Abstract
Symbionts and parasites can manipulate their hosts’ reproduction to their own benefit, profoundly influencing patterns of mate choice and evolution of the host population. Wolbachia is one of the most widespread symbionts among arthropods, and one that alters its hosts’ reproduction in diverse and dramatic ways. While we are beginning to appreciate how Wolbachia’s extreme manipulations of host reproduction can influence species diversification and reproductive isolation, we understand little about how symbionts, and Wolbachia in particular, may affect intrapopulation processes of mate choice. We hypothesized that the maternally transmitted Wolbachia would increase the attractiveness of its female hosts to further its own spread. We therefore tested the effects of Wolbachia removal and microbiome disruption on female attractiveness and male mate choice among ten iso-female lines of Drosophila melanogaster. We found variable effects of general microbiome disruption on female attractiveness, with indications that bacteria interact with hosts in a line-specific manner to affect female attractiveness. However, we found no evidence that Wolbachia influence female attractiveness or male mate choice among these lines. Although the endosymbiont Wolbachia can greatly alter the reproduction of their hosts in many species, there is no indication that they alter mate choice behaviours in D. melanogaster.
Keywords: symbiont, sexual selection, infection, cytoplasmic incompatibility
Introduction
Symbionts and parasites can greatly alter their hosts’ behaviour. Such behaviour modification can increase symbiont transmission and spread, while also impacting the fitness and life history of their hosts (Hoffmann & Turelli, 1997; Hughes et al., 2012). Wolbachia are especially prevalent and influential symbionts in arthropods, infecting up to two-thirds of insect species (Hilgenboecker et al., 2008). These endosymbionts are vertically transmitted from mother to offspring and dramatically manipulate host reproduction in a variety of ways depending on the host species (Hoffmann & Turelli, 1997; Werren, 1997; Miller et al., 2010). For example, Wolbachia can shift sex ratios to be female-skewed by feminizing genetic males (Moreau et al., 2001; Engelstädter & Hurst, 2009), killing males during development (Randerson et al., 2000; Sullivan & Jaenike, 2006), or inducing parthenogenesis in infected females (Schilthuizen & Stouthamer, 1997; Werren et al., 2008). Because Wolbachia are only transmitted by females, each of these reproductive manipulations serves to enhance the spread of Wolbachia in host populations.
The most well-documented reproductive manipulation induced by Wolbachia is cytoplasmic incompatibility, which reduces the viability of the offspring from crosses between either infected males and uninfected females, or between males and females infected with different Wolbachia strains (Hoffmann & Turelli, 1997). In contrast, Wolbachia-infected males produce healthy offspring when they mate with females infected with the same Wolbachia strain. Cytoplasmic incompatibility thus increases the relative fitness of infected females by decreasing the fitness of uninfected females (Jaenike et al., 2006; Miller et al., 2010; Buellesbach et al., 2014). Wolbachia-induced cytoplasmic incompatibility has been shown to dramatically impact the evolution of the host species by promoting reproductive isolation and mate discrimination between populations and species affected by such incompatibility, potentially driving processes of speciation in some insect systems (Jaenike et al., 2006; Koukou et al., 2006; Buellesbach et al., 2014).
However, many Wolbachia infections do not cause cytoplasmic incompatibility or shift sex ratios in their hosts, and there is little understanding of whether or how Wolbachia may affect intrapopulation dynamics in the absence of such extreme manipulations. For example, naturally infected populations of Drosophila melanogaster do not show patterns of strong cytoplasmic incompatibility (Hoffmann, 1988). Still, Wolbachia affect a number of other traits in this species, such as increasing insulin signaling levels (Ikeya et al., 2009), inducing host resistance to viral infections (Teixeira et al., 2008), and increasing female fecundity (Fry & Rand, 2002; Fry et al., 2004). Manipulations of such processes and traits could serve to alter processes underlying host mate choice and host attractiveness. For example, by altering metabolic processes and susceptibility to other infections, Wolbachia could influence activity levels or choosiness. Infections have previously been shown to influence both male mating activity (Champion De Crespigny et al., 2006) and male mate choice (Wittman & Fedorka, 2014). Additionally, male Drosophila, as well as males in many arthropod species, exhibit mating preferences for high fecundity females (Andersson, 1994); therefore, if Wolbachia can influence female fecundity, they may alter female attractiveness, although this possibility has been relatively unexplored to date.
