Abstract
Amyloid fibrils from semen-derived peptide (SEVI) enhance HIV-1 infectivity in vitro but the ability of SEVI to mediate enhancement of HIV infection in vivo has not been tested. In this study we used immunodeficient mice reconstituted with human immune systems to test for in vivo enhancement of HIV-1 transmission. This mouse model supports mucosal transmission of HIV-1 via the intrarectal route leading to productive infection. In separate experiments with humanized mouse cohorts reconstituted with two different donor immune systems, high dose HIV-1JR-CSF that had been incubated with SEVI amyloid fibrils at physiologically relevant concentrations did not show an increased incidence of infection compared to controls. In addition, SEVI failed to enhance rectal transmission with a reduced concentration of HIV-1. Although we confirmed potent SEVI-mediated enhancement of HIV infectivity in vitro, this model showed no evidence that it plays a role in the much more complex situation of in vivo transmission.
Keywords: HIV, Transmission, Semen-derived enhancer of viral infection, SEVI, Amyloid, Humanized mice
Introduction
In the three decades since the start of the AIDS pandemic HIV has killed more than 39 million people, with an additional 35 million people currently infected. The most common mode of transmission is via the sexual route and the risk of sexual transmission increases during the acute phase of infection when viral titers are at their peak (Dimitrov et al., 1993). The presence of human semen has been shown to augment HIV infectivity in vitro (Arnold et al., 2012; Kim et al., 2010; Roan et al., 2011) and in 2007, Münch and colleagues described an amyloidogenic peptide derived from the proteolytic cleavage of a human seminal fluid protein (PAP) that significantly enhanced HIV infectivity in vitro (Munch et al., 2007). Specifically, the infectivity enhancing activity was attributed to PAP peptide 248–286, which was shown to readily aggregate into amyloid fibrils. Formation of amyloid fibrils was integral to enhancement of infectivity and the peptide fibrils were termed SEVI for Semen-derived Enhancer of Viral Infection (Munch et al., 2007). Evidence indicates that SEVI enhances viral infectivity by facilitating virion and target cell interactions, likely by acting as a cationic bridge between negatively charged virions and cells (Roan et al., 2009; Roan et al., 2010), similar to other polycationic enhancers such as polybrene or protamine sulfate (Cornetta and Anderson, 1989; Manning et al., 1971).
The ability of SEVI to enhance not only viral infectivity in vitro but also viral transmission in vivo could have broad implications for HIV prophylaxis. For example, as semen is the major vector for sexual transmission of HIV (Royce et al., 1997), the presence of SEVI in seminal fluid could increase the rate of transmission and thus offer a target for intervention. In fact, several SEVI antagonists have already been described (Capule et al., 2012; Hauber et al., 2009; Olsen et al., 2010; Roan et al., 2010). However, transmission across the mucosal barrier in vivo is much more complex than infection of cell cultures and cationic bridging might have little impact. Thus, it is important to test the ability of SEVI to augment viral transmission in vivo before large investments in developing blocking strategies are made.
Because of the highly selective tropism of HIV for human cells it has not been possible to directly test the ability of SEVI to enhance transmission of HIV in vivo. The effect of SEVI on vaginal transmission of simian immunodeficiency virus (SIV) has been tested in the SIV/rhesus macaque model, albeit with no evidence of enhancement (Munch et al., 2013). It was suggested in that study that SEVI might show better enhancement in intrarectal rather than intravaginal transmission. In the current study we use C57BL/6 Rag2−/−γc−/−CD47−/− BLT-humanized (TKO-BLT) mice to analyze the impact of SEVI on rectal transmission of HIV-1. TKO-BLT mice are highly immunodeficient and irradiated Triple Knock Out mice were transplanted with: 1. human fetal CD34+ hematopoietic stem cells to reconstitute the Bone marrow; 2. Liver as a source of additional CD34+ hematopoietic stem cells; and 3. Thymus for maturation and development of human T cells (Lavender et al., 2014). TKO-BLT mice develop high levels of human immune system reconstitution, are susceptible to HIV-1 infection via intrarectal inoculation and exhibit hallmarks of human HIV infection such as CD4+ T cell depletion and general immune activation (Lavender et al., 2013). The most dramatic enhancing effects of SEVI and semen in vitro have been observed with low doses of HIV-1 irrespective of the virus strain and producer or target cell type (Kim et al., 2010; Munch et al., 2007). Thus for the in vivo experiments we tested both a high dose of HIV-1 that typically infects approximately half of our mice in the absence of SEVI, and also a lower dose of HIV-1 that generally infects only one out of four or five mice. SEVI-mediated enhancement of HIV infection is evident in vitro starting at a concentration of 2μg/mL and increases with concentration up to at least 50μg/mL (Munch et al., 2007). The amount of SEVI reported by Munch et al. to be present in semen was approximately 35μg/mL (Munch et al., 2007). To maximize our chances of observing an enhancing effect in vivo, most of our experiments were done with SEVI at a concentration of 50μg/mL, which is a physiologically relevant concentration that strongly enhances infectivity in vitro.
