Abstract
γδ T cells have been shown to have immunoregulatory functions in several experimental autoimmune models. A mutation of the Foxp3 gene leads to the absence of regulatory T cells (Treg) and a fatal systemic autoimmune disease in scurfy mice. Transfer of scurfy lymphocytes to RAG deficient (−/−) recipients reproduces the inflammatory phenotype of the scurfy donor including hepatitis and pneumonitis. Here, we show that TCRα−/− recipients, that lack αβ T cells, but have γδ T cells and B cells, are significantly protected from the hepatitis and pneumonitis, but not the dermatitis, induced by adoptive transfer of scurfy lymphocytes. Co-transfer of γδ T cells, but not B cells, prevented hepatitis and pneumonitis in RAG−/− recipients of scurfy lymphocytes. γδ T cells in the TCRα−/− recipients of scurfy cells markedly expanded and expressed a highly activated (CD62LloCD44hi) phenotype. The activated γδ T cells expressed high levels of CD39 and NKG2D on their cell surface. A high frequency of scurfy T cells in TCRα−/− recipients produced IL-10 suggesting that γδ T cells may enhance suppressor cytokine production from scurfy T cells in TCRα−/− recipients. This study indicates that γδ T cells may contribute to the maintenance of immunological homeostasis by suppressing autoreactive T cells in liver and lung.
Introduction
γδ T cells are a unique population of lymphocytes that have been shown to play multiple modulatory roles during the course of immune responses including the production of pro-inflammatory cytokines (TNFα IFNγ, IL-17), as well as granzymes capable of lysing infected or stressed cells (1). γδ T cells have also been shown to provide B cell help, trigger dendritic cell maturation, and facilitate the priming of αβ T cells by presenting antigen. In addition to their effects in augmenting immune responses, γδ T cells have also been reported to exert immunoregulatory roles in several different experimental systems (2). In a mouse model of adriamycin–induced nephropathy, Vγ6/Vδ1 T cells exerted a protective function (3) and Vγ6/Vδ1 T cells were also reported to have an inhibitory effect in a model of pulmonary fibrosis induced by chronic inhalation of Bacillus subtilis microorganisms (4). In one model of EAE in B10.PL mice, γδ T cells controlled encephalitogenic T cells by Fas/Fas-ligand-dependent apoptosis of T effector cells (5). Decidual γδ T cells play a protective role in pregnancy by secreting IL-10 (6), while γδ T cells can prevent type I diabetes induced in neonatally thymectomized NOD mice by producing TGF-β1 (7). No single immunosuppressive mechanism can account for the regulatory activity attributed to γδT cells. The potential contribution of γδ T cells to self-tolerance is still largely unknown.
Foxp3+ regulatory T cells (Treg) are potent suppressors of immune activation and play a crucial role in the maintenance of self-tolerance (8). Mutations of Foxp3 result in the fatal immune dysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) syndrome in humans (9), while a two-base pair insertion in Foxp3 leads to the premature termination of translation, resulting in the lymphoproliferative syndrome of the scurfy mice (10, 11). Scurfy mice develop multi-organ inflammation in skin, lungs, pancreas, small intestine and liver associated with splenomegaly, lymphadenopathy, resulting in death at the age of 3 to 5 weeks. In 3–4 wk old scurfy mice, disease is limited to ear skin, tail skin, liver and lung. Transfer of scurfy lymphocytes to RAG deficient (−/−) recipients reproduces the inflammatory phenotype of the scurfy donor, but inflammation is observed in many more organs in the recipient than in the scurfy donor (12). The major reason for the enhanced disease phenotype in the RAG−/− recipients is that scurfy mice only live to an age of 4–5 wk and some organ-specific autoreactive clones may not have had sufficient time to expand in the scurfy donor. Co-transfer of Foxp3+ Treg (12) or in vitro induced Treg (iTreg) completely suppresses the activation and expansion of the scurfy T cells in this transfer model (13). Suppression was mediated by a mechanism that inhibited the expansion of the scurfy cells in secondary lymphoid organs (12).
The development of multi-organ autoimmune disease in the scurfy transfer model offers the opportunity to determine the antigenic targets recognized by autoreactive antibodies and T cells. We previously described a protocol to identify antigens recognized by scurfy T cells in mouse skin by first examining the antigens recognized by scurfy autoantibodies (14). Transfer of total lymphocytes from scurfy mice to RAG−/− mice resulted in the development of a pool of autoantibodies that resembled the antibody repertoire found in the scurfy donor. Scurfy sera were screened for reactivity to skin proteins and we identified several keratins as antigenic targets. As only a small number of scurfy B cells are transferred by this procedure, we attempted to optimize the induction of antibody producing B cells by transferring the scurfy lymphocytes into TCRα−/− mice. Here we demonstrate that γδ T cells present in the TCRα−/− mice inhibited the development of some of the organ-specific diseases seen after transfer of scurfy cells. We characterize several of the potential immunosuppressive mechanisms used by these regulatory γδ T cells and demonstrate that they enhance the percentage of scurfy T cells capable of producing IL-10. Thus, γδ T cells can regulate highly activated scurfy T cells and maintain self-tolerance in vivo.
Materials and Methods
Mice
C57BL/6 were obtained from the National Cancer Institute Mouse Repository (Frederick, MD). C57BL/6 RAG1−/− and C57BL/6 TCRα−/− were obtained from Taconic Farms (Germantown, NY). C57BL/6 TCRβδ−/− mice were purchased from Jackson Laboratories (Bar Harbour, ME). Female heterozygous B6.Cg-Foxp3sf/J (Scurfy) mice were purchased from Jackson Laboratories and bred to C57BL/6 wild type male mice to generate hemizygous male B6 (Cg-Foxp3sf/Y (Scurfy) offspring. All animal protocols were approved by the NIAID Animal Care and Use Committee.
Adoptive transfer for induction of autoimmune disease
Spleen cells were isolated from scurfy mice between 19 and 24 d of age and transferred (1X106) to RAG−/−, TCRα−/− or TCRβδ−/− mice by retroorbital injection. B cells were isolated from spleens of TCRα−/− mice by using anti-CD19 beads and AutoMACS Cell Separater (Miltenyi Biotec) and cotransferred with scurfy spleen cells at a ratio of 1:15. γδ T cells were isolated from spleens of TCRα−/− mice which received scurfy spleen cells 5 wk previously by using TCRγδ T cell isolation kit (Miltenyi Biotec) and co-transferred with scurfy spleen cells to RAG−/− mice at a ratio of 1:5.
