Abstract
In 2010, a new mouse papillomavirus, MmuPV1, was discovered in a colony of NMRI- Foxn1nu /Foxn1nu athymic mice in India. This finding was significant because it was the first papillomavirus to be found in a laboratory mouse. In this paper we report successful infections of both dorsal and ventral surfaces of the rostral tongues of outbred athymic nude mice. We also report the observation that the base of the tongue, the area of the tongue often targeted by cancer-associated high-risk papillomavirus infections in humans, is especially susceptible to infection. A suitable animal model for the study of oral papillomavirus infections, co-infections, and cancers has long been sought. The work presented here suggests that such a model is now at hand.
INTRODUCTION
Human papillomavirus infections are ubiquitous in the general population and a subset of these viruses is associated with significant disease. Papillomavirus infection is an obligate co-factor for most cervical cancers and this cancer is a leading cause of death in women worldwide with in excess of 300,000 deaths reported each yea[1]. The viruses are also increasingly associated with anal and oral cancers [2–4]. While two vaccines are now available that target a few of the viruses most commonly associated with clinical disease including malignant neoplasia, the uptake of these vaccines has been disappointing and the price is out of reach for use in developing nations. Importantly, the vaccines do not eliminate established infections and no effective treatment or therapy is available. More research will be necessary to achieve satisfactory control of these common infections and to assure that the cancers to which they contribute will be minimized in the years to come.
Oropharyngeal cancers associated with papillomavirus infection are increasing, especially in the young male population [5]. The reasons are unclear although it has been postulated that an increased practice of oral sex may be a factor [6]. Papillomaviruses are strictly species specific and consequently no animal can be infected with a human virus. With the demonstration that MmuPV1 can infect oral tissues of the athymic mouse, and with the observation that the virus appears to have a preference for the base of the tongue, we have demonstrated the practicality of this model for the study of oral infections and, potentially, of papillomavirus-associated oral cancers.
MATERIALS AND METHODS
Viral stock
MmuPV1 virus was isolated from lesions on the tails of mice from our previous study [7]. The lesions were scraped from the tail with a scalpel blade and homogenized in phosphate buffered saline (PBS) using a Polytron homogenizer (Brinkman PT10–35) at highest speed for three minutes while chilling in an ice bath. The homogenate was centrifuged at 10,000 rpm and the supernatant was decanted into Eppendorf tubes for storage at −20°C. For these experiments, the virus was diluted 1:5 in PBS and 200μl was passed through a 0.2μm cellulose acetate sterile syringe filter. This was chased by the addition of 200μl PBS. The PBS filtrate was added to the filtered virus to give a total of 250μl sterile virus solution when taking into account loss in the filter. Viral DNA was quantitated following extraction of the DNA from 5μl of this stock. The DNA was isolated in 50 μl water and SYBR green Q PCR assay was conducted on a series of 1:2 dilutions. Dilutions of plasmid DNA of known concentration were used to develop a standard curve. One μl of DNA was determined to contain 0.012ng viral DNA. 1 ng contains 1.2 x108 copies of viral DNA (http://cels.uri.edu/gsc/cndna.html). Thus, 1 μl of the DNA extract contains 1.4x106 copies of viral DNA meaning such that 1 μl of the viral extract contains 1.4 x 107 viral genome equivalents.
MmuPV1 tongue infections
All mouse work was approved by the Institutional Animal Care and Use Committee of the Pennsylvania State University College of Medicine. Outbred athymic nude mice (Hsd: NU-Foxn1nu) were obtained from Harlan Laboratories and were housed in sterile microisolator cages and were fed sterilized food and water. Four mice, ages 8–12 weeks, were sedated i.p. with ketamine/xylazine mixture (100mg/10mg per kg body weight in PBS). Tongues were withdrawn using a sterile forceps and micro needles (Microneedle Systems, LLC, GA) were used to superficially wound both the dorsal and ventral surfaces of the rostral tongues. Some bleeding occurred but care was taken to minimize this bleeding. Animals were allowed to recover overnight. The following day, each animal was again anaesthetized. Tongues were again gently abraded with the microneedles and 10μl of sterile virus (1.4 x 108 viral genome equivalents) was applied to the freshly abraded surfaces. Animals were monitored weekly and photo documentation was performed. All work was conducted in biosafety cabinets.
