Abstract
Target selection is often biased by an observer’s recent experiences. However, not much is known about whether these selection biases influence behavior across different effectors. For example, does looking at a red object make it easier to subsequently reach towards another red object? In the current study, we asked observers to find the uniquely colored target object on each trial. Randomly intermixed pre-trial cues indicated the mode of action: either an eye movement or a visually guided reach movement to the target. In Experiment 1, we found that priming of popout, reflected in faster responses following repetition of the target color on consecutive trials, occurred regardless of whether the effector was repeated from the previous trial or not. In Experiment 2, we examined whether an inhibitory selection bias away from a feature could transfer across effectors. While priming of popout reflects both enhancement of the repeated target features and suppression of the repeated distractor features, the distractor previewing effect isolates a purely inhibitory component of target selection in which a previewed color is presented in a homogenous display and subsequently inhibited. Much like priming of popout, intertrial suppression biases in the distractor previewing effect transferred across effectors. Together, these results suggest that biases for target selection driven by recent trial history transfer across effectors. This indicates that representations in memory that bias attention towards or away from specific features are largely independent from their associated actions.
Keywords: Priming of pop-out, Distractor previewing effect, Visually guided reaching, Eye movements, Intertrial effects
Frequently, multiple objects compete for limited attention resources. The selection of a single object can be guided by an observer’s goals, such that attention is directed towards task-relevant objects (e.g., Green & Anderson, 1956; Posner, 1980). However, sometimes task-irrelevant factors, such as recent experience or physical salience, can instead determine the allocation of attention (e.g., Awh, Belopolsky, & Theeuwes, 2012; Found & Müller, 2010; Itti & Koch, 2001; Theeuwes, 1992; Tipper & Cranston, 1985).
For example, Maljkovic & Nakayama (1994) asked observers to find a uniquely colored diamond target and press a key to indicate which side of that diamond was chipped. Targets were selected randomly on each trial to be either a green diamond among red diamonds, or a red diamond among green diamonds. Keypress response times were faster when the target color was repeated on consecutive trials relative to when it was switched. This occurred regardless of the strategic value of adopting a bias towards recently selected features, suggesting that this was not an explicit, goal-directed approach. This phenomenon, known as priming of popout (PoP), suggests that attention is automatically biased towards recently selected target features. PoP is not limited to psychophysical responses; eye and reach movements towards popout targets are also speeded when target features are repeated on consecutive trials (McPeek, Maljkovic, & Nakayama, 1999; Song & Nakayama, 2006).
In typical PoP studies, only two target features are used. Thus, if the target color is repeated from one trial to the next, the distractor colors are repeated as well. As a result, PoP often reflects a combination of target enhancement and distractor suppression (e.g., Maljkovic & Nakayama, 1994). However, it is possible to isolate a component of intertrial target selection bias that is purely inhibitory. For example, Goolsby, Grabowecky, & Suzuki (2005) had observers perform a similar popout discrimination task. On some trials, however, no target was present; instead, all objects were homogenously colored, and thus no response was required. Responses on the next trial were slower when the target matched the color of the homogenous items, or “previewed color,” from the previous target absent trial. Conversely, responses were faster if the distractors matched the previewed color. Together, this pattern indicates that the previewed color was suppressed, and thus de-prioritized for selection on a subsequent trial. This distractor previewing effect (DPE; see also; Ariga & Kawahara, 2004; Lleras, Kawahara, Wan, & Ariga, 2008) demonstrates a purely inhibitory effect in which selection is automatically biased away from a recently seen feature.
Research on these types of selection biases is typically focused on tasks requiring only a single response effector. However, in the real world, dynamic behavior frequently requires a mixture of multiple types of actions. For example, tasks ranging from making a sandwich (e.g., Hayhoe, Shrivastava, Mruczek, & Pelz, 2003) to driving a car require a series of intermixed eye and hand movements towards a variety of different objects.
Thus, in the present study, we examine whether selection biases from recent experience transfer across effectors. There is a great deal of evidence to suggest that attention and action systems are tightly intertwined, both at the behavioral (e.g., Song & Nakayama, 2006; Spivey, Grosjean, & Knoblich, 2005) and neurophysiological level (e.g., Gallivan, McLean, Smith, & Culham, 2011; Song, Rafal, & McPeek, 2011). Furthermore, PoP shows similar effects for attention, eye movements, and reach movements respectively, and even transfers from one type of hand movement to another (Moher & Song, 2014). Thus, it is plausible that a shared, motor-unspecific priority map (e.g., Zehetleitner, Hengeloh, & Müller, 2011; see also, Song, Takahashi, & McPeek, 2007) is responsible for biasing attention towards recently selected target features regardless of the mode of action required. However, some theories of PoP suggest that it is not just a target feature that is encoded in memory, but rather an entire set of events from a previous trial that is encoded and biases subsequent target selection (e.g., Hillstrom, 2000; Huang, Holcombe, & Pashler, 2004). It could therefore be the case that target selection generates event files in memory (Hommel, 2004) in which features that bias subsequent selection are bound together with their associated effectors. This would result in intertrial repetition effects only when the same mode of action was required on consecutive trials.
We asked observers to either look at or reach to a uniquely colored target object on each trial. The effector to be used (eye or hand) was cued shortly before display onset, and these cues were intermixed randomly. We examined whether PoP (Experiments 1 and 2) and DPE (Experiment 2) transferred across different effectors. In other words, do the properties of stimuli seen on the previous trial affect selection on a subsequent trial even when a different means of action is required?
Experiment 1: Priming of popout transfers across effectors
Method
Participants
Brown University undergraduates and community members (7 female, 11 male, mean age: 19.1 years) participated in exchange for course credit or monetary compensation. All participants were right handed with normal or corrected-to-normal vision and normal color vision. The protocol was approved by the Brown University Institutional Review Board.
