Abstract
Type I interferons (IFN-I) are key innate mediators that create a profound antiviral state and orchestrate the activation of almost all immune cells. Plasmacytoid dendritic cells (pDCs) are the most powerful IFN-I producing cells and play important roles during viral infections, cancer, and autoimmune diseases. By comparing gene expression profiles of murine pDCs and conventional (c) DCs we found that CD28, a prototypic T cell stimulatory receptor, was highly expressed in pDCs. Strikingly, CD28 acted as a negative regulator of pDC IFN-I production upon toll-like receptor stimulation but did not affect pDC survival or maturation. Importantly, cell-intrinsic CD28 expression restrained pDC (and systemic) IFN-I production during in vivo RNA and DNA viral infections, limiting antiviral responses and enhancing viral growth early after exposure. Finally, CD28 also down-regulated IFN-I response upon skin injury. Our study identified a new pDC regulatory mechanism by which the same CD28 molecule that promotes stimulation in most cells that express it is co-opted to negatively regulate pDC IFN-I production and limit innate responses.
Introduction
Type I interferons (IFN-I) play a crucial role in orchestrating the immune response to multiple disease settings, including viral infections, cancers, tissue injury, and autoimmune disease (1). IFN-I are a pleiotropic cytokine family found among mammalian species that includes several IFNα and one IFNβ isoforms that signal through a common ubiquitously expressed receptor (IFNαβ-R), promoting both autocrine and paracrine activation and leading to phosphorylation of STAT 1 and 2. The result of these interactions is a positive feedback loop that drives further IFN-I production as well as the induction of hundreds of IFN-I stimulated genes (ISGs) (2). These ISGs act in concert to create a potent antiviral state and orchestrate the activation of almost all innate and adaptive immune cells. While almost all cell types can produce IFN-I, plasmacytoid dendritic cells (pDCs) are highly specialized to rapidly secrete copious amounts of these cytokines. Not only do pDCs produce up to 1000 times more IFN-I than other cell types, but they also synthesize a broader range of IFN-I isoforms (3). pDCs express endosomal TLR7 and TLR9 which recognize ssRNA and unmethylated CpG-containing motifs (from microbial or self origin), respectively (4). Engagement of TLR7 or TLR9 in pDCs leads to production of IFN-I (both IFNα and IFNβ isoforms) as well as pro-inflammatory cytokines and up-regulation of co-stimulatory molecules such as CD80, CD86 and MHC-II (5–10).
As such, pDCs play an important role during several in vivo viral infections such as those caused by murine cytomegalovirus (MCMV) (11, 12), respiratory syncytial virus (13, 14), and mouse hepatitis virus (15), among others (15–17). Furthermore, persistent viruses such as HIV and hepatitis C virus (HCV) induce substantial IFN-I production upon incubation with pDCs (17, 18) and similar effects are observed early after in vivo infection with persistent strains of lymphocytic choriomeningitis virus (LCMV WE or clone 13; Cl13) (19, 20). However, pDC IFN-I production becomes exhausted during later stages of chronic viral infection, an event accompanied by enhanced susceptibility to opportunistic pathogens (18, 21–23). Similarly, pDC IFN-I production is also attenuated in tumor microenvironments, correlating with cancer progression (24). In contrast, uncontrolled IFN-I production by pDCs is associated with autoimmune diseases such as psoriasis (25), type I diabetes (26) and experimental autoimmune encephalomyelitis (27). In particular, in Systemic Lupus Erythematosus (SLE) patients, pDCs accumulate in target tissues and exhibit sustained IFN-I production, and pDCs were shown to be critical for promoting SLE pathogenesis (28–30). Finally, pDC IFN-I production also promotes innate defenses following tissue injury, playing a critical role in regulating cutaneous wound healing (31). Together, these studies demonstrate the importance of fine-tuning the magnitude of pDC IFN-I response and highlight the significant implications of pDC IFN-I regulation for numerous human illnesses.
In the present study, we compared the gene expression profiles of pDCs and conventional (c) DCs to gain insight on putative pDC IFN-I regulators. Unexpectedly, we found that CD28, a cell surface stimulatory receptor constitutively expressed in T cells (32), was highly and selectively expressed in pDCs but not cDCs. Remarkably, CD28 expression negatively regulated pDC IFN-I production in response to in vitro TLR stimulation and in vivo viral infections or tissue injury. Moreover, bone marrow chimeras revealed a cell-intrinsic effect of CD28 expression in suppressing pDC functions. Thus, our study identified a novel role for the prototypic T cell stimulatory molecule CD28 as a negative regulator of pDC function both in vitro and in vivo. Considering that CD28 is fundamental for T cell priming (32), our work raises the possibility that CD28 may be part of a previously unrecognized molecular pathway that inhibits innate responses while promoting adaptive immunity.
Materials & Methods
Mice and viruses
C57BL/6 (WT), C57BL/6 CD45.1+, CD80/86 double ko (dko), and CD28ko mice were purchased from The Jackson laboratory (Bar Harbor, ME). Mice (6–12 weeks old) were infected intraperitoneally with 1 × 104 PFU MCMV Smith or 2 × 106 LCMV clone 13 (Cl13) intravenously (i.v.). Viruses were propagated and quantified as described before (33, 34).
Mechanical injury of mouse skin and analysis of dermal cells
Mice backs were shaved and depilated (Veet; Reckitt Benckiser, Slough, England) immediately before injury. Mechanical injury was then applied by tape stripping, using 20 strokes of transparent tape (3M; Scotch) across the back. Injured skin was excised, digested with 1 mg/mL Dispase (Sigma-Aldrich, St. Louis, MO), 200 U/mL collagenase type I (Invitrogen, Carlsbad, CA) and 200 U/mL hyaluronidase (SERVA Electrophoresis, Heidelberg, Germany) for 30 min at 37°C and then mechanically dissected to generate a single cell suspension. Cells were stained with 10 μg/mL anti–PDCA-1 APC (JF05-1C2.4.1; Miltenyi, Auburn, CA), anti–CD11c-PE (HL3), anti–B220-FITC (RA3-6B2; BD Biosciences, San Diego, CA), and anti-CD45-PerCP (30-F11; eBiosciences, San Diego, CA). Cells were acquired on a FACS Calibur (BD, San Jose, CA) and analyzed using FlowJo software (Tree Star, Inc., Ashland, OR).
Human Samples and Cell Lines
Residual axillary lymph node samples (n=5), following diagnostic studies, were used for these studies where samples were incubated with RPMI + Collagenase (1mg/mL, Roche, Indianapolis, IN) for 20 min at 37°C and passed through a 100μm strainer to achieve a single cell suspension. Cal-1 cells were kindly provided by Dr. Shimeru Kamihira (Nagasaki University Graduate School of Biomedical Sciences, Nagasaki, Japan). Peripheral blood lymphocytes (n=7 donors) were isolated using standard Ficoll gradient separation as previously described (35). Cells were stained with CD16 (3G8), CD56 (MEM-188), CD14 (HCD14), and CD19 (HIB19)-Pacific Blue, CD3 APC Cy7 (UCHT1), B220 Percp Cy5.5 (RA3-6B2), HLADR PE Cy7 (L243), CD11c Alexa 488 (3.9) and CD123 APC (6H6) (Biolegend, San Diego, CA). pDCs were identified as Lineage (CD16, CD56, CD14, CD19, B220, CD3)−HLADR+CD11c−CD123+. T cells were identified as CD3+. T cells and pDCs (lymph node biopsies) or Cal-1 pDCs were stained for CD28 expression with CD28 PE (CD28.2) or isotype control (mouse IgG1) (Biolegend) and acquired on a BD LSRII and analyzed using FlowJo software.