If Wolbachia do in fact influence the attractiveness of their female hosts, they may alter the number and identity of the males with which females mate. Much theory has suggested that, in addition to seeking out high fecundity partners, individuals assess and choose mates based on genetic fitness (Zahavi, 1975; Iwasa et al., 1991; Rowe & Houle, 1996), and that males and females may mate assortatively by fitness (Rice, 1998; Sharp & Agrawal, 2009). As the endogenous microbiota can also play a role in determining host fitness, it may be possible for these microbes to influence assortative mating and mate choice (Sharon et al. 2010; Najarro et al. 2015). Further, insulin signaling levels have recently been shown to impact both fecundity and attractiveness of D. melanogaster females (Kuo et al., 2012), bolstering the possibility that Wolbachia may increase host females’ attractiveness via their upregulation of host insulin signaling pathways (Ikeya et al. 2009). Finally, natural populations of Drosophila are commonly polymorphic with respect to Wolbachia infection (Hoffman et al., 1994; Mackay et al., 2012), meaning such manipulations of mate choice between infected and uninfected individuals may have significant impacts on the behaviour of natural populations.
However, while manipulations of attractiveness and mate choice within populations could have important effects on host evolution and endosymbiont transmission, we know little about the impact of Wolbachia on these traits. Results from previous investigations of the impact of Wolbachia on intrapopulation mate choice and attractiveness have been mixed, with some finding effects of infection (Vala et al., 2004), while others find no such pattern (Champion De Crespigny & Wedell, 2007; Markov et al., 2009). Furthermore, most of these studies have relied on just one or two inbred host lines and only one Wolbachia strain to make their conclusions, even though the effects of Wolbachia may depend on host genotype (Fry & Rand, 2002; Fry et al., 2004), infection strain (Peng et al., 2008), or on the interaction of the host and Wolbachia genotypes.
Here we tested for the effects of Wolbachia and commensal bacteria on male mating behaviour and female attractiveness among 10 isofemale lines of D. melanogaster. These lines are naturally derived from a population polymorphic for Wolbachia infection, and represent natural host-Wolbachia pairings, which allowed us to evaluate global effects of symbionts on attractiveness in a meaningful context, while also examining potential interactions between host and Wolbachia genotypes. Overall, we did not find an effect of Wolbachia on female attractiveness or male mate choice, but we observed variable effects of naturally occurring microbiota on female attractiveness, depending on host genotype, where altering the microbiota decreased attractiveness in some lines, but increased it in others.
Methods
Drosophila stocks and antibiotic treatments
We used ten isofemale lines from the Drosophila Genetic Reference Panel (DGRP; Mackay et al., 2012) to test for the effects of Wolbachia and the microbiota on female attractiveness and male mate choice. Roughly half of the DGRP lines are naturally infected with Wolbachia, making them an ideal resource for testing the effects of Wolbachia infection and interactions between Wolbachia and genotype. The lines 85, 181, 256, 313, 338, 362, 371, 399, 555, and 738 were obtained from the Bloomington Stock Center in the fall of 2013, and maintained for one year prior to any antibiotic treatment. These DGRP lines were selected to maximize the diversity of the Wolbachia/mitochondrial haplotypes sampled, including representatives from clades I, III, and VI (as designated in Richardson et al., 2012), which contain both wMel-like and wMelCS-like Wolbachia strains.
We then tested the effects of antibiotic treatments on both uninfected (85, 313, 371, 399) and Wolbachia infected lines (181, 256, 338, 362, 555, and 738). We confirmed infection status of all lines (see below). All flies were reared on standard molasses-cornmeal medium at room temperature. Untreated (U) lines were maintained on this food without antibiotic treatment. We first generated the tetracycline-treated (T) lines by treating flies with 0.08 mg/mL tetracycline delivered through the food for three generations, and subsequently cultivating the lines without antibiotics for four generations before testing. Tetracycline dosage varies in published literature, ranging from 0.05 mg/mL (e.g. Texeira et al., 2008) to 0.3 mg/mL (e.g. Harcombe and Hoffman, 2004; Hedges et al., 2012). Recent work has shown that even the low dosage of 0.05 mg/mL can decrease female fecundity outside of tetracycline’s effects on microbial communities (Ridley et al., 2013). We therefore sought to use an antibiotic dose at the lower end of the published range to minimize non-microbial impacts on host physiology. Tetracycline treatment both eliminated Wolbachia but also potentially disrupted other fly microbiota. To restore the gut-associated microbiota that may have been perturbed by the tetracycline treatment without re-introducing the Wolbachia, we generated the treated-and-crossed (TC) flies by crossing Wolbachia-free T females with U males from the same DGRP line, similar to Fast et al. (2011). Thus, relative to the U lines, we expected the T flies to have lost both Wolbachia and other microbiota, while the TC lines should primarily show the effects of Wolbachia alone.
Wolbachia infection status for all lines was assessed by PCR and qPCR. Template DNA was prepared from two male and two female 3 day old adult flies. Both PCR and qPCR assays used the following wsp primers to test for Wolbachia infection: wsp_F1 GTCCAATARSTGATGARGAAAC and wsp_R1 CYGCACCAAYAGYRCTRTAAA (Baldo et al., 2006). For standard PCR, the following program was used: 94°C 2min, 37×(94°C 30s, 59°C 45s, 72°C 1min30s), 72°C 10min. For qPCR, wsp amplification was normalized to the Drosophila Actin 5C gene using these primers: GACGAAGAAGTTGCTGCTCTGGTTG and TGAGGATACCACGCTTGCTCTGC (Osborne et al., 2012).