Harvest and analysis of tissues
Animals were euthanized by ketamine/xylazine anesthesia followed by cervical dislocation and the entire tongue, with submandibular and palatine mucosae attached, was excised. Specimens were routinely processed to paraffin blocks. Sequential sections were cut for H and E analysis, in situ hybridization and immunohistochemistry. Standard conditions were used for H and E staining. A subgenomic fragment of MmuPV1 (3913bp EcoRV/BamH1 fragment) was used as an in situ hybridization probe for the detection of MmuPV1 DNA in tissues. The probe was biotinylated using the random priming method and diluted in a hybridization cocktail described in previous work [7]. Access to target DNA was obtained with 0.2 mg/ml pepsin in 0.1N HCl incubation at 37°C for 8 minutes. After thorough washing, the biotinylated probe was applied and heated to 95°C for 5 minutes to achieve dissociation of target and probe DNA. Reannealing was allowed to occur for 2 hours at 37°C. Target- bound biotin was detected using a streptavidin AP conjugate followed by colorimetric development in BCIP/NBT. For immunohistochemical (IHC) L1 capsid detection, a goat Group Specific Antibody (GSA) to a conserved region of L1 (ViroStat #5001) was used on FFPE sections. Detection was achieved using the ImmPRESS anti-goat IgG polymer system (Vector #MP-7405). Tissues were examined by an ACVP diplomate pathologist blinded to treatment. All images were obtained with an Olympus BX51 microscope and DP71 digital camera using cellSens Standard 1.12 imaging software (Olympus America, Center Valley, PA).
RESULTS
Both the dorsal and ventral surfaces of the rostral tongues of outbred athymic nude mice are susceptible to infection with MmuPV1
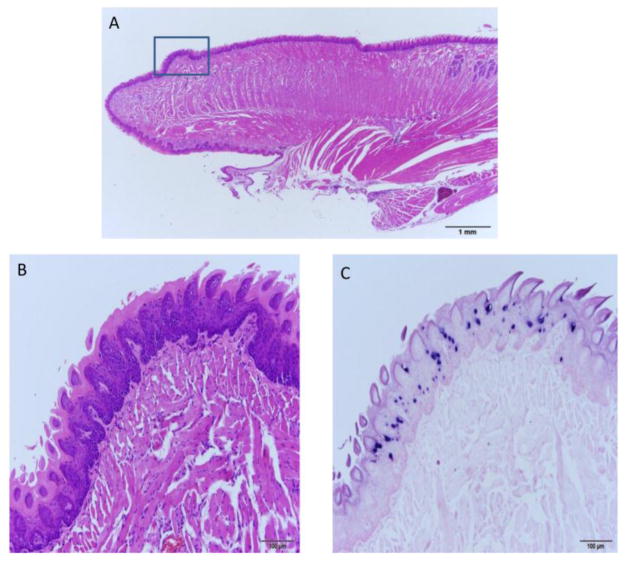
Mice infected on the dorsal and ventral surfaces of the rostral tongues were sacrificed at an early time point of 3.5 months or at later time points of 8, 8.5 and 9 months post infection (one mouse per time point). By 3.5 months, a section of the rostral dorsal surface of the tongue of mouse 3-3L showed significant papillomavirus infection. Figure 1A–C shows H and E, ISH, and IHC respectively of a dorsal lesion of this tongue.
Fig. 1.
A–C The dorsal surface of the rostral tongue is susceptible to MmuPV1 infection. A) 10x sagittal section of the tongue of animal 3-3L sacrificed 3.5 months post infection and stained by H and E. The dorsal lesion on the rostral tongue is boxed. B) H and E (100x) and C) ISH (100x) of the boxed region in A showing a discrete focus of basal cell hyperplasia and strong in situ hybridization signal.
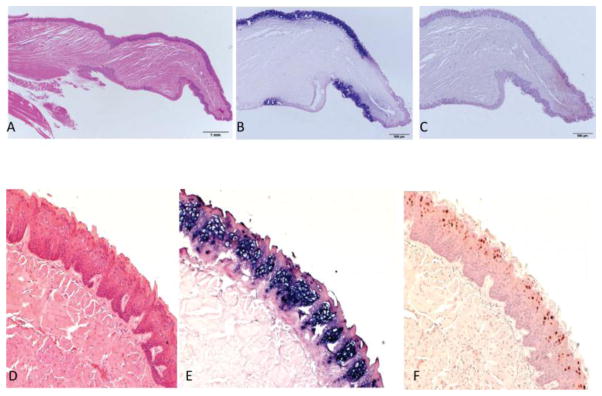
By 8 months post infection, both the dorsal and ventral surfaces of the tongue of animal 3-3R showed very strong hybridization signal and antigen expression. See sagittal sections Figure 2A–C. There was a sharp demarcation between positive and negative signals, the significance of which is not yet understood. Figure 2 D–F shows a higher magnification of a dorsal section of the tongue.
