Abstract
High-throughput techniques are needed to analyze individual virions to understand how viral heterogeneity translates into pathogenesis since in bulk analysis the individual characteristics of virions are lost. Individual Dengue virions (DENV) undergo a maturation that involves a proteolytic cleavage of prM precursor into virion-associated M protein. Here, using a new nanoparticle-based technology, “flow virometry”, we compared the maturation of individual DENV produced by BHK-21 and LoVo cells. The latter lacks the furin-protease that mediates prM cleavage. We found that prM is present on about 50% of DENV particles produced in BHK-21 cells and about 85% of DENV virions produced in LoVo, indicating an increase in the fraction of not fully matured virions. Flow virometry allows us to quantify the number of fully mature particles in DENV preparations and proves to be a useful method for studying heterogeneity of the surface proteins of various viruses.
Keywords: Dengue, Maturation, Flow virometry, prM
Introduction
Viruses pursue different strategies in their infection of the host: while some of them, for example hepatitis A, relies on exact reproduction of their antigenic spectra, others, like HIV are antigenically heterogeneous even when they are produced in homogeneous cell lines [1] or the virus has been cloned [2]. While the characterization of viral preparations by most of the existing bulk techniques effectively describes the composition and properties of an average virion in the preparation, little is known about the variability between virions. Since for enveloped viruses their surface proteins mediate viral interactions with their cellular targets, this heterogeneity may impact biological properties of viruses. Recently, we developed a new technique, “flow virometry” that allows us to analyze the antigenic composition of individual virions [1]. In this study we focus on the surface protein composition of individual DENV, an important human pathogen [3] that is well characterized structurally [4, 5].
DENV is a positive single-stranded RNA Flaviviridae. Its 11Kb genome encodes a single large polyprotein, which is subsequently cleaved into three structural and seven non-structural proteins that are essential for viral replication. The structural proteins are capsid (C), prM/membrane (prM/M) and envelope (E) [6]. The E protein contains three distinct domains, the structurally central domain DI, the dimerization domain DII, which contains the fusion loop, and the immunoglobulin-like carboxy terminal domain DIII involved in the attachment to host cell receptors [7].
DENV and the other flaviviruses assemble in the ER as immature viruses [5, 8]. The mildly acidic conditions of the trans-Golgi network promote virus maturation, in which the E protein changes its conformation allowing the cleavage of prM into pr and M by a furin-like protease [9-12]. The pr portion of prM covers the DII domain of E, preventing premature viral fusion or inactivation in the acidic intracellular compartments through which virions traffic [13]. Similarly to other viruses, an infectious DENV preparation contains immature virions [14]. However, it remains to be understood whether there are any virions that are fully matured, i.e., in which all prM molecules are cleaved, or all the virions are either fully immature with all prM molecules in uncleaved form or “mosaic”, with the same virions carrying both M protein and prM. To answer this question, it is necessary to analyze these proteins on the surface of single virions.
Towards this goal, here, we adapted a high throughput flow virometry technique [1] to characterize the degree of maturation of individual virions in a preparation of infectious DENV. Briefly, flow virometry of DENV includes five main steps: (i) labeling DENV with a fluorescent lipidic dye DiI and then with fluorescent 2H2 antibodies that reveal prM protein; (ii) capturing labeled virions with 15 nm MNPs coupled with 3H5-1 antibodies against E protein; (iii) separating the DENV-MNPs complexes from unbound antibodies using magnetic columns; (iv) eluting DENV-MNPs complexes from the columns, and (v) analyzing the eluted complexes with a flow cytometer. We found that in DENV population produced by BHK-21 cells approximately half of virions are fully mature as no prM could be detected on their surface. In contrast, in DENV population produced in furin-deficient LoVo cells, such a mature fraction constitutes only ~ 15%. The high throughput flow virometry applied here to DENV can be further used for the detailed characterization of the heterogeneity of envelope proteins of various viruses and may prove important in the development of vaccine against DENV.
