Abstract
Instrumental renewal, the return of extinguished instrumental responding after removal from the extinction context, is an important model of behavioral relapse that is poorly understood at the neural level. In two experiments, we examined the role of the dorsomedial prefrontal cortex (dmPFC) and the ventromedial prefrontal cortex (vmPFC) in extinction and ABA renewal of instrumental responding for a sucrose reinforcer. Previous work, exclusively using drug reinforcers, has suggested that the roles of the dmPFC and vmPFC in expression of extinction and ABA renewal may depend at least in part on the type of drug reinforcer used. The current experiments used a food reinforcer because the behavioral mechanisms underlying the extinction and renewal of instrumental responding are especially well worked out in this paradigm. After instrumental conditioning in context A and extinction in context B, we inactivated dmPFC, vmPFC, or a more ventral medial prefrontal cortex region by infusing baclofen/muscimol (B/M) just prior to testing in both contexts. In rats with inactivated dmPFC, ABA renewal was still present (i.e., responding increased when returned to context A); however responding was lower (less renewal) than controls. Inactivation of vmPFC increased responding in context B (the extinction context) and decreased responding in context A, indicating no renewal in these animals. There was no effect of B/M infusion on rats with cannula placements ventral to the vmPFC. Fluorophore-conjugated muscimol was infused in a subset of rats following test to visualize infusion spread. Imaging suggested that the infusion spread was minimal and mainly constrained to the targeted area. Together, these experiments suggest that there is a region of medial prefrontal cortex encompassing both dmPFC and vmPFC that is important for ABA renewal of extinguished instrumental responding for a food reinforcer. In addition, vmPFC, but not dmPFC, is important for expression of extinction of responding for a food reinforcer. The role of the medial prefrontal cortex in renewal in the original conditioning context may depend in part on control over excitatory context-response or context-(response-outcome) relations that might be learned in acquisition. The role of the vmPFC in expression of extinction may depend on its control over inhibitory context-response or context-(response-outcome) relations that are learned in extinction.
Keywords: prelimbic, infralimbic, renewal, extinction, instrumental, operant
Conditioned instrumental (operant) behaviors are voluntary actions that are controlled by their consequences. Animals readily acquire behaviors (e.g., lever pressing) to obtain a desirable outcome (e.g., food pellet or drug delivery) and likewise learn to suppress behavior when the reinforcer is withheld. This extinction of instrumental responding is an important and fundamental component of behavioral change (Bouton, 2014; Bouton & Todd, 2014). However, extinguished behaviors can re-emerge through several manipulations and mechanisms, including renewal. Renewal occurs when an animal is tested in a context different from the extinction context (Bouton & Bolles, 1979), resulting in a return of extinguished responding. This return of responding demonstrates that extinction is not erasure of the original learning. A major challenge in successful treatment of behavioral disorders in humans (e.g., addiction) is the susceptibility of these behaviors to relapse.
Instrumental renewal has been reliably demonstrated with several different reinforcers and across different paradigms. ABA renewal (conditioning in context A, extinction in context B, testing in context A) has been shown with food reinforcers (e.g., Nakajima, Tanaka, Urushihara, & Imada, 2000) and different drug reinforcers (e.g., alcohol, cocaine, heroin) (e.g. (Bossert, Liu, Lu, & Shaham, 2004; Chaudhri, Sahuque, & Janak, 2009; Fuchs, Eaddy, Su, & Bell, 2007; Hamlin, Clemens, & McNally, 2008). Additionally, AAB and ABC renewal have also been observed in instrumental conditioning (Bouton, Todd, Vurbic, & Winterbauer, 2011) and show that removal from the extinction context is sufficient to elicit renewal of responding; return to the acquisition context is not necessary. Such results suggest that extinction at least partly involves learning to inhibit the response in the extinction context.
There are several possible mechanisms that may underlie renewal (e.g., Bouton, 1993). However, Todd (2013) found that when the renewal test occurred in a context that was associated with extinction of a separate response, renewal was not affected. Additionally, in a discriminated operant situation in which responses were controlled by different discriminative stimuli, renewal of a response was reduced if the same response, but not a different response, had previously been extinguished in the test context (Todd, Vurbic, & Bouton, 2014b). Thus, renewal is due at least partly to the release from context specific response inhibition that develops during extinction, rather than negative occasion setting or context-outcome inhibition. It is worth noting that when renewal tests are conducted in context A, the original acquisition context, the test context might also engage excitatory mechanisms learned there during acquisition (see Discussion).
