Abstract
The three major Ras members, Kras, Hras and Nras, are highly homologous and individual Ras gene can have distinct biological functions. Embryonic lethality of Kras-deficient mice precludes study of the biological functions of this Ras family member. Here, we generated and examined mice with hematopoietic-specific deletion of Kras and bone marrow (BM) chimeric mice with B cell-specific targeted deletion of Kras. Hematopoietic-specific deletion of Kras impaired early B cell development at the pre-B-cell stage and late B-cell maturation, resulting in the reduction of BM pre-, immature and mature B cells and peripheral follicular (FO), marginal zone (MZ) and B1 mature B cells. In contrast, Kras deficiency did not affect T cell development. Studies of BM chimeric mice with B cell-specific deletion of Kras demonstrated that Kras deficiency intrinsically impaired B cell development. Kras deficiency reduced BCR-induced B cell proliferation and survival. Further, Kras deficiency specifically impaired pre-BCR- and BCR-induced activation of the Raf-1/MEK/ERK pathway in pre-B and mature B cells, respectively. Thus, Kras is the unique Ras family member that plays a critical role in early B cell development and late B cell maturation through controlling the Raf-1/MEK/ERK pathway.
Keywords: Kras, B cell lymphopoiesis, Signal transduction
Introduction
B cell development occurs through pro-, pre-, immature and mature B cell stages.(1) The pre-B cell receptor (BCR) instructs the transition from pro-B to pre-B cells whereas the BCR directs B cell maturation and subsequent immune responses (2, 3). Both the pre-BCR and BCR initiate signals via transmembrane molecules, Igα and Igβ, and activation of three distinct protein tyrosine kinases, Lyn, Syk and Btk (3). Ultimately, these kinases activate several signaling pathways, including the Ras-Raf-MEK1/2-ERK1/2 cascade (3, 4). A dominant-negative Ras protein that inhibits this pathway blocks the pre-pro to pro-B cell transition (5). In contrast, constitutively active Ras drives Rag1-deficient pro-B cells into pre-B-like cells and promotes maturation of BCR-low immature B cells (6). In addition, ERK1/2-double deficiency blocks pre-BCR-mediated early B cell development (7). These findings demonstrate that the Ras-dependent pathway is critical for B cell development.
Ras protein is a 21 kDa membrane-associated small GTPase that cycles between an active GTP-bound state and an inactive GDP-bound state and functions as a molecular switch relaying signals from cell surface receptors to the Raf/MEK/ERK1/2 pathway (8). Guanine nucleotide exchange factors (GEFs) activate Ras by catalyzing the exchange of GDP for GTP whereas GTPase-activating proteins (GAPs) inactivate Ras via facilitating the hydrolysis of GTP to GDP (9). GTP-bound Ras specifically activates the serine/threonine kinase Raf through direct interaction (10–13). In turn, Raf phosphorylates and activates the dual-specificity threonine/tyrosine kinases MEK1/2, which phosphorylate and activate the serine/threonine kinases ERK1/2 (14, 15). Activation of ERK1/2 leads to up-regulation of c-fos, a component of the transcription factor AP-1, and promotes a wide variety of cellular events (16, 17).
The family of highly conserved GTPases consists of the Ras, Rho, Rab, Ran subfamilies (18). The mammalian Ras subfamily has three highly homologous members, Kras, Hras and Nras, which are ubiquitously expressed (8). Studies of dominant-negative Ras proteins demonstrate a critical role of Ras activity in cell growth and embryogenesis (19). However, mice deficient in either or both of Nras and Hras are viable and largely normal, demonstrating functional redundancy of these ras genes (20, 21). In contrast, Kras-deficient mice are embryonically lethal (22). Although all three Ras isoforms are activated by T cell receptor (TCR) or B cell receptor (BCR) engagement, disruption of a specific Ras isoform has distinct effects (22–26). Deficiency of Hras or Nras does not affect early T-cell development, positive selection or T cell activation, but specifically impairs Th1 response of CD4 T cells (23). Nras deficiency also reduces CD8 thymocyte numbers and impairs CD8 T cell memory (25, 26). These findings demonstrate specific and distinct functions of the individual Ras isoforms.
Embryonic lethality of Kras-deficient mice precludes analysis of the role, if any, of Kras in lymphocyte development and function (22). We report here studies of mice with hematopoieric deletion of Kras and BM chimeric mice with B cell-specific targeted deletion of Kras. Our results demonstrate that Kras is important for B cell development.
Materials and Methods
VavCreKrasfl/fl and BM chimeric mice
VavCreKrasfl/fl mice were generated in Zhang’s laboratory (University of Wisconsin, Madison). Briefly, exon 1 of Kras was flanked with two LoxP sites (Fig. S1). The generated Krasfl/fl mice were crossed with VavCre transgenic mice, in which Cre expression mediates deletion of “floxed” gene throughout the entire hematopoietic compartment. The mouse line was maintained on C57BL/6 genetic background (>N10). Experimental VavCreKrasfl/fl and control VavCreKrasfl/+ or VavCreKras+/+ mice were 8–12 weeks old.
BM chimeric mice were generated. First, BM cells from VavCreKrasfl/fl or control mice were mixed 1:4 with BM cells from µMT mice, and transplanted into sub-lethally irradiated (600 rads) Rag1-deficient or lethally irradiated (1000 rads) µMT mice by intravenous injection (5 × 106 cells/recipient). Eight weeks after transplantation, the recipients were analyzed for B cell development and function.
Rag1-deficient and µMT mice from the Jackson Laboratory were maintained in the Biological Resource Center at the Medical College of Wisconsin (MCW). Animal protocols were approved by the MCW Institutional Animal Care and Use Committee.
Flow Cytometry
Single-cell suspensions from BM and spleen were treated with Gey’s solution to remove red blood cells and resuspended in PBS supplemented with 2% BSA. The cells were then stained with a combination of fluorescence-conjugated antibodies. APC-conjugated anti-B220, anti-IgM, anti-CD4 and anti-CD44, and PE-Cy7-conjugated anti-B220, anti-IFNγ, anti-CD25 and anti-CD23 were purchased from eBioscience. PE-conjugated anti-CD43, anti-CD21, anti-Thy1.2, anti-CD5, anti-CD8, and anti-IgD were purchased from BD Biosciences. Samples were applied to a flow cytometer (LSRII, Becton Dickinson). Data were collected and analyzed using FACSDiva software (Becton Dickinson) or FlowJo software (Tree Star).
