Abstract
Leukocyte trafficking into the central nervous system (CNS) is a prominent feature driving the immunopathogenesis of multiple sclerosis (MS) and its animal model experimental autoimmune encephalomyelitis (EAE). Blocking the recruitment of inflammatory leukocytes into the CNS represents an exploitable therapeutic target; however, the adhesion molecules that specifically regulate the step of leukocyte diapedesis into the CNS remain poorly understood. Here, we report that CD99 is critical for lymphocyte transmigration without affecting adhesion in a human blood-brain barrier model. CD99 blockade in vivo ameliorated EAE and decreased the accumulation of CNS inflammatory infiltrates, including dendritic cells, B-cells and CD4+ and CD8+ T-cells. Anti-CD99 therapy was effective when administered after onset of disease symptoms and blocked relapse when administered therapeutically after disease symptoms had recurred. These findings underscore an important role for CD99 in the pathogenesis of CNS autoimmunity and suggest it may serve as a novel therapeutic target for controlling neuroinflammation.
Keywords: CD99, Central Nervous System, Neuroinflammation, EAE, Autoimmunity, Endothelium, Blood-brain barrier, Transendothelial migration, Diapedesis, Lymphocyte, CD4+ T-cell, Dendritic Cell
INTRODUCTION
Leukocyte diapedesis (transmigration; TEM) is a tightly regulated process that is vital to inflammation and immune responses. TEM is mediated by a set of adhesive molecular interactions between circulating leukocytes (WBCs) and the vascular endothelium. During immune surveillance, a limited number of WBCs enter the CNS by crossing the blood-brain barrier (BBB) to detect potential damaging agents (1, 2); however, exacerbated WBC recruitment into the CNS is a prominent pathological feature driving multiple sclerosis (MS) and its animal model experimental autoimmune encephalomyelitis (EAE) (3, 4).
Before mediating tissue destruction in CNS autoimmunity, lymphocytes first interact with adhesion molecules on the BBB to reach their cognate Ag (5). In addition, the TEM of inflammatory APCs, including inflammatory myeloid-derived dendritic cells (iDCs), macrophages, and B-cells, from the periphery into the CNS is an early event and their accumulation persists throughout the course of disease (6–8). Therefore, elucidating the mechanisms governing the TEM of lymphocytes and inflammatory APC into the CNS is critical to understand the pathogenesis of disease. The integrin VLA-4 mediates firm adhesion to endothelium in the periphery (9–13) and the CNS (14–16) and has been demonstrated to play an integral role in inflammatory WBC accumulation in the CNS (17–20). However, inflammatory WBCs may use alternative mechanisms that specifically govern the step of diapedesis to infiltrate the CNS and mediate disease.
CD99 is a 32-kD surface glycoprotein that is concentrated at cell borders of all endothelium and is expressed diffusely on the surface of WBCs (21, 22). Homophilic WBC-endothelial CD99 interactions are critical for TEM in vitro (21–23) and in inflammatory settings within the periphery in vivo (24–26). Blocking WBC CD99 inhibits TEM to the same extent as blocking endothelial CD99 (21, 22, 24), making it an attractive therapy for inflammatory mediated diseases. Recently, we reported that CD99 mediates monocyte TEM across human BBB models in vitro (23). However, the role of CD99 for other WBC subsets at the BBB may be different and its role in the pathogenesis of CNS disease is unknown.
In the current report, we examined the requirement of CD99 for lymphocyte TEM in the BBB model in vitro and investigated the mechanism and efficacy of anti-CD99 therapy to regulate relapsing-remitting EAE (RR-EAE). RR-EAE in the SJL/J strain shares similar clinical and histopathological hallmarks that are germane to MS (27), and thus serves as a powerful tool for studying the immunopathogenesis of disease and potential therapeutic interventions (28). We show that CD99 is required for lymphocyte diapedesis but not adhesion in a human BBB model in vitro. More important, anti-CD99 therapy reduced the clinical severity of RR-EAE and diminished T-cell, iDC and B-cell accumulation in the CNS. Thus, CD99 is a novel therapeutic target for the management of CNS inflammation and autoimmunity.
MATERIALS AND METHODS
All procedures involving human subjects/materials were approved by the Institutional Review Board of Northwestern University Feinberg School of Medicine.
Antibodies and reagents
Mouse anti-human mAbs for VE-cadherin (clone hec1) and CD99 (clone hec2), Armenian hamster anti-mouse PECAM (clone 2H8) and rat anti-mouse CD99 (clone 3F11) mAbs were generated by hybridoma methodologies as previously described (21, 24, 29–31). Rat anti-mouse CD3 (clone 17A2) mAb was purchased from eBioscience.
Isolation and culture of human endothelial cells for the BBB model
The BBB model was cultured as described previously (23). Prior to TEM experiments, monolayers were activated by TNF (20ng/ml; R&D Systems) for 24h and preincubated with apical CXCL12 (200ng/ml; R&D Systems) for 20–30 min as described previously (32–34).
Isolation of human leukocytes
Lymphocytes were isolated from human PBMC and mitogen activated (1μg/ml PHA; Sigma Aldrich and 20 ng/ml IL-2; R&D Systems) as previously described (33, 34). CD4+ and CD8+ T-cells were isolated from human PBMC by viable-cell sorting using mAb against CD3, CD4 and CD8 by the Robert H. Lurie Comprehensive Cancer Center Flow Cytometry Core Facility.
Transendothelial migration assay
Quantitative endpoint TEM assays were performed as previously described (21, 23, 24, 32–34).
