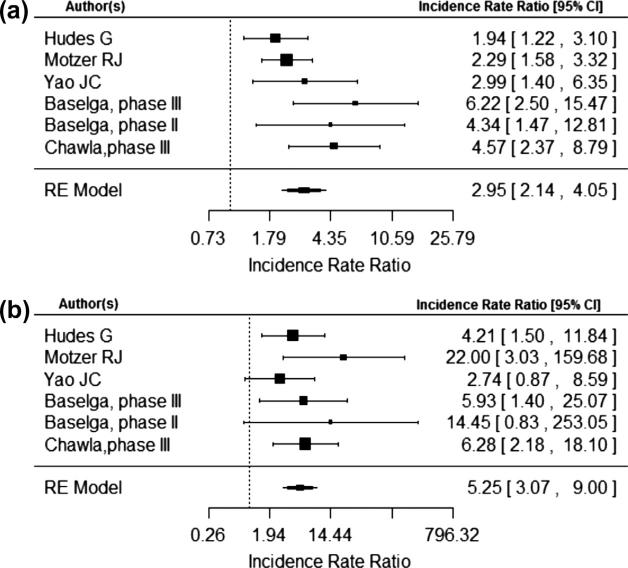
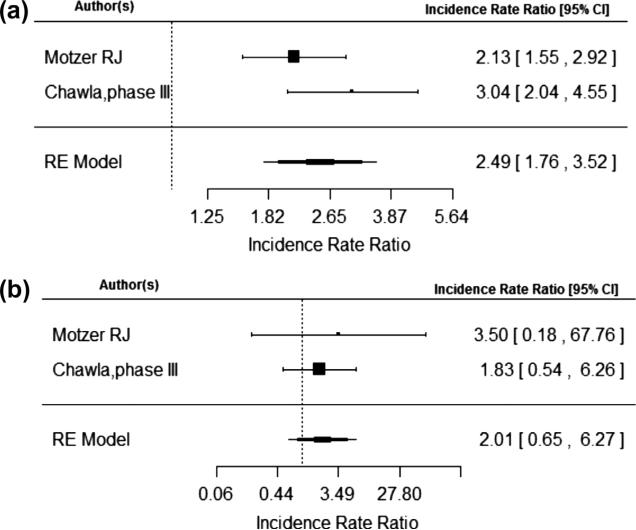
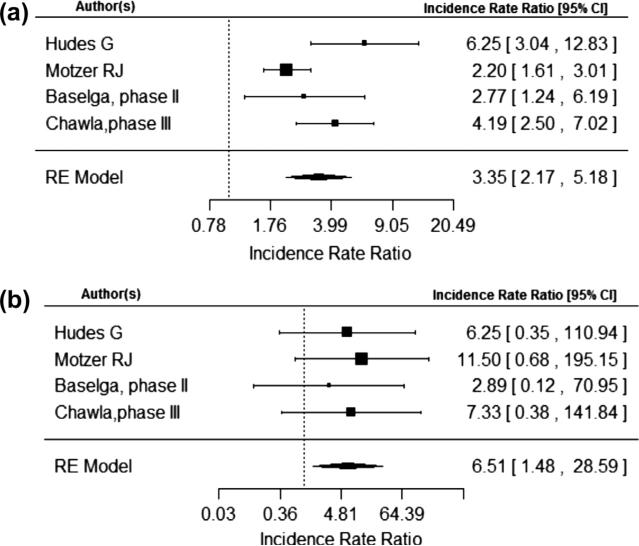
The risk of a metabolic adverse events were calculated individually for hyperglycemia, hypercholesterolemia, and hypertriglyceridemia. The IRR of all grade hyperglycemia was 2.95 (95% CI, 2.14, 4.05) and of grade 3–4 hyperglycemia was 5.25 (95% CI, 3.07, 9.00) as demonstrated in Fig. 3a and b. The IRR of all grade hypertriglyceridemia was 2.49 (95% CI, 1.76, 3.52) and of grade 3–4 hypertriglyceridemia was 2.01 (95% CI, 0.65, 6.27) as demonstrated in Fig. 4a and b. The IRR of all grade hypercholesterolemia was 3.35 (95% CI, 2.17, 5.18) and of grade 3–4 hypercholesterolemia was 6.51 (95% CI, 1.48, 28.59) as seen in Fig. 5a and b. No statistically significant publication bias was detected for the 6 trials included in the meta-analysis for total metabolic event incidence rate ratio (p = 0.27).