Lymphocyte isolation from liver and lung
To isolate lymphocytes from mouse liver, mice were anesthetized, and liver tissues were perfused in situ via the superior vena cava with PBS. Livers were dissected and placed into dishes with complete RPMI media (RPMI supplemented by 10% FBS, 50 μM 2-ME (Sigma-Aldrich), 1% sodium pyruvate, 1% non-essential amino acid, 1% HEPES, 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 mM L-glutamine). The tissues were pressed against a 70-μm cell strainer with the plunger from a 5CC syringe. The cells were centrifuged at 30 g for 3 min to pellet hepatocytes. The supernatants were transferred to new tubes and then centrifuged at 320g for 5 min. The cells were then resuspended in 33% Percoll (Sigma-Aldrich) in PBS and centrifuged at 500g for 15 min. The cell pellet were resuspended in ACK lysis buffer and incubated for 5 min. To isolate lymphocytes from mouse lung, mice were anesthetized, and lungs were perfused in situ with PBS. Lungs were dissected and placed into tubes with 0.5 mg/ml of collagenase from clostridium histolyticum (Sigma-Aldrich) in complete RPMI media. The tissues were dissected into small pieces and incubated for 1 hour at 37 °C. After digestion, the tissues were pressed against a 70-μm cell and washed. The cells were resuspended in 33% Percoll in PBS and centrifuged at 500g for 15 min. The cell pellet was resuspended in ACK lysis buffer and incubated for 5 min.
Flow cytometry
Anti-NK1.1-APC-Cy7 (PK136), anti-CD19-APC (1D3), anti-CD44-Alexa Flour 700 (IM7), anti-TCRβ-V450 (H57-597), anti-Ki-67-PE (MOPC-21), anti-IL-4-PE (11B11) and anti-IL-17A-PE (TC11-18H10) were purchased from BD Biosciences. Anti-TCRδ-FITC (GL3), anti-CD8-PE (53-6.7), anti-CD62L-eFlour 605NC (MEL-14), anti-natural-killer group 2, member D (NKG2D)-APC (CX5), anti-Rae-1d-PE (RD-41), anti-MULT-1-PE (5D10), anti-CD25-PE-Cy7 (PC61.5), anti-Foxp3- Alexa Flour 700 (FJK-16s), anti-CTLA-4-PE (UC10-4B9), anti-GITR-PE (DTA-1), anti-GARP-PE (YGIC86) anti-CD39-PE (24DMS1), anti-IL-10 PE (JES5-16E3) and anti-IFN-γ-PE-Cy7 (XMG1.2) were purchased from eBioscience. Anti-TCRVγ1-PE (2.11), anti-TCRVγ4-APC (UC3-10A6) and anti-CD73-APC (TY/11.8) were purchased from Biolegend. Anti-CD4-Qdot605 (RM4-5) was purchased from Life technologies. Purified mouse anti-mouse latency-associated peptide (LAP) clone TW7-16B4 was provided by H. Weiner (Harvard Medical School, Boston, MA). The LAP Ab was labeled with SureLight-APC at Columbia Biosciences (Columbia, MD). To separate dead cells, cells were stained with Live/Dead fixable aqua dead cell stain Kit (Life technologies) before surface staining. For intracellular staining of Foxp3, CTLA-4, Ki-67, granzyme B and perforin, cells were fixed and permeabilized using the Foxp3 fixation/permeabilization staining kit (eBioscience). For staining of cytokines, cells were stimulated with PMA/ionomycin plus protein transport inhibitors (eBioscience), and then fixed and permeabilized using the Cytofix/Cytoperm kit (BD Bioscience). For intracellular staining of IL-10 in B cells, LPS (10 μg/ml) was also added for the stimulation of B cells. For FACS-analysis, the data were acquired on a FACS LSRII (BD Biosciences) and analyzed using the FlowJo software.
In vitro expansion of γδ T cells
Spleens of TCRα−/− mice were first depleted of B cells using anti-CD19 beads and γδ T cells were purified using the Pan T cell isolation kit II (Miltenyi Biotec). Isolated naïve γδ T cells were expanded as described previously (15) with minor modification. In brief, cells were cultured in complete RPMI media with IL-2 (200 IU/ml) on dishes pre-coated (5 μg/ml) with the γδ T cells-specific mAb GL4 (BD Bioscience) for 3 d. The recovered cells were then expanded in medium containing IL-2 (100 IU/ml) for an additional 6 d.
Histology
The severity of autoimmunity on the liver, lung and skin was scored as described previously (16).
Blockade of NKG2D
NKG2D-specific mAbs HMG2D (BioXcell) and CX5 (generously provided by Dr. Lewis L Lanier, University of California San Francisco) were administered to TCRα−/− recipients of scurfy spleen cells via intraperitoneal injection (400 μg per mouse and 250 μg per mouse, respectively).
Treatment with CD39 inhibitor
POM-1 (Santa Cruz Biotechnology), a chemical inhibitor of NTPDase was administered to TCRα recipients of scurfy spleen cells via intraperitoneal injection at 10 μg per g daily.
Statistical analysis
Statistical analysis was performed with Prism software. Multiple groups were compared by ANOVA followed by Tukey’s multiple comparisons test. Two groups were compared by unpaired two-tailed student’s t-test. Comparisons in Fig. 1B and C were made by Kruskal-Wallis test followed by Dunn’s multiple comparisons test. Comparisons in Fig. 2C and 4B were made by two-tailed Mann Whitney tests. The data are expressed as mean ± SEM. P values <0.05 were considered significant.
FIGURE 1.
The liver and lungs of TCRα−/− recipients are protected from inflammation caused by scurfy lymphocytes. (A) Absolute numbers of CD4+ and CD8+ T cells (pregated on viable NK1.1−TCRβ+ cells) in recipients 5 wk after the scurfy cell transfer. (B–C) Representative images of H&E stained liver (B) and lung (C) sections and histology scores 5 wk after transfer. (D) Serum AST and ALT levels in recipient mice 5 wk after transfer and in wild type mice. (E) Histology scores of ear skin sections 5 wk after transfer. H&E, original magnification ×10. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
FIGURE 2.