A number of brain regions appear to play a role in ABA renewal of extinguished instrumental behavior for drug reinforcers (Bossert et al., 2013). Many of these brain regions are important regardless of the type of drug reinforcer. For example, inactivation of the nucleus accumbens shell, or infusion of a D1R antagonist there, reduced ABA renewal of extinguished lever-pressing for cocaine (Fuchs et al., 2008), alcohol (Chaudhri et al., 2009), or heroin (Bossert et al., 2007). However, the picture is not as clear for the medial prefrontal cortex. For example, inactivation of the dorsomedial prefrontal cortex (primarily the prelimbic cortex) reduced expression of ABA renewal of extinguished lever pressing for cocaine; inactivation of ventromedial prefrontal cortex (primarily the infralmbic cortex) had no effect (Fuchs et al., 2005). Similarly, inactivation of the dmPFC reduced expression of ABA renewal of extinguished nose-poking for alcoholic beer; inactivation of vmPFC had no effect (Willocks & McNally, 2013). In contrast, inactivation of dmPFC had no effect on the expression of ABA renewal of extinguished lever pressing for heroin, whereas inactivation of the vmPFC reduced it (Bossert et al., 2011). Thus, inactivation of dmPFC (but not vmPFC) attenuated ABA renewal of extinguished instrumental behavior for cocaine or alcohol, while inactivation of vmPFC (but not dmPFC) attenuated ABA renewal of extinguished instrumental behavior for heroin. Further evidence that different drug reinforcers might involve different brain regions is provided by results showing that the renewal context activates vmPFC neurons projecting to the nucleus accumbens shell when heroin has been the reinforcer (Bossert et al., 2012) but not when alcohol has been the reinforcer (Hamlin, Clemens, Choi, & McNally, 2009).
While neither the dmPFC nor vmPFC appears to be important for the learning of instrumental extinction (Mendoza, Sanio, & Chaudhri, 2015; Peters, LaLumiere, & Kalivas, 2008), there is some evidence that vmPFC is important for its consolidation and expression. For example, Peters et al. (2008) gave rats 11 sessions of extinction of lever-pressing for cocaine. Inactivation of vmPFC prior to session 12 of extinction impaired expression of extinguished lever-pressing (i.e., increased responding) (Peters et al., 2008). In contrast, inactivation of dmPFC did not produce the same increase in extinguished responding and inactivation of nucleus accumbens shell increased responding on both an extinguished and an inactive lever. LaLumiere, Niehoff, and Kalivas (2010) performed post-session inactivation of the dmPFC or the vmPFC after each of five short (30 minute) sessions of extinction of lever-pressing for cocaine; vmPFC inactivation impaired expression of extinction during subsequent longer (2 hour), inactivation-free sessions of extinction, suggesting impaired consolidation of extinction learning, while dmPFC inactivation was without effect. Although the results of Peters et al. (2008) and LaLumiere et al. (2010) suggest that the vmPFC may be required for suppression of responding in extinction after cocaine reinforcement, Willcocks and McNally (2013) did not find any effect of vmPFC inactivation on expression of extinguished nose-poking for alcoholic beer, and Bossert et al. (2011) did not find any effect of vmPFC inactivation on expression of extinguished lever-pressing for heroin.
In comparison to renewal of extinguished instrumental responding for drug reinforcers, we are reaching a relatively good understanding of the behavioral mechanisms that underlie renewal of extinguished instrumental responding for a food reinforcer (e.g., Bouton & Todd, 2014). This might make the food reinforcement paradigm more analytically powerful for exploring the contributions of different brain regions. Here we therefore extend the investigation of the role of the dmPFC and the vmPFC in relapse by inactivating each of these regions just prior to test in an appetitive instrumental ABA renewal paradigm, using a sucrose pellet reinforcer. Our studies include several controls (e.g., off-site cannula placements; use of fluorescent muscimol to map infusion spread; angled cannula placement in the vmPFC to avoid dmPFC damage) that improve interpretation and application of our results. It was hypothesized that if the vmPFC and dmPFC are underlying suppression and promotion (respectively) of appetitive instrumental behavior, then inactivation of the vmPFC would attenuate expression of extinction, while dmPFC inactivation would attenuate ABA renewal. Both of these predictions were confirmed in the current experiments using a food reinforcer, which represents the first time that both effects have been observed in a single study. However, given previous results showing that vmPFC is important for ABA renewal of extinguished lever-pressing for heroin (Bossert et al., 2011), we were not surprised to also observe an attenuation of ABA renewal with inactivation of the vmPFC.
Material and Methods
Subjects
A total of 95 adult male Wistar rats (57–61 days old at delivery) obtained from Charles River Canada were used. Of this total, 4 rats were eliminated based on an inability to locate one or both cannulas, leaving a total of 91 rats (25 rats in Experiment 1, 42 rats in Experiment 2, 25 rats as off-site controls). Animals were housed in a temperature and humidity controlled colony room, and kept on a 12/12 hr light/dark schedule. Rats were maintained at approximately 90% of their free-feeding weight throughout the experiment.
Apparatus
Two sets of four operant chambers were used in these experiments, which served as context A and context B (counterbalanced). For a detailed description of the apparatus used, see Todd, Winterbauer, and Bouton (2012). Briefly, the chambers were slightly modified versions of Med Associates (St. Albans, VT) model ENV-008-VP chambers. They measured 30.5 x 24.1 x 21.0 cm (l x w x h) and were individually housed in sound attenuation chambers. Chambers in context A and B differed in tactile (staggered stainless steel grid floor/flat stainless steel grid floor), olfactory (lemon scent/pine scent), and visual cues (black stripes on chamber sides/clear chamber sides). Ventilation fans provided background noise of 65 dB. A recessed food cup was centered in the front wall, with retractable levers on either side of the food cup. The right lever remained retracted throughout the experiment, while the left lever was extended following an initial 2-minute interval in each session. Both sets of boxes delivered 45-mg sucrose pellets (TestDiet, Richmond, IN, USA).