Results
In vitro enhancement of HIV infection by SEVI
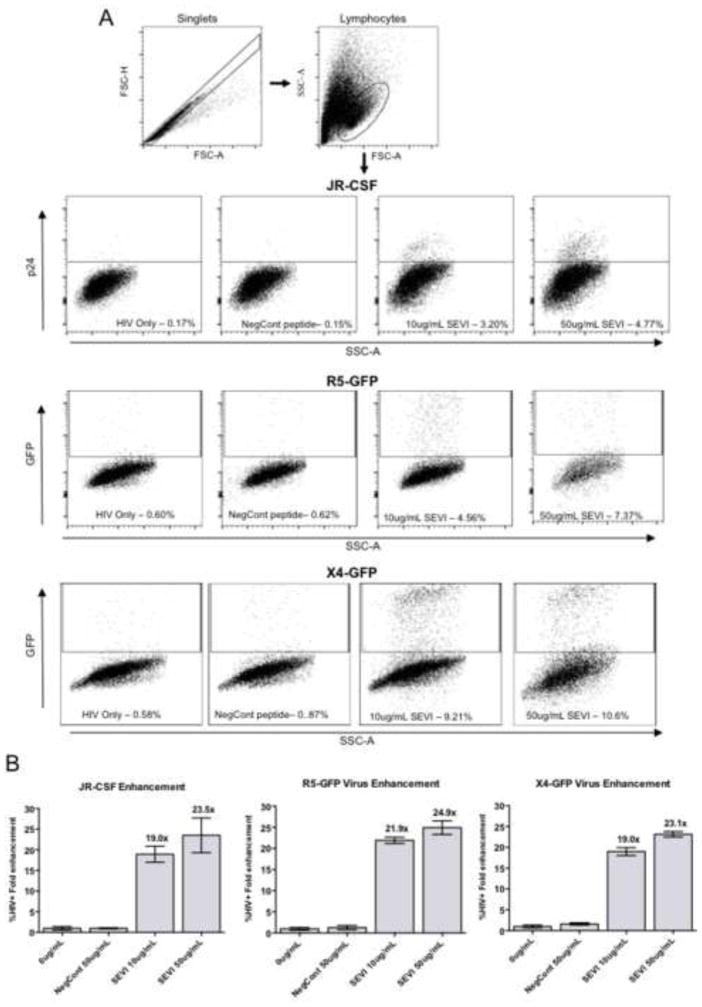
Before testing SEVI in live animals we first confirmed the potency of our preparation of SEVI fibrils on HIV-1 infection in vitro. CD4+ T cells from human peripheral blood mononuclear cells (PBMCs) were stimulated for 3 days in culture and then infected with HIV-1 viruses alone, or HIV-1 pre-incubated with either SEVI (50μg/mL) or a non-amyloidogenic peptide (negative control). At two days post-infection infectivity enhancement was measured for three virus strains: HIV-1JR-CSF, an R5-tropic molecular clone from a primary isolate from the cerebral spinal fluid of an AIDS patient (Cann et al., 1990; Koyanagi et al., 1987; Moore et al., 1995) that was tested by intracellular staining for p24, and two GFP-expressing HIV clones, one an R5-tropic strain and the other an X4-tropic strain. The flow cytometry gating strategy and representative data for each strain are shown in Figure 1A. The mean fold-enhancement of infection compared to HIV-1 infection in the absence of peptide was calculated and the compiled results are shown in Figure 1B. SEVI at 10 μg/mL produced approximately 20-fold enhancement of HIV-1 infectivity and approximately 24-fold enhancement at 50 μg/mL, which is consistent with previous reports (Hauber et al., 2009; Munch et al., 2007; Roan et al., 2009; Roan et al., 2010). These results indicated that SEVI peptide enhanced HIV-1 infectivity in vitro as expected.
Figure 1. SEVI enhances HIV-1 infectivity of activated CD4 T cells in vitro.
(A) Gating strategy for flow cytometric analysis of HIV-1JR-CSF infectivity. Activated CD4+ T cells infected with HIV-1JR-CSF were analyzed for p24 expression by flow cytometry at two days post-infection. Cells were first gated for singlets (FSC-A vs. FSC-H) and live lymphocytes (FSC-A vs. SSC-A). Infectivity was then measured on the gated population by staining for HIV p24 (JR-CSF) or GFP expression (R5-GFP, X4-GFP). Cell populations shown are representative of replicates. The peptide treatment and percentage of HIV-1+ cells are indicated. (B) The presence of SEVI resulted in enhancement of infectivity as determined by the percentage of HIV-1+ cells. Results were consistent between R5-tropic HIV-1JR-CSF (MOI 0.001), X4-tropic GFP-HIV-1 (MOI 0.01), and R5-tropic GFP-HIV-1 (MOI 0.01). Numbers above bars indicate the fold enhancement of infectivity in the presence of peptide compared to virus alone. Data represent the average values +/− SD of triplicate infections. NegCont = the negative control peptide.
Description of in vivo test groups
Next, TKO-BLT humanized mice were produced to test whether SEVI increased HIV-1 transmission across the mucosal barrier of the rectum in vivo. Previous studies on these mice showed that three intrarectal inoculations of 9 x 104 TCIUs of HIV-1JR-CSF over the course of a week resulted in ~50% of the mice becoming infected, which was evident between 2 and 4 weeks post-infection (Lavender et al., 2013). HIV-1JR-CSF was incubated with SEVI or a negative control peptide prior to inoculation and mice were inoculated once daily for three days with mixtures containing 9 x 104 tissue-culture infectious units (TCIU) (high dose) HIV-1 incubated with either SEVI or the negative control peptide. Humanized mice are relatively resistant to intrarectal inoculation of HIV at low doses (Hofer et al., 2008), but the enhancing effect of SEVI might be most evident at low virus concentrations as has been demonstrated in vitro (Munch et al., 2007). To test whether SEVI would enhance infection with lower doses of HIV-1, one group was also infected with a lower dose (1 x 104 TCIU) of HIV-1JR-CSF that had been incubated with SEVI.
Human lymphocyte reconstitution levels and test group distribution
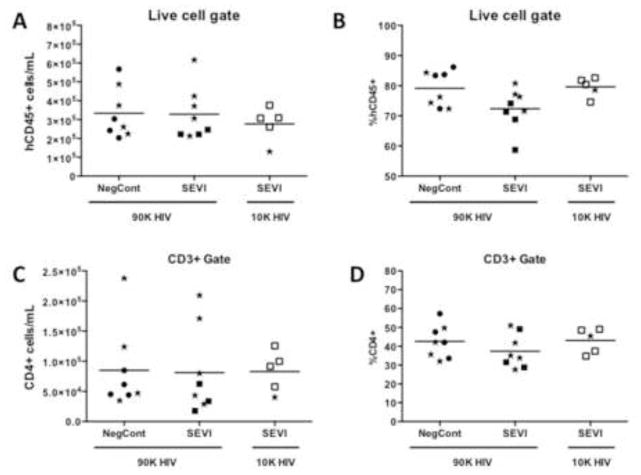
Due to variability in levels of human cell reconstitution of humanized mice it was important to analyze reconstitution levels prior to infection and distribute the mice across the experimental groups as evenly as possible. Human lymphocytes in peripheral blood were detected by flow cytometric staining of human-specific CD45+ cells, and the proportions of T cells (CD3+) and CD4+ and CD8+ T cell subsets were examined. Mice were distributed across the groups to contain roughly equivalent absolute numbers of hCD45+ cells/mL of blood (Fig. 2A). Only mice with >105 human CD45+ cells/mL of blood were included in the study. At this high level of human cell reconstitution, there was no association between whether a mouse eventually became HIV infected and either the absolute number (Fig. 2A,C) or percentages (Fig. 2B,D) of either total human blood cell numbers or CD4+ T cells (mice that eventually became infected in each group are depicted by stars, Fig. 2).
