Abstract
Myeloid cells play a crucial role in the induction and sustained inflammation in neuroinflammatory disorders such as MS. miR-223, a myeloid cell-specific miRNA, is one of the most upregulated miRNAs in MS patients. We demonstrate that miR-223 KO mice display significantly reduced active and adoptive transfer EAE characterized by reduced numbers of myeloid DCs (mDCs) and TH17 cells in the CNS. KO mDCs have increased PD-L1 and decreased IL-1β, IL-6 and IL-23 expression and a reduced capacity to drive TH17, but not TH1, cell differentiation. Thus miR-223 controls mDC-induced activation of pathologic TH17 responses during autoimmune inflammation.
Keywords: MS, EAE, miR-223, mDCs, TH17, Ag presentation
Introduction
Blood-derived myeloid cells comprise a significant proportion of the infiltrating perivascular leukocytes in CNS lesions in MS (1) and its animal model, EAE (2, 3). These APCs have the ability to phagocytize myelin debris and to secrete cytokines which promote the expansion and polarization of CD4+ TH1 and TH17 T cells critical in the initiation and progression of EAE (2).
Ly6chiCD11b+CD11c+ monocyte-derived dendritic cells (Mo-DCs) are a prominent component of CNS-infiltrates cells in EAE (2, 3). Mo-DCs arise from monocytes following their migration into inflamed tissues (4). mDCs are responsible for initiating epitope spreading during EAE within the CNS, inducing the differentiation of TH17 cells (2, 5, 6). In addition, Ly6Chi DCs are critical for TH17 polarization (7).
MicroRNAs (miRNAs) are small non-coding RNAs of 17 to 25 nucleotides that regulate gene expression by inducing mRNA degradation or interfering with mRNA translation (8). More than 60% of protein-coding genes are regulated by miRNAs (9). miRNAs regulate various biological processes including immune cell lineage commitment, differentiation, maturation, and maintenance of immune homeostasis (10) and are involved in the pathogenesis of cancer, inflammatory and autoimmune diseases (11).
Expression studies on tissues from MS patients identified miR-223 as one of the top upregulated miRNAs (12, 13). miR-223 expression is mainly confined to myeloid cells (14) has been shown to regulate the proliferation and activation of neutrophils (15) and M1 macrophages (16). Here we demonstrate that miR-223 regulates EAE pathogenesis by controlling mDC-induced activation of pathogenic TH17 cells.
Materials and Methods
Mice
Female WT C57BL/6 and miR-223−/− mice were purchased from The Jackson Laboratory and housed in the Northwestern Center for Comparative Medicine SPF facility. Identical results were obtained using either littermate or wildtype C57BL/6 mice as controls.
Active and Transfer EAE
8-10 week-old female mice were injected s.c. with 100 μl of an emulsion containing 200 μg of M. tuberculosis H37Ra (Difco, Detroit, MI) and 200 μg of MOG35–55 (Genemed, San Francisco, CA) distributed over three sites on the flank. For transfer EAE, spleen and inguinal lymph nodes collected on day 8 post-MOG35-55 were reactivated with 20 μg/ml of MOG35–55 and 10 ng/ml recombinant murine IL-12 (R&D Systems) for 72 h. 20×106 cells were transferred to recipient mice. In both models, 200 ng pertussis toxin (List, Campbell, CA) were administered i.p on days 0 and 2. 2-3 independent experiments were performed, with ≥5 mice per group. Clinical signs of EAE were assessed daily as previously described (2).
Isolation of CNS leukocytes
CNS-immune cells were isolated by Percoll gradient centrifugation from homogenized combined brain and spinal cords and enumerated as previously described (2).
Flow Cytometry Analysis
Fc receptors were blocked using anti-mouse CD16/32 (0.25 μg; eBioscience) and cells were then stained for 30 min at 4°C using the specified antibodies (BD Biosciences or eBioscience). Cytokine expression was determined in activated T cells (18 h with 1 μg/ml ionomycin and 20 ng/ml PMA) and APCs (18 h with 10 ng/ml E. coli 0111:B4 LPS (Sigma) in the presence of 2 μg/ml brefeldin A (Sigma). Cells were acquired on a BD Canto II and analyzed using BD FACSDiva version 6.1.
