Abstract
Fyn, a major Src family kinase (SFK) member that is densely expressed in striatal neurons, is actively involved in the regulation of cellular and synaptic activities in local neurons. This SFK member is likely regulated by dopamine signaling through a receptor mechanism involving dopamine D2 receptors (D2Rs). In this study, we thus characterized the D2R-dependent regulation of Fyn in the rat striatum in vivo. Moreover, we explored whether D2Rs regulate metabotropic glutamate receptor 5 (mGluR5) in its tyrosine phosphorylation and whether the D2R-SFK pathway modulates trafficking of mGluR5. We found that blockade of D2Rs by a systemic administration of a D2R antagonist eticlopride substantially increased SFK phosphorylation in the striatum. This increase was a transient and reversible event. The eticlopride-induced SFK phosphorylation occurred predominantly in immunopurified Fyn but not another SFK member Src. Eticlopride also elevated tyrosine phosphorylation of mGluR5. In parallel, eticlopride enhanced synaptic delivery of active Fyn and mGluR5. Pretreatment with an SFK inhibitor blocked the eticlopride-induced tyrosine phosphorylation and synaptic trafficking of mGluR5. These results indicate that D2Rs inhibit SFK (mainly Fyn) phosphorylation in the striatum. D2Rs also inhibit tyrosine phosphorylation and synaptic recruitment of mGluR5 through a signaling mechanism likely involving Fyn.
Keywords: metabotropic glutamate receptor, non-receptor tyrosine kinase, Src, SFK, tyrosine phosphorylation, nucleus accumbens, eticlopride, PP2
Graphical Abstract
The Src family kinase (SFK) belongs to a subfamily of non-receptor tyrosine kinases (Neet and Hunter, 1996). At present, nine SFK members have been identified, and five of them are expressed in the mammalian brain, i.e., Src, Fyn (isoform 1; also known as isoform a or FynB), Yes, Lyn, and Lck (Omri et al., 1996; Kalia et al., 2004; Bongiorno-Borbone et al., 2005). Fyn and Src represent two typical SFK members which are particularly enriched at synaptic sites. By phosphorylating a set of synaptic substrates, SFKs vigorously regulate synaptic transmission and plasticity (Kalia et al., 2004; Ohnishi et al., 2011; Schenone et al., 2011). Like many kinases, SFKs are activated through a phosphorylation-dependent mechanism. Constitutive or activity-dependent phosphorylation of a conserved residue, tyrosine 416 (Y416), in the activation loop of SFKs by an autophosphorylation mechanism enables activation of these enzymes (Roskoski, 2005; Okada, 2012).
Dopamine is a principal transmitter in the mesolimbic system involved in various neuropsychiatric and neurodegenerative diseases. In the striatum, dopamine receptors, mainly dopamine D1 receptors (D1Rs) and dopamine D2 receptor (D2Rs), are densely expressed in projection medium spiny neurons (MSNs). Noticeably, these two dopamine receptor subtypes are segregated in two phenotypes of MSNs: D1Rs in striatonigral neurons and D2Rs in striatopallidal neurons (Gerfen et al., 1990; Aubert et al., 2000; Bertran-Gonzalez et al., 2010). Since Src and especially Fyn are enriched in striatonigral and striatopallidal neurons (Pascoli et al., 2011), both D1Rs and D2Rs are believed to impact SFKs. Indeed, in terms of D2Rs, the D2R antagonist haloperidol increased Fyn phosphorylation at Y416 in the mouse striatum (Hattori et al., 2006). This indicates an inhibitory linkage from D2Rs to Fyn, although the detailed role of D2Rs in regulating SFKs has been incompletely characterized.
Glutamate receptors are subject to phosphorylation-dependent modulations by SFKs. Like ionotropic glutamate receptors (Groveman et al., 2012; Trepanier et al., 2012), metabotropic glutamate receptor 5 (mGluR5) is a tyrosine-phosphorylated protein in rat striatal neurons (Orlando et al., 2002). Among eight mGluR subtypes so far cloned, mGluR5 is the mostly abundant subtype in the striatum (Testa et al., 1994; Tallaksen-Greene et al., 1998). Extensive studies have demonstrated that mGluR5 is an important regulator of cellular and synaptic activity in the region (Niswende and Conn, 2010). However, while mGluR5 is tyrosine-phosphorylated, whether D2Rs regulate tyrosine phosphorylation of mGluR5 and whether the D2R-regulated tyrosine phosphorylation of mGluR5 leads to any changes in mGluR5 activity such as synaptic trafficking are unclear.
A series of pharmacological studies was conducted to evaluate and characterize the role of D2Rs in regulating SFK phosphorylation in vivo. We first investigated the dose- and time-dependent effect of a D2R blocker eticlopride on SFK phosphorylation at a common activation site (Y416) in the striatum of adult rats. We then examined whether eticlopride blockade of D2Rs results in tyrosine phosphorylation of striatal mGluR5. Finally, we analyzed whether eticlopride affects synaptic delivery of SFKs and mGluR5 in striatal neurons and whether SFKs play a role in the D2R-regulated trafficking of mGluR5.
MATERIALS AND METHODS
Animals
Adult male Wistar rats weighing 250–340 g (Charles River, New York, NY) were used and housed in pairs. The animal room was on a 12-h/12-h light/dark cycle and was controlled at a constant temperature of 23°C and humidity of 50 ± 10% with food and water available ad libitum. All animal use and procedures were in strict accordance with the US National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the University of Missouri-Kansas City.
