Abstract
Nicotine is a major psychoactive and addictive component of tobacco. Although cessation of tobacco use produces various somatic and affective symptoms, withdrawal-related cognitive deficits are considered to be a critical symptom that predict relapse. Therefore, delineating the cognitive mechanisms of nicotine withdrawal may likely provide gainful insights into the neurobiology of nicotine addiction. The present study was designed to examine the effects of nicotine withdrawal induced by mecamylamine, a non-specific nicotinic receptor (nAChR) antagonist, on cognitive control processes in mice using an operant strategy switching task. Brain-derived neurotrophic factor (BDNF) modulates synaptic transmission in frontostriatal circuits, and these circuits are critical for executive functions. Thus, we examined the effects of mecamylamine-precipitated nicotine withdrawal on prefrontal and striatal BDNF protein expression. Mice undergoing precipitated nicotine withdrawal required more trials to attain strategy switching criterion as compared to the controls. Error analysis indicated that impaired performance in these animals was mostly related to their inability to execute the new strategy. The striatal/prefrontal BDNF ratios robustly increased following precipitated nicotine withdrawal. Moreover, higher BDNF ratios were associated with longer task acquisition. Collectively, our findings illustrate that mecamylamine-induced nicotine withdrawal disrupts cognitive control processes and that these changes are possibly linked to perturbations in frontostriatal BDNF signaling.
Keywords: nicotine withdrawal, addiction, cognitive flexibility, BDNF, mice
1. Introduction
Nicotine addiction is a global health problem and smoking-related illness reigns atop the causes of preventable death worldwide. Even though chronic nicotine exerts very little positive effects on mood and motor/cognitive performance as opposed to other drugs of abuse (Risner and Goldberg, 1983; Epping-Jordan et al., 1998), smokers continue to consume cigarettes presumably to alleviate unpleasant withdrawal-related physiological/affective symptoms and to restore activity in brain reward pathways (Miyata and Yanagita, 2001; Johnson et al., 2008). Despite the availability of treatments for smoking cessation that mostly focus on normalizing the reward function and motivational/affective components of nicotine addiction, relapse to smoking after quit attempts still remains very high (Gonzales et al., 2006; Hughes et al., 2008). Because of the extensive overlap between cognitive and reward-/motivation-related brain processes, chronic drug-induced neuroadaptive changes and possible interactions between these processes are proposed to underlie compulsive drug use (Everitt et al., 2008; Gould, 2010). Moreover, nicotine withdrawal-related cognitive deficits are hypothesized to predict relapse (Ashare et al., 2014). Therefore, delineation of cognitive mechanisms that determine higher rates of relapse during nicotine withdrawal is likely to provide gainful insights into the neurobiology of nicotine addiction.
Loss of cognitive control in drug addicts is primarily manifested as the inability to change responding to stimuli previously associated with drug stimulus or reward, and deficits in cognitive flexibility are critical in triggering drug craving and relapse (Stalnaker et al., 2009; Volkow et al., 2010). Although the effects of nicotine withdrawal on reward/motivation and contextual learning are well studied, how it affects cognitive flexibility and what cellular mechanisms are responsible for the effects are not known. Brain-derived neurotrophic factor (BDNF) plays an important role in activity-dependent regulation of synaptic function, cognition, affect and conditioned reward (Chao, 2003, Nestler and Carlezon, 2006, Lu et al., 2008). BDNF gene polymorphism has been linked to nicotine dependence (Lee et al., 2015). Moreover, frontostriatal circuits involving discrete regions of the prefrontal cortex (PFC) and dorsal striatum are implicated in decision-making (Ragozzino et al., 2007; Balleine et al., 2007) and BDNF regulates corticostriatal synaptic plasticity and cognitive flexibility by activating it cognate receptor, tyrosine kinase B (trkB) (D'Amore et al., 2013; Jia et al., 2010). The present study was designed to assess the effects of nicotine withdrawal on cognitive control processes using an operant strategy switching paradigm in mice. Moreover, we also determined whether alterations in strategy-based decision processes during nicotine withdrawal are tied to changes in prefrontal and striatal BDNF protein levels.
2. Experimental Procedures
2.1. Subjects
Male C57BL/6J mice (8-10 weeks; 20-25g) were purchased from Jackson Laboratories (Bar Harbor, ME). Animals were individually housed in a temperature/humidity-controlled environment with a 12-h light/dark cycle (07:00 lights on). Mice were progressively water-restricted to 5 min of water/day. Operant training was conducted 7 days/week between 9:00 and 16:00 h. Food pellets (PMI LabDiet) were available ad libitum during the experiment. All experimental procedures were authorized by the Institutional Care and Use Committee (IACUC) of Temple University and complied with regulations from the National Institute of Health.
2.2. Operant training procedure
Mice were trained in an operant cognitive flexibility task using standard mouse chambers (Med Associates) as described previously in our studies (Ortega et al., 2013, Cole et al., 2015; D’Amore et al., 2015). Briefly, animals were autoshaped on a FR-1 schedule of reinforcement to acquire lever press responses and subsequent reinforcement of reward (10μl of 0.066% saccharin solution). The animals were then advanced to the pretraining phase. A session began with the illumination of houselight. After an inter-trial interval (ITI) of 9±3 s, a lever (either left or right) was presented and remained active for 10 s or until a lever press response occurred. Lever presentations were completely randomized with no more than 5 activations from the same side. To control for any novelty effects associated with the visual stimulus during later phases of training, trials were randomly associated with unpredictably occurring visual cues (presented only in 50% of trials) that involved illumination of the panel light above the lever. A lever press on the cued trials co-terminated both the visual cue and the lever. Animals that reached pretraining criterion (30 rewards and <20% omissions) were then implanted with mini-osmotic pumps for chronic drug administration (see section 2.3).