Fig. 2.
A–F By 8 months post infection both dorsal and ventral surfaces exhibit strong positivity for MmuPV1. A) Sagittal section of the tongue of mouse 3-3R sacrificed at 8 months post infection. H and E at 10x. B) ISH (20x) of the sagittal section showing strong ISH signal with clear demarcation of positive and negative sites. C) IHC(20x) of same section showing signal in the DNA-positive sites. D) H and E (100x), E) ISH (100x) and F) IHC (100x) illustrating the magnitude of viral infection.
Animals sacrificed at 8.5 and 9 months also exhibited extensive positivity on both dorsal and ventral surfaces of the rostral tongue. (Data not shown).
A Base of tongue lesion was observed in an animal that had been infected on the rostral tongue
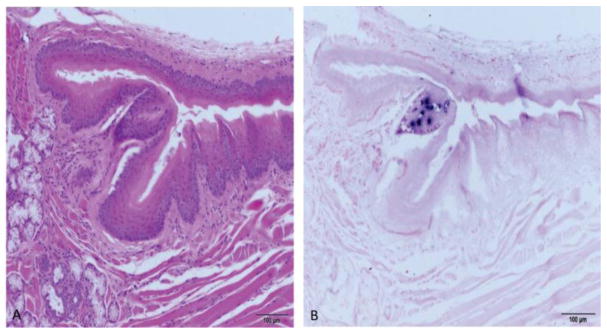
Since tongue infections were initiated on the dorsal and ventral surfaces of the rostral (“mobile”) tongue, we did not expect to see lesions at the caudal (base) of the tongue. However, careful examination of the sections of the entire tongue revealed a base-of-tongue lesion in one of the animals. The base of the tongue is of special interest due to its involvement in papillomavirus-associated human cancers [8]. Figure 3A and B shows H and E and ISH sections of the lesion at the base of the tongue at the dorsal fornix of animal 3-3R. Surrounding tissue is negative for the virus.
Fig. 3.
A and B A secondary back of the tongue lesion was found. A) H and E (100x) of a sagittal section of the caudal tongue encompassing the dorsal fornix of animal 3-3R. B) ISH (100x) of an adjacent section showing a focus of positivity near the dorsal fornix.
Inner cheek infections were found in animals infected on the dorsal and ventral surfaces of the tongue
At the time of sacrifice of the four animals infected on the tongue, inner cheek samples were excised and paraffin-embedded. Our earlier studies had shown that secondary infections can occur and we wished to determine whether this proximal tissue was also infected. Figure S1 A and B shows strong in situ hybridization signal in representative sections of both left and right cheek tissue from animal 3-3L.
The base of the tongue was preferentially targeted by secondary infections
During the course of this work, we also conducted infectivity studies in other animals at mucosal sites such as the vagina and anus as well as cutaneous sites including tail and muzzle. Our observation that primary infection of the rostral tongue yielded a secondary infection at the base of the tongue led us to examine the tongues of some of these animals. Tongues from a total of 18 animals infected at sites other than the oral cavity had been archived as tissue blocks. Of these, ten (56%) were found to have lesions at the base of the tongue. Eight of these involved the caudal dorsal tongue and two involved the caudal ventral tongue. Three of the 18 animals (17%) had small areas of secondary infection on the rostral tongue. Table 1 summarizes the secondary base-of-tongue infections found in animals with primary infections at sites other than the oral cavity.
Table 1.
Secondary oral lesions found in animals infected with MmuPV1 at sites other than the oral cavity.
