Abstract
Objective
Investigate the association between 8-week tumor volume decrease and survival in an independent cohort of EGFR-mutant advanced non-small-cell lung cancer (NSCLC) patients treated with first-line erlotinib or gefitinib, and assess the rate of their volumetric tumor growth after the volume nadir.
Methods
In patients with advanced NSCLC harboring sensitizing EGFR mutations treated with first-line erlotinib or gefitinib, CT tumor volumes of dominant lung lesions were analyzed for 1) the association with survival, and 2) volumetric tumor growth rate after the volume nadir.
Results
In 44 patients with the 8-week follow-up CT, the 8-week tumor volume decrease (%) was significantly associated with longer overall survival (OS) when fitted as a continuous variable in a Cox model (p=0.01). The growth rate of the logarithm of tumor volume (logeV), obtained using a linear mixed-effects model adjusting for time since baseline, was 0.096/month (SE:0.013/month; 95%CI:0.071-0.12/month), which was similar to the rate of 0.12/month (SE:0.015/month; 95%CI: 0.090-0.15/month) observed in the previous report.
Conclusions
The 8-week tumor volume decrease was validated as a marker for longer survival in the independent cohort of EGFR-mutant NSCLC patients treated with first-line erlotinib or gefitinib. Volumetric tumor growth rate after the nadir in this cohort was similar to that of the previous cohort, indicating the reproducibility of the observation among different patient cohorts.
Keywords: lung cancer, non-small-cell, EGFR mutations, tyrosine kinase inhibitors, tumor volume
INTRODUCTION
Discoveries of genomic abnormalities in the tumors from lung cancer patients and the effective treatment with targeted agents have ushered in a new era of therapeutic approaches to lung cancer1, 2. Epidermal growth factor receptor (EGFR) mutations in non-small-cell lung cancer (NSCLC) have been studied as one of the major therapeutic targets since their discovery in 20043-5. NSCLC patients harboring sensitizing EGFR mutations have initial dramatic responses to the EGFR tyrosine kinase inhibitors (TKIs), erlotinib, gefitinib, and afatinib, with response rates of 55-83% and progression-free survival (PFS) of 9.7 to 13.1 months6-12. However, their tumors eventually grow back during EGFR-TKI therapy due to the development of acquired resistance, eventually leading to tumor progression13. The duration of disease control from EGFR-TKI therapy can range from 4 months to 4 years or longer13. In this context, objective early markers of tumor response during EGFR-TKI therapy are needed, in order to identify patients who can safely remain on therapy and those who are unlikely to have long term control and may potentially benefit from an early introduction of additional or alternative agents.
Imaging remains the principal method to objectively characterize the tumor burden during cancer therapy2. Prior studies have demonstrated the limitations of the conventional diameter-based approach according to RECIST, and indicated the need for volumetric tumor assessment2, 14-20. The previous studies evaluated tumor volume measurements in advanced NSCLC patients treated with EGFR-TKIs using FDA-approved, commercially available software and published the high reproducibility of the technique14. By applying this technique to EGFR-mutant NSCLC patients treated with the first-line erlotinib or gefitinib, the study demonstrated that greater tumor volume decrease at 8 weeks of therapy is significantly associated with longer overall survival (OS), with a cut-off value of 38% volume decrease at 8 weeks best differentiating patients with longer OS and PFS21. The 8-week volume change as a predictor of survival has a potential role in identifying patients who may benefit from additional or alternative therapy in the early course of therapy, and help to maximize the benefit of targeted therapy and improve the clinical outcome. Tumor volume analysis was also applied to characterize the rate of tumor growth in EGFR-mutant NSCLC patients after they reach their volume nadir (the smallest tumor volume since baseline), which is another important aspect in assessing benefit of cancer therapy22-27. A prior study reported a reference value of the volumetric tumor growth rate among these patients after their volume nadir, which helps to differentiate slow versus fast progressors among those who are on EGFR-TKI therapy, thus contributing to provide an objective guidance about when to keep patients on the EGFR-TKI after progression28.
Given the promising utility of tumor volume analysis, it is necessary to reproduce the results in independent cohorts, in order to propose the approach to be used in the clinical practice. The purpose of the present study is to validate 1) the association between the 8-week tumor volume decrease and longer OS, and 2) the volumetric tumor growth rate after the volume nadir, in an independent cohort of advanced NSCLC patients harboring sensitizing EGFR mutations treated with first-line erlotinib or gefitinib. Retrospective analysis of an independent cohort also provides an opportunity to assess how these approaches contribute in a real-life clinical setting.
