Abstract
Aim
To evaluate, from a US payer perspective, the cost-effectiveness of treatment strategies for metastatic colorectal cancer (mCRC).
Methods
We performed a systematic review of published cost-effectiveness analyses of treatment strategies for mCRC with pre-specified search criteria.
Results
We identified 14 papers that fulfilled our search criteria and revealed varying levels of value amongst current treatment strategies. Older agents such as 5FU, irinotecan, and oxaliplatin provide high value treatments. More modern agents targeting the EGFR or VEGF pathways, such as bevacizumab, cetuximab and panitumumab do not appear to be cost effective treatments at their current costs. The analytical methods used within the papers varied widely, and likely plays a significant role in the heterogeneity in incremental cost effectiveness ratios.
Conclusions
The cost-effectiveness of current treatment strategies for mCRC is highly variable. Drugs recently approved by the Federal Drug Administration (FDA) for mCRC are not cost-effective, and this is primarily driven by high drug costs.
Introduction
Colorectal cancer is the third most common cancer and the third leading cause of cancer death in men and women in the United States (US).1 In 2010, the US spent $14 billion for the management of colorectal cancer.2 While multidisciplinary care is employed in the treatment of localized colon cancer, systemic chemotherapy is the mainstay of treatment in metastatic colorectal cancer (mCRC). There are currently 10 Food and Drug Administration (FDA) approved drugs for the management of mCRC, which include 5-fluorouracil (5FU), oxaliplatin, irinotecan, capecitabine, bevacizumab, cetuximab, panitumumab, ziv-aflibercept, regorafenib and ramucirumab. There are a variety of regimens with different combinations of these drugs, for use in the first, second and third line settings of treatment. For example, 5FU, oxaliplatin (FOLFOX) and bevacizumab are commonly used as a first line therapy.3 The Rat Sarcoma Viral Oncogene Homolog (RAS) is a family of genes including KRAS and NRAS that are often found to be mutated in colorectal cancer. When a tumor is RAS wild-type (WT), patients derive a benefit from monoclonal antibodies, such as cetuximab or panitumumab, which target the epidermal growth factor receptor (EGFR). Thus, EGFR targeted therapy, in addition to chemotherapy, is a common second line strategy for patients with RAS WT disease.4 The chemotherapy backbone in second-line is often 5FU and irinotecan (FOLFIRI), with regorafenib monotherapy commonly used as a third line option.5
There are multiple different combinations and sequencing strategies available, and this is often dependent on patient and tumor specific factors. Individual patients typically experience different toxicities to individual drugs and the results of RAS testing dictate certain treatment choices. Other strategies include using genetic and pharmacokinetic testing to identify patients that are at a greater risk of developing toxicity from certain systemic agents.6,7 Furthermore, specific liver directed therapies are available to treat hepatic metastases.8,9
In the current economic climate with increasing healthcare costs, policy makers have begun to place greater emphasis on the value, or cost-effectiveness (CE), of management strategies. CE studies can be performed using three different methods: 1) analyzing cost and efficacy data collected prospectively alongside a clinical trial; 2) using large databases such as the Surveillance, Epidemiology and End Results (SEER)-Medicare linked database to evaluate cost-effectiveness retrospectively; 3) developing a Markov model to merge data from clinical trials with financial data, commonly from Medicare sources.10 Efficacy data is often adjusted for quality of life, in order to calculate quality adjusted life years (QALYS) gained. The financial costs of toxicities are incorporated with the final results of the analysis, which then produces an incremental cost effectiveness ratio (ICER) for a specific treatment that is expressed as a dollar amount for each QALY gained.
The objective of this study was to systematically review the value of treatment strategies for mCRC from a US perspective.
Methods
We performed a systematic literature search to identify published cost-effectiveness studies in the MEDLINE database through a series of searches using combinations of the medical subject heading (MeSH) and free text terms “cost, effectiveness and metastatic colorectal cancer” generating an initial pool of 124 articles that were assessed for suitability as shown in Figure 1. Two reviewers (EN and DG) independently performed a study selection and quality assessment using a predefined format. Any disagreement was resolved by another reviewer (CF). Studies were carefully screened for possible duplication of study population based on the author list, participating institutions, and period of patients' diagnosis and accrual. Criteria for inclusion in the systematic review were: 1) articles published in English between January 1st, 2005 and April 1, 2015, with a full set of experimental details; 2) evaluated patients with metastatic colorectal cancer undergoing treatment; 3) involved a direct comparison between two interventions with measurement of costs and effectiveness allowing calculation of an incremental cost-effectiveness ratio (ICER); 4) data included in analysis was not duplicated in any other article included in our systematic review; and 5) studies were performed from a US perspective, with results presented in US dollars.