Drug treatment and protein extraction
Rats received a single intraperitoneal (i.p.) injection of a drug in a volume of 1 ml/kg. The dose of drugs was calculated as the salt. Age-matched rats received an injection of saline (1 ml/kg) and served as a control. Eticlopride was injected at a dose of 0.05 or 0.5 mg/kg. These doses were chosen from early studies in which eticlopride after a systemic injection at 0.5 mg/kg showed behavioral effects derived from its blockade of D2Rs (LaHoste and Marshall, 1990; Wang and McGinty, 1995). After drug injection, rats were deeply anesthetized with sodium pentobarbital (65 mg/kg, i.p.) and decapitated. Brains were removed and were cut into coronal slices. The entire striatum was dissected, which contained the caudate putamen and nucleus accumbens. The dissected brain tissue was homogenized in a radioimmunoprecipitation assay (RIPA) buffer containing 20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% NP-40, 1% sodium deoxycholate, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, and 1 μg/ml leupeptin (Cell Signaling Technology, Danvers, MA) to obtain total homogenates. To enrich postsynaptic density (PSD) proteins, we homogenized brain tissue in isotonic sucrose homogenization buffer (SHB) containing 0.32 M sucrose, 10 mM HEPES, pH 7.4, 2 mM EDTA, a protease inhibitor cocktail, and a phosphatase inhibitor cocktail (Thermo Scientific, Rochester, NY). Homogenates were centrifuged at 800 g (10 min). The supernatant was collected and centrifuged again at 10,000 g (30 min) to obtain P2 pellets, which were washed once and centrifuged at 10,000 g (15 min, 4°C). The pellet was resuspended in the SHB containing 0.5% Triton X-100 (v/v). After slow rotation (20 min, 4°C), the suspension was centrifuged at 32,000 g (20 min, 4°C) to obtain supernatant Triton X-100-soluble non-PSD membranes (peri/extrasynaptic and presynaptic membranes) and the PSD-enriched pellet (Triton X-100-insoluble postsynaptic membranes, also known as PSDI). The PSD pellet was resuspended and solubilized in SHB containing 0.5% Triton X-100, 1% sodium dodecyl sulfate (SDS) and 1% deoxycholic acid with gentle rotation (1 h, 4°C). Protein concentrations were determined. Samples were stored at −80°C until use.
Western blot
Western blots were performed as described previously (Jin et al., 2013). Briefly, we separated proteins on SDS NuPAGE Novex 4–12% gels (Invitrogen, Carlsbad, CA) and then transferred proteins from gels to polyvinylidene fluoride membranes (Millipore, Bedford, MA). After membranes were blocked and washed, membranes were incubated in a buffer containing a primary rabbit or mouse antibody overnight at 4°C. Membranes were then washed and incubated in a horseradish peroxidase-linked secondary antibody against rabbit or mouse (Jackson Immunoresearch Laboratory, West Grove, PA). We visualized Immunoblots by an enhanced chemiluminescence reagent (GE Healthcare Life Sciences, Piscataway, NJ). Optical density of immunoblots was measured using NIH ImageJ analysis software (RRID: nif-0000-30467). Values of optical density of pY416, Src and Fyn were normalized to a loading control (actin or tubulin) and were reported separately. The pY416 values were not normalized to either Src or Fyn.
Immunoprecipitation
Immunoprecipitation procedures have been described previously (Jin et al., 2013; Mao and Wang, 2015). Briefly, for Fyn and Src immunoprecipitation, striatal tissue was homogenized in a RIPA lysis buffer. Samples were centrifuged at 800 g (10 min, 4°C) to remove insoluble materials. The supernatant was used for Fyn and Src immunoprecipitation. For phosphotyrosine protein immunoprecipitation, the P2 pellet was prepared from striatal tissue according to the procedures described above. The P2 pellet was solubilized in SHB containing 1% sodium deoxycholate for 1 h at 4°C. Solubilized proteins were used for phosphotyrosine protein immunoprecipitation. An equal amount of proteins (300–500 μg) was used for immunoprecipitation. Proteins were incubated with a mouse antibody against Src (2 μg), Fyn (3 μg), or phosphotyrosine (pTyr, 6 μg). The protein complex was precipitated with 50% protein A or G agarose/sepharose bead slurry (GE Healthcare). Precipitated proteins were separated on Novex 4–12% gels and probed with a rabbit antibody against Src, Fyn, phospho-Src family at Y416 (pan pY416), mGluR5, or pTyr. Horseradish peroxidase-conjugated secondary antibodies were used to visualize proteins.
Striatal slice preparation
Striatal slices were prepared as described previously (Liu et al., 2009; Jin et al., 2013). Pharmacological agents were added and incubated at 30°C. Slices were frozen after drug treatment and stored at −80°C until assayed.
Antibodies characterization
Table I lists all primary antibodies used in the current study. A set of primary antibodies include rabbit polyclonal antibodies against Src (Cell Signaling), Fyn (Santa Cruz Biotechnology, Santa Cruz, CA), mGluR5 (Millipore), pTyr (Millipore), Rab3A (Abcam, Cambridge, MA), or β-actin (Sigma-Aldrich, St. Louis, MO), or mouse antibodies against Src (Cell Signaling), Fyn (Santa Cruz), pTyr (PY20, BD Biosciences/Transduction Laboratories, Lexington, KY), or tubulin (Millipore). The rabbit antibody against pan pY416 (Cell Signaling) reacts with the Src family members when phosphorylated at the conserved activation residue: Y416 (chicken Src), Y419 (rat Src), and Y420 (rat Fyn). All antibodies have been widely used in research application of Western blot. Incubation of a secondary antibody alone, in the absence of a primary antibody, produced no detectable immunoreactivity.
Table I.