MATERIALS AND METHODS
Patients
The study cohort included 58 patients with advanced EGFR-mutant NSCLC treated with first-line erlotinib or gefitinib monotherapy between 2002 and 2012, who had a baseline CT performed prior to initiating therapy demonstrating at least one measurable lung lesion, and had at least one follow-up chest CT. All patients had histologically or cytologically confirmed NSCLC with sensitizing EGFR mutations, which were defined as deletions, duplications, and deletions-insertions of exon 19, L858R point mutation, L861Q point mutation, and G719 mis-sense point mutations, as described previously21, 28-30. The patients were initially treated with gefitinib or erlotinib and the clinicians made decisions about changing therapies based on the symptoms, signs, and radiographic tumor assessments. Measureable lung lesions were defined as lesions measuring at least 10 mm in the longest diameter, and were chosen based on the review of baseline CT images by a thoracic radiologist (M.N.)21, 28.
CT tumor volume measurements during TKI therapy
Baseline and follow-up chest CT scans were performed to assess response to EGFR-TKI therapy as a part of their clinical care. A thoracic radiologist (M.N.; 10 years of experience in thoracic and oncologic imaging) performed the tumor volume and size (the longest diameter) measurements of a dominant lung lesion (1 lesion per patient) on baseline and all follow-up CT scans during therapy, using the previously validated technique on the volume analysis software (Vitrea 2; Vital Images, Minnetonka, MN)14, 21, 28, 31. In the workflow of tumor volume measurement, axial chest CT images were loaded and displayed in a lung window setting (level = −500; width = 1500). The radiologist (M.N.) manually selected a small region of interest within a lesion on a CT image, which showed the longest diameter of the lesion by a mouse click. The software automatically segmented the lesion from the surrounding normal lung and adjacent structures such as vessels and pleura, using a three-dimensional seed growing algorithm. The boundary of the segmented lesion was then displayed on the CT images. The radiologist visually assessed if the automated algorithm accurately segmented the lesion excluding adjacent structures such as vessels, pleura, atelectasis, and effusion. The radiologist manually adjusted the boundary of the tumor on each image if needed, determining the boundary between the lesion and adjacent structures by visual assessment. After segmentation and manual correction, the volume of the segmented lesion was automatically calculated by the software and the volume of the segmented tumor was provided. The reader also manually measured the longest diameter of the lesion on a CT image using a caliper-type measurement tool on the Vitrea Workstation. The intra- and interobserver measurement variability of the technique has been studied in detail and previously published; therefore, the radiologist measured the lesion once at each timepoint for the present study.
The 8-week tumor volume analysis
The proportional tumor volume and size changes (%) at 8 weeks of therapy were calculated in reference to the baseline tumor volume and size21. Follow-up scans performed at 8±2 weeks of therapy were allowed for the 8-week scans as in the prior study21. Patients who did not have a follow-up CT at 8±2 weeks of therapy were excluded from the 8-week volume analysis.
Volumetric tumor growth after the nadir
Based on the review of the longitudinal tumor volume measurements during therapy, the volume nadir (smallest tumor volume recorded from baseline to TKI termination/last follow-up) was determined in each patient. Patients who experienced volumetric tumor growth after the nadir were eligible for the growth rate analysis28.
Statistical analysis
The association between the proportional tumor volume and size changes (%) at 8 weeks of therapy and outcomes were studied using a landmark analysis, with the landmark time point of 8 weeks21, 32. OS was defined as the time from the date of the 8-week follow-up scan until death from any cause. PFS was defined as the time from the date of 8-week scan until the date of progression or death from any cause, whichever occurred first. Patients not experiencing a progression or death by the time of analyses were censored from each analysis at the last follow-up date. The log-rank test was used to compare event time distributions between groups. Hazard ratios (HRs) were estimated using Cox proportional hazards models, and multivariate analyses were performed using stepwise regression. Differences in clinical characteristics were studied using Fisher's exact test for categorical data and Wilcoxon test for continuous data.
For the volumetric growth rate analysis, all the tumor volume measurements from the nadir to the end of therapy or last follow-up of the eligible patients were analyzed in a linear mixed-effect model, as described previously28, 33. The tumor volume (mm3) was transformed to the natural logarithm scale (logeV)23, 28. A linear mixed-effect model, fitting time from baseline scan as a random effect, was fitted to the repeated measures of tumor volume (logeV) to estimate the effect of time and other prognostic factors on tumor growth28, 33. First, the model was built adjusting only for time in months since baseline. The second model was adjusted for time and the baseline tumor volume (logeV0). The third model was adjusted for time, logeV0, and clinical variables, as in the prior study28. All p values are two-sided at the 0.05 level and no adjustments were made for multiple comparisons.
The approval of the institutional review board has been granted for the study.