Figure 2. Pre-infection reconstitution levels of humanized mice.
At 16 weeks post-transplant surgery humanized mice were analyzed for human immune cell reconstitution (hCD45+) and were distributed across the groups as indicated so that each group had mice with approximately equivalent numbers of CD45+ (human) cells. Since CD4+ T cells are the predominant targets for HIV infection, the numbers and proportions of those cells are also shown. Animals that tested positive for HIV at 4wpi are indicated by stars. Mean values are indicated by horizontal bars. NegCont = negative control peptide; 90K and 10K refer to the HIV JR-CSF challenge dose of either 9 x 104 TCIU s or 1 x 104 TCIU.
Effect of SEVI on rectal transmission of HIV-1
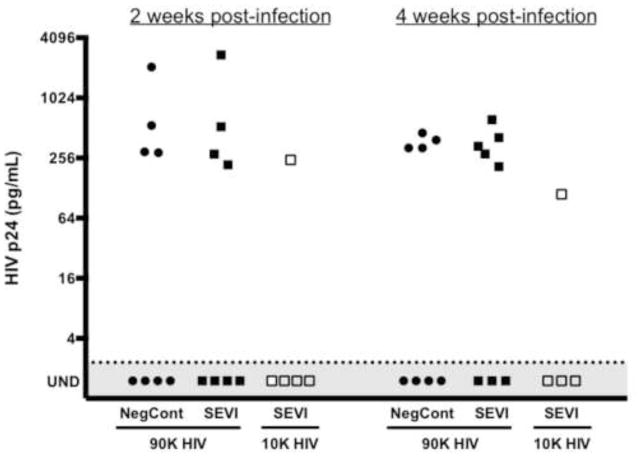
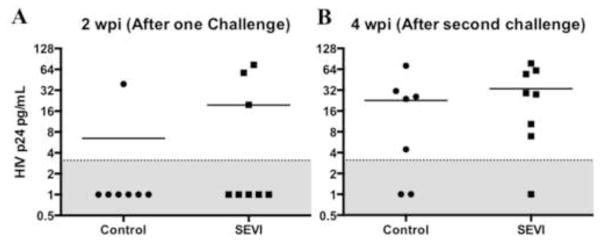
Blood samples were withdrawn from all mice at 2 weeks post-infection (wpi), a time point when HIV p24 antigen usually becomes detectable in the blood of most infected animals. The plasma was tested for HIV p24 by ELISA and the results are shown in Figure 3. At this time point half of the control mice and half of the SEVI test mice were positive for HIV p24. The infections were allowed to progress for an additional two weeks and the animals were then euthanized at 4wpi, the usual peak of infection and a time point after which we’ve not observed conversion to an HIV positive status in 3 different cohorts of mice containing a total of 37 mice (19 HIV+ and 18 HIV−) that were followed to 6 and 9 weeks post infection (data not shown). One mouse in the SEVI-treated group converted to HIV positive between 2 and 4 weeks, but the difference from the untreated controls (4/8 vs 5/8) was not statistically significantly. In mice inoculated with low dose HIV-1/SEVI, only one of five mice tested positive at either time point.
Figure 3. SEVI does not increase viral load or the rate of mucosal transmission of HIV-1 in TKO-BLT mice.
Levels of p24 in plasma of infected humanized mice were determined at 2 and 4 weeks post-infection by p24 ELISA after generating a standard curve for each assay and diluting plasma samples appropriately. The dotted line denotes the limit of detection (2.32 pg/mL for 2wpi and 2.27 pg/mL for 4wpi). The line drawn here is the average of these two detection limits, 2.30 pg/mL, but the limit of detection was defined for each separate ELISA.
To exclude the possibility that some HIV p24 antigen negative mice had low-level infections not detectable by p24 ELISA we used a highly sensitive, quantitative RNA PCR assay to further probe for evidence of HIV infection. All of the plasma samples from the 2wpi time point were consumed for the p24 assays and there was not sufficient plasma from the 4 wpi terminal blood withdrawals to test all the mice individually. Therefore we pooled the samples from the p24 positive animals and the p24 negative animals from each group. Whereas the p24 positive pools had very high titers of HIV RNA, all of the p24 negative pools tested below the limit of detection (Table 1). Thus there was no evidence that SEVI increased either the intrarectal transmissibility or the antigenemia of HIV-1JR-CSF in TKO-BLT mice, certainly not at levels approaching the 20-fold enhancement observed in vitro (Figure 1B).
Table 1. HIV RNA qPCR from pooled plasma samples at 4 weeks post-infection.
