Abstract
Viral vaccines have traditionally protected against disease, but for viruses that establish latent infection, it is desirable for the vaccine to reduce infection to reduce latent infection and reactivation. While seroconversion has been used in clinical trials of herpes simplex virus (HSV) vaccines to measure protection from infection, this has not been modeled in animal infection systems. To measure the ability of a genital herpes vaccine candidate to protect against various aspects of infection, we established a non-lethal murine model of genital HSV-2 infection, an ELISA assay to measure antibodies specific for infected cell protein 8 (ICP8), and a very sensitive qPCR assay. Using these assays, we observed that immunization with HSV-2 dl5-29 virus reduced disease, viral shedding, seroconversion, and latent infection by the HSV-2 challenge virus. Therefore, it may be feasible to obtain protection against genital disease, seroconversion, and latent infection by immunization, even if sterilizing immunity is not achieved.
Introduction
Viral vaccines have traditionally protected against viral disease but have not necessarily protected completely against viral infection, because these pathogens cause acute infection and are cleared by the host immune response (Graham, Crowe, and Ledgerwood, 2013). Viruses such as the herpesviruses and HIV, which establish a latent infection, provide a greater challenge because immunization should ideally reduce infection as much as possible to prevent establishment of latent infection, from which the virus could reactivate and cause disease. However, the chickenpox vaccine is a very successful vaccine as measured by reduction of disease and results in a remarkable decrease in varicella incidence, even though it does not completely prevent acute infection and latent infection by wild-type virus (Gershon, 2013). The goal of HIV vaccines has been to reduce viral load as much as possible. It is widely recognized that there is an immediate need for a genital herpes vaccine because this pathogen presents a great burden on public health. Furthermore, there is a clear co-epidemic of HSV and HIV, and herpes infection significantly increases HIV acquisition and transmission (Barnabas and Celum, 2012). Therefore, reducing or suppressing herpetic disease and/or infection is a desirable goal as it would decrease viral spread and improve herd immunity.
Therapeutic trials of genital herpes vaccine candidates have measured clinical disease such as the rate and number of recurrences, the duration and severity of the first recurrence (Straus et al., 1997). A limited number of clinical trials have tested the efficacy of various HSV-2 vaccine candidates as prophylactic vaccines in humans. First, the Chiron subunit vaccine containing recombinant HSV-2 glycoproteins B and D was administered with MF59 as an adjuvant in a prophylactic vaccine study (Corey et al., 1999). In this study, the endpoints were seroconversion, viral shedding, and manifestation of herpetic lesions. Even though vaccinated subjects developed neutralizing antibodies, this vaccine failed to prevent infection or disease, showing that neutralizing antibodies are not sufficient to control the virus.
In a subsequent study, the GSK HSV-2 glycoprotein D vaccine candidate with alum-MPL as adjuvant was used as the immunogen (Stanberry et al., 2002). The primary endpoint was protection from herpetic disease. Although this vaccine produced humoral and cellular responses, subjects were not protected from herpetic disease. Of note, minor protection was observed but only in women that were HSV-1 and -2 seronegative. In light of these results, a second trial was conducted to evaluate the efficacy of subunit vaccines. This study was conducted on HSV-1 and 2 seronegative women (Belshe et al., 2012) testing the gD-2 alum formulation. Again, the primary endpoint was protection from genital disease and the secondary endpoint was protection against HSV-1 disease or HSV-2 disease. Unfortunately, this trial showed no efficacy of this vaccine candidate against genital herpes disease and no efficacy against HSV-2 disease. Various arguments have been made for prophylactic versus therapeutic vaccines for HSV-2 (Dropulic and Cohen, 2012; Johnston, Koelle, and Wald, 2011). An effective therapeutic vaccine is likely to be more difficult to attain than a prophylactic vaccine, but a therapeutic vaccine trial requires fewer trial subjects, can be conducted more rapidly, and is less expensive (Dropulic and Cohen, 2012). Nevertheless, both prophylactic and therapeutic vaccines for HSV are desirable.
Herpes vaccine studies in animals have aimed at measuring protection from disease or lethality. None of the existing animal infection systems is a perfect model for HSV infection. The mouse models often have lethality as an endpoint, allowing only measurements of protection from acute disease (Johnston, Koelle, and Wald, 2011). The guinea pig model also requires a high dose of challenge virus to elicit disease, and those that do not succumb to the infection show reactivation for a limited period of time (Stanberry et al., 1982). In this model, only protection from herpetic disease, both acute and recurrent, has been measured, and no protection from seroconversion has been shown, although some studies have indicated protection from acute disease as a prophylactic effect (Veselenak et al., 2012).
Recently, the needs for more standardized endpoints and animal models and for more clinically relevant endpoints in the animal studies have been expressed by investigators in the field (Knipe et al., 2014). Here, we present a non-lethal mouse model of genital herpes that allows the comparison of immunized and unimmunized animals after challenge because even unimmunized animals do not succumb to disease. Interestingly, our results indicate that immunization with the dl5-29 vaccine candidate may provide prophylactic protection against infection in this mouse model, as measured by seroconversion.