Surgery
Rats were anesthetized with isoflurane and stereotaxic surgery was performed in order to bilaterally implant guide cannulae (26 gauge, Plastics One) in the dmPFC (Experiment 1) or vmPFC (Experiment 2). Coordinates used were +3.0 mm from bregma, ± 0.75 mm from midline, and −3.0 mm ventral from the skull (dmPFC) and +3.0 mm from bregma, ± 2.66 mm from midline, and −4.71 mm ventral at a 24° angle (vmPFC), and +3.0 mm from bregma, ± 2.66 mm from midline, and −6.10 mm ventral at a 24° angle (ventral off-site) (coordinates adapted from Willcocks & McNally, 2013). Following surgery, rats were given 5–6 days of recovery.
Instrumental Conditioning and Extinction
The two experiments investigating dmPFC and vmPFC were run separately (during different calendar months), but used the same behavioral methods. On day 1, magazine training, rats learned to retrieve sucrose pellets from the food cup. Pellets were delivered independently of the rat’s behavior on average every 30 seconds. Rats underwent two separate 30-minute sessions in both contexts A and B, with sessions separated by 30 minutes. There was no lever present. Rats then underwent 6 daily 30-minute sessions of lever training (acquisition) in context A. During these sessions, following a two-minute interval, the left lever was inserted and a random interval (RI)-30 second reinforcement schedule was in effect. Following acquisition, extinction occurred in the opposite context (context B). During extinction, all parameters were identical except that a lever press no longer resulted in delivery of a sucrose pellet. Extinction took place over 4 daily 30-minute sessions. Following each daily session rats were returned to the home cage and fed an amount of food that maintained them at their target body weight.
Baclofen/Muscimol Infusions and Testing
Testing took place the day after the final extinction session. Rats were given an infusion of 0.9% saline vehicle (control) or baclofen/muscimol (B/M) (1.0mM/0.1mM; Sigma Aldrich, St Louis, MO) dissolved in 0.9% saline to temporarily inactivate the targeted region (dmPFC in Experiment 1; vmPFC in Experiment 2). Internal cannulae (33 gauge, Plastics One) were inserted bilaterally into guide cannulae. Internal cannula tips protruded 1 mm below the guide cannula tip. An infusion of 0.5 μL per side was delivered at a rate of 0.25 μL per minute using a microinfusion pump. Following completion of the infusion, the internal cannulae were left in place for 1 minute to allow diffusion of the drug or saline away from the cannula tips. They were then removed, dummy cannulae replaced, and the rat was placed in the transportation container. Time between the end of infusion and the start of testing ranged from approximately 30–45 minutes.
All animals were tested in a within-subject manner (Bouton et al., 2011). Each rat was tested in two separate 10-minute sessions, one in context A and one in context B (test order counterbalanced). As in extinction sessions, lever presses did not result in a sucrose pellet. The two test sessions were separated by approximately 30 minutes.
Infusion Spread Visualization/Histology
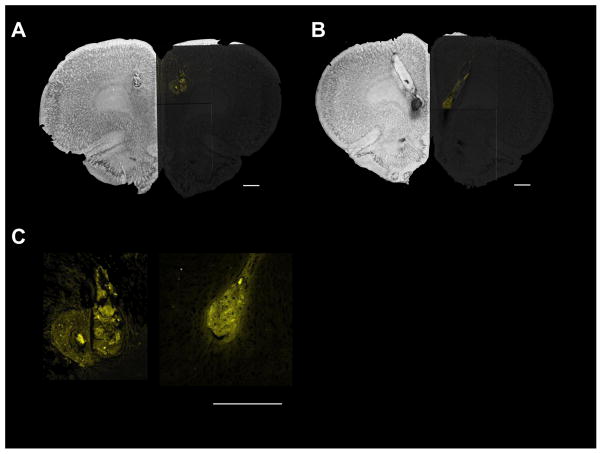
In order to determine the extent of the spread of the B/M infusion, 0.5 μL of 0.5 mg/mL fluorophore-conjugated muscimol (FCM) in 0.9% saline (muscimol, BODIPY®TMR-X conjugate, Molecular Probes) was infused (see Allen, Narayanan, Kholodar-Smith, Zhao, Laubach, & Brown, 2008) in a subset of 4 rats (dmPFC infusion = 2; vmPFC infusion = 2) the day after testing using the same infusion procedure as above. Rats receiving FCM infusions were sacrificed approximately 30 minutes following infusion, to mimic time of testing after drug infusion, and transcardially perfused with 0.9% saline followed by 4% paraformaldehyde. Brains were cryoprotected in 30% sucrose and sectioned at 50μm on a cryostat. FCM was visualized using a Zeiss LSM 7 multiphoton microscope. Images were collected at 10x and a tile scan was used in order to visualize a large field of view (i.e., an entire hemisphere of a coronal section). Fluorescent images were overlaid on brightfield images of cresyl violet stained sections.
All other rats were transcardially perfused with 0.9% saline followed by 10% buffered formalin. An electrolytic lesion was made in order to visualize the cannula tip in rats that did not receive FCM. Brains were cryoprotected in 30% sucrose and sectioned at 50μm on a cryostat. Sections used for cannula verification were stained with cresyl violet. Three independent, blind observers performed cannula verification by marking the location of the electrolytic lesion on corresponding diagrams from a rat brain atlas (Paxinos & Watson, 2007). Data from animals where the location of one or both cannulae tip(s) could not be identified were excluded from analyses. The final number of animals excluded from the study was 4.