Th1/Th17 cell differentiation
Purified CD4+ T cells (≥90%) were cultured with CD4− WT or KO splenocytes (as APCs) plus α-CD3 (1 μg/ml) in Th1- (200 U/ml IL-2, 10 ng/ml IL-12, 1 μg/ml anti-IL-4) or Th17- (10 ng/ml TGF-β, 50 ng/ml IL-6, 10 ng/ml IL-23, 1 μg/ml anti-IFN-γ, 1 μg/ml anti-IL-4, 1 μg/ml anti-IL-2) promoting conditions and analyzed for cytokine expression by flow cytometry.
Bone marrow monocyte isolation
CD11b+ Ly6Chi monocytes were purified (>95%) from BM via magnetic cell sorting (Miltenyi).
Quantitative real-time PCR analysis
CD11b+ myeloid cells, B220+ B cells and CD90+ T lymphocytes were purified using magnetic cell sorting (Miltenyi) and gene expression quantification performed as previously described (15).
Statistical analysis
Statistical analyses were performed using GraphPad PRISM 5.0. Single comparisons of two means were analyzed by Student's t-test. For multiparametric data, two-way ANOVA with a Bonferroni post-test was used. p values < 0.05 were considered significant.
Results and Discussion
miR-223 regulates EAE pathology
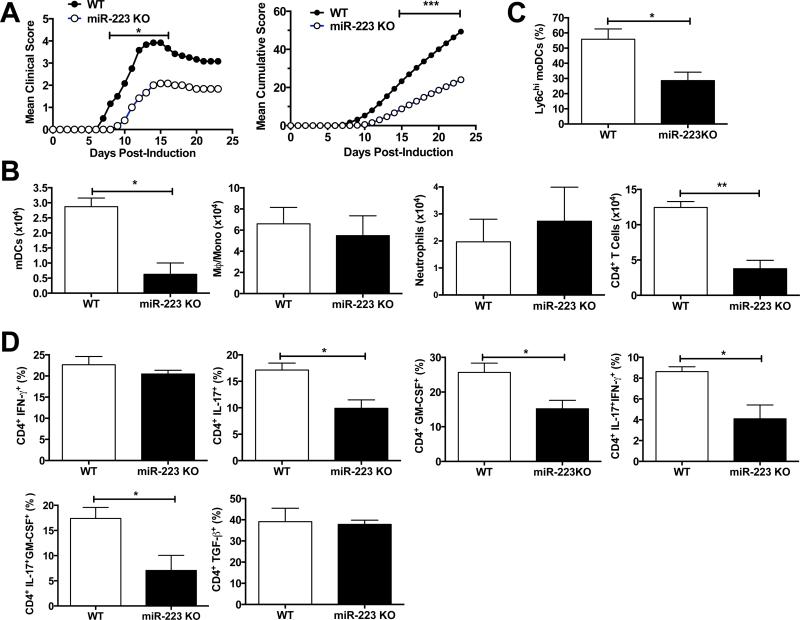
We observed a significant delay of the onset and severity of MOG35-55-induced EAE in miR-223 KO vs. WT mice (Fig. 1A) concomitant with a reduction of CNS infiltration of CD45hiCD11b+Ly6G−CD11c+ mDCs and CD45hiCD11b−CD3+ CD4+ T cells (Fig. 1B). The numbers of macrophages/monocytes, neutrophils, and B cells and pDCs did not differ (Supplemental Fig. S1A). We observed a significant reduction of mo-DCs (Ly6hi) in the CNS of miR-223 KO mice (Fig. 1C) and reduced percentages and numbers of CD4+ IL-17+, CD4+ GM-CSF+, and IL-17+/IFN-γ+ and IL-17+/GM-CSF+ cells (Fig. 1D, Supplemental Fig. S1B) confirming the importance of Ly6Chi mDCs in CNS inflammation (2, 6, 17).
FIGURE 1. Deletion of miR-223 reduces EAE clinical scores and CNS infiltration of mDCs and CD4+ T cells.