Results
Establishment of a non-lethal mouse model for genital HSV-2 infection
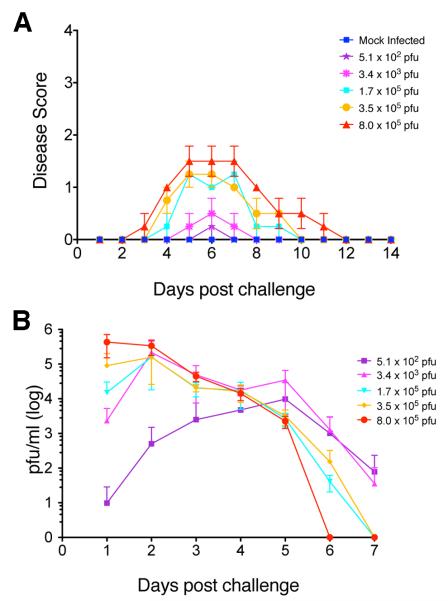
Genital challenge with wild-type (WT) HSV-2 in mice commonly results in the death of the animals. When we reduced the genital challenge dose of WT HSV-2 virus below that of the LD50, we observed that some animals progressed through all stages of herpetic disease including rear leg paralysis, but the animals that did not succumb to the disease did not show any sign of acute or latent infection at these low doses (results not shown). To establish a non-lethal genital infection model, we therefore used a replication-competent mutant of HSV-2, strain 186ΔKpn, which contains a deletion in the UL23 gene encoding the viral thymidine kinase (TK) and is attenuated for neurovirulence (Jones, Taylor, and Knipe, 2000). Following intravaginal infection of BALB/c mice with HSV-2 186ΔKpn virus, we observed that increasing doses of challenge virus resulted in uniform moderate genital disease (Figure 1A). We determined that, in animals receiving doses of 1.7×105 PFU/mouse or greater, every animal showed genital disease. Symptoms did not appear as severe as those from infection with WT HSV-2 (Dudek, Mathews, and Knipe, 2008; Dudek et al., 2011; Morrison, Da Costa, and Knipe, 1998) in that they did not reach the most severe stages of disease, and they subsided within a few days, with animals returning to a normal healthy state within a week after intravaginal challenge (Figure 1A). Similarly, viral shedding was detected in every animal, peaking at 2–3 days post infection (dpi) and decreasing by 6–7 dpi (Figure 1B). Thus, this system provided a non-lethal murine infection model for HSV-2 genital disease that approximated acute human infection and disease.
Figure 1. Disease and viral shedding in mice infected intravaginally with HSV-2 186ΔKpn.
Mice were infected intravaginally with varying doses of HSV-2 186ΔKpn virus, and disease and viral shedding were monitored. Five animals per group were evaluated. A) Disease scores in BALB/c mice infected intravaginally with HSV-2 186ΔKpn. B) Viral shedding in the vaginal cavity of mice infected intravaginally with HSV-2 186ΔKpn.
Quantitative assay for latent HSV-2 DNA
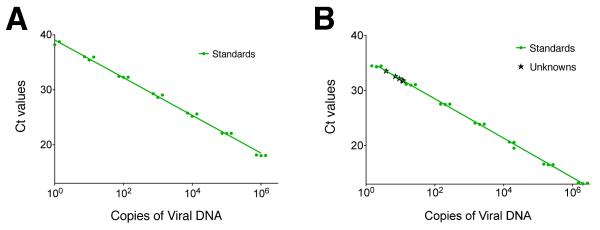
To evaluate latent infection in this animal model, we wished to establish a very sensitive quantitative PCR assay for viral DNA. Based on our previous results (Jones, Taylor, and Knipe, 2000), we expected low copy numbers of latent HSV-2 186ΔKpn in dorsal root ganglia. To distinguish the vaccine DNA from that of the challenge virus, we designed primers for the UL29 gene (deleted in dl5-29) and compared viral DNA purified in different ways as templates for qPCR standard curve reactions. We observed that HSV-2 DNA purified by sodium iodide double-banding (Colgrove et al., 2014) was the best template for standard curves in that it allowed linear detection of viral DNA over 6 logs (1×100 to 1×106 copies) with PCR efficiencies above 90% (Figure 2A). This outperformed HSV-2 DNA purified from nuclear extracts or PCR amplicons that allowed linear ranges of detection of 5 logs (1×101 to 1×106 copies) and efficiencies of 93% and 87% respectively (results not shown). Using this qPCR assay, we were able to detect as few as 3.8 copies per reaction of the UL29 gene in DNA extracted 30 dpi from DRGs of mice intravaginally infected at the dose of challenge virus that produced disease in all mice (1.7×105 PFU/mouse) (Figure 2B).
Figure 2. Development of a sensitive PCR assay to detect latent HSV-2 DNA.
A) A representative qPCR standard curve generated using NaI gradient double-banded HSV-2 DNA and ICP8 primers and probes showing 6 logs of detection with a lower limit of 1 copy/reaction and an efficiency of 96%. B) An independent experiment showing detection of latent HSV-2 DNA in mouse DRGs infected with HSV-2 186ΔKpn. Green circles correspond to standard points ranging from 2 to 2×106 copies. Black crosses correspond to values for experimental samples determined to contain from 3.8 to 12 copies per reaction.