Data Analysis
Behavioral data were analyzed with IBM SPSS version 22.0. Analyses were conducted using mixed model (infusion type X test context) or repeated measures (acquisition and extinction data) ANOVAs with an alpha level of 0.05.
Results
Experiment 1: Dorsomedial Prefrontal Cortex
There were no differences between groups in acquisition (vehicle group mean ± SEM = 16.5 ± 2.0 lever presses per minute in the final session; B/M group mean ± SEM = 19.7 ± 1.5 lever presses per minute in the final session) or extinction (vehicle group mean ± SEM = 6.3 ± 0.6 lever presses per minute in first session and 1.2 ± 0.2 lever presses per minute in the final session; B/M group mean ± SEM = 7.1 ± 0.9 lever presses per minute in the first session and 1.0 ± 0.2 lever presses per minute in the final session), as expected, since no infusions were made during these sessions (all p’s > 0.05). During testing, renewal was observed in both groups but dmPFC inactivation reduced responding in context A (renewal context) compared to controls (Figure 1A). A 2 (B/M, vehicle) X 2 (lever presses A, lever presses B) mixed model ANOVA revealed a significant main effect of context (F(1,23) = 231.21, p < 0.001) and a significant context by infusion type interaction (F(1,23) = 12.24, p = 0.002). Follow-up tests of the interaction revealed significantly less responding in the dmPFC inactivation group in context A (renewal), (F(1,23) = 9.25, p = 0.006), but not in context B (extinction context), (F(1,23) = 0.02, p = 0.88), compared to vehicle controls.
Figure 1.
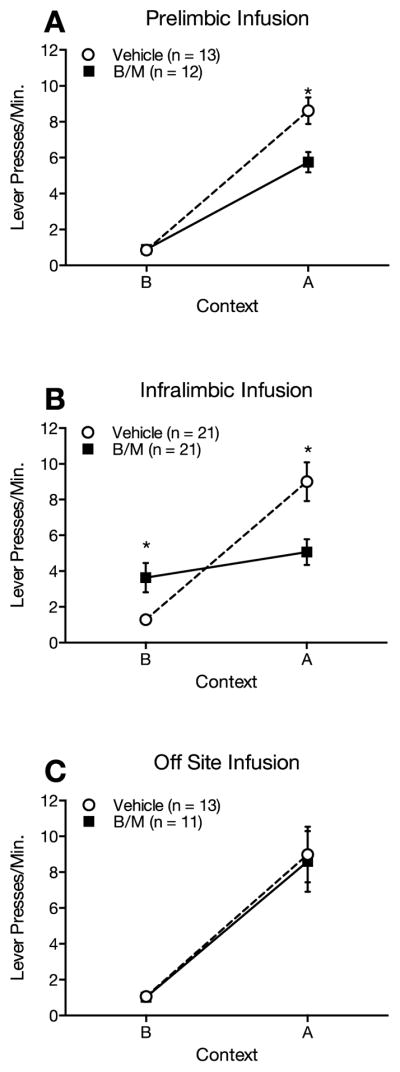
(A) Infusion of baclofen/muscimol (B/M) into dorsomedial prefrontal cortex prior to test attenuates ABA renewal of extinguished lever-pressing for sucrose pellets. (B) Infusion of B/M into ventromedial prefrontal cortex prior to test attenuates ABA renewal of extinguished lever-pressing for sucrose pellets and increases lever-pressing in the extinction context. (C) Infusion of B/M into ventral-most medial prefrontal cortex has no effect on responding in either the extinction or renewal contexts.
Experiment 2: Ventromedial Prefrontal Cortex
Again, as expected, there were no differences between groups in acquisition (vehicle group mean ± SEM = 17.5 ± 3.1 lever presses per minute in the final session; B/M group mean ± SEM = 15.7 ± 1.9 lever presses per minute in the final session) or extinction (vehicle group mean ± SEM = 7.0 ± 0.6 lever presses per minute in the first session and 1.0 ± 0.1 lever presses per minute in the final session; B/M group mean ± SEM = 6.3 ± 0.7 lever presses per minute in the first session and 1.4 ± 0.3 lever presses per minute in the final session) (all p’s > 0.05). During testing, renewal was observed in the vehicle control group but not in the vmPFC inactivation group; vmPFC inactivation reduced responding in context A (renewal context) and increased responding in context B (extinction context) compared to controls (Figure 1B). A 2 (B/M, vehicle) X 2 (lever presses A, lever presses B) mixed model ANOVA revealed a significant main effect of context (F(1,40) = 27.69, p < 0.001) and a significant context by infusion type interaction (F(1,40) = 13.10, p = 0.001). Follow-up tests of the interaction effect revealed significantly less responding in the vmPFC inactivation group in context A (renewal context), (F(1,40) = 9.15, p = 0.008), and significantly more responding in the vmPFC inactivation group in context B (extinction context), (F(1,40) = 7.67, p = 0.004), compared to vehicle controls.
