Abstract
Maternal diabetes is a significant risk factor for structural birth defects, including congenital heart defects and neural tube defects (NTDs). With the rising prevalence of type 2 diabetes and obesity in women of childbearing age, diabetes-induced birth defects have become an increasingly significant public health problem. Maternal diabetes in vivo and high glucose in vitro induce yolk sac injuries by damaging the morphology of cells and altering the dynamics of organelles. The yolk sac vascular system is the first system to develop during embryogenesis, therefore, it is the most sensitive to hyperglycemia. The consequences of yolk sac injuries include impairment of nutrient transportation due to vasculopathy. Although the functional relationship between yolk sac vasculopathy and structural birth defects has not yet been established, a recent study reveals that the quality of yolk sac vasculature is inversely related to embryonic malformation rates. Studies in animal models have uncovered key molecular intermediates of diabetic yolk sac vasculopathy, including hypoxia-inducible factor-1α (HIF-1α), apoptosis signal-regulating kinase 1 (ASK1) and its inhibitor thioredoxin-1 (Trx), c-Jun-N-terminal kinases (JNK), nitric oxide (NO) and nitric oxide synthase (NOS). Yolk sac vasculopathy is also associated with abnormalities in arachidonic acid and myo-inositol. Dietary supplementation with fatty acids that restore lipid levels in the yolk sac lead to reduction in diabetes-induced malformations. Although the role of the human yolk in embryogenesis is less extensive than in rodents, nevertheless, human embryonic vasculogenesis is negatively affected by maternal diabetes. Mechanistic studies have identified potential therapeutic targets for future intervention against yolk sac vasculopathy, birth defects, and other complications associated with diabetic pregnancies.
Keywords: yolk sac, maternal diabetes, embryopathy, vasculopathy
Globally, 60 million women of reproductive age (18–44 year old), and about 3 million American women, have diabetes mellitus, and it has been estimated that this number will double by 20301,2. Due to the large number of women affected by diabetes, embryonic anomalies stemming from maternal diabetes has become a prevalent public health issue3–5. In fact, maternal diabetes-induced embryonic complications have become the leading cause of infant mortality in the United States6. Pregestational type 1 or 2 diabetes is a significant risk factor for structural birth defects, the most common anomalies being congenital heart defects and neural tube defects (NTDs)3–5,7. It has been well established that the rate of birth defects increases linearly with the degree of maternal hyperglycemia, which is the major teratogenic factor in maternal diabetes5,8–13.
The yolk sac is an extra-embryonic membrane derived from the same progenitor cells that produce the embryo14, and it plays an important role in supporting embryonic development14, 15. Pregestational diabetes alters the growth and structure of the human yolk sac 16,17, and abnormalities in human yolk sac structures are associated with embryonic malformations 18,19, suggesting the importance for studying the yolk sac in diabetic embryopathy. During the most critical, vulnerable period of embryogenesis, the rodent yolk sac encompasses the embryo and serves as the primitive placenta14,15,20,21. After implantation and prior to the formation of the placenta, embryonic growth is essentially dependent on the proper development of the yolk sac vasculature, which includes the vitelline circulation. The vitelline circulation serves as the site for the exchange of nutrients, production of red blood cells and blood vessels, and synthesis of essential embryonic proteins20,21. During mouse embryonic development, the yolk sac vascular system is the first system to develop, and it is the most sensitive to hyperglycemia15. Hyperglycemia causes yolk sac vasculopathy that ultimately leads to embryonic malformations or lethality15,22. Diabetes-induced defects in the vascular system have been directly linked to NTDs23, highlighting the importance of studying diabetic yolk sac vasculopathy. This report summarizes the mechanisms underlying maternal diabetes-induced yolk sac injuries and yolk sac vasculopathy, and explores the possible causal relationship between yolk sac vasculopathy and structural anomalies.
The development of yolk sac vasculature
Although the human yolk sac resides outside of the embryo, similar to the rodent yolk sac, it plays an important role in early embryonic vasculogenesis 24. The murine yolk sac is derived from the same progenitor cells that produce the embryo14. In mice, conceptus vasculogenesis starts with the emergence of vascular endothelial growth factor receptor-2-positive (VEGFR2+ or Flk+) cells in the yolk sac25. These Flk1+ progenitor endothelial cells form blood islands that fuse to generate a primary capillary plexus at embryonic day 7.5 (E7.5)25. In addition, extra-embryonic mesodermal cells proliferate to form angioblastic cords on E7.526. At E8.0, blood islands fuse and establish the primary capillary network, which is intimately associated with mural cells27,28. By E9.5, the capillary plexus has remodeled into a complex hierarchy of mature small and large vessels, and functional vitelline circulation is established29. A critical number of Flk1+ cells and blood islands are crucial for normal vasculogenesis25.
Vasculogenesis begins in the yolk sac prior to embryonic vasculogenesis and development of the cardiovascular system. In addition, the yolk sac and embryonic vasculatures are regulated by the same group of angiogenic and survival factors via common mechanisms22,30, 31. Therefore, the elucidation of the mechanism underlying hyperglycemia-induced yolk sac vasculopathy is important in the etiology of diabetic embryopathy.
Maternal diabetes induces yolk sac structure failure and dysfunction
Experimental evidence has elucidated the precise role of the yolk sac in mammalian embryonic development, as well as the relationship between yolk sac injury and embryopathy15, 32. The structures and prostaglandin E2 levels of human yolk sacs are altered by maternal diabetes 15,16,33. Studies have shown that yolk sac development is morphologically impaired under hyperglycemic conditions34. For example, conceptuses exposed to excess glucose demonstrate decreased size and gross malformations34. Furthermore, exposure to excess glucose causes the visceral yolk sac capillaries and vitelline vessels to become sparse, patchy, and non-uniformly located34. Under high glucose conditions, the visceral yolk sac endodermal cells have reduced numbers of rough endoplasmic reticulum, ribosomes, and mitochondria34. These defects in yolk sac structures suggest that hyperglycemia during organogenesis has a primarily deleterious effect on yolk sac functions.
Hyperglycemic conditions also appear to affect the transport function of the yolk sac. For example, experiments using horseradish peroxidase as a tracer protein to examine the transport function of the visceral endodermal yolk sac cells have shown that the cellular uptake of peroxidase is diminished in conceptuses cultured under hyperglycemic conditions35. These findings indicate that hyperglycemia inhibits transport of nutrients from the yolk sac to the embryo. Coupled together with the experiments demonstrating a deleterious effect of hyperglycemia on cell morphology, these data suggest that yolk sac failure is associated with diabetic embryopathy.
Maternal diabetes induces yolk sac vasculopathy
In mice, abnormal development and arrested development of the yolk sac vasculature on E7.5 can result in congenital malformations in a wide variety of organs and tissues, as well as embryonic lethality15,22,30,36. The adverse effects of hyperglycemia on the yolk sac have been documented in maternal diabetic animal models and in vitro cultured rodent embryos15,22,30,36. Under hyperglycemic conditions, development of the blood vessels in the yolk sac is disrupted and the cellular structures in the vessels are altered30,36. Conceptuses display various, profoundly abnormal yolk sac vasculature, with some completely devoid of vasculogenesis, and others having a branched plexus with no apparent arborization or distinction of arteries and veins23,30,36,37.
The adverse effects of hyperglycemia on yolk sac vasculature development can be characterized by arbitrarily assigning morphological scores to individual vasculatures23. Using this rating system, one group showed that the yolk sac vasculature score of the hyperglycemia group was significantly lower than that of the euglycemic group23. Yolk sac vasculature morphologic scores were inversely correlated with embryonic malformation rates, such that the higher the score, the lower the rate of malformations, and vice versa23.
Although the developing yolk sac contains a diverse cell population, evidence shows that vascular endothelial cells are the primary targets of hyperglycemic insults30,37. Platelet-derived endothelial cell adhesion molecule (PECAM-1), an endothelial cell marker, modulates endothelial cell migration, cell-cell adhesion, and in vitro and in vivo angiogenesis38. Under hyperglycemic conditions, the presence of yolk sac vasculopathy is associated with the failure of PECAM-1 tyrosine phosphorylation30,37. Thus, hyperglycemia may adversely impact vascular endothelial cell functions, including apoptosis, proliferation, and differentiation through regulation of endothelial cell specific cellular intermediates and signaling.
Molecular intermediates and signaling pathways contribute to maternal diabetes-induced yolk sac vasculopathy
Studies show that maternal diabetes induces yolk sac vasculopathy through two distinct sets of molecular events. In one set of events, hypoxia-inducible factor 1(HIF-1) and vascular endothelial growth factor (VEGF), two proteins that are typically active in normal vasculogenesis, are down-regulated by maternal diabetes39. In another set of events, maternal diabetes induces activation of a key apoptosis related kinase, known as apoptosis signal regulating kinase 1 (ASK1), which increases induced nitric oxide synthase (iNOS) expression and the promotion of apoptosis 40,41. Inhibition of events downstream of ASK1 activation, such as c-Jun-N-terminal kinases (JNK1/2) signaling, abolishes maternal diabetes-induced vasculopathy23,42. The protective effect of thioredoxin-1, an inhibitor of ASK1, on hyperglycemia-induced vasculopathy has been demonstarted39. The elucidation of the mechanisms underlying hyperglycemia-induced yolk sac vasculopathy can aid in the development of preventative methods for maternal diabetes-induced cardiovascular defects in humans.
The role of HIF-1 in yolk sac vasculopathy
HIF-1 is a key transcriptional regulator for hypoxia regulation of embryonic vascular development. It is an oxygen-sensitive heterodimer consisting of a constitutively expressed HIF-1β subunit, and an oxygen-regulated HIF-1α subunit43. Regulation of HIF-1 activity depends on the degradation of the HIF-1α subunit in normoxic conditions 43. The molecular basis of HIF-1 α degradation is the oxygen-dependent hydroxylation of at least one of the two proline residues in its oxygen-dependent degradation domain by specific prolylhydroxylases (PHD1, PHD2 and PHD3)44–47. In this orm, HIF-1 α binds to the von Hippel-Lindau tumor suppressor protein, which acts as an E3 ubiquitin ligase, and targets HIF-1α for proteasomal degradation 48,49. During conditions of normoxia, HIF-1 β is found in the nucleus, while HIF-1 α is cytoplasmic and rapidly degraded 49. Reduced oxygen levels during embryonic development permit the accumulation of HIF-1α protein in the cytoplasm50. Subsequently, HIF-1α translocates to the nucleus, engages HIF-1β, and forms the HIF-1 complex that initiates transcription50–52.