Primary Antibodies Used
| Antigen | Description of Immunogen | Source, host species, catalog No., clone or lot No., RRID | Concentration used (μg/ml) | Primary antibody blocking solution |
|---|---|---|---|---|
| β-Actin | C-terminal actin fragment: Ser-Gly-Pro-Ser-Ile-Val-His-Arg-Lys-Cys-Phe | Sigma-Aldrich (St. Louis, MO), rabbit polyclonal, A2066, AB_476693 | 0.2 | 3% Nonfat milk-PBS 0.1% Tween-20 |
| Fyn | A peptide mapping at the N-terminus of human Fyn | Santa Cruz Biotechnology (Santa Cruz, CA), rabbit polyclonal, sc-16, AB_631528 | 1 | 3% Nonfat milk-PBS 0.1% Tween-20 |
| Fyn | Amino acids 85-206 of human Fyn | Santa Cruz Biotechnology, mouse monoclonal, sc-434, AB_627642 | 1 | 3% Nonfat milk-PBS 0.1% Tween-20 |
| mGluR5 | KLH-conjugated peptide corresponding to the cytoplasmic domain of mouse mGluR5 | EMD Millipore (Bedford, MA), rabbit polyclonal, ab5675, lot No. 2585810, AB_305042 | 1 | 3% Nonfat milk-PBS 0.1% Tween-20 |
| Src | Recombinant fusion protein corresponding to residues 1-110 of human Src | Cell Signaling Technology (Beverly, MA), rabbit monoclonal, 2123, AB_2106047 | 1 | 3% Nonfat milk-PBS 0.1% Tween-20 |
| Src | Recombinant fusion protein corresponding to residues 1-110 of human Src | Cell Signaling Technology, mouse monoclonal, 2110, AB_2106058 | 1 | 3% Nonfat milk-PBS 0.1% Tween-20 |
| Tubulin | Rat tubulin, beta III | EMD Millipore, mouse monoclonal, MAB5564, lot No. 2266493, AB_570921 | 0.5 | 3% Nonfat milk-PBS 0.1% Tween-20 |
| pTyr | KLH-conjugated to peptides and phosphotyramine corresponding to human phosphotyrosine | EMD Millipore, rabbit polyclonal, 06-427, lot No. Q2495621, AB_310119 | 1 | 3% Nonfat milk-PBS 0.1% Tween-20 |
| pTyr | Phosphotyrosine | BD Biosciences (Lexington, KY), mouse monoclonal, 610000, AB_397423 | 1 | Membrane blocking solution, Invitrogen (Carlsbad, CA), 000105 |
| pY416 | A synthetic phosphopeptide corresponding to residues surrounding Tyr416 of human Src | Cell Signaling Technology, rabbit polyclonal, 6943, AB_10013641 | 1 | 3% Nonfat milk-PBS 0.1% Tween-20 |
| Rab3A | A synthetic peptide corresponding to Humen Rab3A (amino acid 1-18) | Abcam (Cambridge, MA), rabbit polyclonal, ab3335, AB_303714 | 2 | 3% Nonfat milk-PBS 0.1% Tween-20 |
Pharmacological agents
S-(−)-eticlopride hydrochloride was purchased from Sigma. 3-(4-chlorophenyl) 1-(1,1-dimethylethyl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine (PP2) was purchased from Tocris (Bristol, UK). All agents were freshly prepared at the day of experiments. Eticlopride was dissolved in physiological saline. PP2 was dissolved in dimethyl sulfoxide (DMSO). In experiments with PP2, DMSO served as a vehicle control.
Statistics
Data are presented as means ± SEM and were statistically analyzed. A one-way analysis of variance (ANOVA) followed by a Bonferroni (Dunn) comparison of groups using least squares-adjusted means or a two-tailed unpaired Student’s t-test was used. Probability levels of < 0.05 were considered statistically significant.
RESULTS
D2R blocker elevates SFK phosphorylation
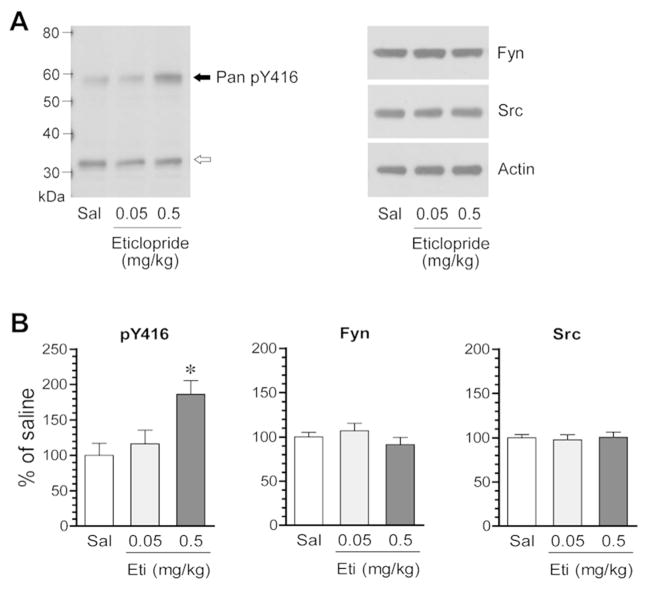
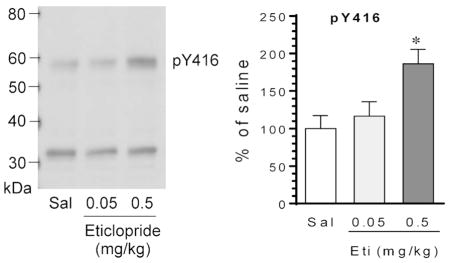
SFKs are activated by phosphorylation at a conserved activation site. To determine the impact of D2R blockade on SFK activation, we investigated the effect of the D2R blocker on SFK phosphorylation. In the first study, we gave rats a single injection of the D2R blocker eticlopride at 0.05 or 0.5 mg/kg (i.p.). We then sacrificed rats 20 min after drug injection. We harvested brain tissue and prepared homogenates for immunoblot analysis of changes in SFK phosphorylation. A pan pY416 antibody that detects phosphorylation of both rat Fyn and Src at their activation sites (Y420/Fyn and Y419/Src) was used. We found that eticlopride significantly altered Y416 phosphorylation in the striatum. While eticlopride at a lower dose (0.05 mg/kg) did not change Y416 phosphorylation, the blocker at a higher dose (0.5 mg/kg) did (Fig. 1A and 1B). The cellular level of total Fyn and Src proteins showed a minimal change in eticlopride-treated rats as compared to saline-treated control rats (Fig. 1A and 1B). These data demonstrate that pharmacological blockade of D2Rs with eticlopride increases SFK phosphorylation in the striatum in vivo.
Figure 1. Effects of eticlopride on SFK phosphorylation in the rat striatum.
(A) Representative immunoblots illustrating the effect of eticlopride on Y416 phosphorylation and Fyn and Src expression. Note that eticlopride at a higher dose induced an increase in Y416 phosphorylation, while the blocker at a lower dose did not. (B) Quantification of the effect of eticlopride on Y416 phosphorylation and Fyn and Src expression. Rats were given a single i.p. injection of saline (Sal) or eticlopride (Eti) at 0.05 or 0.5 mg/kg and were sacrificed 20 min after drug injection for immunoblot analysis. A solid arrow indicates a pan pY416 band, while an open arrow indicates a non-specific band. All values were analyzed with one-way ANOVA: pY416: F(2,9) = 6.42, n = 12, P = 0.018; Fyn: F(2,9) = 1.04, n = 12, P = 0.393; and Src: F(2,9) = 0.07, n = 12, P = 0.933. Data are presented as means ± SEM. *P < 0.05 versus saline.