Figure 1. selection of articles for review.
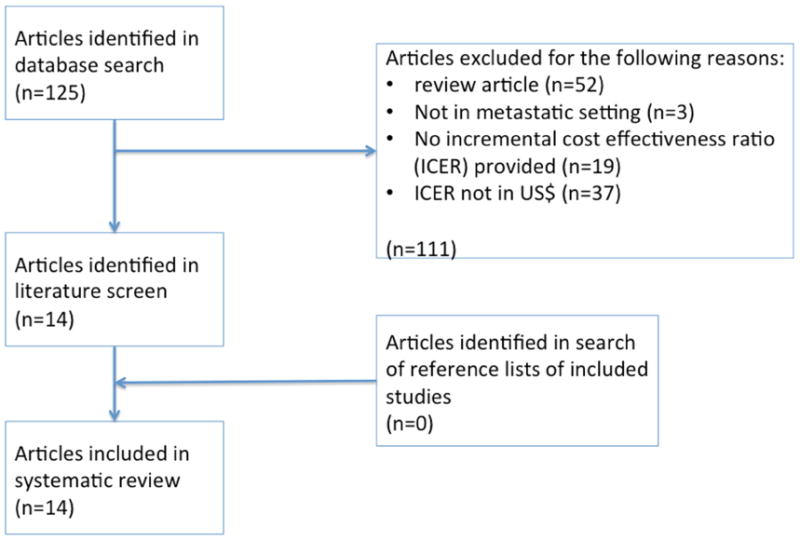
Results
The search strategy generated an initial pool of 124 articles, which was narrowed to 14 using the exclusion criteria depicted in figure 1. These 14 studies are described in table 1.
Table 1. summary of studies evaluating value of treatment strategies.
| Study First author | Pharma funded? | Description | Cost ($) | LYs | QALYs | $/LY | $/QALY |
|---|---|---|---|---|---|---|---|
| Conventional Chemotherapy | |||||||
| Howard15 | no | Value of Irinotecan, capecitabine, oxaliplatin, bevacizumab, and cetuximab. | 37,100 | 0.57 | 66,200 | 99,100 | |
| Tumeh14 | no | FOLFIRI vs FOLFOX | 5,314 | 0.10 | 0.082 | 53,140 | 65,170 |
| Hillner11 | yes | IFL vs FOLFOX | 29,523 | 0.37 | 80,407 | ||
| Wong29 | yes | 5FU/LV vs FOLFIRI followed by FOLFOX | 57,689 | 0.70 | 102,347 | ||
| Wong | Yes | FOLFIRI=>FOLFOX vs FOLFIRI/bevacizumab => FOLFOX => Cetuximab | 67,313 | 0.41 | 170,896 | ||
| Wong | yes | FOLFIRI/bevacizumab => FOLFOX => Cetuximab vs FOLFOX/bevacizumab => irinotecan => cetuximab/irinotecan | 44,388 | 0.16 | 243,096 | ||
| Bevacizumab | |||||||
| Goldstein16 | No | First-line bevacizumab | 60,551 | 0.14 | 0.10 | 438,779 | 571,240 |
| Bevacizumab beyond progression | 39,321 | 0.16 | 0.11 | 235,455 | 364,083 | ||
| Shankaran17 | yes | First-line chemotherapy | 58,776 | 0.78 | 75,354 | ||
| Shankaran | yes | First-line bevacizumab | 32,004 | 0.36 | 88,900 | ||
| KRAS / EGFR inhibitors | |||||||
| Behl19 | no | KRAS/BRAF screening with anti-EGFR therapy | 22,033 | 0.034 | 650,000 | ||
| Vijayaraghavan20 | yes | Irinotecan/cetuximab for KRAS WT and FOLFIRI for KRAS mutated, compared to Irinotecan/cetuximab for KRAS WT and no chemotherapy for KRAS mutated. | 36,148 | - | 35,539 | ||
| Regorafenib | |||||||
| regorafenib | no | Third-line regorafenib | 40,000 | 0.13 | 0.04 | 310,000 | 897,000 |
| Genetic / Pharmacokinetic | |||||||
| Goldstein23 | no | Pharmacokinetically guided FOLFOX | 13,004 | 0.61 | 0.57 | 21,423 | 22,695 |
| Gold22 | no | UGT1A1 screening prior to irinotecan therapy. | 272 | 0.0016 | Dominateda | ||
| Hepatic resections | |||||||
| Abbott26 | no | Resection/RFA vs 2-stage hepatectomy for bilobar liver metastases | Dominatedb | ||||
| Karuna27 | no | Laparoscopy vs Laparotomy prior to resection of hepatic metastases | 500 | 0.02 | Dominatedc | ||
| Bang25 | no | Multi-site cryoablations | 73,900 | 1.97 | 37,513 | ||
UGT1A1 testing dominated no testing ie - costs were reduced while also improving quality of life.