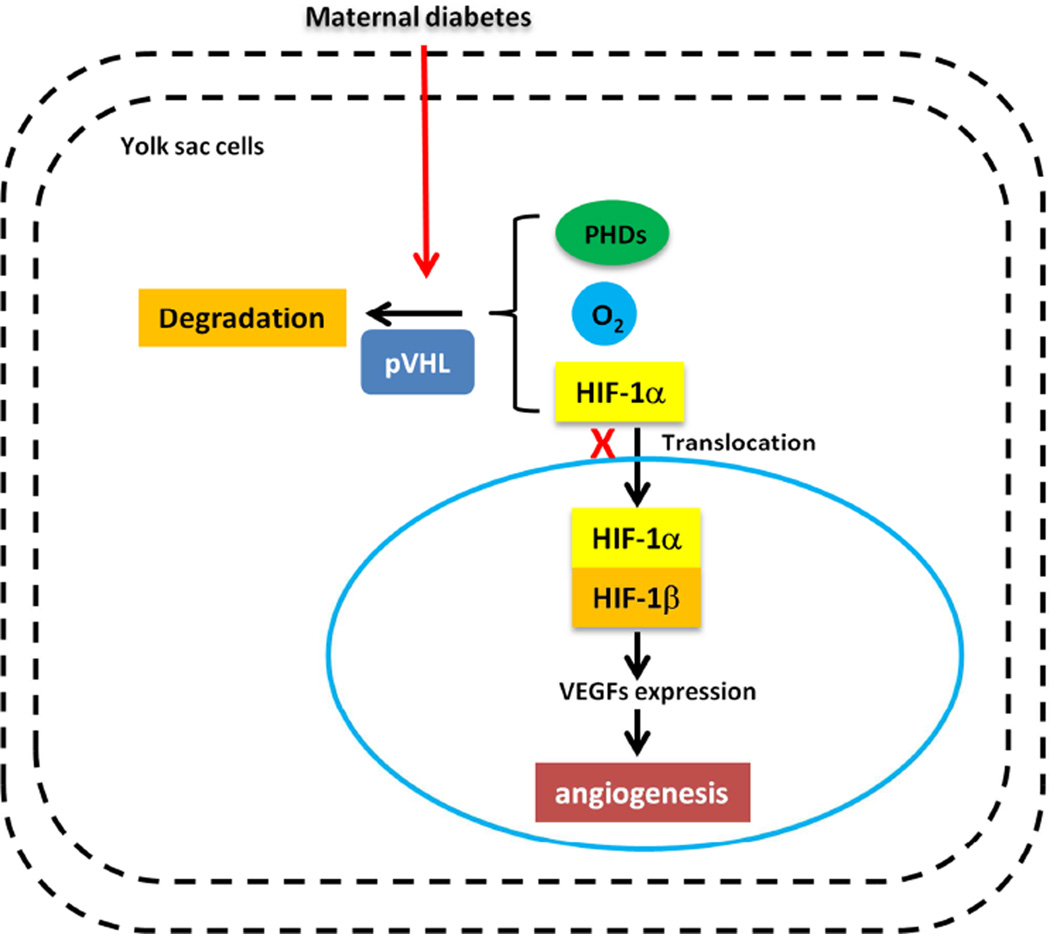
HIF-1 functions as a master regulator of angiogenesis by controlling the expression of multiple angiogenic growth factors52,53. Maternal diabetes has been shown to reduce HIF-1α levels in the embryo, leading to vasculopathy39. Maternal diabetes reduces the embryonic hypoxic environment-induced HIF-1α. AdCA5, an adenovirus encoding a constitutively active form of HIF-1α, blocks diabetes-induced vasculopathy, demonstrating that HIF-1α reduction contributes to diabetes-induced vasculopathy 39. Mice that lack HIF-1 activity due to HIF-1α- or HIF-1β-null mutations develop extensive vascular defects, similar to those observed in diabetic yolk sac vasculopathy, including inadequate vessel formation and aberrant vascular remodeling 54, 55. HIF-1 deficiency also decreases cell survival, leading to abnormal vasculogenesis56. In our previous study, we demonstrated that a decrease in HIF-1α expression is responsible for the VEGF reduction induced by maternal diabetes39. This suggests that the HIF-1α-VEGF signaling pathway plays a role in maternal diabetes-induced vasculopathy (Fig. 1).
Figure 1. Maternal diabetes induces yolk sac vasculopathy via reduction of HIF-1α.
Under normoxic conditions, specific prolylhydroxylases (PHDs) induce oxygen-dependent hydroxylation of HIF-1α. HIF-1α then binds to the von Hippel-Lindau tumor suppressor protein (pVHL), which acts as an E3 ubiquitin ligase and targets HIF-1α for proteasomal degradation. Under hypoxic conditions, HIF-1α translocates to the nucleus, engages HIF-1β, and forms the HIF-1 complex that initiates transcription of downstream genes, including VEGFs. Maternal diabetes reduces HIF-1α levels by enhancing its degradation. The lack of HIF-1α leads to the development of extensive vascular defects, which is similar to diabetic yolk sac vasculopathy.
The pro-apoptotic ASK1-JNK1/2 pathway
Apoptosis has been hypothesized as a primary mechanism of diabetes-induced birth defects 57–59. Under euglycemic conditions, very low basal levels of apoptosis are observed in the embryonic tissues during organogenesis (E7-E11)60. In contrast, compelling evidence demonstrates that maternal hyperglycemia enhances apoptosis in the E7-E11 embryonic tissues 31, 61–66. However, the apoptotic mechanism in this disease process is not well understood. Evidence from clinical and experimental studies has revealed that maternal diabetes leads to an imbalance in intracellular reduction-oxidation (redox) homeostasis, resulting in intracellular oxidative stress57–59,67–70. Recent studies have demonstrated that oxidative stress and ER stress are the main biochemical and molecular mechanisms underlying maternal diabetes- induced apoptosis66,71–75.
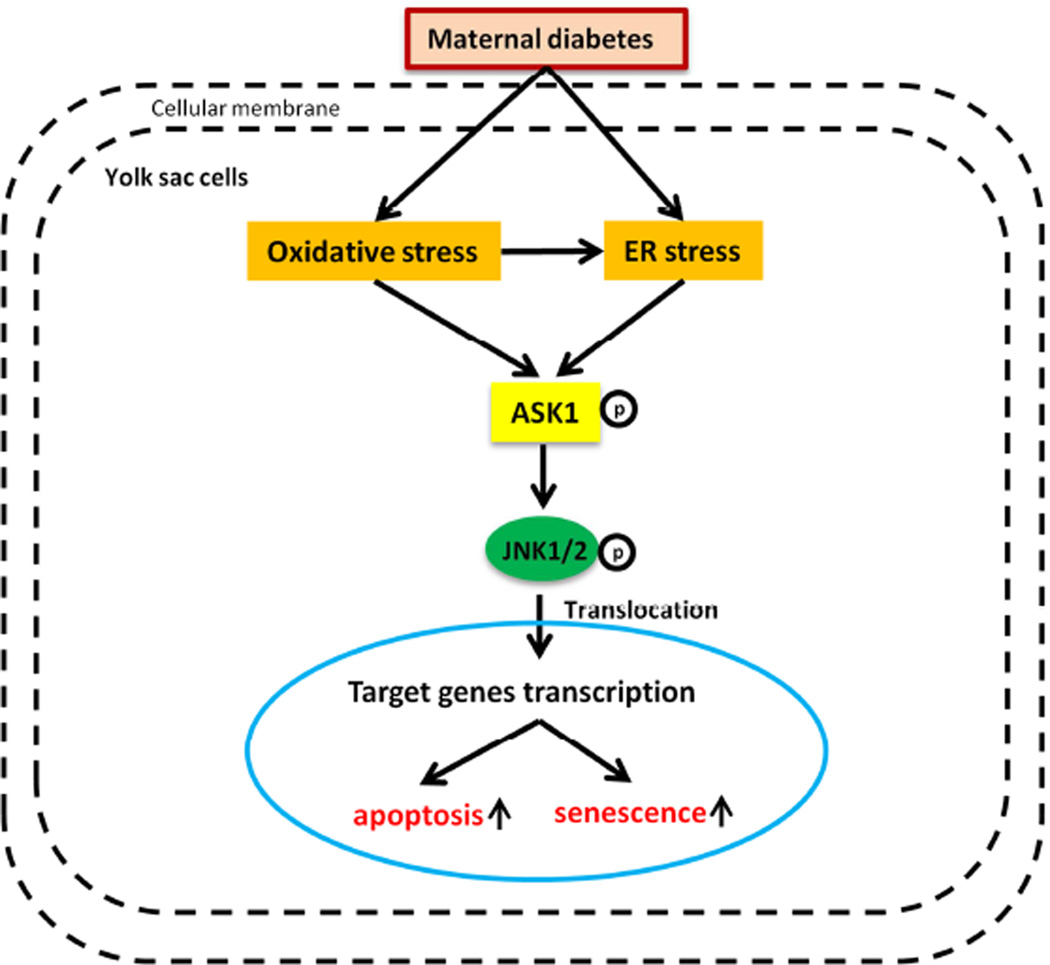
JNK1/2 are pro-apoptotic factors that belong to the mitogen-activated protein kinase (MAPK) family76. MAPKs are members of a complex superfamily of serine/threonine kinases that are activated in response to a variety of extracellular stimuli76,77. The basic assembly of the MAPK signaling pathway is a three component module76, involving sequential activation of MAPK kinase kinase (MAP3K), MAPK kinase (MAPKK), and MAPK 78,79. MAP3K phosphorylates and thereby activates MAPKK, and activated MAPKK in turn phosphorylates and activates MAPK79. Because the activation status of MAPKs largely depends on MAP3Ks, it is important to understand how MAP3Ks are regulated. Fourteen different MAP3Ks have been identified 76. Among them, several MAP3Ks, including ASK1, TAK1 and MLK3, are known to activate the JNK pathway in response to diverse stimuli78–80. In our previous work, we indicated that at a concentration of 800 nM, an inhibitor of JNK1/2 (SP600125), significantly abrogated hyperglycemia-induced yolk sac vasculopathy in both morphologic score and vasculature morphology, strongly suggesting that JNK1/2 activation plays an important role in hyperglycemia-induced yolk sac vasculopathy 23 (Fig. 2).