Plasma samples from the HIV p24 negative mice and the p24 positive mice from the groups indicated above were pooled and HIV-1 RNA levels were measured using the Abbott RealTime HIV-1 assay on the m2000 system. The limit of detection was 600 copies/mL.
| Pooled Samples | HIV dose | RNA copies/mL |
|---|---|---|
| No SEVI p24+ | 90,000 TCIU | 8.3 x 106 |
| No SEVI p24− | 90,000 TCIU | Assay inhibited, no data |
| SEVI p24+ | 90,000 TCIU | 7.0 x 106 |
| SEVI p24− | 90,000 TCIU | <600 |
| SEVI p24+ | 10,000 TCIU | 9.7 x 105 |
| SEVI p24− | 10,000 TCIU | <600 |
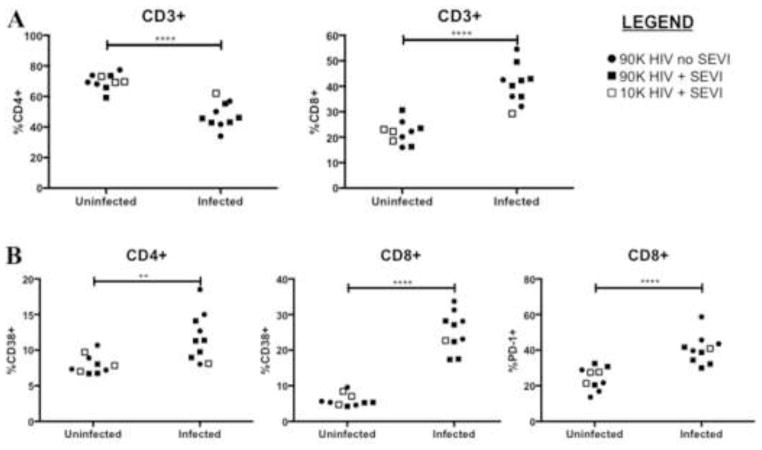
The primary manifestations of HIV-1 infections in both humans (Day et al., 2006; Deeks et al., 2004; Giorgi et al., 1999; Margolick et al., 2006; Orendi et al., 1998) and TKO BLT mice (Lavender et al., 2013) include depletion of CD4+ T cells, increases in CD8+ T cell proportions, and broad activation of T cells as indicated by expression of CD38 and PD-1. To determine if SEVI-treated mice differed from controls in these characteristics, peripheral blood cells from all animals at the 4 wpi time point were assessed by flow cytometry for HIV-associated phenotypes. Across all groups the phenotypic changes including depletion of CD4+ T cells and increased proportions of CD8+ T cells (Figure 4A) as well as the activation levels of the T cells (Figure 4B) were associated with each animal’s HIV status, not with whether SEVI was incubated with the virus. Taken as a whole, the results showed no evidence that SEVI had increased the incidence of HIV transmission, the levels of HIV infection or the pathological changes associated HIV infections.
Figure 4. Immunophenotyping of peripheral blood lymphocytes at 4 weeks post-infection.
Mice were bled at 4wpi for analysis of HIV-associated pathogenic changes. Each point represents one mouse and the points are grouped according to HIV status. (A) Infected mice in all groups had significant decreases in CD4+ T cell proportions and increases in CD8+ T cell proportions compared to uninfected animals. (B) Infected mice had significant increases in proportions of activated CD4+ and CD8+ T cells as measured by CD38 expression. PD-1 is another measure of activation on CD8+ T cells and becomes chronically expressed in exhausted T cells. **P<0.01, ****P<0.0001, ns = not significant by unpaired t test.
Second in vivo test group
To assure that these results were not peculiar to a single virus preparation or a specific tissue donor we prepared a new virus stock and inoculated a second cohort of mice that had been reconstituted with tissues from a separate donor. The human blood cell reconstitution levels between the SEVI-treated and untreated groups were equivalent for total human cells (hCD45+) and also CD3+ and CD4+ subsets (data not shown). Mice were inoculated intrarectally with a high dose of virus (90,000 TCIU) either with or without SEVI, and two weeks later animals were tested for plasma HIV p24 antigen. In the control group one of seven mice tested positive while three of eight mice in the SEVI group tested positive (Fig. 5A). At the two week time point the negative animals were again inoculated and 4 weeks later the mice were euthanized and re-tested for HIV. At that time four more controls and four more SEVI test mice were positive for HIV leaving two controls and one SEVI test mouse remaining negative (Fig. 5B). The difference between groups was not statistically significant at either time point (P = 1.000 and 0.5692, respectively).
Figure 5. SEVI does not increase viral load or the rate of mucosal transmission of HIV-1 in TKO-BLT mice.
Levels of p24 in plasma of infected humanized mice were determined at 2 weeks after the first infection (A) and 4 weeks after the second infection (B) by p24 ELISA as described in Figure 3 and the methods section. The dotted line denotes the limit of detection (3.125 pg/mL). Contingency table analysis by Fisher’s exact test showed no difference between the two groups at either 2wpi (P= 1.0000) or 4wpi (P= 0.5692). The mean levels of human CD45+ cells in the blood before infections exceeded 5 x 105/ml blood for both groups and mean CD4+ T cell levels exceeded 2 x 105/ml blood (data not shown).
Discussion
The in vitro effects of SEVI on HIV infection have been well documented, and this study confirmed potent in vitro enhancement of HIV infection. However, the impact of SEVI amyloid fibrils on the in vivo transmission of HIV has not previously been investigated. An in vivo study on vaginal transmission of SIV in macaques revealed no significant enhancement and some conjectures regarding the lack of enhancement were forwarded (Munch et al., 2013). For example it was speculated that the effect of SEVI on virus transmission across the mucosal barrier might be dependent on low dose virus challenge and that SEVI might be more effective in rectal rather than vaginal transmission (Munch et al., 2013). In the current study we used physiologically relevant infectious doses of both SEVI and HIV (Dyer et al., 1996; Munch et al., 2007) as well as one experiment with low dose HIV. In our first experiment challenging with high dose HIV we achieved infection in about 50% of the mice, so there was plenty of room to observe SEVI-mediated enhancement of transmission. Reducing the virus dose only further reduced transmission. Transmission was higher in the second high dose experiment so the interpretation is not as clear-cut, but still there was no statistically significant enhancement. The result of the low dose challenge experiment, which produced only one infection out of four mice is consistent with a lack of SEVI-mediated enhancement of rectal transmission in this model.