After recovery and following retention of performance, the animals were held on the pretraining phase for two weeks. Mice then progressed to the visual discrimination phase which required making a correct choice by responding to the lever paired with the visual cue light. A trial started with the illumination of a 7s visual cue either from the left or right panel (pseudorandomized sequence across trials), followed by the presentation of both levers 2s later. Both the stimulus light and levers co-terminated together. A lever press response on the cued lever was scored as a “correct response” and was followed by reward (sweetened water) delivery. Responses on the incorrect lever (errors) were not rewarded and resulted in a “time out” (punishment) period characterized by a 10-s extinguishing of the house light. Punishment on incorrect responses was introduced to discourage indiscriminate responding to levers. Following the completion of the punishment phase, the house light was turned “on” and the ITI (9±3s) was reinstated. Failure to respond to any of the levers resulted in omissions. Animals were required to exhibit ≥80% correct responses and <20% omissions for 3 consecutive days to attain criterion following which they were advanced to the testing on strategy switching phase. The experimental parameters for strategy-shifting phase were identical to the previous stage except that the contingencies were altered in such a way that the animals were required to eliminate a visual cue-based strategy and adopt a new spatial response strategy to achieve rewards. Mice were required to press the correct lever (either left or right) to earn a reward irrespective of visual cue presentation, which remained random. Responding on an incorrect lever resulted in an incorrect response (set-shift error) and led to the initiation of the timeout phase. Half of the animals were trained with the reverse set of rules. Performance criterion was defined as ≥80% correct responses and <20% omissions for three consecutive sessions. Each behavioral session for both the visual discrimination phase and the strategy switching phase consisted of 30 trials/day.
The number of correct responses, errors, omissions, response latencies and reward retrieval latencies was obtained for each behavioral session. The total number of performed trials to criterion, errors to criterion and omissions were obtained for each training phase using the above described criteria. Response accuracies were calculated for each session using the formula: correct responses/(correct+incorrect responses)*100. Strategy shifting performance was characterized by distinguishing whether an incorrect response occurred due to the perseverance of a previously learned strategy or failure to acquire/maintain a new strategy. For strategy switching performance, errors were classified as perseverative, regressive and never-reinforced based on criterion reported in previous studies (Cole et al., 2015; D'Amore et al., 2013; Haluk and Floresco, 2009). A perseverative error occurred if the animal responded to the incorrect lever when the visual cue was illuminated above it on ≥ 60% of trials within a session. This is indicative of perseverance to the previously learned strategy. Depending on the training performance in the preceding session, an error was scored as a regressive error if the animal made < 60% incorrect responses on the cue-associated lever in subsequent sessions. At this point, the animals were making fewer errors and are considered to be inhibiting the previously learned strategy and executing the new strategy. Never-reinforced errors occur if an animal responded on the incorrect lever while the visual cue was presented from the opposite side. Both regressive and never-reinforced errors were categorized as “learning errors” as they reflected an index of the acquisition/execution of a new strategy.
2.3. Chronic nicotine administration, induction of withdrawal and experimental design
Mini-osmotic pumps (model 1004; DURECT Corporation, Cupertino, CA) were implanted subcutaneously under isoflurane anesthesia in mice to deliver either saline (control) or nicotine (18mg/kg/day; free base) for 4 weeks (refer to Supplementary Materials for surgery details). After recovery and following retention of presurgery performance, the animals were held on the pretraining phase until 2 weeks and then progressed to the visual discrimination phase. As the acquisition of visual discrimination typically requires 4-7 training sessions, the animals were kept on this phase of the task for a maximum period of 1 week and then split into 4 treatment groups before the assessment of strategy switching performance (see Fig. 1 for Experimental Design).
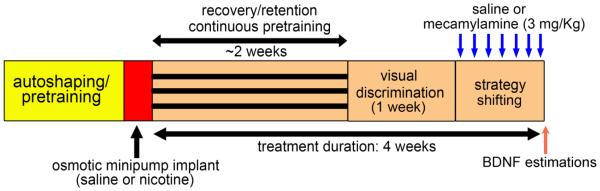
Figure 1.
Schematic representation of the experimental design. Mice were initially autoshaped and then pretrained to press a lever within allotted time to receive reinforcement (refer Methods for details). Animals that reached pretraining criterion were implanted with subcutaneous osmotic minipumps (Alzet, Model #1004) to administer chronic nicotine (18mg/kg/day) or saline for 28 days. Starting day 22, the nicotine withdrawal group received a subcutaneous injection of mecamylamine, a non-specific nicotinic receptor antagonist (3mg/kg), 20 min. before testing in the strategy shifting phase for 7 days. Nicotine control group received saline injections prior to testing. Similar procedure was adopted for chronic saline animals; these animals were challenged with either saline or mecamylamine on testing days. BDNF estimations were conducted in the prefrontal and striatal tissues using ELISA.