Off-site controls
For rats with cannulae implanted ventral or lateral to the vmPFC target region, there were no differences between groups in acquisition (vehicle group mean ± SEM = 19.1 ± 2.6 lever presses per minute in the final session; B/M group mean ± SEM = 19.7 ± 0.5 lever presses per minute in the final session) or extinction (vehicle group mean ± SEM = 6.5 ± 0.8 lever presses per minute in the first session and 1.5 ± 0.2 lever presses per minute in the final session; B/M group mean ± SEM = 7.0 ± 0.3 lever presses per minute in the first session and 1.3 ± 0.1 lever presses per minute in the final session) (all p’s > 0.05). Both groups showed renewal, but infusion type had no effect on responding (Figure 1C). A 2 (B/M, vehicle) X 2 (lever presses A, lever presses B) mixed model ANOVA revealed a main effect of context (F(1,22) = 52.31, p < 0.001), but no significant interaction of context and infusion type (F(1,22) = 0.03, p = 0.87).
Histology
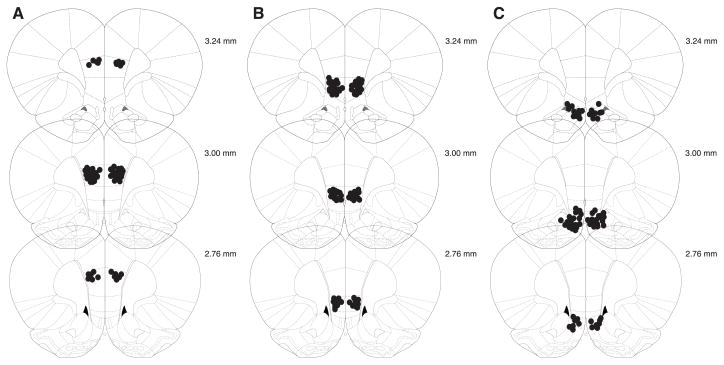
Example images of histological sections taken following test are shown in Figure 2A and 2B for FCM infusions made in dmPFC and vmPFC. FCM images are overlaid on one half of the cresyl violet stained sections. FCM infusion indicated that spread was minimal, and likely did not extend beyond the targeted region. Figure 3 depicts cannula tip placements for all rats used in these experiments.
Figure 2.
Fluorophore-conjugated muscimol (right side of section) overlaid on cresyl violet stained medial prefrontal cortex sections showing (A) dorsomedial prefrontal cortex and (B) ventromedial prefrontal cortex cannula tip location (left side of section; arrow). (C) Infusion spread detail for dorsomedial prefrontal cortex (left) and ventromedial prefrontal cortex (right). Scale bar = 1 mm.
Figure 3.
Cannula tip placements in dorsomedial prefrontal (prelimbic) cortex (A) ventromedial prefrontal (infralimbic) cortex (B) and off-site placements (C) as verified on Nissl-stained sections (modified from Paxinos & Watson, 2007).
Discussion
We examined the role of the dmPFC and the vmPFC in ABA renewal following extinction of instrumental behavior with a sucrose reward. Renewal in context A was reduced by inactivation of the dmPFC (Experiment 1) or vmPFC (Experiment 2). Conversely, although dmPFC inactivation had no impact on responding in context B (extinction context), inactivation of the vmPFC increased responding in this context.
Several controls/techniques were implemented that strengthen the interpretation of our results and contribute to the novelty of this research. First, using FCM, we were able to demonstrate very minimal spread of our experimental drug infusion. It is important to note that the spread of FCM from a 0.5 μL dmPFC infusion in a study by Allen et al. (2008) was 0.5–1.0 mm, which is quite comparable to what we observed here (Figure 3C). In any infusion study, spread of the drug beyond the target region is a concern—particularly when regions of interest are adjacent to one another. However, our FCM infusions suggest minimal spread. One possible criticism is that the FCM infusion underrepresents the actual spread of B/M due to the size of the fluorescent tag (the molecular weight of FCM is 607.46 g/mol; the molecular weight of muscimol hydrobromide is 195.01 g/mol; the molecular weight of baclofen hydrochloride is 250.12 g/mol). While the behavioral effects of FCM versus B/M were not compared in our study, Allen et al. (2008) showed that the behavioral effects of FCM infusion were similar to those of a muscimol (114.10 g/mol) infusion despite their different molecular weights. Second, we included a control group of animals that received B/M infusions in a region ventral to our targeted vmPFC region. This allowed us to demonstrate that our observed results are not due to general effects of inactivation on the mPFC, but are specific to relatively discrete regions of the dmPFC or vmPFC. Finally, small diameter cannulae (26 gauge) aimed at the vmPFC were implanted at a steep angle (24°) in order to minimize incidental damage to the dmPFC.
Previous work has provided us with considerable information about the learning mechanisms underlying instrumental extinction and renewal. Bouton et al. (2011) showed that instrumental renewal can be observed in ABA, AAB, and ABC paradigms. These experiments demonstrated that removal from the extinction context is sufficient for renewal to occur. Renewal also occurs when the associative histories of the extinction and renewal contexts are equated (see Todd, 2013; Todd et al., 2014b). Instead of being dependent upon context-reinforcer associations, Todd (2013; see also Todd et al., 2014b) suggested that renewal is at least partly due to the removal of context-specific response inhibition when the animal is tested outside the extinction context (cf. Rescorla, 1993, 1997). As noted in the Introduction, previous extinction of the same response in the renewal test context reduces renewal (Todd et al., 2014b), whereas previous extinction of a different response does not (Todd, 2013; Todd et al., 2014b). The latter result appears inconsistent with an occasion-setting mechanism, in which the context might signal or set the occasion for a response-no-outcome relationship, because the power of occasion setters is expected to transfer between similarly-trained targets (e.g., Holland, 1992). Extinction may thus instead result from direct inhibition of the specific response.