Western blot analysis
Isolated mature B cells were resuspended in RPMI 1640 medium with 1% BSA. The B cells (10 × 106/mL) were stimulated with anti-IgM (20 µg/mL, Jackson ImmunoResearch Laboratories) at 37°C for the indicated times. B220+CD25+ pre-B cells were sorted from the mouse BM and incubated with biotin-conjugated anti-Igβ (30 µg/ml, anti-CD79B, HM-79-12, Novus Biologicals), followed by adding streptavidin (30 µg/ml, Thermo Fisher Scientific) at 37°C for the indicated times. Cell lysates were subjected to Western blot analysis with the indicated antibodies or Ras-GTP pull-down assay with the Ras activation kit (Millipore). Rabbit polyclonal anti-ERK1/2 (sc-093), and mouse monoclonal anti–phospho-ERK1/2 (pThr202/pTyr204, sc-7383), anti-Nras (sc-31), anti-Kras (sc-30), anti-Akt (sc-8312), anti-JNK (sc-571), anti-MEK1/2 (sc-436), anti-Raf-1 (sc-133), anti-p38 and anti-PLCγ2 (sc-407) antibodies were purchased from Santa Cruz Biotechnology. Rabbit polyclonal anti-phospho-Akt (phospho-Thr308, no. 9275), anti–phospho-JNK (pThr183/pTyr185, no. 4668), anti-phospho-MEK1/2 (pThr180/pTyr182 no. 2338), and mouse monoclonal anti–phospho-p38 (pThr180/pTyr182, no. 9216), and anti-IκBα (no. 9242) antibodies were purchased from Cell Signaling Technology. Mouse monoclonal anti-phospho-Raf1 (Ser338, no. 05-338) and anti-Ras (no. 05-516) antibodies were purchased from EMD Millipore. Anti-Hras (no. 610001) antibody was purchased from BD Biosciences.
Proliferation and cell cycle analysis
Purified mature B cells (2.5 × 104) were stimulated with anti-IgM (10 µg/ml) or anti-IgM (10 µg/ml) plus IL-4 (10 ng/ml) for 48 hours in a 96-well plate. The cells were then stained with propidium iodide for cell cycle analysis or pulsed for 16 hours with 3H-thymidine (1 µCi/well). Thymidine-pulsed samples were collected with the use of a MACH III harvester (TOMEC, Hamden, CT), and the incorporation of 3H-thymidine was determined with a Wallac MicroBeta TriLux scintillation system (PerkinElmer, Waltham, MA).
Gel mobility shift assay
Sorted pre-B cells were stimulated with biotin-conjugated anti-Igβ (30 µg/ml) plus streptavidin (30 µg/ml) at 37°C for the indicated times. The cells (1 × 106) were lysed in the lysis buffer (20 mM HEPES pH 7.9, 350 mM NaCl, 1 mM MgCl2, 0.5 mM EDTA, 0.5 mM DTT, 20% glycerol, 1% NP-40). Cell lysates were incubated with 32P-labeled NF-κB or AP-1 probe (Santa Cruz Biotechnology) for 15 minutes at room temperature and then resolved on a 4% polyacrylamide gel at 4°C.
Calcium flux analysis
Splenocytes (2 × 106) were incubated with indo-1AM (10 µg/ml, Molecular Probes) and APC-conjugated anti-IgM, PE-Cy7-conjugated anti-CD23 and PE-conjugated anti-CD21 antibodies at room temperature for 30 min. Then cells were washed and stimulated with an anti-IgM antibody. Calcium concentrations were determined in mature B cells by flow cytometry.
Statistical analysis
All statistical analysis was performed with the two-tailed unpaired Student’s t test.
Results
Expression of Kras in B and T cells
To determine whether Kras has a distinct role in the development and function of lymphocytes, we compared the expression of Kras with that of Nras and Hras during B and T cell development. Levels of expression of Kras and Hras were higher in pro-B cells and lower in B cells at later developmental stages whereas Nras was expressed at high levels throughout B cell development (Fig. 1A). In thymocytes, all Ras members were expressed at higher level in DN T cells and at lower level in DP, and CD4 or CD8 SP T cells (Fig. 1B). Thus, Kras is expressed in both B and T lymphocytes.
Figure 1.
Expression of Ras members during lymphocyte development and activation. B (A) and T (B) cells at the indicated developmental stages were sorted from the BM or thymus of wild-type mice, respectively. Cell lysates were subjected to direct Western blot analysis with the indicated antibodies.
Kras deficiency impairs B-cell but not T-cell development
To overcome the embryonic lethality associated with Kras deficiency and study the role of Kras in lymphocyte development, mice with “floxed” Kras allele were generated (Fig. S1). Then, Kras “floxed” mice were crossed with VavCre transgenic mice, in which Cre expression mediates deletion of the “floxed” gene throughout the entire hematopoietic compartment (27). Lysates of BM cells from VavCreKrasfl/fl mice displayed a complete deletion of Kras (Fig. 2A).
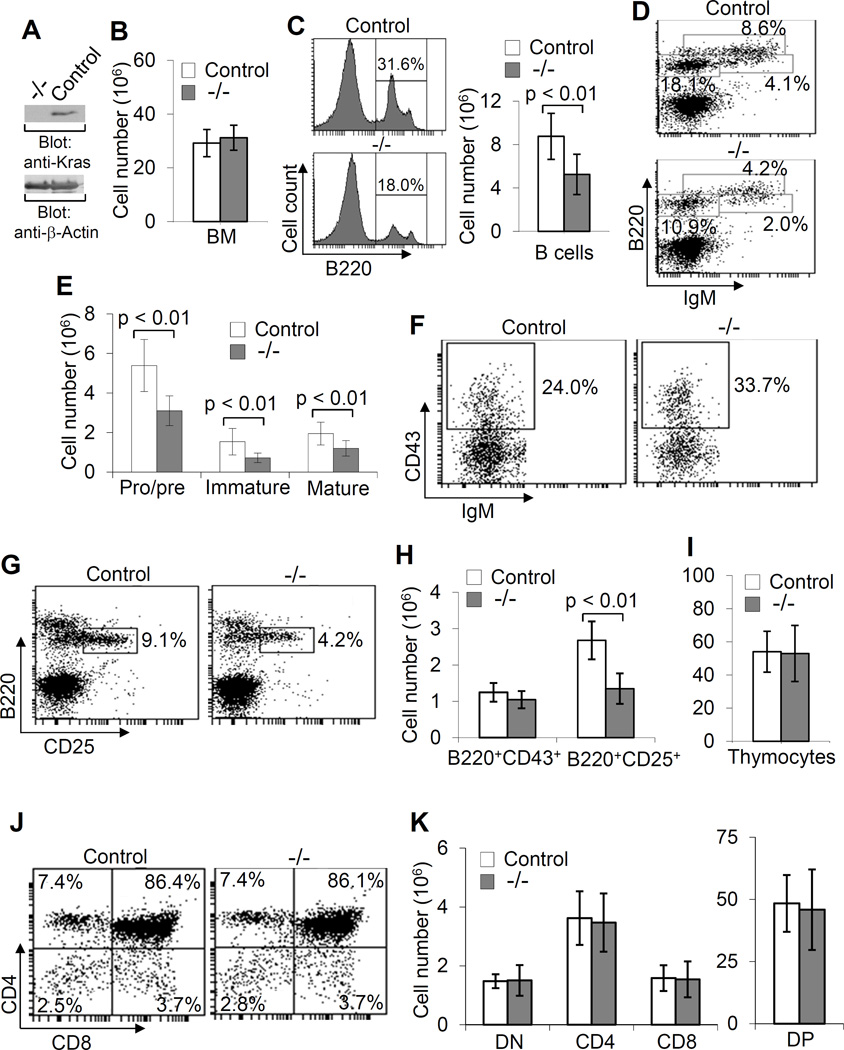
Figure 2.
