Abstract
We describe here a continuous, spectrophotometric, enzyme-coupled assay useful to monitor reactions catalyzed by nucleoside triphosphohydrolases. In particular, using Escherichia coli deoxynucleoside triphosphohydrolase (Dgt), which hydrolyzes dGTP to deoxyguanosine and tripolyphosphate (PPPi), as the enzyme to be tested, we devised a procedure relying on purine nucleoside phosphorylase (PNPase) and xanthine oxidase (XOD) as the auxiliary enzymes. The deoxyguanosine released by Dgt can indeed be conveniently subjected to phosphorolysis by PNPase, yielding deoxyribose-1-phosphate and guanine, which, in turn, can be oxidized to 8-oxoguanine by XOD. By this means, it was possible to continuously detect Dgt activity at 297 nm, at which wavelength the difference between the molar extinction coefficients of 8-oxoguanine (8,000 M−1cm−1) and guanine (1,090 M−1cm−1) is maximal. The initial velocities of Dgt-catalyzed reactions were then determined in parallel with the enzyme-coupled assay and with a discontinuous HPLC method able to selectively detect deoxyguanosine. Under appropriate conditions of excess of auxiliary enzymes, the activities determined with our continuous enzyme-coupled assay were quantitatively comparable with those observed with the HPLC method. Moreover, the enzyme-coupled assay proved to be more sensitive than the chromatographic procedure, permitting reliable detection of Dgt activity at low dGTP substrate concentrations.
Keywords: enzyme-coupled assay, deoxynucleoside triphosphatase, Escherichia coli Dgt, purine nucleoside phosphorylase, xanthine oxidase, 8-oxoguanine
INTRODUCTION
The turnover of dNTPs in vivo is an important factor affecting both the rate of DNA replication and its fidelity. The synthesis of dNTPs starts with the reduction of ribonucleoside diphosphates (rNDPs) to the corresponding dNDPs by ribonucleoside diphosphate reductase, an allosteric enzyme whose regulation ensures a proper balance of the 4 dNDPs [1,2]. Subsequently, the nonspecific action of nucleoside diphosphate kinase (NdpK) yields dNTPs at the expense of ATP and dNDPs [3–5]. The presence of a properly balanced pool of dNTPs is not only important to replication velocity, but is also essential to guarantee high replication fidelity. Interestingly, although NdpK is not an essential enzyme in E. coli, mutator phenotypes were linked to defects of this enzyme [6,7], indicating that unbalances in the dNTP pool are potentially mutagenic. The maintenance of a balanced dNTP pool is also affected by the dephosphorylation of dNTPs by the action of various nucleotidases, including the dNTP triphosphohydrolases [8]. These enzymes catalyze the release of tripolyphosphate and deoxynucleosides at the expense of the corresponding dNTPs [9–14]. In Escherichia coli, Dgt was early recognized as a dNTP triphosphohydrolase [9], and it was also demonstrated that Dgt strongly prefers dGTP as substrate. Remarkably, inactivation of the dgt gene triggers a mutator phenotype, suggesting that an increase in the dGTP pool reduces replication fidelity [15].
At present, the activity of dNTP triphosphohydrolases is determined primarily using discontinuous assays. Usually, aliquots are withdrawn from reaction mixtures as a function of time, the reaction products are separated by chromatographic techniques, and they are finally revealed by UV spectroscopy or radioactivity counting [13,16,17]. A discontinuous high-throughput procedure was also reported [18]. This method relies on the use of Escherichia coli inorganic pyrophosphatase to release orthophosphate from tripolyphosphate, and by the subsequent colorimetric quantitation of orthophosphate.
Recently, an elegant continuous coupled assay of dNTP triphosphohydrolases has been proposed [19]. This method relies on the coupled release of orthophosphate from tripolyphosphate catalyzed by Saccharomyces cerevisiae Ppx1 exopolyphosphatase [19]. The released orthophosphate is then fluorimetrically determined using a modified phosphate-binding protein (N-(2-[1-maleimidyl]ethyl)-7-(diethylamino) coumarin-3-carboxamide-phosphate-binding protein), the fluorescence of which is greatly increased when bound to the target [20,21]. Upon comparison with a reference discontinuous method, this procedure proved to be robust and accurate [19]. However, while the fluorescent phosphate-binding protein (FPBP) is commercially available, one has to overexpress and purify the yeast Ppx1 exopolyphosphatase. In addition, the use of FPBP to detect orthophosphate faces at least two challenges: i) first, due to the low KD of the FPBP-phosphate complex (30–80 nM, depending on pH), homogeneous FPBP has to be further purified to remove the phosphate tightly bound to the protein [20]; ii) the fluorescence increase of the FPBP triggered by the binding of orthophosphate is negatively affected by the ionic strength of the assay solution [22].
We therefore thought it of interest to design a continuous coupled assay of dNTP triphosphohydrolases relying on enzymes commercially available and ready to use. In addition, we searched for continuous method(s) able to detect both reaction products, i.e., the tripolyphosphate and the deoxynucleoside.
Here we report on the continuous detection of E. coli Dgt activity. In particular, the detection of tripolyphosphate or deoxyguanosine was attempted, and the best conditions to perform a continuous coupled assay are described.
MATERIALS AND METHODS
Materials
Buffers, analytical enzymes, and chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA). Analytical enzymes were used without any further purification. Sodium-tripolyphosphate (Na5P3O10) was obtained from Sigma-Aldrich (St. Louis, MO, USA) and Ferrania S.r.l. (Cairo Montenotte, SV, Italy). Spectroscopic experiments and Dgt activity measurements were performed using a Perkin-Elmer λ19 and a Beckman Coulter DU640 spectrophotometer, respectively.
Enzyme Units
One Unit of Purine Nucleoside Phosphorylase (PNPase; EC 2.4.2.1), Xanthine Oxidase (XOD, EC 1.17.3.2), and Alkaline Phosphatase (AP, EC 3.1.3.1) is defined as the amount of enzyme able to convert 1 µMol of substrate per min at pH 7.4 (25 °C), 7.5 (25 °C), and 10.4 (37 °C), respectively. The substrates considered are inosine, hypoxanthine, and p-nitrophenyl phosphate for PNPase, XOD, and AP, respectively.