B cells do not prevent the inflammation in TCRα−/− recipients. (A) Spleen and sLN cells were taken from recipient mice 5 wk after transfer, and stimulated with Cell Stimulation Cocktail (PMA and ionomycin), Protein Transport Inhibitors and LPS for 5 h. The percentage of IL-10+ cells derived from the viable CD19+ cells are shown. (B) Scurfy spleen cells were transferred with or without B cells isolated from untreated TCRα−/− mice into RAG−/− recipients at a ratio of 1:15 (1×106 and 15×106). Absolute numbers of CD4+ and CD8+ T cells (pregated on viable NK1.1−TCRβ+ cells) in recipient mice 5 wk after transfer were shown. (C) Histology scores 5 wk after transfer. (D) Absolute numbers of CD19+ B cells in recipient mice 5 wk after transfer. **p < 0.01.
FIGURE 4.
Co-transfer of γδ T cells with scurfy cells prevents inflammation in RAG−/− recipients. (A) Scurfy spleen cells were transferred with or withoutγδ T cells isolated from Sf-TCRα−/− recipients 5 weeks after the scurfy cell transfer or in vitro cultured γδ T cells (c-γδT) at a ratio of 1:5 (1×106 and 5×106) into RAG−/− recipients. Absolute numbers of CD4+ and CD8+ T cells (pregated on viable NK1.1−TCRβ+ cells) in recipient mice 5 wk after transfer were shown. (B) Histology scores 5 wk after transfer. (C) Absolute numbers of TCRδ+ γδ T cells (pregated on viable NK1.1− cells) in recipient mice 5 wk after transfer. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Results
TCRα−/− recipients are protected from inflammation caused by scurfy lymphocytes
When we examined the three groups of recipients 5 wk after transfer, we were surprised to find that the absolute number of scurfy CD4+ and CD8+ TCR αβ+ T cells was significantly suppressed in the liver and lung, but not in the spleen, in TCRα−/− recipients when compared to RAG−/− and TCRβδ−/− recipients (Fig. 1A). Histologically, liver and lung inflammation was less severe in TCRα−/− recipients than that in RAG−/− and TCRδ−/− recipients, although the differences in histology score between lungs of TCRα−/− and TCRβδ −/− recipients were not statistically significant (Fig. 1B, 1C). Elevated levels of liver enzymes were observed in RAG−/− and TCRδ−/− recipients, but not in TCRα−/− recipients compared to control wild type mice at 5 wk after transfer (Fig. 1D). Interestingly, inflammation in the ear skin was not suppressed in either of TCRα−/− and TCRδ−/− recipients (Fig. 1E). These results demonstrate that TCRα−/− recipients contain an immunoregulatory cell population that specifically protects against the organ-specific autoimmune pathology induced by scurfy T cells in the liver and lung, but not in the skin.
We first checked the different recipient mice for the presence of γδ T cells (TCRδ+NK1.1−) and B cells (CD19+). The absolute number of γδ T cells was higher in the skin draining lymph nodes (sLN) of TCRα−/− mice than wild type (WT) mice and γδ T cells as expected could not be detected in the sLN of TCRβδ−/− mice (Supplemental Fig. 1A). The absolute number of B cells in the three strains was similar. Following transfer of the scurfy cells, expansion of γδ T cells was only observed in TCRα−/− mice indicating that the γδ T cells were of recipient origin and not derived from the scurfy donor (Supplemental Fig. 1B). No B cells could be detected in the RAG−/− recipients, but similar numbers of B cells were present in the organs of the TCRα−/− and TCRβδ−/− recipients indicating that the B cells are of recipient origin. (Supplemental Fig. 1C).
B cells do not contribute to the suppression of inflammation in TCRα−/− recipients
Although both TCRα−/− and TCRβδ−/− mice have similar B cell pools, only the TCRα−/− mice were protected from liver and lung pathology. However, it remained possible that IL-10 producing regulatory B cells (17) mediated protection in the TCRα−/− recipients. There was no difference in the capacity of B cells from TCRα−/− and TCRβδ−/− to produce IL-10 either before or after transfer of scurfy cells (Fig. 2A). We also directly examined the suppressive capacity of B cells in this model by co-transferring scurfy spleen cells and B cells from untreated TCRα−/− mice at a ratio of 1:15 (1×106 and 15×106) into RAG−/− recipients. Co-transfer of B cells did not suppress the expansion of scurfy T cells or pathology in liver and lung in the recipients 5 wk after transfer despite the presence of significantly higher numbers of B cells in the co-transferred recipients (Fig. 2B–2D). It is thus unlikely that B cells play an immunoregulatory role in this model.
γδ T cells are highly activated and proliferative in TCRα−/− recipients
We therefore focused our studies on the endogenous γδ T cells in recipient mice and compared the absolute number of γδ T cells in recipient mice before and after the scurfy cell transfer. γδ T cells markedly expanded in the liver, lung, spleen and sLN in the TCRα−/− recipients (Fig. 3A). The percentage of CD62LloCD44hi activated γδ T cells significantly increased in TCRα−/− recipients after transfer (Fig. 3B, 3C). Of note, the majority of γδ T cells in the liver were CD62LloCD44hi before transfer (Fig. 3C). The percentage of Ki-67+ γδ T cells also increased 5 wk after transfer (Fig. 3D, 3E) consistent with an ongoing expansion process. The expansion of γδ T cells was not restricted to a particular γδ T cell subset, as Vγ1- and Vγ4-expressing γδ T cells were almost equally expanded in TCRα−/− recipients after transfer except for a preferential expansion of Vγ1-expressing γδ T cells in the lung (Fig. 3F, 3G). This may indicate that Vγ1-expressing cells have a unique role in suppressing autoimmune pathology in the lung. These results indicate that endogenous γδ T cells are activated in response to the scurfy cell transfer and polyclonally expanded in the liver, lung, spleen and sLN of TCRα−/− recipients. As scurfy mice also contain γδ T cells, we enumerated the number of γδ T cells in 20–23 d old scurfy mice. However, we did not observe an increase in the number of γδ T cells compared to WT littermates even though the scurfy mice exhibited marked expansion of conventional T cells as this age (Supplemental Fig. 2).
FIGURE 3.