We assessed the infection status of all lines and treatments twice, first six weeks prior to our mating assays via PCR, and again 15 weeks following our mating assay via qPCR. As expected, at both time points, all T and TC lines were not infected with Wolbachia. Additionally, the Wolbachia infection status of the untreated lines agreed completely with previous reports (Richardson et al., 2012), with these lines uninfected: 85, 313, 371, 399. All other untreated (U) lines were infected with Wolbachia.
Female attractiveness
We measured female attractiveness of tetracycline treated (T) and treated + cross (TC) females by subjecting them to mate choice trials in direct competition with untreated (U) females of the same line. We collected virgin females from each of the three treatments among the 10 DGRP lines and held them at 10 females per vial for 2 – 3 days. Virgin Canton-S males were collected at the same time and held at 7 males per vial for 2 – 3 days. The Canton-S line used as the choosing male was not infected with Wolbachia.
Virgin females were labeled with red or green fluorescent powder (Brilliant Group, Inc.) and introduced to experimental arenas 24 hours prior to the start of each mate choice assay. We assessed the effects of our two treatments directly, as each choice trial included one treated female (T or TC) and one untreated female from the same line. This also allowed us to test for the effects of line and treatment × line within our data. Trials were balanced by color, such that the treated female was green in half of trials and red in the other half for each line × treatment combination. Trials were initiated by introducing a Canton-S male into each vial, and observing vials for up to 2 hours. If mating occurred within this period, we noted the color and identity of the mated female. We carried out 20 trials for each of the 20 line × female treatment combinations, divided equally between two blocks separated by one day. The mating status of the untreated female, mated or unmated, was treated as a binomial response variable. We assessed the repeatability of our assay by performing the same measures on four of the lines (85, 181, 313, 555) 10 weeks after the initial assay.
For both the T and TC treatments, we tested whether the overall treatment effects led to departures from a random 50/50 preference by running a logistic regression model of the incidence of mating for treated and untreated females for all lines combined. To test for the effects of line and treatment on female attractiveness, we carried out likelihood ratio tests of generalized linear models with the incidence of the untreated female receiving matings as a binomial response variable, and line and block as fixed effects, testing the T and TC treatments separately. The generalized linear model used to test the TC dataset also included each line’s Wolbachia infection status as a fixed effect. If Wolbachia has a significant impact on female attractiveness, we would expect that antibiotic treatment would cause changes in the incidence of untreated females receiving matings for the six lines infected with Wolbachia, but not the other four lines.
For all lines and treatments, we also collected data on female mass. Several studies have shown that males show mating preferences for larger females, as these females are often more fecund (Byrne & Rice, 2006; Long et al., 2009). We pooled and weighed on an analytical balance 4–6 virgin females aged between 3 and 5 days post-eclosure. We recorded the average weight per female in 4 to 10 pools for each line and treatment. Mass data were analyzed using a linear mixed model, with treatment, line infection status, and their interaction as fixed effects, and line (nested within infection status) as a random effect. We tested the significance of each effect using a series of likelihood ratio tests, removing each effect individually.
Male mate choice
Virgin males of the 10 DGRP lines were collected and held at 7 males per vial for 2 – 3 days. At the same time, virgin females from two different untreated DGRP lines (362 and 774) were collected and held at 10 females per vial for 2 – 3 days. Females from these lines show replicable differences in attractiveness, such that males from different lines (Oregon-R, Canton-S, and several DGRP lines) mate with females from line 362 a majority of the time when given the choice between females from these two lines (unpublished data). Additionally, these two lines differ in their Wolbachia infection status, with line 362 infected, but line 774 uninfected.
To measure male mating rate and choosiness, we gave males the choice between a 362 female and a 774 female, and noted whether the males mated either female as well as the identity of the mated female. High choosiness was indicated by males mating females from line 362 a majority of the time. Females were coloured with fluorescent powder in a balanced design as was done in our measures of female attractiveness, and males were given 2 hours to choose and mate a female before trials were halted. As in the female attractiveness assay, 20 trials were performed for each line × treatment combination. However, unlike the female attractiveness assay, we had to test each of the three treatments (U, T, and TC) separately to measure male choosiness, as we did not put males in direct competition with one another. The incidence of overall mating and males mating the attractive female were analyzed via likelihood ratio tests of generalized linear models with mating or mating of attractive females as a binomial response and male line, treatment, line × treatment interaction, and block as fixed effects.
Results and Discussion
We observed a high overall mating rate among our trials (80%), with 297 trials in which to assess female attractiveness and 394 trials to assess male mating activity. Tetracycline treated (T) flies from line 256 did not produce enough offspring for our mating trials, and so this line × treatment combination was excluded from analysis.