Establishment of an ELISA for anti-ICP8 (UL29) antibodies
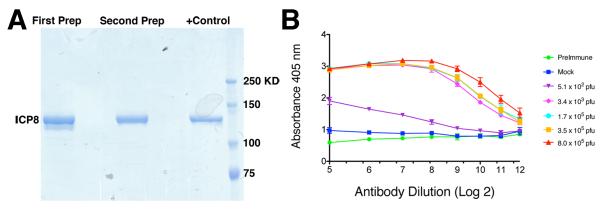
To evaluate protection from infection, we wished to distinguish seroconversion due to immunization from seroconversion generated in response to challenge virus infection. Because the dl5-29 virus does not encode the ICP8 protein (UL29 gene), we set up an ELISA to detect anti-ICP8 antibodies. We produced a recombinant HSV-1 FLAG-tagged ICP8 protein in insect cells (Bryant et al., 2012) as an antigen to coat plates (Figure 3A). Different amounts of ICP8, ranging from 3 to 300 ng/well, were tested in ELISAs using the anti-ICP8 mouse monoclonal antibody 39S (Showalter, Zweig, and Hampar, 1981) as a positive control and sera from uninfected mice as background controls. We determined that 150 ng/well of ICP8 recombinant protein resulted in an optimal assay response based on the signal to background ratio (results not shown). When we tested sera from mice infected intravaginally with increasing doses of challenge virus, we observed increasing titers of anti-ICP8 antibodies (Figure 3B), showing that HSV-2 in general and strain 186ΔKpn in particular can elicit antibodies against ICP8. Although we observed some background with this ELISA that may be due to the purity or nature of our antigen, we were able to measure significant increases above this background level upon infection with the TK− mutant virus. Preimmune sera also showed the same levels of background, which we show as baseline in our results.
Figure 3. Development of an ELISA to detect HSV ICP8-specific antibody.
A) Recombinant ICP8-FLAG protein used for coating ELISA plates. Two independent preparations of HSV-1 ICP8 protein were produced in insect cells and purified with an anti-FLAG antibody. Shown is a gel stained with Simply Blue Coomassie blue staining. The positive control lane is a preparation used in a previous biochemical study (Bryant et al., 2012). B) ELISA titers for anti-ICP8 antibodies from mice inoculated with HSV-2 186ΔKpn virus delivered intravaginally. n = 5 mice/group, plot shows mean titer ± SEM.
Protection from disease and seroconversion
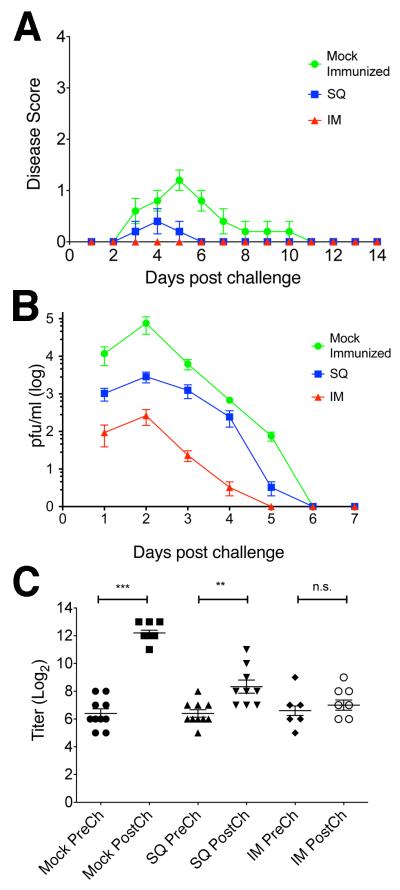
Having established a non-lethal model for genital herpes in mice and assays to measure various aspects of infection, we tested whether immunization with dl5-29 could protect against seroconversion. BALB/c mice were immunized either subcutaneously (SQ) or intramuscularly (IM) with 1×105 PFU of HSV-2 dl5-29. Animals were then challenged with 3×105 PFU of HSV-2 186ΔKpn intravaginally 4 weeks after the second immunization, and we measured disease daily on days 1–14 and viral shedding in the genital tract 1–7 dpi. In animals immunized by the IM route, we observed complete protection from genital disease (Figure 4A) and reduced shedding into the vaginal cavity (Figure 4B). In animals immunized by the SQ route, we observed reduction of genital disease and viral shedding (Figure 4).
Figure 4. Protection against disease, virus shedding, and seroconversion.
Mice were immunized intramuscularly (IM) or subcutaneously (SQ) with HSV-2 dl5-29 or mock-immunized and challenged intravaginally with HSV-2 186ΔKpn virus. A) Disease Scores. B) Viral shedding into the vaginal cavity. C) ELISA titers. n = 10 (Mock-vaccinated) or 9 (vaccinated groups) mice/group, plots show mean ± SEM. p values: Mock = 0.001, SQ = 0.0052, IM = 0.2234.
When we tested sera for anti-ICP8 antibodies, we found that the mock-vaccinated mice showed a significant increase in antibody titers or seroconversion (p value = 0.001) (Figure 4C), which paralleled the observed genital herpetic disease. In contrast, immunized animals showed reduced or no increase in anti-ICP8 antibody titers. Immunization via the IM route protected more efficaciously against vaginal infection (Figure 4C, SQ p value = 0.0052, IM p value = 0.2234).
Protection from latent infection
Having shown that the 186ΔKpn virus can establish latent infection in sacral ganglia, we tested if immunization could protect the mice from latent infection after vaginal challenge with HSV-2 186ΔKpn virus. At 30 dpi, we collected dorsal root ganglia where the cell bodies of neurons innervating the site of infection are located. Quantitative PCR analysis showed that, in mock-vaccinated animals, HSV-2 186ΔKpn established latent infection (Figure 5). In contrast, both SQ and IM immunization greatly reduced latent infection of ganglia when compared to mock immunization (p values =0.0028 and 0.0026, respectively) with the IM route being slightly more effective than the SQ route, although differences between them were not significant (p = 0.8619; Figure 5). Therefore, even when there is viral replication and shedding during acute infection, protection against latent infection can be achieved.