Resection/RFA was better both in terms of cost and efficacy. It cost total of $37,120 for 46.2 months survival. 2-stage hepatectomy cost $62,198 for 35.9 months.
Laparoscopy prior to laparotomy dominates the strategy of proceeding directly to laparotomy. Ie. it is less expensive and yields more QALYs to perform laparoscopy before planned laparotomy in these patients.
Conventional chemotherapy strategies
Although generic versions are now currently available within the United States (irinotecan, 2008; oxaliplatin, 2012), several studies previously evaluated the cost-effectiveness of first line systemic chemotherapy in mCRC. Based upon the clinical data from the N9741 randomized clinical trial, Hillner et al. compared infusional and bolus 5-fluorouracil (5FU) in addition to oxaliplatin (FOLFOX) versus irinotecan and bolus 5FU (IFL).11,12 FOLFOX had an ICER of $80,410 per LY, $111,890 per QALY. When the model incorporated a revised IFL with lower rates of AEs, the ICER was $84,780/LY. Due to chemotherapy toxicity among patients, dose delays and skipped doses were as high as 77% and 85%, in the FOLFOX and IFL treatment arms, respectively. Hypothetically, if patients had received treatment without delays or interruptions, the ICER of FOLFOX would increase to $117,910/LY and $220,200/QALY. Thus, the primary driver of the model was the cost of oxaliplatin and the disutility of oxaliplatin induced peripheral neuropathy and its associated treatment costs.
Using clinical data from a large randomized phase III study of mCRC patients receiving chemotherapy in the first line setting,13 Tumeh et al. developed a Markov model comparing the cost-effectiveness of FOLFOX versus infusional and bolus 5FU with irinotecan (FOLFIRI).14 FOLFOX was associated with an ICER of $65,170/QALY, but ranged from $34,772-$91,968/QALY depending on the number of cycles received (between 4-12 cycles). The primary drivers of this model were the similarity in median OS associated with the two chemotherapy regimens, the probability of death associated with FOLFOX, and the relatively higher cost of oxaliplatin compared with irinotecan. A SEER database study analyzed the cost of mCRC from 1995-2005 among Medicare patients who received chemotherapy and who were not candidates for liver directed therapy or surgical metastectomy.15 The 6.8-month OS improvement among mCRC patients during this timeframe offset the rising treatment-related costs, which was calculated to be $66,200/LY and $99,1000/QALY.
Bevacizumab
Two studies evaluated the incremental benefit of adding bevacizumab to a 5FU based chemotherapy backbone. A Markov model evaluated bevacizumab in the first-line setting, and then when continued beyond progression in the second-line setting.16 The authors found ICERs of $571,240/QALY and $364,083/QALY in the first- and second-line settings, respectively. A retrospective database analysis found that adding bevacizumab to chemotherapy in the first-line setting cost $75,303/LY.17 The lower ICER in the retrospective study, compared to the Markov model, may be due to the greater bevacizumab efficacy seen in the retrospective study.3
RAS testing and cetuximab/panitumumab
The prognosis for patients with BRAF and KRAS mutated mCRC is worse compared to those not harboring these mutations.18 Two studies were identified that assessed the cost-effectiveness of testing for either KRAS and/or BRAF mutations in the context of treatment regimens including anti-EGFR agents. Behl et al. utilized a Markov model of disease progression to assess best supportive care versus anti-EGFR therapy with and without screening for KRAS alone and with BRAF testing. The authors found that screening for both mutations, compared with best supportive care alone, marginally improved the CE of anti-EGFR therapies, with an ICER of $648,396/LY and a cost savings of approximately $7,500 per patient.19
Another Markov model was constructed to determine the value of testing for the KRAS gene mutation in mCRC patients prior to treatment with an EGFR-inhibitor-containing regimen. This group reported cost-savings between $7,500-12,400 per/patient in the US and between €3,900-9,600 per/patient in Germany. Combination therapy (cetuximab + irinotecan in the US / cetuximab + FOLFIRI in Germany) with KRAS testing yielded an ICER of $35,539/LY.20 The lower ICER in the study by Vijayaraghavan et al. may be due to their failure to account for the costs of tumor resection and of second-line therapy.