Purification of Bone Marrow and Spleen DCs
Bone marrow (BM) cells were isolated from femurs and tibias and a single cell suspension was prepared and cultured 7–8 days in the presence of 100ng/ml Flt3L (Amgen, Thousand Oaks, CA & Cell Dex Therapeutics, Needham, MA) as previously described (36). Spleens were incubated with 1mg/mL collagenase D for 20 min at 37°C and passed through a 100μm strainer to achieve a single cell suspension. Splenocytes were enriched with PanDC microbeads using an Automacs system (Miltenyi). PanDC+ fractions were stained with propidium iodide (PI) and FACS-purified using a BD ARIA II (BD) for pDCs (PI−CD11cintermediate/dimCD11b−B220+PDCA+), and CD11b+ cDCs (PI−CD11c+B220−CD11b+) after B (CD19), T (Thy1.2) and NK (Nk1.1) cell exclusion. BM-pDCs and BM-cDCs were stained and sorted as PI−CD11c+CD11b−B220+PDCA+ and PI−CD11c+B220−CD11b+, respectively. Purity of the cells was > 92%. Cells were stimulated with CpG B 1668 (Integrated DNA Technologies, San Diego, CA) at 0.1μM (BM-derived DC) or 1μM (splenic DC), 10μM CpG A 2336 (Invivogen, San Diego, CA), and 100μM Loxoribine (Invivogen).
Cytokine detection
Total IFN-I bioactivity was measured by luciferase bioassay with reference to a recombinant mouse IFNβ standard (Research Diagnostics, Concord, MA) using a L-929 cell line transfected with an interferon-sensitive luciferase as previously described (37). TNFα and IL-6 were measured by ELISA (e-Bioscience). Supernatant cytokine levels were measured 15hr post-stimulation.
Flow Cytometry
The following antibodies were used to stain murine BM or spleen cells: anti-CD3-PerCP-Cy5.5 (145-2C11; BD Biosciences), Thy 1.2-PE (53-2.1), CD19-PE (eBio1D3), NK 1.1-PE (PK136), CD11c-PE or APC (N418), CD11b-PE-Cy7 (M1/70), B220-APC eFluor 780 or efluor 450 (RA3-6B2), PDCA-1-FITC (eBio927), CD45.2-APC-Cy7 (104), CD45.1-PerCy5.5 (A20), MHC class II (I-A/I-E) efluor 450 (M5/114.15.2), CD28-APC (E18) and mouse IgG2b-APC isotype control for anti-CD28-APC (E-bioscience, San Diego, CA), and CD86 PE Cy7 or PE (IT2.2; Biolegend). PI or Ghost dye (Tonbo Biosciences, San Diego, CA) was used to exclude dead cells and to measure cell viability where indicated. Cells were acquired with an LSRII flow cytometer (BD Bioscience). Data were analyzed with FlowJo software.
Generation of mixed WT:CD28ko BM chimeras
WT CD45.1+ C57BL/6 recipient mice were sublethally irradiated with 1000 rads and reconstituted with a 50:50 mix of BM cells from CD45.1+ WT mice and CD45.2+ CD28ko mice. 10×106 BM cells were transferred i.v. into the irradiated recipient mice, which were treated with antibiotics (Trimethoprim 8 mg/ml and Sulfamethoxazole 40 mg/ml supplied in drinking water) for 3 weeks to prevent infection and allow immune reconstitution. Reconstitution was analyzed 8 weeks after BM transfer and the ratio of WT:CD28ko cells was determined by flow cytometry. Mice were then infected with MCMV or LCMV Cl13 as indicated.
Real-time RT-PCR
Total RNA was extracted using RNeasy kits (Qiagen, Redwood City, CA), digested with DNase I (RNase-free DNase set; Qiagen) and reverse transcribed into cDNA using Superscript III RT (Invitrogen). cDNA quantification was performed using SYBR Green PCR kits and a Real-Time PCR Detection System (Applied Biosystems, Carlsbad, CA). The relative transcript levels were normalized against Gapdh as described previously (22). The following primers were used: Ifnα primers recognizing Ifnα 4 and 6: F 5′-TATGTCCTCACAGCCAGCAG-3′ R 5′-TTCTGCAATGACCTCCATCA-3′; Ifnβ1: F 5′-CTGGCTTCCATCATGAACAA-3′ R 5′-GAGGGCTGTGGTGGAGAA-3; Cd28: F 5′-ACAGTTGGGCCACTTGTTGTCCTTT-3′ R 5′-GCTCCCAATGGTGCCTTCTGGA-3′; Mx1: F 5′-CAACTGGAATCCTCCTGGAA-3′ R 5′-GGCTCTCCTCAGAGGTATCA-3′; Rig-I recognizing Ddx58: F 5′-CGGGACCCACTGCCTCAGGT-3′ R 5′-GCATCCAGGGCGGCACAGAG-3′; Tnfα F 5′- CCCTCACACTCAGATCATCTTCT-3′ R 5′-GCTACGACGTGGGCTACAG-3′; MCMV eI: F 5′-GAGTCTGGAACCGAAACCGT-3′ R 5′-GTCGCTGTTATCATTCCCCAC-3′. Transcript levels of Il6 were determined relative to Gapdh using primer and probe sets from the Universal Probe Library (Roche). Cytokine transcript levels were measured 6hr post-stimulation.
Microarray
RNA extracted from FACS-purified splenic pDCs, CD8+DCs and CD11b+ DCs from uninfected WT mice were utilized for DNA microarray using mouse Affymetrix 1.0 ST array genechips (Affymetrix, Santa Clara, CA). Differential gene expression was determined as fold of change (FC) of indicated genes over background intensity. Microarray data has been deposited in the NCBI’s Gene Expression Omnibus (GEO) (38) under the accession number GSE75834 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE75834).
Generation of CD28 retrovirus construct (RVGFP-CD28)
CD28 cDNA clone (MGC premier cDNA; TransOMIC Technologies, Huntsville, AL) was amplified by PCR, where XhoI and HpaI restriction sites were incorporated using primers: F 5′-GACTCGAGGCCGCCACCATGACACTCAGGCTGCTGTTCTTGG-3′ R 5′-TCGTTAACTCAGGGGCGGTACGCTGCAAAGT-3′. The amplicon was gel-purified and digested with XhoI and HpaI restriction enzymes per manufacturer instructions (New England Biolabs, Ipswich, MA) and ligated to previously digested pMIGR (39) (GFP-labeled retroviral vector; RVGFP) (Addgene, Cambridge, MA). 293T cells were transfected with RVGFP-CD28 or empty vector for control, LT1 transfection reagent (Mirus Bio, Madison, WI) and the pcl-Eco packaging plasmid required to produce viral vectors. 72hrs later, supernatants were harvested and stored at −80°C until transfection of pDCs.
CD28 overexpression by retrovirus
At day 3 post-Flt3L culture, CD28ko BM cells were transduced with RVGFP-CD28 or empty vector control (RVGFP) and polybrene reagent (Fisher Scientific), and spin-infected room temperature at 1000g 90min. Cells were incubated 37°C overnight. On day 4 post-Flt3L culture, cells were again transduced with RVGFP-CD28 or RVGFP and incubated 3hr at 37°C. Cells were then washed in PBS and placed in fresh DC media+ Flt3L. On day 8 post-culture, cells were harvested and FACS-purified for GFP+ pDC fractions. Purified cells were stimulated with 0.1μM CpG B 1668 6hr and harvested for mRNA detection of IFN-I and pro-inflammatory cytokine transcripts relative to Gapdh.
Statistics
Unpaired student’s t-tests or ANOVA tests were performed using GraphPad Prism software (GraphPad, La Jolla, CA). Error bars represent mean ± SEM. P<0.05 was considered statistically significant.
Study Approval
Human axillary lymph node biopsies sent to the UC San Diego Clinical Flow Cytometry Laboratory were de-identified and used for research according to UC San Diego institutional review board (IRB) approved protocol #130973X. Peripheral blood samples were collected from healthy volunteers (written informed consent was obtained prior to study inclusion) at the UC San Diego CFAR Clinical Investigation Core Antiviral Research Center according to UC San Diego IRB approved protocol #110522. Mice were bred and maintained in a closed breeding facility and mouse handling conformed to the requirements of the National Institute of Health and the Institutional Animal Care and Use Guidelines of UC San Diego according to approved protocol S07315.