γδ T cells in TCRα−/− mice become activated and proliferate after transfer of scurfy cells. (A) Comparison of absolute numbers of TCRδ+ γδ T cells (pregated on viable NK1.1− cells) from untreated TCRα−/− mice and TCRα−/− recipients 5 wk after the scurfy cell transfer (n = 3–10). (B) Flow cytometry of cells from the liver and spleen of TCRα−/− mice and TCRα−/− recipients 5 wk after transfer. Numbers on top left in each panel indicate percent CD62LloCD44hi cells among TCRδ+ γδ T cells. (C) Quantification of the frequency of CD62LloCD44hi γδ T cells from untreated TCRα−/− mice and TCRα−/− recipients 5 wk after transfer. (D) Flow cytometry of cells from the liver and spleen of untreated TCRα−/− mice and TCRα−/− recipients 5 wk after transfer. Numbers on top right in each panel indicate percent CD44+Ki-67+ cells among TCRδ+ γδ T cells. (E) Quantification of the frequency of CD44+Ki-67+ γδ T cells from untreated TCRα−/− mice and TCRα−/− recipients 5 wk after transfer. (F) Flow cytometry of cells from the liver and spleen of untreated TCRα−/− mice and TCRα−/− recipients 5 wk after transfer. Percentages of TCRVγ1+ cells and TCRVγ4+ cells among TCRδ+ γδ T cells are shown at bottom right and top left in each panel, respectively. (G) Quantification of the frequency of TCRVγ1+/TCRVγ4+ γδ T cells from untreated TCRα−/− mice and TCRα−/− recipients 5 wk after transfer. **p < 0.01, ***p < 0.001.
Co-transfer of γδ T cells with scurfy cells prevents inflammation in RAG−/− recipients
To directly examine the suppressive capacity of γδT cells in this model, scurfy spleen cells were co-transferred into RAG−/− recipients with γδT cells (1:5) from TCRα−/− recipient that received scurfy spleen cells 5 wk previously. Co-transfer of γδT cells suppressed the expansion of scurfy T cells and decreased inflammation in the liver and lung, but not in the spleen or sLNs 5 wk after transfer (Fig. 4A, 4B). In addition to freshly explanted γδ T cells from treated mice, we expanded γδT cells from untreated TCRα−/− mice by stimulation with anti-TCRδ mAb and IL-2. Co-transfer of in vitro cultured γδ T cells (c-γδT) also suppressed the expansion of scurfy T cells in the lung, but not the liver (Fig. 4A). Marked expansion of the co-transferred freshly explanted or in vitro expanded γδ T cells was seen when they were co-transferred with the scurfy cells, but no expansion was seen when the c-γδT cells were transferred alone (Fig. 4C).
Phenotype of immunoregulatory γδ T cells
As the suppressive function of the γδ T cells in this model closely resembled that of Foxp3+ Treg, we first examined the expression of a number of markers that are preferentially expressed on Treg. Very low to undetectable levels of CD25, Foxp3, CTLA-4, LAP, or GARP were detected on γδ T cells in TCRα−/− mice and these levels were unchanged in the spleens of TCRα−/− mice following transfer of the scurfy cells. The GITR was expressed at high levels on γδ T cells and its expression was slightly decreased after transfer of the scurfy cells (Fig. 5A). The immunosuppressive cytokine, IL-10, was expressed by a low percentage of γδ T cells in TCRα−/− mice and the percent of IL-10 producing γδ T cells did not increase after scurfy cell transfer (Fig. 5B). The expression of granzyme B was increased in γδ T cells following scurfy cell transfer, but perforin expression could not be detected either before or after transfer (Fig. 5C). We cannot exclude the possibility that some of the suppressive effects of the γδ T cells may be secomdary to granzyme B mediated cytolysis.
FIGURE 5.
Phenotype of immunoregulatory γδ T cells. (A) Histograms shown represent the expression of CD25, Foxp3, intracellular CTLA-4, GITR, LAP and GARP on TCRδ+ γδ T cells (pregated on viable NK1.1− cells) from the spleen of TCRα−/− mice (dotted lines) and TCRα−/− recipients 5 wk after transfer (black lines). (B) Spleen cells were obtained from untreated TCRα−/− mice and TCRα−/− recipients 5 wk after the scurfy cell transfer and were activated with Cell Stimulation Cocktail and Protein Transport Inhibitors for 4 h. Frequency of IL-10+ TCRδ+ γδ T cells is shown. (B) Histograms shown represent the expression of granzyme B and perforin on TCRδ+ γδ T cells from the spleen of TCRα−/− mice (dotted lines) and TCRα−/− recipients 5 wk after transfer (black lines).
Activated γδ T cells express high levels of CD39/CD73
One other potential pathway which has been postulated to be involved in the immunosuppressive function of Treg cells and other cell types is the ectoenzyme-mediated degradation of ATP to adenosine by CD39 and CD73 (18). CD73 was constitutively expressed at high levels on most γδ T cells in TCRα−/− mice before transfer (Fig. 6A). In contrast, CD39 was expressed at low levels on splenic and lung γδ T cells and markedly upregulated after scurfy cell transfer, while CD39 was expressed on liver γδ T cells at high levels constitutively and its expression was not changed after scurfy cell transfer (Fig. 6A, 6B). Thus, the majority of γδ T cells in the liver of untreated TCRα−/− mice expressed both CD39 and CD73 in a pattern that resembled that seen on Foxp3+ Tregs (18), while most γδ T cells in the lung expressed CD73 alone (Fig. 6C).
FIGURE 6.
Activated γδ T cells express high levels of CD39/CD73. (A) Histograms shown represent the expression of CD39 and CD73 on TCRδ+ γδ T cells (pregated on viable NK1.1− cells) from the spleen of untreated TCRα−/− mice (dotted lines) and TCRα−/− recipients 5 wk after the scurfy cell transfer (black lines). (B) Histograms shown represent the expression of CD39 on TCRδ+ γδ T cells from the liver and lung of untreated TCRα−/− mice (left) and TCRα−/− recipients (right) 5 wk after transfer. (C) Expression of CD39 and CD73 on TCRδ+ γδ T cells from the liver and lung of untreated TCRα−/− mice. (D) Histograms shown represent the expression of CD39 on CD62LloCD44hi (left), CD62LhiCD44hi (center) and CD62LhiCD44lo (right) TCRδ+ γδ T cells from the liver and lung of untreated TCRα−/− mice. (E) Absolute numbers of CD4+ and CD8+ T cells (pregated on viable NK1.1−TCRβ+ cells) in POM-1 treated TCRα−/− recipients 5 wk after transfer.