| Animal | Primary infection | Location of secondary infection | Figure |
|---|---|---|---|
| 5-2L | Tail | Caudodorsal tongue; | ND |
| 7-2L | Back and tail | Caudodorsal tongue extending into the duct of the serous lingual accessory salivary gland | 5 E, F |
| 8-2R | Anus | Caudodorsal tongue extending into the duct of the serous lingual accessory salivary gland | 4 A–C |
| 10-2L | Vagina | Caudodorsal tongue extending into the duct of the serous lingual accessory salivary gland | 4 D–F |
| 1-3R | Anus | Dorsal/pharynx in area of palate | S 2 A, B |
| 4-3L | Vagina | Caudal margin of dorsal tongue near reflection to palatine mucosa; lingual salivary gland duct involvement | 5 A, B |
| 5-3R | Vagina | Caudal margin of dorsal tongue near reflection to palatine mucosa; lingual salivary gland duct involvement | ND |
| 6-3R | Muzzle, tail, anus, vagina | Caudoventral tongue near reflection to sublingual oral mucosa | 6 A, B |
| 7-3L | Muzzle, tail, anus, vagina | Caudoventral tongue near reflection to sublingual oral mucosa | 6 C–E |
| 10-3 | Muzzle, tail, anus, vagina | Caudodorsal tongue extending into the duct of the serous lingual accessory salivary gland | 5 C, D |
The circumvallate papilla and adjacent Von Ebners glands represent a preferred locus for MmuPV1 infection in mice secondarily infected in the oral cavity
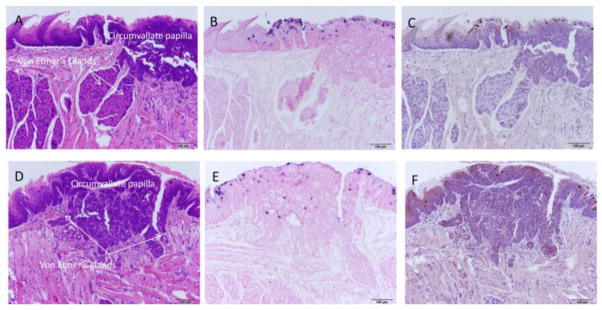
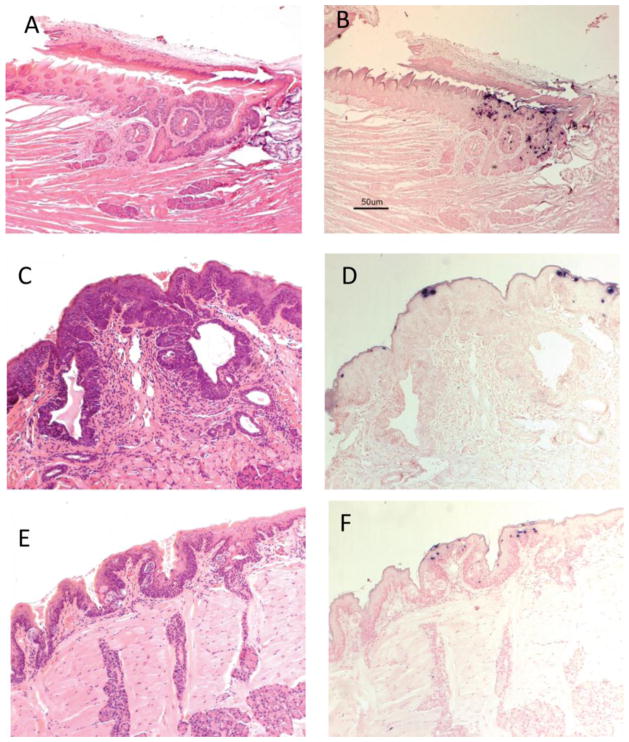
Careful observation of the tongue sections positive for secondary MmuPV1 infection identified the location of seven of the eight dorsal tongue infections as the single circumvallate papilla of the mouse tongue and the adjacent Von Ebners glands. More advanced infections were associated with significant hyperplasia and dysplasia (Figures 4A–F; 5A,B). Figure 5 C–F shows sites in two of four additional tongues with less advanced lesions. Supplemental figure 2 A, B shows the single dorsal back of the tongue lesion not found in the area of the circumvallate papilla. This lesion was located in the dorsal pharynx/palate area of the caudal tongue.
Fig. 4.
A–F Back of the tongue secondary lesions involved the circumvallate papilla and adjacent Von Ebner’s glands and exhibited marked dysplasia. A) H and E (100x) of a sagittal section of the caudodorsal tongue of animal 8-2R demonstrating florid pseudocarcinomatous hyperplasia of the epithelium of the circumvallate papilla of the dorsal tongue adjacent to and extending into the duct of the serous lingual accessory salivary (Von Ebner’s) glands. Adjacent duct of the mucinous salivary gland is unaffected. B) ISH (100x) of an adjacent section demonstrating strong staining in the superficial epithelial cells, correlating with viral cytopathic effect seen on H&E section. C) IHC (100x) of an adjacent section showing capsid antigen presence in the same areas positive for MmuPV1 DNA. D) H and E (100x) of a sagittal section of the caudodorsal tongue of animal 10-2L demonstrating florid pseudocarcinomatous hyperplasia of the epithelium of the circumvallate papilla of the dorsal tongue adjacent to and extending into the duct of the serous lingual accessory salivary (Von Ebner’s) glands. Adjacent duct of the mucinous salivary gland is unaffected. E) ISH (100x) of an adjacent section demonstrating strong staining in the superficial epithelial cells, correlating with viral cytopathic effect seen on H&E section. F) IHC (100x) of an adjacent section showing capsid antigen presence in the same areas positive for MmuPV1 DNA.