Fig. 3.
(a) and (b) are number of total hyperglycemia events and total number of hyperglycemia events grade 3 or 4 respectively.
Fig. 4.
(a) and (b) are number of total hypertriglyceridemia events and total number of hypertriglyceridemia events grade 3 or 4 respectively.
Fig. 5.
(a) and (b) are number of total hypercholesterolemia events and total number of hypercholesterolemia events grade 3 or 4 respectively.
Discussion
The development of molecular targeted therapies has ushered in an era in which clinical oncologists are required to be familiar with, and manage, adverse events not traditionally associated with cancer therapy. Many patients with cancer have comorbidities such as diabetes and cardiovascular disease that may be exacerbated by the side effects of these new classes of agents. Additionally, as these treatments are moved earlier in the course of disease, and subsets of patients experience prolonged disease-free survival, the implications of such chronic toxicities may become even more significant.
In this study we sought to determine the incidence and risk of metabolic adverse events as a consequence of targeting the mTOR pathway. Our analysis demonstrates an almost 4.5-fold increase in the risk of serious grade 3 and 4 metabolic adverse events in patients with advanced solid tumors receiving mTOR inhibitor therapy compared with control.
This study has several potential limitations. There was some inconsistency in the terms used to report metabolic events with some papers using the term hyperlipidemia while others using the term hypertriglyceridemia. Quality and transparency in reporting was inconsistent for individual studies. For example, baseline information on lipidemia or glycemia was not available for most studies although some studies commented on whether patients with uncontrolled lipid or glycemic disease were excluded. We included patients with a variety of different solid tumors and the risk of metabolic related adverse events could theoretically vary among tumor types. The small sample sizes precluded subset analyses. We included patients treated with three different mTOR inhibitors. While each of these drugs inhibits mTOR, there may be relevant differences with regards to potency and pharmacology. Aggregate data was utilized rather than individual patient level data. However, studies have suggested similar results with both approaches and the latter approach is associated with a unique set of methodological issues and potential biases.32
In summary, the current analysis suggests an increased relative risk of overall and severe metabolic adverse events, specifically hyperglycemia, hypertriglyceridemia and hypercholesterolemia, for patients with advanced malignancies treated with mTOR inhibitors. These results are particularly important as these drugs are moved from clinical trial populations, with restrictive eligibility criteria, to the general population of cancer patients with comorbidities that might be severely impacted by these side effects. Oncologists, primary care physicians, and endocrinologists should be aware of the metabolic side effects associated with mTOR inhibitors in an attempt to appropriately manage these toxicities and mitigate risk.
Footnotes
Conflict of interest
The authors declare no conflict of interest and all authors approved the final submission of this manuscript.