Impaired early B cell development in VavCreKrasfl/fl mice. Direct Western blot analysis of cell lysates from BM cells of VavCreKrasfl/fl (−/−) and control (control) mice with the indicated antibodies demonstrates complete deletion of Kras in BM cells of VavCreKrasfl/fl mice (A). Bar graphs show the numbers of total BM cells isolated from two femurs of VavCre Krasfl/fl and control mice (B). Histograms and bar graphs show the percentages and numbers of total B cells in BM of VavCreKrasfl/fl and control mice (C). BM cells from VavCreKrasfl/fl and control mice were stained with anti-B220 and anti-IgM and percentages indicate cells in the gated live cells (D). Bar graphs show the numbers of pro/pre-, immature and mature B cells in BM (E). BM cells from VavCreKrasfl/fl and control mice were stained with anti-B220, anti-IgM and anti-CD43 and percentages indicate pro-B cells (IgM−CD43+) in the gated B220+IgM− population (F). BM cells from VavCreKrasfl/fl and control mice were stained with anti-B220 and anti-CD25 and percentages indicate pre-B cells (B220+CD25+) in the gated live cells (G). Bar graphs show the numbers of pro- and pre-B cells in the BM of VavCreKrasfl/fl and control mice (H). Bar graphs show the numbers of total thymocytes in VavCreKrasfl/fl and control mice (I). Thymocytes from VavCreKrasfl/fl and control mice were stained with anti-CD4 and anti-CD8 and percentages indicate cells in the gated live cells (J). Bar graphs show the numbers of DN, DP and SP T cells in the thymuses of VavCreKrasfl/fl and control mice (K). Data shown are obtained from or representative of 10 (B–H) or 8 (I–K) VavCreKrasfl/fl and 11 (B–H) or 9 (I–K) control mice.
BM cell-specific deletion of Kras did not affect total BM cell numbers in VavCreKrasfl/fl relative to control (VavCreKras+/+ and VavCreKrasfl/+) mice (Fig. 2B). In contrast, the percentages and absolute numbers of BM B cells were markedly reduced in VavCreKrasfl/fl relative to control mice (Fig. 2C). In addition, the percentages and numbers of pro/pre- (B220+IgM−), immature (B220+IgM+) and mature (B220hiIgM+) B cells were all reduced in the BM of VavCreKrasfl/fl relative to control mice (Fig. 2D, 2E). Within pro/pre- (B220+IgM−) population, the percentage of IgM−CD43+ pro-B cells was increased in VavCreKrasfl/fl relative to control mice (Fig. 2F). In contrast, the percentage of B220+CD25+ pre-B cells was decreased in VavCreKrasfl/fl relative to control mice (Fig. 2G). The absolute number of pre-B, but not pro-B, cells was markedly reduced in VavCreKrasfl/fl mice (Fig. 2H). Within the population of B220+CD43+ pro-B cells, the percentages and numbers of pre-pro-B (fraction A, BP-1−CD24−), early pro-B (fraction B, BP-1−CD24+), late pro- (fraction C, BP-1+CD24+), and large pre-B (fraction C′, BP-1+CD24hi) cells were comparable between VavCreKrasfl/fl and control mice (Fig. S2). Thus, Kras deficiency impairs early B cell development at the pro- to pre-B cell transition stage.
Early T-cell development was also examined in VavCreKrasfl/fl mice. Thymic T-cell development was normal in VavCreKrasfl/fl relative to control mice, based on the presence of comparable populations of total, DN, DP and SP thymocytes (Fig. 2I, 2J, 2K). In addition, among DN thymocytes, the populations of CD44+CD25− (DN1), CD44+CD25+ (DN2), CD44−CD25+ (DN3), and CD44−CD25− (DN4) were comparable between VavCreKrasfl/fl and control mice (data not shown). Thus, Kras deficiency has no effect on early T cell development.
The effect of Kras deficiency on lymphocyte maturation was also examined. The number of total splenocytes was comparable between VavCreKrasfl/fl and control mice (Fig. 3A). However, the percentage and number of B220+ B cells in the spleens of VavCreKrasfl/fl relative to control mice were reduced whereas the population of Thy1.2+ splenic T cells was comparable between the two types of mice (Fig. 3B, 3C). In addition, the percentages of transitional 1 (T1) (IgMhiIgDlo), transitional 2 (T2) (IgMhiIgDhi), and follicular (FO) mature (IgMloIgDhi) B cells were reduced in VavCreKrasfl/fl relative to control mice (Fig. 3D). Based on the expression of IgM, CD21 and CD23, splenic B cells can also be separated into different subsets of immature and mature B cells. Among CD23+ B cells, the population of FO (CD23+ CD21intIgMlo) but not T2 (CD23+CD21hiIgMhi) B cells was reduced in VavCreKrasfl/fl mice relative to control mice (Fig. 3E, 3F). In CD23− B cells, the population of MZ (CD23+CD21hiIgMhi) but not T1 (CD23+CD21loIgMhi) B cells was dramatically reduced in VavCreKrasfl/fl mice (Fig. 3E, 3F). Of note, the populations of CD4+ and CD8+ T cells in the spleen were comparable between VavCreKrasfl/fl relative to control mice (Fig. 3G). The self-renewing mature B1 B cells reside mainly in the peritoneal and pleural cavities. Within peritoneal lymphocytes, the population of B1 B cells (CD5+IgM+) was largely reduced in VavCreKrasfl/fl relative to control mice whereas the population of B2 B cells (CD5−IgM+) was normal in VavCre Krasfl/fl mice (Fig. 3H). Therefore, Kras deficiency severely impairs B-cell maturation, resulting in a marked reduction of FO, MZ and B1 B cells, but has no effect on T cell maturation.
Figure 3.