Mice
Female SJL/J (Jackson Laboratories; Bar harbor, ME); were housed under specific pathogen-free conditions in the Northwestern University Center for Comparative Medicine Animal Facility. Northwestern University Animal Care and Use Committee approved all protocols.
Active induction and clinical evaluation of PLP139–151 mediated RR-EAE
6–8 week-old female SJL/J were immunized s.c. over three sites on the flank with 100 μl of an emulsion containing 50 μg proteolipid protein (PLP)139–151 and CFA as previously described (7, 8). Disease was scored on a 0 to 5 scale, as previously described (8, 35).
In vivo antibody treatment
RR-EAE mice were randomized at the onset of clinical symptoms and treated i.p. with 100 μg of rat-anti-CD99 mAb (clone 3F11) or control-IgG (rat IgG; JacksonImmuno Research Laboratories) beginning either at onset of symptoms or at relapse and every other day thereafter.
In situ whole mount immunostaining
Whole mount immunostaining was performed, acquired, and analyzed as previously described (24, 36). Spinal cords (SC) were harvested intact at disease peak and relapse by flushing the vertebral column with saline (37), then fixed in 4% paraformaldehyde overnight at 4°C. This method removes the meninges. SC were blocked and permeabilized in a solution of 0.2% TritonX-100, 2.5% BSA, 2% FBS and 2.5% host-species serum in PBS overnight at 4°C. SC were incubated overnight at 4°C with primary mAb against PECAM-1 and CD3 in blocking solution. SC were incubated with DyLight 550 goat anti-Armenian hamster and DyLight 488 goat anti-rat secondary antibody in PBS at room temperature for 4h. SCs were mounted in sagittal orientation and parenchymal postcapillary venules (identified by PECAM and 20–40 μm in diameter) in the lumbar region associated with perivascular lymphocyte infiltrates (identified by CD3) were imaged using an Ultraview Vox imaging system equipped with a Yokogawa CSU-1 spinning disk. Images were acquired using Volocity software (Perkin Elmer), which renders the optical sections into 3D images. Image processing and analysis was performed with ImageJ. The figure shows representative projections of the whole stack using the maximum intensity method.
Cell isolation
WBCs were isolated from digested spinal cord and brain of individual PBS perfused mice via Percoll gradient as described previously (8, 38).
FACS
CNS cells were stained with the indicated antibodies (eBioscience and BD) as described previously (8, 38, 39): CD45, CD39, CD11b, CD11c, Ly6c, CD19, plasmacytoid dendritic cell antigen (PDCA)-1, F4/80, CD3, CD4, CD8 and CD25. Intracellular staining for FoxP3 was performed per manufacturer’s instructions with eBioscience staining buffer kit. Aqua/VID Live-dead cell stain was purchased from Life Technologies.
Statistical analysis
All statistical analyses were done using GraphPad Prism software (GraphPad, San Diego, CA).
RESULTS and DISCUSSION
CD99 blockade restricts lymphocyte TEM but not adhesion at the BBB
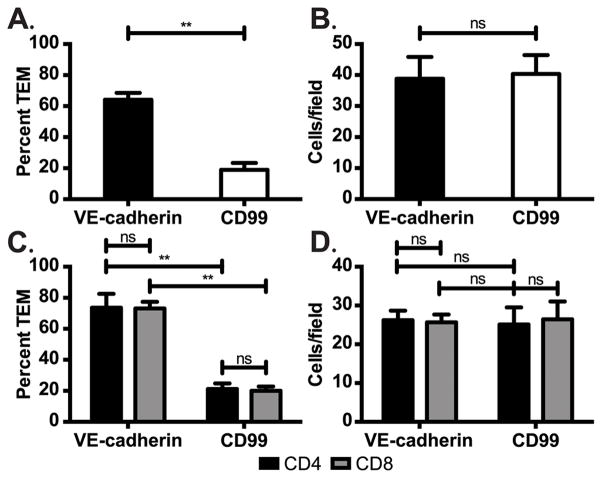
Studies in EAE have demonstrated that CNS infiltrating lymphocytes play a critical role in CNS autoimmune disease. We investigated the role of CD99 for lymphocyte TEM at the inflamed BBB model in vitro. BBB model monolayers were activated as described in the Materials and Methods. TEM assays with polyclonal activated lymphocytes were performed in the presence of blocking mAb against CD99 or non-blocking control mAb against VE-cadherin. Under control conditions 65% of adherent lymphocytes transmigrated, while anti-CD99 blocked TEM down to ~19% (>70% block in TEM) (Fig 1A). Anti-CD99 blocked TEM of both CD4+ and CD8+ T-cells to a similar extent (Fig 1C). Under all of the conditions examined, none of the mAb treatments significantly affected lymphocyte adhesion to the monolayers (Fig 1B,D), demonstrating that our observations are specifically due to a block in TEM, as observed for monocytes (21, 23, 24) and neutrophils (22, 24, 36). This is in contrast to ICAM-1 (40), VCAM-1 (17), ALCAM (41) and Ninjurin-1 (42), which have been demonstrated to play a role in both adhesion and subsequent diapedesis of inflammatory WBCs subsets across the BBB.
Figure 1. CD99 is required for lymphocyte TEM at the human BBB model in vitro.