Systemic administration of mecamylamine, a non-specific nAChR antagonist, in nicotine-treated rodents is a well-established model to produce somatic signs of nicotine withdrawal (Damaj et al., 2003, Salas et al., 2004) as well as withdrawal-related decreases in brain reward function typically observed in abstinent smokers (Watkins et al., 2000, Miyata et al., 2011, Hilario et al., 2012). Another advantage of using this model is that induction of withdrawal symptoms could be specifically timed prior to the onset of behavioral session. Moreover, withdrawal symptoms could be induced over multiple behavioral sessions during the acquisition of strategy switching. Therefore, the mecamylamine-precipitated nicotine withdrawal model was used in our study. Starting on day 22, the nicotine withdrawal group received a daily subcutaneous injection of mecamylamine, (3mg/kg), 20-min prior to task onset for one week (nic-mec; n=12). The dose of mecamylamine used to precipitate withdrawal was based on previous studies (Damaj et al., 2003; Salas et al., 2004). The nicotine control group received saline injections prior to testing for the same duration (nic-sal; n=7). Likewise, chronic saline-treated animals received either a saline (sal-sal; n=8) or mecamylamine (sal-mec; n=12) challenge. The animals were monitored for somatic signs of withdrawal on the first and seventh day of the saline or mecamylamine injection (see Supplementary Information). Most of the animals attained strategy shifting criterion within 7 days. Animals that did reach criterion by this time continued on task for 2 additional days.
2.4. BDNF ELISA
All mice were decapitated following the completion of last behavioral session and brains were removed rapidly. Tissues from the PFC and striatum (both dorsal and ventral) from the same animals were dissected on ice and pooled from both hemispheres. Samples were homogenized in 1 mL of ice-cold buffer HEPES NaOH buffer containing a protease inhibitor cocktail (1.0 μg/ml leupeptin, 1.0 μg/ml aprotinin, 1.0 μg/ml pepstatin and 250 μg/ml phenylmethylsulfonyl fluoride). Homogenates were stored on ice for 30 min and centrifuged for 15 min at 13,000 g to precipitate tissue lysates. Pellets and supernatants were separated and stored at −80°C until further analysis. A mouse BDNF ELISA kit (IBL-America, Minneapolis, MN) was used to quantify BDNF levels in the tissue samples as per manufacture recommendations. Protein estimations were conducted using BCA protein assay kit (Pierce Biotechnology Inc., Waltham, MA). BDNF values were calculated as pg/mg protein and expressed as percent change with respect to the control (sal-sal) group.
2.5. Statistical analysis
Statistical analysis was performed with SPSS/PC+V21.0 (IBM SPSS Software, Armonk, NY). Data for trials to criterion, errors to criterion, error types, omissions, correct and incorrect response latencies, and BDNF levels were analyzed using one-way ANOVAs determined a priori. Learning analyses were conducted by comparing response accuracies using mixed-factor repeated measures ANOVA. Post hoc tests were applied where necessary using Bonferroni corrections for multiple comparisons. Pearson’s correlation coefficient was calculated for all correlative analyses. Values of p<0.05 were considered significant.
3. Results
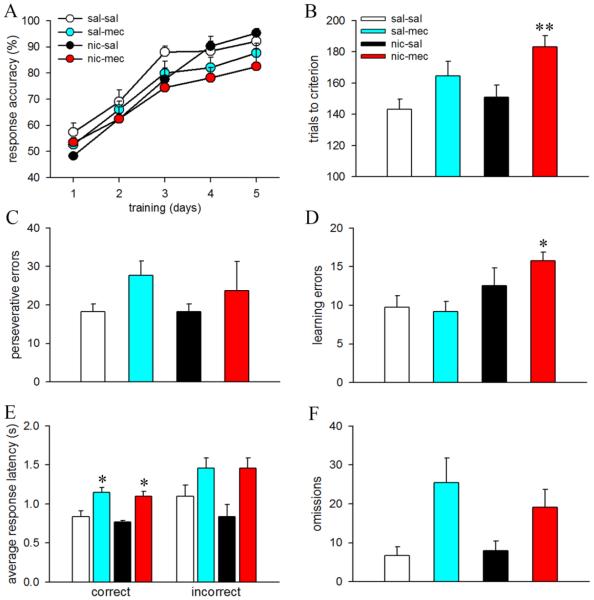
Visual discrimination learning remained similar between animals assigned to either the saline- or mecamylamine-challenge groups (see Supplementary Results). Mecamylamine-challenge robustly increased somatic symptoms reported to associated with nicotine withdrawal and remained persistent through the duration of the strategy shift (F3,35=21.44; p<0.001, η2=.65; see Supplementary Results). The main results on strategy switching performance are summarized in Fig. 2. The rate of task acquisition was analyzed by comparing response accuracy for the first five behavioral sessions across all treatment groups. Mixed factor ANOVAs for this behavioral measure show a session-dependent learning effect (F4,140=88.69; p<0.001, η2=.72; Fig. 2A). Although, response accuracies in nic-mec animals appear to be lower illustrating slower task acquisition in these animals (Fig 2A), ANOVA measure failed to detect a significant difference between groups (F3,35=1.72, p=0.18) as well as the session × group interaction (F12,140=1.17, p=0.31), plausibly due to high variability in the data. The data on trials, errors, response latencies and omissions are presented as cumulative values over multiple behavioral sessions. Statistical comparisons show significant group differences in the trials to criterion during the strategy switching phase (F3,35=6.06, p=0.002,η2=.34). Post hoc analysis revealed that animals that underwent mecamylamine-precipitated withdrawal required more trials to attain criterion performance (p=0.002 vs sal-sal; Fig. 2B). Trials to criterion in the sal-mec and nic-sal groups remained comparable to the sal-sal group (both p’s>0.26).
Figure 2.
Effect of mecamylamine-precipitated withdrawal on cognitive flexibility. Data are Mean ± SEM.