RESULTS
The 8-week tumor volume decrease and survival
Among the 58 patients studied, 44 had a follow-up chest CT at 8 +/− 2 weeks since the initiation of therapy and were eligible for the 8-week volume analysis. The remaining 14 patients were excluded for this part of analysis because their follow-up chest CT scans were not performed within the specified window. Table 1 shows the demographics and disease characteristics of the 44 patients with the 8-week scan.
Table 1.
Demographics and disease characteristics of 44 patients with 8-week scan
| Variables | Category | |
|---|---|---|
| Age | Median (range) | 66.5 years (26-88) |
| Sex | Female | 30 |
| Male | 14 | |
| Race | White | 37 |
| Black | 3 | |
| Asian | 2 | |
| Other | 2 | |
| Smoking status | Never | 42 |
| Former | 1 | |
| Current | 1 | |
| Pathology | Adenocarcinoma | 43 |
| Unknown | 1 | |
| ECOG PS | 0 | 18 |
| 1 | 16 | |
| 2 | 5 | |
| 3 | 1 | |
| Unknown | 4 | |
| Extrathoracic metastasis | Present | 26 |
| Absent | 18 | |
| EGFR-TKI | Erlotinib | 33 |
| Gefitinib | 11 | |
| EGFR mutations | Exon19 del | 22 |
| L858R | 20 | |
| L861Q | 1 | |
| G719 | 1 |
The values represent the number of patients unless otherwise specified.
ECOG, Eastern Cooperative Oncology Group, PS, performance status; EGFR, epidermal growth factor receptor; TKI, tyrosine kinase inhibitor.
When fitted as a continuous variable in a Cox model, the 8-week tumor volume change (%) was also significantly associated with OS (p=0.01). The 8-week size change (%) was not associated with OS in a Cox model (p=0.99) (Figs. 1, 2). The OS distribution was assessed by a recursive partitioning model as before, however, no optimal cutpoint was identified.
Fig. 1.
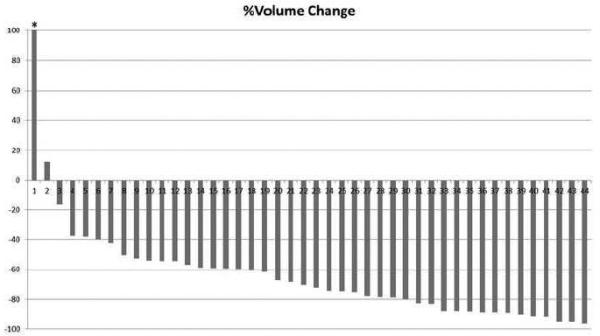
A waterfall plot of the 8-week volume decrease (%) in the 44 patients.
Each bar represents the percent change of tumor volume on the 8-week scan compared to the baseline volume in each patient. The patient indicated by an asterisk (*) had 189.7% increase in tumor volume at 8-week follow-up scan.
Fig. 2.
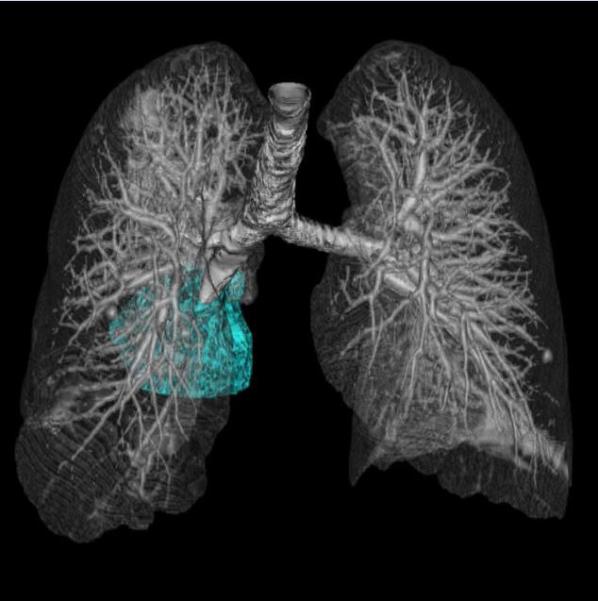
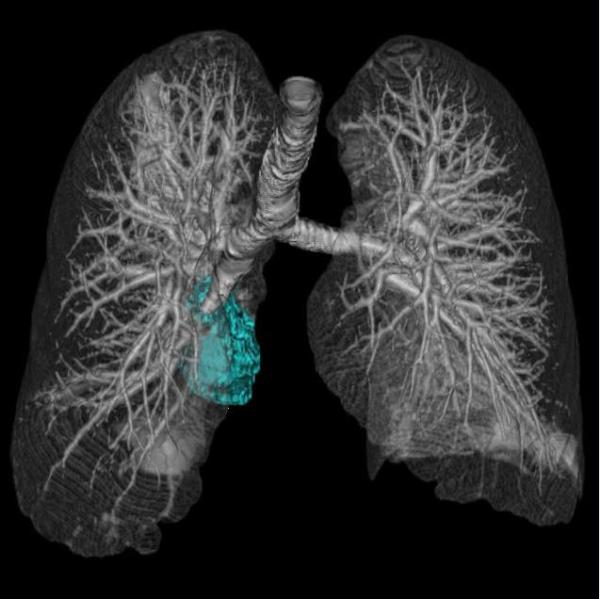
A representative case of tumor volume decrease at 8-week scan with longer survival in a 66-year-old female with stage IV NSCLC harboring EGFR L858R mutation treated with gefitinib.