EAE was induced in C57BL/6 WT mice and miR-223 KO mice by active immunization with MOG35-55. (A) miR-223 KO mice (○) show a significant reduction in clinical scores as compared to WT animals (●). Mean cumulative scores were calculated as the mean of the sum of daily clinical scorers observed for the duration of the experiment (n = 6 mice per group; *, p < 0.05; ***, p < 0.001). (B) Number of CD45hi CD11b+ Ly6G− CD11c+ mDCs, CD45hi CD11b+ Ly6G− CD11c− macrophages/monocytes, CD45hi CD11b+ Ly6G+ Ly6C+ neutrophils, and CD45hi CD11b− CD3+ CD4+ T cells in the CNS of EAE miR-223 KO mice (black bars), or WT mice (white bars) at day 18 post-immunization (n = 3 mice per group; *, p < 0.05, **, p < 0.01). (C) Proportion of Ly6Chi mo-DCs is reduced in the CNS (brain and spinal cord homogenates) of MOG35-55-immunized miR-223 KO mice (black bar), as compared to WT animals (white bar) at day 18 post-immunization (n = 3 mice per group; *, p < 0.05). (D) Percentages of IL-17, IFN-γ, GM-CSF and TGF-β-expressing CD4+ T cells in the CNS of EAE animals revealed a significant reduction of T cells producing IL-17-related cytokines in miR-223 KO animals (black bars), as compared to WT animals (white bar) at day 18 post-immunization (n = 3 mice per group; *p < 0.05). Data shown are representative of three independent experiments performed on ≥5 mice per group.
miR-223 is expressed in myeloid cells
CD11b+ myeloid cells had the highest basal level of miR-223 expression (Fig. 2A) and expression is upregulated during EAE with comparable levels at disease onset and peak. miR-223 was not expressed in B and T cells. In transfer EAE, disease onset is delayed and severity significantly reduced in miR-223 recipients of WT MOG35-55-specific CD4+ T cells (Fig. 2B). Collectively, these results indicate a critical role for miR-223 in peripheral and CNS myeloid APCs required for EAE initiation. Interestingly, the fact that miR-223 controls astrocyte expression of CXCL12, a chemokine which attracts CD4+ T cells and myeloid cells (18), may provide a potential mechanistic basis for the reduced disease severity and immune cell infiltration observed in KO mice.
FIGURE 2. miR-223 is expressed in myeloid cells and is upregulated during EAE.
(A) CD11b+ myeloid cells, B220+ B cells and CD90+ T cells were isolated from the spleen of naïve WT mice and EAE mice at onset (day 8 post-immunization) and peak of the disease (day 15 post-immunization) and miR-223 gene expression was quantified by real-time PCR. A significant increase of miR-223 gene expression was observed at EAE onset and peak in the myeloid cell compartment (white bars) (n = 3 to 5 mice per time-point; ***, p < 0.001). (B) 20 × 106 MOG35-55-reactivated spleen and lymph nodes collected at day 8 post-immunization of WT C57BL/6 animals with MOG35-55/CFA were injected i.p. into miR-223 KO or WT recipient mice. miR-223 KO mice (○) display a significant reduction in clinical scores as compared to WT animals (●) (***p < 0.001). Data shown are representative of 2 independent experiments performed on ≥5 mice per group.
Reduced TH17 polarizing cytokines in miR-223 KO mDCs
PD-L1, but not PD-L2, expression was significantly higher on mDCs and BM-monocytes (Fig. 3A, Supplemental Fig. S2A) and CD40 and CD80 expression was enhanced on splenic macrophage/monocytes, but not pDCs, of MOG35-55-primed KO mice (Supplemental Fig. S2B). These results are in direct accordance with the fact that miR-223 regulates STAT1 expression (19), which is a crucial regulator of PD-L1 expression on mDCs (20). Spleen mDCs from the KO mice expressed significantly lower levels IL-1β, IL-23p19 and IL-6, but control levels of IL-12p40 and TNF (Fig. 3B and Supplemental Fig. S2C). Splenic macrophages/monocytes from miR-223 KO mice had increased expression of TNF, but there were no differences in cytokine expression in splenic pDCs. These data suggest that mDCs from miR-223 KO have a lower capacity to activate T cells due to the PD-L1 increase and, in particular, TH17 cells due to the decreased expression of TH17-promoting cytokines (IL-1β, IL-6 and IL-23). These findings are particularly interesting in that miR-223 regulates Roquin-1 expression by binding to the 3’ UTR (21). Roquin-1 is an important regulator of expression of TH17-related cytokines and TH17 differentiation (22) and its loss leads to overproduction of IL-6 and increases TH17 cell differentiation. Mice expressing a M199R mutation in the Roquin protein (Roquinsan/san mice) develop autoimmune pathologies (23) and Roquin-1 was shown to regulate innate and inflammatory responses in autoimmunity (24). In addition, miR-223 regulates genes involved in the inflammasome pathway, cytokine-cytokine receptor interactions, and signaling via the TLR, JAK-Stat, FcεRI, NOD-like receptor, and NF-κb pathway, pathways (25). We are currently analyzing these possibilities with an emphasis on Roquin.