Apparatus
The methods for the current study were adapted largely from that of Moher & Song (2013, 2014). Stimuli were projected from behind a plexiglass display that was arranged upright on a table perpendicular to the observer’s line of vision, facing the seated observer at a distance of approximately 48 cm. Three-dimensional hand position was recorded at a rate of approximately 240 Hz using an electromagnetic position and orientation recording system (Liberty, Polhemus; http://polhemus.com/) with a measuring error of .03 cm root mean square. A motion tracking marker was secured with a Velcro strap near the tip of each observer’s right index finger. The observer’s index finger was rested on a Styrofoam block placed in front of them on the table, located 27 cm from the screen along the z-dimension (i.e., the axis that is bounded by the observer and the display). The finger was aligned with the bottom of the display along the y-dimension (i.e. the axis that is bounded by the top and bottom of the display), and the horizontal midline of the display along the x-dimension (i.e., the axis that is bounded by the left and right sides of the display). Simultaneously, eye position was recorded with a head-mounted Eyelink II eyetracker (SR Research, Ottawa, ON) at a rate of approximately 240 Hz. Stimulus presentation was conducted using custom software designed with MATLAB (Mathworks, http://uk.mathworks.com/) and Psychtoolbox (Brainard, 1997).
Stimuli
All stimuli appeared on a black background. A fixation cross appeared at the center with a width and length of 0.7 cm (0.8° of visual angle). Three diamonds appeared during each trial, each with a 3 cm diameter (4.0°). On each trial, the diamonds were placed at 4, 8, and 12 o’clock equally spaced on an imaginary circle surrounding fixation with a radius of 11 cm (13.1°), with an inter-item distance of 18.9 cm (measured from center to center). The diamonds were rendered in either red or green. The two colors were approximately equiluminant using photometer calibration (green: 28.5 cd/m2, red: 32 cd/m2). On each trial, one diamond appeared in the randomly selected target color and the remaining diamonds were rendered in the other color. The target location was selected randomly on each trial.
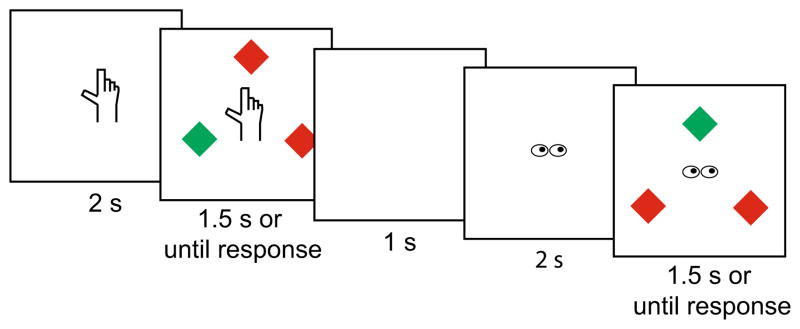
Cues preceding each trial indicated the effector required on the upcoming trial (Fig. 1). An equal number of trials for each effector (eye and hand) were presented in a randomly intermixed order in each block. Cues were either a cartoon image of a pair of eyes (from http://www.proprofs.com/), measuring approximately 1.9 cm vertically (2.3°) and 2. 7 cm horizontally (3.2°), or a cartoon image of a hand (from http://www.clker.com/) measuring approximately 2.6 cm vertically (3.1°) and 2 cm horizontally (2.4°).
Fig. 1.
A sample sequence of two trials from Experiment 1. The target was the uniquely colored object. The second trial in this example is an effector switch, since the previous trial cued a reach movement response but the current trial cues an eye movement response. The second trial is also a target color repeat, because in both trials the target is a green color singleton
Procedure
Nine-point calibration was conducted for eye and hand position, and drift correction for eye movements was executed throughout the experiment as needed. Participants were instructed to keep the index finger on their right hand in the starting position and their eyes fixated at the center. Each trial began with the presentation of the response cue informing participants whether they should respond with an eye or reach movement to the unique target on the upcoming trial. After 2 s, the three diamonds appeared (Fig. 1) and the response cue remained onscreen. Participants either reached to or looked at the uniquely colored diamond.
If the participant moved their eyes during a reach trial or vice versa, or they did not respond within 1.5 s following stimulus onset, the trial was counted as incorrect. The display remained on the screen for an additional 200 ms after the participant’s response to encourage participants to rest their finger briefly on the target. Participants were given auditory feedback following every trial—a high beep for accurate responses, low beep for inaccurate responses, two low beeps if the time limit elapsed before a response was executed, and a very low-pitched beep if they moved the wrong effector. There was a 1-s intertrial interval during which a blank black screen was presented
The experiment began with 24 practice trials, followed by 8 blocks of 50 trials each.1 Each session lasted approximately 1 h.
Data analysis
When the participant’s finger came within approximately 1.3 cm of the display on the z-dimension and simultaneously within approximately 2 cm of the target diamond on the x and y dimensions within the 1.5-s time limit, a response was considered correct. If this threshold was passed for a non-target diamond (a selection error) or no response was registered during the time limit (a timeout error), the trial was counted as incorrect.
Reach movement data were analyzed offline using custom MATLAB (Mathworks) software. Three-dimensional resultant speed scalars were created for each trial using a differentiation procedure in MATLAB. These scalars were then submitted to a 2nd order, low-pass Butterworth filter with a cutoff of 10 Hz. Movement onset was calculated as the first time point on each trial after stimulus onset at which reach movement speed exceeded 25.4 cm/s (consistent with previous research in our lab; see e.g., Moher & Song, 2013, Moher & Song, 2014). Movement offset was defined as the first subsequent measurement on each trial when speed decreased to below 25.4 cm/s. Initiation latency was defined as the time elapsed between stimulus onset and movement onset. Movement time was defined as the time elapsed between movement onset and movement offset. Similar measures were calculated using 2D eye movement data with a speed threshold for defining the beginning and end of a movement set at 35°/s (similar to Godijn & Theeuwes, 2004; Ludwig & Gilchrist, 2002) using a 40 Hz filter cutoff for the Butterworth filter (based on visual inspection of Butterworth filter ranges, done blind to conditions, for minimum distortion of event timing).
Trajectories for calculating curvature were measured in two-dimensional XY space by calculating a line from the start to the end point of the movement, and measuring the orthogonal deviation of the actual movement from that line at each sample throughout the movement. Curvature was defined as the maximum point of deviation (unsigned) in centimeters divided by the length of the line from the start to the end points of the eye or reach movement.
All dependent measures not including selection error rates were calculated for correct trials only. For both eye and hand movements, each individual trial was inspected visually (e.g., Song & Nakayama, 2006; Moher & Song, 2013, 2014) for trials where the default threshold clearly missed part of the movement or included substantial movement back to the starting point. Thresholds were adjusted manually to more appropriate levels when necessary (1.6 % of all trials in Experiment 1, 1.4 % in Experiment 2).