The expression of CD39 in both liver and lung was correlated with an activated phenotype (CD62LloCD44hi) even before transfer (Fig. 6D) and this prompted us to examine the expression of CD39 on γδ T cells in wild type mice. In wild type mice, γδ T cells expressed high levels of CD39 in the liver, but not in the lung, spleen, or sLN (Supplemental Fig. 3A). As seen in the TCRα−/− mice, high levels of CD39 expression correlated with the activation state of the cells (Supplemental Fig. 3B). It is possible that γδ T cells may be involved in the maintenance of immune homeostasis in mouse liver in the steady state.
To determine whether the CD39/CD73 pathway contributed to the immunosuppressive function of γδ T cells, we treated TCRα−/− recipients of scurfy T cells with the nucleoside triphosphate diphosphatase inhibitor POM-1 (19). POM-1 was administered intraperitoneally (10mg per kg daily for 5 wk), but did not reverse the γδ T cell-mediated suppression of liver and lung inflammation in the recipients (Fig. 6E).
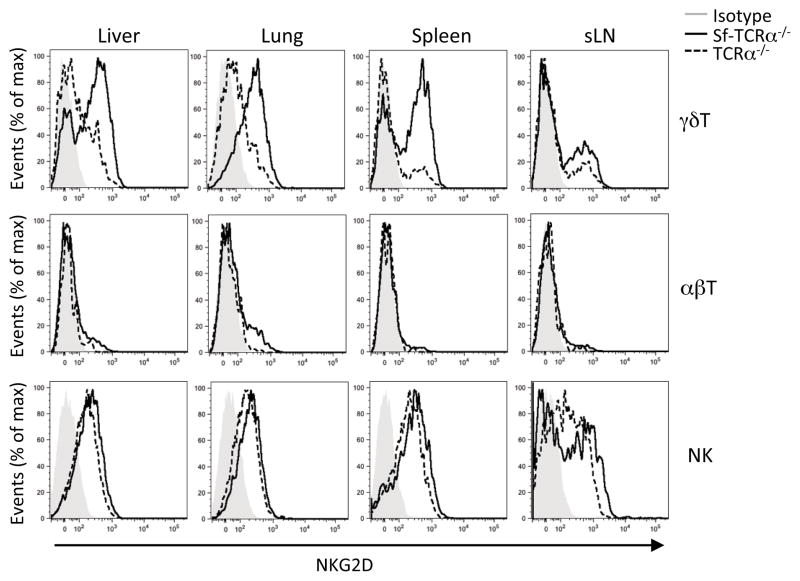
NKG2D is upregulated on γδ T cells in response to scurfy cell transfer
Another potential pathway by which γδ T cells might mediate suppression in this model is via the lectin-type activating receptor, NKG2D, that is expressed by most NK cells and NKT cells, by many γδ T cells and by antigen-experienced CD8+ αβ T cells (1). NKG2D recognizes a diverse family of major histocompatibility complex class I-related proteins such as retinoic acid early transcript 1 (Rae-1), murine UL16-binding protein transcript 1 (MULT1) and histocompatibility 60 protein (H60) in mice, which are expressed on the surface of infected, stressed or transformed cells (1). We hypothesized that NKG2D on γδ T cells in TCRα−/− recipients might recognize NKG2D ligands on scurfy T cells or on damaged cells in affected organs. As expected, most NK cells expressed NKG2D in untreated TCRα−/− mice (Fig. 7). Some γδ T cells expressed NKG2D in the liver, lung and spleen of untreated TCRα−/− mice and the expression level was up-regulated after the scurfy cell transfer (Fig. 7). About a half of the γδ T cells and a few CD8+ γδ T cells also expressed NKG2D in untreated wild type mice (Supplemental Fig. 4). Our attempts to examine the expression of NKG2D ligands (Rae-1δ and MULT1) on scurfy T cells by flow cytometry and on liver tissues from scurfy mice by immunofluorescence were unsuccessful (data not shown). Lastly, we treated TCRα−/− recipients of scurfy cells with a blocking NKG2D-specific mAb (CX5 at 250 μg per mouse one d prior to the scurfy cell transfer and twice weekly starting on day 3) (20), which resulted in partial reduction of NKG2D expression on γδ T cells, but failed to reverse the suppressive effects of the γδT cells on the liver and lung inflammation in TCRα−/− recipients (data not shown).
FIGURE 7.
NKG2D is up-regulated on γδ T cells in response to the scurfy cell transfer. Histograms shown represent the expression of NKG2D on TCRδ+ γδ T cells (pregated on viable NK1.1− cells) from untreated TCRα−/− mice (dotted lines) and TCRα−/− recipients 5 wk after the scurfy cell transfer (black lines).
γδ T cells may modulate the pathogenic potential of scurfy T cells by enhancing their capacity to produce IL-10
CD4+ and CD8+ T cells from the spleen and lymph nodes of symptomatic scurfy mice primarily represent Th1 cells with high percentages of IFNγ-producing cells (Fig. 8A), but also contained a significant percentage of IL-10 producing CD4+ T cells. Scurfy CD4+ and CD8+ T cells from RAG−/−, TCRα−/− and TCRβδ−/− recipients contained even higher percentages of IFNγ-producing cells and very few IL-4 and IL-17 producing CD4+ T cells Fig. 8A, 8C–E.). Surprisingly, CD4+ and CD8+ scurfy cells from spleen, liver and lung recovered from the TCRα−/− recipients contained a 2–3-fold increase in the percentage of cells producing IL-10 (Fig. 8B). Almost all the IL-10-producing CD4+ and CD8+ cells also produced IFN-γ (Fig. 8A). These results suggest that γδ T cells may protect TCRα−/− recipients by enhancing IL-10 production from scurfy T cells.
FIGURE 8.