Baseline chest CT prior to therapy (A) demonstrated a large dominant lung lesion in the right lower lobe, measuring 105,157 mm3. The 8-week follow-up CT (B) showed a significant volume deacrease of the lesion, measuring 42,914 mm3, demonstrating 59.2% decrease in reference to the baseline scan. The patient had an overall survival of 45.4 months after the 8-week scan.
Volumetric tumor growth after the nadir
Among the overall cohort, 50 of the 58 patients had reached their nadir and experienced volumetric tumor growth on CT, and thus were eligible for the growth rate analysis. A total of 285 scans from the nadir to the end of therapy or last follow-up from the 50 patients were analyzed in the liner mixed-effect model. The median time on TKI monotherapy was 13.1 months. Nine patients were on therapy for more than 2 years, including 4 patients on therapy for longer than 3 years. The median time from baseline to tumor volume nadir was 4.8 months. Figure 3 demonstrates the volumetric tumor growth from the nadir to the therapy termination or last follow-up scan in these 50 patients.
Fig. 3.
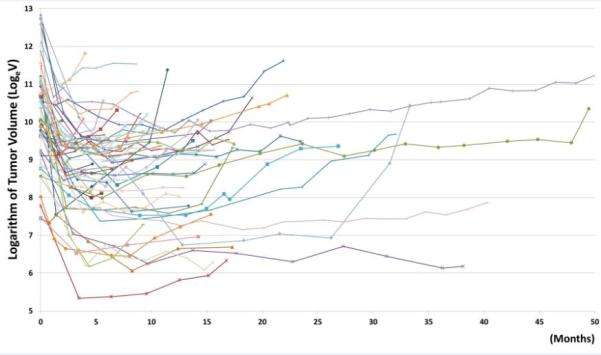
The spider plot representing the tumor volume changes during EGFR-TKI therapy.
Each line represents tumor volume changes in a patient during therapy, starting from the baseline. The x-axis shows time in months since baseline.
The first model, estimating logeV as a function of time from baseline, provided the following formula: logeV = 0.096*time + 7.95. Here, time represents the number of months from baseline. The regression coefficient for time, 0.096/month, represents the growth rate of logeV (standard error (SE): 0.013/month; 95%CI: 0.071-0.12/month; p<0.001)28.
The second model adjusting for logeV0 as a fixed effect, logeV was estimated as follows: logeV=0.093*time+0.62 logeV0+1.58. Baseline volume (logeV0) was a significant predictor of logeV (p<0.001). A regression coefficient for time was 0.93/month (SE: 0.013; 95%CI: 0.068-0.12; p<0.001), representing the growth rate after adjusting for logeV0.
The third model adjusted for predefined clinical variables based on the prior results including stage at diagnosis (stage IV or others), TKI (gefitinib or erlotinib), and smoking status (current/former versus never smoker), and logeV0. The logeV was estimated as follows: logeV=0.093* time + 0.62*logeV0+ 0.39*stage + 0.39*TKI −0.11*smoking +0.10. (p<0.001 for logeV, p<0.001 for logeV0, p=0.58 for stage, p=0.41 for TKI, and p=0.79 for smoking. The growth rate was 0.093/months (SE: 0.013; 95%CI: 0.068-0.12; p<0.001). While logeV0 was a significant predictor of logeV, other clinical variables did not significantly affect the tumor volume after the nadir.
DISCUSSION
Greater tumor volume decrease at 8 weeks of EGFR-TKI therapy was associated with longer OS in the independent cohort of advanced NSCLC patients harboring EGFR mutations, validating the previous observation21, which further provide the basis for the potential utility of tumor volume analysis for early identification of patients who may require additional or alternate therapy. The volumetric tumor growth rate after reaching the nadir for this cohort was 0.096/month for logeV (SE: 0.013/month, 95%CI: 95%CI:0.071-0.12/month), which was consistent with the growth rate in the prior cohort of 0.12/month (SE: 0.015/month; 95%CI: 0.090-0.15/month)28, demonstrating the reproducibility of this observation as well. The approach using the tumor volume analysis can be potentially beneficial because the 8-week tumor volume decrease may serve as an earlier objective marker of efficacy of agents, compared to the conventional PFS endpoint which takes more than a year for data maturation. The early assessment of the efficacy can also be useful in the ongoing trials of newer agents, including third-generation EGFR-TKIs with PFS of approximately a year and their combination therapies with other agents34-36. The validated results of tumor growth rate further indicate its utility in providing an aid for therapeutic decision making as to when the patients can safely remain on EGFR-TKI therapy after initial response.