In examining error responses, we focused exclusively on selection errors and did not include timeout errors or device errors as these were frequently attributable to drift in the eyetracking camera, making those error types difficult to interpret. We did not conduct drift correction in between trials because we did not want to interfere with intertrial effects of target color and response effector.
For all analyses, we removed all trials in which participants used the wrong effector on the current or previous trial, trials where the previous response was inaccurate, and the first trial of each block (16.6 % of all trials in Experiment 1 and 12.7 % of all trials in Experiment 2). In addition, using a recursive trimming procedure that defined outliers as at least 3.5 standard deviations above or below the mean within each condition for each participant (exact number of standard deviations was determined dynamically according to the number of observations per condition; adopted from Van Selst & Jolicoeur, 1994), we removed any trial that was considered an outlier in each experimental condition along measures of initiation latency, movement time, or curvature for eye and reach movements (3.7 % of all trials in Experiment 1 and 2.1 % of all trials in Experiment 2). Finally, we removed trials for which a large number of movement samples were dropped due to computer error (0.7 % of all trials in Experiment 1 and 0.3 % of all trials in Experiment 2). However, this criterion was not applied to selection error analyses, as the online calculation of accuracy was unaffected by missing data in the trajectory of the movement.
Results
Below we report the results of 2×2 ANOVAs for effector (repeat vs. switch) and target color (repeat vs. switch) on initiation latency for eye and reach movement trials separately. All direct comparisons involve measures from the same effector; in other words, responses on eye movement trials are compared only to responses on eye movement trials. The results sections in the present manuscript focus primarily on initiation latency, as this is a measure that has been shown consistently to be sensitive to variations in target selection processes and intertrial priming effects across eye and hand movements in previous studies (e.g., Caddigan & Lleras, 2010; McPeek, Maljkovic, & Nakayama, 1999; Moher & Song, 2014). Furthermore, latencies fall along relatively similar timescales for eye and hand movements, whereas measures of movement time and curvature differ a great deal in magnitude between eye and hand movements (see e.g., Table 1). However, descriptive statistics and statistical outcome for 2×2 ANOVAS for other dependent variables, including movement time, curvature, and selection errors for both eye and reach movements, are reported for both experiments in Tables 1, 2 and 3.
Table 1.
Data from Experiment 1. Error terms reflect standard error of the mean (SEM)
| Dependent variable | Response mode | Color
|
Main effects
|
Interaction | ||
|---|---|---|---|---|---|---|
| Repeat | Switch | Effector | Color | |||
| Eye movements | ||||||
| Initiation latency | *** | * | ||||
| Repeat | 404±14 ms | 436±15 ms | ||||
| Switch | 418±15 ms | 438±18 ms | ||||
| Movement Time | ||||||
| Repeat | 72±2 ms | 73±3 ms | ||||
| Switch | 70±2 ms | 71±3 ms | ||||
| Movement curvature | ||||||
| Repeat | 0.043±.003 | 0.047±.005 | ||||
| Switch | 0.044±.003 | 0.049±.007 | ||||
| Selection error rate | ** | |||||
| Repeat | 0.7±0.3 % | 3.8±1.1 % | ||||
| Switch | 0.2±0.02 % | 2.8±0.7 % | ||||
| Reach movements | ||||||
| Initiation latency | * | *** | ||||
| Repeat | 439±10 ms | 457±12 ms | ||||
| Switch | 453±12 ms | 467±13 ms | ||||
| Movement time | ||||||
| Repeat | 450±11 ms | 448±10 ms | ||||
| Switch | 452±11 ms | 452±11 ms | ||||
| Movement curvature | * | |||||
| Repeat | 0.094±.006 | 0.103±.007 | ||||
| Switch | 0.094±.006 | 0.102±.007 | ||||
| Selection error rate | ||||||
| Repeat | 0 | 0 | ||||
| Switch | 0 | 0 | ||||
p<.05,
p<.01,
p<.001
Table 2.
Priming of popout (PoP) data from Experiment 2. Error terms reflect SEM
| Dependent variable | Response mode | Color
|
Main effects
|
Interaction | ||
|---|---|---|---|---|---|---|
| Repeat | Switch | Effector | Color | |||
| Eye movements | ||||||
| Initiation latency | *** | |||||
| Repeat | 410±10 ms | 444±9 ms | ||||
| Switch | 434±14 ms | 454±12 ms | ||||
| Movement time | ||||||
| Repeat | 70±4 ms | 69±4 ms | ||||
| Switch | 67±4 ms | 71±5 ms | ||||
| Movement curvature | ||||||
| Repeat | 0.050±.005 | 0.057±.007 | ||||
| Switch | 0.047±.004 | 0.055±.007 | ||||
| Selection error rate | ||||||
| Repeat | 0.4±0.4 % | 1.3±0.6 % | ||||
| Switch | 1.0±0.5 % | 1.0±0.4 % | ||||
| Reach movements | ||||||
| Initiation latency | *** | |||||
| Repeat | 502±15 ms | 531±15 ms | ||||
| Switch | 506±17 ms | 529±18 ms | ||||
| Movement time | * | |||||
| Repeat | 455±11 ms | 447±10 ms | ||||
| Switch | 457±10 ms | 458±11 ms | ||||
| Movement curvature | ||||||
| Repeat | 0.096±.006 | 0.104±.008 | ||||
| Switch | 0.096±.005 | 0.098±005 | ||||
| Selection error rate | ||||||
| Repeat | 0 | 0 | ||||
| Switch | 0 | 0 | ||||
p<.05,
p<.01,
p<.001
Table 3.