(A) Response accuracies depicting session-dependent learning in animals performing the strategy switching task. (B) Nicotine-treated mice challenged with mecamylamine (nic-mec) required more trials to reach criterion. Trials to criterion for sal-mec and nic-sal mice remained similar to the sal-sal mice. Bar charts depicting perseverative errors (C) and learning errors (D) in all treatment groups. Nic-mec mice committed more learning errors. (E) Both sal-mec and nic-mec mice exhibited higher latencies for correct responses. (F) Total omission during the strategy switching phase of the task. *, ** p < 0.05, 0.01 vs. saline treated mice challenged with saline (sal-sal).
Error analysis show similar perseverative errors across groups (F3,28=1.34, p=0.28; Fig 2C). However, significant group differences were observed in learning errors (F3,35=4.77, p=0.01, η2=.29). Group comparisons show that nic-mec animals committed more learning errors as compared to the sal-sal group (p=0.04; Fig. 2D). Learning errors remained similar between sal-mec/nic-sal and the control animals (both p=1.0). Response latencies differed significantly between treatment groups (correct responses: F3,35=7.32, p<0.001, η2= .39; incorrect responses: F3,35=3.96, p=0.02). Multiple comparisons show higher reaction times for correct responses in both sal-mec and nic-mec groups as compared to controls (p=0.02 and p=0.04 respectively; Fig. 2E). Although incorrect response latencies also remained higher for these treatment groups, the effect did not reach significance (both p=0.53 vs. sal-sal mice). Interestingly, when examining omissions during strategy-shifting we uncovered a significant effect of group (F3,35=3.10, p=0.04, η2=.21). Subsequent post hoc analysis indicated a trend for higher omitted trials in the sal-mec group (p=0.08 vs sal-sal). Omissions for the nic-sal and nic-mec groups remained comparable to the control group (both p>0.57).
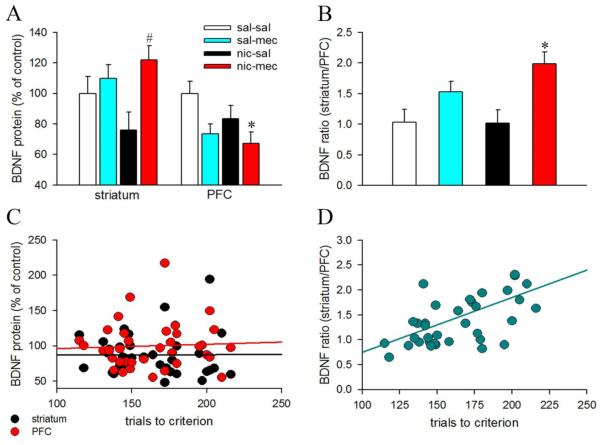
BDNF concentration estimated using ELISA varied between 312.28 – 1227.89 pg/mg protein for the striatum and 227.72 – 734.55 pg/mg protein for the PFC, respectively. Group comparisons show significant alterations in BDNF protein expression with drug treatment in both brain regions (striatum: F3,34=3.26, p=0.03, η2=.22; PFC: F3,33=3.41, p=0.03, η2=.24). However, post hoc comparisons did not reveal significant changes in striatal BDNF levels of sal-mec/nic-sal/nic-mec mice vs. sal-sal animals (all p>0.46; Fig. 3A) presumably due to higher variability in the data. Interestingly, striatal BDNF change in nic-sal mice remained significantly lower than the nic-mec animals (p=0.02). Our results do not concord with a previous study by Varani and colleagues (2014) that reported lower BDNF in the dorsal striatum of mecamylamine-precipitated withdrawal mice. This inconsistency may either be due to different duration of nicotine exposure prior to mecamylamine challenge (7 days vs. 21 days) or different methodologies to detect striatal BDNF levels (immunohistochemistry vs. ELISA) used between the two studies. Multiple comparisons show that mecamylamine treatment in nicotine-exposed mice robustly reduced prefrontal BDNF expression p=0.03 vs. sal-sal). Prefrontal BDNF levels did not differ in the sal-mec and nic-sal groups (both p>0.09 vs sal-sal). BDNF is anterogradely transported from cortical neurons to the striatum, and BDNF pools in the dorsal striatum are primarily present in the corticostriatal afferents (Altar et al., 1997). Moreover, cortical activity-dependent release of BDNF in the dorsal striatum facilitated long-term potentiation (Jia et al., 2010). Because, frontostriatal circuits are recruited during behavioral flexibility (Ragozzino et al., 2007), and striatal BDNF modulates strategy switching (D’Amore et al., 2013), the distribution of BDNF in the striatum and PFC may be associated with alterations in cognitive flexibility. Therefore, we calculated the ratios of striatal/prefrontal BDNF in all animals. As expected, we noted a robust increase in the striatuml/PFC BDNF ratios in the nic-mec mice as compared to the saline control animals (p=0.01; Fig 3B). Mecamylamine treatment per se did not affect the striatum/PFC BDNF ratios (p=0.43). Fig. 3C and 3D illustrates population data for strategy switching performance from all groups and how it varied with respect to changes in the striatal and prefrontal BDNF levels and striatal/PFC BDNF ratios. The acquisition performance did not correlate with either prefrontal or striatal BDNF levels (both p’s>0.18; Fig 3C). However, striatal/prefrontal BDNF ratios positively correlated with the trials to attain strategy shifting criterion (r=0.43, p=0.01; Fig 3D).
Figure 3.