The present results are the first to indicate roles for the dmPFC and the vmPFC in expression of extinction and ABA renewal of instrumental responding in a method involving food reinforcement. The importance of studying the phenomenon in a food preparation is that the behavioral mechanisms underlying the effects have been intensively studied there. In contrast to our experiments using a food reinforcer, the roles of the dmPFC and the vmPFC in extinction and ABA renewal of drug-seeking may depend upon which drug serves as the reinforcer (Bossert et al., 2013; Peters et al., 2013). For example, as noted in the Introduction, Bossert et al. (2011) found that ABA renewal of lever-pressing for heroin was decreased following inactivation of the vmPFC while inactivation of the dmPFC had no effect. In contrast, Willcocks and McNally (2013), examining nose-poking for alcoholic beer, and Fuchs et al. (2005), examining lever-pressing for cocaine, found no effect of vmPFC inactivation on ABA renewal, instead demonstrating that dmPFC inactivation reduced ABA renewal. None of those studies reported an effect of dmPFC or vmPFC inactivation on expression of extinction, although Peters et al. (2008) found that inactivation of the vmPFC after extended extinction training reduced expression of extinction.
As noted earlier, the behavioral mechanisms of the extinction and renewal of food-reinforced behavior have been studied more intensively (e.g., Bouton & Todd, 2014). One possible role of the dmPFC is in expression of the original learning that occurred during acquisition in context A. Because all rats underwent acquisition and extinction before receiving any infusion, and all animals acquired and extinguished responding to the same level, we can conclude that this relative reduction of responding is not due to a failure to learn or consolidate information about the context, the response, or the reward. Bouton and colleagues have provided evidence that, unlike a context switch after Pavlovian conditioning, which usually does not affect the expression of the conditioned response to a conditioned stimulus, a context switch after instrumental conditioning causes a decrement in the expression of the instrumental response (Bouton et al., 2011; Bouton, Todd, & Leon, 2014; Thrailkill & Bouton, 2015; Todd, 2013; see Bouton & Todd, 2014, for a review). Such a result could occur if the conditioning context had entered into a direct association with the instrumental response (Thrailkill & Bouton, 2015) or if the context had come to signal the relationship between the response and the reinforcing outcome in a more “hierarchical” manner (Trask & Bouton, 2014; see Bouton & Todd, 2014, for more discussion). In principle, either of these processes could have been affected by inactivation of the dmPFC here. It is worth noting that the ability for a context to signal the response-reinforcer relationship (Trask & Bouton, 2014) may depend on complex discrimination training with several response-outcome relations; when animals are given simple acquisition training with a single response-outcome relation in one context, the response decrement that occurs with a context switch appears to result from the loss of the direct context-response association (Thrailkill & Bouton, 2015). One implication might be that dmPFC inactivation could have reduced control by a context-response association in the relatively simple situation studied here. Other evidence suggests that lesioning or inactivation of the dmPFC prior to instrumental conditioning can discourage expression of response-outcome learning (Balleine & Dickinson, 1998; Corbit & Balleine, 2003; Coutureau, Marchand, & Di Scala, 2009; Killcross & Coutureau, 2003; Ostlund & Balleine, 2005; Tran-Tu-Yen, Marchand, Pape, Di Scala, & Coutureau, 2009). Although the present data suggest that dmPFC inactivation reduces excitatory control of the response that occurs when the animal is returned to the conditioning context after extinction, they are consistent with the dmPFC influencing the expression of either an excitatory context-response association or a hierarchical context-(response-outcome) relation.
Inactivation of the vmPFC increased responding in the extinction context and decreased responding in the renewal context relative to control animals. Again, because we know that both the B/M and saline vehicle groups learned equivalently during acquisition and extinction, the increased responding in the extinction context may indicate that without the vmPFC, these animals are impaired at expressing an inhibitory context-response association. If we assume that the extinction context forms an inhibitory association with the response (Todd, 2013; Todd et al., 2014), then inactivation of the vmPFC may impair the expression of this inhibitory context-response association, resulting in more responding in the context in which lever pressing was extinguished. The additional reduction of responding in the acquisition context (i.e., reduction in ABA renewal) with inactivation of the vmPFC may be due to impairment in expression of an excitatory context-response association or a context-(response-outcome) relation, as with the dmPFC. vmPFC lesioning or inactivation before or after instrumental conditioning can discourage expression of stimulus-response learning (Coutureau & Killcross, 2003; Killcross & Coutureau, 2003).
Here we examined basic reinforcement processes using a sucrose pellet reward. There is some suggestion that the processes underlying renewal of instrumental responding are not identical in food-reinforced and drug-reinforced paradigms. ABA, AAB, and ABC renewal have all been convincingly demonstrated using food reinforcers, while only ABA renewal has been shown in drug reinforced paradigms (Todd et al., 2014a). Our data suggest that the dmPFC does indeed play a role in ABA renewal of extinguished lever pressing for sucrose, but is not the sole substrate underlying this behavior. Interestingly, we found that inactivation of the vmPFC affected both extinction and ABA renewal expression. Without the vmPFC, expression of both an excitatory and an inhibitory context-response association may be impaired. Together, these data suggest that a region of mPFC encompassing dmPFC and vmPFC is important in ABA renewal of extinguished instrumental behavior for a food reinforcer.