Assay for tripolyphosphate (PPPi) hydrolysis by Alkaline Phosphatase (AP)
The hydrolysis of tripolyphosphate by AP was assayed using a previously described enzyme-coupled assay for inorganic phosphate (Pi) [23]. The reactions contained 100 mM Tris-HCl (pH 8), 5 mM MgCl2, 0.25 mM inosine, 50 mU/mL Purine nucleoside phosphorylase (PNPase) and 500 mU/mL xanthine oxidase (XOD). PPPi and AP concentrations were 100 µM and 50 mU/mL, respectively. The hydrolysis was monitored by quantitating the generated phosphate (Pi) via absorbance changes at 293 nm [23].
Difference spectra of guanine and its XOD-generated oxidation product
To determine the spectroscopic properties of oxidized guanine produced by XOD, the UV spectrum of 0.3 mM guanine in 100 mM Tris-HCl (pH 8) was recorded between 220 and 320 nm against a reference containing buffer only. XOD (5 mU/mL, final concentration) was then added to both cuvettes and spectra were recorded 5, 10, 20, and 40 minutes after enzyme addition.
Assay of Xanthine Oxidase (XOD) with guanine
The oxidation of guanine by XOD (125 mU/mL) was assayed in 100 mM Tris-HCl (pH 8). Initial velocities were determined as a function of guanine concentration, and reactions were monitored at 297 nm.
Assay of Purine Nucleoside Phosphorylase (PNPase) with deoxyguanosine and xanthine oxidase (XOD)
The phosphorolysis of deoxyguanosine catalyzed by PNPase (50 mU/mL) was assayed in 100 mM Tris-HCl (pH 8), 5 mM KH2PO4, and 500 mU/mL of XOD. Initial velocities were determined as a function of deoxyguanosine concentration. The detection wavelength was 297 nm.
Expression and purification of Escherichia coli Dgt
Dgt was overexpressed using E. coli BL21(DE3) transformed with the pET30-dgt vector [10]. Transformants were grown at 37 °C until mid-log phase. To trigger overexpression, the cultures were shifted to 18 °C, and 0.5 mM isopropyl-β-D-thiogalactoside (IPTG) was added. After overnight incubation, cells were harvested and resuspended in 25 mM Tris-HCl (pH 8), 1 M NaCl, 10 % (w/v) sucrose, 5 mM EDTA, 1 mM β-mercaptoethanol, lysozyme, and EDTA-free protease inhibitor cocktail (Roche, Basel, Switzerland). The cell suspension was sonicated at 4°C, polyethyleneimine was added (0.3 %, v/v), and the protein extract centrifuged at 16,000 rpm for 30 min. Dgt was precipitated by adding ammonium sulfate at 50 % (w/v) and centrifuging the sample. The protein pellet was resuspended in 25 mM Tris-HCl (pH 8), 2 mM EDTA, 15 % glycerol (v/v) and 1 mM β-mercaptoethanol. The dissolved pellet was then dialyzed against 25 mM Tris-HCl (pH 8), 300 mM NaCl. The protein solution was mixed in batch with Ni-NTA agarose resin (Qiagen, Venlo, Holland), previously equilibrated with 25 mM phosphate buffer (pH 8), 300 mM NaCl (buffer A). After a 45 min incubation, the resin was washed with buffer A supplemented with 2 M NaCl and 5 mM imidazole. Dgt was then eluted from the resin using buffer A containing 200 mM imidazole. The hexahistidine tag was cleaved with Enterokinase, and the tag-less protein dialyzed against 25 mM Tris-HCl (pH 8), 10 mM MgCl2, 75 mM sodium citrate. The dialyzed sample was concentrated using ultrafiltration cells equipped with a 10 kDa molecular weight cut-off membrane.
Assay of Dgt activity using enzyme-coupled assay
The reaction was performed in 1.5-ml quartz cuvettes (1-cm path length) placed in a Beckman Coulter DU 640 spectrophotometer at ambient temperature. Reaction mixtures contained 100 mM Tris·HCl (pH 8.0), 5 mM MgCl2, 5 mM inorganic phosphate (Pi), 50 mU/ml PNPase, and 500 mU/ml XOD. dGTP substrate was included at a series of concentrations between 2 and 120 µM. Dgt enzyme was pre-activated at 37°C for 20 min, and the reactions started by adding Dgt enzyme at 4 nM final (hexamer) concentration. Automated absorbance readings were performed at 297 nm every 5 or 10 s. The absorbance was plotted against time, and the linear portion of the response was used to determine the initial velocity of the reaction. The calculated velocities were plotted as a function of dGTP concentration and fitted to the Hill equation [24] by non-linear regression using Kaleidagraph software version 3.6..
Determination of Dgt activity using HPLC analysis
The assay was performed by combining two different pre-mixtures each containing 20 mM Tris-HCl pH 8.0, 5 mM MgCl2. One contained in addition the dGTP substrate at a desired concentration, while the other contained Dgt enzyme. Both mixtures were pre-incubated at 37 °C for 20 minutes prior to the initiation of the reaction. Final enzyme concentration was 0.2 nM. Reactions were incubated at 30 °C and were terminated at the desired time by addition of EDTA (10 mM final concentration). Analysis of deoxyguanosine product was by ion-pair reverse phase chromatography using a Zorbax Eclipse Plus C18 column (Agilent Technologies) on an Agilent Technologies 1200 series HPLC instrument. Separation of the deoxyguanosine product peak and the dGTP substrate peak was achieved by using a water-acetonitrile buffer system. The column was equilibrated with 100 % water and the samples were eluted with a 0–25 % linear acetonitrile gradient. The volume of the deoxyguanosine peak was quantified by UV absorbance at 274 nm using ChemStation software (Agilent Technologies). Enzyme activity as function of the dGTP concentration was fit to the Michaelis-Menten equation using non-linear regression on Kaleidagraph software (version 3.6).