Male mate choice
Male mating rate (the incidence of a male mating either presented female) was affected by the male line (, p < 0.0001), but not by male treatment (, p = 0.73), or their interaction (, p = 0.32). Therefore, some lines were more active in seeking out and achieving matings than others, but mating rate was not influenced by tetracycline treatment or crosses. If Wolbachia influenced male mating rate, we would expect to see a treatment × line interaction, because only the Wolbachia-infected lines would behave differently when Wolbachia were removed (TC) while no such removal takes place in the uninfected lines. Therefore, counter to the findings of Champion De Crespigny et al., 2006, we did not find any effect of Wolbachia infection on mating rate among several natural host-Wolbachia pairings. Male choosiness (measured as the rate at which males selected the more attractive female) was similarly unaffected by line (, p = 0.16), treatment (, p = 0.72), or their interaction (, p = 0.15). In addition to differences in attractiveness, the females that were offered to males differed in their Wolbachia infection status. Therefore, our results show that male mate choice is not influenced by antibiotic treatments or Wolbachia infection, both with respect to female attractiveness, and with respect to female infection status. However, we note that though both the male mate choice and female attractiveness assays set out to measure the effects of microbiome disruption and Wolbachia removal on female attractiveness and male mate choice, it is possible that intrasexual competition between females also plays a role in these trials.
Tetracycline treatment, commensal bacteria, and female attractiveness
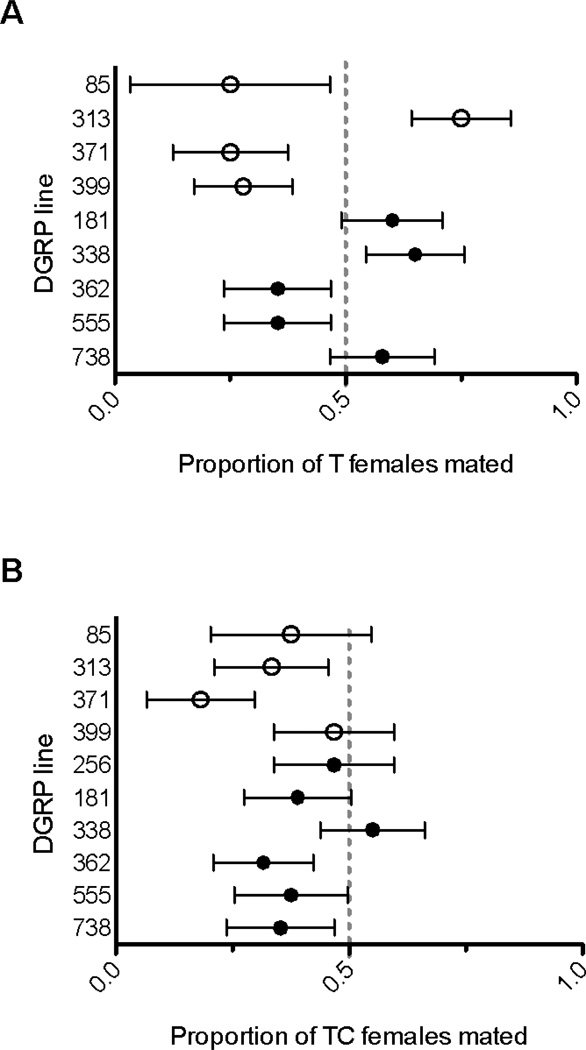
To address our questions regarding the effects of antibiotic treatment, the microbiota, and Wolbachia on female attractiveness, we analyzed the data from the T and TC treatments separately. Our mating trials involving females treated with tetracycline without backcrossing (T) allowed us to evaluate the effects of generally perturbing the microbiota on female attractiveness. Overall, there was no universal change in attractiveness with antibiotic treatment across the nine tested lines (Logistic Regression; z8 = 0.42, p = 0.68). However, in our first assay, line identity had a significant effect on the change in attractiveness in response to antibiotic treatment (LRT; p = 0.02; Fig. 1a). The disruption of some resident bacteria appeared to decrease female attractiveness relative to untreated females for some lines (e.g. 371, 399) while increasing attractiveness in others (e.g. 313, 338).
Figure 1. Tetracycline treatment alters female attractiveness independent of Wolbachia infection.
Antibiotic-treated female flies competed with untreated females of the same DGRP line for matings with Canton-S males. The proportion of tetracycline-treated (T, panel A) versus untreated or treated-and-crossed (TC, panel B) versus untreated females mated is shown. A value of 0.5 represents no effect of the antibiotic treatment and equal matings between treated and untreated females. Error bars show the standard error. DGRP lines are indicated as naturally Wolbachia-infected (filled circles) or uninfected (open circles) in the untreated lines.