Figure 5. Protection against latent infection.
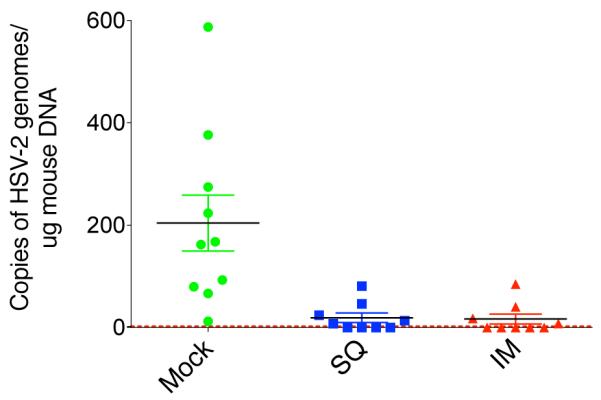
At 30 days after challenge with HSV-2 186ΔKpn, DNA was collected from the dorsal root ganglia of BALB/c mice. In two independent experiments, we measured latent viral DNA in all mock-vaccinated animals. Results are expressed as copies of viral DNA per microgram of total DNA. ICP8 was used to measure viral DNA copies. Murine adipsin was used as an internal control. Dotted line shows the lower limit of detection of 2 copies/reaction. n = 10 (Mock-vaccinated) or 9 (vaccinated groups) mice/group, plot shows mean ± SEM. Mock-vaccinated group was significantly higher that both SQ and IM vaccinated groups (p = 0.0028 and 0.0026 respectively).
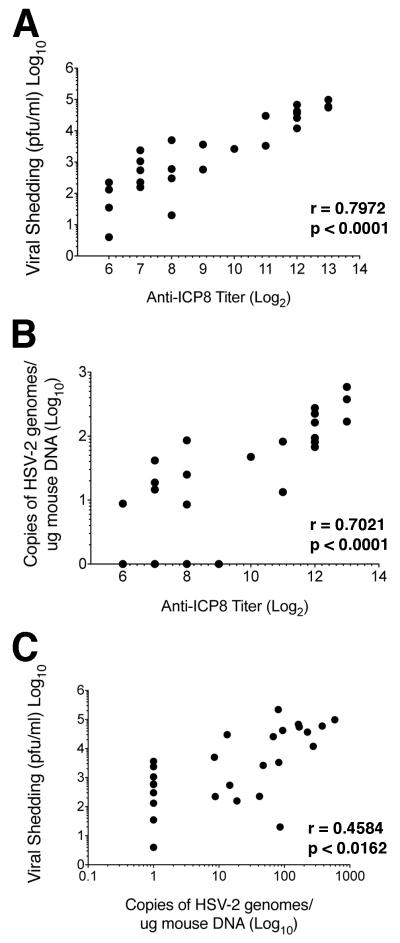
We had hypothesized that the three parameters of protection were related; therefore, we performed pairwise comparisons of the values for protection. We observed a significant correlation between viral shedding and anti-ICP8 antibody titers (p<0.001) with a moderately strong linear correlation (r=0.7972) (Figure 6A). We also observed a statistically significant correlation between latent viral DNA load and anti-ICP8 antibody titers (p<0.0001) with a moderately strong linear correlation (r=0.7021) (Figure 6B). Finally, we observed a statistically significant correlation between viral shedding and latent viral DNA load (p<0.0162) with a moderate linear correlation (r=0.4582). Therefore, in general, there was a correlation between the three values used for measuring protection from infection.
Figure 6. Correlation of protection from acute and latent infection.
Pairwise comparisons of the three protection parameters were evaluated for all animals in this study. A) Viral shedding versus anti-ICP8 titers. B) Copies of latent viral DNA in DRGs versus anti-ICP8 titers. C) Viral shedding versus copies of latent viral DNA in DRGs. Pearson coefficients (r) and p-values (p) were calculated as described in Materials and Methods.
Discussion
Viral vaccines have usually protected against viral disease but have not protected completely against infection. The existing viral vaccines are used mostly for viruses that cause an acute infection, which could be cleared by the host immune response. In contrast, viruses that establish a latent infection, such as the herpesviruses and HIV, are more difficult vaccine targets because, even if disease is prevented, latent infection may still be established, from which the virus could reactivate and cause disease. For herpesviral vaccines, there has been debate about the type of protection that should be measured in clinical trials, i.e., whether it should be protection against disease or infection, the latter being measured by seroconversion (Dropulic and Cohen, 2012; Gershon, 2013; Johnston, Koelle, and Wald, 2014; Knipe et al., 2014).
In this study we established a non-lethal model of genital HSV-2 infection that allowed measurement of long-term protection, including protection against disease, viral replication at the primary mucosal site, seroconversion, and latent infection. We established a sensitive PCR assay that can detect very low levels of HSV-2 DNA, and we used this system to assess protection against various aspects of HSV-2 genital infection. We used the HSV-2 dl5-29 replication-defective viral vaccine candidate for immunization, and, because this virus does not encode the HSV-2 ICP8 protein (UL29 gene product), we established an ELISA system to measure antibody responses to ICP8 as evidence of wildtype virus infection. We observed that immunization with dl5-29 reduces disease, viral shedding, seroconversion and latent infection. Therefore, it is feasible to model a non-lethal infection in mice.