Figure 2. Maternal diabetes induces endothelial progenitor apoptosis via ASK1 activation.
Maternal diabetes induces oxidative stress, which causes ER stress by aggravating unfolding protein response (UPR) events in the ER. Oxidative stress and ER stress induce phosphorylation of the Thr-845 present on the activation loop of ASK1, thereby activating ASK1. ASK1 activation then leads to the phosphorylation of JNK1/2, which activates several transcription factors. These transcription factors ultimately induce endothelial progenitor cell apoptosis and senescence.
ASK1-mediated apoptosis is involved in the pathogenesis of several oxidative stress-related diseases such as brain ischemia81, ischemic heart disease82, and Alzheimer’s disease83. ASK1 activation leads to apoptosis via the JNK or the p38MAP kinase pathways80. ASK1 is activated by phosphorylation of Thr-845 in its activation loop, and ASK1 is required for reactive oxygen species (ROS)- and endoplasmic reticulum (ER) stress-induced JNK activation and apoptosis58,59,80,84–86. Recently, it has been shown that high glucose-induced activation of ASK1 mediates hyperglycemia-induced endothelial cell senescence87. We have demonstrated that ASK1 is activated in diabetic yolk sac vasculopathy, and that ASK1 deletion morphologically ameliorates diabetic yolk sac vasculopathy23. This indicates that ASK1 mediates maternal diabetes-induced endothelial progenitor apoptosis or senescence by JNK1/2, and that activation of the ASK1-JNK1/2 pathway leads to vasculopathy (Fig. 2).
Altered nitric oxide and nitric oxide synthase (NOS) in yolk sac vasculopathy
Nitric oxide (NO) is a small multifunctional gaseous molecule that acts as a vasoactive modulator, signaling molecule, and free radical in mammalian systems. NO is synthesized from oxidation of L-arginine by three distinct NO synthases (NOS): neuronal (nNOS), endothelial (eNOS), and inducible (iNOS), using the cofactors, NADPH, FAD, and tetrahydrobiopterin (BH4)88,89. nNOS and eNOS are constitutively expressed at low levels88. iNOS generates very high concentrations of NO only when induced 90. NO has been shown to be involved in cell differentiation, proliferation, and apoptosis, and the effect of NO is both physiologically essential and cytotoxic 91–93. Upon generation, NO freely diffuses through the cell membrane into the exracellular space, and subsequently modifies protein thiols or cysteine residues. In addition, NO induces a variety of biological responses by interacting with free radicals94–97. NO interacts with several signaling pathways to mediate these responses, including MAPK, Janus kinase (JAK), and JNK pathways, as well as reactive oxygen depending on signaling pathways98–100.
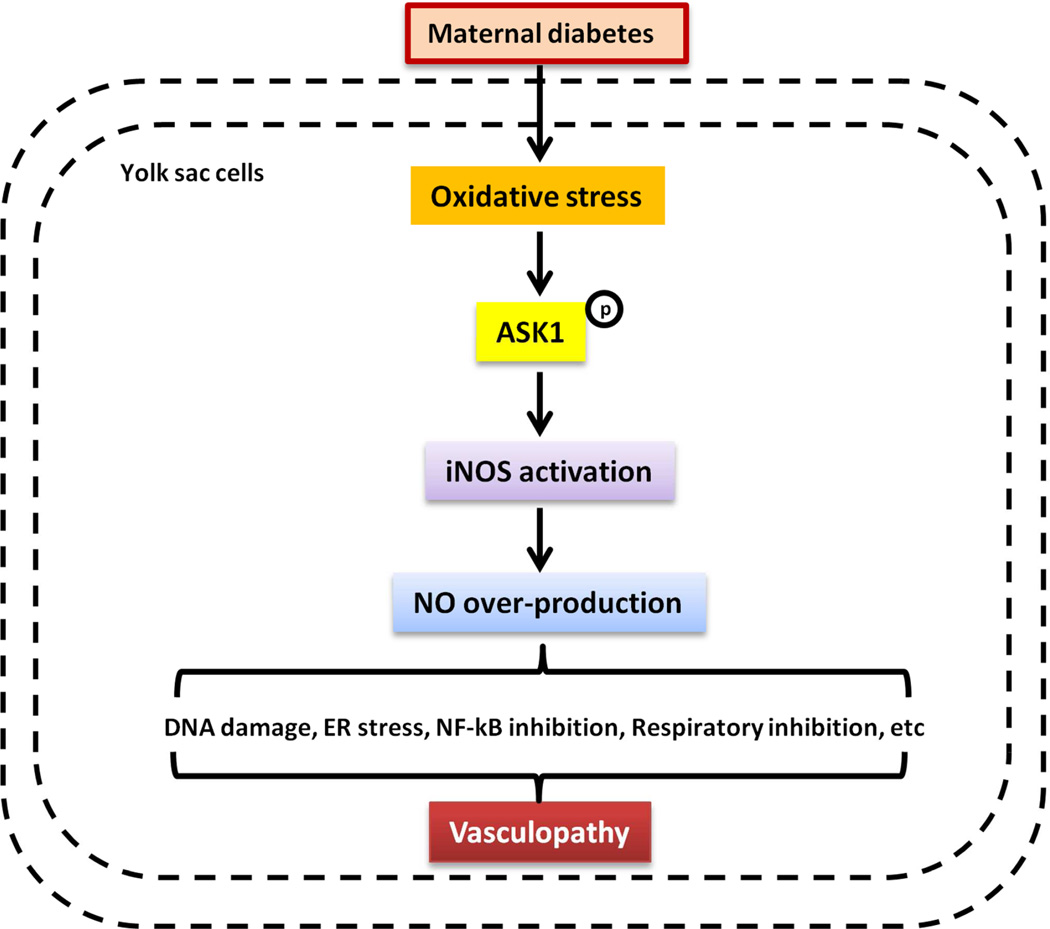
During blood island formation in diabetic pregnancies, the endoderm produces NO which inhibits NOS. Inhibition of NOS, L-NG-monomethyl arginine citrate (L-NMMA), leads to developmental arrest at the primary plexus stage, and ultimately vasculopathy22. Administration of an NO donor reverses these adverse effects on yolk sac vasculature22. Additionally, it has been reported that NO derived from iNOS plays a detrimental role in human disease101. Moreover, iNOS and eNOS are expressed during early embryonic vasculogenesis, and the alteration of NO expression induces yolk sac vasculopathy22. Hyperglycemia increases iNOS protein expression and activity through ASK140,41. The increase of iNOS leads to over-production of NO that causes DNA damage, ER stress, NF-kB and respiratory inhibition102 that may play a vital role on embryonic malformation (Fig. 3).
Figure 3. Overproduction of NO mediates maternal diabetes-induced yolk sac vasculopathy.
Maternal diabetes-induced oxidative stress activates ASK1. The phosphorylation of ASK1 stimulates iNOS gene expression, which generates very high concentrations of NO. The detrimental role of NO derived from iNOS includes DNA damage, ER stress, NF-kB inhibition, and respiratory inhibition, all of which contribute to yolk sac vasculopathy.
The protective effect of the ASK1 inhibitor thioredoxin-1 in yolk sac vasculopthay
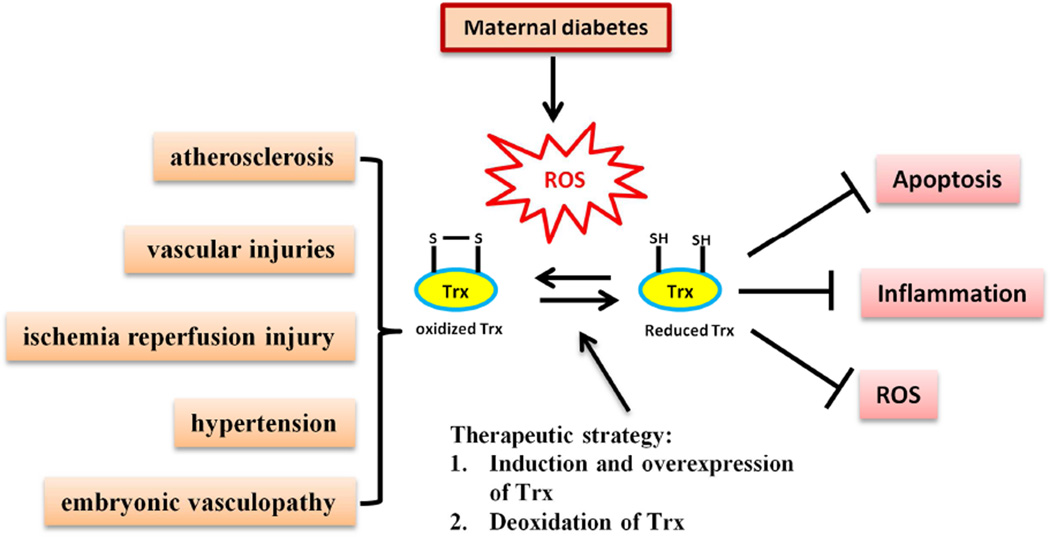
Thioredoxin-1 (Trx) is a 12-kDa protein with a redox-active dithiol in the active site (-Cys-Gly-Pro-Cys-) and constitutes a major thiol reducing system103. Trx is a potent antioxidant and reduces ROS through interactions with its redox-active center, which protects cells from stress-induced damage through anti-oxidative, anti-apoptotic, and anti-inflammatory effects103. Trx shows an anti-apoptotic function by inhibiting cell death signals104, activating survival signaling pathways105,106, or scavenging ROS107. Diabetic yolk sac vasculopathy is an oxidative stress and apoptotic disease process39–41,58,59,71. Therefore, Trx is able to reduce diabetic yolk sac vasculopathy via its anti-oxidative and anti-apoptotic functions (Fig. 4).