FIGURE 3. Splenic mDCs from miR-223 KO mice express reduced levels of TH17 polarizing cytokines during EAE.
At day 16 post MOG35-55 immunization, spleens from miR-223 KO mice and WT mice were homogenized and immune cells were isolated. Cells were gated on CD11b+ B220− CD3− CD11c+ to analyze the profile of mDCs by flow cytometry. (A) Expression levels of PD-L1 and PD-L2 on splenic mDCs of WT mice (left panel) and miR-223 KO mice (right panel) was examined. (B) IL-23p19, IL-1β and IL-6 expression on splenic mDCs are decreased in miR-223 KO mice (right panels) compared to WT mice (left panels). No difference in TNF and TGF-β were observed. Data shown are representative of 6 mice in 2 independent experiments.
miR-223 KO APCs fail to drive differentiation of TH17 cells
Naïve CD4+ T cells from WT mice were cultured with anti-CD3 and CD4− splenic APCs from naïve WT or miR-223 KO mice in both TH1 or TH17 driving conditions and T cell differentiation was assessed at days 1, 3 and 5. In TH1 skewing conditions, although the percentage of IFN-γ+ cells was reduced at day 3 with KO APCs, WT and KO APCs induced equivalent numbers of TH1 cells at day 5 (Fig. 4A). In contrast, KO APCs were significantly less capable of inducing differentiation of both IL-17- and GM-CSF-producing TH17 cells with virtually no GM-CSF, a cytokine reported to be essential in the pathogenic process of EAE (26), produced at day 5. The low levels of IFN-γ observed in TH17 conditions confirm the specific skewing of the TH17 differentiation.
FIGURE 4. APCs from miR-223 KO mice are deficient in driving differentiation of TH17 cells.
CD4+ T cells were purified from spleens of naïve miR-223 KO mice. The cells were co-cultured with CD4− splenic APCs from naïve WT mice or miR-223 KO mice in (A) TH1-promoting [IL-2 (200 U/ml), IL-12 (10 ng/ml), and anti-IL-4 (1 μg/ml)] or (B) TH17-promoting [TGF-β (10 ng/ml), IL-6 (50 ng/ml), IL-23 (10 ng/ml), anti-IL-2, anti-IFN-γ, and anti-IL-4 (1 μg/ml)] conditions. Flow cytometry analyses for IFN-γ, IL-17 and GM-CSF expression by live CD3+ CD4+ T cells was performed after 1, 3 and 5 days of culture. The origin of the APCs had no effect on differentiation of IFN-γ producing T cells in TH1-driving conditions. However, in TH17-driving conditions, CD4+ T cells showed a significant decrease in IL-17 and GM-CSF expression when co-cultured with miR-223 KO APCs, as compared to co-culture with WT APCs. Data shown are representative of 3 independent experiments (*p < 0.05; ***p < 0.001).
Collectively, our results uncover a new role for miR-223 in mDCs and emphasize the important role it plays in the development of CNS inflammation by its ability to regulate induction of TH17 responses by controlling levels of Th17 polarizing cytokines, including IL-1β, IL-6 and IL-23. Additionally, miR-223 may participate in regulation of monocyte differentiation into mo-DCs in inflamed environments. In the absence of miR-223, C57BL/6 mice exhibit significantly lower clinical signs of EAE and associated peripherally-derived CNS-infiltrating inflammatory cells, confirming the importance of this miRNA in the disease pathology and identifying a new potential autoimmune regulatory target.
Supplementary Material
Acknowledgments
Supported by NIH Grants NS-026543 (SDM) and CA149669 (BZ). II was supported by NMSS Postdoc Fellowship FG 2065-A-1.
Abbreviations
- mDC
myeloid dendritic cell
- miRNAs
micro-RNAs
- moDC
monocyte-derived dendritic cell
- PMA
phorbol myristate acid
Footnotes
Disclosures
The authors have no financial conflicts.