RESULTS AND DISCUSSION
Alkaline phosphatase and assay of Dgt activity
To assay the activity of Dgt with AP, we first investigated the activity of AP towards tripolyphosphate, using PNPase and XOD (Scheme 1) to detect the phosphate released according to our previously reported continuous enzyme-coupled assay [23]. However, in agreement with previous observations [25], we observed that AP does catalyze the release of orthophosphate from dGTP (data not shown). Therefore, the action of AP towards tripolyphosphate could not be coupled to Dgt to assay the activity of this enzyme in the presence of dGTP or other dNTPs. Accordingly, we searched for an alternative route to assay Dgt by means of a continuous and spectrophotometric enzyme-coupled procedure.
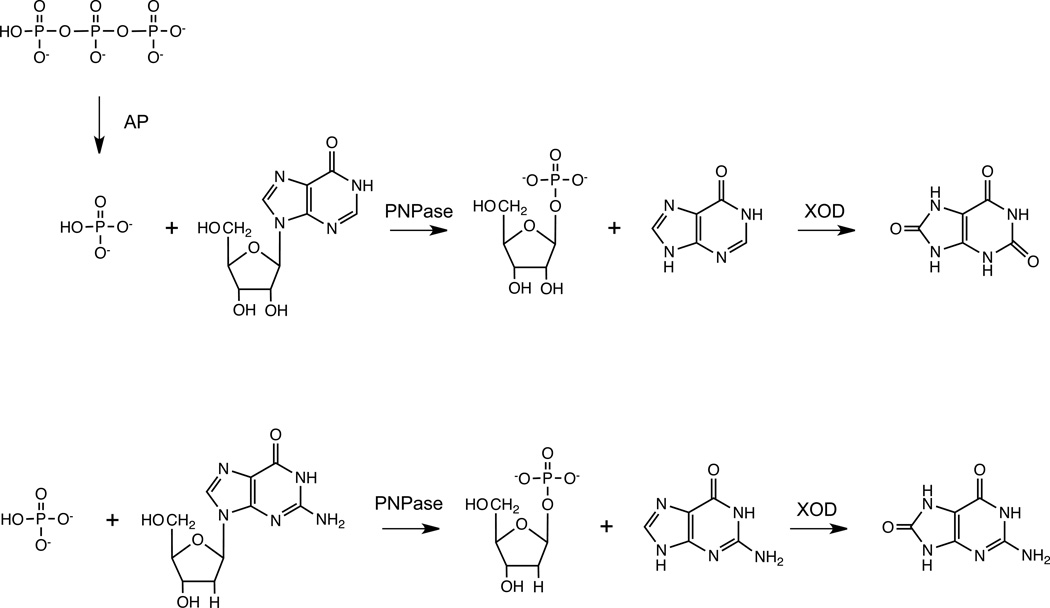
Scheme 1.
Reaction paths starting from the products (PPPi and dG) released by the catalytic action of Dgt yielding spectrophotometrically detectable compounds. Upper path, the tripolyphosphate (PPPi) produced by Dgt is hydrolyzed by alkaline phosphatase (AP), yielding orthophosphate (Pi), which, in the presence of inosine and PNPase, is converted to ribose-1-phosphate and hypoxanthine. Finally, hypoxanthine is oxidized by XOD, yielding uric acid. Lower path, the deoxyguanosine released by Dgt is phosphorolysed by PNPase, producing deoxyribose-1-phosphate and guanine. Guanine is then converted by XOD into 8-oxoguanine.
PNPase, XOD and assay of Dgt activity
We reasoned that Dgt activity could be assayed by detecting the release of deoxyguanosine using a continuous coupled enzyme assay. In particular, we hypothesized that deoxyguanosine (dG) could be a substrate for PNPase, which would accordingly catalyze the phosphorolysis of dG, yielding deoxyribose-1-phosphate and guanine (Scheme 1). This reaction could then be coupled to the oxidation of guanine by XOD, the product of this reaction being finally detected spectrophotometrically (Scheme 1).