Distractor previewing effect (DPE) data from Experiment 2. Error terms reflect SEM
| Dependent variable | Response mode | Previewed color
|
Main effects
|
Interaction | ||
|---|---|---|---|---|---|---|
| Repeat | Switch | Effector | Color | |||
| Eye movements | ||||||
| Initiation latency | * | * | ||||
| Repeat | 440±15 ms | 469±12 ms | ||||
| Switch | 421±10 ms | 435±10 ms | ||||
| Movement time | ||||||
| Repeat | 70±5 ms | 68±4 ms | ||||
| Switch | 70±4 ms | 73±6 ms | ||||
| Movement curvature | ||||||
| Repeat | 0.052±.006 | 0.051±.005 | ||||
| Switch | 0.052±.005 | 0.053±.007 | ||||
| Selection error rate | ||||||
| Repeat | 0 | 0 | ||||
| Switch | 0.3±0.3 % | 0.7±0.5 % | ||||
| Reach movements | ||||||
| Initiation latency | * | ** | ||||
| Repeat | 524±17 ms | 565±20 ms | ||||
| Switch | 503±16 ms | 541±22 ms | ||||
| Movement time | *** | |||||
| Repeat | 454±11 ms | 449±10 ms | ||||
| Switch | 464±9 ms | 467±11 ms | ||||
| Movement curvature | ||||||
| Repeat | 0.100±.007 | 0.111±.009 | ||||
| Switch | 0.098±.007 | 0.090±.006 | ||||
| Selection error rate | ||||||
| Repeat | 0 | 0 | ||||
| Switch | 0 | 0 | ||||
p<.05,
p<.01,
p<.001
Eye movement trials
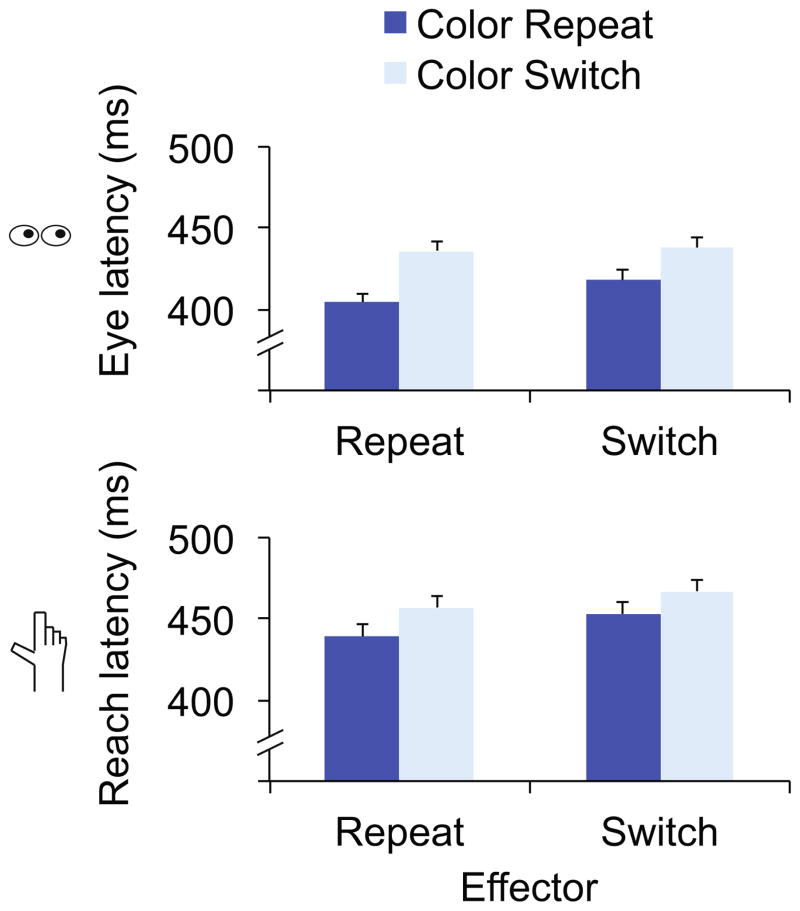
Initiation latencies for eye movements were faster when the target color was repeated from the previous trial (411 ms) relative to when it was switched (437 ms), F(1,17)=38.7, p<.001, ηp2=.70. There was no main effect of whether the effector was repeated from the previous trial, F(1,17)=1.8, p=.2. We did, however, find an interaction between effector and target color, F(1,17)=4.5, p=.05, ηp2=.21 (Figure 2, top; see Table 1 for more detailed description of data).
Fig. 2.
Eye movement initiation latencies (top) and reach movement initiation latencies (bottom) for Experiment 1. Data are separated by effector (repeat or switch from the previous trial) and target color (repeat or switch from the previous trial). Error bars Within-subject error term (Loftus & Masson, 1994)
To further parse this interaction, we conducted simple main effects analyses for target color repetition on effector repeat and effector switch trials separately. Eye movement initiation latencies were 32 ms faster on effector repeat trials, F(1,17)=39.9, p<.001, ηp2=.70. Furthermore, eye movement initiation latencies were 20 ms faster on effector switch trials, F(1,17)=15.6, p=.001, ηp2=.48. Thus, while the interaction reflects a reduction in the magnitude of the color repetition benefit on effector switch trials relative to effector repeat trials, these simple main effects analyses reveal that repeating the target color speeds eye movements even when the effector was switched from the previous trial. Thus, PoP transfers from reach to eye movement responses.
Reach movement trials
A similar pattern of results was observed in the reach movement data. Initiation latencies were faster on target color repeat trials (446 ms) than switch trials (462 ms), F(1,17)=21.43, p<.001, ηp2=.25. Latencies were also faster when the previous trial also required a reach movement (448 ms) rather than an eye movement (460 ms), F(1,17)=5.74, p=.028, ηp2=.56. This suggests the possibility of a slight overall cost in responding, regardless of target color, when switching effectors from the previous trial.
For reach movement latencies, however, we found no interaction between effector and target color, F(1,17)<1 (Fig. 2, bottom). Thus, for both eye and reach movements, biases towards the color of a recently selected target impact behavior regardless of whether the effector is repeated from the previous trial.
Experiment 2: Distractor previewing effect transfers across effectors
The results of Experiment 1 demonstrate that target selection biases transfer across different response effectors. This suggests that representations of target features are stored in memory largely independently from their associated actions. This result builds on previous work in which we showed transfer of PoP from one type of hand movement (a keypress) to another (a reach movement; Moher & Song, 2014; see also Makovski & Jiang, 2011).
However, while PoP is typically thought to reflect a bias toward recently selected target features, it may also reflect inhibition of recently non-selected distractor features when distractor colors are also repeated, as in the present design (e.g., Maljkovic & Nakayama, 1994). Although inhibition plays a critical role in guiding attention and action (e.g., Moher, Abrams, Egeth, Yantis, & Stuphorn, 2011; Tipper, Meegan, & Howard, 2002; Watson & Humphreys, 1997), less is known about whether and how inhibitory biases transfer when movement outputs differ from one moment to the next.