When we repeated these experiments for four of the lines 10 weeks later, we found that these antibiotic-induced changes in female attractiveness were not stable over time (Table 1). Lines that initially showed significant differences in attractiveness between the U and T lines generally showed no change in the latter assay, and effect of line determining whether untreated females received matings disappeared (, p = 0.75). Because the microbiome of Drosophila melanogaster is known to vary widely over time even in flies reared in constant laboratory conditions (Wong et al., 2013), it is likely that the variable attractiveness observed in the T lines is due to changes in either the make-up or the effects of each line’s microbiome. It is also possible that the microbiome effects we detected in the first assay arose because the lines had not yet recovered from the tetracycline treatment four generations prior, while the five additional generations between the first and second assay allowed for sufficient time for recovery.
Table 1.
Repeatability of female attractiveness measures in four DGRP lines
| Proportion treated females mated (+/− SE) | ||||
|---|---|---|---|---|
| T vs U | TC vs U | |||
| DGRP Line | Assay 1 | Assay 2 | Assay 1 | Assay 2 |
| 85 | 0.25 (0.22) | 0.57 (0.19) | 0.38 (0.17) | 0.33 (0.19) |
| 181 | 0.6 (0.11) | 0.52 (0.10) | 0.39 (0.11) | 0.54 (0.10) |
| 313 | 0.75 (0.11) | 0.38 (0.12) | 0.33 (0.12) | 0.22 (0.10) |
| 555 | 0.35 (0.12) | 0.52 (0.10) | 0.38 (0.12) | 0.61 (0.10) |
As a whole, commensal bacteria could affect their host’s sexual signals (e.g. chemical attractants) to either increase or decrease attractiveness (Burand et al., 2005; Sharon et al., 2010). Sharon et al. (2010) found that diet could influence the prevalence of certain commensal gut microbes in D. melanogaster, which in turn altered mate choice within only two generations of switching populations to a new diet. Our experiments reveal that the impact of altered microbiota on host attractiveness can vary based on both the fly line analyzed and the timepoint investigated. However, the particular signals responsible for the variation in attractiveness, as well as the contributions of the microbiome, host genotype, or their interaction remain to be elucidated.
Wolbachia and female attractiveness
We assessed the importance of Wolbachia in female attractiveness by separately analyzing the data of the TC treatment females. The backcrosses performed in this treatment were intended to restore any perturbed gut microbes, allowing us to measure the effects of Wolbachia while limiting disruptions to other commensal bacteria. This TC treatment largely normalized the effects of tetracycline treatment with respect to attractiveness, as there was no line effect on the incidence of untreated females receiving matings (Fig. 1b; LRT, , p = 0.72), a result that was repeated in our second assay (LRT, , p = 0.78). Therefore, the variable response of lines to microbiome disruption was eliminated when crosses were carried out to restore each line’s microbiome. In our initial experiments, the TC lines exhibited mildly decreased female attractiveness as a whole, as untreated females received the majority of matings among nine out of ten tested lines (Logistic Regression; z9 = 2.72, p = 0.007; Fig. 1b). The reason for this decrease in attractiveness is unknown, but this minor decrease in attractiveness was not repeated in our later assay on four lines (t3 = 0.82, p = 0.47).
Nevertheless, our experimental design allowed us to specifically evaluate the influence of Wolbachia on female attractiveness. We compared the effects of the TC treatment between uninfected and Wolbachia-infected lines. For the four uninfected lines, the U and TC competing females should harbor highly similar microbiota. In contrast, for the six infected lines, the mate choice assays included competition between females with (U) and without Wolbachia (TC). We found that the Wolbachia infection status of a line had no effect on the incidence of untreated females receiving matings (Logistic Regression; z1 = 0.05, p = 0.96). Even in our initial assay when the TC treatment decreased attractiveness, the TC treatment affected all lines similarly, whether Wolbachia was initially present or not. Although we used previously described backcrossing methods to reintroduce non-Wolbachia microbes (Fast et al., 2011), the effect of the TC treatment on the attractiveness of Wolbachia-uninfected lines suggests that this treatment did not perfectly reintroduce each line’s microbiome, or, alternatively, that the antibiotic treatment had long-lasting effects. However, regardless of the efficacy of the TC treatment, our methods allow us to conclude that Wolbachia infection did not have a significant effect on female attractiveness in Drosophila melanogaster, neither in a global sense nor in a line-specific manner. This result is unexpected given the results of previous studies showing that Wolbachia can influence fecundity (Fry & Rand, 2002; Fry et al., 2004) as well as insulin signaling levels (Ikeya et al., 2009), which can influence female attractiveness in fruit flies (Kuo et al., 2012).
We also collected data on female mass in all lines and treatments (Table 2). We found a significant effect of line on mass (LRT: , p < 0.0001), but no significant effect of treatment (, p = 0.21) or the Wolbachia infection status of lines (, p = 0.11). However, we did find a treatment × infection status effect (, p = 0.001), where Wolbachia infected lines showed a significant decrease in mass under the T and TC treatments vs. the untreated controls, while this pattern was absent in lines that lack Wolbachia symbionts. This pattern is consistent with a Wolbachia-induced increase in female body size, possibly via their manipulation of the insulin signaling pathway (Ikeya et al., 2009). While such a manipulation would be consistent with our hypothesis that Wolbachia can manipulate the attractiveness of their female hosts, our behavioural measurements show that such increased size does not manifest in increased attractiveness. However, we should note that the Wolbachia present in our D. melanogaster lines is a relatively young symbiont (Riegler et al., 2005; Richardson et al., 2012), and may have not had sufficient time to adapt to their fruit fly hosts to the level of manipulating a trait as complex as female attractiveness.