Regarding aspects of the model that may have affected this outcome, the levels of human CD45 and CD4+ T cell reconstitution before infection were roughly equivalent in all groups and were not limiting factors determining whether any given mouse became infected or not (Fig. 2). It must be noted that although TKO BLT mice contain a human immune system including gut-associated lymphoid tissue, the GI tract itself is of mouse origin, which could potentially affect transmission enhancement. Mucosal infection by HIV in humans is not fully understood and appears able to proceed either directly or indirectly through different cell types including CD4+ T cells, macrophages and dendritic cells (reviewed in (Wu, 2008)). While HIV infection obviously occurs in this mouse model, it may not proceed through quite the same mechanisms as in humans. That said, the current results are consistent with the previous failure to observe enhancement of infection in the SIV/macaque experiments (Munch et al., 2013).
There are a number of differences between in vitro infection of cells and in vivo transmission of HIV-1 that could explain the lack of in vivo enhancement. For instance, an important mechanism of mucosal transmission may involve the transfer of HIV from DC’s to CD4+ T cells rather than direct infection of the CD4+ T cells (Wang et al., 2007). There could be differences in target cell populations between in vitro cultures and the genital tract or rectal mucosa. For example, one study found the enhancing effect of SEVI to be diminished in CD4 T+ cells derived from the female genital tract compared to CD4+ T cells derived from PBMCs (Roan et al., 2009). Additionally, elements in the in vivo environment could be inhibiting the effect of SEVI on HIV-1 virions. Various polyanionic compounds in the in vivo environment have been shown to neutralize the ability of SEVI to enhance infectivity by disrupting the cationic bridge formed between virions and the cell surface (Roan et al., 2009). Furthermore, physiological concentrations of zinc and copper have been shown to inhibit fibrilization of SEVI in vitro (Sheftic et al., 2012). While not directly applicable to our experiments, one study found the enhancing activity of SEVI to be neutralized in the presence of human seminal fluid through the action of enzymes that degrade SEVI into non-enhancing fragments (Martellini et al., 2011). Another factor that might affect SEVI-mediated enhancement of HIV transmission is whether transmission occurs predominantly through free virus or cell-associated virus, a subject not clearly understood at this time. However, the enhancement of SEVI has not yet been studied in the context of cell-associated virus. Finally, a major factor may be differences in accessibility of target cells to SEVI between in vitro cultures and the mucosa. SEVI might not even penetrate the mucosal layer or the epithelium to reach the cellular targets for infection. It is possible that sexual activity that causes bleeding such that HIV could directly enter the blood stream might produce very different results and that SEVI enhancement might be important in that situation. Likewise, the presence of sexually transmitted diseases that cause lesions in the mucosal epithelium could also affect the ability of SEVI to cause enhancement.
While the current results do not exclude the possibility of SEVI having an enhancing effect during normal human sexual transmission of HIV they certainly cast some doubt on the issue. Given the lack of in vivo enhancement in either SIV-infected macaques or HIV-infected humanized mice it would seem prudent to reconsider whether further development of therapeutics targeting SEVI is warranted until this issue is resolved.
Materials and Methods
Peptides
SEVI and a non-amyloidogenic peptide (negative control), without chemical N- or C-terminal modifications, were purchased from Eurogentec/Anaspec. The sequence of synthetic semen-derived peptide PAP248–286 (GIHKQKEKSRLQGGVLVNEILNHMKRATQIPSYKKLIMY) (SEVI) has been described previously (Munch et al., 2007). Purity as assessed by HPLC was >97% for SEVI, and >99% for control peptide, respectively. Quality control was performed by means of mass spectrometry. Lyophilized synthetic peptides were resuspended in PBS at final concentrations of 2.5 mg/mL (SEVI) and 10 mg/mL (control peptide). The peptide solutions were agitated at 37°C for 36 hrs until SEVI was turbid indicative for fibril formation and then aliquoted and stored at −20°C until use.
Virus stocks
Virus stocks of HIV-1 were generated in human embryonic kidney 293FT cells (Invitrogen, Grand Island, NY). The cells were grown in DMEM containing 10% (v/v) Fetal Bovine Serum (FBS), 100 U/mL penicillin, 100 μg/mL streptomycin, 1 mM sodiumpyruvate, and non-essential amino acids, and maintained at 37°C and 5% CO2. When cultures reached ~80% confluence, the cells were transiently transfected with the infectious molecular clones pYK-JRCSF (an infectious molecular clone of HIV-1JR-CSF, a primary isolate from cerebral spinal fluid obtained from an AIDS patient (https://www.aidsreagent.org/reagentdetail.cfm?t=molecular_clones&id=851) obtained from Drs Irvin S. Y. Chen and Yoshio Koyanagi through the NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID), pBRNL43_005pf135(R5)nef+_IRES_eGFP clone 1872 to express R5-tropic GFP-HIV-1 (Munch et al., 2002), or NLENG1-IRES to express X4-tropic GFP-HIV-1 (Kutsch et al., 2002; Levy et al., 2004). Briefly, 60 μg plasmid DNA in Opti-Mem I medium was mixed with 180 μL Lipofectamine-2000 in Opti-Mem I medium and incubated at room temperature for 20 minutes. Trypsinized 293FT cells were added to the DNA/Lipofectamine mixture and incubated for 16 h at 37°C and 5% CO2 for transfection. Transfected cells were then washed once with PBS and incubated for an additional 48 hours in supplemented DMEM medium. HIV-1 stock from cell culture supernatant was concentrated on Amicon Ultra-15 centrifugal filter units (EMD Millipore, Darmstadt, Germany) and frozen at −70°C. Infectious titer of stock concentrations was determined using TZM-Bl (JC53-bl) reporter cells (NIH AIDS Research and Reference Reagent Program from Drs John Kappes and Xiaoyun Wu and Tranzyme, Inc.) maintained in DMEM supplemented with 5% (v/v) FBS.