Fig. 5.
A–F Additional back of the tongue lesions targeted the circumvallate papilla. A) H and E (40x) of a longitudinal parasagittal section of the tongue of animal 4-3L experimentally infected vaginally. At the caudal margin of the dorsal tongue near the reflection to the palatine mucosa and involving the ducts of the lingual salivary (Von Ebner’s) glands and associated circumvallate papilla, there is focally extensive epithelial hyperplasia with dysplasia and viral cytopathic effect (CPE). B) ISH (40x) of an adjacent section showing strong DNA positivity in the same dysplastic area. C) H and E of lesion targeting the circumvallate papilla of animal 10-3(100x). D) ISH of the same lesion showing DNA positivity. E) H and E of lesion targeting the circumvallate papilla of animal 7-2L (100x). F) ISH of the same lesion showing DNA positivity(100x).
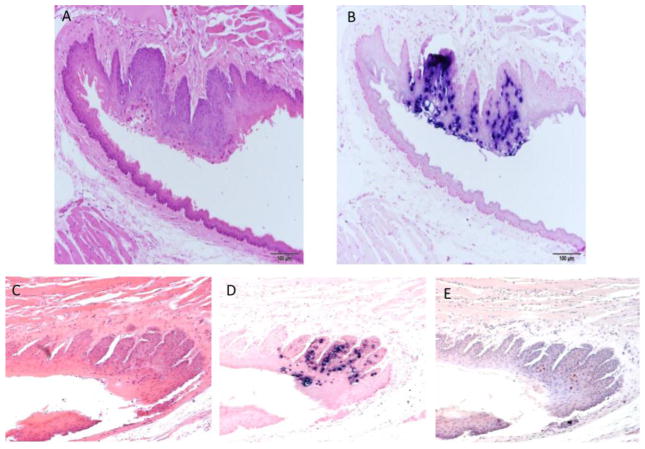
The two secondary infections at the caudoventral tongue were found to target the same locus
Only two secondary infections targeted the caudoventral tongue and both of these were located in an area near the reflection to the sublingual oral mucosa. The limited number of samples does not allow the conclusion that this is a preferred site on the ventral tongue but the data are suggestive. Figure 6A–E.
Fig. 6.
A–E Two lesions were found at the caudal ventral tongue. A) H and E (100x) of a sagittal/oblique section of the caudoventral tongue of animal 6-3R showing the area near the reflection to the sublingual oral mucosa. The animal was experimentally infected at several non-oral mucosal and cutaneous sites. B) ISH (100x) of an adjacent section showing strong staining near the reflection to the sublingual oral mucosa. C) H and E (100x) of a longitudinal parasagittal section of the tongue of animal 7-3L showing the reflection to the sublingual oral mucosa. The animal was originally infected at several non-oral mucosal and cutaneous sites. D) ISH (100x) of an adjacent section showing strong positivity at the reflection to the sublingual oral mucosa. E) IHC (100x) of an adjacent section showing capsid antigen presence in the same areas positive for MmuPV1 DNA.
DISCUSSION
Papillomavirus-associated diseases pose a major problem throughout the world. To date, more than 150 species of human PVs have been fully sequenced. Most of these are relatively benign but a number are implicated in cancers, especially anogenital cancers, as well as some skin cancers and head and neck malignancies. Others do not cause cancer under normal conditions but do present significant medical challenges (reviewed in [9]). Because human papillomaviruses do not infect animals, finding a suitable in vivo model to study the disease has been challenging. Our laboratory [10–16] and those of others [17–21] have used the cottontail rabbit papillomavirus (CRPV) model to good advantage. We have also pioneered the rabbit oral papillomavirus (ROPV) model [22,23]. Due to limited reagents for the rabbit and technical challenges with ROPV, we sought another small laboratory animal model to complement our studies. We were especially interested in a mouse model because of the abundance of reagents available for this common laboratory animal. When the new mouse virus, MmuPV1, was reported [24] we initiated studies to see if this virus would aid our studies on preclinical papillomavirus infections.
MmuPV1 was reported to be a strictly cutaneous pathogen [24] but our early studies revealed mucosal secondary infections [7,25]. This observation was later corroborated by Sundberg et al [26]. We have shown that MmuPV1 has a broad tropism in athymic Foxn1nu mice and that it infects anal, vaginal and cervical tissues [25]. In this paper we extend our observations to demonstrate that the virus can also infect oral mucosal sites, specifically both the dorsal and ventral surfaces of the rostral tongue, the inner cheek, and the oropharyngeal cavity. We show that the oropharyngeal cavity, including base of tongue and surrounding tissues, is especially susceptible to infection and we have localized most of the infections to the circumvallate papilla and associated Von Ebner’s glands. The more advanced lesions (figures 4 A–F; 5A,B) exhibit florid pseudocarcinomatosis and cytopathic effect (CPE) associated with papillomavirus infections.