Regorafenib
Regorafenib is a tyrosine kinase inhibitor that is approved for patients whose disease has progressed on multiple prior lines of systemic therapy. It provides an additional 6 weeks of life for these patients with advanced disease and was recently shown to cost $900,000/QALY.21
Genetic and pharmacokinetic testing
A significant amount of research has been performed analyzing the UGT1A1 gene as a biomarker for potential irinotecan toxicity.22 Patients with genetic mutations received a 25% irinotecan dose reduction and the study found that 11% of patients would be dose reduced to avoid 84.5/10,000 cases of severe neutropenia and save a total of $2.7 million in treatment costs or an average saving of $272 per patient.
Another technique to personalize the dosing of 5FU is through pharmacokinetically (PK) guided dosing. This strategy has been shown to improve response rates, decrease toxicity,7 and although limited by its reliance on a non-randomized study, was shown to be cost-effective with an ICER of $23,000/QALY.23,24
Liver directed therapies
Other studies have evaluated the cost-effectiveness of liver directed therapies and surgery in metastatic CRC. One group looked at multi-site cryoablation (MCA) of oligo-metastatic CRC (sites: liver, lung, soft tissue, retroperitoneal, intraperitoneal, bone), and with a median OS of 24 months, MCA was calculated to cost $39,661-$85,580/LY.25 Similarly, Abbott et al. looked at the cost-effectiveness of simultaneous resection and radiofrequency ablation (RFA) versus two-stage hepatectomy among highly selected patients with bilobar colorectal liver metastasis.26 The total cost of resection with RFA was estimated to be $37,120 and was associated with a 46.2 month OS, while the cost of a two-stage resection was estimated to be $62,198 and was associated with a 35.9 month OS. In addition to health utility values and associated QALYs, the cost of office visits, laboratory evaluation, and treatment related complications were not incorporated into this model. Based upon the clinical data from five prior cohort studies, another group reported on the cost-effectiveness of laparoscopy preceding laparotomy versus laparotomy alone for the initial surgical evaluation and treatment of potentially resectable hepatic metastasis.27 Due to the high rate of patients found to have intra-abdominal metastases intra-operatively despite the use of advanced imaging modalities (∼10%),28 laparoscopy followed by laparotomy was found to be cost-effective when compared to laparotomy alone, with an ICER of $40,000/QALY.
Different Sequences of therapy
Wong et al. obtained clinical data from previous multicenter phase II and randomized phase III trials and devised a Markov model that incorporated nine possible mCRC treatment strategies and sequences.29 The following outcomes were observed: FOLFOX, when compared to 5FU in the first-line setting, had an ICER of $102,347; FOLFOX with bevacizumab, when compared to FOLFOX in the first-line setting, had an ICER of $170,896; Cetuximab and irinotecan when compared to cetuximab alone in the third-line setting, had an ICER of $243,096. The cost of targeted agents and their associated efficacy, rather than the sequence of first-line therapy, were found to be the primary drivers of the model. Although not likely to have had a substantial impact on the results, the model was limited by the failure to incorporate significant adverse effects, health utilities, and QALYs.
Discussion
With the rising drug costs in the US, cost-effective treatment strategies for mCRC are becoming increasingly important. Our analysis revealed varying levels of value amongst current treatment strategies. Older agents that are now available in generic form such as 5FU, irinotecan, and oxaliplatin appear to be high value, cost-effective treatments. More modern agents, targeting the EGFR or VEGF pathways such as bevacizumab, cetuximab and panitumumab appear to not be cost-effective treatments and are deemed to be of low value at their current cost. However, with the use of biomarkers such as RAS mutational analysis, EGFR targeted agents can potentially be more cost-effective.
Our analysis also revealed the difficulties in making comparisons between different cost-effectiveness studies. We attempted to reduce these limitations by excluding international data that were not presented in US$.30 However, even with this simplification, the scope of costs considered in different studies was highly variable, which makes comparisons exceedingly difficult.