References
- 1.Sabatini DM. mTOR and cancer: insights into a complex relationship. Nat Rev Cancer. 2006;6(9):729–34. doi: 10.1038/nrc1974. [DOI] [PubMed] [Google Scholar]
- 2.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12(1):21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Okuno S, Bailey H, Mahoney MR, Adkins D, Maples W, Fitch T, et al. A phase 2 study of temsirolimus (CCI-779) in patients with soft tissue sarcomas: a study of the Mayo phase 2 consortium (P2C). Cancer. 2011;117(15):3468–75. doi: 10.1002/cncr.25928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chawla SP, Staddon AP, Baker LH, Schuetze SM, Tolcher AW, D'Amato GZ, et al. Phase II study of the mammalian target of rapamycin inhibitor ridaforolimus in patients with advanced bone and soft tissue sarcomas. J Clin Oncol. 2012;30(1):78–84. doi: 10.1200/JCO.2011.35.6329. [DOI] [PubMed] [Google Scholar]
- 5.Duran I, Kortmansky J, Singh D, Hirte H, Kocha W, Goss G, et al. A phase II clinical and pharmacodynamic study of temsirolimus in advanced neuroendocrine carcinomas. Br J Cancer. 2006;95(9):1148–54. doi: 10.1038/sj.bjc.6603419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang SM, Wen P, Cloughesy T, Greenberg H, Schiff D, Conrad C, et al. Phase II study of CCI-779 in patients with recurrent glioblastoma multiforme. Invest New Drugs. 2005;23(4):357–61. doi: 10.1007/s10637-005-1444-0. [DOI] [PubMed] [Google Scholar]
- 7.Galanis E, Buckner JC, Maurer MJ, Kreisberg JI, Ballman K, Boni J, et al. Phase II trial of temsirolimus (CCI-779) in recurrent glioblastoma multiforme: a North central cancer treatment group study. J Clin Oncol. 2005;23(23):5294–304. doi: 10.1200/JCO.2005.23.622. [DOI] [PubMed] [Google Scholar]
- 8.Tarhini A, Kotsakis A, Gooding W, Shuai Y, Petro D, Friedland D, et al. Phase II study of everolimus (RAD001) in previously treated small cell lung cancer. Clin Cancer Res. 2010;16(23):5900–7. doi: 10.1158/1078-0432.CCR-10-0802. [DOI] [PubMed] [Google Scholar]
- 9.Slomovitz BM, Lu KH, Johnston T, Coleman RL, Munsell M, Broaddus RR, et al. A phase 2 study of the oral mammalian target of rapamycin inhibitor, everolimus, in patients with recurrent endometrial carcinoma. Cancer. 2010;116(23):5415–9. doi: 10.1002/cncr.25515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doi T, Muro K, Boku N, Yamada Y, Nishina T, Takiuchi H, et al. Multicenter phase II study of everolimus in patients with previously treated metastatic gastric cancer. J Clin Oncol. 2010;28(11):1904–10. doi: 10.1200/JCO.2009.26.2923. [DOI] [PubMed] [Google Scholar]
- 11.Yao JC, Lombard-Bohas C, Baudin E, Kvols LK, Rougier P, Ruszniewski P, et al. Daily oral everolimus activity in patients with metastatic pancreatic neuroendocrine tumors after failure of cytotoxic chemotherapy: a phase II trial. J Clin Oncol. 2010;28(1):69–76. doi: 10.1200/JCO.2009.24.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ellard SL, Clemons M, Gelmon KA, Norris B, Kennecke H, Chia S, et al. Randomized phase II study comparing two schedules of everolimus in patients with recurrent/metastatic breast cancer: NCIC clinical trials group IND.163. J Clin Oncol. 2009;27(27):4536–41. doi: 10.1200/JCO.2008.21.3033. [DOI] [PubMed] [Google Scholar]
- 13.Amato RJ, Jac J, Giessinger S, Saxena S, Willis JP. A phase 2 study with a daily regimen of the oral mTOR inhibitor RAD001 (everolimus) in patients with metastatic clear cell renal cell cancer. Cancer. 2009;115(11):2438–46. doi: 10.1002/cncr.24280. [DOI] [PubMed] [Google Scholar]
- 14.Seront E, Rottey S, Sautois B, Kerger J, D'Hondt LA, Verschaeve V, et al. Phase II study of everolimus in patients with locally advanced or metastatic transitional cell carcinoma of the urothelial tract: clinical activity, molecular response, and biomarkers. Ann Oncol. 2012;10:2663–70. doi: 10.1093/annonc/mds057. [DOI] [PubMed] [Google Scholar]