Protection against Disease
Protection against disease in murine genital infection models usually has involved measurement of protection against death (Parr et al., 1994). In this system we have defined a dose that causes genital disease in all mice but with 100% survival. This is based on the inability of TK− mutants to replicate in sensory neurons and cause neurological disease (Coen et al., 1989). This may not be non-physiological if human neurons are more non-permissive for HSV replication than mouse neurons.
Protection against Infection
Protection against infection can be measured at various levels. In experimental models, it is feasible to measure viral infection by assessment of viral shedding from the genital tract. In this and probably all murine systems, immunization reduces shedding from the genital tract but does not prevent recovery of infectious virus from the genital tract (Morrison, Da Costa, and Knipe, 1998). While some of this virus at day 1 may represent input virus, increased levels of virus at day 2 are likely due to viral replication. Therefore, sterilizing immunity is not achieved, but reduced replication at the primary site of infection reduces or prevents latent infection as described below.
Measurement of viral shedding is usually not feasible in large clinical trials. Instead, seroconversion can be measured. However, this has not been measured in murine genital infection systems because HSV-2 infections are lethal in mice. In our study employing a TK− mutant virus, we observed that immunization with dl5-29 did prevent increased antibody responses specific to ICP8. Thus, protection against seroconversion is feasible in mice, even when sterilizing immunity is not achieved.
Protection against Latent Infection
In this study we observed reduction or even prevention of latent infection of sacral ganglia in immunized animals but not in the control group. The TK− challenge virus, although attenuated, is capable of establishing latent infection in mock-vaccinated mice. Therefore, it is possible, at least in this mouse model, to achieve protection against latent infection without completely preventing viral replication at the primary site of infection. Concerns have been expressed about the possibility of preventing disease without reducing latent infection, which could raise the incidence of asymptomatic infection (Rupp and Bernstein, 2008). Studies in mice and guinea pigs utilizing dl5-29 for immunization have found a reduction in latent infection and recurrent disease when disease and viral shedding are reduced (Hoshino et al., 2005; Hoshino et al., 2009; Hoshino et al., 2008; Jones, Taylor, and Knipe, 2000). In this study we observed a correlation between reductions in viral shedding, anti-ICP8 antibody titers, and latent viral DNA load. Therefore, these results predict that reduction in viral acute infection and seroconversion will reduce HSV latent infection, but this will need to be examined in the human situation.
In summary, immunization of mice with the HSV-2 dl5-29 virus can provide protection against seroconversion and latent infection, even if viral replication does occur in the genital tract. Thus, it may be feasible to use a reduction of seroconversion as a clinical endpoint in a clinical trial of an HSV-2 vaccine. Nevertheless, it still seems prudent to have disease as the primary endpoint and seroconversion as a secondary endpoint because approval of products by the FDA is based on clinical parameters. Measurement of protection against seroconversion using dl5-29 virus as the immunogen presents a challenge in that it expresses all but two viral gene products. We used an ICP8 antibody ELISA measurement in this study, but the ELISA as constituted is not optimal because it has a limited dynamic range. Antibody responses to ICP8 may be more robust in humans, but, if not, a different assay for ICP8-specific antibodies such as the luciferase immunoprecipitation assay (Burbelo et al., 2009) might be used. Alternatively, additional viral genes could be deleted from the dl5-29 virus, an approach that we are now attempting.
Methods
Cells and Viruses
Vero cells (ATCC CCL-81) were used for propagation and titration of replication-competent virus stocks (Spang, Godowski, and Knipe, 1983). The V5-29 complementing cell line (Da Costa et al., 2000) was used for propagation and titration of replication-defective viruses. The HSV-2 dl5-29 replication-defective virus was generated from HSV-2 186syn+ by deletions of the essential UL5 and UL29 ORFs (Da Costa et al., 2000; Da Costa et al., 1997). The HSV-2 186ΔKpn UL23 gene (TK−) deletion mutant virus was described previously (Jones, Taylor, and Knipe, 2000).
Animal Studies
Animal housing and experiments were conducted according to protocols approved by the Harvard Medical Area Standing Committee on Animals.
Immunization and Genital Infections
Genital infections
Five-week-old female BALB/c mice were bled from the tail vein before any injection and then administered two doses of 3 mg of Depo-Provera 1 week apart. One day after the second dose of Depo-Provera, the vaginal cavities were then preswabbed, and the indicated doses of the attenuated HSV-2 strain 186ΔKpn were delivered in a 10 μl volume using a micropipettor as described (Morrison, Da Costa, and Knipe, 1998). Animals were monitored for herpetic disease daily on days 1–14. At day 21 post challenge, mice were bled again.
Immunization
For protection assays, 5-week-old female BALB/c mice were immunized twice subcutaneously (SQ) in the rear flank or intramuscularly (IM) in the gastrocnemius muscle, on days 0 and 28, with 105 plaque-forming units (PFU) of extracellular dl5-29 virus in a 50 μl volume in PBS as described previously (Delagrave et al., 2012; Dudek et al., 2011; Morrison, Da Costa, and Knipe, 1998). On days 48 and 55, mice were injected subcutaneously in the scruff of the neck with 3 mg of Depo-Provera in a 100 μl volume. At 4 weeks after the second immunization (day 56), the vaginal cavities were pre-swabbed with a wet polyester swab, and mice were challenged intravaginally with the indicated doses of HSV-2 186ΔKpn TK− virus.