TEM assays were performed with total lymphocytes (A) or purified CD4+ and CD8+ T-cells (C) on inflamed BBB model monolayers in the presence of anti-CD99 or control mAbs (non-blocking anti-VE-cadherin). (B,D) The number of lymphocytes that adhered to the monolayer were scored for multiple high power fields in each monolayer. Data represent the mean ± SD of hundreds of TEM events from at least six replicates from two (C,D) and three (A,B) independent experiments. **p< 0.001 (unpaired t-Test and two-way ANOVA).
CD99 reduces clinical severity and histopathological burden of RR-EAE
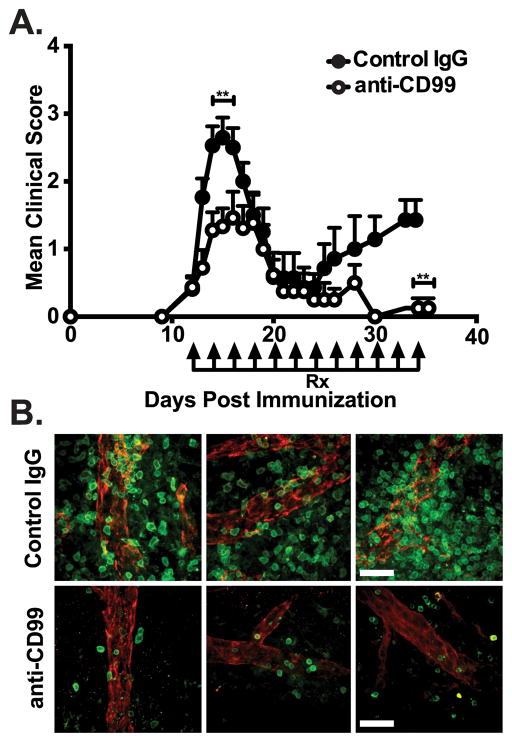
To date, the role of CD99 in EAE is unknown. We determined the role of CD99 for WBC recruitment to the CNS in vivo using PLP139–151 induced RR-EAE in SJL/J mice by administering anti-CD99 or control IgG i.p. once mice became symptomatic. Anti-CD99 mAb given i.p. was able to reach the CNS as it labeled endothelial junctions of the BBB (data not shown). Mice receiving anti-CD99 mAb displayed significantly lower clinical scores than controls (p ≤0.001) at both peak disease and relapse (Fig 2A and Table I). The majority of anti-CD99 treated mice displayed mild disease and were even protected against the development of relapse (Fig 2A and Table II). Whole mount immunostaining revealed reduced histopathological burden of CD3+ T-cells in the SC of anti-CD99 treated mice compared to controls (Fig 2B). Qualitatively similar results were seen in SC of mice examined at the peak of relapse (not shown).
Figure 2. Anti-CD99 ameliorates RR-EAE and reduces histopathological burden.
(A) Comparison of mean clinical score between mice treated (arrows) with anti-CD99 (open circles) or control IgG (closed circles) at onset of clinical symptoms and every other day thereafter. (B) Whole mount immunostaining of spinal cord parenchymal postcapillary venules (PECAM-1; red) and CD3+ T-cell (green) infiltrates from anti-CD99 (lower panels) or control-IgG (upper panels) treated mice at peak of disease. Data shown are representative of 3 independent experiments (A,B) with a minimum of 5 mice per group and from >20 immunostainings performed on post-mortem material from 3 animals/group (B). Scale bar = 26μm. Error bars indicate SEM. **p< 0.001 (Mann-Whitney U test).
Table I. Clinical scores of RR-EAE mice.
The mean clinical scores of anti-CD99 or control IgG treated mice from three independent experiments were quantitated. Mean ± SEM.
| Treatment | Onset | Peak | Remission | Relapse |
|---|---|---|---|---|
| Control IgG | 0.34 ± 0.12 | 2.50 ± 0.24 | 0.71 ± 0.22 | 1.50 ± 0.31 |
| Anti-CD99 | 0.36 ± 0.12 ns | 1.39 ± 0.21 ** | 0.61 ± 0.24 ns | 0.46 ± 0.14 ** |
ns= non-significant
p≤0.001 compared to control IgG by Mann-Whitney U test
Table II. EAE severity of RR-EAE mice.
The percentages of mice receiving anti-CD99 or control IgG with no clinical score, mild (score 1–2) or severe disease (score 3–5).
| Peak | |||
|---|---|---|---|
| Treatment | No clinical score | Mild (1–2) | Severe (3–5) |
| Control IgG | 10% (4/40) | 35% (14/40) | 55% (22/40) |
| Anti-CD99 | 25% (10/40) | 62.5% (25/40) | 12.5% (5/40) |
| Relapse | |||
|---|---|---|---|
| Treatment | No clinical score | Mild (1–2) | Severe (3–5) |
| Control IgG | 11% (2/18) | 83% (15/18) | 6% (1/18) |
| Anti-CD99 | 50% (9/18) | 50% (9/18) | 0% (0/18) |
Anti-CD99 treatment diminishes inflammatory leukocyte infiltration in the CNS of RR-EAE mice
The in vitro findings from Fig 1 and whole mount staining in Fig 2 suggests that the therapeutic efficacy of anti-CD99 was due to its ability to block the recruitment of lymphocytes from entering the CNS. However, similar to MS, RR-EAE is also characterized by extensive CNS perivascular infiltration of inflammatory APCs that perpetuate damage (3, 8, 43). In particular, iDCs are major players in CNS autoimmune pathology (8, 44), and their numbers correlate with disease severity and progression (5, 8, 42, 43, 45–48). The molecular mechanisms governing the TEM of this pathogenic subset into the CNS are poorly understood.