Impaired B cell maturation in VavCreKrasfl/fl mice. Bar graphs show the numbers of total splenocytes in VavCreKrasfl/fl (−/−) and control (control) mice (A). Splenocytes from VavCre Krasfl/fl and control mice were stained with anti-B220 and anti-Thy1.2 and percentages indicate cells in the gated live cells (B). Bar graphs show the numbers of B and T cells in the spleens of VavCreKrasfl/fl and control mice (C). Splenocytes from VavCreKrasfl/fl and control mice were stained with anti-B220, anti-IgD and anti-IgM (D) or anti-IgM, anti-CD21 and anti-CD23 (E), and percentages indicate cells in the gated live cells. Bar graphs show the numbers of T1, T2, FO and MZ B cells in the spleens of VavCreKrasfl/fl and control mice (F). Splenocytes from VavCreKrasfl/fl and control mice were stained with anti-CD4 and anti-CD8. Percentages indicate cells in the gated live cells and bar graphs show the numbers of CD4 and CD8 T cells in the spleens of VavCreKrasfl/fl and control mice (G). Cells from the peritoneal cavity of VavCre Krasfl/fl and control mice were stained with anti-IgM and anti-CD5. Percentages indicate cells in the gated lymphoid population and bar graphs show the percentages of B1 and B2 B cells in the peritonea of VavCreKrasfl/fl and control mice (H). Data shown are obtained from or representative of 15 (A–F) or 4 (G and H) VavCreKrasfl/fl and 16 (A–F) or 4 (G and H) control mice.
Impairment of the development of Kras-deficient B cells is B-cell intrinsic
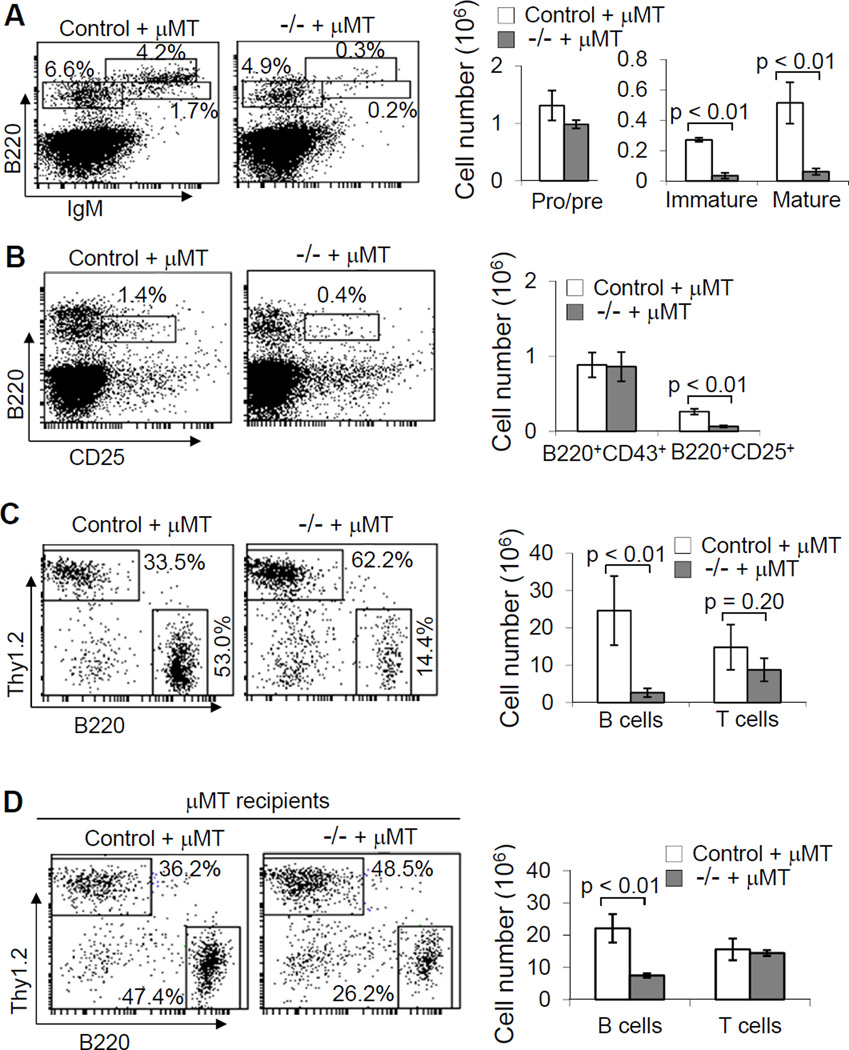
We examined whether the defective B-cell development in VavCreKrasfl/fl mice is the result of an intrinsic abnormality of the B cells. We generated BM chimeric mice with B cell-specific deficiency of Kras by mixing BM cells from VavCreKrasfl/fl or control mice with BM cells from B cell-deficient µMT mice, and transplanting the mixture into sub-lethally irradiated B and T cell-null Rag1-deficient or lethally irradiated µMT mice. Subsequently, B cell development in the recipients was examined. The populations of pre-, immature and mature B cells in the BM were markedly reduced in the Rag1-deficient or µMT recipients of a mixture of VavCreKrasfl/fl and µMT BM cells relative to the recipients of a mixture of control and µMT BM cells (Fig. 4A, 4B and data not shown). Moreover, the populations of splenic B cells in the recipients of a mixture of VavCreKrasfl/fl and µMT BM cells were dramatically reduced compared with the recipients received control and µMT BM cells (Fig. 4C, 4D and data not shown). Of note, the total number of splenocytes was markedly reduced in the Rag1-deficient but not µMT recipients of VavCreKrasfl/fl and µMT BM cells relative to the corresponding recipients of a mixture of control and µMT BM cells (data not shown). Thus, the total numbers of splenic T cells was reduced in the B-cell specific Kras-deficient BM chimeric Rag1-deficient but not µMT recipients, although the reduction was not significant (Fig. 4C, 4D). In addition, BM cells from VavCreKrasfl/fl or control mice were transplanted into lethally irradiated CD45.1 congenic mice. The populations of splenic B but not T cells were dramatically reduced in the CD45.1 recipients of VavCreKrasfl/fl BM cells relative to the corresponding recipients of control BM cells (data not shown). Taken together, these data demonstrate that the defective B-cell development in VavCreKrasfl/fl mice is B-cell autonomous.
Figure 4.
Kras deficiency intrinsically impairs B cell development. BM cells from VavCreKrasfl/fl or control mice were mixed with those from µMT mice and transplanted into sub-lethally irradiated Rag1-deficient mice. Two months after transplantation, BM cells from the chimeric mice that received the mixed BM from control and µMT (control + µMT) or VavCreKrasfl/fl and µMT (−/− + µMT) mice were stained with anti-B220 and anti-IgM (A) or anti-B220 and anti-CD25 (B). Percentages indicate cells in the gated live cells. Bar graphs show the numbers of pro/pre-, immature and mature B cells (A) or pre-B cells (B) in the BM of the chimeric mice. Splenocytes from the BM chimeric mice were stained with anti-B220 and anti-Thy1.2 (C). BM cells from VavCreKrasfl/fl or control mice were mixed with those from µMT mice and transplanted into lethally irradiated µMT mice. Splenocytes from the BM chimeric mice were stained with anti-B220 and anti-Thy1.2 (D). Percentages indicate cells in the gated live cells. Bar graphs show the numbers of B and T cells in the spleens of the chimeric mice. Data shown are representative of or obtained from 3 (A–C) or 4 (D) BM chimeric mice of each type.