Measurement of Viral Shedding
On days 1–7 post-intravaginal challenge, the vaginal cavities of the mice were successively swabbed twice with pre-wetted polyester swabs (Puritan, #25-800D50). The swabs were placed in 1 ml of assay medium (DMEM, 0.1% glucose, 1% FCS, 5% glycerol) and stored at −80°C. Viral titers were determined by standard plaque assay on Vero cells (Morrison, Da Costa, and Knipe, 1998).
Clinical Disease Measurement
Mice that were challenged with wild-type HSV-2 were observed on days 1-14 post infection for signs of genital lesions and systemic illness, as described previously (Morrison, Da Costa, and Knipe, 1998). The severity of disease was scored as follows: 0 = no sign of disease; 1 = slight genital erythema and edema; 2 = moderate genital inflammation; 3 = purulent genital lesions; 4 = hind-limb paralysis. Mice were euthanized with CO2 at the first sign of paralysis.
Measurement of Seroconversion
Assay for anti-ICP8 (UL29) antibodies
The ICP8 (UL29) protein from HSV-1 was produced in Sf21 insect cells infected with a baculovirus encoding a FLAG-tagged version of ICP8 as described previously (Bryant et al., 2012). The purity of the protein was assessed by running an aliquot from each step of the purification protocol in a polyacrylamide gel and staining with SimplyBlue stain (Life Technologies LC6060). MaxiSorp plates were coated with varying amounts of ICP8-FLAG (3–300 ng/well) and used for ELISA with the 39S mouse monoclonal anti-ICP8 antibody, preimmune mouse serum, or serum from mice infected intravaginally with HSV-2 186ΔKpn. All subsequent ICP8 ELISA plates were coated with 150 ng/well of ICP8-FLAG protein. ELISA for ICP8 was carried as below.
ELISA
Mouse sera were prepared using BD Microtainer tubes (BD, Ref# 365956). Total anti-HSV-2 and anti-ICP8 antibodies titers were determined by ELISA on 96-well flat-bottomed plates (Nunc, MaxiSorp) coated with 50 ng/well of purified HSV-2 (Advanced Biotech, Cat# 10-146-000) or 150 ng/well of ICP8-FLAG in 50 μl of carbonate-bicarbonate buffer pH 9.6. Bound antibodies in sera were detected by alkaline phosphatase-conjugated rabbit anti-mouse antibody followed by p-Nitrophenyl phosphate (Sigma, Cat# A4312, N2770) and measuring absorbance at 405nm according to the manufacturer instructions. The endpoint titer was determined according to Frey et al. (Frey, Di Canzio, and Zurakowski, 1998).
Measurement of latent viral DNA load
Dorsal root ganglia were harvested at 30 dpi (Malin, Davis, and Molliver, 2007), and DNA was purified from 10 DRGs per sample with the DNeasy blood and tissue kit (Qiagen, #69506). Levels of latent viral DNA in DRGs were measured using TaqMan real-time PCR to detect the HSV-2 UL29 (ICP8) gene, which is present in the challenge virus HSV-2 186ΔKpn but not in the vaccine vector dl5-29, and the mouse adipsin gene as an internal control. Standard curve samples were prepared using HSV-2 DNA purified by double banding in sodium iodide gradients as described previously (Colgrove et al., 2014; Walboomers and ter Schegget, 1976) and mouse genomic DNA as the source of the adipsin gene. The standard curve and DRG samples were run in triplicate using the following primers and probes in duplex reactions, mouse adipsin primers: forward 5′-GCAGTCGAAGGTGTGGTTACG-3′; reverse 5′-GGTATAGACGCCCGGCTTTT-3′. The probe for adipsin, with reporter dye, was 5′- VIC-CTGTGGCAATGGC-MGBNFQ-3′ (MGBNFQ, minor groove binder non-fluorescent quencher) (Awasthi et al., 2011). Primers for ICP8 with nucleotide positions based on HSV-2 strain HG52: forward 5′-TTTTGGCTTCTCGGACTACG-3′ (62082-62063); reverse 5′-ATCACCAGATACAGCAAGGC-3′ (61966-61985). The probe for ICP8 was 5′- 6FAM-TGCGACCTAAAACAC-MGBNFQ-3′ (62051-62037). Reactions were performed on 5 μl of DNA (250–300 ng) in 25 μl reaction mixtures, using TaqMan Gene Expression Master Mix (Life Technologies Cat#: 4369016) and ABI 7300 and StepOne Plus Real Time PCR Systems.
Statistical analysis
All statistical analyses were performed with GraphPad Prism. Statistical significance was evaluated with Student t-tests and the mean ± SEM is plotted in all the figures. Correlation coefficients (r) were calculated with a two-tailed Pearson test, and correlation significance is reported based on the p value.
Research Highlights.
A nonlethal genital infection model was established for herpes simplex virus 2.
An ELISA was established to measure ICP8-specific antibodies.
A sensitive PCR assay was established to measure latent HSV-2 DNA.
Immunization reduced antibody responses, virus shedding, and latent viral DNA.