The reproduced observation of association between 8-week tumor volume decrease and OS in the present cohort further supports the importance of volumetric tumor shrinkage as an early response marker for longer OS among EGFR-mutant NSCLC patients treated with EGFR-TKIs. On the other hand, the lack of association between tumor size changes and OS, as in the previous study, indicates that diameter measurements alone do not provide as much information to use as surrogate for survival in these patients. No optimal cutpoint was identified in a recursive partitioning analysis in this cohort. The previously identified cutpoint (>38% volume decrease at 8 weeks) differentiated patients with longer versus shorter OS (median OS: 27.3 vs. 16.4 months, respectively; p= 0.02) and remained significant in multivariable analysis (HR=0.15, p=0.006) after adjusting for other variables, while the small number of patients in <38% decrease group (n=4) in this cohort is a limitation. The results provide a basis for further studying the volumetric response marker for longer survival among EGFR-mutant NSCLC patients receiving EGFR-TKI therapy in future prospective studies.
The approach using the 8-week volume decrease as an early marker is also applicable to cohorts treated with third-generation EGFR-TKIs, including rociletinib, AZD9291, and HM61713, in ongoing trials35, 36. The now validated association between the 8-week volume decrease and survival in the erlotinib or gefitinib treated cohorts may contribute to early assessment of the efficacy and therapeutic benefit of these novel agents, by providing additional information beyond the RECIST-based evaluations. The approach can also be utilized in other genomically-characterized cohorts of NSCLC patients, such as ALK (anaplastic lymphoma kinase)-positive patients treated with ALK inhibitors2, 37-39. Multiple second generation ALK inhibitors including ceritinib and alectinib are being tested with prolonged PFS. The primary endpoint of PFS can be longer than 2 years as observed with alectinib and thus requiring longer observation period before reaching the endpoint 34, 40, indicating the need for an early marker of therapeutic efficacy and longer survival to facilitate the drug development and clinical translation.
Continuation of EGFR-TKIs beyond RECIST progression is another important issue during the course of treatment of EGFR-mutant patients29, and was shown to be feasible with additional 3.1 months of PFS among EGFR-mutant patients treated with first-line erlotinib in the ASPIRATION study41. The ability to keep patients on EGFR-TKIs beyond progression prompts the need for objective guidelines to identify patients who can safely continue EGFR-TKI therapy, and volumetric tumor growth rate is a promising candidate for this purpose, along with the clinical characteristics identified in their exploratory analysis. Such guidance is particularly important given the recent results of another study, the IMPRESS trial, which found no benefit of continuing gefitinib in addition to chemotherapy compared to chemotherapy alone for EGFR-mutant patients who had disease progression on gefitinib42. While the study provided the first results based on randomized controlled trial to address this actively debated issue of continuation of EGFR-TKI, it is possible that a more specific selection of patients who are more likely to benefit from continued EGFR-TKI therapy may result in different results.
The growth rate for logeV in the present cohort was very consistent with the rate obtained in the prior study of EGFR-mutant patients. Notably, given the growth rate of 0.096/month with SE of 0.013, the 95% confidence interval for the rate is 0.071-0.12/month, which includes the prior results of 0.12/month (SE: 0.015, 95%CI: 0.090-0.15/month)28. The previously proposed tumor growth rate for slow growth, ≤0.15/month for the logarithm of tumor volume (logeV) based on the upper 95% CI from the previous cohort, could be a conservative threshold given the overlapping CIs from 2 cohorts. As in the prior studies, the models adjusting for baseline tumor volume (logeV0) with or without clinical variables did not have much impact on proposed tumor growth rate for keeping patients on EGFR-TKIs. While logeV0 was a significant predictor of logeV, the predefined clinical variables (tumor stage, types of EGFR-TKI, and smoking history) were not significant in predicting logeV in the present cohort, which is expected given the marginal significance in the prior cohort28. The consistent results of volumetric tumor growth rate further indicate the utility of this approach for providing objective criteria for safely keeping patients on the TKIs beyond progression, which may help to prolong their survival. Such objective information can also be important in other genomically-defined cohorts with effective targeted therapy, such as ALK-positive patients treated with ALK inhibitors, where a similar concept of therapy continuation has been applied in the recent trials and patients can be kept on therapy if judged by investigators as receiving “clinical benefit”15, 37, indicating the wider applicability of the objective guidelines.