The frequency of IL-10-producing scurfy T cells is higher in TCRα−/− recipients than that in RAG−/− and TCRβδ−/− recipients. (A) Spleen cells were obtained from RAG−/−, TCRα−/− and TCRβδ−/− recipients 5 wk after the scurfy cell transfer and activated with Cell Stimulation Cocktail and Protein Transport Inhibitors for 4 h. Intracellular staining of IFN-γ and IL-10 production from CD4+ and CD8+ T cells (pregated on viable NK1.1− TCRβ+ cells) are shown. Results of scurfy spleen cells are shown as a control. (B) Quantification of the frequency of IL-10-producing CD4+ and CD8+ T cells from the liver, lung and spleen of recipient mice 5 weeks after transfer. (C) Quantification of the frequency of IFN-γ-producing CD4+ and CD8+ T cells from the spleens of recipients 5 wk after transfer. (D) Quantification of the frequency of IL-4- and IL-17A-producing CD4+ T cells from the spleens of recipient mice 5 wk after transfer. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Discussion
In this study we have demonstrated the γδ T cells can exert potent immunoregulatory effects and protect the liver and lung of TCRα−/− mice from the immunopathology seen when scurfy T cells are transferred to immunodeficient recipients. The scurfy defect, together with loss of TGF-β1 (21) and CTLA-4 (22), represents one of the most severe defects resulting in the development of systemic and organ-specific autoimmune disease. The scurfy cells used to induce disease were obtained from animals older than 21 d of age at which time almost all the CD4+ and CD8+ exhibit a highly activated phenotype and a high percentage of IFNγ producing T cells. In general, Foxp3+ Treg, with rare exception (23), are the only suppressor T cell population capable of suppressing scurfy T effector cells. We were therefore surprised at the potent suppressive capacity of the γδT cells for liver and lung inflammation that we observed by comparing the spectrum of disease induced by transfer of scurfy cells to RAG−/−. TCRα−/− and TCRβδ−/− mice. We then confirmed these results in co-transfer studies with purified γδT cells isolated from TCRα−/− that had previously received scurfy cells and with in vitro expanded γδT cells from naive mice. It remains possible that the development of γδ T cells is abnormal in TCRα−/− mice and that suppression might not be a property of γδ that develop in normal mice. It is also possible that differences in the microbiome of the recipient strains may have played some role in augmenting the function of the γδ T cells, as the TCRα−/− and TCRβδ−/− strains were obtained from different sources.
We do not believe that B cells in the TCRα−/− mice played a significant immunoregulatory role in this model, as B cells were recently reported to be critical for autoimmune pathology (24) rather than for autoimmune regulation in scurfy mice. Co-transfer of B cells failed to suppress the expansion of scurfy T cells in RAG−/− recipients. Furthermore, no increase in IL-10 producing B cells was observed in the TCRα−/− recipients. It is also not likely that NK cells played significant roles in suppression of the transferred scurfy cells, as the numbers of NK cells in livers and lungs were less in TCRα/− recipients than in RAG−/− or TCRβδ−/− recipients (data not shown).
We observed marked expansion of the γδcells in the liver and lung, but only when co-transferred with scurfy T cells. No homeostatic expansion of γδcells was observed when they were transferred alone. We do not understand why the γδT cells failed to expand when transferred alone in the lymphopenic environment, but the γδT cells used in these studies were previously expanded in vitro by stimulation with anti-γδ TCR and IL-2. However, the marked expansion when co-transferred with the scurfy cells raised the possibility that the γδ T cells were activated by a ligand expressed on the scurfy cells. NKG2D (25) is expressed by γδ T cells and enables them to promptly respond to stressed or damaged cells that express NKG2D ligands. We found that about a half of γδ T cells in the liver and lung of untreated TCRα−/− mice and wild type mice expressed NKG2D and observed marked up-regulation of NKG2D expression in response to the scurfy cell transfer. This suggested a possible contribution of NKG2D to the activation of γδ T cells in our experimental system although both TCR and NKG2D might be involved as reported previously (26). The levels of NKG2D were positively correlated with the expression of CD44 on γδ T cells (data not shown). We failed in our attempts to identify NKG2D ligands on scurfy T cells by flow cytometry or in the inflamed liver by immunofluorescence. Although NKG2D ligands were detected on hepatocytes in a chronic HBV infection model after stimulation with ConA (27), their expression was transient. Similar transient expression of NKG2D ligands was observed on epidermal cells in a tape-stripping model (28). These findings suggest that the timing after cell-stress is important for the detection of NKG2D ligands and we cannot rule out the possibility that NKG2D ligands were expressed at some time point after scurfy cell transfer. However, we also attempted to use a blocking antibody to NKG2D (20) to reverse the suppressive effects of the γδ T cells, but did not observe an increase in the immunopathology in the liver.
Numerous other suppressive mechanisms (29–33) have been proposed to account for the immunoregulatory effects of γδ T cells and some studies have suggested that immunoregulatory γδ T cells resembled Foxp3+ Tregs. The activated γδ T cells in this model did not express Foxp3, Fas-ligand, cell surface TGF-β, or elevated levels of IL-4. Interestingly, both CD39 and CD73, which are frequently expressed on Tregs (18, 34), were markedly up-regulated on γδ T cells in liver and lung in response to the scurfy cell transfer. CD39 has recently been reported as a surface marker of mouse regulatory γδ T cells and CD39+ γδ T cells have been shown to suppress contact hypersensitivity although the mechanism utilized by these cells was unclear (35). The major function of CD39 is to convert ATP into AMP. CD73 then dephosphorylates AMP into adenosine that acts via A2a receptors to potently inhibit T cell responses (36, 37). Because the CD39/CD73 pathway seemed to be a likely mediator of suppression in our model, we attempted to block the function of CD39 with the nucleoside triphosphate diphosphatase inhibitor POM-1 (19). However, we did not observe a decrease in the suppressive function of the γδ T cells in treated mice at the doses of drug we used.
It is unclear why γδT cells could suppress scurfy induced disease in TCR αβ−/−recipients, but failed to expand or exert suppression in scurfy mice. One explanation for the failure to observe any effects of γδT cells in the scurfy donor is that in the scurfy mouse γδT cells are overwhelmed by the early and extensive influx of T effector cells. One of the more puzzling aspects of our results was the selective suppression of effector T cell expansion and immunopathology in liver and lung, but not in skin, spleen or other secondary lymphoid tissues. It was previously noted that T cells doubly deficient for the scurfy gene and IL-2 also produced a disease phenotype distinct from that of T cells with only the scurfy mutation in that the doubly deficient T cells failed to induce skin and lung pathology. This difference in organ specificity of disease induction was later shown to be secondary to the IL-2 mediated regulation of genes controlling trafficking/chemotaxis/retention and Th2 differentiation (38). It is unlikely that the pattern of organ specificity observed in the present study was similarly regulated as we observed regulation of liver and lung immunopathology, but not skin pathology by γδ T cells. It remains possible that the “organ-specificity” of protection by γδ T cells may be related to the normal physiologic function of γδ T cells in those organs. In wild type mice, γδ T cells in the liver had an activated phenotype and expressed high levels of CD39 and NKG2D. γδ T cells, particularly in the liver, may contribute to what has been termed liver tolerance, an environment promoting T cell tolerance to antigens delivered via the portal vein (39).