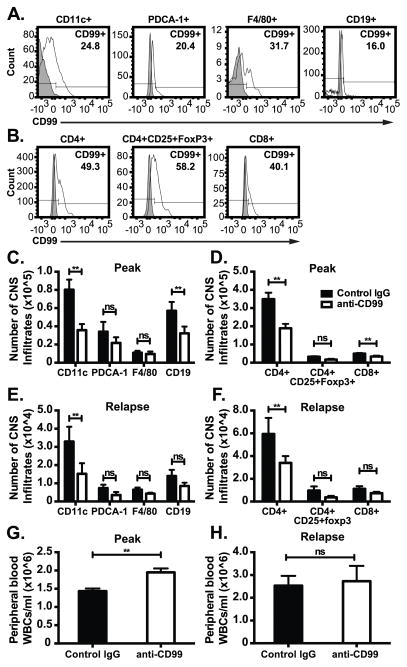
The frequency of CNS infiltrating WBC subsets known to play a role in the pathogenesis of disease that express CD99 was characterized by FACs on cells from dissociated brains and spinal cords harvested at disease peak (Figure 3A–B) from 5 individual mice. We next enumerated the different CNS infiltrating inflammatory WBC populations in anti-CD99 or control mice by performing FACS at disease peak and relapse. There was a significant reduction in the absolute numbers of CNS CD45hi-infiltrating B-cells, iDC, CD4+ and CD8+ T cells at disease peak and relapse in CD99 treated mice (Fig 3C–F). CD3+CD4+ Th1 and Th17 cells were both reduced to an equal extent in anti-CD99 treated mice (data not shown). There were no significant differences in the absolute numbers of microglia (CD45lo CD11blo CD11c+), macrophages or plasmacytoid DCs between anti-CD99 and control IgG treated RR-EAE mice. In contrast to the CNS, we observed no significant differences in absolute cell numbers in spleen and lymph nodes between anti-CD99 treated mice and controls at any time point assessed (data not shown). In addition, at disease onset and remission there were no significant differences in cell numbers between anti-CD99 and control in any of the tissues examined. Importantly, peripheral blood WBC counts were slightly higher in CD99 treated animals compared to control IgG (Figure 3G–H). Taken together, these data suggest that anti-CD99 therapy specifically inhibited WBC migration into the CNS rather than depleting WBCs or inhibiting emigration from bone marrow.
Figure 3. Inhibiting CD99 function prevents accumulation of inflammatory infiltrates in the CNS of RR-EAE mice.
(A) The percentage of CD45hi infiltrating iDCs (CD11b+CD11c+Ly6chi), macrophages (CD11b+CD11c−F4/80+), plasmacytoid DCs (CD11b−CD11c+PDCA-1+) and B-cells (CD19+), and (B) T-helper cells (CD3+CD4+), cytotoxic T-cells (CD3+CD8+) and T-regulatory cells (CD3+CD4+CD25+Foxp3+) expressing CD99 from the brain and spinal cord of individual mice at disease peak was quantitated by FACS. Histograms are representative of 5 individual mice. Filled bar indicates fluorescence minus one control and open line represents CD99 expressing cells. (C–F). The number of CD45hi infiltrating CD11c+ iDCs, F4/80+ macrophages, PDCA-1+ plasmacytoid DCs and CD19+ B-cells, and CD4+ T cells, CD8+ T-cells and CD4+CD25+Foxp3+ T-regulatory cells at disease peak (C–D) and relapse (E–F) were enumerated from brain and spinal cords of individual anti-CD99 (open bars) or control IgG (closed bars) treated mice by FACS. Bolded antigens denote respective labeling on the bar graphs. Data shown in C–F are mean absolute number of CD45hi CNS infiltrating cells pooled from 3 independent experiments obtained from at least 12 mice/group. Error bars indicate standard error of the mean. **p< 0.05 by two-way ANOVA. (G–H) Peripheral blood leukocytes were from anti-CD99 or control IgG treated RR-EAE mice were collected via cardiac puncture at disease peak and relapse and manually counted (from Fig 2A). Error bars indicate SEM. **p< 0.05; ns= not significant by t-Test.
Anti-CD99 blocks disease relapse in ongoing EAE and reduces leukocyte accumulation in the CNS
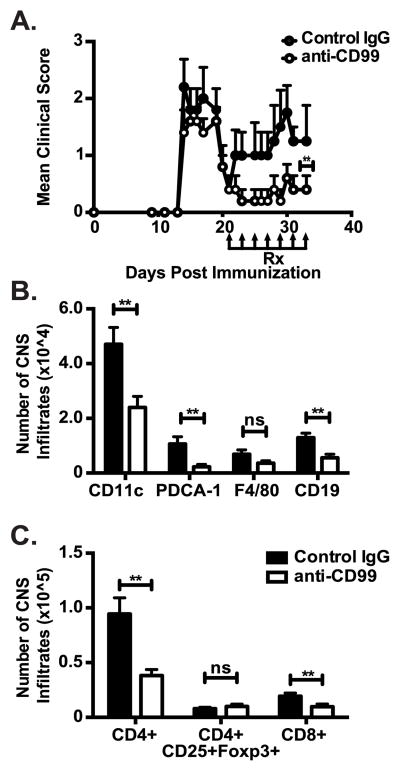
In the clinic most patients with MS receive treatment following disease exacerbations, rather than prophylactically. Therefore, we took a more clinically relevant approach by treating mice with established EAE only during the secondary phase of disease at relapse onset (~23 DPI). Anti-CD99 therapy ameliorated the development and severity of the secondary phase of disease (Fig 4A). Similar to our observations in Fig 3, CD45hi CNS infiltrating iDCs, B-cells, and CD4+ and CD8+ T-cells were all significantly reduced in mice that received anti-CD99 therapy at the onset of relapse compared to controls. Importantly, as observed in Figure 3B,D there was no reduction of CD3+CD4+CD25+Foxp3+ T-regulatory cell accumulation in the CNS following treatment with anti-CD99 (Fig 4C). Selective inhibition of effector but not regulatory T cell TEM by anti-CD99 has important implications for translation into the clinical setting.