Figure 4. Thioredoxin-1 (Trx) reduces diabetic yolk sac vasculopathy by scavenging ROS.
Reduced Trx is a potent antioxidant that decreases ROS levels through the function of its redox-active center. Trx ultimately protects cells from stress-induced damage by anti-oxidative, anti-apoptosis, and anti-inflammation processes. Maternal diabetes-induced oxidative stress disturbs the redox balance of Trx, leading to a disproportionate increase in oxidized Trx. High levels of oxidized Trx are associated with several cardiovascular diseases, including atherosclerosis, vascular injuries, ischemia reperfusion injury, hypertension, and yolk sac vasculopathy. The therapeutic strategy for maternal diabetes-associated embryopathy may be through induction or overexpression, and deoxidation of Trx.
Trx is expressed ubiquitously in mammalian cells and its expression is essential for early differentiation and morphogenesis of the mouse embryo108. Genetic deletion of Trx leads to an early embryonic lethal phenotype109. Trx-deficient embryos die shortly after implantation, and the conceptuses are resorbed prior to gastrulation109. When preimplantation, Trx-null embryos are placed in culture, the inner mass cells of the homozygous embryos fail to proliferate109. This indicates that proper levels of Trx are essential for normal embryogenesis. Trx levels are reduced in embryonic tissues exposed to diabetes39, implying that Trx reduction is involved in the pathogenesis of diabetic emrbyopathy.
Trx is expressed ubiquitously in endothelial cells110 and protects them from ROS-induced apoptosis111. Trx is active in the vessel wall and functions either as an important endogenous antioxidant, or interacts directly with signaling molecules to influence cell growth, apoptosis, and inflammation112,113. Recent evidence implicates that Trx is involved in cardiovascular diseases associated with oxidative stress, such as atherosclerosis110; vascular injuries114, ischemia reperfusion injury115, and hypertension116. In vivo studies have shown a protective role of Trx in different cardiovascular diseases114,115. Thus, Trx is considered an important target for therapeutic intervention of cardiovascular disorders.
It has also been reported that Trx stimulates angiogenesis via induction of angiogenic factors117. For example, hyperglycemia-induced yolk sac vasculopathy in mice can be ameliorated by treating with exogenous human Trx recombinant protein39. Based on the profound beneficial effects of Trx on vascular functions and diabetic vasculopathy, induction or overexpression and deoxidation of Trx is able to reverse hyperglycemia-induced yolk sac vasculopathy (Fig. 4).
Therapeutic implications of targeting the yolk sac
The leading intervention strategy currently applied to prevent diabetic embryopathy is rigorous glycemic control with lifestyle modifications and various anti-diabetic agents, such as insulin, and other therapies, such as anti-hypertensives, as needed57,71. Unfortunately, continuous euglycemic control is difficult to achieve and maintain, and even transient exposure to hyperglycemia causes embryonic malformation118.
Our group has shown that fatty acid supplements have some beneficial effects on the outcome of diabetic pregnancies119. We analyzed the fatty acid composition in major lipid groups of the yolk sac in rats119, and found that maternal diabetes induces quantitative and qualitative abnormalities in major lipid groups of the yolk sac119. This implies that the teratogenic mechanism of diabetic embryopathy may be related to a deficiency in essential fatty acids in the yolk sac119. In addition, we used dietary supplementation of arachidonic acid and myo-inositol, in vitro and in vivo, and showed that these substrates can reduce the incidence of diabetes-related malformation in offspring120.
Previous work also has indicated that arachidonic acid prevents hyperglycemia-associated yolk sac damage and embryopathy119–122. When rodent conceptuses were cultured in normal, arachidonic acid-supplemented normal, and arachidonic acid-supplemented hyperglycemic rat serum122, the addition of 20 mg/ml of arachidonic acid prevented open neural tubes, increased number of lysosome-like structures in the visceral endodermal yolk sac cells, advanced neuropil formation in the neuroepithelium, significant reduction of ER, and decreased size and number of lipid droplets in embryos cultured under high glucose conditions122.
Dietary myo-inositol supplements also appear to significantly decrease the incidence of NTDs in offspring of diabetic dams123. The results of a previous study showed that dietary therapy successfully restored myo-inositol levels in the yolk sac and reduced malformation123. These therapies hold promise for use as a dietary prophylaxis against diabetic embryopathy in humans.
Future perspectives and clinical relevance
Investigating the mechanisms underlying yolk sac vasculopathy in animal models may reveal the pathophysiology of adverse pregnancy outcomes in diabetic women, and may provide a strategy for preventing and treating diabetic embryopathy.
Pathological studies have revealed that placental vascular dysfunction and placental infarction occur in diabetic pregnancies124–129. While most of these studies have only reported findings after birth, we and others hypothesize that the vasculopathy actually starts as early as the yolk sac period. The primary yolk sac in humans is formed in the beginning of the second week of pregnancy (ADD BACK REF 142). Although human and murine embryonic dependence on the yolk sac differs, findings in animal models do suggest that preventing vasculopathy in the human yolk sac may influence the subsequent development of the placenta and, thus, the outcome of the pregnancy. Indeed, placental vasculopathy in humans increases the need for obstetric intervention, the rates of preterm birth, stillbirth and miscarriage130–137.
Implementing the earliest possible interventions that can prevent aberrant embryogenesis remains a significant hurdle to improving the outcomes and reducing the healthcare costs associated with diabetic pregnancies138–140. Although most international guidelines recommend intensive glycemic control during diabetic pregnancy, most of the current guidelines do not stress the importance of pre-pregnancy glucose control. Unless a woman has diagnosed diabetes prior to pregnancy or a medical history of metabolic syndrome, some women may not even be screened for diabetes until 24 to 28 weeks of gestation141–144. International guidelines also suggest that target glucose levels be based on glycated hemoglobin, which only represents a general blood sugar level within the past three months. However, even short spikes in glucose can be detrimental to the fetus. In reality, normalization of glucose metabolism using daily mean glucose level is preferable and desirable.
In addition, many pregnancies are unplanned145. Therefore, intervention strategies often miss the most important phase of organogenesis, the first weeks of the first trimester of pregnancy.. This may be a reason why there is such a high incidence of diabetes related birth defects despite modern prenatal care. Thus pregnancy education in women who currently have or who are at high-risk for diabetes should be implemented prior to pregnancy139,146.
Because the fetuses are extremely vulnerable to hyperglycemia during the yolk sac period, it is pivotal to maintain the glucose stability very early in pregnancy. Different types of insulin are used clinically to control glucose, and insulin analogues are often used to treat type 1 or 2 diabetic patients147. For women whose blood glucose is poorly controlled by daily insulin injections, subcutaneous insulin pumps might be useful in such settings148,149. Although insulin and insulin analogues have been shown to improve HbA1c, with less risk of hypoglycaemia and with little or no adverse effects on the developing fetus147,150–152, use of anti-diabetic therapeutics alone has not completely eliminated the incidence of hyperglycemia-induced birth defects153.
To date, there has been no single, “best” approach to control glucose in pregnant, diabetic women. Studies in animal models have suggested that, in addition to anti-pharmaceutical interventions, dietary supplements that improve the lipid content of the yolk sac can reduce congenital malformations in offspring of diabetic dams118. However, only isolated clinical trials in humans have been performed to date. Large-scale, multicenter clinical trials are needed to determine if targeting the health of the yolk sac, either by using nutritional supplements or therapeutics that improve yolk sac vasculogenesis, can prevent diabetic embryopathy.
Acknowledgements
We are grateful to Dr. Julie Wu, Offices of the Dean and Public Affairs & Communications, University of Maryland School of medicine, for critical reading and editing.
Source of financial support: This research is supported by NIH R01DK083243, R01DK101972 (to Peixin Yang), and R01DK103024 (to P. Y and E. A. R).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: None of the authors have a conflict of interest.