In Experiment 2, we examine whether exclusively inhibitory biases reflected in the DPE transfer from eye to reach movements and vice versa. The DPE has a number of empirical characteristics that suggest it draws on distinct mechanisms from PoP (e.g., Ariga & Kawahara, 2004). Furthermore, whereas PoP appears to be driven by the attentional selection made on the previous trial, DPE appears to be independent of attention allocation on the preview trial (e.g., Goolsby & Suzuki, 2001; Goolsby et al., 2005). Thus, while we might see similar transfer across effectors with the DPE as we did with PoP, it is also possible that the mechanisms involved in DPE might be more effector-dependent than those involved in PoP, precluding transfer across effectors. Because the paradigm for DPE requires only occasional target absent trials, Experiment 2 also provides an opportunity to directly replicate the PoP results from Experiment 1.
Method
Except where otherwise noted, the methods were identical to Experiment 1.
Participants
Brown University undergraduates and community members (18 female, 5 male, mean age: 20.8 years) participated in exchange for monetary compensation or course credit. All participants were right handed with normal or corrected-to-normal vision and normal color vision. The protocol was approved by the Brown University Institutional Review Board.
Procedure
On 25 % of all trials, no target was present; all three objects were instead rendered in the same color. There were eight possible two-trial sequences used. In these sequences, the first trial was either a typical trial (red or green target) or a target absent trial (all red or all green), and the second trial was always typical trial (red or green target). Each block included four instances of each of these eight sequences in completely randomized order, resulting in 64 total trials per block. There were an equivalent number of randomly assigned and intermixed eye and reach movement trials in each block.
Four participants were removed from analysis because they were unable to complete at least seven blocks of trials due to discomfort and fatigue. An additional participant was removed for low overall accuracy (67 %, more than three standard deviations below the mean).
Results
Below we present two analyses each for eye and reach movement data. The first is identical to the PoP analyses presented in Experiment 1. The second is a 2×2 ANOVA with factors of previewed color (target vs. distractor) and effector (repeat vs. switch) on initiation latency. The latter analysis is only examining two trial sequences in which the first trial was a target absent trial.
Eye movement trials
Priming of popout
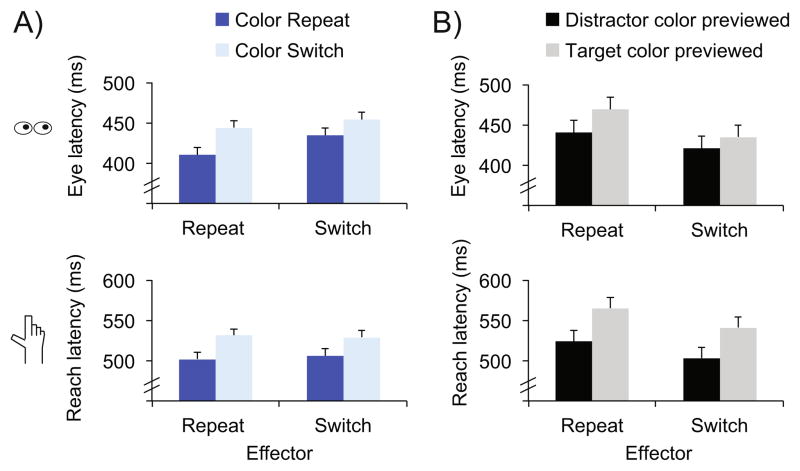
We again found faster eye movement initiation latencies when the color repeated (422 ms) than when it did not (449 ms), F(1,17)=20.7, p<.001, ηp2=.55. We again found no cost for switching effectors, F(1,17) = 2.4, p = .14. Unlike in Experiment 1, we found no interaction between effector and target color, F(1,17)=2.4, p=.14 (Fig. 3a, top; see Table 2 for more detailed description of data). However, the pattern of results was similar; there was a greater magnitude benefit on eye movement initiation latencies for repeating the target color when the effector was repeated (34 ms) than when it was switched (20 ms). Furthermore, we conducted a cross-experiment ANOVA with within-subject factors of effector and target color, and a between-subject factor of experiment. This revealed an interaction between target color and effector, F(1,34)=6.0, p=.019, ηp2=.15, but no three-way interaction with experiment, F(1,34)<1. Thus, we largely replicated the eye movement results of Experiment 1, demonstrating a transfer of PoP across effectors, though with a reduced magnitude when the effector is switched relative to when it is repeated.
Fig. 3.
a, b Data from Experiment 2. a Comparison of movement latencies for eye movements (top) and reach movements (bottom) as a function of effector (repeat vs. switch from the previous trial) and target color (repeat vs. switch from the previous trial). b Comparison of movement latencies for eye movements (top) and reach movements (bottom) as a function of effector (repeat vs. switch from the previous trial) and previewed color (distractor vs. target). Error bars Within-subject error term (Loftus & Masson, 1994)
Distractor previewing effect
Saccade initiation latencies were faster when the previewed color appeared on the next trial as the distractor color (431 ms) than as the target color (452 ms), F(1,17)=6.8, p=.018, ηp2=.29. Consistent with the previous literature on the distractor previewing effect, this supports the notion that the previewed color, to which observers must withhold a response, is subsequently inhibited for eye movements (Caddigan & Lleras, 2010). This makes it harder to select a subsequent target in that color and easier to ignore subsequent distractors in that color.
We also found that responses were slower when the effector was repeated (455 ms) than when it was switched (428 ms), F(1,17)=7.5, p=.014, ηp2=.31. This suggests that when a trial requires withholding a response, not only is the color that appears inhibited, the cued effector is inhibited as well. Thus, if a reach movement response is cued and no target appears, it is easier to make a subsequent eye movement to a target than if an eye movement cue had appeared on the previous target absent trial.
Finally, we found no interaction between effector and preview color, F(1,17)<1 (Fig. 3b, top; see Table 3 for more detailed description of data). This indicates that the DPE transfers from reach to eye movements. Thus, inhibitory biases away from colors appearing on target absent trials transfer from reach to eye movements.
Reach movement trials
Priming of popout
The reach movement data also largely replicated Experiment 1. Responses were faster on color repeat trials (504 ms vs. 530 ms), F(1,17) = 30.0, p < .001, ηp2 = .64. Unlike Experiment 1, we found no main effect of effector repetition, F(1,17)< 1. The lack of effector repetition effect in Experiment 2 might be attributable to the inclusion of trials in which a movement had to be withheld because no target was present, which may have changed overall response strategies. Indeed, overall reach movement latencies were longer in Experiment 2 (517 ms) relative to Experiment 1 (454 ms), t(34)=3.3, p=.002. Finally, as in Experiment 1, we found no interaction between effector and target color, F(1,17)<1 (Fig. 3a, bottom). Thus, across both experiments, for both eye and reach movement responses, repeating the color of a popout target on consecutive trials sped responses regardless of whether the effector was also repeated from the previous trial.