Nicotine withdrawal and frontostriatal BDNF. Data are Mean ± SEM. All BDNF values were expressed as percent change with respect to the control (sal-sal) group. Bar charts depicting changes in the BDNF levels in the striatum and PFC (A) and the striatal/prefrontal ratios (B), across all treatment groups. Striatal BDNF levels were higher in the mecamylamine precipitated nicotine withdrawal group (nic-mec) as compared to the nicotine control group (nic-sal). Prefrontal BDNF levels declined in nic-mec mice. Moreover, the ratio of striatal to prefrontal BDNF levels robustly increased in nic-mec animals. (C) Scatter plots show no association between the strategy switching performance and either the striatal or prefrontal BDNF levels. (D) Striatal/prefrontal BDNF ratios exhibited a significant positive correlation with trial to attain strategy switching criterion. Higher ratios illustrate poor performance. *, p < 0.05 vs. sal-sal; #, p <0.05 vs. nic-sal
4. Discussion
In the present study, mecamylamine-precipitated nicotine withdrawal hampered strategy set-shifting in mice. These cognitive deficits were mostly related to the animals’ inability to execute a new learning strategy manifested as increases in learning errors. Previous studies on abstinent smokers reflected deficits in response inhibition in various tasks such as the stop signal task, go/no-go task, and the continuous performance task (Harrison et al., 2009, Kozink et al., 2010, Ashare and Hawk, 2012). However, we did not observe any increases in perseverative responding to the previously reinforced lever in the withdrawal animals. If the deficits in strategy switching occurred due to a response inhibition mechanism, we would have seen an increase in both perseverative and learning errors. One possibility that we may not rule out is that the activation of inhibitory control mechanisms under conflicting conditions are task-specific and these mechanisms are not disrupted by nicotine withdrawal in our cognitive flexibility task that mostly engages attentional set-shifting functions as a component process.
Increases in learning errors in nicotine withdrawal animals indicate that these animals inappropriately focused attention to environmental cues that were not been consistently paired with the reward. This might suggest that learning abilities required to adopt or execute an optimal decision strategy under conditions of stimulus-response conflicts were impacted in these animals. Nicotine withdrawal has been demonstrated to disrupt hippocampus-dependent learning processes such as contextual and trace fear conditioning (Davis et al., 2005, Raybuck and Gould, 2009), and incidental learning of spatial information (Kenney et al., 2011). Moreover, attentional deficits have also been reported during nicotine withdrawal in rats performing the 5-choice serial reaction time task (Semenova et al., 2007; Shoaib and Bizarro, 2005).Thus, our findings that mecamylamine-precipitated withdrawal affects decision-making processes are in line with previous work indicating that multiple cognitive domains are affected during nicotine withdrawal. Extinction studies employing nicotine self-administration paradigm have shown that withdrawal may contribute to nicotine seeking during early abstinence and that removal of nicotine associated cues facilitate extinction to nicotine seeking (Cohen et al., 2005; O’Dell et al., 2007; Harris et al., 2011). Whether deficits in cognitive flexibility predict abstinence-induced nicotine craving and relapse remains to be investigated.
Consistent with our previous findings (Ortega et al., 2013, Cole et al., 2015), we did not observe any effects of chronic nicotine (nic-sal group) on strategy switching. Likewise, chronic nicotine did not affect contextual fear memory and instrumental learning (Davis et al., 2005; Leach et al., 2013). Prior studies reported that chronic nicotine may improve attentional performance (Semenova et al., 2007). However, as noted previously, withdrawal from chronic nicotine impaired these cognitive processes. Therefore, the behavioral effects of chronic nicotine and nicotine withdrawal appears to be distinct and possibly engage different cellular mechanisms that may linked to different activation and desensitization states of nAChRs (Picciotto et al., 2008; Gould et al., 2014).
We noted increased reaction times in both sal-mec and nic-mec animals. Our response latency data are in line with a previous study that reported slower reaction times with mecamylamine in non-human primates performing a visual-spatial associative memory task (Katner et al., 2004). Striatal nAChRs are critical to maintain motor behavior (Livingstone and Wonnacott, 2009). Therefore, it is possible that higher response latencies observed in sal-mec and nic-mec groups may just represent a mecamylamine effect and is related to motor dysfunction presumably due to striatal nAChR antagonism. Mecamylamine treatment per se increased omissions during strategy switching in our study. Activation of nAChRs has been shown to benefit attention (Newhouse et al., 2004; Howe et al., 2010). Moreover, prior studies reported that higher omissions observed in mecamylamine treated rodents may indicate impairments in sensory and attentional processing (Newman and Mair, 2007; Turchi et al., 1995). Additionally, nAChRs are also implicated in regulating reward-motivated behavior (Lof et al., 2010) and it is possible that sal-mec animals omitted more trials due to reduced motivation to perform the task. Taken together, our data indicate that central nAChR function may be critical for attentional, sensorimotor and reward processing. However, the effects of mecamylamine on these processes appear to be moderate and not sufficient to affect the strategy switching performance in sal-mec mice.
Our findings that striatal/prefrontal BDNF ratios increased in nic-mec indicate a possible association between mecamylamine-precipitated nicotine withdrawal and frontostriatal BDNF. Although lower prefrontal BDNF noted in the nic-mec group would explain higher striatal/prefrontal ratios in these animals, trials required to acquire strategy shifting criterion did not exhibit a positive correlation either with prefrontal or striatal BDNF levels individually, but only with the regional ratios. This finding further illustrates that perhaps a balance between prefrontal and striatal BDNF levels is critical for the maintenance of cognitive control processes. We previously found that exogenous BDNF infusion into the dorsal striatum modulated strategy shifting in an inverted-U fashion (D’Amore et al., 2013). Moreover, genetic overexpression of the mature form of BDNF impaired learning and memory (Cunha et al., 2009; Papaleo et al., 2011), suggesting that sustained activation of BDNF-trkB signaling may exert detrimental effects on cognitive abilities. As noted above, major pools of BDNF reside in the corticostriatal afferents and this neurotrophin is secreted from the presynaptic terminals in an activity dependent fashion (Altar et al., 1997; Kohara et al., 2001). Therefore, it is possible that nicotine withdrawal-related maladaptive frontostriatal activation may produce enhanced anterograde transport and secretion of BDNF from the PFC to the dorsal striatum leading to impairments in adaptive learning and decision processes.