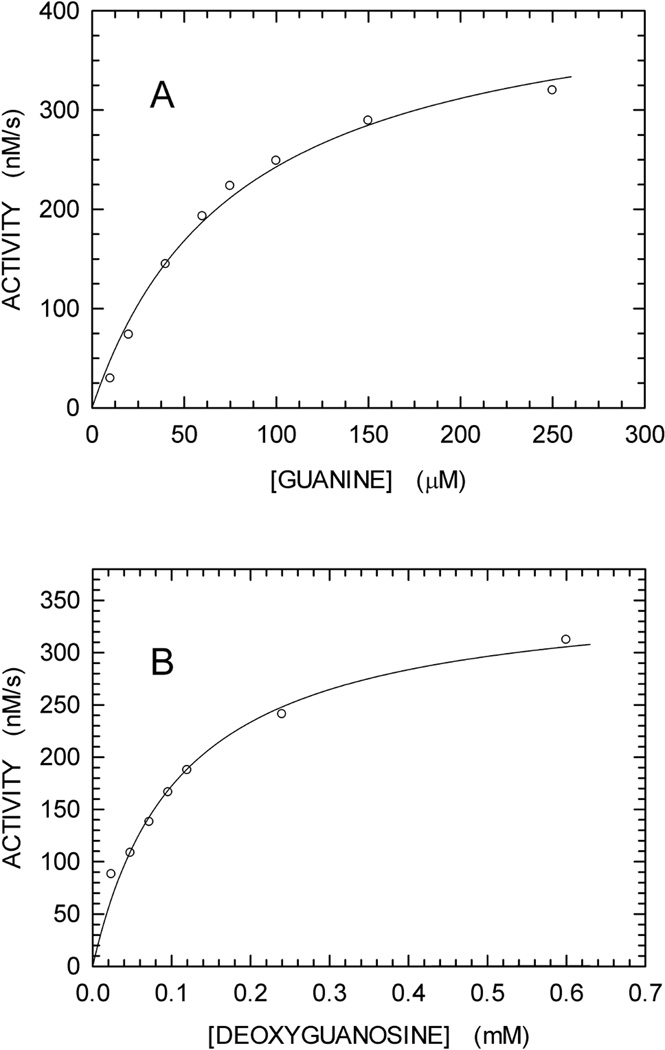
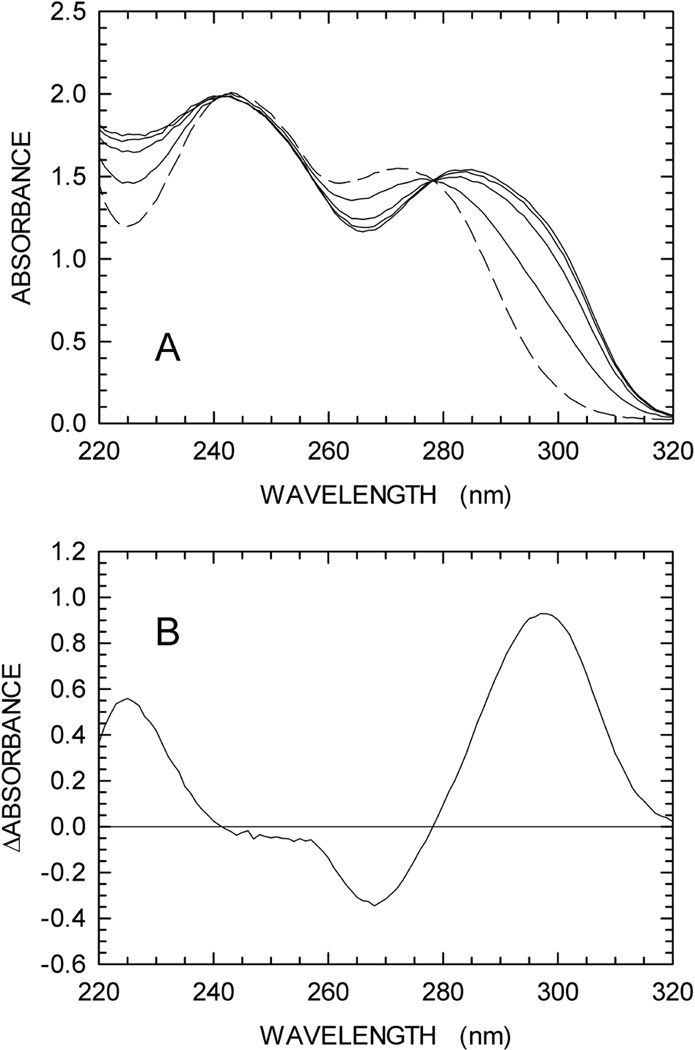
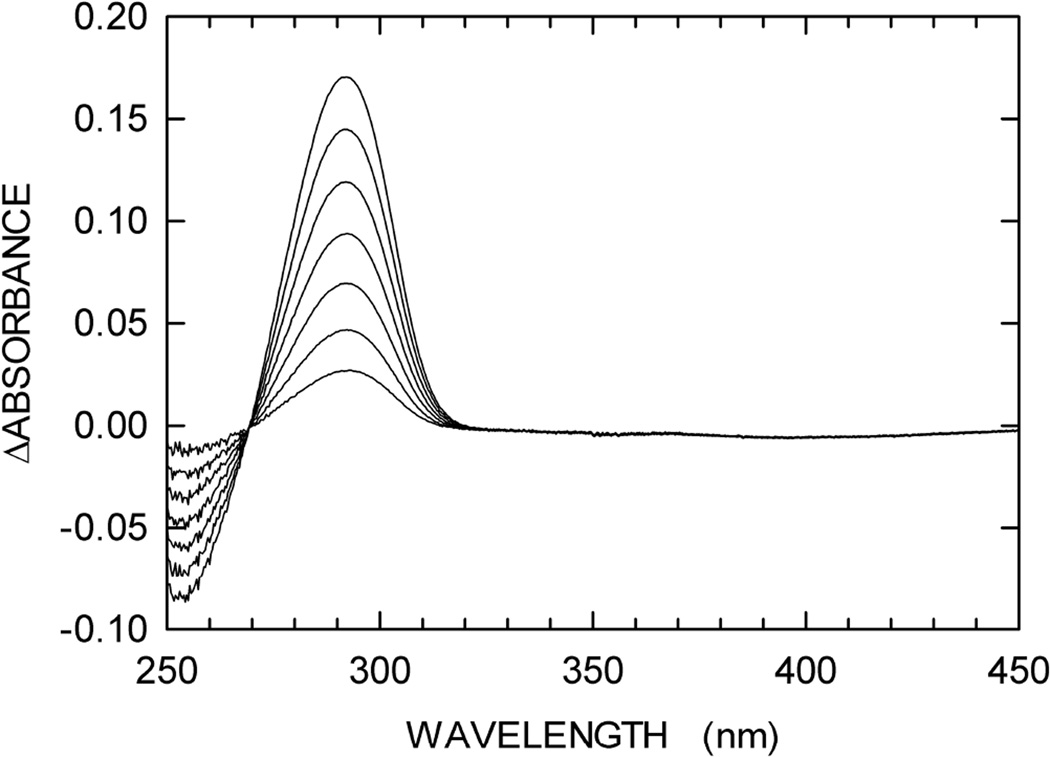
According to previous observations reported for Streptomyces cyanogenus and Enterobacter cloacae enzymes, guanine is converted to 8-oxoguanine by XOD [26,27]. We therefore tested if any spectroscopic difference can be detected when guanine is oxidized to 8-oxoguanine by XOD. To this aim, we recorded a series of difference spectra (between 220 and 320 nm) as a function of time. In particular, we prepared two solutions containing 100 mM Tris-HCl (pH 8), to one of which 0.3 mM guanine was also added. After a first difference spectrum (t0) was recorded using 1 cm path-length cells, XOD was added to both cuvettes. Further, difference spectra were recorded 5, 10, 20, and 40 min (t40) after XOD addition. By this means, the absorption spectrum of guanine was determined in the absence of XOD (t0), yielding an extinction coefficient for this base equal to 5,167 and 1,090 M−1cm−1 at 272 and 297 nm, respectively (fig. 2A). When XOD was added, a significant red-shift was observed, the magnitude of which was maximal 40 min after enzyme addition (Fig. 1A). The difference between the spectra at t40 and t0 was calculated, revealing a maximum at 297 nm (Fig. 1B). Remarkably, this difference spectrum is in good agreement with the absorption spectrum of 8-oxoguanosine, the molar extinction coefficient (ε) of which is equal to 8,000 M−1cm−1 at 297 nm [28]. Accordingly, the Δε at 297 nm when guanine is oxidized to 8-oxoguanine should be equal to 6,910 M−1cm−1. We then assayed the activity of XOD (125 mU/mL) as a function of guanine concentration, monitoring the absorbance increase at 297 nm due to oxidation of guanine, and using 6,910 M−1cm−1 as the Δε to calculate initial reaction velocities. XOD was find to nicely obey Michaelis-Menten kinetics, featuring Km and Vmax equal to 79.3 ± 10.9 µM and 435.4 ± 25.9 nM/s, respectively (Fig. 2A).