Figure 4. Anti-CD99 ameliorates relapse in ongoing RR-EAE and reduces CNS inflammatory infiltrates.
(A) Animals with ongoing RR-EAE were treated with anti-CD99 (open circles) or control IgG (closed circles) during the secondary phase of disease at the onset of relapse. **p< 0.001 by Mann-Whitney U test. (B–C) CNS tissues were harvested at the end of the experiment to enumerate inflammatory APC (B) and T-cell (C) infiltrates in the CNS of RR-EAE mice as described in Fig 3. Data in A are representative of 3 independent experiments (n= 8 mice/group for each experiment; **p< 0.001 by Mann-Whitney U test). Data in B–C are pooled from 12-mice/treatment group. Error bars indicate SEM. **p< 0.05 compared to control IgG by two way ANOVA (B,C).
Blocking WBC extravasation into the CNS is an exploitable and effective therapeutic target for treating CNS-directed autoimmune disease. Anti-VLA-4 (Natalizumab) is a highly potent and efficacious therapy for the management of MS. Anti-VLA-4 prevents the binding of leukocyte α4 integrin to its endothelial ligand VCAM-1, thereby inhibiting adhesion and subsequent TEM into the CNS. The S1P receptor modulator FTY720 (Fingolimod) sequesters WBCs in the lungs and secondary lymphoid organs (49), thus impeding homing of WBC to the CNS. In the present report, our data demonstrate that targeting CD99 interrupts leukocyte extravasation into the CNS. Anti-CD99 specifically disrupts the step of diapedesis but not adhesion or trafficking, making it a unique therapeutic target. Unlike the steps preceding TEM in the inflammatory response, diapedesis is arguably the only committed, non-reversible step of inflammation (50). Once WBCs undergo TEM, they are committed to carrying out their effector function in the inflammatory response (51).
Preventing WBC infiltration into the CNS reduces the extent of CNS inflammation and thereby ameliorates MS symptoms; however this does not come without risk (49, 52). Both anti-VLA-4 and Fingolimod therapies have been reported to impede the ability to protect against latent and chronic CNS viral infections by impairing immune surveillance (53–56). It is unknown whether anti-CD99 therapy would increase the risk of opportunistic infection, but fortunately recent advances in the field have made it possible to assess the likelihood with simple antibody tests (49).
Taken together, our report reveals a novel role for CD99 in the TEM of inflammatory WBCs across the BBB in vivo and further emphasizes the importance of WBC recruitment in the pathogenesis of CNS autoimmune disease. Furthermore, because anti-CD99 specifically blocks the step of diapedesis, anti-CD99 or small molecule antagonists of CD99 signaling (24) might serve as exploitable therapeutic targets for suppressing ongoing neuroinflammation in CNS autoimmune disease.
Acknowledgments
We wish to thank Clifford D. Carpenter for excellent technical assistance.
This work was supported by NIH T32 AI7476-17 and Heartland Affiliate AHA 15PRE22710025 to R.C. Winger, NIH grants R37 HL064774 and R01 HL046849 to W.A. Muller, R01 NS026543 to S.D. Miller and NCI CA060553 to The Robert H. Lurie Comprehensive Cancer Center Flow Cytometry Core Facility
Abbreviations used in this article
- TEM
transendothelial migration
- BBB
blood-brain barrier
- iDC
inflammatory myeloid-derived dendritic cell
- RR-EAE
relapsing remitting-experimental autoimmune encephalomyelitis
- SC
spinal cord
References
- 1.Hickey WF. Basic principles of immunological surveillance of the normal central nervous system. Glia. 2001;36:118–124. doi: 10.1002/glia.1101. [DOI] [PubMed] [Google Scholar]
- 2.Ousman SS, Kubes P. Immune surveillance in the central nervous system. Nature neuroscience. 2012;15:1096–1101. doi: 10.1038/nn.3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frohman EM, Racke MK, Raine CS. Multiple sclerosis--the plaque and its pathogenesis. N Engl J Med. 2006;354:942–955. doi: 10.1056/NEJMra052130. [DOI] [PubMed] [Google Scholar]
- 4.Lucchinetti C, Bruck W, Parisi J, Scheithauer B, Rodriguez M, Lassmann H. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Annals of neurology. 2000;47:707–717. doi: 10.1002/1531-8249(200006)47:6<707::aid-ana3>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 5.Ifergan I, Kebir H, Bernard M, Wosik K, Dodelet-Devillers A, Cayrol R, Arbour N, Prat A. The blood-brain barrier induces differentiation of migrating monocytes into Th17-polarizing dendritic cells. Brain: a journal of neurology. 2008;131:785–799. doi: 10.1093/brain/awm295. [DOI] [PubMed] [Google Scholar]
- 6.Henderson AP, Barnett MH, Parratt JD, Prineas JW. Multiple sclerosis: distribution of inflammatory cells in newly forming lesions. Annals of neurology. 2009;66:739–753. doi: 10.1002/ana.21800. [DOI] [PubMed] [Google Scholar]