Highlights.
Dorsomedial and ventromedial prefrontal cortex may be critical in ABA renewal of extinguished instrumental behavior when a food reinforcer is used
Inactivation of dorsomedial prefrontal cortex attenuated expression of ABA renewal but did not affect expression of extinction
Inactivation of ventromedial prefrontal cortex attenuated expression of ABA renewal and expression of extinction
There were no effects of inactivation of more ventral medial prefrontal cortex
Infusion spread was verified using fluorophore-conjugated muscimol
Acknowledgments
Supported by NIH R01 MH082893 to JTG and NIH 5 P30 RR032135 from the COBRE Program of the National Center for Research Resources and 8 P30 GM 103498 from the National Institute of General Medical Sciences.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen TA, Narayanan NS, Kholodar-Smith DB, Zhao Y, Laubach M, Brown TH. Imaging the spread of reversible brain inactivations using fluorescent muscimol. Journal of Neuroscience Methods. 2008;171(1):30–38. doi: 10.1016/j.jneumeth.2008.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine BW, Dickinson A. Goal-directed instrumental action: Contingency and incentive learning and their cortical substrates. Neuropharmacology. 1998;37:407–419. doi: 10.1016/s0028-3908(98)00033-1. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Liu SY, Lu L, Shaham Y. A role of ventral tegmental area glutamate in contextual cue-induced relapse to heroin seeking. Journal of Neuroscience. 2004;24(47):10726–10730. doi: 10.1523/JNEUROSCI.3207-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Marchant NJ, Calu DJ, Shaham Y. The reinstatement model of drug relapse: Recent neurobiological findings, emerging research topics, and translational research. Psychopharmacology. 2013;229:453–476. doi: 10.1007/s00213-013-3120-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Poles GC, Wihbey KA, Koya E, Shaham Y. Differential effects of blockade of dopamine D1-family receptors on nucleus accumbens core or shell on reinstatement of heroin seeking induced by contextual and discrete cues. Journal of Neuroscience. 2007;27(46):12655–12663. doi: 10.1523/JNEUROSCI.3926-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Stern AL, Theberge FR, Cifani C, Koya E, Hope BT, Shaham Y. Ventral medial prefrontal cortex neuronal ensembles mediate context-induced relapse to heroin. Nature Neuroscience. 2011;14(4):420–422. doi: 10.1038/nn.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Stern AL, Theberge FR, Marchant NJ, Wang HL, Morales M, Shaham Y. Role of projections from ventral medial prefrontal cortex to nucleus accumbens shell in context-induced reinstatement of heroin seeking. Journal of Neuroscience. 2012;32(14):4982–4991. doi: 10.1523/JNEUROSCI.0005-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME. Context, time, and memory retrieval in the interference paradigms of Pavlovian learning. Psychological Bulletin. 1993;114(1):80–99. doi: 10.1037/0033-2909.114.1.80. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Why behavior change is difficult to sustain. Preventative Medicine. 2014;68:29–36. doi: 10.1016/j.ypmed.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME, Bolles RC. Role of conditioned contextual stimuli in reinstatement of extinguished fear. Journal of Experimental Psycholology: Animal Behavior Processes. 1979;5(4):368–378. doi: 10.1037//0097-7403.5.4.368. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Todd TP. A fundamental role for context in instrumental learning and extinction. Behavioural Processes. 2014;104:13–19. doi: 10.1016/j.beproc.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME, Todd TP, Leon SP. Contextual control of discriminated operant behavior. Journal of Experimental Psycholology: Animal Behavior Processes. 2014;40(1):92–105. doi: 10.1037/xan0000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME, Todd TP, Vurbic D, Winterbauer NE. Renewal after the extinction of free operant behavior. Learning and Behavior. 2011;39(1):57–67. doi: 10.3758/s13420-011-0018-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Sahuque LL, Janak PH. Ethanol seeking triggered by environmental context is attenuated by blocking dopamine D1 receptors in the nucleus accumbens core and shell in rats. Psychopharmacology. 2009;207(2):303–314. doi: 10.1007/s00213-009-1657-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Balleine BW. The role of prelimbic cortex in instrumental conditioning. Behavioural Brain Research. 2003;146:145–157. doi: 10.1016/j.bbr.2003.09.023. [DOI] [PubMed] [Google Scholar]
- Coutureau E, Killcross S. Inactivation of the infralimbic prefrontal cortex reinstates goal-directed responding in overtrained rats. Behavioural Brain Research. 2003;146:167–174. doi: 10.1016/j.bbr.2003.09.025. [DOI] [PubMed] [Google Scholar]