CD4+ T cells from the scurfy donors contained an elevated percentage of IL-10 producing T cells. One of the most surprising findings in this study was the marked upregulation of IL-10 production by both CD4+ and CD8+ by the scurfy T cells following transfer to TCR αβ−/− recipients. These results suggest that γδT cells modulate IL-10 production by scurfy effectors and that the enhanced production of IL-10 may be responsible for the suppressor function of the γδT cells. The mechanism by which γδ T cells modulate cytokine production by effector T cells and the role of the IL-10 producing scurfy cells in mediating protection from immunopathology remain to be determined.
Supplementary Material
Acknowledgments
This work was supported by the intramural program of the National Institute of Allergy and Infectious Diseases, NIH. H.U. was also supported by the Japan Society for the Promotion of Science Postdoctoral Fellowships for Research Abroad program, the Nakatomi Foundation, and the Sumitomo Life Welfare and Culture Foundation.
We would like to thank Dr. L. Lanier for the generous gift of the anti-NKG2D blocking mAb.
Abbreviations used in this article
- Treg
regulatory T cells
- sLN
skin draining lymph nodes
- WT
wild type
- c-γδT
in vitro cultured γδT cells
- LAP
latency-associated peptide
Footnotes
Disclosures
The authors declare no financial conflicts of interest
References
- 1.Vantourout P, Hayday A. Six-of-the-best: unique contributions of gammadelta T cells to immunology. Nat Rev Immunol. 2013;13:88–100. doi: 10.1038/nri3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kabelitz D, Peters C, Wesch D, Oberg HH. Regulatory functions of gammadelta T cells. Int Immunopharmacol. 2013;16:382–387. doi: 10.1016/j.intimp.2013.01.022. [DOI] [PubMed] [Google Scholar]
- 3.Wu H, Knight JF, Alexander SI. Regulatory gamma delta T cells in Heymann nephritis express an invariant Vgamma6/Vdelta1 with a canonical CDR3 sequence. Eur J Immunol. 2004;34:2322–2330. doi: 10.1002/eji.200324780. [DOI] [PubMed] [Google Scholar]
- 4.Simonian PL, Roark CL, Diazdel Valle F, Palmer BE, Douglas IS, Ikuta K, Born WK, O'Brien RL, Fontenot AP. Regulatory role of gammadelta T cells in the recruitment of CD4+ and CD8+ T cells to lung and subsequent pulmonary fibrosis. J Immunol. 2006;177:4436–4443. doi: 10.4049/jimmunol.177.7.4436. [DOI] [PubMed] [Google Scholar]
- 5.Ponomarev ED, Dittel BN. Gamma delta T cells regulate the extent and duration of inflammation in the central nervous system by a Fas ligand-dependent mechanism. J Immunol. 2005;174:4678–4687. doi: 10.4049/jimmunol.174.8.4678. [DOI] [PubMed] [Google Scholar]
- 6.Nagaeva O, Jonsson L, Mincheva-Nilsson L. Dominant IL-10 and TGF-beta mRNA expression in gammadeltaT cells of human early pregnancy decidua suggests immunoregulatory potential. Am J Reprod Immunol. 2002;48:9–17. doi: 10.1034/j.1600-0897.2002.01131.x. [DOI] [PubMed] [Google Scholar]
- 7.Locke NR, Stankovic S, Funda DP, Harrison LC. TCR gamma delta intraepithelial lymphocytes are required for self-tolerance. J Immunol. 2006;176:6553–6559. doi: 10.4049/jimmunol.176.11.6553. [DOI] [PubMed] [Google Scholar]
- 8.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 9.Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 10.Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF, Ramsdell F. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 11.Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova JL, Buist N, Levy-Lahad E, Mazzella M, Goulet O, Perroni L, Bricarelli FD, Byrne G, McEuen M, Proll S, Appleby M, Brunkow ME. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001;27:18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 12.Sharma R, Jarjour WN, Zheng L, Gaskin F, Fu SM, Ju ST. Large functional repertoire of regulatory T-cell suppressible autoimmune T cells in scurfy mice. J Autoimmun. 2007;29:10–19. doi: 10.1016/j.jaut.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huter EN, Punkosdy GA, Glass DD, Cheng LI, Ward JM, Shevach EM. TGF-beta-induced Foxp3+ regulatory T cells rescue scurfy mice. Eur J Immunol. 2008;38:1814–1821. doi: 10.1002/eji.200838346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huter EN, Natarajan K, Torgerson TR, Glass DD, Shevach EM. Autoantibodies in scurfy mice and IPEX patients recognize keratin 14. J Invest Dermatol. 2010;130:1391–1399. doi: 10.1038/jid.2010.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drobyski WR, Vodanovic-Jankovic S, Klein J. Adoptively transferred gamma delta T cells indirectly regulate murine graft-versus-host reactivity following donor leukocyte infusion therapy in mice. J Immunol. 2000;165:1634–1640. doi: 10.4049/jimmunol.165.3.1634. [DOI] [PubMed] [Google Scholar]
- 16.Mayer CT, Tian L, Hesse C, Kuhl AA, Swallow M, Kruse F, Thiele M, Gershwin ME, Liston A, Sparwasser T. Anti-CD4 treatment inhibits autoimmunity in scurfy mice through the attenuation of co-stimulatory signals. J Autoimmun. 2014;50:23–32. doi: 10.1016/j.jaut.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 17.Candando KM, Lykken JM, Tedder TF. B10 cell regulation of health and disease. Immunol Rev. 2014;259:259–272. doi: 10.1111/imr.12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen JF, Enjyoji K, Linden J, Oukka M, Kuchroo VK, Strom TB, Robson SC. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun X, Wu Y, Gao W, Enjyoji K, Csizmadia E, Muller CE, Murakami T, Robson SC. CD39/ENTPD1 expression by CD4+Foxp3+ regulatory T cells promotes hepatic metastatic tumor growth in mice. Gastroenterology. 2010;139:1030–1040. doi: 10.1053/j.gastro.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim J, Chang CK, Hayden T, Liu FC, Benjamin J, Hamerman JA, Lanier LL, Kang SM. The activating immunoreceptor NKG2D and its ligands are involved in allograft transplant rejection. J Immunol. 2007;179:6416–6420. doi: 10.4049/jimmunol.179.10.6416. [DOI] [PubMed] [Google Scholar]