Kras deficiency impairs BCR-induced cell proliferation and survival but marginally affects B-cell humoral immune response
We studied the effect of Kras deficiency on B-cell proliferation and survival. We examined BCR-induced proliferation of Kras-deficient B cells using 3H-thymidine incorporation. Mature splenic B cells were sorted from VavCreKrasfl/fl or control mice and cultured in the presence of anti-IgM or anti-IgM plus IL-4. The 3H-thymidine incorporation rate of Kras-deficient, relative to control, mature B cells in response to anti-IgM or anti-IgM plus IL-4 was markedly reduced (Fig. 5A). To determine whether the reduction of BCR-induced 3H-thymidine incorporation in Kras-deficient B cells was due to a decrease of cell proliferation and/or an increase of cell apoptosis, propidium iodide (PI) staining in combination with FACS analysis was used to detect the stimulation-induced cell proliferation and apoptosis of experimental B cells. Following anti-IgM or anti-IgM plus IL-4 stimulation, the entry into S and G2/M phase of Kras-deficient B cells was markedly reduced compared with that of control B cells (Fig. 5B). In addition, the population of apoptotic cells (subG0) was obviously increased in Kras-deficient relative to control B cells in the absence or presence of stimulation (Fig. 5B). The marked increase of BCR-induced apoptosis in Kras-deficient B cells was further confirmed by a short period of anti-IgM stimulation (Fig. 5C). Thus, Kras deficiency impairs BCR-induced B cell proliferation and survival.
Figure 5.
Kras deficiency impairs BCR-induced cell proliferation and survival. Mature B cells (CD23+CD21intIgMlo) were sorted from VavCreKrasfl/fl (−/−) or control (control) mice. Cells were stimulated with medium, anti-IgM or anti-IgM plus IL-4 for 48 hours and proliferative responses were determined by [3H] thymidine incorporation (A). Mature B cells isolated from VavCre Krasfl/fl or control mice were cultured with medium alone, anti-IgM, or anti-IgM plus IL-4 for 40 hours, stained with PI, and analyzed for cell-cycle profile by FACS (B). Mature B cells isolated from VavCreKrasfl/fl or control mice were stimulated with anti-IgM and at the indicated time points, cell apoptotic rates were determined by PI staining (C). Data shown are obtained from or representative of 5 (A), 3 (B) or 2 (C) independent experiments.
We examined the effect of Kras deficiency on B-cell humoral immune response. The basal levels of serum immunoglobulins in VavCreKrasfl/fl mice were measured. The levels of IgM, IgG1, IgG2b, IgG2c and IgG3 in the sera were comparable between naïve VavCre Krasfl/fl and control mice (Fig. 6A). In addition, antigen-specific antibody responses to T cell-dependent (TD) or T cell-independent (TI) antigens were examined in the B-cell specific Kras-deficient BM chimeric mice. Following TD antigen NP-CGG or TI antigen TNP-Ficoll immunization, the levels of NP-specific IgG1 and TNP-specific IgM were slightly but not significant less in the chimeric mice that received a mixture of BM cells from VavCreKrasfl/fl and µMT mice relative to those that received a mixture of BM cells from control and µMT mice (Fig. 6B, 6C). Thus, Kras deficiency slightly, but not significantly, impairs B-cell immune responses.
Figure 6.
The effect of Kras deficiency on serum immunoglobulin levels and antibody response to TD and TI antigens. Basal serum immunoglobulin isotype levels in VavCreKrasfl/fl and control mice were determined by ELISA (A). BM chimeric mice that received the mixed BM of VavCreKrasfl/fl and µMT (−/− + µMT) or control and µMT (control + µMT) mice were immunized with the TD antigen, NP-CGG (B), or the TI antigen, TNP-Ficoll (C). At the indicated time points after immunization, the serum NP-specific IgG1 or TNP-specific IgM response in the mice was determined by ELISA. Each dot represents an individual mouse and horizontal bars indicate mean values.
Kras is critical for BCR- and pre-BCR-induced activation of the ERK pathway in B and pre-B cells, respectively
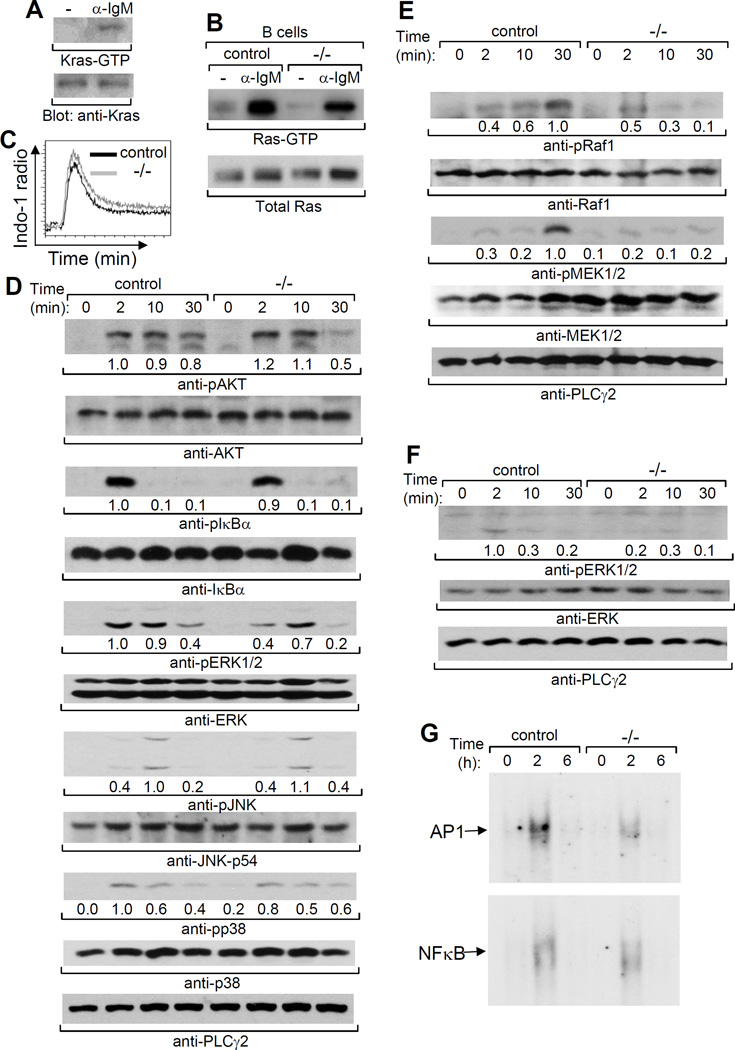
Kras deficiency impaired BCR-mediated functions. We further examined the role of Kras in BCR signaling. Consistent with the previous studies with a B cell line (24), Kras was activated by BCR ligation in primary B cells (Fig. 7A). Importantly, the level of total BCR-activated Ras (Ras-GTP) was largely reduced in Kras-deficient B cells (Fig. 7B). These data indicate a potential role of Kras in BCR signaling in B cells. Ras is able to regulate multiple signaling pathways, including the PI3K/AKT, PLC/Ca2+/PKC, Raf/MEK/Erk, et al, in many types of cells. We further investigated the downstream pathways that are controlled by Kras during BCR signaling. BCR-induced Ca2+ flux was comparable between Kras-deficient and control B cells (Fig. 7C). BCR-induced phosphorylation of Akt and IκBα was normal in mutant relative to control B cells (Fig. 7D). However, BCR-induced activation of ERK1/2 but not JNK or p38 was impaired in Kras-deficient relative to control B cells (Fig. 7D). Consistently, BCR-induced activation of Raf-1 and MEK1/2, the upstream activators of ERK1/2, was markedly reduced in mutant B cells (Fig. 7E). Of note, TCR-induced AKT and Raf/MEK/ERK activation was normal in Kras-deficient CD4 T cells (data not shown). Thus, Kras plays an important role in BCR-mediated activation of the ERK pathway in B cells.