Fig. 2.
Initial velocities of reactions catalyzed by XOD and PNPase. (A) Oxidation of guanine by XOD as a function of substrate concentration. The continuous line represents the best fit to the Michaelis-Menten equation. (B) Phosphorolysis of deoxyguanosine by PNPase as a function of substrate concentration. The continuous line represents the best fit to the Michaelis-Menten equation.
Fig. 1.
Oxidation of guanine by xanthine oxidase (XOD). The reactions were performed as described in Materials and methods. (A) The absorption spectrum of 0.3 mM guanine was first determined in 100 mM Tris-HCl pH 8 (black line). XOD was then added to the cuvette, and spectra were recorded at 5, 10, 20, and 40 min after enzyme addition (green, blue, red, and dark-green lines, respectively). A reference cuvette was used containing 100 mM Tris-HCl pH 8 and XOD. (B) Difference between the spectra recorded before and after 40 min of XOD addition.
We finally tested the activity of PNPase (50 mU/mL) at the expense of deoxyguanosine. To this aim, we monitored absorbance changes at 297 nm of solutions containing 5 mM orthophosphate, PNPase, XOD (500 mU/mL), and variable concentrations of deoxyguanosine. When initial velocities were determined under these conditions, we obtained for PNPase Km and Vmax values equal to 110 ± 13 µM and 361.5 ± 17.5 nM/s, respectively (Fig. 2B).
Overall, our observations suggest that the reaction catalyzed by Dgt, e.g., at the expense of dGTP, could be spectrophotometrically and continuously monitored by the coupling with PNPase and XOD as auxiliary enzymes, and using 297 nm as the detection wavelength for the oxidized guanine product. According to McClure [29], the lag time observed with enzyme-coupled assays should approach a minimum when the first-order rate constant (Vmax/Km) of the secondary auxiliary enzyme is about 4-fold the Vmax/Km of the secondary coupling enzyme. We therefore tested our enzyme-coupled assay, using Dgt as the primary enzyme, dGTP as substrate, and PNPase (50 mU/mL) and XOD (500 mU/mL) as coupling enzymes. Under these conditions, the first-order rate constant (Vmax/Km) of XOD is 6–7 times higher than the corresponding rate constant of PNPase.
Analysis of Dgt activity using enzyme-coupled assay and comparison to a discontinuous, HPLC-based assay
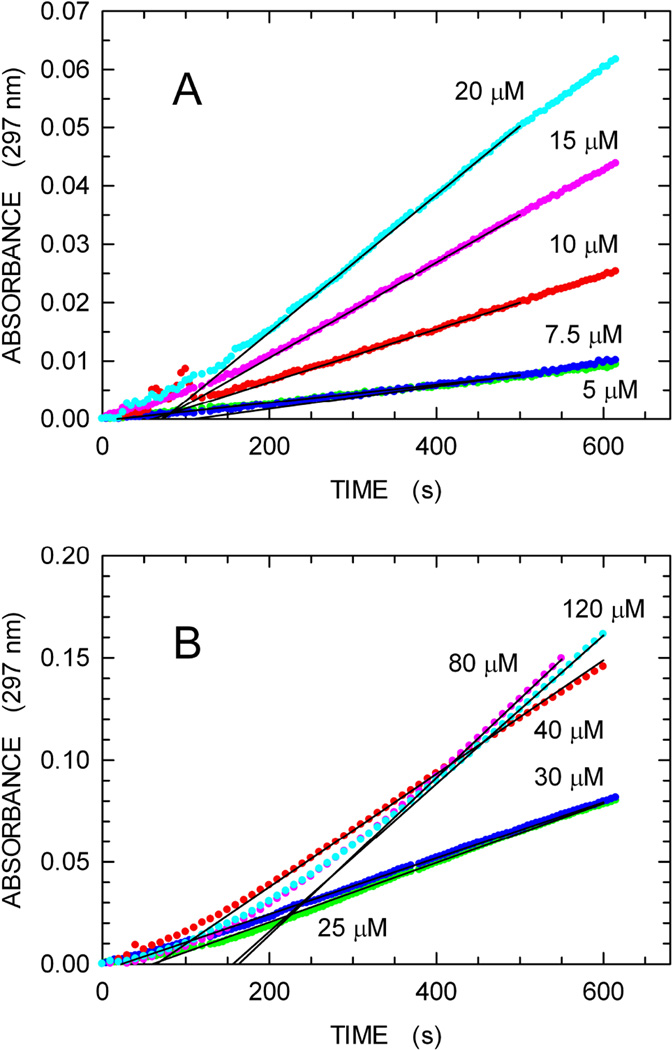
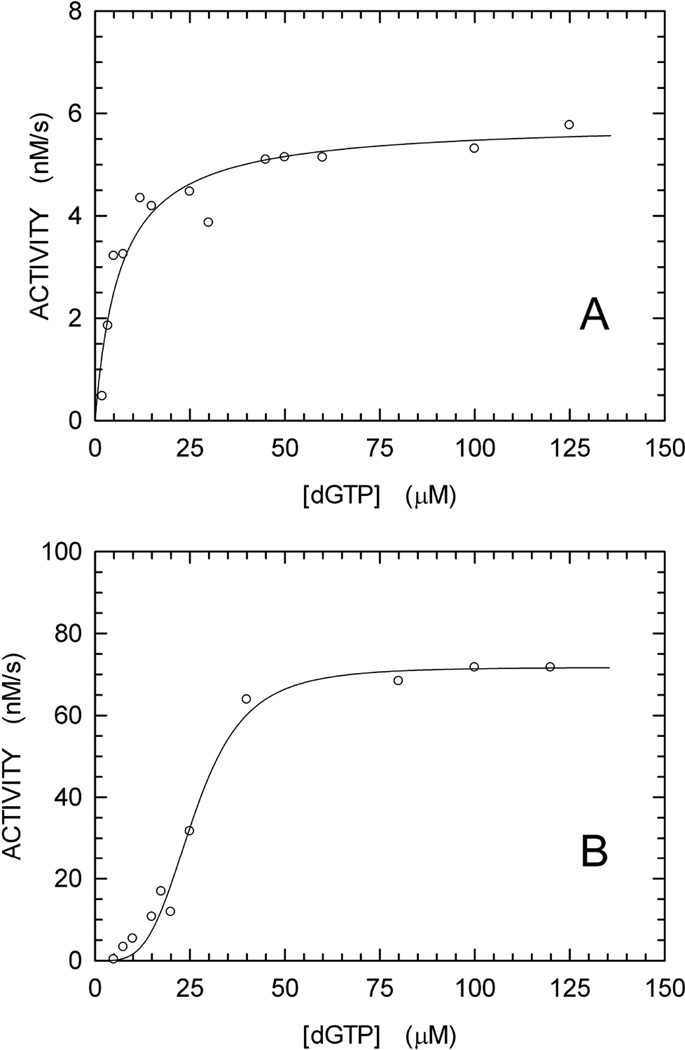
In Fig. 3 we show time courses for dGTP hydrolysis using the continuous enzyme-coupled assay for a wide series of dGTP concentrations. Linear increases in absorbance are observed, permitting ready determination of the initial reaction velocities. In particular, our enzymecoupled assay performed well under conditions (5 or 7.5 µM substrate) yielding an absorbance increase equal to 0.01 in 10 min (Fig. 3). This corresponds to the detection of 1.4 µM of 8-oxoguanine. This sensitivity is of the same order of magnitude when compared with the lower detection limit, i.e. 0.5 µM phosphate, of the continuous assay relying on the fluorescent phosphate-binding protein [20]. In Fig. 4, panel B, we have plotted the rate data as a function of the dGTP concentration. For comparison, in panel A we show data for Dgt activity measurements using an alternative, discontinuous HPLC-based method (see Materials and Methods). The HPLC method, which was developed first, lacks sensitivity in the lower dGTP concentration range, such that rates for dGTP concentrations less than 10 µM cannot be reliably measured. As a result, the best fit to the data is a simple Michaelis-Menten curve. However, the greater sensitivity of the continuous assay reveals a clearly sigmoidal response in the low dGTP substrate concentration range (Fig. 4B). This sigmoidal behavior presumably reflects an allosteric cooperative interaction, in which binding of one dGTP substrate molecule facilitates binding of a second dGTP.