Furthermore, the “threshold” for the definition for a cost-effective healthcare intervention remains undefined. Previous analyses suggested that the threshold was $50,000/QALY based on the cost-effectiveness of dialysis.31 More recent analysis revealed that this value should be updated to approximately $130,000/QALY.32 In other fields of medicine we note some excellent examples of treatments that are highly cost-effective. For example, hip or knee replacement surgery is estimated to cost less than $20,000 per QALY.33 Cataract surgery has also been found to cost less than $20,000/QALY.34 In the field of global health, the distribution of mosquito nets is frequently mentioned as a highly cost-effective health intervention at a cost of just $34 per life year gained.35
The question of how to make new cancer drugs more cost-effective is multifactorial. More impressive survival benefits as recently proposed by the American Society of Clinical Oncology would improve the cost-effectiveness profile.36 Using biomarkers to treat only the patients likely to get a significant survival benefit would also improve cost-effectiveness. Furthermore, reduced prices would of course improve the value of such therapies. Variable dosing strategies, including the duration of therapy also impact the overall cost of care, and thus the cost-effectiveness. Attention should be paid to these important factors in the design and development of clinical trials.
Ultimately however, currently in the US, neither clinicians nor policy-makers make decisions based on the cost-effectiveness of a certain therapy. A recent editorial in the Journal of Clinical Oncology noted that “…cost effectiveness research has become an academic exercise of no meaningful consequence…”.37 While the findings of cost-effectiveness analyses regarding patients with mCRC provide variable findings regarding the value of these interventions, the approaches utilized offer benchmarks for assessing and comparing the merits of therapy using common terminology. Future approaches should use a standardized method to evaluate the cost-effectiveness of new drugs and technologies prior to their arrival in the US market-place. This will help to provide an appropriate cost for the benefit that new treatments provide.
Acknowledgments
Funding: NIH T32 Grant - T32CA160040-02
Footnotes
Conflicts of interest: G, SZ, CB, EN declare no conflicts of interest
- Consultancy (paid) - Optum Rx;
- Consultancy (unpaid) – Genentech/Biogen-Idec/Roche, Millenium/Takeda;
- Research Funding – Millenium/Takeda, Gilead; TG Therapeutics, Abbvie, Janssen, Pharmacyclics for studies in hematological malignancies
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA: a cancer journal for clinicians. 2014;64(2):104–117. doi: 10.3322/caac.21220. [DOI] [PubMed] [Google Scholar]
- 2.Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010-2020. Journal of the National Cancer Institute. 2011;103(2):117–128. doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saltz LB, Clarke S, Diaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26(12):2013–2019. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- 4.Seymour MT, Brown SR, Middleton G, et al. Panitumumab and irinotecan versus irinotecan alone for patients with KRAS wild-type, fluorouracil-resistant advanced colorectal cancer (PICCOLO): a prospectively stratified randomised trial. The lancet oncology. 2013;14(8):749–759. doi: 10.1016/S1470-2045(13)70163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381(9863):303–312. doi: 10.1016/S0140-6736(12)61900-X. [DOI] [PubMed] [Google Scholar]
- 6.Innocenti F, Undevia SD, Iyer L, et al. Genetic variants in the UDP-glucuronosyltransferase 1A1 gene predict the risk of severe neutropenia of irinotecan. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2004;22(8):1382–1388. doi: 10.1200/JCO.2004.07.173. [DOI] [PubMed] [Google Scholar]
- 7.Gamelin E, Delva R, Jacob J, et al. Individual fluorouracil dose adjustment based on pharmacokinetic follow-up compared with conventional dosage: results of a multicenter randomized trial of patients with metastatic colorectal cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26(13):2099–2105. doi: 10.1200/JCO.2007.13.3934. [DOI] [PubMed] [Google Scholar]