- 7.Miller SD, McMahon EJ, Schreiner B, Bailey SL. Antigen presentation in the CNS by myeloid dendritic cells drives progression of relapsing experimental autoimmune encephalomyelitis. Ann N Y Acad Sci. 2007;1103:179–191. doi: 10.1196/annals.1394.023. [DOI] [PubMed] [Google Scholar]
- 8.Bailey SL, Schreiner B, McMahon EJ, Miller SD. CNS myeloid DCs presenting endogenous myelin peptides ‘preferentially’ polarize CD4+ T(H)-17 cells in relapsing EAE. Nat Immunol. 2007;8:172–180. doi: 10.1038/ni1430. [DOI] [PubMed] [Google Scholar]
- 9.Elices MJ, Osborn L, Takada Y, Crouse C, Luhowskyj S, Hemler ME, Lobb RR. VCAM-1 on activated endothelium interacts with the leukocyte integrin VLA-4 at a site distinct from the VLA-4/Fibronectin binding site. Cell. 1990;60:577–584. doi: 10.1016/0092-8674(90)90661-w. [DOI] [PubMed] [Google Scholar]
- 10.Siegelman MH, Stanescu D, Estess P. The CD44-initiated pathway of T-cell extravasation uses VLA-4 but not LFA-1 for firm adhesion. J Clin Invest. 2000;105:683–691. doi: 10.1172/JCI8692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berlin C, Bargatze RF, Campbell JJ, von Andrian UH, Szabo MC, Hasslen SR, Nelson RD, Berg EL, Erlandsen SL, Butcher EC. Alpha 4 integrins mediate lymphocyte attachment and rolling under physiologic flow. Cell. 1995;80:413–422. doi: 10.1016/0092-8674(95)90491-3. [DOI] [PubMed] [Google Scholar]
- 12.Chan JR, Hyduk SJ, Cybulsky MI. Chemoattractants induce a rapid and transient upregulation of monocyte alpha4 integrin affinity for vascular cell adhesion molecule 1 which mediates arrest: an early step in the process of emigration. J Exp Med. 2001;193:1149–1158. doi: 10.1084/jem.193.10.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huo Y, Hafezi-Moghadam A, Ley K. Role of vascular cell adhesion molecule-1 and fibronectin connecting segment-1 in monocyte rolling and adhesion on early atherosclerotic lesions. Circ Res. 2000;87:153–159. doi: 10.1161/01.res.87.2.153. [DOI] [PubMed] [Google Scholar]
- 14.Laschinger M, Engelhardt B. Interaction of alpha4-integrin with VCAM-1 is involved in adhesion of encephalitogenic T cell blasts to brain endothelium but not in their transendothelial migration in vitro. J Neuroimmunol. 2000;102:32–43. doi: 10.1016/s0165-5728(99)00156-3. [DOI] [PubMed] [Google Scholar]
- 15.Jain P, Coisne C, Enzmann G, Rottapel R, Engelhardt B. Alpha4beta1 integrin mediates the recruitment of immature dendritic cells across the blood-brain barrier during experimental autoimmune encephalomyelitis. J Immunol. 2010;184:7196–7206. doi: 10.4049/jimmunol.0901404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vajkoczy P, Laschinger M, Engelhardt B. Alpha4-integrin-VCAM-1 binding mediates G protein-independent capture of encephalitogenic T cell blasts to CNS white matter microvessels. J Clin Invest. 2001;108:557–565. doi: 10.1172/JCI12440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yednock TA, Cannon C, Fritz LC, Sanchez-Madrid F, Steinman L, Karin N. Prevention of experimental autoimmune encephalomyelitis by antibodies against alpha 4 beta 1 integrin. Nature. 1992;356:63–66. doi: 10.1038/356063a0. [DOI] [PubMed] [Google Scholar]
- 18.Baron JL, Madri JA, Ruddle NH, Hashim G, Janeway CA., Jr Surface expression of alpha 4 integrin by CD4 T cells is required for their entry into brain parenchyma. J Exp Med. 1993;177:57–68. doi: 10.1084/jem.177.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Theien BE, Vanderlugt CL, Eagar TN, Nickerson-Nutter C, Nazareno R, Kuchroo VK, Miller SD. Discordant effects of anti-VLA-4 treatment before and after onset of relapsing experimental autoimmune encephalomyelitis. J Clin Invest. 2001;107:995–1006. doi: 10.1172/JCI11717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lehmann-Horn K, Sagan SA, Bernard CC, Sobel RA, Zamvil SS. B-cell very late antigen-4 deficiency reduces leukocyte recruitment and susceptibility to central nervous system autoimmunity. Annals of neurology. 2015;77:902–908. doi: 10.1002/ana.24387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schenkel AR, Mamdouh Z, Chen X, Liebman RM, Muller WA. CD99 plays a major role in the migration of monocytes through endothelial junctions. Nat Immunol. 2002;3:143–150. doi: 10.1038/ni749. [DOI] [PubMed] [Google Scholar]
- 22.Lou O, Alcaide P, Luscinskas FW, Muller WA. CD99 is a key mediator of the transendothelial migration of neutrophils. J Immunol. 2007;178:1136–1143. doi: 10.4049/jimmunol.178.2.1136. [DOI] [PubMed] [Google Scholar]