Table 2.
Female body mass (± SE). Mass data represent the average mass of 4–6 females weighed together in milligrams.
| Treatment | |||
|---|---|---|---|
| DGRP line | U | T | TC |
| 85 | 0.56 (0.03) | 1.19 (0.04) | 1.03 (0.01) |
| 313 | 0.94 (0.01) | 0.71 (0.01) | 0.82 (0.02) |
| 371 | 0.99 (0.02) | 0.92 (0.01) | 0.88 (0.01) |
| 399 | 1.04 (0.02) | 1.07 (0.01) | 1.03 (0.02) |
| 181 | 0.90 (0.01) | 0.87 (0.02) | 0.89 (0.01) |
| 338 | 1.31 (0.03) | 1.03 (0.03) | 1.39 (0.03) |
| 362 | 1.25 (0.01) | 1.14 (0.02) | 1.18 (0.01) |
| 555 | 1.19 (0.01) | 0.89 (0.02) | 0.57 (0.02) |
| 738 | 0.99 (0.01) | 1.18 (0.01) | 1.17 (0.01) |
Our results also demonstrate the importance of including proper controls in such tests, as we would have improperly concluded that Wolbachia increased female attractiveness had we not included uninfected, antibiotic-treated lines in our assay. Wolbachia are well known for manipulating host reproduction to further their spread in the host population (Werren, 1997; Miller & Schneider, 2012). We initially hypothesized that in the absence of such drastic manipulations, Wolbachia could manipulate the reproductive opportunities of their female hosts by increasing their attractiveness. However, such behavioural manipulations appear to be absent in this species.
Conclusions
Commensal bacteria and symbionts can significantly affect the attractiveness and mate choice of their hosts (Sharon et al., 2010; Wittman & Fedorka, 2014). However, our data show that the impact of resident microbes on host attractiveness is more complex than has been previously appreciated, in that microbiome disruption may affect female attractiveness in a genotype- or even time-dependent manner. The effects of the microbiome on attractiveness likely depend on the bacterial composition and/or the host genotype, although the particular mechanisms underlying this relationship are not yet known. Our results also demonstrate the importance of testing multiple genotypes to make conclusions relevant to natural populations, rather than relying on just one or two genotypes to make broad-scale conclusions. Even though Wolbachia are extraordinarily prevalent arthropod symbionts that are known to manipulate their hosts extensively, we did not find any evidence that Wolbachia alter female attractiveness or male mating behaviour in D. melanogaster, either through a universal manipulation or through a strain × host dependent interaction.
Acknowledgements
We thank Erin Tudor, Quynh Tran, and Sharon Ornelas for help with the mate choice assays, Ganeshkumar Miriyala for assisting with the diagnostic PCR and qPCR, and Harmit Malik for helpful discussion. DA and DP were supported in part by NIH grant GM102279.
References
- Andersson M. Sexual Selection. Princeton, NJ: Princeton University Press; 1994. [Google Scholar]
- Baldo L, Hotopp JCD, Jolley Ka, Bordenstein SR, Biber Sa, Choudhury RR, et al. Multilocus sequence typing system for the endosymbiont Wolbachia pipientis. Appl. Environ. Microbiol. 2006;72:7098–7110. doi: 10.1128/AEM.00731-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buellesbach J, Greim C, Raychoudhury R, Schmitt T. Asymmetric assortative mating behaviour reflects incomplete pre-zygotic isolation in the Nasonia species complex. Ethology. 2014;120:834–843. [Google Scholar]
- Burand JP, Tan W, Kim W, Nojima S, Roelofs W. Infection with the insect virus Hz-2v alters mating behavior and pheromone production in female Helicoverpa zea moths. J. Insect Sci. 2005;5:6. doi: 10.1093/jis/5.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne PG, Rice WR. Evidence for adaptive male mate choice in the fruit fly Drosophila melanogaster. Proc. R. Soc. Lond. B Biol. Sci. 2006;273:917–922. doi: 10.1098/rspb.2005.3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champion De Crespigny FE, Pitt TD, Wedell N. Increased male mating rate in Drosophila is associated with Wolbachia infection. J. Evol. Biol. 2006;19:1964–1972. doi: 10.1111/j.1420-9101.2006.01143.x. [DOI] [PubMed] [Google Scholar]
- Champion De Crespigny FE, Wedell N. Mate preferences in Drosophila infected with Wolbachia? Behav. Ecol. Sociobiol. 2007;61:1229–1235. [Google Scholar]
- Engelstädter J, Hurst GDD. The Ecology and Evolution of Microbes that Manipulate Host Reproduction. Annu. Rev. Ecol. Evol. Syst. 2009;40:127–149. [Google Scholar]