The limitations of the study include a relatively small number of patients treated at a single institution. Retrospective design is also a limitation, however, such design can also be beneficial in assessing the performance of the approach in the real-life clinical setting; of note, the cohort was independent from the prior study and the findings were reproduced. Only a part of the patients in the overall cohort was eligible for the 8-week landmark analysis because of the lack of follow-up scans within the timeframe, which also delineates the challenges of the clinical setting where follow-up scans are performed per care providers’ discretion without predefined intervals. Further studies are planned to prospectively validate the observations in larger cohorts treated in multicenter trials. Tumor measurements were performed in a dominant lung lesion per patients, and smaller lung lesions and extrathoracic lesions were not taken into account for volume analysis. The study was designed this way because the purpose was to validate the observations in the prior studies that followed the same strategy and demonstrated a significant association between volume and survival. The prior studies by others also demonstrated that the single-lesion approach provides concordant response assessment with multiple-lesion assessment in lung and other cancers43-45. We believe that tumor volume should be used as an additive method to the conventional measures such as RECIST, which is designed to capture the systemic tumor burden with a simple and practical method15, 21, 46. While the current technology allows to measure volumes of multiple lesions in multiple organs, the strategy for tumor response assessment should be chosen carefully after considering the trade-off between the amount of time and effort needed for measurements, cost, and potential limitations associated with the advanced techniques such as increased measurement variability and difficulty in standardizing the technique2, 30. Based on the present study validating the association between single-lesion tumor volume analysis and survival, this approach, used in combination with RECIST-based assessment, may be optimal for cohorts of EGFR-mutant NSCLC patients treated with EGFR-TKIs.
In conclusion, the 8-week tumor volume decrease is associated with longer survival in this independent validation cohort of advanced EGFR-mutant NSCLC patients treated with first-line erlotinib or gefitinib, and therefore can be used as a reliable marker for therapeutic benefit during the early course of EGFR-TKI therapy. Volumetric tumor growth rate in these patients were also consistent with the previously obtained results, indicating the utility of the approach to define slow progression. The quantitative imaging markers provided by tumor volume analysis in the present study may contribute to therapeutic decision making in clinical practice if prospectively validated.
Acknowledgement
The investigators were supported by 1K23CA157631 (NCI) (M.N.), Grants 1RO1CA114465-07 (B.E.J.) and 5R21 CA11627-02 (H.H.) from the National Institutes of Health, Grant No. 2P50CA090578-10 (B.E.J.) from the National Cancer Institute Specialized Program of Research Excellence in Lung Cancer, and a grant from Genentech Inc, as well as by the Doris and William Krupp Research Fund in Thoracic Oncology, the Gallup Fund in Thoracic Oncology, and American Society of Clinical Oncology Translational Research Professorship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest:
Mizuki Nishino: Consultant, Bristol-Myers Squibb, Grant from Canon Inc
Suzanne E. Dahlberg, Linnea E. Fulton, Subba R. Digumarthy: Nothing to disclose
Hiroto Hatabu: Grants from Toshiba Medical, AZE Ltd, Canon Inc.
Bruce E. Johnson: Consultant: AstraZeneca, Genentech, GE Healthcare, Ariad, Novartis, Synta, Chugai, Teva, Puma, Transgenomic, Genzyme; Expert Testimony: Expert witness for Genetech; Stock Ownership: KEW Group; Other: DFCI post marketing patent received for EGFR testing
Lecia V. Sequist: Non-compensated consulting for Boehringer-Ingelheim, Clovis, AstraZeneca, Novartis, Genentech, Merrimack, Taiho
REFERENCES
- 1.Kris MG, Johnson BE, Berry LD, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA. 2014;311:1998–2006. doi: 10.1001/jama.2014.3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nishino M, Hatabu H, Johnson BE, McLoud TC. State of the art: Response assessment in lung cancer in the era of genomic medicine. Radiology. 2014;271:6–27. doi: 10.1148/radiol.14122524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 4.Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 5.Pao W, Miller V, Zakowski M, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A. 2004;101:13306–13311. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12:735–742. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 7.Yang JKD, Kim D, Planchard D, et al. Updated safety and efficacy from a phase I study of AZD9291 in patients (pts) with EGFR-TKI-resistant non-small cell lung cancer (NSCLC). ESMO. 2014 [Google Scholar]