Acknowledgments
We are grateful to Hector Hernandez and John Hamberger for critical assistance with dissection of the dorsal root ganglia. This research was supported by NIH grant AI 057552 to DMK and a research award from Sanofi Pasteur.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Awasthi S, Lubinski JM, Shaw CE, Barrett SM, Cai M, Wang F, Betts M, Kingsley S, Distefano DJ, Balliet JW, Flynn JA, Casimiro DR, Bryan JT, Friedman HM. Immunization with a vaccine combining herpes simplex virus 2 (HSV-2) glycoprotein C (gC) and gD subunits improves the protection of dorsal root ganglia in mice and reduces the frequency of recurrent vaginal shedding of HSV-2 DNA in guinea pigs compared to immunization with gD alone. J Virol. 2011;85:10472–86. doi: 10.1128/JVI.00849-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnabas RV, Celum C. Infectious co-factors in HIV-1 transmission herpes simplex virus type-2 and HIV-1: new insights and interventions. Curr HIV Res. 2012;10:228–37. doi: 10.2174/157016212800618156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belshe RB, Leone PA, Bernstein DI, Wald A, Levin MJ, Stapleton JT, Gorfinkel I, Morrow RL, Ewell MG, Stokes-Riner A, Dubin G, Heineman TC, Schulte JM, Deal CD. Efficacy results of a trial of a herpes simplex vaccine. N Engl J Med. 2012;366:34–43. doi: 10.1056/NEJMoa1103151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant KF, Yan Z, Dreyfus DH, Knipe DM. Identification of a divalent metal cation binding site in herpes simplex virus 1 (HSV-1) ICP8 required for HSV replication. J Virol. 2012;86:6825–34. doi: 10.1128/JVI.00374-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbelo PD, Hoshino Y, Leahy H, Krogmann T, Hornung RL, Iadarola MJ, Cohen JI. Serological diagnosis of human herpes simplex virus type 1 and 2 infections by luciferase immunoprecipitation system assay. Clin Vaccine Immunol. 2009;16:366–71. doi: 10.1128/CVI.00350-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen DM, Kosz-Vnenchak M, Jacobson JG, Leib DA, Bogard CL, Schaffer PA, Tyler KL, Knipe DM. Thymidine kinase-negative herpes simplex virus mutants establish latency in mouse trigeminal ganglia but do not reactivate. Proc Natl Acad Sci U S A. 1989;86:4736–4740. doi: 10.1073/pnas.86.12.4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgrove R, Diaz F, Newman R, Saif S, Shea T, Young S, Henn M, Knipe DM. Genomic sequences of a low passage herpes simplex virus 2 clinical isolate and its plaque-purified derivative strain. Virology. 2014;450-451:140–5. doi: 10.1016/j.virol.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey L, Langenberg AG, Ashley R, Sekulovich RE, Izu AE, Douglas JM, Jr., Handsfield HH, Warren T, Marr L, Tyring S, DiCarlo R, Adimora AA, Leone P, Dekker CL, Burke RL, Leong WP, Straus SE. Recombinant glycoprotein vaccine for the prevention of genital HSV-2 infection: Two randomized controlled trials. Chiron HSV Vaccine Study Group. JAMA. 1999;28:331–340. doi: 10.1001/jama.282.4.331. [DOI] [PubMed] [Google Scholar]
- Da Costa X, Kramer MF, Zhu J, Brockman MA, Knipe DM. Construction, phenotypic analysis, and immunogenicity of a UL5/UL29 double deletion mutant of herpes simplex virus 2. J Virol. 2000;74:7963–71. doi: 10.1128/jvi.74.17.7963-7971.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Costa XJ, Bourne N, Stanberry LR, Knipe DM. Construction and characterization of a replication-defective herpes simplex virus 2 ICP8 mutant strain and its use in immunization studies in a guinea pig model of genital herpes. Virology. 1997;232:1–12. doi: 10.1006/viro.1997.8564. [DOI] [PubMed] [Google Scholar]
- Delagrave S, Hernandez H, Zhou C, Hamberger JF, Mundle ST, Catalan J, Baloglu S, Anderson SF, DiNapoli JM, Londono-Hayes P, Parrington M, Almond J, Kleanthous H. Immunogenicity and efficacy of intramuscular replication-defective and subunit vaccines against herpes simplex virus type 2 in the mouse genital model. PLoS One. 2012;7:e46714. doi: 10.1371/journal.pone.0046714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dropulic LK, Cohen JI. The challenge of developing a herpes simplex virus 2 vaccine. Expert Rev Vaccines. 2012;11:1429–40. doi: 10.1586/erv.12.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek T, Mathews LC, Knipe DM. Disruption of the U(L)41 gene in the herpes simplex virus 2 dl5-29 mutant increases its immunogenicity and protective capacity in a murine model of genital herpes. Virology. 2008;372:165–75. doi: 10.1016/j.virol.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek TE, Torres-Lopez E, Crumpacker C, Knipe DM. Evidence for differences in immunologic and pathogenesis properties of herpes simplex virus 2 strains from the United States and South Africa. J Infect Dis. 2011;203:1434–41. doi: 10.1093/infdis/jir047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey A, Di Canzio J, Zurakowski D. A statistically defined endpoint titer determination method for immunoassays. J Immunol Methods. 1998;221:35–41. doi: 10.1016/s0022-1759(98)00170-7. [DOI] [PubMed] [Google Scholar]