Fig. 3.
Enzyme-coupled assay for formation of dG from dGTP. The reaction contained dGTP substrate, Dgt enzyme, Purine Nucleoside Phosphorylase and Xanthine Oxidase, as described in Materials and Methods. The reaction was monitored by increase in absorbance at 297 nm. The concentration of dGTP was varied from 2 to 120 µM. Panels A and B show the reactions for the 2–20 µM and 25–120 µM range, respectively. For each reaction, the zero-order reaction velocity (continuous black lines) was determined and used to derive the kinetics parameters of Fig. 4B. The lag-time before steady-state intermediate concentrations are reached, as observed here, is well known for enzyme-coupled assays [29].
Fig. 4.
dGTPase activity of E. coli Dgt measured using two different techniques. (A) HPLC method. The Dgt (hexamer) concentration was 0.2 nM; the kcat was calculated at 29.2 ± 1.1 s−1. (B) Enzyme-coupled assay. The protein concentration used was 4 nM; the kcat was calculated at 17.9 ± 0.6 s−1. The data were fitted to the Hill equation [23]. For the curve of Fig. 4B, an N value > 1 was obtained, indicating a cooperative mechanism for dGTP hydrolysis.
Assay of pyrimidine nucleoside triphosphate triphosphohydrolases
To test the usefulness of our enzyme-coupled assay to detect the activity of pyrimidine nucleoside triphosphate triphosphohydrolases, we investigated the competence of XOD in catalyzing the oxidation of thymine. Despite a prolonged incubation (1 h), we did not observe any significant oxidation of 0.3 mM thymine by 500 mU/mL of XOD (data not shown). Accordingly, we attempted to define a reaction path relying on the same auxiliary enzymes (PNPase and XOD) previously used to assay Dgt activity, but directed to the quantitation of pyrimidine nucleosides by means of the oxidation of hypoxanthine to uric acid as the detection step. To this aim, we decided to test the transferase activity of PNPase, which is known to catalyze the transfer of a base to a nucleoside [30]. In particular, we assayed PNPase in the presence of 0.15 mM thymine, 0.25 mM inosine, and 500 mU/mL each of PNPase and XOD. It is important to note that, to prevent the phosphorolysis of inosine by PNPase, phosphate was omitted in this assay mixture. As shown in Fig. 5, under these conditions the transferase activity of PNPase was readily detected. In particular, by recording absorption spectra of the assay mixture as a function of time we were able to observe a progressive increase of the band centered at 292 nm, which is due to uric acid, and a concomitant decrease in absorbance centered at 254 nm, which is due to the oxidation of inosine (Fig. 5). Accordingly, pyrimidine nucleosides generated by the enzyme of interest could be detected by the coupled action of: i) the nucleoside hydrolase from Pseudomonas fluorescens, which is known to discriminate against purine nucleosides, e.g. the activity towards inosine is only 5–6 % when compared to the lyase activity in the presence of uridine or cytidine [31]; ii) PNPase, which, in the presence of an excess of inosine, would generate hypoxanthine and ribose-1-phosphate (scheme 1); iii) XOD, the action of which would oxidize hypoxanthine to uric acid (scheme 1), detectable at 293 nm.