- 8.Sequist LV, Martins RG, Spigel D, et al. First-line gefitinib in patients with advanced non-small-cell lung cancer harboring somatic EGFR mutations. J Clin Oncol. 2008;26:2442–2449. doi: 10.1200/JCO.2007.14.8494. [DOI] [PubMed] [Google Scholar]
- 9.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 10.Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 11.Jackman DM, Yeap BY, Sequist LV, et al. Exon 19 deletion mutations of epidermal growth factor receptor are associated with prolonged survival in non-small cell lung cancer patients treated with gefitinib or erlotinib. Clin Cancer Res. 2006;12:3908–3914. doi: 10.1158/1078-0432.CCR-06-0462. [DOI] [PubMed] [Google Scholar]
- 12.Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–246. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 13.Sequist LV, Waltman BA, Dias-Santagata D, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med. 2011;3:75ra26. doi: 10.1126/scitranslmed.3002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nishino M, Guo M, Jackman DM, et al. CT tumor volume measurement in advanced non-small-cell lung cancer: Performance characteristics of an emerging clinical tool. Acad Radiol. 2011;18:54–62. doi: 10.1016/j.acra.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nishino M, Jagannathan JP, Krajewski KM, et al. Personalized tumor response assessment in the era of molecular medicine: cancer-specific and therapy-specific response criteria to complement pitfalls of RECIST. AJR Am J Roentgenol. 2012;198:737–745. doi: 10.2214/AJR.11.7483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Erasmus JJ, Gladish GW, Broemeling L, et al. Interobserver and intraobserver variability in measurement of non-small-cell carcinoma lung lesions: implications for assessment of tumor response. J Clin Oncol. 2003;21:2574–2582. doi: 10.1200/JCO.2003.01.144. [DOI] [PubMed] [Google Scholar]
- 17.Mozley PD, Bendtsen C, Zhao B, et al. Measurement of tumor volumes improves RECIST-based response assessments in advanced lung cancer. Transl Oncol. 2012;5:19–25. doi: 10.1593/tlo.11232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mozley PD, Schwartz LH, Bendtsen C, Zhao B, Petrick N, Buckler AJ. Change in lung tumor volume as a biomarker of treatment response: a critical review of the evidence. Ann Oncol. 2010;21:1751–1755. doi: 10.1093/annonc/mdq051. [DOI] [PubMed] [Google Scholar]
- 19.Zhao B, James LP, Moskowitz CS, et al. Evaluating variability in tumor measurements from same-day repeat CT scans of patients with non-small cell lung cancer. Radiology. 2009;252:263–272. doi: 10.1148/radiol.2522081593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao B, Schwartz LH, Moskowitz CS, Ginsberg MS, Rizvi NA, Kris MG. Lung cancer: computerized quantification of tumor response--initial results. Radiology. 2006;241:892–898. doi: 10.1148/radiol.2413051887. [DOI] [PubMed] [Google Scholar]
- 21.Nishino M, Dahlberg SE, Cardarella S, et al. Tumor volume decrease at 8 weeks is associated with longer survival in EGFR-mutant advanced non-small-cell lung cancer patients treated with EGFR TKI. J Thorac Oncol. 2013;8:1059–1068. doi: 10.1097/JTO.0b013e318294c909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Looney WB, Trefil JS, Hopkins HA, Kovacs CJ, Ritenour R, Schaffner JG. Solid tumor models for assessment of different treatment modalities: therapeutic strategy for sequential chemotherapy with radiotherapy. Proc Natl Acad Sci U S A. 1977;74:1983–1987. doi: 10.1073/pnas.74.5.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Demicheli R, Foroni R, Ingrosso A, Pratesi G, Soranzo C, Tortoreto M. An exponential-Gompertzian description of LoVo cell tumor growth from in vivo and in vitro data. Cancer Res. 1989;49:6543–6546. [PubMed] [Google Scholar]