Oropharyngeal squamous cell carcinomas have been on the rise in much of the world in the past few decades [8]. Two different populations of individuals have been identified as having this disease and it appears to present differently in these two populations [27]. One of the groups is older on average and has risk factors such as heavy drinking and smoking. The other group tends to be younger and healthier. In the latter case, disease is frequently associated with papillomavirus infection, commonly HPV16 but also other types. These infections tend to be located at the base of the tongue and in the tonsillar region [28]. We have demonstrated in this study that the base of the tongue of the nude mouse appears to be a preferred target for oral cavity MmuPV1 infection. We postulate that the MmuPV1/mouse model could be very useful for the study of papillomavirus infection at this anatomical site. We also anticipate that the model will be useful for studies of cancer initiation and progression, studies that will have significant relevance to the human condition. Extensive hyperplasia and dysplasia as well as viral CPE were noted in some of the lesions reported here. Cancers develop over time and we hypothesize that given more time these lesions might have progressed to cancer. Addition of a cofactor such as tobacco smoke or ozone could accelerate malignant conversion. Our research with the rabbit model has shown that synonymous codon modifications in the oncogenes E6 and E7 of CRPV result in genomes that yield infections that rapidly progress to cancer [14]. MmuPV1 genomes with codon modifications may similarly enhance the malignant potential of this virus.
Another use for this model will be to study papillomavirus infection under conditions of immune suppression, conditions that are naturally present in the Hsd: NU-Foxn1nu mouse. As the population of individuals infected with the Human Immunodeficiency Virus (HIV) increases and as more patients receive organ transplants, papillomavirus infections are becoming more prevalent as are associated oral cancers. [29,30]. Until now, there has been no in vivo model to study the disease under these unique conditions. The MmuPV1 /athymic mouse model is ideally suited for these studies.
CONCLUSIONS
We have shown in this study that mouse papillomavirus 1(MmuPV1) infects the oral cavity of outbred nude mice and has a preference for the base of the tongue. Of the 18 tongues of animals infected at sites other than the oral cavity, 10 (56%) had secondary infections at the base of the tongue. The circumvallate papilla and associated Von Ebner’s glands on the caudal dorsal tongue was the preferred site of infection with 7 (70%) of infected animals demonstrating secondary infections only at this locus and not at other oral sites. Two animals (20%) had lesions on the caudal ventral tongue and these lesions targeted the same location suggesting that this might also be a susceptible site. A single lesion was found on the caudal dorsal tongue at the pharynx/palate area. To date, there has been no suitable in vivo model to study oral papillomavirus-associated lesions. Human lesions associated with papillomavirus-induced cancers also tend to occur at the back of the tongue. The MmuPV1 model for papillomavirus infection should provide a viable option for gaining insights into human lesions and associated cancers.
Supplementary Material
Fig. S1 A, B Inner cheeks were secondarily infected. A) ISH(40x) of left inner cheek of animal 3-3L. B) ISH(40x) of the right inner cheek of the same animal. Both show strong signals for viral DNA indicating that inner cheek tissue is permissive for secondary infections.
Fig. S2 A,B A single back of the tongue lesion was found at the dorsal area near the palate. A) H and E (40x) of a secondary infection occurring in the dorsal pharynx/palate area at the caudal tongue of animal 1-3R. This animal was originally experimentally infected anally. B) ISH (100x) of adjacent section showing a small region of strong DNA positivity near the dorsal pharynx.
Highlights.
Mouse papillomavirus 1 (MmuPV1) preferentially targets the back of the mouse tongue.
Human oral cancers associated with papillomavirus also target the back of the tongue.
The MmuPV1 model will allow the study of oral papillomavirus infections in an in vivo animal model.