References
- 1.Lassmann H. Multiple sclerosis pathology: evolution of pathogenetic concepts. Brain Pathol. 2005;15:217–222. doi: 10.1111/j.1750-3639.2005.tb00523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bailey SL, Schreiner B, McMahon EJ, Miller SD. CNS myeloid DCs presenting endogenous myelin peptides 'preferentially' polarize CD4+ T(H)-17 cells in relapsing EAE. Nat. Immunol. 2007;8:172–180. doi: 10.1038/ni1430. [DOI] [PubMed] [Google Scholar]
- 3.King IL, Dickendesher TL, Segal BM. Circulating Ly-6C+ myeloid precursors migrate to the CNS and play a pathogenic role during autoimmune demyelinating disease. Blood. 2009;113:3190–3197. doi: 10.1182/blood-2008-07-168575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dominguez PM, Ardavin C. Differentiation and function of mouse monocyte-derived dendritic cells in steady state and inflammation. Immunol. Rev. 2010;234:90–104. doi: 10.1111/j.0105-2896.2009.00876.x. [DOI] [PubMed] [Google Scholar]
- 5.McMahon EJ, Bailey SL, Castenada CV, Waldner H, Miller SD. Epitope spreading initiates in the CNS in two mouse models of multiple sclerosis. Nat. Med. 2005;11:335–339. doi: 10.1038/nm1202. [DOI] [PubMed] [Google Scholar]
- 6.Greter M, Heppner FL, Lemos MP, Odermatt BM, Goebels N, Laufer T, Noelle RJ, Becher B. Dendritic cells permit immune invasion of the CNS in an animal model of multiple sclerosis. Nat. Med. 2005;11:328–334. doi: 10.1038/nm1197. [DOI] [PubMed] [Google Scholar]
- 7.Schlitzer A, Sivakamasundari V, Chen J, Sumatoh HR, Schreuder J, Lum J, Malleret B, Zhang S, Larbi A, Zolezzi F, Renia L, Poidinger M, Naik S, Newell EW, Robson P, Ginhoux F. Identification of cDC1- and cDC2-committed DC progenitors reveals early lineage priming at the common DC progenitor stage in the bone marrow. Nat. Immunol. 2015;16:718–728. doi: 10.1038/ni.3200. [DOI] [PubMed] [Google Scholar]
- 8.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 9.Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dai R, Ahmed SA. MicroRNA, a new paradigm for understanding immunoregulation, inflammation, and autoimmune diseases. Transl. Res. 2011;157:163–179. doi: 10.1016/j.trsl.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Connell RM, Rao DS, Baltimore D. microRNA regulation of inflammatory responses. Annu. Rev. Immunol. 2012;30:295–312. doi: 10.1146/annurev-immunol-020711-075013. [DOI] [PubMed] [Google Scholar]
- 12.Keller A, Leidinger P, Lange J, Borries A, Schroers H, Scheffler M, Lenhof HP, Ruprecht K, Meese E. Multiple sclerosis: microRNA expression profiles accurately differentiate patients with relapsing-remitting disease from healthy controls. PloS One. 2009;4:e7440. doi: 10.1371/journal.pone.0007440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Junker A, Krumbholz M, Eisele S, Mohan H, Augstein F, Bittner R, Lassmann H, Wekerle H, Hohlfeld R, Meinl E. MicroRNA profiling of multiple sclerosis lesions identifies modulators of the regulatory protein CD47. Brain. 2009;132:3342–3352. doi: 10.1093/brain/awp300. [DOI] [PubMed] [Google Scholar]
- 14.Fukao T, Fukuda Y, Kiga K, Sharif J, Hino K, Enomoto Y, Kawamura A, Nakamura K, Takeuchi T, Tanabe M. An evolutionarily conserved mechanism for microRNA-223 expression revealed by microRNA gene profiling. Cell. 2007;129:617–631. doi: 10.1016/j.cell.2007.02.048. [DOI] [PubMed] [Google Scholar]
- 15.Johnnidis JB, Harris MH, Wheeler RT, Stehling-Sun S, Lam MH, Kirak O, Brummelkamp TR, Fleming MD, Camargo FD. Regulation of progenitor cell proliferation and granulocyte function by microRNA-223. Nature. 2008;451:1125–1129. doi: 10.1038/nature06607. [DOI] [PubMed] [Google Scholar]