Effect of amyloid fibrils on HIV-1 infection in vitro
To purify CD4+ T cells, mononuclear cells derived from whole human blood by Ficoll-Paque PLUS centrifugal elutriation (GE Healthcare Life Sciences, Little Chalfont, UK) were depleted of CD8+ and CD56+ cells using MACS Microbeads and LD columns (Miltenyi Biotec Inc., Cologne, Germany) following the manufacturers instructions. Cells were stained with anti-human CD8-APC-eFluor780 (eBioscience, San Diego, CA) and anti-human CD56-A700 (Biolegend, San Diego, CA) to ensure <0.5% of depleted cell types. Purified CD4+ T cells were then stimulated with 500 ng/mL anti-CD3, 100 ng/mL anti-CD28, and 20 U/mL IL-2 for 3 days at 37°C and 5% CO2. After 3 days, virus was incubated with 10 μg/mL or 50 μg/ml of SEVI, 50 μg/mL of the negative control peptide, or virus alone in 10μL PBS for 10 minutes at 37°C and transferred to cells for infection. Cells were incubated for 4 hours at 37°C and 5% CO2 with 5 U/mL IL-2, washed once with PBS, and the virus/peptide mixture was replaced with 200 μL fresh RPMI +10% FBS, 100 U/mL penicillin, 100 μg/mL streptomycin, 2 mM L-glutamine, and 25 U/mL IL-2. After 2 days, cells were harvested and analyzed for infection. For analysis of infection with GFP-expressing virus, cells were washed once with 100 μL PBS + 2% FBS, resuspended in 150 μL 2% PFA, and analyzed for GFP expression by flow cytometry. For cells infected with HIV-1JR-CSF, infection was determined by staining intracellular p24. Infected cells were fixed for 90 minutes using 4% PFA, permeabilized using BD Permeabilization buffer (Becton Dickinson, East Rutherford, NJ) for 20 minutes at 25°C, and intracellular p24 was stained using KC57-RD1 (Beckman Coulter, Pasadena, CA) (1:100) in 50 μL BD Permeabilization buffer for 30 minutes at 4°C. Cells were washed once with BD Permeabilization buffer, resuspended in 150 μL PBS and analyzed for p24 expression by flow cytometry.
Mice
BLT-humanized C57BL/6 Rag2−/−γc−/− CD47−/− (TKO) mice were generated by engrafting human fetal thymus and liver tissues and fetal liver-derived CD34+ hematopoietic progenitor cells (HPC) as previously described (Lavender et al., 2014; Lavender et al., 2013). Both male and female mice were used for experiments and no differences in susceptibility were observed. Animals were housed in filter-cover cages under specific pathogen-free conditions, and all experiments were performed in accordance with the regulations and guidelines of the Animal Care and Use Committee of Rocky Mountain Laboratories, National Institute of Allergy and Infectious Disease, National Institutes of Health (ASP #2014-33).
Effect of amyloid fibrils on transmission of HIV in humanized mice
Twenty-one mice were humanized from the same donor as previously described (Lavender et al., 2013; Sun et al., 2007) and infected starting 16 weeks post-transplantation. Only mice with sufficient levels of human reconstitution, as determined by number of hCD45+ cells/mL of peripheral blood, were chosen for the study. Mucosal infection was achieved by rectal inoculation. Prior to infection, 9.0 x 104 TCIUs HIV-1JR-CSF was incubated with 50 ug/ml SEVI or the negative control peptide and 1.0 x 104 TCIUs HIV-1JR-CSF was incubated with 50 ug/ml SEVI for 10 minutes at 37°C in a final volume of 20 uL per dose. For the first experiment, rectal inoculations were performed as previously described (Lavender et al., 2013) daily over a 3-day period. In the second experiment, the mice only received 2 inoculations, the second 2 weeks after the first. Mice were bled at 2 and 4 wpi for immunophenotyping and to determine HIV-1 viral load in plasma by p24 enzyme-linked immunosorbent assay (ELISA; Advanced Bioscience Laboratories, Rockville, MD). One mouse was found dead from unknown reasons on day 18 in the low dose HIV plus SEVI group.
Isolation and phenotypic analysis of human blood leukocytes
Blood leukocytes were purified as previously described (Lavender et al., 2013). Briefly, the blood volume was measured and the red blood cells were lysed with RBC Lysis Buffer (BioLegend). Following lysis, the leukocytes were counted using a hemocytometer with trypan blue exclusion. The number of leukocytes per mL of blood was calculated by dividing the total number of leukocytes by the bleed volume. Human leukocytes were distinguished within samples using a directly conjugated anti-human CD45-V500 antibody (clone HI30; BD Biosciences, San Jose, CA). Additional immunophenotyping used anti-human CD4-PE-Cy7 and CD8-APC-eFluor780 (eBioscience, San Diego, CA); CD3-V450 and CD38-PE (Becton Dickinson); and PD-1-FITC (BioLegend, San Diego, CA). Isolated leukocytes were stained for 15 minutes at 4°C, washed once with 100 uL PBS + 2% FBS, fixed in 2% PFA for at least 20 minutes, and analyzed by flow cytometry using a BD LSR II flow cytometer system (Becton Dickinson). Lymphocytes from peripheral blood were stained with anti-hCD45, -CD3, -CD4 and -CD8 fluorescent antibodies and analyzed after gating on physical parameters (forward and side scatters). CD3+ cells were analyzed after gating on hCD45+. Likewise, CD4+ and CD8+ cells were analyzed after gating on CD3+ cells. Absolute counts of CD45+ and CD4+ cells were determined by multiplying the percentages of these populations among total lymphocytes by the number of Lymphocytes/mL blood (as described above).
HIV RNA detection
Plasma HIV-1 RNA in peripheral blood was measured by using the Abbott RealTime HIV-1 assay on the m2000 system. In short, viral RNA was isolated from 200μl plasma applying the manual sample preparation protocol from the manufacturer followed by RT-PCR in the m2000 system. The HIV-RNA detection limit for an input volume of 200μl plasma was 600 copies/mL.
Data analysis
All statistical calculations were performed using GraphPad Prism (GraphPad Software, La Jolla, CA). Analysis of flow cytometry data was performed using FlowJo X software (Treestar, Inc., Ashland, OR).
Highlights.
semen-derived peptide (SEVI) enhances HIV-1 infectivity in vitro
SEVI failed to enhance rectal transmission in two separate humanized mouse cohorts
differences between in vitro infection of cells and in vivo transmission of HIV-1 likely explain the lack of in vivo enhancement
Acknowledgments
This work was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases of the National Institutes of Health, USA. O.T. K. acknowledges financial support from the Goethe University and from the Robert Koch-Institute of the National Reference Center for Retroviruses. U.D. acknowledges support from the German Research Association (Transregio 60 and RTG 1949). HIV-1JR-CSF was obtained through the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH: HIV-1JR-CSF from Dr. Irvin Chen.