Distractor previewing effect
Previous work has focused largely on the distractor previewing effect in keypress responses and eye movements. Here, we examine whether target absent trials produce feature inhibition in goal-directed action.
The reach movement data largely mirrored the eye movement data. Movement initiation latencies were faster when the previewed color became the distractor (513 ms) than when it became the target (553 ms), F(1,17)=14.2, p=.002, ηp2=.46. This demonstrates that the DPE occurs in goal-directed action, producing inhibition of a previewed feature for a subsequent visually guided reach movement. As with the eye movement data, initial latencies were also slowed when the effector was repeated vs. switched (545 ms vs. 522 ms), F(1,17)=8.2, p=.011, ηp2=.33.2 Therefore, when a reach response is withheld on a target absent trial, subsequent reach movement initiation is delayed. Finally, we again found no interaction between response mode and preview color, F(1,17)<1 (Fig, 3b, bottom), indicating transfer of the DPE from eye to reach movements.
Together, both the eye and reach movement data suggest that the distractor previewing effect transfers across effectors. That is, target absent trials in popout search generate inhibitory tags of the previewed color, and this inhibition affects subsequent responses regardless of the mode of action used.
General discussion
In two experiments, we found that initiation latencies of both eye and reach movements were faster when popout target colors were repeated from the previous trial relative to when they switched, even when the response effector was not repeated from the previous trial. Additionally, we found that previewing the target color slowed both eye and reach movement responses; again, this effect persisted even when the mode of action was switched. Together, these results suggest that intertrial biases towards and away from target features transfer across effectors. In other words, both PoP and DPE transfer from eye to reach movements and vice versa.
In a previous study (Moher & Song, 2014), we found that PoP transferred from keypress responses to reach movements and vice versa. The current results expand on this finding in a number of ways. First, the previous study examined two different types of responses that both involved the hand. Here, we found transfer across two different effectors (eyes and hands), suggesting that biases towards recently selected target features transfers across effectors as well. Second, to our knowledge, the present study provides the first demonstration of the DPE in a visually guided reaching task. This may provide a useful basis for future studies, particularly since movement trajectories can provide insight into otherwise internal cognitive processes involved in inhibition (e.g., Song & Nakayama, 2009). Third, we found that inhibitory biases away from a recently seen feature, as indexed by the DPE, transfer across modes of action. This provides clear evidence that inhibitory biases in target selection are largely independent from their associated action responses. Furthermore, because the DPE and PoP involve at least partially distinct mechanisms (e.g., Ariga & Kawahara, 2004; Goolsby et al., 2005), these results suggest that the transfer of selection biases across effectors is a relatively robust phenomenon.
Finally, we found that response initiation latencies were slower when the effector was repeated on consecutive trials if the first of those trials was a target absent trial requiring no response. In other words, if a mode of action is cued, and then a trial occurs in which no response is required, that effector is inhibited and thus harder to initiate relative to a new mode of action on a subsequent trial. These results have several interesting implications. Firstly, even if a given mode of action is not executed, the preparation cue is sufficient to generate a representation in memory that affects subsequent responses. Second, the withholding of a response on target absent trials not only generates inhibition of the previewed feature, but also of the cued effector. This result indicates that while the effector is not bound in memory with its associated target feature, it is nonetheless represented in memory at some level and is capable of influencing subsequent responses. Thus, much like target selection history, action history can impact behavior. Whether this impact is automatic in the way that selection history is understood to be is one of many important issues that future research can address.
We found that PoP effects were reduced, but still significant, when the effector was switched on eye movement trials. In all other comparisons, we found no significant interactions between effector and target color. However, we must be cautious in interpreting null effects, as it may be the case that there are small reductions in the magnitude of intertrial effects when the effector is switched that might be revealed in future studies targeted at this issue. Furthermore, across all experiments, the magnitude of PoP or DPE was greater when the effector was repeated relative to when it was switched for both eye and hand movement initiation latencies. In a previous study examining transfer of PoP from hand movements to keypresses and vice versa, we found a similar pattern (Moher & Song, 2014). That is, while we did not find significant interactions between the mode of action and the target color, we did find a consistent reduction in magnitude in PoP when the mode of action was switched across trials. Thus, while we can confidently claim that PoP and the DPE transfer across effectors, and that the effect appears to be largely effector-independent, we cannot rule out the possibility that there is a small effector-dependent effect that the present study cannot reveal.
Methodology and future research
In our previous work (Moher & Song, 2014), we found transfer of PoP from a no-go trial to either a keypress or hand movement trial. There, we did not find strong evidence for suppression of the mode of response following no-go trials; however, that study did not involve switching across effectors. Those results, combined with the present results, have implications for understanding motor inhibition as often studied in paradigms requiring the withholding of a response, such as the stop-signal paradigm (e.g., Logan et al., 1984) or go/no-go paradigm (e.g., Rubia et al., 2001). Specifically, it suggests that a representation of the inhibited response effector, but not necessarily the specific type of response cued for that effector, affects subsequent behavior. Furthermore, examining intertrial effects following withheld responses as we have done in the present study may prove useful in untangling brain activation associated with motor inhibition from activation involved in higher-level attentional processes, which has previously been a challenge in traditional stop-signal and go/no-go paradigms (e.g., Criaud & Boulinguez, 2013). In other words, the present paradigm might prove useful because we have demonstrated that a withheld response on a single trial can produce measurable behavioral effects that reflect inhibition of a specific effector on a subsequent trial; for example, we found that inhibiting a hand movement on one trial resulted in slower responses on the next trial if a hand movement (rather than an eye movement) response was required. Thus, this approach allows for temporal separation of the act of motor inhibition itself from higher-level cognitive processes that arise from that inhibition.