- 16.Zhuang G, Meng C, Guo X, Cheruku PS, Shi L, Xu H, Li H, Wang G, Evans AR, Safe S, Wu C, Zhou B. A novel regulator of macrophage activation: miR-223 in obesity-associated adipose tissue inflammation. Circulation. 2012;125:2892–2903. doi: 10.1161/CIRCULATIONAHA.111.087817. [DOI] [PubMed] [Google Scholar]
- 17.Mildner A, Mack M, Schmidt H, Bruck W, Djukic M, Zabel MD, Hille A, Priller J, Prinz M. CCR2+Ly-6Chi monocytes are crucial for the effector phase of autoimmunity in the central nervous system. Brain. 2009;132:2487–2500. doi: 10.1093/brain/awp144. [DOI] [PubMed] [Google Scholar]
- 18.Shin JH, Park YM, Kim DH, Moon GJ, Bang OY, Ohn T, Kim HH. Ischemic brain extract increases SDF-1 expression in astrocytes through the CXCR2/miR-223/miR-27b pathway. Biochim Biophys Acta. 2014;1839:826–836. doi: 10.1016/j.bbagrm.2014.06.019. [DOI] [PubMed] [Google Scholar]
- 19.Moles R, Bellon M, Nicot C. STAT1: a novel target of miR-150 and miR-223 Is involved in the proliferation of HTLV-I-transformed and ATL cells. Neoplasia. 2015;17:449–462. doi: 10.1016/j.neo.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loke P, Allison JP. PD-L1 and PD-L2 are differentially regulated by Th1 and Th2 cells. Proc. Natl. Acad. Sci. USA. 2003;100:5336–5341. doi: 10.1073/pnas.0931259100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schaefer JS, Montufar-Solis D, Vigneswaran N, Klein JR. Selective upregulation of microRNA expression in peripheral blood leukocytes in IL-10−/− mice precedes expression in the colon. J. Immunol. 2011;187:5834–5841. doi: 10.4049/jimmunol.1100922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeltsch KM, Hu D, Brenner S, Zoller J, Heinz GA, Nagel D, Vogel KU, Rehage N, Warth SC, Edelmann SL, Gloury R, Martin N, Lohs C, Lech M, Stehklein JE, Geerlof A, Kremmer E, Weber A, Anders HJ, Schmitz I, Schmidt-Supprian M, Fu M, Holtmann H, Krappmann D, Ruland J, Kallies A, Heikenwalder M, Heissmeyer V. Cleavage of roquin and regnase-1 by the paracaspase MALT1 releases their cooperatively repressed targets to promote T(H)17 differentiation. Nat. Immunol. 2014;15:1079–1089. doi: 10.1038/ni.3008. [DOI] [PubMed] [Google Scholar]
- 23.Vinuesa CG, Cook MC, Angelucci C, Athanasopoulos V, Rui L, Hill KM, Yu D, Domaschenz H, Whittle B, Lambe T, Roberts IS, Copley RR, Bell JI, Cornall RJ, Goodnow CC. A RING-type ubiquitin ligase family member required to repress follicular helper T cells and autoimmunity. Nature. 2005;435:452–458. doi: 10.1038/nature03555. [DOI] [PubMed] [Google Scholar]
- 24.Pratama A, Ramiscal RR, Silva DG, Das SK, Athanasopoulos V, Fitch J, Botelho NK, Chang PP, Hu X, Hogan JJ, Mana P, Bernal D, Korner H, Yu D, Goodnow CC, Cook MC, Vinuesa CG. Roquin-2 shares functions with its paralog Roquin-1 in the repression of mRNAs controlling T follicular helper cells and systemic inflammation. Immunity. 2013;38:669–680. doi: 10.1016/j.immuni.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 25.Baek D, Villen J, Shin C, Camargo FD, Gygi SP, Bartel DP. The impact of microRNAs on protein output. Nature. 2008;455:64–71. doi: 10.1038/nature07242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Codarri L, Gyulveszi G, Tosevski V, Hesske L, Fontana A, Magnenat L, Suter T, Becher B. RORgammat drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat. Immunol. 2011;12:560–567. doi: 10.1038/ni.2027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.