Fig. 5.
Glycosyltransferase activity of PNPase. An assay mixture containing 100 mM Tris (pH 8), 0.15 mM thymine, 0.25 mM inosine, and 500 mU/mL of PNPase was prepared, added to sample and reference cuvettes to record a baseline in the 250–450 nm wavelength interval. 500 mU/mL of XOD were then added to the sample cuvette, and difference spectra were recorded every 30 min after enzyme addition.
CONCLUDING REMARKS
We have described here a convenient, sensitive, and continuous enzyme-coupled assay useful to detect the activity of deoxynucleoside triphosphohydrolases. In particular, we have shown here that the activity of E. coli Dgt was readily detected by subjecting the deoxyguanosine released by Dgt to the action of PNPase, producing deoxyribose-1-phosphate and guanine, which, in turn, was oxidized to 8-oxoguanine by XOD. By this means, Dgt was assayed at 297 nm, taking advantage of the Δε between the molar extinction coefficients of guanine and 8-oxoguanine. It should be noted that the assay described here could be applied to a larger set of enzymes. First, when considering that PNPase does not significantly discriminate among ribo-and deoxynucleosides [32], it is conceivable to assay ribonucleoside phosphohydrolases [33], e.g. apyrase (EC 3.6.1.5) [34]. Moreover, considering that Homo sapiens XOD is active towards adenine [35], the activity of enzymes releasing deoxyadenosine or adenosine could be assayed using the procedure described here. It is therefore our hope that the commercial repertoire of XODs from different sources will be expanded, giving the opportunity to widen the applications of the enzyme-coupled assay described here.
ACKNOWLEDGMENTS
We thank Drs. Mark Itsko and Clinton Orebaugh of the NIEHS for helpful comments on a draft for this paper. This work was supported, in part, by project number Z01 ES101905 of the Intramural Research Program of the National Institutes of Health, National Institute of Environmental Health Sciences.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Brignole EJ, Ando N, Zimanyi CM, Drennan CL. The prototypic class Ia ribonucleotide reductase from Escherichia coli : still surprising after all these years. Biochem. Soc. Trans. 2012;40:523–530. doi: 10.1042/BST20120081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmad MF, Dealwis CG. The structural basis for the allosteric regulation of ribonucleotide reductase. Prog. Mol. Biol. Transl. Sci. 2013;177:389–410. doi: 10.1016/B978-0-12-386931-9.00014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lascu I, Gonin P. The catalytic mechanism of nucleoside diphosphate kinases. J. Bioenerg. Biomembr. 2000;32:237–246. doi: 10.1023/a:1005532912212. [DOI] [PubMed] [Google Scholar]
- 4.Lascu L, Giartosio A, Ransac S, Erent M. Quaternary structure of nucleoside diphosphate kinases. J. Bioenerg. Biomembr. 2000;32:227–236. doi: 10.1023/a:1005580828141. [DOI] [PubMed] [Google Scholar]
- 5.Bernard MA, Ray NB, Olcott MC, Hendricks SP, Mathews CK. Metabolic functions of microbial nucleoside diphosphate kinases. J. Bioenerg. Biomembr. 2000;32:259–267. doi: 10.1023/a:1005537013120. [DOI] [PubMed] [Google Scholar]
- 6.Nordman J, Wright A. The relationship between dNTP pool levels and mutagenesis in an Escherichia coli NDP kinase mutant. Proc. Natl. Acad. Sci. USA. 2008;105:10197–10202. doi: 10.1073/pnas.0802816105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nordman J, Wright A. Escherichia coli nucleoside diphosphate kinase mutants depends on translesion DNA synthesis to prevent mutagenesis. J. Bacteriol. 2011;193:4531–4533. doi: 10.1128/JB.05393-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rampazzo C, Miazzi C, Franzolin E, Pontarin G, Ferraro P, Frangini M, Reichard P, Bianchi V. Regulation by degradation, a cellular defense against deoxyribonucleotide pool imbalances. Mutat. Res. 2010;703:2–10. doi: 10.1016/j.mrgentox.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 9.Seto D, Bhatnagar SK, Bessman MJ. The purification and properties of deoxyguanosine triphosphate triphosphohydrolase from Escherichia coli. J. Biol. Chem. 1988;263:1494–1499. [PubMed] [Google Scholar]
- 10.Singh D, Gawel D, Itsko M, Hochkoeppler A, Krahn JM, London RE, Schaaper RM. Structure of Escherichia coli dGTP triphosphohydrolase: a hexameric enzyme with DNA effector molecules. J. Biol. Chem. 2015;290:10418–10129. doi: 10.1074/jbc.M115.636936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vorontsov II, Minasov G, Kiryukhina O, Brunzelle JS, Shuvalova L, Anderson WF. Characterization of the deoxynucleotide triphosphate triphosphohydrolase (dNTPase) activity of the EF1143 protein from Enterococcus faecalis and crystal structure of the activator-substrate complex. J. Biol. Chem. 2011;286:33158–33166. doi: 10.1074/jbc.M111.250456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vorontsov II, Wu Y, DeLucia M, Minasov G, Mehrens J, Shuvalova L, Anderson WF, Ahn J. Mechanism of allosteric activation and inhibition of the deoxyribonucleoside triphosphate triphosphohydrolase from Enterococcus faecalis. J. Biol. Chem. 2014;289:2815–2824. doi: 10.1074/jbc.M113.524207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mega R, Kondo N, Nakagawa N, Kuramitsu S, Masui R. Two dNTP triphosphohydrolases from Pseudomonas aeruginosa possess diverse substrate specificities. FEBS J. 2009;276:3211–3221. doi: 10.1111/j.1742-4658.2009.07035.x. [DOI] [PubMed] [Google Scholar]