This work was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institute of Health, USA and by the German Research Association (Transregio 60 and RTG 1949)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arnold F, Schnell J, Zirafi O, Sturzel C, Meier C, Weil T, Standker L, Forssmann WG, Roan NR, Greene WC, Kirchhoff F, Munch J. Naturally occurring fragments from two distinct regions of the prostatic acid phosphatase form amyloidogenic enhancers of HIV infection. Journal of virology. 2012;86:1244–1249. doi: 10.1128/JVI.06121-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cann AJ, Zack JA, Go AS, Arrigo SJ, Koyanagi Y, Green PL, Koyanagi Y, Pang S, Chen IS. Human immunodeficiency virus type 1 T-cell tropism is determined by events prior to provirus formation. Journal of virology. 1990;64:4735–4742. doi: 10.1128/jvi.64.10.4735-4742.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capule CC, Brown C, Olsen JS, Dewhurst S, Yang J. Oligovalent amyloid-binding agents reduce SEVI-mediated enhancement of HIV-1 infection. Journal of the American Chemical Society. 2012;134:905–908. doi: 10.1021/ja210931b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornetta K, Anderson WF. Protamine sulfate as an effective alternative to polybrene in retroviral-mediated gene-transfer: implications for human gene therapy. Journal of virological methods. 1989;23:187–194. doi: 10.1016/0166-0934(89)90132-8. [DOI] [PubMed] [Google Scholar]
- Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, Mncube Z, Duraiswamy J, Zhu B, Eichbaum Q, Altfeld M, Wherry EJ, Coovadia HM, Goulder PJ, Klenerman P, Ahmed R, Freeman GJ, Walker BD. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- Deeks SG, Kitchen CM, Liu L, Guo H, Gascon R, Narvaez AB, Hunt P, Martin JN, Kahn JO, Levy J, McGrath MS, Hecht FM. Immune activation set point during early HIV infection predicts subsequent CD4+ T-cell changes independent of viral load. Blood. 2004;104:942–947. doi: 10.1182/blood-2003-09-3333. [DOI] [PubMed] [Google Scholar]
- Dimitrov DS, Willey RL, Sato H, Chang LJ, Blumenthal R, Martin MA. Quantitation of human immunodeficiency virus type 1 infection kinetics. Journal of virology. 1993;67:2182–2190. doi: 10.1128/jvi.67.4.2182-2190.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer JR, Gilliam BL, Eron JJ, Jr, Grosso L, Cohen MS, Fiscus SA. Quantitation of human immunodeficiency virus type 1 RNA in cell free seminal plasma: comparison of NASBA with Amplicor reverse transcription-PCR amplification and correlation with quantitative culture. Journal of virological methods. 1996;60:161–170. doi: 10.1016/0166-0934(96)02063-0. [DOI] [PubMed] [Google Scholar]
- Giorgi JV, Hultin LE, McKeating JA, Johnson TD, Owens B, Jacobson LP, Shih R, Lewis J, Wiley DJ, Phair JP, Wolinsky SM, Detels R. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. The Journal of infectious diseases. 1999;179:859–870. doi: 10.1086/314660. [DOI] [PubMed] [Google Scholar]
- Hauber I, Hohenberg H, Holstermann B, Hunstein W, Hauber J. The main green tea polyphenol epigallocatechin-3-gallate counteracts semen-mediated enhancement of HIV infection. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:9033–9038. doi: 10.1073/pnas.0811827106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer U, Baenziger S, Heikenwalder M, Schlaepfer E, Gehre N, Regenass S, Brunner T, Speck RF. RAG2−/− gamma(c)−/− mice transplanted with CD34+ cells from human cord blood show low levels of intestinal engraftment and are resistant to rectal transmission of human immunodeficiency virus. Journal of virology. 2008;82:12145–12153. doi: 10.1128/JVI.01105-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KA, Yolamanova M, Zirafi O, Roan NR, Staendker L, Forssmann WG, Burgener A, Dejucq-Rainsford N, Hahn BH, Shaw GM, Greene WC, Kirchhoff F, Munch J. Semen-mediated enhancement of HIV infection is donor-dependent and correlates with the levels of SEVI. Retrovirology. 2010;7:55. doi: 10.1186/1742-4690-7-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyanagi Y, Miles S, Mitsuyasu RT, Merrill JE, Vinters HV, Chen IS. Dual infection of the central nervous system by AIDS viruses with distinct cellular tropisms. Science. 1987;236:819–822. doi: 10.1126/science.3646751. [DOI] [PubMed] [Google Scholar]
- Kutsch O, Benveniste EN, Shaw GM, Levy DN. Direct and quantitative single-cell analysis of human immunodeficiency virus type 1 reactivation from latency. Journal of virology. 2002;76:8776–8786. doi: 10.1128/JVI.76.17.8776-8786.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavender KJ, Messer RJ, Race B, Hasenkrug KJ. Production of bone marrow, liver, thymus (BLT) humanized mice on the C57BL/6 Rag2(−/−)gammac(−/−)CD47(−/−) background. Journal of immunological methods. 2014;407:127–134. doi: 10.1016/j.jim.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavender KJ, Pang WW, Messer RJ, Duley AK, Race B, Phillips K, Scott D, Peterson KE, Chan CK, Dittmer U, Dudek T, Allen TM, Weissman IL, Hasenkrug KJ. BLT-humanized C57BL/6 Rag2−/−gammac−/−CD47−/− mice are resistant to GVHD and develop B and T cell immunity to HIV infection. Blood. 2013 doi: 10.1182/blood-2013-06-506949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy DN, Aldrovandi GM, Kutsch O, Shaw GM. Dynamics of HIV-1 recombination in its natural target cells. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:4204–4209. doi: 10.1073/pnas.0306764101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning JS, Hackett AJ, Darby NB., Jr Effect of polycations on sensitivity of BALD-3T3 cells to murine leukemia and sarcoma virus infectivity. Applied microbiology. 1971;22:1162–1163. doi: 10.1128/am.22.6.1162-1163.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolick JB, Gange SJ, Detels R, O’Gorman MR, Rinaldo CR, Jr, Lai S. Impact of inversion of the CD4/CD8 ratio on the natural history of HIV-1 infection. Journal of acquired immune deficiency syndromes. 2006;42:620–626. doi: 10.1097/01.qai.0000223028.55080.9d. [DOI] [PubMed] [Google Scholar]