- 23.Winger RC, Koblinski JE, Kanda T, Ransohoff RM, Muller WA. Rapid remodeling of tight junctions during paracellular diapedesis in a human model of the blood-brain barrier. J Immunol. 2014;193:2427–2437. doi: 10.4049/jimmunol.1400700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watson RL, Buck J, Levin LR, Winger RC, Wang J, Arase H, Muller WA. Endothelial CD99 signals through soluble adenylyl cyclase and PKA to regulate leukocyte transendothelial migration. The Journal of Experimental Medicine. 2015 doi: 10.1084/jem.20150354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dufour EM, Deroche A, Bae Y, Muller WA. CD99 is essential for leukocyte diapedesis in vivo. Cell Commun Adhes. 2008;15:351–363. doi: 10.1080/15419060802442191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bixel G, Kloep S, Butz S, Petri B, Engelhardt B, Vestweber D. Mouse CD99 participates in T-cell recruitment into inflamed skin. Blood. 2004;104:3205–3213. doi: 10.1182/blood-2004-03-1184. [DOI] [PubMed] [Google Scholar]
- 27.Brown AM, McFarlin DE. Relapsing experimental allergic encephalomyelitis in the SJL/J mouse. Laboratory investigation; a journal of technical methods and pathology. 1981;45:278–284. [PubMed] [Google Scholar]
- 28.Robinson AP, Harp CT, Noronha A, Miller SD. The experimental autoimmune encephalomyelitis (EAE) model of MS: utility for understanding disease pathophysiology and treatment. Handbook of clinical neurology. 2014;122:173–189. doi: 10.1016/B978-0-444-52001-2.00008-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ali J, Liao F, Martens E, Muller WA. Vascular endothelial cadherin (VE-cadherin): cloning and role in endothelial cell-cell adhesion. Microcirculation. 1997;4:267–277. doi: 10.3109/10739689709146790. [DOI] [PubMed] [Google Scholar]
- 30.Muller WA, Ratti CM, McDonnell SL, Cohn ZA. A human endothelial cell-restricted, externally disposed plasmalemmal protein enriched in intercellular junctions. J Exp Med. 1989;170:399–414. doi: 10.1084/jem.170.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schenkel AR, Chew TW, Muller WA. Platelet endothelial cell adhesion molecule deficiency or blockade significantly reduces leukocyte emigration in a majority of mouse strains. J Immunol. 2004;173:6403–6408. doi: 10.4049/jimmunol.173.10.6403. [DOI] [PubMed] [Google Scholar]
- 32.Mamdouh Z, Mikhailov A, Muller WA. Transcellular migration of leukocytes is mediated by the endothelial lateral border recycling compartment. J Exp Med. 2009;206:2795–2808. doi: 10.1084/jem.20082745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mamdouh Z, Kreitzer GE, Muller WA. Leukocyte transmigration requires kinesin-mediated microtubule-dependent membrane trafficking from the lateral border recycling compartment. J Exp Med. 2008;205:951–966. doi: 10.1084/jem.20072328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carman CV, Springer TA. A transmigratory cup in leukocyte diapedesis both through individual vascular endothelial cells and between them. J Cell Biol. 2004;167:377–388. doi: 10.1083/jcb.200404129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCarthy DP, Richards MH, Miller SD. Mouse models of multiple sclerosis: experimental autoimmune encephalomyelitis and Theiler’s virus-induced demyelinating disease. Methods Mol Biol. 2012;900:381–401. doi: 10.1007/978-1-60761-720-4_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weber EW, Han F, Tauseef M, Birnbaumer L, Mehta D, Muller WA. TRPC6 is the endothelial calcium channel that regulates leukocyte transendothelial migration during the inflammatory response. J Exp Med. 2015;212:1883–1899. doi: 10.1084/jem.20150353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Terry RL, Ifergan I, Miller SD. Experimental Autoimmune Encephalomyelitis in Mice. Methods Mol Biol. 2014 doi: 10.1007/7651_2014_88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McMahon EJ, Bailey SL, Castenada CV, Waldner H, Miller SD. Epitope spreading initiates in the CNS in two mouse models of multiple sclerosis. Nat Med. 2005;11:335–339. doi: 10.1038/nm1202. [DOI] [PubMed] [Google Scholar]
- 39.Bailey-Bucktrout SL, Caulkins SC, Goings G, Fischer JA, Dzionek A, Miller SD. Cutting edge: central nervous system plasmacytoid dendritic cells regulate the severity of relapsing experimental autoimmune encephalomyelitis. J Immunol. 2008;180:6457–6461. doi: 10.4049/jimmunol.180.10.6457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bullard DC, Hu X, Schoeb TR, Collins RG, Beaudet AL, Barnum SR. Intercellular adhesion molecule-1 expression is required on multiple cell types for the development of experimental autoimmune encephalomyelitis. J Immunol. 2007;178:851–857. doi: 10.4049/jimmunol.178.2.851. [DOI] [PubMed] [Google Scholar]
- 41.Cayrol R, Wosik K, Berard JL, Dodelet-Devillers A, Ifergan I, Kebir H, Haqqani AS, Kreymborg K, Krug S, Moumdjian R, Bouthillier A, Becher B, Arbour N, David S, Stanimirovic D, Prat A. Activated leukocyte cell adhesion molecule promotes leukocyte trafficking into the central nervous system. Nat Immunol. 2008;9:137–145. doi: 10.1038/ni1551. [DOI] [PubMed] [Google Scholar]