- 14.Ji X, Wu Y, Yan J, Mehrens J, Yang H, DeLucia M, Hao C, Gronenborn AM, Skowronski J, Ahn J, Xiong Y. Mechanism of allosteric activation of SAMHD1 by dGTP. Nat. Struct. Mol. Biol. 2013;20:1304–1309. doi: 10.1038/nsmb.2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gawel D, Hamilton MD, Schaaper RM. A novel mutator of Escherichia coli carrying a defect in the dgt gene encoding a dGTP triphosphohydrolase. J. Bacteriol. 2008;190:6931–6939. doi: 10.1128/JB.00935-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miazzi C, Ferraro P, Pontarin G, Rampazzo C, Reichard P, Bianchi V. Allosteric regulation of the human and mouse deoxyribonucleotide triphosphohydrolase sterile α-motif/histidine-aspartate domain-containing protein 1 (SAMHD1) J. Biol. Chem. 2014;289:18339–18346. doi: 10.1074/jbc.M114.571091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hansen EC, Seamon KJ, Cravens SL, Stivers JT. GTP activator and dNTP substrates of HIV-1 restriction factor SAMHD1 generate a long-lived activated state. Proc. Natl. Acad. Sci. USA. 2014;111:E1843–E1851. doi: 10.1073/pnas.1401706111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seamon KJ, Stivers JT. A high-throughput enzyme-coupled assay for SAMDH1 dNTPase. J. Biomol. Screen. 2015;20:801–809. doi: 10.1177/1087057115575150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arnold LH, Kunzelmann S, Webb MR, Taylor IA. A continuous enzyme-coupled assay for triphosphohydrolase activity of HIV-1 restriction factor SAMHD1. Antimicrob. Agents Chemother. 2015;59:186–192. doi: 10.1128/AAC.03903-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brune M, Hunter JL, Corrie JE, Webb MR. Direct, real-time measurement of rapid inorganic phosphate release using a novel fluorescent probe and its application to actomyosin subfragment 1 ATPase. Biochemistry. 1994;33:8262–8271. doi: 10.1021/bi00193a013. [DOI] [PubMed] [Google Scholar]
- 21.Brune M, Hunter JL, Howell SA, Martin SR, Hazlett TL, Corrie JE, Webb MR. Mechanism of inorganic phosphate interaction with phosphate binding protein from Escherichia coli. Biochemistry. 1998;37:10370–10380. doi: 10.1021/bi9804277. [DOI] [PubMed] [Google Scholar]
- 22.Wang Z, Choudhary A, Ledvina PS, Quiocho FA. Fine tuning the specificity of the periplasmic phosphate transport receptor. Site-directed mutagenesis, ligand binding, and crystallographic studies. J. Biol. Chem. 1994;269:25091–25094. doi: 10.2210/pdb1pbp/pdb. [DOI] [PubMed] [Google Scholar]
- 23.Guillén Suárez AS, Stefan A, Lemma S, Conte E, Hochkoeppler A. Continuous enzyme-coupled assay of phosphate- or pyrophosphate-releasing enzymes. BioTechniques. 2012;53:99–103. doi: 10.2144/000113905. [DOI] [PubMed] [Google Scholar]
- 24.Hill AV. The possible effects of the aggregation of the molecules of haemoglobin on its dissociation curve. J. Physiol. 1910;40:iv–vii. [Google Scholar]
- 25.Mössner E, Boll M, Pfleiderer G. Purification of human and bovine alkaline phosphatases by affinity chromatography. Hoppe Seylers Z. Physiol. Chem. 1980;361:543–549. doi: 10.1515/bchm2.1980.361.1.543. [DOI] [PubMed] [Google Scholar]
- 26.Ohe T, Watanabe Y. Purification and properties of xanthine oxidase from Streptomyces cyanogenus. J. Biochem. 1979;86:45–53. [PubMed] [Google Scholar]
- 27.Machida Y, Nakanishi T. Purification and properties of xanthine oxidase from Enterobacter cloacae. Agr. Biol. Chem. 1981;45:425–432. [Google Scholar]
- 28.Jayanth N, Ramachandran S, Puranik M. Solution structure of the DNA damage lesion 8-oxoguanosine from ultraviolet resonance Raman spectroscopy. J. Phys. Chem. 2009;113:1459–1471. doi: 10.1021/jp8071519. [DOI] [PubMed] [Google Scholar]
- 29.McClure WR. Kinetic analysis of coupled enzyme assays. Biochemistry. 1969;8:2782–2786. doi: 10.1021/bi00835a014. [DOI] [PubMed] [Google Scholar]
- 30.Bzowska A, Kulikowska E, Shugar D. Purine nucleoside phosphorylases: properties, functions, and clinical aspects. Pharmacol. & Ther. 2000;88:349–425. doi: 10.1016/s0163-7258(00)00097-8. [DOI] [PubMed] [Google Scholar]
- 31.Terada M, Tatibana M, Hayaishi O. Purification and properties of nucleoside hydrolase from Pseudomonas fluorescens. J. Biol. Chem. 1967;242:5578–5585. [PubMed] [Google Scholar]
- 32.Bennett EM, Li C, Allan PW, Parker WB, Ealick SE. Structural basis for substrate specificity of Escherichia coli purine nucleoside phosphorylase. J. Biol. Chem. 2003;278:47110–47118. doi: 10.1074/jbc.M304622200. [DOI] [PubMed] [Google Scholar]
- 33.Arch JRS, Newsholme EA. Activities and some properties of 5’-nucleotidase, adenosine kinase, and adenosine deaminase in tissues from vertebrates and invertebrates in relation to the control of the concentration and the physiological role of adenosine. Biochem. J. 1978;174:965–977. doi: 10.1042/bj1740965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leal DB, Streher CA, Neu TN, Bittencourt FP, Leal CA, da Silva JE, Morsch VM, Schetinger MR. Characterization of NTPDase (NTPDase1; ecto-apyrase; ectodiphosphohydrolase; CD39; EC 3.6.1.5) activity in human lymphocytes. Biochim. Biophys. Acta. 2005;1721:9–15. doi: 10.1016/j.bbagen.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 35.Krenitsky TA, Spector T, Hall WW. Xanthine oxidase from human liver: purification and characterization. Arch. Biochem. Biophys. 1986;247:108–119. doi: 10.1016/0003-9861(86)90539-4. [DOI] [PubMed] [Google Scholar]