References
- 1.Centers for Disease C, Prevention. Update on overall prevalence of major birth defects--Atlanta, Georgia, 1978–2005. MMWR Morbidity and mortality weekly report. 2008;57:1–5. [PubMed] [Google Scholar]
- 2.FEig DS, Palda VA. Type 2 diabetes in pregnancy: a growing concern. Lancet. 2002;359:1690–1692. doi: 10.1016/S0140-6736(02)08599-9. [DOI] [PubMed] [Google Scholar]
- 3.Reece EA, Homko C, Miodovnik M, Langer O. A consensus report of the Diabetes in Pregnancy Study Group of North America Conference, Little Rock, Arkansas, May 2002. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2002;12:362–364. doi: 10.1080/jmf.12.6.362.364. [DOI] [PubMed] [Google Scholar]
- 4.Sever L, Lynberg MC, Edmonds LD. The impact of congenital malformations on public health. Teratology. 1993;48:547–549. doi: 10.1002/tera.1420480603. [DOI] [PubMed] [Google Scholar]
- 5.Eriksson UJ, Cederberg J, Wentzel P. Congenital malformations in offspring of diabetic mothers--animal and human studies. Reviews in endocrine & metabolic disorders. 2003;4:79–93. doi: 10.1023/a:1021879504372. [DOI] [PubMed] [Google Scholar]
- 6.Heron M, Tejada-Vera B. Deaths: leading causes for 2005. National vital statistics reports : from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System. 2009;58:1–97. [PubMed] [Google Scholar]
- 7.Cai GJ, Sun XX, Zhang L, Hong Q. Association between maternal body mass index and congenital heart defects in offspring: a systematic review. American journal of obstetrics and gynecology. 2014;211:91–117. doi: 10.1016/j.ajog.2014.03.028. [DOI] [PubMed] [Google Scholar]
- 8.Greene MF, Hare JW, Cloherty JP, Benacerraf BR, Soeldner JS. First-trimester hemoglobin A1 and risk for major malformation and spontaneous abortion in diabetic pregnancy. Teratology. 1989;39:225–231. doi: 10.1002/tera.1420390303. [DOI] [PubMed] [Google Scholar]
- 9.Rose BI, Graff S, Spencer R, Hensleigh P, Fainstat T. Major congenital anomalies in infants and glycosylated hemoglobin levels in insulin-requiring diabetic mothers. Journal of perinatology : official journal of the California Perinatal Association. 1988;8:309–311. [PubMed] [Google Scholar]
- 10.Lucas MJ, Leveno KJ, Williams ML, Raskin P, Whalley PJ. Early pregnancy glycosylated hemoglobin, severity of diabetes, and fetal malformations. American journal of obstetrics and gynecology. 1989;161:426–431. doi: 10.1016/0002-9378(89)90536-x. [DOI] [PubMed] [Google Scholar]
- 11.Key TC, Giuffrida R, Moore TR. Predictive value of early pregnancy glycohemoglobin in the insulin-treated diabetic patient. American journal of obstetrics and gynecology. 1987;156:1096–1100. doi: 10.1016/0002-9378(87)90117-7. [DOI] [PubMed] [Google Scholar]
- 12.Miller E, Hare JW, Cloherty JP, et al. Elevated maternal hemoglobin A1c in early pregnancy and major congenital anomalies in infants of diabetic mothers. The New England journal of medicine. 1981;304:1331–1334. doi: 10.1056/NEJM198105283042204. [DOI] [PubMed] [Google Scholar]
- 13.Ylinen K, Aula P, Stenman UH, Kesaniemi-Kuokkanen T, Teramo K. Risk of minor and major fetal malformations in diabetics with high haemoglobin A1c values in early pregnancy. British medical journal. 1984;289:345–346. doi: 10.1136/bmj.289.6441.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carney EW, Scialli AR, Watson RE, DeSesso JM. Mechanisms regulating toxicant disposition to the embryo during early pregnancy: an interspecies comparison. Birth Defects Res C Embryo Today. 2004;72:345–360. doi: 10.1002/bdrc.20027. [DOI] [PubMed] [Google Scholar]
- 15.Reece EA, Pinter E, Homko C, Wu YK, Naftolin F. The yolk sac theory: closing the circle on why diabetes-associated malformations occur. Journal of the Society for Gynecologic Investigation. 1994;1:3–13. [PubMed] [Google Scholar]
- 16.Cosmi E, Piazze JJ, Ruozi A, et al. Structural-tridimensional study of yolk sac in pregnancies complicated by diabetes. J Perinat Med. 2005;33:132–136. doi: 10.1515/JPM.2005.025. [DOI] [PubMed] [Google Scholar]
- 17.Ivanisevic M, Djelmis J, Jalsovec D, Bljajic D. Ultrasonic morphological characteristics of yolk sac in pregnancy complicated with type-1 diabetes mellitus. Gynecol Obstet Inves. 2006;61:80–86. doi: 10.1159/000088933. [DOI] [PubMed] [Google Scholar]
- 18.Barzilai M, Lyons EA, Levi CS, Lindsay DJ. Vitelline Duct Cyst or Double Yolk-Sac. J Ultras Med. 1989;8:523–526. doi: 10.7863/jum.1989.8.9.523. [DOI] [PubMed] [Google Scholar]
- 19.Berdahl DM, Blaine J, Van Voorhis B, Dokras A. Detection of enlarged yolk sac on early ultrasound is associated with adverse pregnancy outcomes. Fertil Steril. 2010;94:1535–1537. doi: 10.1016/j.fertnstert.2009.12.064. [DOI] [PubMed] [Google Scholar]
- 20.Huyhn A, Dommergues M, Izac B, et al. Characterization of hematopoietic progenitors from human yolk sacs and embryos. Blood. 1995;86:4474–4485. [PubMed] [Google Scholar]
- 21.Baron MH. Embryonic origins of mammalian hematopoiesis. Exp Hematol. 2003;31:1160–1169. doi: 10.1016/j.exphem.2003.08.019. [DOI] [PubMed] [Google Scholar]
- 22.Nath AK, Enciso J, Kuniyasu M, Hao XY, Madri JA, Pinter E. Nitric oxide modulates murine yolk sac vasculogenesis and rescues glucose induced vasculopathy. Development. 2004;131:2485–2496. doi: 10.1242/dev.01131. [DOI] [PubMed] [Google Scholar]
- 23.Yang P, Zhao Z, Reece EA. Blockade of c-Jun N-terminal kinase activation abrogates hyperglycemia-induced yolk sac vasculopathy in vitro. American journal of obstetrics and gynecology. 2008;198:321 e1–321 e7. doi: 10.1016/j.ajog.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 24.Luckett WP. Origin and differentiation of the yolk sac and extraembryonic mesoderm in presomite human and rhesus monkey embryos. The American journal of anatomy. 1978;152:59–97. doi: 10.1002/aja.1001520106. [DOI] [PubMed] [Google Scholar]
- 25.Drake CJ, Fleming PA. Vasculogenesis in the day 6.5 to 9.5 mouse embryo. Blood. 2000;95:1671–1679. [PubMed] [Google Scholar]
- 26.Palis J, McGrath KE, Kingsley PD. Initiation of hematopoiesis and vasculogenesis in murine yolk sac explants. Blood. 1995;86:156–163. [PubMed] [Google Scholar]
- 27.Flamme I, Frolich T, Risau W. Molecular mechanisms of vasculogenesis and embryonic angiogenesis. Journal of cellular physiology. 1997;173:206–210. doi: 10.1002/(SICI)1097-4652(199711)173:2<206::AID-JCP22>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 28.Risau W, Flamme I. Vasculogenesis. Annual review of cell and developmental biology. 1995;11:73–91. doi: 10.1146/annurev.cb.11.110195.000445. [DOI] [PubMed] [Google Scholar]
- 29.Loughman MS, Chatzistefanou K, Gonzalez EM, et al. Experimental corneal neovascularisation using sucralfate and basic fibroblast growth factor. Australian and New Zealand journal of ophthalmology. 1996;24:289–295. doi: 10.1111/j.1442-9071.1996.tb01596.x. [DOI] [PubMed] [Google Scholar]
- 30.Pinter E, Mahooti S, Wang Y, Imhof BA, Madri JA. Hyperglycemia-induced vasculopathy in the murine vitelline vasculature: correlation with PECAM-1/CD31 tyrosine phosphorylation state. The American journal of pathology. 1999;154:1367–1379. doi: 10.1016/S0002-9440(10)65391-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eriksson UJ, Borg LA, Cederberg J, et al. Pathogenesis of diabetes-induced congenital malformations. Upsala journal of medical sciences. 2000;105:53–84. doi: 10.1517/03009734000000055. [DOI] [PubMed] [Google Scholar]
- 32.Reece EA, Homko CJ, Wu YK. Multifactorial basis of the syndrome of diabetic embryopathy. Teratology. 1996;54:171–182. doi: 10.1002/(SICI)1096-9926(199610)54:4<171::AID-TERA1>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 33.Schoenfeld A, Warchaizer S, Erman A, Hod M. Prostaglandin metabolism in the yolk sacs of normal and diabetic pregnancies. Early pregnancy : biology and medicine : the official journal of the Society for the Investigation of Early Pregnancy. 1996;2:129–132. [PubMed] [Google Scholar]
- 34.Pinter E, Reece EA, Leranth CZ, et al. Yolk sac failure in embryopathy due to hyperglycemia: ultrastructural analysis of yolk sac differentiation associated with embryopathy in rat conceptuses under hyperglycemic conditions. Teratology. 1986;33:73–84. doi: 10.1002/tera.1420330110. [DOI] [PubMed] [Google Scholar]
- 35.Reece EA, Pinter E, Leranth C, Hobbins JC, Mahoney MJ, Naftolin F. Yolk sac failure in embryopathy due to hyperglycemia: horseradish peroxidase uptake in the assessment of yolk sac function. Obstetrics and gynecology. 1989;74:755–762. [PubMed] [Google Scholar]
- 36.Pinter E, Haigh J, Nagy A, Madri JA. Hyperglycemia-induced vasculopathy in the murine conceptus is mediated via reductions of VEGF-A expression and VEGF receptor activation. The American journal of pathology. 2001;158:1199–1206. doi: 10.1016/S0002-9440(10)64069-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pinter E, Barreuther M, Lu T, Imhof BA, Madri JA. Platelet-endothelial cell adhesion molecule-1 (PECAM-1/CD31) tyrosine phosphorylation state changes during vasculogenesis in the murine conceptus. The American journal of pathology. 1997;150:1523–1530. [PMC free article] [PubMed] [Google Scholar]
- 38.DeLisser HM, Christofidou-Solomidou M, Strieter RM, et al. Involvement of endothelial PECAM-1/CD31 in angiogenesis. The American journal of pathology. 1997;151:671–677. [PMC free article] [PubMed] [Google Scholar]