- Coutureau E, Marchand AR, Di Scala G. Goal-directed responding is sensitive to lesions to the prelimbic cortex or basolateral nucleus of the amygdala but not to their disconnection. Behavioral Neuroscience. 2009;123(2):443–448. doi: 10.1037/a0014818. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Eaddy JL, Su ZI, Bell GH. Interactions of the basolateral amygdala with the dorsal hippocampus and dorsomedial prefrontal cortex regulate drug context-induced reinstatement of cocaine-seeking in rats. European Journal of Neuroscience. 2007;26(2):487–498. doi: 10.1111/j.1460-9568.2007.05674.x. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Ledford CC, Parker MP, Case JM, Mehta RH, See RE. The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology. 2005;30(2):296–309. doi: 10.1038/sj.npp.1300579. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Ramirez DR, Bell GH. Nucleus accumbens shell and core involvement in drug context-induced reinstatement of cocaine seeking in rats. Psychopharmacology. 2008;200:545–556. doi: 10.1007/s00213-008-1234-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlin AS, Clemens KJ, McNally GP. Renewal of extinguished cocaine-seeking. Neuroscience. 2008;151(3):659–670. doi: 10.1016/j.neuroscience.2007.11.018. [DOI] [PubMed] [Google Scholar]
- Hamlin AS, Clemens KJ, Choi EA, McNally GP. Paraventricular thalamus mediates context-induced reinstatement (renewal) of extinguished reward seeking. European Journal of Neuroscience. 2009;29:802–812. doi: 10.1111/j.1460-9568.2009.06623.x. [DOI] [PubMed] [Google Scholar]
- Holland PC. Occasion setting in Pavlovian conditioning. In: Medin D, editor. The psychology of learning and motivation. Vol. 28. Orlando, FL: Academic Press; 1992. pp. 69–125. [Google Scholar]
- Killcross S, Coutureau E. Coordination of actions and habits in the medial prefrontal cortex of rats. Cerebral Cortex. 2003;13:400–408. doi: 10.1093/cercor/13.4.400. [DOI] [PubMed] [Google Scholar]
- LaLumiere RT, Niehoff KE, Kalivas PW. The infralimbic cortex regulates the consolidation of extinction after cocaine administration. Learning & Memory. 2010;17:168–175. doi: 10.1101/lm.1576810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza J, Sanio C, Chaudhri N. Inactivating the infralimbic but not prelimbic medial prefrontal cortex facilitates the extinction of appetitive Pavlovian conditioning in Long-Evans rats. Neurobiology of Learning and Memory. 2015;118:198–208. doi: 10.1016/j.nlm.2014.12.006. [DOI] [PubMed] [Google Scholar]
- Nakajima S, Tanaka S, Urushhihara K, Imada H. Renewal of extinguished lever-press responses upon return to the training context. Learning and Motivation. 2000;31:416–431. doi: 10.1006/lmot.2000.1064. [DOI] [Google Scholar]
- Ostlund SB, Balleine BW. Lesions of medial prefrontal cortex disrupt the acquisition but not the expression of goal-directed learning. Journal of Neuroscience. 2005;25(34):7763–7770. doi: 10.1523/JNEUROSCI.1921-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6. Boston: Academic Press/Elsevier; 2007. [Google Scholar]
- Peters J, LaLumiere RT, Kalivas PW. Infralimbic prefrontal cortex is responsible for inhibiting cocaine seeking in extinguished rats. Journal of Neuroscience. 2008;28(23):6046–6053. doi: 10.1523/JNEUROSCI.1045-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Pattij T, De Vries TJ. Targeting cocaine versus heroin memories: Divergent roles within ventromedial prefrontal cortex. Trends in Pharmacological Sciences. 2013;34:689–695. doi: 10.1016/j.tips.2013.10.004. [DOI] [PubMed] [Google Scholar]
- Rescorla. Inhibitory associations between S and R in extinction. Animal Learning & Behavior. 1993;21(4):327–336. [Google Scholar]
- Rescorla. Response inhibition in extinction. Quarterly Journal of Experimental Psychology. 1997;50B:238–252. [Google Scholar]
- Thrailkill EA, Bouton ME. Contextual control of instrumental actions and habits. Journal of Experimental Psychology: Animal Learning and Cognition. 2015;41(1):69–80. doi: 10.1037/xan0000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd TP. Mechanisms of renewal after the extinction of instrumental behavior. Journal of Experimental Psychology: Animal Behavior Processes. 2013;39(3):193–207. doi: 10.1037/a0032236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd TP, Vurbic D, Bouton ME. Behavioral and neurobiological mechanisms of extinction in Pavlovian and instrumental learning. Neurobiology of Learning and Memory. 2014a;108:52–64. doi: 10.1016/j.nlm.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd TP, Vurbic D, Bouton ME. Mechanisms of renewal after the extinction of discriminated operant behavior. Journal of Experimental Psycholology: Animal Learning and Cognition. 2014b;40(3):355–368. doi: 10.1037/xan0000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd TP, Winterbauer NE, Bouton ME. Contextual control of appetite. Renewal of inhibited food-seeking behavior in sated rats after extinction. Appetite. 2012;58(2):484–489. doi: 10.1016/j.appet.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran-Tu-Yen DAS, Marchand AR, Pape JR, Di Scala G, Coutureau E. Transient role of the rat prelimbic cortex in goal-directed behaviour. European Journal of Neuroscience. 2009;30:464–471. doi: 10.1111/j.1460-9568.2009.06834.x. [DOI] [PubMed] [Google Scholar]
- Trask S, Bouton ME. Contextual control of operant behavior: Evidence for hierarchical associations in instrumental learning. Learning & Behavior. 2014;42:281–288. doi: 10.3758/s13420-014-0145-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcocks AL, McNally GP. The role of medial prefrontal cortex in extinction and reinstatement of alcohol-seeking in rats. European Journal of Neuroscience. 2013;37(2):259–268. doi: 10.1111/ejn.12031. [DOI] [PubMed] [Google Scholar]