Figure 7.
Kras deficiency impairs BCR- and pre-BCR-induced activation of the Raf1/MEK1/2 /ERK1/2 pathway. Splenic mature B cells from wild-type mice were stimulated with anti-IgM. Kras-GTP or total Kras proteins in cell lysates were detected by Raf-RBD agarose bead pull-down and subsequent Western blotting with anti-Kras (upper) or direct Western blotting with anti-Kras (lower), respectively (A). Splenic mature B cells from VavCreKrasfl/fl (−/−) or control (control) were stimulated with anti-IgM. Ras-GTP or total Ras proteins in cell lysates were detected by Raf-RBD agarose bead pull-down and subsequent Western blotting with anti-Ras (upper) or direct Western blotting with anti-Ras (lower), respectively (B). Splenocytes from VavCre Krasfl/fl or control mice were labeled with Indo-1 and stained with anti-IgM, anti-CD21 and anti-CD23. The cells were stimulated with anti-IgM, and Ca2+ flux in mature B cells was measured by flow cytometry and the lines were drawn at levels of constant cell number (C). Splenic mature B cells were stimulated with anti-IgM and cell lysates were subjected to direct Western blot analysis with the indicated antibodies (D–E). BM pre-B cells sorted from VavCreKrasfl/fl or control mice were stimulated with anti-Igβ. Cell lysates were subjected to direct Western blot analysis with indicated antibodies (F) or AP-1 and NF-kB gel mobility shift analysis (G). Data shown are representative of 3 (A–C,F), 4 (D–E) or 2 (G) independent experiments. The number under a band position indicates the intensity of the corresponding band.
Signals from the pre-BCR control the expansion of pre-B cells. Kras deficiency resulted in a marked reduction of pre-B cells (Fig. 2G). We investigated whether Kras is the Ras member that controls pre-BCR-mediated activation of the Raf-1/MEK/ERK pathway. Pre-B cells FACS sorted from VavCreKrasfl/fl mice were stimulated with anti-Igβ. Pre-BCR-induced ERK1/2 activation was clearly impaired in Kras-deficient relative to control pre-B cells (Fig. 7F). Consistently, pre-BCR-induced activation of AP-1, the transcription factors downstream of ERK1/2, but not NF-κB was decreased in mutant relative to control pre-B cells (Fig. 7G). Thus, Kras critically controls pre-BCR-mediated activation of the ERK pathway in pre-B cells.
Discussion
Signals emanating from the pre-BCR direct the expansion and differentiation of pre-B cells and a lack of a functional pre-BCR arrests early B cell development at the pre-B cell stage (2). The pre-BCR initiates several signaling pathways, including the ERK pathway. ERK1/2-double deficiency or dominant-negative Ras can block pre-BCR-mediated B cell development (7, 28). Constitutively active Ras compensates for pre-BCR deficiency to drive Rag1-deficient pro-B cells to differentiate into pre-B-like cells (6). Thus, the Ras-dependent ERK pathway is critical for pre-BCR-mediated pre-B cell development. The ERK pathway is activated by Kras, Nras or Hras, through the Raf/MEK1/2 signaling cascade (10–15). Nras and Hras single- or Nras/Hras double-deficient mice are largely normal whereas Kras-deficient mice are embryonic lethal (20–22). Although Nras and Hras, similar to Kras, are highly expressed in B cell progenitors as we find here, no defective B cell development is reported in mice deficient in either or both of Nras and Hras (20, 21). However, overexpression of a dominant-negative form of Hras in early B cells completely arrests B cell development at the pre-pro-B to pro-B cell transition prior to pre-BCR signaling, suggesting an important role of the Ras pathway in the function of other receptors, such as the IL-7 receptor, during early B cell development (5). Mice with overexpression of the dominant-negative Hras in late B cell precursors display a more severe reduction in the numbers of late pre-B cells than Kras-deficient mice do, strongly indicating the involvement of other Ras members in pre-BCR-mediated early B cell development (29). Our current study demonstrates that Kras functions uniquely among Ras family members as its ability to contribute to pre-BCR-mediated activation of Raf/MEK/ERK pathway to control pre-B cell development.
The BCR also activates the ERK pathway and constitutively active Ras rescues the differentiation of immature B cells with low levels of BCR expression (30). As we report here, B cells at the later developmental stages, relative to pro-B cells, express lower levels of Kras. Nonetheless, Kras largely accounts for BCR-induced Ras activities, which might be due to its up-regulation by BCR stimulation. Kras deficiency leads to a reduction of FO, MZ and B1, but not transitional T1 or T2 B cells, indicating that the reduction of mutant mature B cells is a result of impaired BCR function but not decreased pre-B cells. Indeed, BCR-induced B cell proliferation and survival are impaired in Kras-deficient B cells. Of note, Kras deficiency impairs but does not completely block ERK activation, indicating potential roles of Nras and Hras in BCR signaling. Indeed, overexpression of the dominant-negative Hras in B cells markedly reduces the populations of peripheral mature B cells, including B1 B cells (5). Nonetheless, no defective B cell maturation is reported in mice with deletion of either or both of Nras and Hras (20, 21). Thus, studies of mice with compound deficiencies of Kras, Nras and Hras are required to reveal any contributions of Nras and/or Hras to BCR-mediated ERK activation and B cell maturation.