Results
CD28 is constitutively expressed in pDCs
Previous analyses have demonstrated that, although pDCs and cDC subsets (including CD8+ and CD11b+ cDCs) are derived from a distinct branch of the leukocyte family tree and exhibit functional differences, they maintain an evolutionarily conserved transcriptional signature (40, 41). Therefore, we surmised that a comparison between pDCs and cDCs may highlight regulatory molecules that selectively modulate pDC function. We noted that the expression of the gene encoding the prototypic T cell co-stimulatory molecule CD28 was 59 and 57 times higher in pDCs compared with CD11b+ and CD8+ cDCs, respectively (Fig. 1A). Other CD28 family receptors (42) were either undetectable in pDCs (i.e. Ctla4, Pdcd1 and Icos) or equally expressed in all DC subsets (i.e. Btla). Notably, DC subset or T cell specific gene transcripts were selectively expressed or absent, respectively. To confirm CD28 expression in pDCs we first determined Cd28 transcript levels by quantitative PCR (qPCR) in both murine bone marrow (BM)-derived DCs 7 days post-culture with Flt3L and splenic DC subsets. We observed that Cd28 transcripts were undetectable in cDCs but were significantly expressed in both BM-derived and splenic pDCs, albeit to a lesser extent than in splenic T cells (Fig. 1B and Supplemental Fig. 1A–B). Surface expression of CD28 protein was also present in pDCs freshly obtained from spleen, BM, blood (Fig. 1C) and BM-Flt3L cultures (Fig. 1D), with levels lower than in T cells but contrasting the background expression in cDCs. Of note, basal surface and intracellular CD28 expression were similar, and CD28 expression did not increase in response to TLR stimulation (data not shown). Finally, we examined CD28 protein expression in human pDCs. For this, we first analyzed the Cal-1 human pDC cell line (43) and compared it with non-pDC human cell lines A549 (lung epithelial cells) (44) and Thp-1 (monocytic cells) (45). Similar to murine pDCs, CD28 expression was observed in Cal-1 but not A549 or Thp-1 cells (Fig. 1E). Furthermore, analysis of primary human pDCs revealed that, while CD28 was undetectable in blood pDCs (data not shown), it was consistently expressed above isotype levels in pDCs obtained from lymph node biopsies (Fig. 1F & Supplemental Fig. 1C). Altogether, these data indicated that the prototypic T cell co-stimulatory molecule CD28 is constitutively expressed in pDCs.
Figure 1. pDCs constitutively express CD28.
(a) Splenic pDCs, CD11b+cDCs, and CD8α+cDCs were FACS-purified from wild type (WT) mice and processed for DNA microarray analysis. Heat map depicts fold of change (FC) of indicated genes over background. (b) Cd28 expression relative to Gapdh was determined in pDCs and cDCs FACS-purified from BM cultured for 8 days in the presence of Flt3L and from spleens. CD4+T cells (Thy1.2+CD4+) and CD8+T cells (Thy1.2+CD8+) purified from spleens were also evaluated. Statistical analysis performed between pDCs and other cell types. (c & d) Surface expression of CD28 was measured by flow cytometry in WT murine T cells, pDCs, and cDCs from spleen, BM, and blood ex vivo (c) and BM 8 days post-Flt3L cultures (d). (e & f) CD28 expression was evaluated in human cell lines A549 (lung epithelial cells), Cal-1 (pDCs), and Thp-1 (monocytes) (e) and T cells (CD3+) and pDCs (Lineage−HLADR+CD11c−CD123+) from human axillary lymph nodes (f). Representative histograms of CD28 expression (black open) overlayed with isotype (gray filled) are shown. Bar graphs depict mean values ± SEM of mean fluorescence intensity (MFI) for CD28. Data are derived from one experiment with cells pooled from 5–10 mice (a), or are representative of 4–6 independent experiments with n=3–5 mice per group (b–d), or from n=3 independent experiments of cells lines (e) and n=5 human lymph node samples (f). *p<0.05, **p<0.01, ***p<0.001.
CD28 down-regulates pDC cytokine production upon TLR stimulation
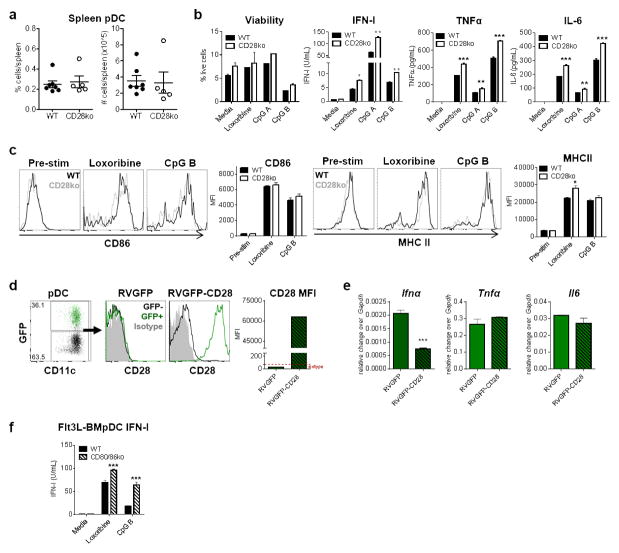
To investigate a putative role for CD28 in pDC differentiation, survival and/or function, we first examined pDCs obtained from wild type (WT) versus CD28ko BM-Flt3L cultures. Similar percentages and numbers of live pDCs were obtained at day 7–8 post-culture, indicating normal pDC and cDC development from BM progenitors (Supplemental Fig. 2A–B). In contrast, significantly higher levels of IFN-I were detected upon stimulation of FACS-purified CD28ko BM-derived pDCs with the TLR7 agonist Loxoribine or TLR9 agonist CpG (Supplemental Fig. 2C). We next sought to extend these findings to pDCs freshly isolated from spleens of WT and CD28ko mice. Consistent with normal development of DCs in the absence of CD28, we observed comparable percentages and numbers of pDCs and cDCs in WT and CD28ko spleens (Fig. 2A & data not shown, respectively). Importantly, as described for BM-Flt3L cultures, significantly increased levels of IFN-I were detected when FACS-purified splenic pDCs from CD28ko mice were stimulated with loxoribine, CpG A or CpG B. Moreover, quantification of TNF-α and IL-6 in these same culture conditions revealed a similarly increased production of these pro-inflammatory cytokines by CD28ko compared to WT pDCs, whereas viability of both WT and CD28ko pDCs was similar following agonist stimulation (Fig. 2B). Despite changes in cytokine response, CD86 (a hallmark of DC maturation (46)) and MHC-II were equally expressed in WT versus CD28ko pDCs before and after TLR stimulation, although a minor increase in MHC-II was observed in loxoribine-treated CD28ko pDCs (Fig. 2C). Furthermore, although incubation of murine WT pDCs and human Cal-1 pDCs with agonistic anti-CD28 antibody (Ab) or recombinant CTLA4-Ig (47, 48) showed no effect on cytokine production (data not shown), we did observe reduced Ifna in CD28ko pDCs upon reconstitution of CD28 levels (Fig. 2D), ruling out any off-target effects in IFN-I production by CD28ko pDC. The levels of Tnfa and Il6 transcripts were, however, unchanged in CD28ko pDCs with restored CD28 expression, raising the possibility that the effect of CD28 may be more profound and/or selective for IFN-I than for pro-inflammatory cytokines.
Figure 2. CD28 down-regulates pDC IFN-I production upon TLR stimulation.