- 21.Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold RJ, Yin M, Allen R, Sidman C, Proetzel G, Calvin D, Annunziata N, Doetschman T. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature. 1992;359:693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 23.Collison LW, Chaturvedi V, Henderson AL, Giacomin PR, Guy C, Bankoti J, Finkelstein D, Forbes K, Workman CJ, Brown SA, Rehg JE, Jones ML, Ni HT, Artis D, Turk MJ, Vignali DA. IL-35-mediated induction of a potent regulatory T cell population. Nat Immunol. 2010;11:1093–1101. doi: 10.1038/ni.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aschermann S, Lehmann CH, Mihai S, Schett G, Dudziak D, Nimmerjahn F. B cells are critical for autoimmune pathology in Scurfy mice. Proc Natl Acad Sci U S A. 2013;110:19042–19047. doi: 10.1073/pnas.1313547110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL, Spies T. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285:727–729. doi: 10.1126/science.285.5428.727. [DOI] [PubMed] [Google Scholar]
- 26.He W, Hao J, Dong S, Gao Y, Tao J, Chi H, Flavell R, O'Brien RL, Born WK, Craft J, Han J, Wang P, Zhao L, Wu J, Yin Z. Naturally activated V gamma 4 gamma delta T cells play a protective role in tumor immunity through expression of eomesodermin. J Immunol. 2010;185:126–133. doi: 10.4049/jimmunol.0903767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen Y, Wei H, Sun R, Dong Z, Zhang J, Tian Z. Increased susceptibility to liver injury in hepatitis B virus transgenic mice involves NKG2D-ligand interaction and natural killer cells. Hepatology. 2007;46:706–715. doi: 10.1002/hep.21872. [DOI] [PubMed] [Google Scholar]
- 28.Strid J, Sobolev O, Zafirova B, Polic B, Hayday A. The intraepithelial T cell response to NKG2D-ligands links lymphoid stress surveillance to atopy. Science. 2011;334:1293–1297. doi: 10.1126/science.1211250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han G, Wang R, Chen G, Wang J, Xu R, Wang L, Feng J, Li X, Guo R, Fu L, Shen B, Li Y. Interleukin-17-producing gammadelta+ T cells protect NOD mice from type 1 diabetes through a mechanism involving transforming growth factor-beta. Immunology. 2010;129:197–206. doi: 10.1111/j.1365-2567.2009.03166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hao J, Dong S, Xia S, He W, Jia H, Zhang S, Wei J, O'Brien RL, Born WK, Wu Z, Wang P, Han J, Hong Z, Zhao L, Yin Z. Regulatory role of Vgamma1 gammadelta T cells in tumor immunity through IL-4 production. J Immunol. 2011;187:4979–4986. doi: 10.4049/jimmunol.1101389. [DOI] [PubMed] [Google Scholar]
- 31.Liang D, Zuo A, Shao H, Born WK, O'Brien RL, Kaplan HJ, Sun D. IL-23 receptor expression on gammadelta T cells correlates with their enhancing or suppressive effects on autoreactive T cells in experimental autoimmune uveitis. J Immunol. 2013;191:1118–1125. doi: 10.4049/jimmunol.1300626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seo N, Tokura Y, Takigawa M, Egawa K. Depletion of IL-10- and TGF-beta-producing regulatory gamma delta T cells by administering a daunomycin-conjugated specific monoclonal antibody in early tumor lesions augments the activity of CTLs and NK cells. J Immunol. 1999;163:242–249. [PubMed] [Google Scholar]
- 33.Zhao N, Hao J, Ni Y, Luo W, Liang R, Cao G, Zhao Y, Wang P, Zhao L, Tian Z, Flavell R, Hong Z, Han J, Yao Z, Wu Z, Yin Z. Vgamma4 gammadelta T cell-derived IL-17A negatively regulates NKT cell function in Con A-induced fulminant hepatitis. J Immunol. 2011;187:5007–5014. doi: 10.4049/jimmunol.1101315. [DOI] [PubMed] [Google Scholar]
- 34.Borsellino G, Kleinewietfeld M, Di Mitri D, Sternjak A, Diamantini A, Giometto R, Hopner S, Centonze D, Bernardi G, Dell'Acqua ML, Rossini PM, Battistini L, Rotzschke O, Falk K. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood. 2007;110:1225–1232. doi: 10.1182/blood-2006-12-064527. [DOI] [PubMed] [Google Scholar]
- 35.Otsuka A, Hanakawa S, Miyachi Y, Kabashima K. CD39: a new surface marker of mouse regulatory gammadelta T cells. J Allergy Clin Immunol. 2013;132:1448–1451. doi: 10.1016/j.jaci.2013.05.037. [DOI] [PubMed] [Google Scholar]
- 36.Antonioli L, Pacher P, Vizi ES, Hasko G. CD39 and CD73 in immunity and inflammation. Trends Mol Med. 2013;19:355–367. doi: 10.1016/j.molmed.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ohta A, Sitkovsky M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature. 2001;414:916–920. doi: 10.1038/414916a. [DOI] [PubMed] [Google Scholar]
- 38.Sharma R, Sharma PR, Kim YC, Leitinger N, Lee JK, Fu SM, Ju ST. IL-2-controlled expression of multiple T cell trafficking genes and Th2 cytokines in the regulatory T cell-deficient scurfy mice: implication to multiorgan inflammation and control of skin and lung inflammation. J Immunol. 2011;186:1268–1278. doi: 10.4049/jimmunol.1002677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crispe IN, Giannandrea M, Klein I, John B, Sampson B, Wuensch S. Cellular and molecular mechanisms of liver tolerance. Immunol Rev. 2006;213:101–118. doi: 10.1111/j.1600-065X.2006.00435.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.