- 39.Yang P, Reece EA. Role of HIF-1alpha in maternal hyperglycemia-induced embryonic vasculopathy. American journal of obstetrics and gynecology. 2011;204:332 e1–332 e7. doi: 10.1016/j.ajog.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weng H, Li X, Reece EA, Yang P. SOD1 suppresses maternal hyperglycemia-increased iNOS expression and consequent nitrosative stress in diabetic embryopathy. American journal of obstetrics and gynecology. 2012;206:448 e1–448 e7. doi: 10.1016/j.ajog.2012.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang P, Cao Y, Li H. Hyperglycemia induces inducible nitric oxide synthase gene expression and consequent nitrosative stress via c-Jun N-terminal kinase activation. American journal of obstetrics and gynecology. 2010;203:185 e5–185 e11. doi: 10.1016/j.ajog.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang P, Zhao Z, Reece EA. Involvement of c-Jun N-terminal kinases activation in diabetic embryopathy. Biochemical and biophysical research communications. 2007;357:749–754. doi: 10.1016/j.bbrc.2007.04.023. [DOI] [PubMed] [Google Scholar]
- 43.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bruick RK, McKnight SL. A conserved family of prolyl-4-hydroxylases that modify HIF. Science. 2001;294:1337–1340. doi: 10.1126/science.1066373. [DOI] [PubMed] [Google Scholar]
- 45.Epstein AC, Gleadle JM, McNeill LA, et al. C elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107:43–54. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- 46.Ivan M, Kondo K, Yang H, et al. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 47.Jaakkola P, Mole DR, Tian YM, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 48.Kallio PJ, Wilson WJ, O‘Brien S, Makino Y, Poellinger L. Regulation of the hypoxia-inducible transcription factor 1alpha by the ubiquitin-proteasome pathway. The Journal of biological chemistry. 1999;274:6519–6525. doi: 10.1074/jbc.274.10.6519. [DOI] [PubMed] [Google Scholar]
- 49.Huang LE, Gu J, Schau M, Bunn HF. Regulation of hypoxia-inducible factor 1alpha is mediated by an O2-dependent degradation domain via the ubiquitin-proteasome pathway. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:7987–7992. doi: 10.1073/pnas.95.14.7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee YM, Jeong CH, Koo SY, et al. Determination of hypoxic region by hypoxia marker in developing mouse embryos in vivo: a possible signal for vessel development. Developmental dynamics : an official publication of the American Association of Anatomists. 2001;220:175–186. doi: 10.1002/1097-0177(20010201)220:2<175::AID-DVDY1101>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 51.Liu LX, Lu H, Luo Y, et al. Stabilization of vascular endothelial growth factor mRNA by hypoxia-inducible factor 1. Biochemical and biophysical research communications. 2002;291:908–914. doi: 10.1006/bbrc.2002.6551. [DOI] [PubMed] [Google Scholar]
- 52.Liu W, Shen SM, Zhao XY, Chen GQ. Targeted genes and interacting proteins of hypoxia inducible factor-1. International journal of biochemistry and molecular biology. 2012;3:165–178. [PMC free article] [PubMed] [Google Scholar]
- 53.Hirota K, Semenza GL. Regulation of angiogenesis by hypoxia-inducible factor 1. Critical reviews in oncology/hematology. 2006;59:15–26. doi: 10.1016/j.critrevonc.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 54.Ryan HE, Lo J, Johnson RS. HIF-1 alpha is required for solid tumor formation and embryonic vascularization. The EMBO journal. 1998;17:3005–3015. doi: 10.1093/emboj/17.11.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Adelman DM, Maltepe E, Simon MC. Multilineage embryonic hematopoiesis requires hypoxic ARNT activity. Genes & development. 1999;13:2478–2483. doi: 10.1101/gad.13.19.2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ramirez-Bergeron DL, Runge A, Adelman DM, Gohil M, Simon MC. HIF-dependent hematopoietic factors regulate the development of the embryonic vasculature. Developmental cell. 2006;11:81–92. doi: 10.1016/j.devcel.2006.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gabbay-Benziv R, Reece EA, Wang F, Yang P. Birth defects in pregestational diabetes: Defect range, glycemic threshold and pathogenesis. World journal of diabetes. 2015;6:481–488. doi: 10.4239/wjd.v6.i3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang F, Reece EA, Yang P. Advances in revealing the molecular targets downstream of oxidative stress-induced proapoptotic kinase signaling in diabetic embryopathy. American journal of obstetrics and gynecology. 2015;213:125–134. doi: 10.1016/j.ajog.2015.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang P, Reece EA, Wang F, Gabbay-Benziv R. Decoding the oxidative stress hypothesis in diabetic embryopathy through proapoptotic kinase signaling. American journal of obstetrics and gynecology. 2015;212:569–579. doi: 10.1016/j.ajog.2014.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sun F, Akazawa S, Sugahara K, et al. Apoptosis in normal rat embryo tissues during early organogenesis: the possible involvement of Bax and Bcl-2. Archives of histology and cytology. 2002;65:145–157. doi: 10.1679/aohc.65.145. [DOI] [PubMed] [Google Scholar]
- 61.Moley KH. Hyperglycemia and apoptosis: mechanisms for congenital malformations and pregnancy loss in diabetic women. Trends in endocrinology and metabolism: TEM. 2001;12:78–82. doi: 10.1016/s1043-2760(00)00341-6. [DOI] [PubMed] [Google Scholar]
- 62.Fine EL, Horal M, Chang TI, Fortin G, Loeken MR. Evidence that elevated glucose causes altered gene expression, apoptosis, and neural tube defects in a mouse model of diabetic pregnancy. Diabetes. 1999;48:2454–2462. doi: 10.2337/diabetes.48.12.2454. [DOI] [PubMed] [Google Scholar]
- 63.Forsberg H, Eriksson UJ, Welsh N. Apoptosis in embryos of diabetic rats. Pharmacology & toxicology. 1998;83:104–111. doi: 10.1111/j.1600-0773.1998.tb01452.x. [DOI] [PubMed] [Google Scholar]
- 64.Sun F, Kawasaki E, Akazawa S, et al. Apoptosis and its pathway in early post-implantation embryos of diabetic rats. Diabetes research and clinical practice. 2005;67:110–118. doi: 10.1016/j.diabres.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 65.Gu H, Yu J, Dong D, Zhou Q, Wang JY, Yang P. The miR-322-TRAF3 circuit mediates the pro-apoptotic effect of high glucose on neural stem cells. Toxicological sciences : an official journal of the Society of Toxicology. 2015;144:186–196. doi: 10.1093/toxsci/kfu271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang P, Li X, Xu C, et al. Maternal hyperglycemia activates an ASK1-FoxO3a–caspase 8 pathway that leads to embryonic neural tube defects. Science signaling. 2013;6 doi: 10.1126/scisignal.2004020. ra74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dincer Y, Akcay T, Alademir Z, Ilkova H. Assessment of DNA base oxidation and glutathione level in patients with type 2 diabetes. Mutation research. 2002;505:75–81. doi: 10.1016/s0027-5107(02)00143-4. [DOI] [PubMed] [Google Scholar]
- 68.Sakamaki H, Akazawa S, Ishibashi M, et al. Significance of glutathione-dependent antioxidant system in diabetes-induced embryonic malformations. Diabetes. 1999;48:1138–1144. doi: 10.2337/diabetes.48.5.1138. [DOI] [PubMed] [Google Scholar]
- 69.Wolff SP. Diabetes mellitus free radicals Free radicals, transition metals and oxidative stress in the aetiology of diabetes mellitus and complications. British medical bulletin. 1993;49:642–652. doi: 10.1093/oxfordjournals.bmb.a072637. [DOI] [PubMed] [Google Scholar]
- 70.Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991;40:405–412. doi: 10.2337/diab.40.4.405. [DOI] [PubMed] [Google Scholar]
- 71.Yang P, Zhao Z, Reece EA. Activation of oxidative stress signaling that is implicated in apoptosis with a mouse model of diabetic embryopathy. American journal of obstetrics and gynecology. 2008;198:130 e1–130 e7. doi: 10.1016/j.ajog.2007.06.070. [DOI] [PubMed] [Google Scholar]
- 72.Wang F, Reece EA, Yang P. Superoxide dismutase 1 overexpression in mice abolishes maternal diabetes-induced endoplasmic reticulum stress in diabetic embryopathy. American journal of obstetrics and gynecology. 2013;209:345 e1–345 e7. doi: 10.1016/j.ajog.2013.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li X, Xu C, Yang P. c-Jun NH2-terminal kinase 1/2 and endoplasmic reticulum stress as interdependent and reciprocal causation in diabetic embryopathy. Diabetes. 2013;62:599–608. doi: 10.2337/db12-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li X, Weng H, Reece EA, Yang P. SOD1 overexpression in vivo blocks hyperglycemia-induced specific PKC isoforms: substrate activation and consequent lipid peroxidation in diabetic embryopathy. American journal of obstetrics and gynecology. 2011;205:84 e1–84 e6. doi: 10.1016/j.ajog.2011.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang F, Reece EA, Yang PX. Superoxide dismutase 1 overexpression in mice abolishes maternal diabetes-induced endoplasmic reticulum stress in diabetic embryopathy. American journal of obstetrics and gynecology. 2013;209 doi: 10.1016/j.ajog.2013.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kyosseva SV. Mitogen-activated protein kinase signaling. International review of neurobiology. 2004;59:201–220. doi: 10.1016/S0074-7742(04)59008-6. [DOI] [PubMed] [Google Scholar]
- 77.Torres M, Forman HJ. Redox signaling and the MAP kinase pathways. BioFactors (Oxford, England) 2003;17:287–296. doi: 10.1002/biof.5520170128. [DOI] [PubMed] [Google Scholar]
- 78.Widmann C, Gibson S, Jarpe MB, Johnson GL. Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiological reviews. 1999;79:143–180. doi: 10.1152/physrev.1999.79.1.143. [DOI] [PubMed] [Google Scholar]
- 79.Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiological reviews. 2001;81:807–869. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- 80.Tobiume K, Saitoh M, Ichijo H. Activation of apoptosis signal-regulating kinase 1 by the stress-induced activating phosphorylation of pre-formed oligomer. Journal of cellular physiology. 2002;191:95–104. doi: 10.1002/jcp.10080. [DOI] [PubMed] [Google Scholar]