B cell development in the BM chimeric recipients of a mixture of VavCreKrasfl/fl and µMT BM cells is more severely impaired than that in hematopoietic-specific Kras-deficient (VavCreKrasfl/fl) mice. This is likely due to the inability of Kras-deficient B cell progenitors to outcompete µMT B cell progenitors in the transplanted recipients. In fact, defective B cell development in the CD45.1 congenic recipients that receive Kras-deficient BM cells without BM cells from µMT mice is similar to that in the non-transplanted Kras-deficient mice (VavCreKrasfl/fl). In addition, due to unclear reason, the total number of splenocytes in Rag1-deficient BM chimeric mice with B cell-specific Kras deficiency is markedly reduced compared to control Rag1-deficient BM chimeric mice. Consequently, the total number of splenic T cells in the experimental relative to control Rag1-deficient BM chimeric mice was reduced, although the reduction was not statistically significant. However, µMT BM chimeric mice with B cell-specific deficiency of Kras display a marked reduction of splenic B cells but a normal population of splenic T cells compared to control µMT BM chimeric mice. Moreover, Kras deficiency results in a marked reduction of all three subsets of mature B cells and an impairment of BCR-induced cell proliferation and survival, but only slightly affects B-cell humoral immune response in vivo. Kras-deficient mature B cells with the remaining ERK activities might be enough to drive antibody production following antigen challenge in vivo.
The molecular mechanism underlying the distinct functions of the closely related Ras family members is not clear. The posttranslational modification differences of the carboxy termini of Ras lead to their differential localization. The palmitoylated carboxy terminus of H-Ras directs its localization to lipid rafts in the plasma membrane whereas a stretch of basic residues in the carboxy terminus of Kras guides its localization outside rafts (31, 32). The differential cellular localization of different Ras members could result in their differential activation and engagement of diverse downstream effector pathways. There are two types of RasGEFs, mSOS and RasGRP (33, 34). The BCR can activate Ras through mSos that is regulated by the Grb2/Shc complex or through RasGRP that is controlled by DAG, a product of PLCγ (4, 35). However, the contribution of differential localization of Ras members and differential activation of RasGEFs by the BCR or pre-BCR to distinct Ras activation in B cells requires further investigation.
Supplementary Material
Acknowledgments
This work is supported in part by NIH grants PO1 HL44612 (D.W.), R01 AI079087 (D.W.), R01 CA152108 (J.Z.), R01 HL113066 (J.Z.), and a Scholar Award from the Leukemia & Lymphoma Society (J.Z.).
Abbreviations used
- BM
bone marrow
- FO
follicular
- MZ
marginal zone
- BCR
B cell receptor
- TCR
T cell receptor
- GEF
guanine nucleotide exchange factor
- GAP
GTPase-activating protein
References
- 1.Hardy RR, Hayakawa K. B cell development pathways. Annu. Rev. Immunol. 2001;19:595–621. doi: 10.1146/annurev.immunol.19.1.595. [DOI] [PubMed] [Google Scholar]
- 2.Monroe JG. ITAM-mediated tonic signalling through pre-BCR and BCR complexes. Nat. Rev. Immunol. 2006;6:283–294. doi: 10.1038/nri1808. [DOI] [PubMed] [Google Scholar]
- 3.Kurosaki T, Shinohara H, Baba Y. B cell signaling and fate decision. Annu. Rev. Immunol. 2010;28:21–55. doi: 10.1146/annurev.immunol.021908.132541. [DOI] [PubMed] [Google Scholar]
- 4.Oh-hora M, Johmura S, Hashimoto A, Hikida M, Kurosaki T. Requirement for Ras guanine nucleotide releasing protein 3 in coupling phospholipase C-gamma2 to Ras in B cell receptor signaling. J. Exp. Med. 2003;198:1841–1851. doi: 10.1084/jem.20031547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iritani BM, Forbush KA, Farrar MA, Perlmutter RM. Control of B cell development by Ras-mediated activation of Raf. EMBO J. 1997;16:7019–7031. doi: 10.1093/emboj/16.23.7019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shaw AC, Swat W, Ferrini R, Davidson L, Alt FW. Activated Ras signals developmental progression of recombinase-activating gene (RAG)-deficient pro-B lymphocytes. J. Exp. Med. 1999;189:123–129. doi: 10.1084/jem.189.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yasuda T, Sanjo H, Pages G, Kawano Y, Karasuyama H, Pouyssegur J, Ogata M, Kurosaki T. Erk kinases link pre-B cell receptor signaling to transcriptional events required for early B cell expansion. Immunity. 2008;28:499–508. doi: 10.1016/j.immuni.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 8.Barbacid M. ras genes. Annu. Rev. Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- 9.Lowy DR, Willumsen BM. Function and regulation of ras. Annu. Rev. Biochem. 1993;62:851–891. doi: 10.1146/annurev.bi.62.070193.004223. [DOI] [PubMed] [Google Scholar]
- 10.Moodie SA, Willumsen BM, Weber MJ, Wolfman A. Complexes of Ras.GTP with Raf-1 and mitogen-activated protein kinase kinase. Science. 1993;260:1658–1661. doi: 10.1126/science.8503013. [DOI] [PubMed] [Google Scholar]
- 11.Zhang XF, Settleman J, Kyriakis JM, Takeuchi-Suzuki E, Elledge SJ, Marshall MS, Bruder JT, Rapp UR, Avruch J. Normal and oncogenic p21ras proteins bind to the amino-terminal regulatory domain of c-Raf-1. Nature. 1993;364:308–313. doi: 10.1038/364308a0. [DOI] [PubMed] [Google Scholar]
- 12.Warne PH, Viciana PR, Downward J. Direct interaction of Ras and the amino-terminal region of Raf-1 in vitro. Nature. 1993;364:352–355. doi: 10.1038/364352a0. [DOI] [PubMed] [Google Scholar]
- 13.Vojtek AB, Hollenberg SM, Cooper JA. Mammalian Ras interacts directly with the serine/threonine kinase Raf. Cell. 1993;74:205–214. doi: 10.1016/0092-8674(93)90307-c. [DOI] [PubMed] [Google Scholar]
- 14.Kyriakis JM, App H, Zhang XF, Banerjee P, Brautigan DL, Rapp UR, Avruch J. Raf-1 activates MAP kinase-kinase. Nature. 1992;358:417–421. doi: 10.1038/358417a0. [DOI] [PubMed] [Google Scholar]