Time-dependent effects of eticlopride on SFK phosphorylation
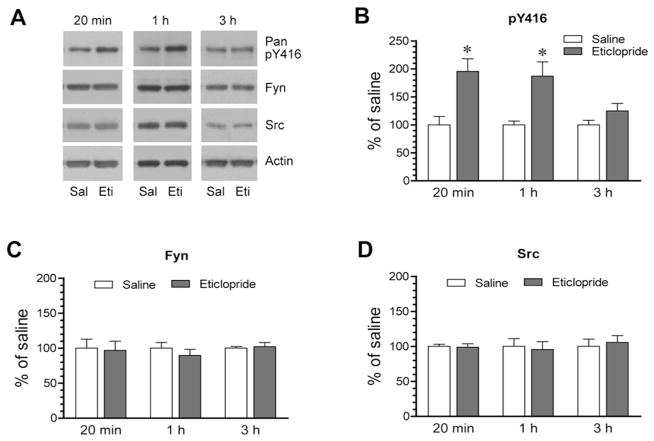
Given the significant impact of eticlopride on Y416 phosphorylation, we set forth to determine the temporal property of its action. To this end, we subjected rats to a single effective dose of eticlopride (0.5 mg/kg). We then sacrificed rats at different time points (20 min, 1 h, and 3 h) after eticlopride injection to analyze changes in Y416 phosphorylation and Fyn and Src expression in the striatum. At an early time point (20 min), as detected earlier, eticlopride enhanced an amount of Y416-phosphorylated proteins (Fig. 2A and 2B). At 1 h, the increase in Y416 phosphorylation persisted in eticlopride-treated rats compared to saline-treated rats. However, at a later time point (3 h), this increase returned to a level insignificantly different from that observed in saline-treated rats. At all time points surveyed, total Fyn and Src proteins were not significantly altered following eticlopride administration (Fig. 2C for Fyn quantification and Fig. 2D for Src quantification). Thus, eticlopride induced a transient and reversible increase in Y416 phosphorylation in the striatum.
Figure 2. Time-dependent effects of eticlopride on SFK phosphorylation in the rat striatum.
(A) Representative immunoblots showing time-dependent effects of eticlopride on Y416 phosphorylation. (B–D) Quantification analysis of effects of eticlopride on Y416 phosphorylation (B) and expression of total Fyn (C) and Src (D) proteins. Note that eticlopride induced a rapid and reversible increase in Y416-phosphorylated protein levels. Rats were given a single i.p. dose of saline (Sal) or eticlopride (Eti) at 0.5 mg/kg and were sacrificed at different time points (20 min, 1 h, and 3 h) after drug injection for immunoblot analysis. Data are presented as means ± SEM and were analyzed by Student’s t-test (n = 3–8 per group). *P < 0.05 versus saline at the same time point.
Eticlopride selectively phosphorylates Fyn
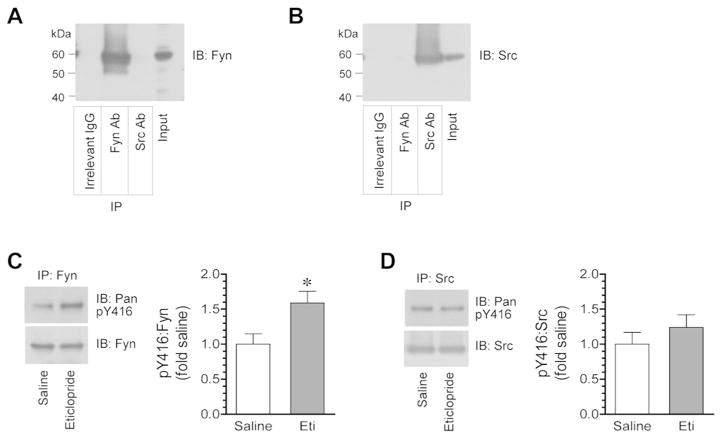
To determine whether Fyn and/or Src are phosphorylated by eticlopride, we immunoprecipitated Fyn and Src proteins from striatal lysates and assayed changes in Y416 phosphorylation in these immunoprecipitated Fyn and Src in response to eticlopride. Strong Fyn immunoreactivity was detected in immunoprecipitates produced by using an antibody against Fyn but not Src (Fig. 3A). An irrelevant IgG did not precipitate a detectable level of Fyn. Similarly, Src was detected in Src antibody-immunoprecipitated samples (Fig. 3B). No Src was found in precipitates obtained with the Fyn antibody or an irrelevant IgG. These data validate the selectivity of immunoprecipitation with Fyn and Src antibodies. We then tested the effect of eticlopride on phosphorylation of immunoprecipitated Fyn and Src. Eticlopride at 0.5 mg/kg (1 h prior to tissue collection) elevated Y416 phosphorylation in Fyn precipitates from the striatum (Fig. 3C). In contrast, eticlopride did not alter Y416 phosphorylation in Src precipitates in the same rats (Fig. 3D). These results support that D2R blockade results in selective activation of Fyn in the striatum.
Figure 3. Effects of eticlopride on Y416 phosphorylation in immunoprecipitated Fyn and Src proteins from the rat striatum.
(A and B) Representative immunoblots showing immunoprecipitation of Fyn (A) and Src (B) from striatal lysates. Note that Fyn and Src antibodies (Ab) only precipitated their respective proteins, while an irrelevant IgG did not precipitate Fyn and Src. (C and D) Effects of eticlopride on phosphorylation of immunopurified Fyn (C) and Src (D) from the striatum. Note that eticlopride selectively increased Fyn phosphorylation. Representative immunoblots are shown left to the quantified data (C and D). Rats were given a single dose of eticlopride (Eti, 0.5 mg/kg, i.p.) and were sacrificed 1 h after drug injection for immunoprecipitation (IP) of Fyn and Src, followed by immunoblot (IB) analysis of Fyn and Src phosphorylation. Data are presented as means ± SEM and were analyzed by Student’s t-test (n = 5 per group). *P < 0.05 versus saline.