(a) Graphs depict the proportion and number of splenic pDCs from WT and CD28ko mice where mice are plotted individually and mean ± SEM are shown. (b & c) Splenic pDCs were FACS-purified and stimulated with Loxoribine, CpG A, CpG B, or media alone for 15hrs. Cells were analyzed for viability post-stimulation and supernatants were analyzed for IFN-I bioactivity by bioassay and TNFα and IL-6 levels by ELISA (b). Cells were analyzed for CD86 and MHC II expression by flow cytometry. Representative histograms are shown where WT (black open) and CD28ko (gray open) pDCs are overlayed. Bar graphs depict MFI for CD86 and MHCII (c). (d & e) Total BM cells from CD28ko mice were transduced with retroviral constructs containing CD28 overexpression plasmid (RVGFP-CD28) or empty vector control (RVGFP). Retroviral-transduced (GFP+) or un-transduced (GFP−) pDCs were analyzed at day 8 post-Flt3L culture and analyzed for CD28 protein expression by flow cytometry. FACS plot depicts proportion of pDCs transduced by RVGFP-CD28, indicated by GFP+ cells. Representative histograms are shown where GFP+ pDCs (retrovirus incorporated; green open) are overlayed with GFP− pDCs (retrovirus not incorporated; black open) and shown with respect to CD28 isotype control (gray filled) for RVGFP and RVGFP-CD28. Bar graph depicts CD28 MFI (d). FACS-purified GFP+ pDCs were stimulated with CpG B for 6hrs and evaluated for Ifnα, Tnfα, and Il6 relative to Gapdh by qPCR (e). (f) BM from WT and CD80/86 dko mice was cultured in Flt3L for 7–8 days and pDCs were FACS-purified and stimulated with Loxoribine, CpG B, or media alone 15hrs. Supernatants were analyzed for IFN-I bioactivity by bioassay. Data are representative of 2–4 independent experiments with 3–6 mice/group. Bar graphs depict mean ± SEM. *p<0.05, **p<0.01, ***p<0.001.
Given that CD80 and CD86 engage CD28 to promote T cell activation and are up-regulated in pDCs upon TLR stimulation (Fig. 2C and (8–10)), we next evaluated their putative role in pDC IFN-I production. Interestingly, FACS-purified pDCs from CD80/86 double knockout (dko) BM-Flt3L cultures exhibited enhanced IFN-I production in response to loxoribine and CpG stimulation when compared with WT BMpDCs (Fig. 2F), although this effect was not observed with FACS-purified splenic pDCs from CD80/86 dko mice (data not shown), possibly due to other anomalies in CD80/86 dko mice. Altogether, these data demonstrated that, in the absence of CD28, pDC development and maturation were unchanged but IFN-I production was significantly increased in response to TLR stimulation, revealing a novel regulatory role for CD28 in limiting the magnitude of pDC cytokine responses. Furthermore, our data suggest that, CD80/CD86 molecules, which are natural CD28 ligands, may also mediate IFN-I downregulation in pDCs.
CD28 limits pDC-derived IFN-I and antiviral defense early after in vivo viral infection
We next investigated the effect of CD28 signaling on pDC IFN-I response and host defense to viral infection in vivo. We first infected WT and CD28ko mice with Lymphocytic Choriomeningitis Virus (LCMV) Clone 13 (Cl13), a ssRNA virus in which pDCs, via TLR7, contribute to peak IFN-I response at 24hr post-infection (p.i.) (20, 22). At this timepoint p.i., we detected higher IFN-I in serum from CD28ko versus WT mice infected with LCMV, while no difference in systemic TNFα levels were observed (Fig. 3A–B). Importantly, splenic pDCs isolated at this same timepoint from LCMV-infected CD28ko mice showed significantly higher levels of Ifnα and Ifnβ transcripts compared to pDCs from WT infected mice, while cDCs from both WT and CD28ko mice demonstrated low to undetectable IFN-I levels, as previously described (Fig. 3C & (20)). However, we observed no differences in LCMV Cl13 replication in CD28ko versus WT pDCs or liver homogenates at day 1 p.i. (data not shown), potentially due to abundant and sufficient IFN-I already present at this timepoint in WT controls.
Figure 3. CD28 restricts IFN-I production and early virus control during in vivo viral infection.
(a–c) WT and CD28ko mice were infected with LCMV Cl13 for 24hr. Serum IFN-I and TNFα levels were evaluated by bioassay (a) and ELISA (b), respectively, and FACS-purified splenic pDCs and cDCs were evaluated for Ifnα and Ifnβ transcript levels relative to Gapdh by qPCR (c). (d–h) WT and CD28ko mice were infected with MCMV for 36hr. Serum IFN-I and TNFα levels were determined at 24hr and 36hr p.i by bioassay (d) and ELISA (e), respectively. FACS-purified splenic pDCs and cDCs were evaluated by qPCR for Ifnα, Ifnβ (f), Mx1, and RigI (g) transcript levels relative to Gapdh. MCMV titers were quantified by plaque assay and depicted as plaque forming units (pfu) per gram (g) of tissue and expression of MCMV eI gene relative to Gapdh was determined by qPCR in livers (h). Bar graphs show mean value ± SEM. Data are representative of 2–3 independent experiments with 4 mice/group. *p<0.05, **p<0.01, ***p<0.001.
To further validate the inhibitory role of CD28 on pDC antiviral function in vivo and to examine its biological relevance in a model where the contribution of pDCs to viral control is well-established, we next evaluated WT and CD28ko mice infected with Murine Cytomegalovirus (MCMV) (11, 12). During MCMV infection, pDCs recognize and respond to viral infection in a TLR9-dependent manner and are essential for the systemic IFN-I peak observed at 36hr p.i. (11, 12). We observed that, while systemic IFN-I was undetectable in WT and CD28ko mice at 24hr p.i., they were enhanced at 36hr p.i. and that this elevation was significantly higher in CD28ko compared to WT infected mice (Fig. 3D), while serum TNFα levels were similar between both groups (Fig. 3E). Of note, at 36hr p.i., pDC numbers were similar between WT and CD28ko mice, demonstrating that the difference in systemic IFN-I levels was not the result of increased numbers of pDCs in CD28ko mice (data not shown). Consistently, FACS-purified splenic pDCs isolated from CD28ko MCMV-infected mice 36hr p.i. demonstrated higher levels of Ifnα and Ifnβ transcripts than their WT counterparts (Fig. 3F). As expected, undetectable levels of Ifnα and Ifnβ transcripts were observed in WT and CD28ko cDCs at 36hr p.i. (Fig. 3F & (36)). Consistent with the enhanced IFN-I response in CD28ko mice, expression of prototypic ISGs (i.e. Mx1 and RigI) were up-regulated in both pDCs and cDCs from CD28ko compared to WT MCMV-infected mice (Fig. 3G). Importantly, in the absence of CD28, we detected lower viral replication, as indicated by decreased numbers of plaque forming units (pfu) and transcript levels of the MCMV early inducible (eI) gene in the livers of CD28ko compared to WT infected mice (Fig. 3H). Together, these results indicate that CD28 inhibited pDC and systemic IFN-I production during in vivo viral infection with both RNA (LCMV) and DNA (MCMV) viruses. Furthermore, this effect was associated with a restricted antiviral state and influenced viral control early after MCMV exposure, supporting the biologically relevant role of CD28 on pDC IFN-I response.
Cell-intrinsic CD28 signaling suppresses pDC-IFN-I response upon in vivo viral exposure
To investigate whether the inhibitory effect of CD28 on pDC-IFN-I production during viral infection was cell-intrinsic or resulted from CD28 deficiency on other cell types, we generated WT:CD28ko mixed chimeras (Fig. 4A). Chimeric mice showed similar proportions of total lymphocytes, pDCs and cDCs in WT vs. CD28ko compartments (Supplemental Fig. 3). Notably, pDCs isolated from LCMV-infected WT:CD28ko mixed chimeric mice 24hr p.i. showed enhanced expression of Ifnα and Ifnβ transcripts when derived from the CD28ko compared to WT origin (Fig. 4B). Moreover, 36hr after MCMV infection, FACS-purified splenic pDCs from the CD28ko compartment showed an even more dramatic increase of Ifnα and Ifnβ expression compared to WT pDCs (Fig. 4C). Similarly, pro-inflammatory cytokine levels were elevated in CD28ko pDCs compared to their WT counterpart (Fig. 4D). Interestingly, pDC Mx1 expression was also enhanced in CD28ko pDCs during MCMV infection, suggesting autocrine IFN-I signaling in this setting (Fig. 4E). Furthermore, expression of the MCMV eI gene was greatly down-regulated in pDCs (but not cDCs) from the CD28ko origin compared to their WT counterparts, indicating that intrinsic CD28 signaling (potentially through suppression of an autocrine IFN-I effect) was promoting MCMV replication in pDCs (Fig. 4F). Altogether these data indicate that cell-intrinsic CD28 signaling on pDCs significantly down-regulated their IFN-I and pro-inflammatory cytokine production in response to in vivo RNA and DNA viral infections. In the case of MCMV infection, cell-intrinsic CD28 signaling also resulted in a restricted antiviral state and increased viral replication, indicating that CD28 signaling directly regulated pDC antiviral defense during in vivo infection.