We previously reported reduced BDNF levels in the dorsal striatum following chronic nicotine exposure (Ortega et al., 2013). Although striatal BDNF levels remained more variable in nicotine-treated animals (lower BDNF in nic-sal vs nic-mec; see Results), chronic nicotine per se did not produce a significant decrease in striatal BDNF. There could be two potential explanations for this disparity. First, the striatal tissue isolated in the present study constituted regions from both the dorsal striatum and nucleus accumbens. It is possible that chronic nicotine reduces BDNF levels specifically in the dorsal striatum and the effects might have been diluted in the whole striatum. Second, chronic nicotine-induced alterations in striatal BDNF observed in performing mice may be task-related. In this context, it is important to note that nicotine-treated mice were performing a reversal learning task in our previous study when tissues were extracted for BDNF analysis which is different from the current study where the animals were performing the strategy shifting task.
To conclude, our data adds new information to the current literature that mecamylamine-precipitated nicotine withdrawal affects decision-making processes and that deficits are not related to impairments in behavioral inhibition but rather due to an inability to execute new learning strategies under conditions of stimulus-response conflicts. It remains to be seen whether similar cognitive mechanisms account for decision-making deficits in a spontaneous nicotine withdrawal model that mimics human abstinence syndrome in smokers. Strategy switching deficits observed in precipitated withdrawal animals may possibly be linked to perturbations in frontostriatal BDNF signaling. Future research will address whether downregulation of prefrontal BDNF during nicotine abstinence occur as a compensatory response to balance BDNF signaling in discrete striatal regions and plausibly corticolimbic circuits, and whether these neuroadapations are manifested as disturbances in affective homeostasis leading to executive deficits. As chronic nicotine desensitizes nAChRs, whether alterations in the activation/desensitization states of nAChRs account for BDNF-dependent plastic changes in the frontostriatal circuits and cognitive control processes also needs to be investigated.
Supplementary Material
Highlights.
Nicotine withdrawal impaired strategy switching in mice.
Animals undergoing nicotine withdrawal committed higher learning errors.
The striatal/prefrontal BDNF ratios increased during nicotine withdrawal.
Higher BDNF ratios were associated with longer task acquisition.
Cognitive control deficits in nicotine withdrawal may relate to perturbations in frontostriatal BDNF signaling.
Acknowledgements
This research was supported by the Brain and Behavior Research Foundation (V.P.) and partly by grants from the National Institute of Health (NIH) DA 037421 (V.P. and T.J.G.) and DA 017949 (T.J.G.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altar CA, Cai N, Bliven T, Juhasz M, Conner JM, Acheson AL, Lindsay RM, Wiegand SJ. Anterograde transport of brain-derived neurotrophic factor and its role in the brain. Nature. 1997;389:856–860. doi: 10.1038/39885. [DOI] [PubMed] [Google Scholar]
- Ashare RL, Falcone M, Lerman C. Cognitive function during nicotine withdrawal: Implications for nicotine dependence treatment. Neuropharmacology. 2014;76:581–591. doi: 10.1016/j.neuropharm.2013.04.034. Pt B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashare RL, Hawk LW., Jr. Effects of smoking abstinence on impulsive behavior among smokers high and low in ADHD-like symptoms. Psychopharmacology. 2012;219:537–547. doi: 10.1007/s00213-011-2324-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine BW, Delgado MR, Hikosaka O. The role of the dorsal striatum in reward and decision-making. J Neurosci. 2007;27:8161–8165. doi: 10.1523/JNEUROSCI.1554-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao MV. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nature Reviews Neuroscience. 2003;4:299–309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- Cohen C, Perrault G, Griebel G, Soubrie P. Nicotine-associated cues maintain nicotine-seeking behavior in rats several weeks after nicotine withdrawal: reversal by the cannabinoid (CB1) receptor antagonist, rimonabant (SR141716) Neuropsychopharmacology. 2005;30:145–155. doi: 10.1038/sj.npp.1300541. [DOI] [PubMed] [Google Scholar]
- Cole RD, Poole RL, Guzman DM, Gould TJ, Parikh V. Contributions of beta2 subunit-containing nAChRs to chronic nicotine-induced alterations in cognitive flexibility in mice. Psychopharmacology. 2015;232:1207–1217. doi: 10.1007/s00213-014-3754-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha C, Angelucci A, D'Antoni A, Dobrossy MD, Dunnett SB, Berardi N, Brambilla R. Brain-derived neurotrophic factor (BDNF) overexpression in the forebrain results in learning and memory impairments. Neurobiology of Disease. 2009;33:358–368. doi: 10.1016/j.nbd.2008.11.004. [DOI] [PubMed] [Google Scholar]
- D'Amore DE, Tracy BA, Parikh V. Exogenous BDNF facilitates strategy set-shifting by modulating glutamate dynamics in the dorsal striatum. Neuropharmacology. 2013;75:312–323. doi: 10.1016/j.neuropharm.2013.07.033. [DOI] [PubMed] [Google Scholar]