- Fast EM, Toomey ME, Panaram K, Danielle D, Kolaczyk ED, Frydman HM. Wolbachia enhance Drosophila stem cell proliferation and target the germline stem cell niche. Science. 2011;334:990–992. doi: 10.1126/science.1209609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry aJ, Palmer MR, Rand DM. Variable fitness effects of Wolbachia infection in Drosophila melanogaster. Heredity. 2004;93:379–389. doi: 10.1038/sj.hdy.6800514. [DOI] [PubMed] [Google Scholar]
- Fry AJ, Rand DM. Wolbachia interactions that determine Drosophila melanogaster survival. Evolution. 2002;56:1976–1981. doi: 10.1111/j.0014-3820.2002.tb00123.x. [DOI] [PubMed] [Google Scholar]
- Harcombe W, Hoffmann AA. Wolbachia effects in Drosophila melanogaster: in search of fitness benefits. J. Invertebr. Pathol. 2004;87:45–50. doi: 10.1016/j.jip.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Hedges LM, Yamada R, O’Neill SL, Johnson KN. The small interfering RNA pathway is not essential for Wolbachia-mediated antiviral protection in Drosophila melanogaster. Appl. Environ. Microb. 2012;78:6773–6776. doi: 10.1128/AEM.01650-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgenboecker K, Hammerstein P, Schlattmann P, Telschow A, Werren JH. How many species are infected with Wolbachia? - A statistical analysis of current data. FEMS Microbiol. Lett. 2008;281:215–220. doi: 10.1111/j.1574-6968.2008.01110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann AA. Partial cytoplasmic incompatibility between two Australian populations of Drosophila melanogaster. Entomoligia Exp. Appl. 1988;48:61–67. [Google Scholar]
- Hoffmann AA, Turelli M. Cytoplasmic incompatibility in insects. In: O’Neill SL, Hoffmann AA, Werren JH, editors. Influential Passengers: Inherited Microorganisms and Arthropod Reproduction. Oxford: Oxford University Press; 1997. pp. 42–80. [Google Scholar]
- Hughes DP, Brodeur J, Thomas F. Host Manipulations by Parasites. Oxford: Oxford University Press; 2012. [Google Scholar]
- Ikeya T, Broughton S, Alic N, Grandison R, Partridge L. The endosymbiont Wolbachia increases insulin/IGF-like signalling in Drosophila. Proc. Biol. Sci. 2009;276:3799–3807. doi: 10.1098/rspb.2009.0778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasa Y, Pomiankowski A, Nee S. The evolution of costly mate preferences II. The handicap principle. Evolution. 1991;45:1431–1442. doi: 10.1111/j.1558-5646.1991.tb02646.x. [DOI] [PubMed] [Google Scholar]
- Jaenike J, Dyer Ka, Cornish C, Minhas MS. Asymmetrical reinforcement and Wolbachia infection in Drosophila. PLoS Biol. 2006;4:1852–1862. doi: 10.1371/journal.pbio.0040325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koukou K, Pavlikaki H, Kilias G, Werren JH, Bourtzis K, Alahiotis SN. Influence of antibiotic treatment and Wolbachia curing on sexual isolation among Drosophila melanogaster cage populations. Evolution. 2006;60:87–96. [PubMed] [Google Scholar]
- Kuo TH, Fedina TY, Hansen I, Dreisewerd K, Dierick Ha, Yew JY, et al. Insulin signaling mediates sexual attractiveness in Drosophila. PLoS Genet. 2012;8 doi: 10.1371/journal.pgen.1002684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long TaF, Pischedda A, Stewart AD, Rice WR. A cost of sexual attractiveness to high-fitness females. PLoS Biol. 2009;7:e1000254. doi: 10.1371/journal.pbio.1000254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay TFC, Richards S, Stone Ea, Barbadilla A, Ayroles JF, Zhu D, et al. The Drosophila melanogaster Genetic Reference Panel. Nature. 2012;482:173–178. doi: 10.1038/nature10811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markov aV, Lazebny OE, Goryacheva II, Antipin MI, Kulikov aM. Symbiotic bacteria affect mating choice in Drosophila melanogaster. Anim. Behav. 2009;77:1011–1017. [Google Scholar]
- Miller WJ, Ehrman L, Schneider D. Infectious speciation revisited: Impact of symbiont-depletion on female fitness and mating behavior of Drosophila paulistorum. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1001214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WJ, Schneider D. Endosymbiotic microbes as adaptive manipulators of arthropod behavior and natural driving sources of host speciation. In: Hughes DP, Brodeur J, Thomas F, editors. Host Manipulation by Parasites. Oxford: Oxford University Press; 2012. pp. 119–137. [Google Scholar]