Eticlopride increases tyrosine phosphorylation of mGluR5
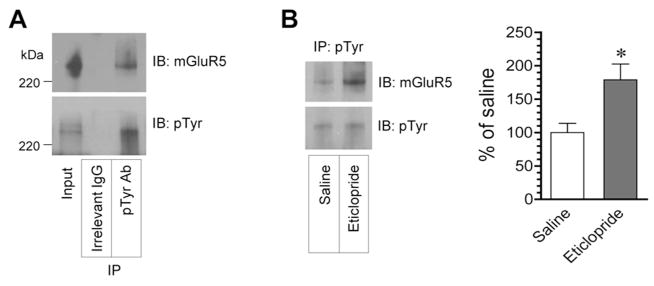
mGluR5 was a phosphotyrosine protein in the adult rat striatum in vivo and in striatal slices (Orlando et al., 2002). Tyrosine-phosphorylated mGluR5 was also seen in the mouse forebrain (Giuffrida et al., 2005). To determine whether D2R antagonism impacts tyrosine phosphorylation of mGluR5, we tested the effect of eticlopride on the level of tyrosine-phosphorylated mGluR5 in the striatum. A phosphotyrosine antibody was used to precipitate tyrosine-phosphorylated proteins from striatal synaptosomal proteins. A small amount of mGluR5 proteins was found in phosphotyrosine immunoprecipitates (Fig. 4A), consistent with those reported previously (Orlando et al., 2002; Giuffrida et al., 2005). This indicates a low level of tyrosine phosphorylation of mGluR5 in striatal neurons under normal conditions. Noticeably, eticlopride (0.5 mg/kg, i.p., 1 h) enhanced an amount of tyrosine-phosphorylated mGluR5 (Fig. 4B). Thus, blocking D2Rs leads to the upregulation of mGluR5 tyrosine phosphorylation in striatal neurons.
Figure 4. Effects of eticlopride on tyrosine phosphorylation of mGluR5 in the rat striatum.
(A) Tyrosine phosphorylation of mGluR5 in the striatum. Striatal synaptosomal proteins were immunoprecipitated with phosphotyrosine (pTyr) antibodies. mGluR5 immunoreactivity was then detected in immunoprecipitates. (B) Effects of eticlopride on tyrosine phosphorylation of mGluR5 in the striatum. Representative immunoblots are shown left to the quantified data. Rats were given a single injection of eticlopride (0.5 mg/kg, i.p.) and were sacrificed 1 h after drug injection for immunoprecipitation (IP) with phosphotyrosine antibodies (Ab), followed by immunoblot (IB) analysis of mGluR5. Data are presented as means ± SEM and were analyzed by Student’s t-test (n = 5 per group). *P < 0.05 versus saline.
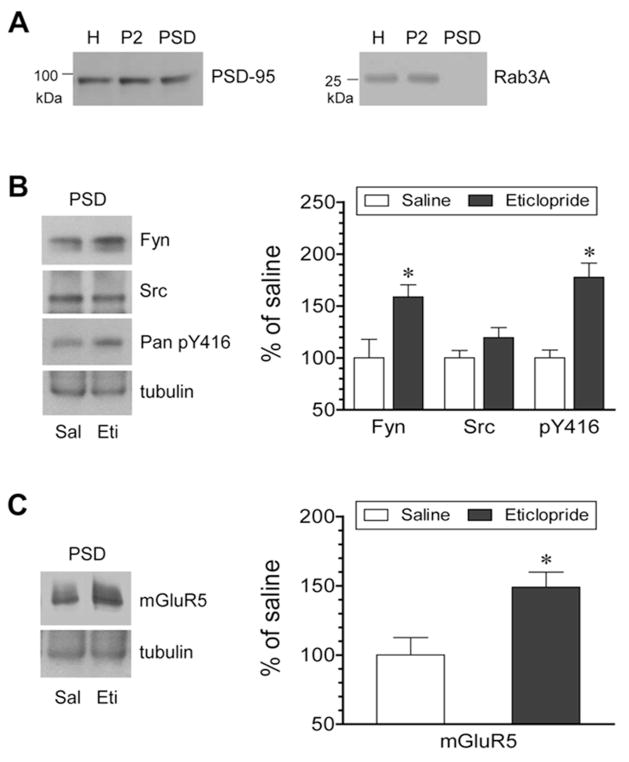
Eticlopride enhances synaptic recruitment of Fyn and mGluR5
We carried out this study to determine whether blocking D2Rs alters synaptic expression of SFKs and mGluR5. Changes in the abundance of Fyn, Src, and mGluR5 proteins were analyzed in purified PSD samples from the striatum following an injection of eticlopride (0.5 mg/kg, i.p., 1 h). Extraction of the PSD fraction was achieved by demonstrating the distribution of PSD-95 and the lack of the distribution of Rab3A, a presynaptically located protein, in the PSD (Fig. 5A). We observed a moderate increase in Fyn proteins in PSD fractions after eticlopride injection (Fig. 5B). Y416-phosphorylated proteins were also elevated in the PSD. Src proteins were however not significantly altered. Like Fyn, the mGluR5 abundance in the PSD was enhanced in eticlopride-treated rats relative to saline-treated rats (Fig. 5C). These data indicate that D2Rs limit the number of Fyn and mGluR5 in the PSD location. Blocking D2Rs increases PSD levels of mGluR5 in striatal neurons, accompanied by a parallel increase in synaptic trafficking of Fyn.
Figure 5. Effects of eticlopride on phosphorylation of SFKs and expression of SKFs and mGluR5 in the PSD of rat striatal neurons.
(A) Expression of PSD-95 and Rab3A in homogenate (H), crude synaptosome (P2), and PSD fractions from rat striatal neurons. (B) Effects of eticlopride on Fyn and Src expression and Y416 phosphorylation in the PSD. (C) Effects of eticlopride on mGluR5 expression in the PSD. Note that eticlopride enhanced Fyn and mGluR5 expression and Y416 phosphorylation in PSD fractions. Representative immunoblots are shown left to the quantified data (B and C). Rats were given an injection of saline (Sal) or eticlopride (Eti, 0.5 mg/kg, i.p.) and were sacrificed 1 h after drug injection for biochemical fractionation of PSD proteins (B and C). Data are presented as means ± SEM and were analyzed by Student’s t-test (n = 5 per group). *P < 0.05 versus saline.