- 81.Zhang Q, Zhang G, Meng F, Tian H. Biphasic activation of apoptosis signal-regulating kinase 1-stress-activated protein kinase 1-c-Jun N-terminal protein kinase pathway is selectively mediated by Ca2+-permeable alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate receptors involving oxidative stress following brain ischemia in rat hippocampus. Neuroscience letters. 2003;337:51–55. doi: 10.1016/s0304-3940(02)01295-8. [DOI] [PubMed] [Google Scholar]
- 82.Watanabe T, Otsu K, Takeda T, et al. Apoptosis signal-regulating kinase 1 is involved not only in apoptosis but also in non-apoptotic cardiomyocyte death. Biochemical and biophysical research communications. 2005;333:562–567. doi: 10.1016/j.bbrc.2005.05.151. [DOI] [PubMed] [Google Scholar]
- 83.Kadowaki H, Nishitoh H, Urano F, et al. Amyloid beta induces neuronal cell death through ROS-mediated ASK1 activation. Cell death and differentiation. 2005;12:19–24. doi: 10.1038/sj.cdd.4401528. [DOI] [PubMed] [Google Scholar]
- 84.Tobiume K, Matsuzawa A, Takahashi T, et al. ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO reports. 2001;2:222–228. doi: 10.1093/embo-reports/kve046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang F, Wu Y, Quon MJ, Li X, Yang P. ASK1 mediates the teratogenicity of diabetes in the developing heart by inducing ER stress and inhibiting critical factors essential for cardiac development. American journal of physiology Endocrinology and metabolism. 2015 doi: 10.1152/ajpendo.00121.2015. ajpendo 00121 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang F, Wu Y, Gu H, et al. Ask1 gene deletion blocks maternal diabetes-induced endoplasmic reticulum stress in the developing embryo by disrupting the unfolded protein response signalosome. Diabetes. 2015;64:973–988. doi: 10.2337/db14-0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yokoi T, Fukuo K, Yasuda O, et al. Apoptosis signal-regulating kinase 1 mediates cellular senescence induced by high glucose in endothelial cells. Diabetes. 2006;55:1660–1665. doi: 10.2337/db05-1607. [DOI] [PubMed] [Google Scholar]
- 88.Forstermann U, Gath I, Schwarz P, Closs EI, Kleinert H. Isoforms of nitric oxide synthase Properties, cellular distribution and expressional control. Biochemical pharmacology. 1995;50:1321–1332. doi: 10.1016/0006-2952(95)00181-6. [DOI] [PubMed] [Google Scholar]
- 89.Albina JE. On the expression of nitric oxide synthase by human macrophages. Why no NO? Journal of leukocyte biology. 1995;58:643–649. doi: 10.1002/jlb.58.6.643. [DOI] [PubMed] [Google Scholar]
- 90.Hosogi S, Iwasaki Y, Yamada T, et al. Effect of inducible nitric oxide synthase on apoptosis in Candida-induced acute lung injury. Biomedical research (Tokyo, Japan) 2008;29:257–266. doi: 10.2220/biomedres.29.257. [DOI] [PubMed] [Google Scholar]
- 91.Forrester K, Ambs S, Lupold SE, et al. Nitric oxide-induced p53 accumulation and regulation of inducible nitric oxide synthase expression by wild-type p53. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:2442–2447. doi: 10.1073/pnas.93.6.2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bennett M, Macdonald K, Chan SW, Luzio JP, Simari R, Weissberg P. Cell surface trafficking of Fas: a rapid mechanism of p53-mediated apoptosis. Science. 1998;282:290–293. doi: 10.1126/science.282.5387.290. [DOI] [PubMed] [Google Scholar]
- 93.Bossi G, Griffiths GM. Degranulation plays an essential part in regulating cell surface expression of Fas ligand in T cells and natural killer cells. Nature medicine. 1999;5:90–96. doi: 10.1038/4779. [DOI] [PubMed] [Google Scholar]
- 94.Lacza Z, Pankotai E, Csordas A, et al. Mitochondrial NO and reactive nitrogen species production: does mtNOS exist? Nitric oxide : biology and chemistry / official journal of the Nitric Oxide Society. 2006;14:162–168. doi: 10.1016/j.niox.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 95.Nicotera P, Bernassola F, Melino G. Nitric oxide (NO), a signaling molecule with a killer soul. Cell death and differentiation. 1999;6:931–933. doi: 10.1038/sj.cdd.4400583. [DOI] [PubMed] [Google Scholar]
- 96.Stamler JS. Redox signaling: nitrosylation and related target interactions of nitric oxide. Cell. 1994;78:931–936. doi: 10.1016/0092-8674(94)90269-0. [DOI] [PubMed] [Google Scholar]
- 97.Stamler JS, Singel DJ, Loscalzo J. Biochemistry of nitric oxide and its redox-activated forms. Science. 1992;258:1898–1902. doi: 10.1126/science.1281928. [DOI] [PubMed] [Google Scholar]
- 98.Brunet LR. Nitric oxide in parasitic infections. International immunopharmacology. 2001;1:1457–1467. doi: 10.1016/s1567-5769(01)00090-x. [DOI] [PubMed] [Google Scholar]
- 99.Pineda-Molina E, Lamas S. Nitric oxide as a regulator of gene expression: studies with the transcription factor proteins cJun and p50. BioFactors (Oxford, England) 2001;15:113–115. doi: 10.1002/biof.5520150213. [DOI] [PubMed] [Google Scholar]
- 100.Nath AK, Madri JA. The roles of nitric oxide in murine cardiovascular development. Developmental biology. 2006;292:25–33. doi: 10.1016/j.ydbio.2005.12.039. [DOI] [PubMed] [Google Scholar]
- 101.Pieper GM, Roza AM. The complex role of iNOS in acutely rejecting cardiac transplants. Free radical biology & medicine. 2008;44:1536–1552. doi: 10.1016/j.freeradbiomed.2008.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Taylor EL, Megson IL, Haslett C, Rossi AG. Nitric oxide: a key regulator of myeloid inflammatory cell apoptosis. Cell death and differentiation. 2003;10:418–430. doi: 10.1038/sj.cdd.4401152. [DOI] [PubMed] [Google Scholar]
- 103.Kaimul AM, Nakamura H, Masutani H, Yodoi J. Thioredoxin and thioredoxin-binding protein-2 in cancer and metabolic syndrome. Free radical biology & medicine. 2007;43:861–868. doi: 10.1016/j.freeradbiomed.2007.05.032. [DOI] [PubMed] [Google Scholar]
- 104.Saitoh M, Nishitoh H, Fujii M, et al. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. The EMBO journal. 1998;17:2596–2606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kaimul Ahsan M, Nakamura H, Tanito M, Yamada K, Utsumi H, Yodoi J. Thioredoxin-1 suppresses lung injury and apoptosis induced by diesel exhaust particles (DEP) by scavenging reactive oxygen species and by inhibiting DEP-induced downregulation of Akt. Free radical biology & medicine. 2005;39:1549–1559. doi: 10.1016/j.freeradbiomed.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 106.Widlansky ME, Biegelsen ES, Hamburg NM, Duffy SJ, Keaney JF, Jr, Vita JA. Coronary endothelial dysfunction is not rapidly reversible with ascorbic acid. Free radical biology & medicine. 2004;36:123–130. doi: 10.1016/j.freeradbiomed.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 107.Matsuda M, Masutani H, Nakamura H, et al. Protective activity of adult T cell leukemia-derived factor (ADF) against tumor necrosis factor-dependent cytotoxicity on U937 cells. J Immunol. 1991;147:3837–3841. [PubMed] [Google Scholar]
- 108.Laurent TC, Moore EC, Reichard P. Enzymatic Synthesis of Deoxyribonucleotides. Iv. Isolation and Characterization of Thioredoxin, the Hydrogen Donor from Escherichia Coli B. The Journal of biological chemistry. 1964;239:3436–3444. [PubMed] [Google Scholar]
- 109.Yodoi J, Okada M, Tagaya Y, et al. IL-2 receptor gene activation by ATL-derived factor (ADF) Advances in experimental medicine and biology. 1987;213:139–148. doi: 10.1007/978-1-4684-5323-2_14. [DOI] [PubMed] [Google Scholar]
- 110.Okuda M, Inoue N, Azumi H, et al. Expression of glutaredoxin in human coronary arteries: its potential role in antioxidant protection against atherosclerosis. Arteriosclerosis, thrombosis, and vascular biology. 2001;21:1483–1487. doi: 10.1161/hq0901.095550. [DOI] [PubMed] [Google Scholar]
- 111.Nakamura H, Matsuda M, Furuke K, et al. Adult T cell leukemia-derived factor/human thioredoxin protects endothelial F-2 cell injury caused by activated neutrophils or hydrogen peroxide. Immunology letters. 1994;42:75–80. doi: 10.1016/0165-2478(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 112.Collet JF, Messens J. Structure, function, and mechanism of thioredoxin proteins. Antioxidants & redox signaling. 2010;13:1205–1216. doi: 10.1089/ars.2010.3114. [DOI] [PubMed] [Google Scholar]
- 113.Arner ES, Holmgren A. Physiological functions of thioredoxin and thioredoxin reductase. European journal of biochemistry / FEBS. 2000;267:6102–6109. doi: 10.1046/j.1432-1327.2000.01701.x. [DOI] [PubMed] [Google Scholar]
- 114.Takagi Y, Gon Y, Todaka T, et al. Expression of thioredoxin is enhanced in atherosclerotic plaques and during neointima formation in rat arteries. Laboratory investigation; a journal of technical methods and pathology. 1998;78:957–966. [PubMed] [Google Scholar]
- 115.Turoczi T, Chang VW, Engelman RM, Maulik N, Ho YS, Das DK. Thioredoxin redox signaling in the ischemic heart: an insight with transgenic mice overexpressing Trx1. Journal of molecular and cellular cardiology. 2003;35:695–704. doi: 10.1016/s0022-2828(03)00117-2. [DOI] [PubMed] [Google Scholar]
- 116.Tanito M, Nakamura H, Kwon YW, et al. Enhanced oxidative stress and impaired thioredoxin expression in spontaneously hypertensive rats. Antioxidants & redox signaling. 2004;6:89–97. doi: 10.1089/152308604771978381. [DOI] [PubMed] [Google Scholar]
- 117.Welsh SJ, Bellamy WT, Briehl MM, Powis G. The redox protein thioredoxin-1 (Trx-1) increases hypoxia-inducible factor 1alpha protein expression: Trx-1 overexpression results in increased vascular endothelial growth factor production and enhanced tumor angiogenesis. Cancer research. 2002;62:5089–5095. [PubMed] [Google Scholar]
- 118.Reece EA, Wiznitzer A, Homko CJ, Hagay Z, Wu YK. Synchronization of the factors critical for diabetic teratogenesis: an in vitro model. American journal of obstetrics and gynecology. 1996;174:1284–1288. doi: 10.1016/s0002-9378(96)70672-5. [DOI] [PubMed] [Google Scholar]