- Gershon AA. Varicella zoster vaccines and their implications for development of HSV vaccines. Virology. 2013;435:29–36. doi: 10.1016/j.virol.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham BS, Crowe JE, Ledgerwood JE. Immunization Against Viral Diseases. In: Knipe DM, Howley PM, editors. Fields Virology. 6th ed Vol. 1. Lippincott Williams & Wilkins; Philadelphia: 2013. pp. 374–413. [Google Scholar]
- Hoshino Y, Dalai SK, Wang K, Pesnicak L, Lau TY, Knipe DM, Cohen JI, Straus SE. Comparative efficacy and immunogenicity of replication-defective, recombinant glycoprotein, and DNA vaccines for herpes simplex virus 2 infections in mice and guinea pigs. J Virol. 2005;79:410–8. doi: 10.1128/JVI.79.1.410-418.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino Y, Pesnicak L, Dowdell KC, Burbelo PD, Knipe DM, Straus SE, Cohen JI. Protection from Herpes Simplex Virus (HSV)-2 Infection with Replication-Defective HSV-2 or Glycoprotein D2 Vaccines in HSV-1-Seropositive and HSV-1-Seronegative Guinea Pigs. J Infect Dis. 2009;200:1088–95. doi: 10.1086/605645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino Y, Pesnicak L, Dowdell KC, Lacayo J, Dudek T, Knipe DM, Straus SE, Cohen JI. Comparison of immunogenicity and protective efficacy of genital herpes vaccine candidates herpes simplex virus 2 dl5-29 and dl5-29-41L in mice and guinea pigs. Vaccine. 2008;26:4034–40. doi: 10.1016/j.vaccine.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston C, Koelle DM, Wald A. HSV-2: In pursuit of a vaccine. J Clin Invest. 2011;121:4600–9. doi: 10.1172/JCI57148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston C, Koelle DM, Wald A. Current status and prospects for development of an HSV vaccine. Vaccine. 2014;32:1553–60. doi: 10.1016/j.vaccine.2013.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CA, Taylor TJ, Knipe DM. Biological properties of herpes simplex virus 2 replication-defective mutant strains in a murine nasal infection model. Virology. 2000;278:137–150. doi: 10.1006/viro.2000.0628. [DOI] [PubMed] [Google Scholar]
- Knipe DM, Corey L, Cohen JI, Deal CD. Summary and recommendations from a National Institute of Allergy and Infectious Diseases (NIAID) workshop on “Next Generation Herpes Simplex Virus Vaccines”. Vaccine. 2014;32:1561–2. doi: 10.1016/j.vaccine.2014.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malin SA, Davis BM, Molliver DC. Production of dissociated sensory neuron cultures and considerations for their use in studying neuronal function and plasticity. Nat Protoc. 2007;2:152–60. doi: 10.1038/nprot.2006.461. [DOI] [PubMed] [Google Scholar]
- Morrison LA, Da Costa XJ, Knipe DM. Influence of mucosal and parenteral immunization with a replication-defective mutant of HSV-2 on immune responses and protection from genital challenge. Virology. 1998;243:178–187. doi: 10.1006/viro.1998.9047. [DOI] [PubMed] [Google Scholar]
- Parr MB, Kepple L, McDermott MR, Drew MD, Bozzola JJ, Parr EL. A mouse model for studies of mucosal immunity to vaginal infection by herpes simplex virus type 2. Lab Invest. 1994;70:369–380. [PubMed] [Google Scholar]
- Rupp R, Bernstein DI. The potential impact of a prophylactic herpes simplex vaccine. Expert Opin Emerg Drugs. 2008;13:41–52. doi: 10.1517/14728214.13.1.41. [DOI] [PubMed] [Google Scholar]
- Showalter SD, Zweig M, Hampar B. Monoclonal antibodies to herpes simplex virus type 1 proteins, including the immediate-early protein ICP 4. Infect Immun. 1981;34:684–92. doi: 10.1128/iai.34.3.684-692.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spang AE, Godowski PJ, Knipe DM. Characterization of herpes simplex virus 2 temperature-sensitive mutants whose lesions map in or near the coding sequences for the major DNA-binding protein. J Virol. 1983;45:332–342. doi: 10.1128/jvi.45.1.332-342.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanberry LR, Kern ER, Richards JT, Abbott TM, Overall JC., Jr. Genital herpes in guinea pigs: Pathogenesis of the primary infection and description of recurrent disease. J Infect Dis. 1982;146:397–404. doi: 10.1093/infdis/146.3.397. [DOI] [PubMed] [Google Scholar]
- Stanberry LR, Spruance SL, Bernstein DI, Mindel A, Sacks S, Tyring S, Aoki FY, Slaoui M, Denis M, Vandepapeliere P, Dubin G, GlaxoSmithKline Herpes Vaccine Efficacy Study Group Glycoprotein-D-adjuvant vaccine to prevent genital herpes. N Engl J Med. 2002;347:1652–61. doi: 10.1056/NEJMoa011915. [DOI] [PubMed] [Google Scholar]
- Straus SE, Wald A, Kost RG, McKenzie R, Langenberg AG, Hohman P, Lekstrom J, Cox E, Nakamura M, Sekulovich R, Izu A, Dekker C, Corey L. Immunotherapy of recurrent genital herpes with recombinant herpes simplex virus type 2 glycoproteins D and B: Results of a placebo-controlled vaccine trial. J Infect Dis. 1997;176:1129–1134. doi: 10.1086/514103. [DOI] [PubMed] [Google Scholar]
- Veselenak RL, Shlapobersky M, Pyles RB, Wei Q, Sullivan SM, Bourne N. A Vaxfectin((R))-adjuvanted HSV-2 plasmid DNA vaccine is effective for prophylactic and therapeutic use in the guinea pig model of genital herpes. Vaccine. 2012;30:7046–51. doi: 10.1016/j.vaccine.2012.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walboomers JM, ter Schegget J. A new method for the isolation of herpes simplex virus type 2 DNA. Virology. 1976;74:256–258. doi: 10.1016/0042-6822(76)90151-3. [DOI] [PubMed] [Google Scholar]