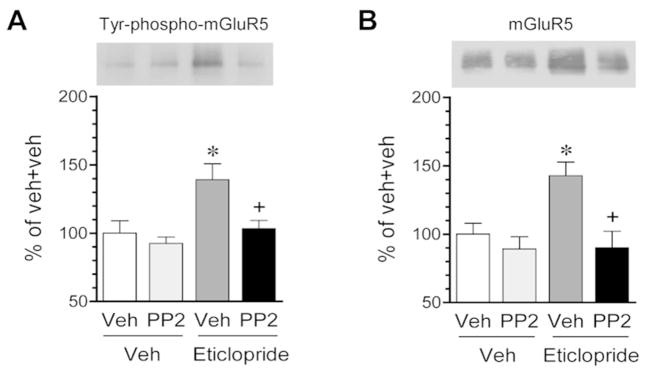
PP2 blocks effects of eticlopride on mGluR5
Eticlopride-activated Fyn may mediate the effect of eticlopride on mGluR5. To determine this, we investigated the effect of inhibition of Fyn with an SFK inhibitor PP2 on the eticlopride-induced increases in tyrosine phosphorylation and synaptic delivery of mGluR5. In striatal slices, adding eticlopride (1 μM, 1 h) induced a small but significant increase in tyrosine phosphorylation of mGluR5 as detected by immunoprecipitation of phosphotyrosine proteins followed by immunoblot analysis of mGluR5 in precipitates (Fig. 6A). This is consistent with the above data observed from the striatum of adult rats treated with eticlopride and solidifies an existence of a D2R tone in inhibiting mGluR5 tyrosine phosphorylation under basal conditions. In slices pretreated with PP2 (2.5 μM), eticlopride no longer induced an increase in tyrosine-phosphorylated mGluR5. In assays with purified PSD fractions, eticlopride elevated the amount of mGluR5 in the PSD (Fig. 6B). PP2 completely blocked this elevation as no increase in PSD mGluR5 expression was seen in the presence of PP2. These data suggest that a PP2-sensitive SFK, likely Fyn, mediates the effect of eticlopride on mGluR5 in its tyrosine phosphorylation and synaptic recruitment.
Fig. 6. Effects on PP2 on eticlopride-induced tyrosine phosphorylation and synaptic delivery of mGluR5 in rat striatal slices.
(A) Effects of PP2 on the eticlopride-induced tyrosine phosphorylation of mGluR5. Tyrosine phosphoproteins were immunoprecipitated by an anti-phosphotyrosine antibody. Tyrosine-phosphorylated mGluR5 was detected in precipitates using an anti-mGluR5 antibody. (B) Effects of PP2 on eticlopride-induced synaptic trafficking of mGluR5. Note that PP2 blocked the eticlopride-induced tyrosine phosphorylation and synaptic expression of mGluR5. PP2 (2.5 μM) was added to striatal slices 20 min prior to and during eticlopride incubation (1 μM, 1 h). All values were analyzed with one-way ANOVA: Tyr-phospho-mGluR5: F(3,16) = 6.27, n = 20, P = 0.005; and mGluR5: F(3,18) = 7.55, n = 22, P = 0.002. Data are presented as means ± SEM. *P < 0.05 versus vehicle + vehicle. +P < 0.05 versus vehicle + eticlopride.
DISCUSSION
In this study, we investigated the role of D2Rs in the regulation of Fyn and mGluR5 phosphorylation in the rat striatum. We found that blockade of D2Rs with an antagonist eticlopride enhanced SFK phosphorylation at an activation site. This enhancement was transient and reversible. Between Fyn and Src surveyed, eticlopride selectively increased Fyn but not Src phosphorylation. Interestingly, eticlopride also increased tyrosine phosphorylation of mGluR5 and synaptic delivery of the receptor. The SFK inhibitor PP2 blocked these eticlopride-induced events. These results indicate that D2Rs inhibit Fyn in striatal neurons under normal conditions. D2Rs also suppress tyrosine phosphorylation and synaptic accumulation of mGluR5, in which SFKs (likely Fyn) play an important role.
Dopamine is a major transmitter in the striatum and is believed to participate in the regulation of local SFKs. D1Rs have been studied in this regard. It was found that D1R agonists enhanced SFK phosphorylation in mouse cultured striatal neurons (Pascoli et al., 2011) and the adult rat striatum (Mao and Wang, 2015), indicating a role of D1Rs in upregulating SFK phosphorylation. Unlike D1R agonists, the D2R agonist quinpirole did not alter SFK phosphorylation (Mao and Wang, 2015). In contrast, the D2R antagonist haloperidol elevated SFK phosphorylation (Hattori et al., 2006). Another D2R antagonist eticlopride produced the same effect (this study). Thus, D2Rs as opposed to D1Rs inhibit SFK phosphorylation. It is noteworthy that both D1Rs and D2Rs selectively regulate Fyn but not Src phosphorylation (Pascoli et al., 2011; Mao and Wang, 2015, this study). This is in accordance with the fact that Fyn is much more abundant than Src in the striatum (Pascoli et al., 2011).
Based on the segregation of D1Rs and D2Rs in different MSNs, the D1R agonist and the D2R antagonist may increase SFK phosphorylation in the D1R-bearing striatonigral and D2R-bearing striatopallidal MSNs, respectively. D1Rs and D2Rs are known to activate and inhibit the cAMP-protein kinase A (PKA) pathway, respectively (Neve, 2004). Thus, the cAMP-PKA pathway is reasoned to link these receptors to SFKs. In fact, PKA phosphorylated a specific residue (Fyn S21/Src S17) to allow Y416 autophosphorylation (Schmitt and Stork, 2002; Yeo et al., 2011). Forskolin, a PKA activator, activated Fyn although not Src in mouse spinal dorsal horn neurons (Yang et al., 2011). Future studies need to further characterize the role of the cAMP-PKA pathway in linking dopamine receptors to SFKs in a cell type-specific manner.
Striatal-enriched protein tyrosine phosphatase (STEP) may also link D2Rs to Fyn. STEP is present in the PSD of striatal neurons and bound to Fyn, but not other synapse-enriched SFKs (Nguyen et al., 2002). Through dephosphorylating Fyn at the conserved activation site, Y416 as detected in this study, STEP inactivated the kinase (Nguyen et al., 2002). Given that PKA phosphorylates and thus inactivates STEP (Paul et al., 2000; Xu et al., 2015), the D2R antagonist can enhance PKA activity to achieve a PKA-dependent inactivation of STEP (Carty et al., 2012), leading to an increase in Fyn Y416 phosphorylation.
An important finding is that the D2R antagonist elevated tyrosine phosphorylation of mGluR5. Tyrosine phosphorylation of mGluR5 has been established in early studies. In the striatum of adult rats and in striatal slices, mGluR5 was among phosphotyrosine-precipitated proteins (Orlando et al., 2002). In the mouse forebrain, similar results were seen (Giuffrida et al., 2005). Consistent with those early studies, a reliable amount of mGluR5 proteins was observed in phosphotyrosine proteins precipitated from the striatum (this study). It is notably that constitutive tyrosine phosphorylation of mGluR5 in striatal neurons is usually at a low level under normal conditions (Orlando et al., 2002; Giuffrida et al., 2005; this study). This provides a sufficient room for upregulation in response to changing dopamine signals. In fact, we observed that pharmacological removal of D2R influence by a D2R antagonist elevated tyrosine phosphorylation of striatal mGluR5. This for the first time indicates a tonic inhibition of mGluR5 tyrosine phosphorylation by D2Rs. It seems that D2R signals provide a major inhibitory drive in keeping homeostasis of mGluR5 in its tyrosine phosphorylation. Of note, the effect of D1R and D2R agonists on tyrosine phosphorylation of striatal mGluR5 is less clear at present. Given the fact that the D1R but not D2R agonist activated Fyn in striatal neurons (Mao and Wang, 2015), the D1R agonist is reasoned to elevate tyrosine phosphorylation of substrates of Fyn, including mGluR5, although it needs to be proven experimentally.