Figure 4. Cell-intrinsic CD28 signaling limits pDC cytokine production and their antiviral defense during in vivo viral infection.
(a–f) CD45.1+ WT mice were sublethally irradiated, reconstituted with a 50:50 ratio of CD45.1+ WT and CD28ko (CD45.2+) BM cells for eight weeks (a) and infected with LCMV Cl13 for 24hr (b) or MCMV for 36hr (c–f). (b–e). Expression of Ifnα, Ifnβ (b & c), Tnfα, Il-6 (d), Mx1 (e), and MCMV eI gene (f) relative to Gapdh were determined by qPCR in splenic FACS-purified pDCs and cDCs. Bar graphs show mean value ± SEM. Data are representative of 2 independent experiments with 4–5 mice/group. * p<0.05, ** p<0.01, ***p<0.001.
CD28 deficiency enhances the IFN-I signature in a wound healing model
Aside from its role in antiviral defense, pDC IFN-I production also promotes innate defense during tissue injury (31, 49). Indeed, pDCs sense host-derived nucleic acids that are released following common skin injuries, migrate to the site of cutaneous lesion, and secrete IFN-I, promoting tissue re-epithelialization and wound repair (31). To investigate the role of CD28 in pDC IFN-I response triggered by tissue injury, we compared pDC infiltration and IFN-I levels in WT and CD28ko mice following mechanical injury where the upper epidermal layer of the skin is removed (50). No differences were observed in pDC infiltration between WT and CD28ko mice at 24hr or 48hr post-injury, the time at which pDC infiltrate peaks (data not shown and Fig. 5A, respectively). Interestingly, despite no change in pDC number, CD28ko mice demonstrated an enhanced IFN-I signature which, in this model, is known to be fully dependent on pDCs (31). In fact, 48hr post-injury, we observed increased transcript levels of Ifnα5 and Ifnα6 (Fig. 5B) and the IFN-I response gene Ifi202b. Irf7 and Isg15 also trended towards increased levels in CD28ko mice but differences did not reach statistical significance (Fig. 5C). These data indicate that CD28 suppression of pDC IFN-I response was not restricted to viral infection but instead also influenced non-viral innate responses such as those following tissue injuries.
Figure 5. CD28 deficiency enhances pDC IFN-I signature in response to skin injury.

Skin injury was induced in WT and CD28ko mice by tape stripping (Tape stripped) or left uninjured (No Tx) and evaluated 48hr post-injury. (a) Dermal cell suspensions were isolated from uninjured and injured skin and viable pDCs were counted (CD11c+B220+PDCA+). Representative FACS plots are shown for injured mice. (b & c) Transcript levels of Ifnα2, Ifnα5, Ifnα6 (b) and Ifi202b, Irf7, and Isg15 (c) were determined relative to Gapdh by qPCR. Graphs depict mean ± SEM, where symbols represent individual mice. Data is representative of 2 independent experiments with 4–5 mice per group. *p<0.05, **p<0.01, ***p<0.001.
Discussion
pDCs are highly specialized to produce IFN-I, which dramatically influence antiviral and anti-cancer defense, autoimmunity, and wound healing, among other human illnesses (1). Therefore, understanding the regulation of pDC IFN-I production at the molecular level is of great importance to ultimately fine-tune the magnitude of IFN-I responses as well as their multiple (and often opposing) biological consequences (1). We found that pDCs constitutively expressed the prototypic T cell stimulatory molecule CD28, which unexpectedly restrained (rather than stimulated) pDC IFN-I production in response to TLR stimulation. Importantly, CD28-mediated pDC suppression was also observed upon infection with RNA and DNA viruses and following skin injury in vivo, indicating that it is a general mechanism that down-regulates pDC innate response in different immune settings.
CD28 co-stimulation in T cells, through interaction with CD80 and/or CD86 molecules on antigen presenting cells, is one of the best established events that bridge innate and adaptive immunity following pathogen recognition (32, 51). Interestingly, CD28 expression has been previously reported in other immune cells including NK cells, eosinophils, neutrophils, and plasma cells. For example, engagement of CD28 on a subset of NK cells promotes activation and NK-mediated cytotoxicity (52), while CD28 activity on eosinophils promotes their activation and secretion of IL-2, IFNγ, and IL-13 (53, 54). Furthermore, CD28 ligation on neutrophils enhances CXCR-1 expression, promoting their migration (55), and also induces neutrophil IFNγ secretion during Leishmania major infection (56). More recently, plasma cells were found to express CD28 (57), but the role of CD28 in regulating plasma cell survival and function is unclear as evidence for both promoting and limiting plasma cell survival and antibody production have been reported (58, 59). Importantly, our in vivo experiments with BM-mixed chimeras indicated that CD28 dampened pDC function and its antiviral state, promoting viral replication in a cell-intrinsic manner, rather than through signaling via the aforementioned non-pDC cells that also express CD28. This is consistent with the enhanced cytokine production in CD28ko pDCs differentiated in BM-Flt3L cultures where pDCs are the only CD28 expressing cells. Of note, reconstitution of CD28 in CD28ko pDCs from such BM-Flt3L cultures down-regulated their IFN-I production, ruling out any off-target effects in IFN-I production by CD28ko pDC. Overall, it is striking that while CD28 appears to induce stimulatory signals that promote activation, enhanced function and/or survival in most cells that express it, it exerts a suppressive role in pDCs. It is tempting to speculate that such opposing roles of CD28 in pDCs versus other immune cells might have evolved to counter-balance the immune response to best fight infections with minimal collateral tissue damage.
Interestingly, our findings suggest the possibility that CD80/CD86 interaction with CD28 could regulate the ability of pDCs to produce IFN-I. Since we observed enhanced pDC-IFN-I production in FACS-purified CD80/86 double deficient BMpDCs, it is possible that CD80/CD86 can act in a pDC-autonomous manner. However, it is also conceivable that other CD80/86 expressing cells (such as conventional DCs) might also modulate pDC IFN-I production via CD28 signaling. Moreover, although it is still unclear at what stage of the pDC lifespan CD28 suppression becomes effective, failure to alter IFN-I production by ex vivo CD28 stimulation or blockade in fully differentiated WT pDCs (concomitantly or 1–2 hours before TLR stimulation) suggests that CD28 may homeostatically regulate pDCs (i.e. before pDCs encounter pathogen-associated molecular patterns). Similarly, a recent study demonstrated homeostatic regulation of pDCs by the microRNA miR-126, which (in steady-state conditions) is required for pDC survival and optimal expression of molecules involved in pDC TLR responses, affects subsequent pDC antiviral responses (60). Interestingly, miR-126 regulates pDCs through the VEGFR2 protein, which, similar to CD28, is undetectable in human pDCs isolated from blood but significantly expressed in pDCs purified from tissues (60). Furthermore, the T cell regulatory molecule Lymphocyte Activating Gene 3 (Lag3) (61, 62) also controls the homeostatic proliferation and expansion of pDCs (63). Together with CD28, these and other homeostatic pDC regulators such as E2-2 (64), Flt3 (65), and the PI3k/mTOR pathway (66) highlight the important and intricate nature of pDC control under steady-state, where these multiple pathways may partially overlap but may also regulate distinct aspects of pDC biology, pre-conditioning subsequent pDC responses.