Acknowledgments
Financial support. The authors are appreciative of funding provided by NIH grant R01 CA047622 as well as contributions from the Jake Gittlen Memorial Golf Tournament.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Arbyn M, Castellsague X, de Sanjose S, Bruni L, Saraiya M, Bray F, Ferlay J. Worldwide burden of cervical cancer in 2008. Annals of Oncology. 2011;22:2675–2686. doi: 10.1093/annonc/mdr015. [DOI] [PubMed] [Google Scholar]
- 2.D’Souza G, Kreimer AR, Viscidi R, Pawlita M, Fakhry C, Koch WM, Westra WH, Gillison ML. Case-control study of human papillomavirus and oropharyngeal cancer. New England Journal of Medicine. 2007;356:1944–1956. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 3.Grulich AE, Poynten IM, Machalek DA, Jin FY, Templeton DJ, Hillman RJ. The epidemiology of anal cancer. Sexual Health. 2012;9:504–508. doi: 10.1071/SH12070. [DOI] [PubMed] [Google Scholar]
- 4.Ouhoummane N, Steben M, Coutlee F, Vuong T, Forest P, Rodier C, Louchini R, Duarte E, Brassard P. Squamous anal cancer: Patient characteristics and HPV type distribution. Cancer Epidemiology. 2013;37:807–812. doi: 10.1016/j.canep.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 5.Gillison ML, Koch WM, Capone RB, Spafford M, Westra WH, Wu L, Zahurak ML, Daniel RW, Viglione M, Symer DE, Shah KV, Sidransky D. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92:709–720. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 6.Gillison ML, D’Souza G, Westra W, Sugar E, Xiao W, Begum S, Viscidi R. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst. 2008;100:407–420. doi: 10.1093/jnci/djn025. djn025 [pii]; [DOI] [PubMed] [Google Scholar]
- 7.Cladel NM, Budgeon LR, Cooper TK, Balogh KK, Hu J, Christensen ND. Secondary infections, expanded tissue tropism, and evidence for malignant potential in immunocompromised mice infected with Mus musculus papillomavirus 1 DNA and virus. J Virol. 2013;87:9391–9395. doi: 10.1128/JVI.00777-13. JVI.00777-13 [pii]; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woods R, Sr, O’Regan EM, Kennedy S, Martin C, O’Leary JJ, Timon C. Role of human papillomavirus in oropharyngeal squamous cell carcinoma: A review. World J Clin Cases. 2014;2:172–193. doi: 10.12998/wjcc.v2.i6.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doorbar J, Egawa N, Griffin H, Kranjec C, Murakami I. Human papillomavirus molecular biology and disease association. Reviews in Medical Virology. 2015;25:2–23. doi: 10.1002/rmv.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu J, Cladel NM, Balogh K, Budgeon L, Christensen ND. Impact of genetic changes to the CRPV genome and their application to the study of pathogenesis in vivo. Virology. 2006 doi: 10.1016/j.virol.2006.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christensen ND, Reed CA, Cladel NM, Han R, Kreider JW. Immunization with viruslike particles induces long-term protection of rabbits against challenge with cottontail rabbit papillomavirus. J Virol. 1996;70:960–965. doi: 10.1128/jvi.70.2.960-965.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han R, Cladel NM, Reed CA, Peng XW, Budgeon LR, Pickel M, Christensen ND. DNA vaccination prevents and/or delays carcinoma development of papillomavirus-induced skin papillomas on rabbits1. Journal of Virology. 2000;74:9712–9716. doi: 10.1128/jvi.74.20.9712-9716.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu J, Peng X, Cladel NM, Pickel MD, Christensen ND. Large cutaneous rabbit papillomas that persist during cyclosporin A treatment can regress spontaneously after cessation of immunosuppression. J Gen Virol. 2005;86:55–63. doi: 10.1099/vir.0.80448-0. [DOI] [PubMed] [Google Scholar]
- 14.Cladel NM, Budgeon LR, Hu JF, Balogh KK, Christensen ND. Synonymous codon changes in the oncogenes of the cottontail rabbit papillomavirus lead to increased oncogenicity and immunogenicity of the virus. Virology. 2013;438:70–83. doi: 10.1016/j.virol.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu J, Budgeon LR, Cladel NM, Culp TD, Balogh KK, Christensen ND. Detection of L1, infectious virions and anti-L1 antibody in domestic rabbits infected with cottontail rabbit papillomavirus. Journal of General Virology. 2007;88:3286–3293. doi: 10.1099/vir.0.82879-0. [DOI] [PubMed] [Google Scholar]
- 16.Han R, Cladel NM, Reed CA, Christensen ND. Characterization of transformation function of cottontail rabbit papillomavirus E5 and E8 genes. Virology. 1998;251:253–263. doi: 10.1006/viro.1998.9416. [DOI] [PubMed] [Google Scholar]