SFKs seem to mediate the effect of the D2R blocker on mGluR5 tyrosine phosphorylation. This is supported by the finding that the SFK inhibitor PP2 blocked the effect of the D2R antagonist on tyrosine phosphorylation of mGluR5. Among SFKs, Fyn but not Src is likely to carry out this activity-dependent event. This is because eticlopride selectively activated Fyn rather than Src. However, future studies need to define the role of other neuron-enriched SFKs in this event, including Yes, Lyn and Lck which are present in synaptic locations (Kalia and Salter, 2003). Regarding the consequence of elevated tyrosine phosphorylation of mGluR5 after removing the inhibitory D2R tone, we monitored changes in synaptic expression of mGluR5 in response to eticlopride. We found that eticlopride induced a concurrent increase in the abundance of mGluR5 in the PSD. This increase was completely blocked by PP2. Thus, D2Rs seem to normally inhibit synaptic delivery of mGluR5 by limiting its SFK-mediated tyrosine phosphorylation level. Blocking D2Rs releases SFKs to tyrosine-phosphorylate mGluR5 and thus drive the receptor to the PSD microdomain. These findings add mGluR5 as a new substrate of SFKs in addition to previously established ionotropic glutamate receptor substrates of SFKs, such as NMDA receptors (Kalia et al., 2004; Ohnishi et al., 2011; Groveman et al., 2012; Trepanier 2012). Synaptic SFKs by regulating these glutamate receptors participate in the control of excitatory synaptic transmission and plasticity.
SIGNIFICANCE.
In this study, we found that blockade of a dopamine receptor (D2 subtype) by injecting a D2 receptor blocker activated a protein tyrosine kinase named Fyn in the rat brain region called the striatum. The D2 blocker also increased tyrosine phosphorylation of a receptor called metabotropic glutamate receptor 5 (mGluR5) and enhanced delivery of Fyn and mGluR5 proteins to a subcellular region called synapse. These results reveal the ability of D2 receptors to regulate Fyn and mGluR5. Malfunction of this D2 receptor activity may be linked to various psychiatric and neurological disorders such as drug addiction and depression.
Acknowledgments
This work was supported by NIH grants R01DA10355 (JQW) and R01MH61469 (JQW). Authors wish to thank Drs. Bing Xue and Elton Chen for their technical assistance.
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
Role of authors
All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: LMM and JQW. Acquisition of data: LMM and JQW. Analysis and interpretation of data: LMM and JQW. Drafting and critical revision of the manuscript: LMM and JQW.
References
- Aubert I, Ghorayeb I, Normand E, Bloch B. Phenotypical characterization of the neurons expressing the D1 and D2 dopamine receptors in the monkey striatum. J Comp Neurol. 2000;418:22–32. [PubMed] [Google Scholar]
- Bertran-Gonzalez J, Herve D, Girault JA, Valjent E. What is the degree of segregation between striatonigral and striatopallidal projections? Front Neuroanat. 2010;4:136. doi: 10.3389/fnana.2010.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bongiorno-Borbone L, Kadare G, Benfenati F, Girault JA. FAK and PYK2 interact with SAP90/PSD-95-associated protein-3. Biochem Biophys Res Commun. 2005;337:641–646. doi: 10.1016/j.bbrc.2005.09.099. [DOI] [PubMed] [Google Scholar]
- Carty NC, Xu J, Kurup P, Brouillette J, Goebel-Goody SM, Austin DR, Yuan P, Chen G, Correa PR, Haroutunian V, Pittenger C, Lombroso PJ. The tyrosine phosphatase STEP: implications in schizophrenia and the molecular mechanism underlying antipsychotic medications. Transl Psychiatry. 2012;2:e137. doi: 10.1038/tp.2012.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ, Jr, Sibley DR. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250:1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- Giuffrida R, Musumeci S, D’Antoni S, Bonaccorso CM, Giuffrida-Stella AM, Oostra BA, Catania MV. A reduced number of metabotropic glutamate subtype 5 receptors are associated with constitutive Homer proteins in a mouse model of Fragile X Syndrome. J Neurosci. 2005;25:8908–8916. doi: 10.1523/JNEUROSCI.0932-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groveman BR, Feng S, Fang XQ, Plueger M, Lin SX, Bienkiewicz EA, Yu X. The regulation of N-methyl-D-aspartate receptors by Src kinase. FEBS J. 2012;279:20–28. doi: 10.1111/j.1742-4658.2011.08413.x. [DOI] [PubMed] [Google Scholar]
- Hattori K, Uchino S, Isosaka T, Maekawa M, Iyo M, Sato T, Kohsaka S, Yagi T, Yuasa S. Fyn is required for haloperiodol-induced catalepsy in mice. J Biol Chem. 2006;281:7129–7135. doi: 10.1074/jbc.M511608200. [DOI] [PubMed] [Google Scholar]
- Jin DZ, Guo ML, Xue B, Fibuch EE, Choe ES, Mao LM, Wang JQ. Phosphorylation and feedback regulation of metabotropic glutamate receptor 1 by calcium/calmodulin-dependent protein kinase II. J Neurosci. 2013;33:3402–3412. doi: 10.1523/JNEUROSCI.3192-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalia LV, Gingrich JR, Salter MW. Src in synaptic transmission and plasticity. Oncogene. 2004;23:8007–8016. doi: 10.1038/sj.onc.1208158. [DOI] [PubMed] [Google Scholar]
- Kalia LV, Salter MW. Interactions between Src family protein tyrosine kinases and PSD-95. Neuropharmacology. 2003;45:720–728. doi: 10.1016/s0028-3908(03)00313-7. [DOI] [PubMed] [Google Scholar]