Although CD28 signaling in pDCs may be critical for homeostatic pDC function, it also holds the potential for therapeutic exploitation to limit excessive IFN-I responses that contribute to autoimmunity or immunopathology. In fact, in several autoimmune diseases, pDCs play a pathogenic role via TLR recognition of self nucleic acids, resulting in excessive IFN-I production that promotes activation and survival of autoreactive T and B cells (26, 27, 29, 30, 67). Given that CD28 promotes activation of conventional T cells while also expanding regulatory T cells and dampening pDC innate responses, it is not surprising that the impact of ubiquitous CD28 inhibition or stimulation varies in different autoimmune disease settings. Indeed, while studies have shown that CD28-mediated signaling prevents spontaneous development of autoimmune diabetes (68) and reduces experimental autoimmune neuritis (69), others have shown that CD28 promotes autoimmune diseases such as collagen-induced arthritis, experimental autoimmune encephalomyelitis, and systemic lupus erythematosus (70–72). While these studies have primarily focused on the effects of CD28 on conventional or regulatory T cells, our results open the possibility that altered IFN-I production by pDCs may also play a role in the abovementioned autoimmune disease models. Future studies with cell-specific and temporally controlled ablation of CD28 may illuminate how to best harness CD28 and its downstream signaling pathways towards therapeutically attenuating autoimmune responses while promoting host defense and wound healing.
In conclusion, our study revealed an unexpected role for the prototypic stimulatory receptor CD28 in suppressing innate immunity through cell-intrinsic inhibition of pDC IFN-I production. These findings broaden our understanding of the molecular networks that coordinate innate responses and may aid future pDC-related therapies.
Supplementary Material
Acknowledgments
This work was funded by the Lupus Research Institute, American Cancer Society and NIH grant A1081923 to E.I.Z. E.I.Z. is a Leukemia and Lymphoma Society Scholar. M.M. was supported by NIH supplemental award A1081923. M.A.T. was supported by a postdoctoral fellowship from the Swedish Research Foundation.
We are grateful to Dr. Jack Bui for facilitating studies with human biopsies and critically reading the manuscript.
Footnotes
Competing financial interests: The authors declare no competing financial interests.
References
- 1.Tomasello E, Pollet E, Vu Manh TP, Uze G, Dalod M. Harnessing Mechanistic Knowledge on Beneficial Versus Deleterious IFN-I Effects to Design Innovative Immunotherapies Targeting Cytokine Activity to Specific Cell Types. Front Immunol. 2014;5:526. doi: 10.3389/fimmu.2014.00526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garcia-Sastre A, Biron CA. Type 1 interferons and the virus-host relationship: a lesson in detente. Science. 2006;312:879–882. doi: 10.1126/science.1125676. [DOI] [PubMed] [Google Scholar]
- 3.Reizis B, Bunin A, Ghosh HS, Lewis KL, Sisirak V. Plasmacytoid dendritic cells: recent progress and open questions. Annu Rev Immunol. 2011;29:163–183. doi: 10.1146/annurev-immunol-031210-101345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Y, Swiecki M, McCartney SA, Colonna M. dsRNA sensors and plasmacytoid dendritic cells in host defense and autoimmunity. Immunological reviews. 2011;243:74–90. doi: 10.1111/j.1600-065X.2011.01049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asselin-Paturel C, Brizard G, Pin JJ, Briere F, Trinchieri G. Mouse strain differences in plasmacytoid dendritic cell frequency and function revealed by a novel monoclonal antibody. J Immunol. 2003;171:6466–6477. doi: 10.4049/jimmunol.171.12.6466. [DOI] [PubMed] [Google Scholar]
- 6.Asselin-Paturel C, Trinchieri G. Production of type I interferons: plasmacytoid dendritic cells and beyond. J Exp Med. 2005;202:461–465. doi: 10.1084/jem.20051395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takeuchi O, Akira S. Innate immunity to virus infection. Immunol Rev. 2009;227:75–86. doi: 10.1111/j.1600-065X.2008.00737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McKenna K, Beignon AS, Bhardwaj N. Plasmacytoid dendritic cells: linking innate and adaptive immunity. Journal of virology. 2005;79:17–27. doi: 10.1128/JVI.79.1.17-27.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ito T, Amakawa R, Kaisho T, Hemmi H, Tajima K, Uehira K, Ozaki Y, Tomizawa H, Akira S, Fukuhara S. Interferon-alpha and interleukin-12 are induced differentially by Toll-like receptor 7 ligands in human blood dendritic cell subsets. The Journal of experimental medicine. 2002;195:1507–1512. doi: 10.1084/jem.20020207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lore K, Betts MR, Brenchley JM, Kuruppu J, Khojasteh S, Perfetto S, Roederer M, Seder RA, Koup RA. Toll-like receptor ligands modulate dendritic cells to augment cytomegalovirus- and HIV-1-specific T cell responses. Journal of immunology. 2003;171:4320–4328. doi: 10.4049/jimmunol.171.8.4320. [DOI] [PubMed] [Google Scholar]
- 11.Dalod M, Salazar-Mather TP, Malmgaard L, Lewis C, Asselin-Paturel C, Briere F, Trinchieri G, Biron CA. Interferon alpha/beta and interleukin 12 responses to viral infections: pathways regulating dendritic cell cytokine expression in vivo. J Exp Med. 2002;195:517–528. doi: 10.1084/jem.20011672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krug A, French AR, Barchet W, Fischer JA, Dzionek A, Pingel JT, Orihuela MM, Akira S, Yokoyama WM, Colonna M. TLR9-dependent recognition of MCMV by IPC and DC generates coordinated cytokine responses that activate antiviral NK cell function. Immunity. 2004;21:107–119. doi: 10.1016/j.immuni.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 13.Smit JJ, Rudd BD, Lukacs NW. Plasmacytoid dendritic cells inhibit pulmonary immunopathology and promote clearance of respiratory syncytial virus. J Exp Med. 2006;203:1153–1159. doi: 10.1084/jem.20052359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang H, Peters N, Schwarze J. Plasmacytoid dendritic cells limit viral replication, pulmonary inflammation, and airway hyperresponsiveness in respiratory syncytial virus infection. J Immunol. 2006;177:6263–6270. doi: 10.4049/jimmunol.177.9.6263. [DOI] [PubMed] [Google Scholar]
- 15.Cervantes-Barragan L, Lewis KL, Firner S, Thiel V, Hugues S, Reith W, Ludewig B, Reizis B. Plasmacytoid dendritic cells control T-cell response to chronic viral infection. Proc Natl Acad Sci U S A. 2012 doi: 10.1073/pnas.1117359109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lund JM, Linehan MM, Iijima N, Iwasaki A. Cutting Edge: Plasmacytoid dendritic cells provide innate immune protection against mucosal viral infection in situ. J Immunol. 2006;177:7510–7514. doi: 10.4049/jimmunol.177.11.7510. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi K, Asabe S, Wieland S, Garaigorta U, Gastaminza P, Isogawa M, Chisari FV. Plasmacytoid dendritic cells sense hepatitis C virus-infected cells, produce interferon, and inhibit infection. Proc Natl Acad Sci U S A. 2010;107:7431–7436. doi: 10.1073/pnas.1002301107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fitzgerald-Bocarsly P, Jacobs ES. Plasmacytoid dendritic cells in HIV infection: striking a delicate balance. J Leukoc Biol. 2010;87:609–620. doi: 10.1189/jlb.0909635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jung A, Kato H, Kumagai Y, Kumar H, Kawai T, Takeuchi O, Akira S. Lymphocytoid choriomeningitis virus activates plasmacytoid dendritic cells and induces a cytotoxic T-cell response via MyD88. J Virol. 2008;82:196–206. doi: 10.1128/JVI.01640-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Macal M, Lewis GM, Kunz S, Flavell R, Harker JA, Zuniga EI. Plasmacytoid Dendritic Cells Are Productively Infected and Activated through TLR-7 Early after Arenavirus Infection. Cell Host Microbe. 2012;11:617–630. doi: 10.1016/j.chom.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee LN, Burke S, Montoya M, Borrow P. Multiple mechanisms contribute to impairment of type 1 interferon production during chronic lymphocytic choriomeningitis virus infection of mice. J Immunol. 2009;182:7178–7189. doi: 10.4049/jimmunol.0802526. [DOI] [PubMed] [Google Scholar]