- 42.Ifergan I, Kebir H, Terouz S, Alvarez JI, Lecuyer MA, Gendron S, Bourbonniere L, Dunay IR, Bouthillier A, Moumdjian R, Fontana A, Haqqani A, Klopstein A, Prinz M, Lopez-Vales R, Birchler T, Prat A. Role of Ninjurin-1 in the migration of myeloid cells to central nervous system inflammatory lesions. Annals of neurology. 2011;70:751–763. doi: 10.1002/ana.22519. [DOI] [PubMed] [Google Scholar]
- 43.Greter M, Heppner FL, Lemos MP, Odermatt BM, Goebels N, Laufer T, Noelle RJ, Becher B. Dendritic cells permit immune invasion of the CNS in an animal model of multiple sclerosis. Nat Med. 2005;11:328–334. doi: 10.1038/nm1197. [DOI] [PubMed] [Google Scholar]
- 44.Chastain EM, Duncan DS, Rodgers JM, Miller SD. The role of antigen presenting cells in multiple sclerosis. Biochim Biophys Acta. 2011;1812:265–274. doi: 10.1016/j.bbadis.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clarkson BD, Walker A, Harris MG, Rayasam A, Sandor M, Fabry Z. CCR2-dependent dendritic cell accumulation in the central nervous system during early effector experimental autoimmune encephalomyelitis is essential for effector T cell restimulation in situ and disease progression. J Immunol. 2015;194:531–541. doi: 10.4049/jimmunol.1401320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zozulya AL, Ortler S, Lee J, Weidenfeller C, Sandor M, Wiendl H, Fabry Z. Intracerebral dendritic cells critically modulate encephalitogenic versus regulatory immune responses in the CNS. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29:140–152. doi: 10.1523/JNEUROSCI.2199-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Serafini B, Rosicarelli B, Magliozzi R, Stigliano E, Capello E, Mancardi GL, Aloisi F. Dendritic cells in multiple sclerosis lesions: maturation stage, myelin uptake, and interaction with proliferating T cells. Journal of neuropathology and experimental neurology. 2006;65:124–141. doi: 10.1097/01.jnen.0000199572.96472.1c. [DOI] [PubMed] [Google Scholar]
- 48.Serafini B, Columba-Cabezas S, Di Rosa F, Aloisi F. Intracerebral recruitment and maturation of dendritic cells in the onset and progression of experimental autoimmune encephalomyelitis. Am J Pathol. 2000;157:1991–2002. doi: 10.1016/S0002-9440(10)64838-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Steinman L. Immunology of relapse and remission in multiple sclerosis. Annu Rev Immunol. 2014;32:257–281. doi: 10.1146/annurev-immunol-032713-120227. [DOI] [PubMed] [Google Scholar]
- 50.Muller WA. PECAM: Regulating the start of diapedesis. In: Ley K, editor. Adhesion Molecules: Function and Inhibition. Birkhauser Verlag AG; Basel: 2007. pp. 201–220. [Google Scholar]
- 51.Sumagin R, I, Sarelius H. Intercellular adhesion molecule-1 enrichment near tricellular endothelial junctions is preferentially associated with leukocyte transmigration and signals for reorganization of these junctions to accommodate leukocyte passage. J Immunol. 2010;184:5242–5252. doi: 10.4049/jimmunol.0903319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hussain RZ, Hayardeny L, Cravens PC, Yarovinsky F, Eagar TN, Arellano B, Deason K, Castro-Rojas C, Stuve O. Immune surveillance of the central nervous system in multiple sclerosis--relevance for therapy and experimental models. J Neuroimmunol. 2014;276:9–17. doi: 10.1016/j.jneuroim.2014.08.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Langer-Gould A, Atlas SW, Green AJ, Bollen AW, Pelletier D. Progressive multifocal leukoencephalopathy in a patient treated with natalizumab. N Engl J Med. 2005;353:375–381. doi: 10.1056/NEJMoa051847. [DOI] [PubMed] [Google Scholar]
- 54.Stuve O, Marra CM, Jerome KR, Cook L, Cravens PD, Cepok S, Frohman EM, Phillips JT, Arendt G, Hemmer B, Monson NL, Racke MK. Immune surveillance in multiple sclerosis patients treated with natalizumab. Annals of neurology. 2006;59:743–747. doi: 10.1002/ana.20858. [DOI] [PubMed] [Google Scholar]
- 55.del Pilar Martin M, Cravens PD, Winger R, Frohman EM, Racke MK, Eagar TN, Zamvil SS, Weber MS, Hemmer B, Karandikar NJ, Kleinschmidt-DeMasters BK, Stuve O. Decrease in the numbers of dendritic cells and CD4+ T cells in cerebral perivascular spaces due to natalizumab. Archives of neurology. 2008;65:1596–1603. doi: 10.1001/archneur.65.12.noc80051. [DOI] [PubMed] [Google Scholar]
- 56.Calic Z, Cappelen-Smith C, Hodgkinson SJ, McDougall A, Cuganesan R, Brew BJ. Treatment of progressive multifocal leukoencephalopathy-immune reconstitution inflammatory syndrome with intravenous immunoglobulin in a patient with multiple sclerosis treated with fingolimod after discontinuation of natalizumab. Journal of clinical neuroscience: official journal of the Neurosurgical Society of Australasia. 2015;22:598–600. doi: 10.1016/j.jocn.2014.08.016. [DOI] [PubMed] [Google Scholar]