- 17.Brandsma JL, Yang ZH, DiMaio D, Barthold SW, Johnson E, Xiao W. The putative E5 open reading frame of cottontail rabbit papillomavirus is dispensable for papilloma formation in domestic rabbits. J Virol. 1992;66:6204–6207. doi: 10.1128/jvi.66.10.6204-6207.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Du MJ, Fan XL, Hanada T, Gao H, Lutchman M, Brandsma JL, Chishti AH, Chen JJ. Association of cottontail rabbit papillornavirus E6 oncoproteins with the hDlg/SAP97 tumor suppressor1. Journal of Cellular Biochemistry. 2005;94:1038–1045. doi: 10.1002/jcb.20383. [DOI] [PubMed] [Google Scholar]
- 19.Peh WL, Brandsma JL, Christensen ND, Cladel NM, Wu X, Doorbar J. The viral E4 protein is required for the completion of the cottontail rabbit papillomavirus productive cycle in vivo. J Virol. 2004;78:2142–2151. doi: 10.1128/JVI.78.4.2142-2151.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han R, Breitburd F, Marche PN, Orth G. Linkage of regression and malignant conversion of rabbit viral papillomas to MHC class II genes. Nature. 1992;356:66–68. doi: 10.1038/356066a0. [DOI] [PubMed] [Google Scholar]
- 21.Salmon J, Nonnenmacher M, Caze S, Flamant P, Croissant O, Orth G, Breitburd F. Variation in the nucleotide sequence of cottontail rabbit papillomavirus a and b subtypes affects wart regression and malignant transformation and level of viral replication in domestic rabbits. J Virol. 2000;74:10766–10777. doi: 10.1128/jvi.74.22.10766-10777.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Christensen ND, Cladel NM, Reed CA, Han RC. Rabbit oral papillomavirus complete genome sequence and immunity following genital infection5. Virology. 2000;269:451–461. doi: 10.1006/viro.2000.0237. [DOI] [PubMed] [Google Scholar]
- 23.Wilgenburg BJ, Budgeon LR, Lang CM, Griffith JW, Christensen ND. Characterization of immune responses during regression of rabbit oral papillomavirus infections1. Comparative Medicine. 2005;55:431–439. [PubMed] [Google Scholar]
- 24.Ingle A, Ghim S, Joh J, Chepkoech I, Bennett JA, Sundberg JP. Novel laboratory mouse papillomavirus (MusPV) infection. Vet Pathol. 2011;48:500–505. doi: 10.1177/0300985810377186. 0300985810377186 [pii]; [DOI] [PubMed] [Google Scholar]
- 25.Cladel NM, Budgeon LR, Balogh KK, Cooper TK, Hu J, Christensen ND. A novel pre-clinical murine model to study the life cycle and progression of cervical and anal papillomavirus infections. PLoS ONE. 2015;10:e0120128. doi: 10.1371/journal.pone.0120128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sundberg JP, Stearns TM, Joh J, Proctor M, Ingle A, Silva KA, Dadras SS, Jenson AB, Ghim SJ. Immune Status, Strain Background, and Anatomic Site of Inoculation Affect Mouse Papillomavirus (MmuPV1) Induction of Exophytic Papillomas or Endophytic Trichoblastomas. PLoS ONE. 2014;9:e113582. doi: 10.1371/journal.pone.0113582. PONE-D-14-23798 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gillison ML, D’Souza G, Westra W, Sugar E, Xiao WH, Begum S, Viscidi R. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. Journal of the National Cancer Institute. 2008;100:407–420. doi: 10.1093/jnci/djn025. [DOI] [PubMed] [Google Scholar]
- 28.Ramqvist T, Grun N, Dalianis T. Human papillomavirus and tonsillar and base of tongue cancer. Viruses. 2015;7:1332–1343. doi: 10.3390/v7031332. v7031332 [pii]; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Syrjanen S. Human papillomavirus infection and its association with HIV. Adv Dent Res. 2011;23:84–89. doi: 10.1177/0022034511399914. 23/1/84 [pii]; [DOI] [PubMed] [Google Scholar]
- 30.Gaester K, Fonseca LAM, Luiz O, Assone T, Fontes AS, Costa F, Duarte AJS, Casseb J. Human papillomavirus infection in oral fluids of HIV-1-positive men: prevalence and risk factors. Scientific Reports. 2014;4 doi: 10.1038/srep06592. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 A, B Inner cheeks were secondarily infected. A) ISH(40x) of left inner cheek of animal 3-3L. B) ISH(40x) of the right inner cheek of the same animal. Both show strong signals for viral DNA indicating that inner cheek tissue is permissive for secondary infections.
Fig. S2 A,B A single back of the tongue lesion was found at the dorsal area near the palate. A) H and E (40x) of a secondary infection occurring in the dorsal pharynx/palate area at the caudal tongue of animal 1-3R. This animal was originally experimentally infected anally. B) ISH (100x) of adjacent section showing a small region of strong DNA positivity near the dorsal pharynx.