- 22.Zuniga EI, Liou LY, Mack L, Mendoza M, Oldstone MB. Persistent virus infection inhibits type I interferon production by plasmacytoid dendritic cells to facilitate opportunistic infections. Cell Host Microbe. 2008;4:374–386. doi: 10.1016/j.chom.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dolganiuc A, Chang S, Kodys K, Mandrekar P, Bakis G, Cormier M, Szabo G. Hepatitis C virus (HCV) core protein-induced, monocyte-mediated mechanisms of reduced IFN-alpha and plasmacytoid dendritic cell loss in chronic HCV infection. J Immunol. 2006;177:6758–6768. doi: 10.4049/jimmunol.177.10.6758. [DOI] [PubMed] [Google Scholar]
- 24.Sisirak V, Faget J, Gobert M, Goutagny N, Vey N, Treilleux I, Renaudineau S, Poyet G, Labidi-Galy SI, Goddard-Leon S, Durand I, Le Mercier I, Bajard A, Bachelot T, Puisieux A, Puisieux I, Blay JY, Menetrier-Caux C, Caux C, Bendriss-Vermare N. Impaired IFN-a production by Plasmacytoid dendritic cells favors regulatory T cell expansion and contributes to breast cancer progression. Cancer Res. 2012 doi: 10.1158/0008-5472.CAN-11-3468. [DOI] [PubMed] [Google Scholar]
- 25.Nestle FO, Conrad C, Tun-Kyi A, Homey B, Gombert M, Boyman O, Burg G, Liu YJ, Gilliet M. Plasmacytoid predendritic cells initiate psoriasis through interferon-alpha production. J Exp Med. 2005;202:135–143. doi: 10.1084/jem.20050500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Q, Xu B, Michie SA, Rubins KH, Schreriber RD, McDevitt HO. Interferon-alpha initiates type 1 diabetes in nonobese diabetic mice. Proc Natl Acad Sci U S A. 2008;105:12439–12444. doi: 10.1073/pnas.0806439105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Isaksson M, Ardesjo B, Ronnblom L, Kampe O, Lassmann H, Eloranta ML, Lobell A. Plasmacytoid DC promote priming of autoimmune Th17 cells and EAE. Eur J Immunol. 2009;39:2925–2935. doi: 10.1002/eji.200839179. [DOI] [PubMed] [Google Scholar]
- 28.Guiducci C, Gong M, Xu Z, Gill M, Chaussabel D, Meeker T, Chan JH, Wright T, Punaro M, Bolland S, Soumelis V, Banchereau J, Coffman RL, Pascual V, Barrat FJ. TLR recognition of self nucleic acids hampers glucocorticoid activity in lupus. Nature. 2010;465:937–941. doi: 10.1038/nature09102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rowland SL, Riggs JM, Gilfillan S, Bugatti M, Vermi W, Kolbeck R, Unanue ER, Sanjuan MA, Colonna M. Early, transient depletion of plasmacytoid dendritic cells ameliorates autoimmunity in a lupus model. J Exp Med. 2014 doi: 10.1084/jem.20132620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sisirak V, Ganguly D, Lewis KL, Couillault C, Tanaka L, Bolland S, D’Agati V, Elkon KB, Reizis B. Genetic evidence for the role of plasmacytoid dendritic cells in systemic lupus erythematosus. J Exp Med. 2014 doi: 10.1084/jem.20132522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gregorio J, Meller S, Conrad C, Di Nardo A, Homey B, Lauerma A, Arai N, Gallo RL, Digiovanni J, Gilliet M. Plasmacytoid dendritic cells sense skin injury and promote wound healing through type I interferons. J Exp Med. 2010;207:2921–2930. doi: 10.1084/jem.20101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–258. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 33.Ahmed R, Salmi A, Butler LD, Chiller JM, Oldstone MB. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J Exp Med. 1984;160:521–540. doi: 10.1084/jem.160.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tabeta K, Georgel P, Janssen E, Du X, Hoebe K, Crozat K, Mudd S, Shamel L, Sovath S, Goode J, Alexopoulou L, Flavell RA, Beutler B. Toll-like receptors 9 and 3 as essential components of innate immune defense against mouse cytomegalovirus infection. Proc Natl Acad Sci U S A. 2004;101:3516–3521. doi: 10.1073/pnas.0400525101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Macal M, Sankaran S, Chun TW, Reay E, Flamm J, Prindiville TJ, Dandekar S. Effective CD4+ T-cell restoration in gut-associated lymphoid tissue of HIV-infected patients is associated with enhanced Th17 cells and polyfunctional HIV-specific T-cell responses. Mucosal Immunol. 2008;1:475–488. doi: 10.1038/mi.2008.35. [DOI] [PubMed] [Google Scholar]
- 36.Zuniga EI, McGavern DB, Pruneda-Paz JL, Teng C, Oldstone MB. Bone marrow plasmacytoid dendritic cells can differentiate into myeloid dendritic cells upon virus infection. Nat Immunol. 2004;5:1227–1234. doi: 10.1038/ni1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang Z, Georgel P, Du X, Shamel L, Sovath S, Mudd S, Huber M, Kalis C, Keck S, Galanos C, Freudenberg M, Beutler B. CD14 is required for MyD88-independent LPS signaling. Nat Immunol. 2005;6:565–570. doi: 10.1038/ni1207. [DOI] [PubMed] [Google Scholar]
- 38.Edgar R, Domrachev M, Lash AE. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic acids research. 2002;30:207–210. doi: 10.1093/nar/30.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pear WS, Miller JP, Xu L, Pui JC, Soffer B, Quackenbush RC, Pendergast AM, Bronson R, Aster JC, Scott ML, Baltimore D. Efficient and rapid induction of a chronic myelogenous leukemia-like myeloproliferative disease in mice receiving P210 bcr/abl-transduced bone marrow. Blood. 1998;92:3780–3792. [PubMed] [Google Scholar]
- 40.Dalod M, Chelbi R, Malissen B, Lawrence T. Dendritic cell maturation: functional specialization through signaling specificity and transcriptional programming. EMBO J. 2014;33:1104–1116. doi: 10.1002/embj.201488027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robbins SH, Walzer T, Dembele D, Thibault C, Defays A, Bessou G, Xu H, Vivier E, Sellars M, Pierre P, Sharp FR, Chan S, Kastner P, Dalod M. Novel insights into the relationships between dendritic cell subsets in human and mouse revealed by genome-wide expression profiling. Genome Biol. 2008;9:R17. doi: 10.1186/gb-2008-9-1-r17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Riley JL, June CH. The CD28 family: a T-cell rheostat for therapeutic control of T-cell activation. Blood. 2005;105:13–21. doi: 10.1182/blood-2004-04-1596. [DOI] [PubMed] [Google Scholar]
- 43.Maeda T, Murata K, Fukushima T, Sugahara K, Tsuruda K, Anami M, Onimaru Y, Tsukasaki K, Tomonaga M, Moriuchi R, Hasegawa H, Yamada Y, Kamihira S. A novel plasmacytoid dendritic cell line, CAL-1, established from a patient with blastic natural killer cell lymphoma. Int J Hematol. 2005;81:148–154. doi: 10.1532/ijh97.04116. [DOI] [PubMed] [Google Scholar]
- 44.Giard DJ, Aaronson SA, Todaro GJ, Arnstein P, Kersey JH, Dosik H, Parks WP. In vitro cultivation of human tumors: establishment of cell lines derived from a series of solid tumors. J Natl Cancer Inst. 1973;51:1417–1423. doi: 10.1093/jnci/51.5.1417. [DOI] [PubMed] [Google Scholar]