- Damaj MI, Kao W, Martin BR. Characterization of spontaneous and precipitated nicotine withdrawal in the mouse. Journal of Pharmacology and Experimental Therapeutics. 2003;307:526–534. doi: 10.1124/jpet.103.054908. [DOI] [PubMed] [Google Scholar]
- Davis JA, James JR, Siegel SJ, Gould TJ. Withdrawal from chronic nicotine administration impairs contextual fear conditioning in C57BL/6 mice. The Journal of neuroscience. 2005;25:8708–8713. doi: 10.1523/JNEUROSCI.2853-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epping-Jordan MP, Watkins SS, Koob GF, Markou A. Dramatic decreases in brain reward function during nicotine withdrawal. Nature. 1998;393:76–79. doi: 10.1038/30001. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW. Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences. 2008;363:3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, Watsky EJ, Gong J, Williams KE, Reeves KR. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA. 2006;296:47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- Gould TJ. Addiction and cognition. Addiction Science and Clinical Practice. 2010;5:4–14. [PMC free article] [PubMed] [Google Scholar]
- Haluk DM, Floresco SB. Ventral striatal dopamine modulation of different forms of behavioral flexibility. Neuropsychopharmacology. 2009;34:2041–2052. doi: 10.1038/npp.2009.21. [DOI] [PubMed] [Google Scholar]
- Harris AC, Pentel PR, Burroughs D, Staley MD, Lesage MG. A lack of association between severity of nicotine withdrawal and individual differences in compensatory nicotine self-administration in rats. Psychopharmacology. 2011;217:153–166. doi: 10.1007/s00213-011-2273-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison EL, Coppola S, McKee SA. Nicotine deprivation and trait impulsivity affect smokers' performance on cognitive tasks of inhibition and attention. Experimental and clinical psychopharmacology. 2009;17:91–98. doi: 10.1037/a0015657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilario MR, Turner JR, Blendy JA. Reward sensitization: effects of repeated nicotine exposure and withdrawal in mice. Neuropsychopharmacology. 2012;37:2661–2670. doi: 10.1038/npp.2012.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe WM, Ji J, Parikh V, Williams S, Mocaer E, Trocme-Thibierge C, Sarter M. Enhancement of attentional performance by selective stimulation of alpha4beta2(*) nAChRs: underlying cholinergic mechanisms. Neuropsychopharmacology. 2010;35:1391–1401. doi: 10.1038/npp.2010.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR, Peters EN, Naud S. Relapse to smoking after 1 year of abstinence: a meta-analysis. Addictive Behaviors. 2008;33:1516–1520. doi: 10.1016/j.addbeh.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y, Gall CM, Lynch G. Presynaptic BDNF promotes postsynaptic long-term potentiation in the dorsal striatum. The Journal of neuroscience. 2010;30:14440–14445. doi: 10.1523/JNEUROSCI.3310-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PM, Hollander JA, Kenny PJ. Decreased brain reward function during nicotine withdrawal in C57BL6 mice: evidence from intracranial self-stimulation (ICSS) studies. Pharmacology, Biochemistry, and Behavior. 2008;90:409–415. doi: 10.1016/j.pbb.2008.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katner SN, Davis SA, Kirsten AJ, Taffe MA. Effects of nicotine and mecamylamine on cognition in rhesus monkeys. Psychopharmacology. 2004;175:225–240. doi: 10.1007/s00213-004-1804-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney JW, Adoff MD, Wilkinson DS, Gould TJ. The effects of acute, chronic, and withdrawal from chronic nicotine on novel and spatial object recognition in male C57BL/6J mice. Psychopharmacology. 2011;217:353–365. doi: 10.1007/s00213-011-2283-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohara K, Kitamura A, Morishima M, Tsumoto T. Activity-dependent transfer of brain-derived neurotrophic factor to postsynaptic neurons. Science. 2001;291:2419–2423. doi: 10.1126/science.1057415. [DOI] [PubMed] [Google Scholar]
- Kozink RV, Lutz AM, Rose JE, Froeliger B, McClernon FJ. Smoking withdrawal shifts the spatiotemporal dynamics of neurocognition. Addiction Biology. 2010;15:480–490. doi: 10.1111/j.1369-1600.2010.00252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BG, Anastasia A, Hempstead BL, Lee FS, Blendy JA. Effects of the BDNF Val66Met Polymorphism on Anxiety-Like Behavior Following Nicotine Withdrawal in Mice. Nicotine and Tobacco Research. 2015 doi: 10.1093/ntr/ntv047. (epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach PT, Cordero KA, Gould TJ. The effects of acute nicotine, chronic nicotine, and withdrawal from chronic nicotine on performance of a cued appetitive response. Behavioral Neuroscience. 2013;127:303–310. doi: 10.1037/a0031913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone PD, Wonnacott S. Nicotinic acetylcholine receptors and the ascending dopamine pathways. Biochemical Pharmacology. 2009;78:744–755. doi: 10.1016/j.bcp.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Lof E, Olausson P, Stomberg R, Taylor JR, Soderpalm B. Nicotinic acetylcholine receptors are required for the conditioned reinforcing properties of sucrose associated cues. Psychopharmacology. 2010;212:321–328. doi: 10.1007/s00213-010-1957-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Christian K, Lu B. BDNF: a key regulator for protein synthesis-dependent LTP and long-term memory? Neurobiology of Learning and Mmory. 2008;89:312–323. doi: 10.1016/j.nlm.