- 15.Dent P, Haser W, Haystead TA, Vincent LA, Roberts TM, Sturgill TW. Activation of mitogen-activated protein kinase kinase by v-Raf in NIH 3T3 cells and in vitro. Science. 1992;257:1404–1407. doi: 10.1126/science.1326789. [DOI] [PubMed] [Google Scholar]
- 16.Whitmarsh AJ, Shore P, Sharrocks AD, Davis RJ. Integration of MAP kinase signal transduction pathways at the serum response element. Science. 1995;269:403–407. doi: 10.1126/science.7618106. [DOI] [PubMed] [Google Scholar]
- 17.Marais R, Wynne J, Treisman R. The SRF accessory protein Elk-1 contains a growth factor-regulated transcriptional activation domain. Cell. 1993;73:381–393. doi: 10.1016/0092-8674(93)90237-k. [DOI] [PubMed] [Google Scholar]
- 18.Macara IG, Lounsbury KM, Richards SA, McKiernan C, Bar-Sagi D. The Ras superfamily of GTPases. FASEB J. 1996;10:625–630. doi: 10.1096/fasebj.10.5.8621061. [DOI] [PubMed] [Google Scholar]
- 19.Yamauchi N, Kiessling AA, Cooper GM. The Ras/Raf signaling pathway is required for progression of mouse embryos through the two-cell stage. Mol. Cell. Biol. 1994;14:6655–6662. doi: 10.1128/mcb.14.10.6655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Umanoff H, Edelmann W, Pellicer A, Kucherlapati R. The murine N-ras gene is not essential for growth and development. Proc. Natl. Acad. Sci. USA. 1995;92:1709–1713. doi: 10.1073/pnas.92.5.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Esteban LM, Vicario-Abejon C, Fernandez-Salguero P, Fernandez-Medarde A, Swaminathan N, Yienger K, Lopez E, Malumbres M, McKay R, Ward JM, Pellicer A, Santos E. Targeted genomic disruption of H-ras and N-ras, individually or in combination, reveals the dispensability of both loci for mouse growth and development. Mol. Cell. Biol. 2001;21:1444–1452. doi: 10.1128/MCB.21.5.1444-1452.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson L, Greenbaum D, Cichowski K, Mercer K, Murphy E, Schmitt E, Bronson RT, Umanoff H, Edelmann W, Kucherlapati R, Jacks T. K-ras is an essential gene in the mouse with partial functional overlap with N-ras. Genes Dev. 1997;11:2468–2481. doi: 10.1101/gad.11.19.2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iborra S, Soto M, Stark-Aroeira L, Castellano E, Alarcon B, Alonso C, Santos E, Fernandez-Malave E. H-ras and N-ras are dispensable for T-cell development and activation but critical for protective Th1 immunity. Blood. 2011;117:5102–5111. doi: 10.1182/blood-2010-10-315770. [DOI] [PubMed] [Google Scholar]
- 24.Ehrhardt A, David MD, Ehrhardt GR, Schrader JW. Distinct mechanisms determine the patterns of differential activation of H-Ras, N-Ras, K-Ras 4B, and M-Ras by receptors for growth factors or antigen. Mol. Cell. Biol. 2004;24:6311–6323. doi: 10.1128/MCB.24.14.6311-6323.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perez de Castro I, Diaz R, Malumbres M, Hernandez MI, Jagirdar J, Jimenez M, Ahn D, Pellicer A. Mice deficient for N-ras: impaired antiviral immune response and T-cell function. Cancer Res. 2003;63:1615–1622. [PubMed] [Google Scholar]
- 26.Iborra S, Ramos M, Arana DM, Lazaro S, Aguilar F, Santos E, Lopez D, Fernandez-Malave E, Del Val M. N-ras couples antigen receptor signaling to Eomesodermin and to functional CD8+ T cell memory but not to effector differentiation. J. Exp. Med. 2013;210:1463–1479. doi: 10.1084/jem.20112495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stadtfeld M, Graf T. Assessing the role of hematopoietic plasticity for endothelial and hepatocyte development by non-invasive lineage tracing. Development. 2005;132:203–213. doi: 10.1242/dev.01558. [DOI] [PubMed] [Google Scholar]
- 28.Mandal M, Powers SE, Ochiai K, Georgopoulos K, Kee BL, Singh H, Clark MR. Ras orchestrates exit from the cell cycle and light-chain recombination during early B cell development. Nat. Immunol. 2009;10:1110–1117. doi: 10.1038/ni.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagaoka H, Takahashi Y, Hayashi R, Nakamura T, Ishii K, Matsuda J, Ogura A, Shirakata Y, Karasuyama H, Sudo T, Nishikawa S, Tsubata T, Mizuochi T, Asano T, Sakano H, Takemori T. Ras mediates effector pathways responsible for pre-B cell survival, which is essential for the developmental progression to the late pre-B cell stage. J. Exp. Med. 2000;192:171–182. doi: 10.1084/jem.192.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rowland SL, DePersis CL, Torres RM, Pelanda R. Ras activation of Erk restores impaired tonic BCR signaling and rescues immature B cell differentiation. J. Exp. Med. 2010;207:607–621. doi: 10.1084/jem.20091673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Apolloni A, Prior IA, Lindsay M, Parton RG, Hancock JF. H-ras but not K-ras traffics to the plasma membrane through the exocytic pathway. Mol. Cell. Biol. 2000;20:2475–2487. doi: 10.1128/mcb.20.7.2475-2487.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prior IA, Harding A, Yan J, Sluimer J, Parton RG, Hancock JF. GTP-dependent segregation of H-ras from lipid rafts is required for biological activity. Nat. Cell Biol. 2001;3:368–375. doi: 10.1038/35070050. [DOI] [PubMed] [Google Scholar]
- 33.Ebinu JO, Bottorff DA, Chan EY, Stang SL, Dunn RJ, Stone JC. RasGRP, a Ras guanyl nucleotide-releasing protein with calcium- and diacylglycerol-binding motifs. Science. 1998;280:1082–1086. doi: 10.1126/science.280.5366.1082. [DOI] [PubMed] [Google Scholar]
- 34.Buday L, Downward J. Epidermal growth factor regulates p21ras through the formation of a complex of receptor, Grb2 adapter protein, and Sos nucleotide exchange factor. Cell. 1993;73:611–620. doi: 10.1016/0092-8674(93)90146-h. [DOI] [PubMed] [Google Scholar]
- 35.Saxton TM, van Oostveen I, Bowtell D, Aebersold R, Gold MR. B cell antigen receptor cross-linking induces phosphorylation of the p21ras oncoprotein activators SHC and mSOS1 as well as assembly of complexes containing SHC, GRB-2, mSOS1, and a 145-kDa tyrosine-phosphorylated protein. J. Immunol. 1994;153:623–636. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.