- 15.Wolpin BM, Hezel AF, Abrams T, Blaszkowsky LS, Meyerhardt JA, Chan JA, et al. Oral mTOR inhibitor everolimus in patients with gemcitabine-refractory metastatic pancreatic cancer. J Clin Oncol. 2009;27(2):193–8. doi: 10.1200/JCO.2008.18.9514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356(22):2271–81. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 17.Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, et al. Phase 3 trial of everolimus for metastatic renal cell carcinoma: final results and analysis of prognostic factors. Cancer. 2010;116(18):4256–65. doi: 10.1002/cncr.25219. [DOI] [PubMed] [Google Scholar]
- 18.Yao JC, Shah MH, Ito T, Bohas CL, Wolin EM, Van Cutsem E, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364(6):514–23. doi: 10.1056/NEJMoa1009290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baselga J, Campone M, Piccart M, Burris HA, 3rd, Rugo HS, Sahmoud T, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366(6):520–9. doi: 10.1056/NEJMoa1109653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baselga J, Semiglazov V, van Dam P, Manikhas A, Bellet M, Mayordomo J, et al. Phase II randomized study of neoadjuvant everolimus plus letrozole compared with placebo plus letrozole in patients with estrogen receptor-positive breast cancer. J Clin Oncol. 2009;27(16):2630–7. doi: 10.1200/JCO.2008.18.8391. [DOI] [PubMed] [Google Scholar]
- 21.Chan S, Scheulen ME, Johnston S, Mross K, Cardoso F, Dittrich C, et al. Phase II study of temsirolimus (CCI-779), a novel inhibitor of mTOR, in heavily pretreated patients with locally advanced or metastatic breast cancer. J Clin Oncol. 2005;23(23):5314–22. doi: 10.1200/JCO.2005.66.130. [DOI] [PubMed] [Google Scholar]
- 22.Pandya KJ, Dahlberg S, Hidalgo M, Cohen RB, Lee MW, Schiller JH, et al. A randomized, phase II trial of two dose levels of temsirolimus (CCI-779) in patients with extensive-stage small-cell lung cancer who have responding or stable disease after induction chemotherapy: a trial of the eastern cooperative oncology group (E1500). J Thorac Oncol. 2007;2(11):1036–41. doi: 10.1097/JTO.0b013e318155a439. [DOI] [PubMed] [Google Scholar]
- 23.Atkins MB, Hidalgo M, Stadler WM, Logan TF, Dutcher JP, Hudes GR, et al. Randomized phase II study of multiple dose levels of CCI-779, a novel mammalian target of rapamycin kinase inhibitor, in patients with advanced refractory renal cell carcinoma. J Clin Oncol. 2004;22(5):909–18. doi: 10.1200/JCO.2004.08.185. [DOI] [PubMed] [Google Scholar]
- 24.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36(3):1–48. [Google Scholar]
- 26.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36(3):1–48. [Google Scholar]
- 28.Javle MM, Shroff RT, Xiong H, Varadhachary GA, Fogelman D, Reddy SA, et al. Inhibition of the mammalian target of rapamycin (mTOR) in advanced pancreatic cancer: results of two phase II studies. BMC Cancer. 2010;10:368. doi: 10.1186/1471-2407-10-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Margolin K, Longmate J, Baratta T, Synold T, Christensen S, Weber J, et al. CCI-779 in metastatic melanoma: a phase II trial of the California cancer consortium. Cancer. 2005;104(5):1045–8. doi: 10.1002/cncr.21265. [DOI] [PubMed] [Google Scholar]
- 30.Chawla SP, Blay J, Ray-Coquard IL, Le Cesne A, Staddon AP, Milhelm MM, et al. Results of the phase III, placebo-controlled trial (SUCCEED) evaluating the mTOR inhibitor ridaforolimus (R) as maintenance therapy in advanced sarcoma patients (pts) following clinical benefit from prior standard cytotoxic chemotherapy (CT). J Clin Oncol. 2011;29(Suppl.) abstr 10005. [Google Scholar]
- 31.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 32.Sud S, Douketis J. ACP journal club. The devil is in the details..or not? A primer on individual patient data meta-analysis. Ann Intern Med. 2009;151(2):JC1–3. doi: 10.7326/0003-4819-151-2-200907210-02002. [DOI] [PubMed] [Google Scholar]