- LaHoste GJ, Marshall JF. Nigral D1 and striatal D2 receptors mediate the behavioral effects of dopamine agonists. Behav Brain Res. 1990;38:233–242. doi: 10.1016/0166-4328(90)90178-h. [DOI] [PubMed] [Google Scholar]
- Liu XY, Mao LM, Zhang GC, Papasian CJ, Fibuch EE, Lan HX, Zhou HF, Xu M, Wang JQ. Activity-dependent modulation of limbic dopamine D3 receptors by CaMKII. Neuron. 2009;61:425–438. doi: 10.1016/j.neuron.2008.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao LM, Wang JQ. Dopaminergic and cholinergic regulation of Fyn tyrosine kinase phosphorylation in the rat striatum in vivo. Neuropharmacology. 2015;99:491–499. doi: 10.1016/j.neuropharm.2015.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neet K, Hunter T. Vertebrate non-receptor protein-tyrosine kinase families. Genes Cell. 1996;1:147–169. doi: 10.1046/j.1365-2443.1996.d01-234.x. [DOI] [PubMed] [Google Scholar]
- Neve KA, Seamans JK, Trantham-Davidson H. Dopamine receptor signaling. J Recept Signal Transduct Res. 2004;24:165–205. doi: 10.1081/rrs-200029981. [DOI] [PubMed] [Google Scholar]
- Nguyen TH, Liu J, Lombroso PJ. Striatal enriched phosphatase 61 dephosphorylates Fyn at phosphotyrosine 420. J Biol Chem. 2002;277:24274–24279. doi: 10.1074/jbc.M111683200. [DOI] [PubMed] [Google Scholar]
- Niswende CM, Conn PJ. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol. 2010;50:295–322. doi: 10.1146/annurev.pharmtox.011008.145533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi H, Murata Y, Okazawa H, Matozaki T. Src family kinases: modulators of neurotransmitter receptor function and behavior. Trends Neurosci. 2011;34:629–637. doi: 10.1016/j.tins.2011.09.005. [DOI] [PubMed] [Google Scholar]
- Okada M. Regulation of the Src family kinase by Csk. Int J Biol Sci. 2012;8:1385–1397. doi: 10.7150/ijbs.5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omri B, Crisanti P, Marty MC, Alliot F, Fagard R, Molina T, Pessac B. The Lck tyrosine kinase is expressed in brain neurons. J Neurochem. 1996;67:1360–1364. doi: 10.1046/j.1471-4159.1996.67041360.x. [DOI] [PubMed] [Google Scholar]
- Orlando LR, Dunah AW, Standaert DG, Young AB. Tyrosine phosphorylation of the metabotropic glutamate receptor mGluR5 in striatal neurons. Neuropharmacology. 2002;43:161–173. doi: 10.1016/s0028-3908(02)00113-2. [DOI] [PubMed] [Google Scholar]
- Pascoli V, Besnard A, Herve D, Pages C, Heck N, Girault JA, Caboche J, Vanhoutte P. Cyclic adenosine monophosphate-independent tyrosine phosphorylation of NR2B mediates cocaine-induced extracellular signal-regulated kinase activation. Biol Psychiatry. 2011;69:218–227. doi: 10.1016/j.biopsych.2010.08.031. [DOI] [PubMed] [Google Scholar]
- Paul S, Snyder GL, Yokakura H, Picciotto MR, Nairn AC, Lombroso PJ. The Dopamine/D1 receptor mediates the phosphorylation and inactivation of the protein tyrosine phosphatase STEP via a PKA-dependent pathway. J Neurosci. 2000;20:5630–5638. doi: 10.1523/JNEUROSCI.20-15-05630.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roskoski R., Jr Src kinase regulation by phosphorylation and dephosphorylation. Biochem Biophys Res Commun. 2005;331:1–14. doi: 10.1016/j.bbrc.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Schenone S, Brullo C, Musumeci F, Biava M, Falchi F, Botta M. Fyn kinase in brain diseases and cancer: the search for inhibitors. Curr Med Chem. 2011;18:2921–2942. doi: 10.2174/092986711796150531. [DOI] [PubMed] [Google Scholar]
- Schmitt JM, Stork PJ. PKA phosphorylation of Src mediates cAMP’s inhibition of cell growth via Rap1. Mol Cell. 2002;9:85–94. doi: 10.1016/s1097-2765(01)00432-4. [DOI] [PubMed] [Google Scholar]
- Tallaksen-Greene SJ, Kaatz KW, Romano C, Albin RL. Localization of mGluR1a-like immunoreactivity and mGluR5a-like immunoreactivity in identified population of striatal neurons. Brain Res. 1998;780:210–217. doi: 10.1016/s0006-8993(97)01141-4. [DOI] [PubMed] [Google Scholar]
- Testa CM, Standaert DG, Young AB, Penney JB., Jr Metabotropic glutamate receptor mRNA expression in the basal ganglia of the rat. J Neurosci. 1994;14:3005–3018. doi: 10.1523/JNEUROSCI.14-05-03005.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trepanier CH, Jackson MF, MacDonald JF. Regulation of NMDA receptors by the tyrosine kinase Fyn. FEBS J. 2012;279:12–19. doi: 10.1111/j.1742-4658.2011.08391.x. [DOI] [PubMed] [Google Scholar]
- Wang JQ, McGinty JF. Differential effects of D1 and D2 dopamine receptor antagonists on acute amphetamine- or methamphetamine-induced up-regulation of zif/268 mRNA expression in rat forebrain. J Neurochem. 1995;65:2706–2715. doi: 10.1046/j.1471-4159.1995.65062706.x. [DOI] [PubMed] [Google Scholar]
- Xu J, Kurup P, Foscue E, Lombroso PJ. Striatal-enriched protein tyrosine phosphatase regulates the PTPα/Fyn signaling pathway. J Neurochem. 2015;134:629–641. doi: 10.1111/jnc.13160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HB, Yang X, Cao J, Li S, Liu YN, Suo ZW, Cui HB, Guo Z, Hu XD. cAMP-dependent protein kinase activated Fyn in spinal dorsal horn to regulate NMDA receptor function during inflammatory pain. J Neurochem. 2011;116:93–104. doi: 10.1111/j.1471-4159.2010.07088.x. [DOI] [PubMed] [Google Scholar]
- Yeo MG, Oh HJ, Cho HS, Chun JS, Marcantonio EE, Song WK. Phosphorylation of Ser 21 in Fyn regulates its kinase activity, focal adhesion targeting, and is required for cell migration. J Cell Physiol. 2011;226:236–247. doi: 10.1002/jcp.22335. [DOI] [PubMed] [Google Scholar]