- 8.Wicherts DA, Miller R, de Haas RJ, et al. Long-term results of two-stage hepatectomy for irresectable colorectal cancer liver metastases. Ann Surg. 2008;248(6):994–1005. doi: 10.1097/SLA.0b013e3181907fd9. [DOI] [PubMed] [Google Scholar]
- 9.Bilchik AJ, Faries M. Radiofrequency ablation of hepatic malignancies: inexpensive and minimally invasive but should it replace resection? Annals of surgical oncology. 2003;10(9):1002–1004. doi: 10.1245/aso.2003.09.915. [DOI] [PubMed] [Google Scholar]
- 10.Tumeh JW, Moore SG, Shapiro R, Flowers CR. Practical approach for using Medicare data to estimate costs for cost-effectiveness analysis. Expert review of pharmacoeconomics & outcomes research. 2005;5(2):153–162. doi: 10.1586/14737167.5.2.153. [DOI] [PubMed] [Google Scholar]
- 11.Hillner BE, Schrag D, Sargent DJ, Fuchs CS, Goldberg RM. Cost-effectiveness projections of oxaliplatin and infusional fluorouracil versus irinotecan and bolus fluorouracil in first-line therapy for metastatic colorectal carcinoma. Cancer. 2005;104(9):1871–1884. doi: 10.1002/cncr.21411. [DOI] [PubMed] [Google Scholar]
- 12.Goldberg RM, Sargent DJ, Morton RF, et al. Randomized controlled trial of reduced-dose bolus fluorouracil plus leucovorin and irinotecan or infused fluorouracil plus leucovorin and oxaliplatin in patients with previously untreated metastatic colorectal cancer: a North American Intergroup Trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24(21):3347–3353. doi: 10.1200/JCO.2006.06.1317. [DOI] [PubMed] [Google Scholar]
- 13.Colucci G, Gebbia V, Paoletti G, et al. Phase III randomized trial of FOLFIRI versus FOLFOX4 in the treatment of advanced colorectal cancer: a multicenter study of the Gruppo Oncologico Dell'Italia Meridionale. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23(22):4866–4875. doi: 10.1200/JCO.2005.07.113. [DOI] [PubMed] [Google Scholar]
- 14.Tumeh JW, Shenoy PJ, Moore SG, Kauh J, Flowers C. A Markov model assessing the effectiveness and cost-effectiveness of FOLFOX compared with FOLFIRI for the initial treatment of metastatic colorectal cancer. American journal of clinical oncology. 2009;32(1):49–55. doi: 10.1097/COC.0b013e31817c6a4d. [DOI] [PubMed] [Google Scholar]
- 15.Howard DH, Kauh J, Lipscomb J. The value of new chemotherapeutic agents for metastatic colorectal cancer. Arch Intern Med. 2010;170(6):537–542. doi: 10.1001/archinternmed.2010.36. [DOI] [PubMed] [Google Scholar]
- 16.Goldstein DA, Chen Q, Ayer T, et al. First- and Second-Line Bevacizumab in Addition to Chemotherapy for Metastatic Colorectal Cancer: A United States-Based Cost-Effectiveness Analysis. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015 doi: 10.1200/JCO.2014.58.4904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shankaran V, Mummy D, Koepl L, et al. Survival and lifetime costs associated with first-line bevacizumab use in older patients with metastatic colorectal cancer. The oncologist. 2014;19(8):892–899. doi: 10.1634/theoncologist.2013-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Roock W, Claes B, Bernasconi D, et al. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: a retrospective consortium analysis. The lancet oncology. 2010;11(8):753–762. doi: 10.1016/S1470-2045(10)70130-3. [DOI] [PubMed] [Google Scholar]
- 19.Behl AS, Goddard KA, Flottemesch TJ, et al. Cost-effectiveness analysis of screening for KRAS and BRAF mutations in metastatic colorectal cancer. Journal of the National Cancer Institute. 2012;104(23):1785–1795. doi: 10.1093/jnci/djs433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vijayaraghavan A, Efrusy MB, Goke B, Kirchner T, Santas CC, Goldberg RM. Cost-effectiveness of KRAS testing in metastatic colorectal cancer patients in the United States and Germany. International journal of cancer Journal international du cancer. 2012;131(2):438–445. doi: 10.1002/ijc.26400. [DOI] [PubMed] [Google Scholar]