- 24.Gomez-Roca C, Koscielny S, Ribrag V, et al. Tumour growth rates and RECIST criteria in early drug development. Eur J Cancer. 2011;47:2512–2516. doi: 10.1016/j.ejca.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 25.Stein WD, Gulley JL, Schlom J, et al. Tumor regression and growth rates determined in five intramural NCI prostate cancer trials: the growth rate constant as an indicator of therapeutic efficacy. Clin Cancer Res. 2011;17:907–917. doi: 10.1158/1078-0432.CCR-10-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stein WD, Wilkerson J, Kim ST, et al. Analyzing the pivotal trial that compared sunitinib and IFN-alpha in renal cell carcinoma, using a method that assesses tumor regression and growth. Clin Cancer Res. 2012;18:2374–2381. doi: 10.1158/1078-0432.CCR-11-2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferte C, Fernandez M, Hollebecque A, et al. Tumor growth rate is an early indicator of antitumor drug activity in phase I clinical trials. Clin Cancer Res. 2014;20:246–252. doi: 10.1158/1078-0432.CCR-13-2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nishino M, Dahlberg SE, Cardarella S, et al. Volumetric tumor growth in advanced non-small cell lung cancer patients with EGFR mutations during EGFR-tyrosine kinase inhibitor therapy: developing criteria to continue therapy beyond RECIST progression. Cancer. 2013;119:3761–3768. doi: 10.1002/cncr.28290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nishino M, Cardarella S, Dahlberg SE, et al. Radiographic assessment and therapeutic decisions at RECIST progression in EGFR-mutant NSCLC treated with EGFR tyrosine kinase inhibitors. Lung Cancer. 2013;79:283–288. doi: 10.1016/j.lungcan.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nishino M, Cardarella S, Jackman DM, et al. RECIST 1.1 in NSCLC patients with EGFR mutations treated with EGFR tyrosine kinase inhibitors: comparison with RECIST 1.0. AJR Am J Roentgenol. 2013;201:W64–71. doi: 10.2214/AJR.12.9668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nishino M, Jackman DM, DiPiro PJ, Hatabu H, Janne PA, Johnson BE. Revisiting the relationship between tumour volume and diameter in advanced NSCLC patients: An exercise to maximize the utility of each measure to assess response to therapy. Clin Radiol. 2014;69:841–848. doi: 10.1016/j.crad.2014.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gold KA, Kim ES, Lee JJ, Wistuba, Farhangfar CJ, Hong WK. The BATTLE to personalize lung cancer prevention through reverse migration. Cancer Prev Res (Phila) 2011;4:962–972. doi: 10.1158/1940-6207.CAPR-11-0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- 34.McKeage K. Alectinib: A Review of Its Use in Advanced ALK-Rearranged Non-Small Cell Lung Cancer. Drugs. 2014 doi: 10.1007/s40265-014-0329-y. [DOI] [PubMed] [Google Scholar]
- 35.Sato T, Watanabe A, Kondo H, et al. Long-term results and predictors of survival after surgical resection of patients with lung cancer and interstitial lung diseases. J Thorac Cardiovasc Surg. 2015;149:64–69, 70 e61-62. doi: 10.1016/j.jtcvs.2014.08.086. [DOI] [PubMed] [Google Scholar]
- 36.Kato M, Shukuya T, Takahashi F, et al. Pemetrexed for advanced non-small cell lung cancer patients with interstitial lung disease. BMC Cancer. 2014;14:508. doi: 10.1186/1471-2407-14-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Camidge DR, Bang YJ, Kwak EL, et al. Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. Lancet Oncol. 2012;13:1011–1019. doi: 10.1016/S1470-2045(12)70344-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363:1693–1703. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368:2385–2394. doi: 10.1056/NEJMoa1214886. [DOI] [PubMed] [Google Scholar]
- 40.Seto T, Kiura K, Nishio M, et al. CH5424802 (RO5424802) for patients with ALK-rearranged advanced non-small-cell lung cancer (AF-001JP study): a single-arm, open-label, phase 1-2 study. Lancet Oncol. 2013;14:590–598. doi: 10.1016/S1470-2045(13)70142-6. [DOI] [PubMed] [Google Scholar]
- 41.Park KAM, Ahn MJ, Yu CJ, et al. ASPIRATION: first-line erlotinib until and beyond RECIST progression in Asian patients with EGFR mutation-positive NSCLC. ESMO. 2014 [Google Scholar]
- 42.Soria JC, Wu YL, Nakagawa K, et al. Gefitinib plus chemotherapy versus placebo plus chemotherapy in EGFR-mutation-positive non-small-cell lung cancer after progression on first-line gefitinib (IMPRESS): a phase 3 randomised trial. Lancet Oncol. 2015;16:990–998. doi: 10.1016/S1470-2045(15)00121-7. [DOI] [PubMed] [Google Scholar]
- 43.Kim HS, Kim JH. Ideal number of target lesions per organ to measure in metastatic colorectal cancer. Oncol Lett. 2014;8:1896–1900. doi: 10.3892/ol.2014.2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim HS, Kim JH, Yang I. Tumor response assessment by measuring the single largest lesion per organ in patients with advanced non-small cell lung cancer. Lung Cancer. 2014;85:385–389. doi: 10.1016/j.lungcan.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 45.Kim HS, Kim JW, Kim JH, et al. Single-Lesion Measurement per Organ for Assessing Tumor Response in Advanced Gastric Cancer. Oncology. 2014;88:69–75. doi: 10.1159/000367810. [DOI] [PubMed] [Google Scholar]
- 46.Nishino M, Jagannathan JP, Ramaiya NH, Van den Abbeele AD. Revised RECIST guideline version 1.1: What oncologists want to know and what radiologists need to know. AJR Am J Roentgenol. 2010;195:281–289. doi: 10.2214/AJR.09.4110. [DOI] [PubMed] [Google Scholar]