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata H, Yanagita T. Neurobiological mechanisms of nicotine craving. Alcohol. 2001;24:87–93. doi: 10.1016/s0741-8329(01)00144-6. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Carlezon WA., Jr. The mesolimbic dopamine reward circuit in depression. Biological Psychiatry. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Newhouse PA, Potter A, Singh A. Effects of nicotinic stimulation on cognitive performance. Current Opinion in Pharmacology. 2004;4:36–46. doi: 10.1016/j.coph.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Newman LA, Mair RG. Cholinergic modulation of visuospatial responding in central thalamus. European Journal of Neuroscience. 2007;26:3543–3552. doi: 10.1111/j.1460-9568.2007.05961.x. [DOI] [PubMed] [Google Scholar]
- O'Dell LE, Chen SA, Smith RT, Specio SE, Balster RL, Paterson NE, Markou A, Zorrilla EP, Koob GF. Extended access to nicotine self-administration leads to dependence: Circadian measures, withdrawal measures, and extinction behavior in rats. Journal of Pharmacology and Experimental Therapeutics. 2007;320:180–193. doi: 10.1124/jpet.106.105270. [DOI] [PubMed] [Google Scholar]
- Ortega LA, Tracy BA, Gould TJ, Parikh V. Effects of chronic low- and high-dose nicotine on cognitive flexibility in C57BL/6J mice. Behavioural Brain Research. 2013;238:134–145. doi: 10.1016/j.bbr.2012.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaleo F, Silverman JL, Aney J, Tian Q, Barkan CL, Chadman KK, Crawley JN. Working memory deficits, increased anxiety-like traits, and seizure susceptibility in BDNF overexpressing mice. Learning and Memory. 2011;18:534–544. doi: 10.1101/lm.2213711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto MR, Addy NA, Mineur YS, Brunzell DH. It is not “either/or”: activation and desensitization of nicotinic acetylcholine receptors both contribute to behaviors related to nicotine addiction and mood. Progress in neurobiology. 2008;84:329–342. doi: 10.1016/j.pneurobio.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME. The contribution of the medial prefrontal cortex, orbitofrontal cortex, and dorsomedial striatum to behavioral flexibility. Annals of the New York Academy of Sciences. 2007;1121:355–375. doi: 10.1196/annals.1401.013. [DOI] [PubMed] [Google Scholar]
- Raybuck JD, Gould TJ. Nicotine withdrawal-induced deficits in trace fear conditioning in C57BL/6 mice--a role for high-affinity beta2 subunit-containing nicotinic acetylcholine receptors. European journal of neuroscience. 2009;29:377–387. doi: 10.1111/j.1460-9568.2008.06580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risner ME, Goldberg SR. A comparison of nicotine and cocaine self -administration in the dog: fixed-ratio and progressive-ratio schedules of intravenous drug infusion. Journal of Pharmacology and Experimental Therapeutics. 1983;224:319–326. [PubMed] [Google Scholar]
- Roegge CS, Levin ED. Nicotinic Receptor Antagonists in Rats. In: Levin ED, Buccafusco JJ, editors. Animal Models of Cognitive Impairment. Boca Raton (FL): 2006. [Google Scholar]
- Salas R, Pieri F, De Biasi M. Decreased signs of nicotine withdrawal in mice null for the beta4 nicotinic acetylcholine receptor subunit. The Journal of neuroscience. 2004;24:10035–10039. doi: 10.1523/JNEUROSCI.1939-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenova S, Stolerman IP, Markou A. Chronic nicotine administration improves attention while nicotine withdrawal induces performance deficits in the 5-choice serial reaction time task in rats. Pharmacology, Biochemistry and Behavior. 2007;87:360–368. doi: 10.1016/j.pbb.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoaib M, Bizzaro L. Deficits in sustained attention task following nicotine withdrawal in rats. Psychopharmacology. 2005;178:211–222. doi: 10.1007/s00213-004-2004-6. [DOI] [PubMed] [Google Scholar]
- Stalnaker TA, Takahashi Y, Roesch MR, Schoenbaum G. Neural substrates of cognitive inflexibility after chronic cocaine exposure. Neuropharmacology. 2009;56(Suppl 1):63–72. doi: 10.1016/j.neuropharm.2008.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turchi J, Holley LA, Sarter M. Effects of nicotinic acetylcholine receptor ligands on behavioral vigilance in rats. Psychopharmacology. 1995;118:195–205. doi: 10.1007/BF02245840. [DOI] [PubMed] [Google Scholar]
- Varani AP, Machado, LM, Balerio GN. Baclofen prevented the changes in c-Fos and brain-derived neutrophic factor expressions during mecamylamine-precipitated nicotine withdrawal in mice. Synapse. 2014;68:508–517. doi: 10.1002/syn.21763. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Telang F, Logan J, Jayne M, Ma Y, Pradhan K, Wong C, Swanson JM. Cognitive control of drug craving inhibits brain reward regions in cocaine abusers. NeuroImage. 2010;49:2536–2543. doi: 10.1016/j.neuroimage.2009.10.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins SS, Stinus L, Koob GF, Markou A. Reward and somatic changes during precipitated nicotine withdrawal in rats: centrally and peripherally mediated effects. Journal of Pharmacology and Experimental Therapeutics. 2000;292:1053–1064. [PubMed] [Google Scholar]
- Wonnacott S, Barik J, Dickinson J, Jones IW. Nicotinic receptors modulate transmitter cross talk in the CNS: nicotinic modulation of transmitters. 2006 doi: 10.1385/JMN:30:1:137. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.