- 119.Pinter E, Reece EA, Ogburn PL, Jr, et al. Fatty acid content of yolk sac and embryo in hyperglycemia-induced embryopathy and effect of arachidonic acid supplementation. American journal of obstetrics and gynecology. 1988;159:1484–1490. doi: 10.1016/0002-9378(88)90579-0. [DOI] [PubMed] [Google Scholar]
- 120.Reece EA, Homko CJ, Wu YK, Wiznitzer A. The role of free radicals and membrane lipids in diabetes-induced congenital malformations. Journal of the Society for Gynecologic Investigation. 1998;5:178–187. doi: 10.1016/s1071-5576(98)00008-2. [DOI] [PubMed] [Google Scholar]
- 121.Reece EA, Eriksson UJ. The pathogenesis of diabetes-associated congenital malformations. Obstetrics and gynecology clinics of North America. 1996;23:29–45. doi: 10.1016/s0889-8545(05)70243-6. [DOI] [PubMed] [Google Scholar]
- 122.Pinter E, Reece EA, Leranth CZ, et al. Arachidonic acid prevents hyperglycemia-associated yolk sac damage and embryopathy. American journal of obstetrics and gynecology. 1986;155:691–702. doi: 10.1016/s0002-9378(86)80001-1. [DOI] [PubMed] [Google Scholar]
- 123.Khandelwal M, Reece EA, Wu YK, Borenstein M. Dietary myo-inositol therapy in hyperglycemia-induced embryopathy. Teratology. 1998;57:79–84. doi: 10.1002/(SICI)1096-9926(199802)57:2<79::AID-TERA6>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 124.Edwards A, Springett A, Padfield J, Dorling J, Bugg G, Mansell P. Differences in post-mortem findings after stillbirth in women with and without diabetes. Diabetic medicine : a journal of the British Diabetic Association. 2013;30:1219–1224. doi: 10.1111/dme.12272. [DOI] [PubMed] [Google Scholar]
- 125.Starikov R, Inman K, Chen K, et al. Comparison of placental findings in type 1 and type 2 diabetic pregnancies. Placenta. 2014;35:1001–1006. doi: 10.1016/j.placenta.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 126.Beauharnais CC, Roberts DJ, Wexler DJ. High rate of placental infarcts in type 2 compared with type 1 diabetes. The Journal of clinical endocrinology and metabolism. 2012;97:E1160–E1164. doi: 10.1210/jc.2011-3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Leach L. Placental vascular dysfunction in diabetic pregnancies: intimations of fetal cardiovascular disease? Microcirculation. 2011;18:263–269. doi: 10.1111/j.1549-8719.2011.00091.x. [DOI] [PubMed] [Google Scholar]
- 128.Ecker AM, Donnellan NM, Shepherd JP, Lee TT. Abdominal wall endometriosis: 12 years of experience at a large academic institution. American journal of obstetrics and gynecology. 2014;211:363 e1–363 e5. doi: 10.1016/j.ajog.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 129.Yanit KE, Snowden JM, Cheng YW, Caughey AB. The impact of chronic hypertension and pregestational diabetes on pregnancy outcomes. American journal of obstetrics and gynecology. 2012;207:333 e1–333 e6. doi: 10.1016/j.ajog.2012.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cooley SM, Reidy FR, Mooney EE, McAuliffe FM. Antenatal suspicion of ischemic placental disease and coexistence of maternal and fetal placental disease: analysis of over 500 cases. American journal of obstetrics and gynecology. 2011;205:576 e1–576 e6. doi: 10.1016/j.ajog.2011.06.061. [DOI] [PubMed] [Google Scholar]
- 131.Baker AM, Braun JM, Salafia CM, et al. Risk factors for uteroplacental vascular compromise and inflammation. American journal of obstetrics and gynecology. 2008;199:256 e1–256 e9. doi: 10.1016/j.ajog.2008.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Darling AM, McDonald CR, Conroy AL, et al. Angiogenic and inflammatory biomarkers in midpregnancy and small-for-gestational-age outcomes in Tanzania. American journal of obstetrics and gynecology. 2014;211:509 e1–509 e8. doi: 10.1016/j.ajog.2014.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Guzman ER, Shen-Schwarz S, Benito C, Vintzileos AM, Lake M, Lai YL. The relationship between placental histology and cervical ultrasonography in women at risk for pregnancy loss and spontaneous preterm birth. American journal of obstetrics and gynecology. 1999;181:793–797. doi: 10.1016/s0002-9378(99)70303-0. [DOI] [PubMed] [Google Scholar]
- 134.Haeri S, Khoury J, Kovilam O, Miodovnik M. The association of intrauterine growth abnormalities in women with type 1 diabetes mellitus complicated by vasculopathy. American journal of obstetrics and gynecology. 2008;199:278 e1–278 e5. doi: 10.1016/j.ajog.2008.06.066. [DOI] [PubMed] [Google Scholar]
- 135.Stanek J. Comparison of placental pathology in preterm, late-preterm, near-term, and term births. American journal of obstetrics and gynecology. 2014;210:234 e1–234 e6. doi: 10.1016/j.ajog.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 136.Stevens DU, Al-Nasiry S, Fajta MM, et al. Cardiovascular and thrombogenic risk of decidual vasculopathy in preeclampsia. American journal of obstetrics and gynecology. 2014;210:545 e1–545 e6. doi: 10.1016/j.ajog.2013.12.029. [DOI] [PubMed] [Google Scholar]
- 137.Chaiworapongsa T, Romero R, Korzeniewski SJ, et al. Maternal plasma concentrations of angiogenic/antiangiogenic factors in the third trimester of pregnancy to identify the patient at risk for stillbirth at or near term and severe late preeclampsia. American journal of obstetrics and gynecology. 2013;208:287 e1–287 e15. doi: 10.1016/j.ajog.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Boghossian NS, Yeung E, Albert PS, et al. Changes in diabetes status between pregnancies and impact on subsequent newborn outcomes. American journal of obstetrics and gynecology. 2014;210:431 e1–431 e14. doi: 10.1016/j.ajog.2013.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Peterson C, Grosse SD, Li R, et al. Preventable health and cost burden of adverse birth outcomes associated with pregestational diabetes in the United States. American journal of obstetrics and gynecology. 2015;212:74 e1–74 e9. doi: 10.1016/j.ajog.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Schwartz N, Nachum Z, Green MS. The prevalence of gestational diabetes mellitus recurrence-effect of ethnicity and parity: a metaanalysis. American journal of obstetrics and gynecology. 2015 doi: 10.1016/j.ajog.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 141.Maresh MJ, Holmes VA, Patterson CC, et al. Glycemic targets in the second and third trimester of pregnancy for women with type 1 diabetes. Diabetes care. 2015;38:34–42. doi: 10.2337/dc14-1755. [DOI] [PubMed] [Google Scholar]
- 142.Diabetes in Pregnancy: Management of Diabetes and Its Complications from Preconception to the Postnatal Period. London: 2008. [PubMed] [Google Scholar]
- 143.Skupien J, Cyganek K, Malecki MT. Diabetic pregnancy: an overview of current guidelines and clinical practice. Current opinion in obstetrics & gynecology. 2014;26:431–437. doi: 10.1097/GCO.0000000000000111. [DOI] [PubMed] [Google Scholar]
- 144.Baxi LV, Dziadosz M. Use of hemoglobin A1c as an early predictor of gestational diabetes mellitus. American journal of obstetrics and gynecology. 2015;212:826–827. doi: 10.1016/j.ajog.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 145.Finer LB, Zolna MR. Unintended pregnancy in the United States: incidence and disparities, 2006. Contraception. 2011;84:478–485. doi: 10.1016/j.contraception.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Oza-Frank R, Kachoria R, Keim SA, Klebanoff MA. Provision of specific preconception care messages and associated maternal health behaviors before and during pregnancy. American journal of obstetrics and gynecology. 2015;212:372 e1–372 e8. doi: 10.1016/j.ajog.2014.10.027. [DOI] [PubMed] [Google Scholar]
- 147.Bacon S, Schmid J, McCarthy A, et al. The clinical management of hyperglycemia in pregnancy complicated by maturity-onset diabetes of the young. American journal of obstetrics and gynecology. 2015;213:236 e1–236 e7. doi: 10.1016/j.ajog.2015.04.037. [DOI] [PubMed] [Google Scholar]
- 148.Egerman RS, Ramsey RD, Kao LW, et al. Perinatal outcomes in pregnancies managed with antenatal insulin glargine. American journal of perinatology. 2009;26:591–595. doi: 10.1055/s-0029-1220782. [DOI] [PubMed] [Google Scholar]
- 149.Gabbe SG, Holing E, Temple P, Brown ZA. Benefits, risks, costs, and patient satisfaction associated with insulin pump therapy for the pregnancy complicated by type 1 diabetes mellitus. American journal of obstetrics and gynecology. 2000;182:1283–1291. doi: 10.1067/mob.2000.106182. [DOI] [PubMed] [Google Scholar]
- 150.Roeder HA, Moore TR, Ramos GA. Insulin pump dosing across gestation in women with well-controlled type 1 diabetes mellitus. American journal of obstetrics and gynecology. 2012;207:324 e1–324 e5. doi: 10.1016/j.ajog.2012.06.029. [DOI] [PubMed] [Google Scholar]
- 151.Bismuth E, Bouche C, Caliman C, et al. Management of pregnancy in women with type 1 diabetes mellitus: guidelines of the French-Speaking Diabetes Society (Societe francophone du diabete [SFD]) Diabetes & metabolism. 2012;38:205–216. doi: 10.1016/j.diabet.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 152.Herrera KM, Rosenn BM, Foroutan J, et al. Randomized controlled trial of insulin detemir versus NPH for the treatment of pregnant women with diabetes. American journal of obstetrics and gynecology. 2015 doi: 10.1016/j.ajog.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 153.Parker SE, Yazdy MM, Tinker SC, Mitchell AA, Werler MM. The impact of folic acid intake on the association among diabetes mellitus, obesity, and spina bifida. American journal of obstetrics and gynecology. 2013;209 doi: 10.1016/j.ajog.2013.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]