- 45.Tsuchiya S, Yamabe M, Yamaguchi Y, Kobayashi Y, Konno T, Tada K. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1) Int J Cancer. 1980;26:171–176. doi: 10.1002/ijc.2910260208. [DOI] [PubMed] [Google Scholar]
- 46.Liu YJ. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu Rev Immunol. 2005;23:275–306. doi: 10.1146/annurev.immunol.23.021704.115633. [DOI] [PubMed] [Google Scholar]
- 47.Harding FA, McArthur JG, Gross JA, Raulet DH, Allison JP. CD28-mediated signalling co-stimulates murine T cells and prevents induction of anergy in T-cell clones. Nature. 1992;356:607–609. doi: 10.1038/356607a0. [DOI] [PubMed] [Google Scholar]
- 48.Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, Thompson CB, Griesser H, Mak TW. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 49.Bond E, Liang F, Sandgren KJ, Smed-Sorensen A, Bergman P, Brighenti S, Adams WC, Betemariam SA, Rangaka MX, Lange C, Wilkinson RJ, Andersson J, Lore K. Plasmacytoid dendritic cells infiltrate the skin in positive tuberculin skin test indurations. J Invest Dermatol. 2012;132:114–123. doi: 10.1038/jid.2011.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wojcik SM, Bundman DS, Roop DR. Delayed wound healing in keratin 6a knockout mice. Mol Cell Biol. 2000;20:5248–5255. doi: 10.1128/mcb.20.14.5248-5255.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Medzhitov R, Janeway CA., Jr Innate immune recognition and control of adaptive immune responses. Seminars in immunology. 1998;10:351–353. doi: 10.1006/smim.1998.0136. [DOI] [PubMed] [Google Scholar]
- 52.Galea-Lauri J, Darling D, Gan SU, Krivochtchapov L, Kuiper M, Gaken J, Souberbielle B, Farzaneh F. Expression of a variant of CD28 on a subpopulation of human NK cells: implications for B7-mediated stimulation of NK cells. Journal of immunology. 1999;163:62–70. [PubMed] [Google Scholar]
- 53.Woerly G, Lacy P, Younes AB, Roger N, Loiseau S, Moqbel R, Capron M. Human eosinophils express and release IL-13 following CD28-dependent activation. Journal of leukocyte biology. 2002;72:769–779. [PubMed] [Google Scholar]
- 54.Woerly G, Roger N, Loiseau S, Dombrowicz D, Capron A, Capron M. Expression of CD28 and CD86 by human eosinophils and role in the secretion of type 1 cytokines (interleukin 2 and interferon gamma): inhibition by immunoglobulin a complexes. The Journal of experimental medicine. 1999;190:487–495. doi: 10.1084/jem.190.4.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Venuprasad K, Parab P, Prasad DV, Sharma S, Banerjee PR, Deshpande M, Mitra DK, Pal S, Bhadra R, Mitra D, Saha B. Immunobiology of CD28 expression on human neutrophils. I. CD28 regulates neutrophil migration by modulating CXCR-1 expression. European journal of immunology. 2001;31:1536–1543. doi: 10.1002/1521-4141(200105)31:5<1536::AID-IMMU1536>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 56.Venuprasad K, Banerjee PP, Chattopadhyay S, Sharma S, Pal S, Parab PB, Mitra D, Saha B. Human neutrophil-expressed CD28 interacts with macrophage B7 to induce phosphatidylinositol 3-kinase-dependent IFN-gamma secretion and restriction of Leishmania growth. Journal of immunology. 2002;169:920–928. doi: 10.4049/jimmunol.169.2.920. [DOI] [PubMed] [Google Scholar]
- 57.Delogu A, Schebesta A, Sun Q, Aschenbrenner K, Perlot T, Busslinger M. Gene repression by Pax5 in B cells is essential for blood cell homeostasis and is reversed in plasma cells. Immunity. 2006;24:269–281. doi: 10.1016/j.immuni.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 58.Njau MN, Kim JH, Chappell CP, Ravindran R, Thomas L, Pulendran B, Jacob J. CD28-B7 interaction modulates short- and long-lived plasma cell function. J Immunol. 2012;189:2758–2767. doi: 10.4049/jimmunol.1102728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rozanski CH, Arens R, Carlson LM, Nair J, Boise LH, Chanan-Khan AA, Schoenberger SP, Lee KP. Sustained antibody responses depend on CD28 function in bone marrow-resident plasma cells. J Exp Med. 2011;208:1435–1446. doi: 10.1084/jem.20110040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Agudo J, Ruzo A, Tung N, Salmon H, Leboeuf M, Hashimoto D, Becker C, Garrett-Sinha LA, Baccarini A, Merad M, Brown BD. The miR-126-VEGFR2 axis controls the innate response to pathogen-associated nucleic acids. Nat Immunol. 2013 doi: 10.1038/ni.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hannier S, Tournier M, Bismuth G, Triebel F. CD3/TCR complex-associated lymphocyte activation gene-3 molecules inhibit CD3/TCR signaling. J Immunol. 1998;161:4058–4065. [PubMed] [Google Scholar]
- 62.Workman CJ, Vignali DA. Negative regulation of T cell homeostasis by lymphocyte activation gene-3 (CD223) J Immunol. 2005;174:688–695. doi: 10.4049/jimmunol.174.2.688. [DOI] [PubMed] [Google Scholar]
- 63.Workman CJ, Wang Y, El Kasmi KC, Pardoll DM, Murray PJ, Drake CG, Vignali DA. LAG-3 regulates plasmacytoid dendritic cell homeostasis. J Immunol. 2009;182:1885–1891. doi: 10.4049/jimmunol.0800185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cisse B, Caton ML, Lehner M, Maeda T, Scheu S, Locksley R, Holmberg D, Zweier C, den Hollander NS, Kant SG, Holter W, Rauch A, Zhuang Y, Reizis B. Transcription factor E2-2 is an essential and specific regulator of plasmacytoid dendritic cell development. Cell. 2008;135:37–48. doi: 10.1016/j.cell.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Waskow C, Liu K, Darrasse-Jeze G, Guermonprez P, Ginhoux F, Merad M, Shengelia T, Yao K, Nussenzweig M. The receptor tyrosine kinase Flt3 is required for dendritic cell development in peripheral lymphoid tissues. Nat Immunol. 2008;9:676–683. doi: 10.1038/ni.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sathaliyawala T, O’Gorman WE, Greter M, Bogunovic M, Konjufca V, Hou ZE, Nolan GP, Miller MJ, Merad M, Reizis B. Mammalian target of rapamycin controls dendritic cell development downstream of Flt3 ligand signaling. Immunity. 2010;33:597–606. doi: 10.1016/j.immuni.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gilliet M, Cao W, Liu YJ. Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nat Rev Immunol. 2008;8:594–606. doi: 10.1038/nri2358. [DOI] [PubMed] [Google Scholar]
- 68.Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, Bluestone JA. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–440. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 69.Schmidt J, Elflein K, Stienekemeier M, Rodriguez-Palmero M, Schneider C, Toyka KV, Gold R, Hunig T. Treatment and prevention of experimental autoimmune neuritis with superagonistic CD28-specific monoclonal antibodies. J Neuroimmunol. 2003;140:143–152. doi: 10.1016/s0165-5728(03)00182-6. [DOI] [PubMed] [Google Scholar]
- 70.Finck BK, Linsley PS, Wofsy D. Treatment of murine lupus with CTLA4Ig. Science. 1994;265:1225–1227. doi: 10.1126/science.7520604. [DOI] [PubMed] [Google Scholar]
- 71.Peterson KE, Sharp GC, Tang H, Braley-Mullen H. B7.2 has opposing roles during the activation versus effector stages of experimental autoimmune thyroiditis. J Immunol. 1999;162:1859–1867. [PubMed] [Google Scholar]
- 72.Tada Y, Nagasawa K, Ho A, Morito F, Ushiyama O, Suzuki N, Ohta H, Mak TW. CD28-deficient mice are highly resistant to collagen-induced arthritis. J Immunol. 1999;162:203–208. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.