- Martellini JA, Cole AL, Svoboda P, Stuchlik O, Chen LM, Chai KX, Gangrade BK, Sorensen OE, Pohl J, Cole AM. HIV-1 enhancing effect of prostatic acid phosphatase peptides is reduced in human seminal plasma. PloS one. 2011;6:e16285. doi: 10.1371/journal.pone.0016285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore JP, Cao Y, Qing L, Sattentau QJ, Pyati J, Koduri R, Robinson J, Barbas CF, 3rd, Burton DR, Ho DD. Primary isolates of human immunodeficiency virus type 1 are relatively resistant to neutralization by monoclonal antibodies to gp120, and their neutralization is not predicted by studies with monomeric gp120. Journal of virology. 1995;69:101–109. doi: 10.1128/jvi.69.1.101-109.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munch J, Rucker E, Standker L, Adermann K, Goffinet C, Schindler M, Wildum S, Chinnadurai R, Rajan D, Specht A, Gimenez-Gallego G, Sanchez PC, Fowler DM, Koulov A, Kelly JW, Mothes W, Grivel JC, Margolis L, Keppler OT, Forssmann WG, Kirchhoff F. Semen-derived amyloid fibrils drastically enhance HIV infection. Cell. 2007;131:1059–1071. doi: 10.1016/j.cell.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Munch J, Sauermann U, Yolamanova M, Raue K, Stahl-Hennig C, Kirchhoff F. Effect of semen and seminal amyloid on vaginal transmission of simian immunodeficiency virus. Retrovirology. 2013;10:148. doi: 10.1186/1742-4690-10-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munch J, Standker L, Pohlmann S, Baribaud F, Papkalla A, Rosorius O, Stauber R, Sass G, Heveker N, Adermann K, Escher S, Kluver E, Doms RW, Forssmann WG, Kirchhoff F. Hemofiltrate CC chemokine 1[9–74] causes effective internalization of CCR5 and is a potent inhibitor of R5-tropic human immunodeficiency virus type 1 strains in primary T cells and macrophages. Antimicrobial agents and chemotherapy. 2002;46:982–990. doi: 10.1128/AAC.46.4.982-990.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen JS, Brown C, Capule CC, Rubinshtein M, Doran TM, Srivastava RK, Feng C, Nilsson BL, Yang J, Dewhurst S. Amyloid-binding small molecules efficiently block SEVI (semen-derived enhancer of virus infection)- and semen-mediated enhancement of HIV-1 infection. The Journal of biological chemistry. 2010;285:35488–35496. doi: 10.1074/jbc.M110.163659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orendi JM, Bloem AC, Borleffs JC, Wijnholds FJ, de Vos NM, Nottet HS, Visser MR, Snippe H, Verhoef J, Boucher CA. Activation and cell cycle antigens in CD4+ and CD8+ T cells correlate with plasma human immunodeficiency virus (HIV-1) RNA level in HIV-1 infection. The Journal of infectious diseases. 1998;178:1279–1287. doi: 10.1086/314451. [DOI] [PubMed] [Google Scholar]
- Roan NR, Muller JA, Liu H, Chu S, Arnold F, Sturzel CM, Walther P, Dong M, Witkowska HE, Kirchhoff F, Munch J, Greene WC. Peptides released by physiological cleavage of semen coagulum proteins form amyloids that enhance HIV infection. Cell host & microbe. 2011;10:541–550. doi: 10.1016/j.chom.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roan NR, Munch J, Arhel N, Mothes W, Neidleman J, Kobayashi A, Smith-McCune K, Kirchhoff F, Greene WC. The cationic properties of SEVI underlie its ability to enhance human immunodeficiency virus infection. Journal of virology. 2009;83:73–80. doi: 10.1128/JVI.01366-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roan NR, Sowinski S, Munch J, Kirchhoff F, Greene WC. Aminoquinoline surfen inhibits the action of SEVI (semen-derived enhancer of viral infection) The Journal of biological chemistry. 2010;285:1861–1869. doi: 10.1074/jbc.M109.066167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royce RA, Sena A, Cates W, Jr, Cohen MS. Sexual transmission of HIV. The New England journal of medicine. 1997;336:1072–1078. doi: 10.1056/NEJM199704103361507. [DOI] [PubMed] [Google Scholar]
- Sheftic SR, Snell JM, Jha S, Alexandrescu AT. Inhibition of semen-derived enhancer of virus infection (SEVI) fibrillogenesis by zinc and copper. European biophysics journal : EBJ. 2012;41:695–704. doi: 10.1007/s00249-012-0846-0. [DOI] [PubMed] [Google Scholar]
- Sun Z, Denton PW, Estes JD, Othieno FA, Wei BL, Wege AK, Melkus MW, Padgett-Thomas A, Zupancic M, Haase AT, Garcia JV. Intrarectal transmission, systemic infection, and CD4+ T cell depletion in humanized mice infected with HIV-1. The Journal of experimental medicine. 2007;204:705–714. doi: 10.1084/jem.20062411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JH, Janas AM, Olson WJ, Wu L. Functionally distinct transmission of human immunodeficiency virus type 1 mediated by immature and mature dendritic cells. Journal of virology. 2007;81:8933–8943. doi: 10.1128/JVI.00878-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L. Biology of HIV mucosal transmission. Curr Opin HIV AIDS. 2008;3:534–540. doi: 10.1097/COH.0b013e32830634c6. [DOI] [PMC free article] [PubMed] [Google Scholar]