- 21.Goldstein DA, Ahmad BB, Chen Q, et al. Cost-Effectiveness Analysis of Regorafenib for Metastatic Colorectal Cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015 doi: 10.1200/JCO.2015.61.9569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gold HT, Hall MJ, Blinder V, Schackman BR. Cost effectiveness of pharmacogenetic testing for uridine diphosphate glucuronosyltransferase 1A1 before irinotecan administration for metastatic colorectal cancer. Cancer. 2009;115(17):3858–3867. doi: 10.1002/cncr.24428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldstein DA, Chen Q, Ayer T, et al. Cost Effectiveness Analysis of Pharmacokinetically-Guided 5-Fluorouracil in FOLFOX Chemotherapy for Metastatic Colorectal Cancer. Clinical colorectal cancer. 2014 doi: 10.1016/j.clcc.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 24.Capitain O, Asevoaia A, Boisdron-Celle M, Poirier AL, Morel A, Gamelin E. Individual fluorouracil dose adjustment in FOLFOX based on pharmacokinetic follow-up compared with conventional body-area-surface dosing: a phase II, proof-of-concept study. Clinical colorectal cancer. 2012;11(4):263–267. doi: 10.1016/j.clcc.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 25.Bang HJ, Littrup PJ, Currier BP, et al. Percutaneous Cryoablation of Metastatic Lesions from Colorectal Cancer: Efficacy and Feasibility with Survival and Cost-Effectiveness Observations. ISRN Minim Invasive Surg. 2012;2012 doi: 10.5402/2012/942364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abbott DE, Sohn VY, Hanseman D, Curley SA. Cost-effectiveness of simultaneous resection and RFA versus 2-stage hepatectomy for bilobar colorectal liver metastases. J Surg Oncol. 2014;109(6):516–520. doi: 10.1002/jso.23539. [DOI] [PubMed] [Google Scholar]
- 27.Karuna ST, Thirlby R, Biehl T, Veenstra D. Cost-effectiveness of laparoscopy versus laparotomy for initial surgical evaluation and treatment of potentially resectable hepatic colorectal metastases: a decision analysis. J Surg Oncol. 2008;97(5):396–403. doi: 10.1002/jso.20964. [DOI] [PubMed] [Google Scholar]
- 28.Selzner M, Hany TF, Wildbrett P, McCormack L, Kadry Z, Clavien PA. Does the novel PET/CT imaging modality impact on the treatment of patients with metastatic colorectal cancer of the liver? Ann Surg. 2004;240(6):1027–1034. doi: 10.1097/01.sla.0000146145.69835.c5. discussion 1035-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wong YN, Meropol NJ, Speier W, Sargent D, Goldberg RM, Beck JR. Cost implications of new treatments for advanced colorectal cancer. Cancer. 2009;115(10):2081–2091. doi: 10.1002/cncr.24246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yabroff KR, Borowski L, Lipscomb J. Economic studies in colorectal cancer: challenges in measuring and comparing costs. Journal of the National Cancer Institute Monographs. 2013;2013(46):62–78. doi: 10.1093/jncimonographs/lgt001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Winkelmayer WC, Weinstein MC, Mittleman MA, Glynn RJ, Pliskin JS. Health economic evaluations: the special case of end-stage renal disease treatment. Medical decision making : an international journal of the Society for Medical Decision Making. 2002;22(5):417–430. doi: 10.1177/027298902236927. [DOI] [PubMed] [Google Scholar]
- 32.Lee CP, Chertow GM, Zenios SA. An empiric estimate of the value of life: updating the renal dialysis cost-effectiveness standard. Value in health : the journal of the International Society for Pharmacoeconomics and Outcomes Research. 2009;12(1):80–87. doi: 10.1111/j.1524-4733.2008.00401.x. [DOI] [PubMed] [Google Scholar]
- 33.Daigle ME, Weinstein AM, Katz JN, Losina E. The cost-effectiveness of total joint arthroplasty: a systematic review of published literature. Best Pract Res Clin Rheumatol. 2012;26(5):649–658. doi: 10.1016/j.berh.2012.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lansingh VC, Carter MJ, Martens M. Global cost-effectiveness of cataract surgery. Ophthalmology. 2007;114(9):1670–1678. doi: 10.1016/j.ophtha.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 35.Wiseman V, Hawley WA, ter Kuile FO, et al. The cost-effectiveness of permethrin-treated bed nets in an area of intense malaria transmission in western Kenya. Am J Trop Med Hyg. 2003;68(4 Suppl):161–167. [PubMed] [Google Scholar]
- 36.Ellis LM, Bernstein DS, Voest EE, et al. American Society of Clinical Oncology perspective: Raising the bar for clinical trials by defining clinically meaningful outcomes. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32(12):1277–1280. doi: 10.1200/JCO.2013.53.8009. [DOI] [PubMed] [Google Scholar]
- 37.Saltz LB. Can Money Really Be No Object When Cancer Care Is the Subject? Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015 doi: 10.1200/JCO.2014.60.1401. [DOI] [PubMed] [Google Scholar]