More broadly, using the present approach in future studies may prove useful in identifying the underlying neural substrates involved in effector-independent target selection. Previous work has identified a number of brain regions, including intraparietal sulcus (IPS), frontal eye fields (FEF), and the superior colliculus (e.g., Gallivan et al., 2011; Song & McPeek, 2015; Song et al., 2011), that are involved in effector-independent target selection. However, it is not known whether these same regions encode selection history. Similar regions, including the IPS and FEF, do encode target selection history in a simple keypress response task (Kristjánsson, Vuilleumier, Schwartz, Macaluso, & Driver, 2006). Thus, it may be the case that effector-independent target selection history involved in reach and eye movements is encoded in similar regions. Action history, however, is represented quite broadly in the brain (e.g., Gallivan & Culhmam, 2015), and thus there may be many candidate areas that encode action history independently from effector-independent target selection history. These questions remain open, and are critical to our understanding of the link between brain and behavior for target selection across different modes of action.
Finally, there are additional behavioral questions that might be addressed using the current paradigm. For example, in a recent study (Moher & Song, 2013), we found that the trajectory of a reach movement to a popout target on a single trial predicted movement trajectory on subsequent responses. Specifically, movements that were deviated towards distractors, referred to as partial errors, were likely to be followed by similarly curved movements, though only when the task context (i.e., target color) was repeated. Because we examined only reaching movements, we were unable to distinguish whether these trial history effects were influencing performance at the level of target selection, or whether they simply reflected a tendency to reproduce similar motor output on consecutive trials. However, if a partial error on a hand movement trial affected performance on an immediately following eye movement trial, we could infer that intertrial effects of partial errors reflect disruption of the target selection process. Thus, the methodology used in the current experiments has the potential to distinguish between these kinds of competing theoretical explanations.
Conclusions
The results of the present study build on an expanding literature that examines the role of action in target selection. Specifically, we emphasize the intertwined roles of action history and target selection history in guiding behavior, finding that target selection history biases subsequent performance across effectors. Continued research in understanding this relationship is critical, as everyday behavior frequently involves a dynamic mixture of action responses to objects in the surrounding world.
Acknowledgments
This project was supported by NIGMS-NIH (P20GM103645) to J.H.S, and J.M. is supported by the Center for Vision Research fellowship and the Brown Training Program in Systems and Behavioral Neuroscience NIH T32MH019118. We thank Jennifer Flaherty for help with participant recruitment.
Footnotes
Two participants in Experiments 1 and 2 completed only seven blocks due to technical difficulties and/or discomfort.
We note that an opposite effect was observed in the movement time data (see Table 3), with shorter movement time on effector repeat trials (451 ms) relative to effector switch trials (466 ms), F(1,17)=31.0, p<.001, ηp2=.65. Thus, there is a tradeoff following target absent trials during goal-directed action, in which initiation latency is slower when the effector is repeated, but subsequent movement times are faster, presumably because target selection has reached a later stage prior to movement initiation due to longer initiation latencies (e.g., Song & Nakayama, 2008).
References
- Ariga A, Kawahara JI. The perceptual and cognitive distractor-previewing effect. Journal of Vision. 2004;4(10):891–903. doi: 10.1167/4.10.5. [DOI] [PubMed] [Google Scholar]
- Awh E, Belopolsky AV, Theeuwes J. Top-down versus bottom-up attentional control: a failed theoretical dichotomy. Trends in Cognitive Sciences. 2012;16(8):437–443. doi: 10.1016/j.tics.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard D. The psychophysics toolbox. Spatial Vision. 1997;10(4):433–436. [PubMed] [Google Scholar]
- Caddigan E, Lleras A. Saccadic repulsion in pop-out search: how a target’s dodgy history can push the eyes away from it. Journal of Vision. 2010;10(14):1–9. doi: 10.1167/10.14.9. [DOI] [PubMed] [Google Scholar]
- Criaud M, Boulinguez P. Have we been asking the right questions when assessing response inhibition in go/no-go tasks with fMRI? A meta-analysis and critical review. Neuroscience and Biobehavioral Reviews. 2013;37(1):11–23. doi: 10.1016/j.neubiorev.2012.11.003. [DOI] [PubMed] [Google Scholar]
- Found A, Müller HJ. Searching for unknown feature targets on more than one dimension: investigating a dimension-weighting account. Perception & Psychophysics. 2010;58(1):1–14. doi: 10.3758/bf03205479. [DOI] [PubMed] [Google Scholar]
- Gallivan JP, Culham JC. Neural coding within human brain areas involved in actions. Current Opinion in Neurobiology. 2015;33:141–149. doi: 10.1016/j.conb.2015.03.012. [DOI] [PubMed] [Google Scholar]
- Gallivan JP, McLean DA, Smith FW, Culham JC. Decoding effector-dependent and effector-independent movement intentions from human parieto-frontal brain activity. Journal of Neuroscience. 2011;31(47):17149–17168. doi: 10.1523/JNEUROSCI.1058-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godijn R, Theeuwes J. The relationship between inhibition of return and saccade trajectory deviations. Journal of Experimental Psychology Human Perception and Performance. 2004;30(3):538–554. doi: 10.1037/0096-1523.30.3.538. [DOI] [PubMed] [Google Scholar]
- Goolsby B, Grabowecky M, Suzuki S. Adaptive modulation of color salience contingent upon global form coding and task relevance. Vision Research. 2005;45(7):901–930. doi: 10.1016/j.visres.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Goolsby BA, Suzuki S. Understanding priming of color-singleton search: roles of attention at encoding and “retrieval”. Attention, Perception, & Psychophysics. 2001;63(6):929–944. doi: 10.3758/bf03194513. [DOI] [PubMed] [Google Scholar]
- Green BF, Anderson LK. Color coding in a visual search task. Journal of Experimental Psychology. 1956;51(1):19–24. doi: 10.1037/h0047484. [DOI] [PubMed] [Google Scholar]