- Moreau J, Bertin a, Caubet Y, Rigaud T. Sexual selection in an isopod with Wolbachia-induced sex reversal: Males prefer real females. J. Evol. Biol. 2001;14:388–394. [Google Scholar]
- Osborne SE, Iturbe-Ormaetxe I, Brownlie JC, O’Neill SL, Johnson KN. Antiviral protection and the importance of Wolbachia density and tissue tropism in Drosophila simulans. Appl. Environ. Microbiol. 2012;78:6922–6929. doi: 10.1128/AEM.01727-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y, Nielsen JE, Cunningham JP, McGraw Ea. Wolbachia infection alters olfactory-cued locomotion in Drosophila spp. Appl. Environ. Microbiol. 2008;74:3943–3948. doi: 10.1128/AEM.02607-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randerson JP, Jiggins FM, Hurst LD. Male killing can select for male mate choice: a novel solution to the paradox of the lek. Proc. R. Soc. Lond. 2000;267:867–874. doi: 10.1098/rspb.2000.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice WR. Requisite mutational load, pathway epistasis, and deterministic mutation accumulation in sexual versus asexual populations. Genetica. 1998;102:71–81. [PubMed] [Google Scholar]
- Richardson MF, Weinert La, Welch JJ, Linheiro RS, Magwire MM, Jiggins FM, et al. Population Genomics of the Wolbachia Endosymbiont in Drosophila melanogaster. PLoS Genet. 2012;8 doi: 10.1371/journal.pgen.1003129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley EV, Wong ACN, Douglas AE. Microbe-dependent and nonspecific effects of procedures to eliminate resident microbiota from Drosophila melanogaster. Appl. Environ. Microb. 2013;79:3209–3214. doi: 10.1128/AEM.00206-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riegler M, Sidhu M, Miller WJ, O’Neill SL. Evidence for a global Wolbachia replacement in Drosophila melanogaster. Curr. Biol. 2005;15:1428–1433. doi: 10.1016/j.cub.2005.06.069. [DOI] [PubMed] [Google Scholar]
- Rowe L, Houle D. The lek paradox and the capture of genetic variance by condition dependent traits. Proc. R. Soc. Lond. 1996;263:1415–1421. [Google Scholar]
- Schilthuizen M, Stouthamer R. Horizontal transmission of parthenogenesis-inducing microbes in Trichogramma wasps. Proc. R. Soc. Lond. 1997;264:361–366. doi: 10.1098/rspb.1997.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon G, Segal D, Ringo JM, Hefetz A, Zilber-Rosenberg I, Rosenberg E. Commensal bacteria play a role in mating preference of Drosophila melanogaster. Proc. Natl. Acad. Sci. 2010;107:20051–20056. doi: 10.1073/pnas.1009906107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp NP, Agrawal AF. Sexual selection and the random union of gametes: testing for a correlation in fitness between mates in Drosophila melanogaster. Am. Nat. 2009;174:613–622. doi: 10.1086/605960. [DOI] [PubMed] [Google Scholar]
- Sullivan J, Jaenike J. Male-killing Wolbachia and male mate choice: A test with Drosophila innubila. Evol. Ecol. Res. 2006;8:91–102. [Google Scholar]
- Teixeira L, Ferreira Á, Ashburner M. The bacterial symbiont Wolbachia induces resistance to RNA viral infections in Drosophila melanogaster. PLoS Biol. 2008;6:2753–2763. doi: 10.1371/journal.pbio.1000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vala F, Egas M, Breeuwer JaJ, Sabelis MW. Wolbachia affects oviposition and mating behaviour of its spider mite host. J. Evol. Biol. 2004;17:692–700. doi: 10.1046/j.1420-9101.2003.00679.x. [DOI] [PubMed] [Google Scholar]
- Werren JH. Biology of Wolbachia. Annu. Rev. Entomol. 1997;42:587–609. doi: 10.1146/annurev.ento.42.1.587. [DOI] [PubMed] [Google Scholar]
- Werren JH, Baldo L, Clark ME. Wolbachia: master manipulators of invertebrate biology. Nat. Rev. Microbiol. 2008;6:741–751. doi: 10.1038/nrmicro1969. [DOI] [PubMed] [Google Scholar]
- Wittman T, Fedorka KM. Male mate choice for unparasitized females in Drosophila melanogaster. J. Insect Behav. 2014;28:37–43. [Google Scholar]
- Wong AC-N, Chaston JM, Douglas AE. The inconstant gut microbiota of Drosophila species revealed by 16S rRNA gene analysis. ISME J. 2013;7:1922–1932. doi: 10.1038/ismej.2013.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahavi A. Mate selection - a selection for a handicap. J. Theor. Biol. 1975;53:205–214. doi: 10.1016/0022-5193(75)90111-3. [DOI] [PubMed] [Google Scholar]