- Hayhoe MM, Shrivasta A, Mruczek R, Pelz JB. Visual memory and motor planning in a natural task. Journal of Vision. 2003;3:49–63. doi: 10.1167/3.1.6. [DOI] [PubMed] [Google Scholar]
- Hillstrom A. Repetition effects in visual search. Perception & Psychophysics. 2000;62(4):800–817. doi: 10.3758/bf03206924. [DOI] [PubMed] [Google Scholar]
- Hommel B. Event files: feature binding in and across perception and action. Trends in Cognitive Sciences. 2004;8(11):494–500. doi: 10.1016/j.tics.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Huang L, Holcombe AO, Pashler H. Repetition priming in visual search: episodic retrieval, not feature priming. Memory and Cognition. 2004;32(1):12–20. doi: 10.3758/bf03195816. [DOI] [PubMed] [Google Scholar]
- Itti L, Koch C. Computational modelling of visual attention. Nature Reviews Neuroscience. 2001;2(3):194–203. doi: 10.1038/35058500. [DOI] [PubMed] [Google Scholar]
- Kristjánsson A, Vuilleumier P, Schwartz S, Macaluso E, Driver J. Neural basis for priming of pop-out during visual search revealed with fMRI. Cerebral Cortex. 2006;17(7):1612–1624. doi: 10.1093/cercor/bhl072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lleras A, Kawahara JI, Wan XI, Ariga A. Intertrial inhibition of focused attention in pop-out search. Perception and Psychophysics. 2008;70(1):114–131. doi: 10.3758/PP.70.1.114. [DOI] [PubMed] [Google Scholar]
- Loftus G, Masson M. Using confidence intervals in within-subject designs. Psychonomic Bulletin and Review. 1994;1(4):476–490. doi: 10.3758/BF03210951. [DOI] [PubMed] [Google Scholar]
- Logan GD, Cowan WB, Davis KA. On the ability to inhibit simple and choice reaction time responses: a model and a method. Journal of Experimental Psychology: Human Perception and Performance. 1984;10(2):276–291. doi: 10.1037//0096-1523.10.2.276. [DOI] [PubMed] [Google Scholar]
- Ludwig CJH, Gilchrist ID. Stimulus-driven and goal-driven control over visual selection. Journal of Experimental Psychology Human Perception and Performance. 2002;28(4):902–912. [PubMed] [Google Scholar]
- Makovski T, Jiang YV. Investigating the role of response in spatial context learning. The Quarterly Journal of Experimental Psychology. 2011;64(8):1563–1579. doi: 10.1080/17470218.2011.564291. [DOI] [PubMed] [Google Scholar]
- Maljkovic V, Nakayama K. Priming of pop-out: I. Role of features. Memory and Cognition. 1994;22(6):657–672. doi: 10.3758/bf03209251. [DOI] [PubMed] [Google Scholar]
- McPeek R, Maljkovic V, Nakayama K. Saccades require focal attention and are facilitated by a short-term memory system. Vision Research. 1999;39(8):1555–1566. doi: 10.1016/s0042-6989(98)00228-4. [DOI] [PubMed] [Google Scholar]
- Moher J, Song JH. Context-dependent sequential effects of target selection for action. Journal of Vision. 2013;13(8):1–13. doi: 10.1167/13.8.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher J, Song JH. Target selection bias transfers across different response actions. Journal of Experimental Psychology Human Perception and Performance. 2014;40(3):1117–1130. doi: 10.1037/a0035739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher J, Abrams J, Egeth HE, Yantis S, Stuphorn V. Trial-by-trial adjustments of top-down set modulate oculomotor capture. Psychonomic Bulletin and Review. 2011;18(5):897–903. doi: 10.3758/s13423-011-0118-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner M. Orienting of attention. The Quarterly Journal of Experimental Psychology. 1980;32(1):3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Song JH, McPeek RM. Neural correlates of target selection for reaching movements in superior colliculus. Journal of Neurophysiology. 2015;113(5):1414–1422. doi: 10.1152/jn.00417.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JH, Nakayama K. Role of focal attention on latencies and trajectories of visually guided manual pointing. Journal of Vision. 2006;6(9):982–995. doi: 10.1167/6.9.11. [DOI] [PubMed] [Google Scholar]
- Song J, Nakayama K. Target selection in visual search as revealed by movement trajectories. Vision Research. 2008;48(7):853–861. doi: 10.1016/j.visres.2007.12.015. [DOI] [PubMed] [Google Scholar]
- Song JH, Nakayama K. Hidden cognitive states revealed in choice reaching tasks. Trends in Cognitive Sciences. 2009;13(8):360–366. doi: 10.1016/j.tics.2009.04.009. [DOI] [PubMed] [Google Scholar]
- Song JH, Rafal RD, Mcpeek RM. Deficits in reach target selection during inactivation of the midbrain superior colliculus. Proceedings of the National Academy of Sciences. 2011;108(51):E1433–E1440. doi: 10.1073/pnas.1109656108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JH, Takahashi N, Mcpeek RM. Target selection for visually guided reaching in macaque. Journal of Neurophysiology. 2007;99(1):14–24. doi: 10.1152/jn.01106.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spivey MJ, Grosjean M, Knoblich G. Continuous attraction toward phonological competitors. Proceedings of the National Academy of Sciences. 2005;102(29):10393–10398. doi: 10.1073/pnas.0503903102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theeuwes J. Perceptual selectivity for color and form. Perception & Psychophysics. 1992;51(6):599–606. doi: 10.3758/bf03211656. [DOI] [PubMed] [Google Scholar]
- Tipper SP, Cranston M. Selective attention and priming: inhibitory and facilitatory effects of ignored primes. The Quarterly Journal of Experimental Psychology a, Human Experimental Psychology. 1985;37(4):591–611. doi: 10.1080/14640748508400921. [DOI] [PubMed] [Google Scholar]
- Tipper SP, Meegan D, Howard LA. Action-centred negative priming: evidence for reactive inhibition. Visual Cognition. 2002;9(4–5):591–614. doi: 10.1080/13506280143000593. [DOI] [Google Scholar]
- Rubia K, Russell T, Overmeyer S, Brammer MJ, Bullmore ET, Sharma T, et al. Mapping motor inhibition: conjunctive brain activations across different versions of go/no-go and stop tasks. NeuroImage. 2001;13(2):250–261. doi: 10.1006/nimg.2000.0685. [DOI] [PubMed] [Google Scholar]
- Selst MV, Jolicoeur P. A solution to the effect of sample size on outlier elimination. The Quarterly Journal of Experimental Psychology. 1994;47(3):631–650. [Google Scholar]
- Watson DG, Humphreys GW. Visual marking: prioritizing selection for new objects by top-down attentional inhibition of old objects. Psychological Review. 1997;104(1):90–122. doi: 10.1037/0033-295x.104.1.90. [DOI] [PubMed] [Google Scholar]
- Van Selst M, Jolicoeur P. A solution to the effect of sample size on outlier elimination. The Quarterly Journal of Experimental Psychology. 1994;47(3):631–650. [Google Scholar]
- Zehetleitner M, Hegenloh M, Muller HJ. Visually guided pointing movements are driven by the salience map. Journal of Vision. 2011;11(1):1–18. doi: 10.1167/11.1.24. [DOI] [PubMed] [Google Scholar]