Results
For this flow virometry study we defined DENV as a membrane particle that carries E protein. Therefore, to reveal DENV we first labeled our preparation with a fluorescent lipidic dye DiI (adapted from [15]) and then stained virions for the presence of prM with fluorescently labeled 2H2 anti-prM antibodies [16]. To capture DiI fluorescent particles that carry E protein we used MNPs coupled with 3H5-1 antibodies [17] specific for the DIII domain of E protein. The DENV–MNPs complexes were separated from free antibodies on magnetic columns: due to the magnetic properties of MNPs these complexes were retained while free antibodies were not. Preparations of DENV were analyzed with a flow cytometer triggered on viral fluorescence.
Visualization of single particles
To verify that the events registered in flow analysis represent single virions rather than their aggregates, we first analyzed in a flow cytometer free DiI-labeled DENV (Fig.1) prepared as described in Methods.
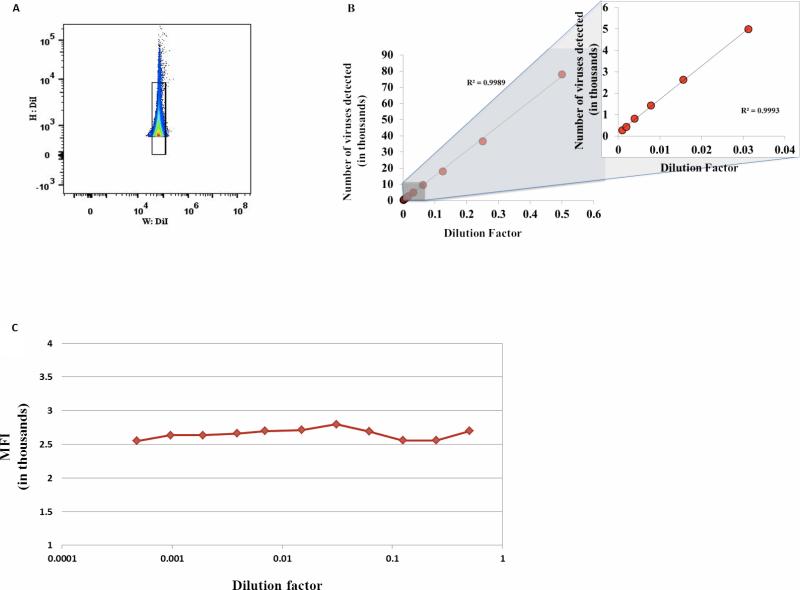
Figure 1. Analysis of individual virions.
DiI-stained DENV preparation was serially diluted two fold from 1:2 to 1:2048. The events were acquired in a fixed volume of 160 μl in duplicate using a High Throughput Sampler (HTS) at a flow rate of 0.5 μl per second on a LSRII flow cytometer, set to be triggered by DiI fluorescence. (A) Gating strategy to exclude aggregates. A singlet gate was defined by plotting fluorescence height versus fluorescence width. This gate excludes events with a high width and high height that represent aggregates. (B) Number of particles as a function of the dilution factor. DiI-labeled virions were serially diluted and the number of events for each dilution was evaluated by flow cytometry based on volumetric control. Right panel is a blow up of the left part of the X axis. (C) Median fluorescence intensity (MFI). DiI-labeled DENV was serially diluted with a factor two and MFI was evaluated for each dilution.
Note that the number of events is inversely linearly dependent on the number of dilutions and the MFI is constant in the large range of dilutions suggesting that the events correspond to single particles detection and not to swarm detection.
In general, while the number of single particles measured by flow cytometry depends linearly on the dilution of the preparation, aggregates or coincident detection do not show a linear relation with concentration [18]. To distinguish between DENV aggregates and single virions, we diluted our preparation in serial two-fold dilutions and evaluated the relation between event frequencies and dilution factors. We excluded aggregates from our analysis by the gating strategy presented in Fig.1A. Then we plotted the number of single events as a function of the dilution factor (Fig.1B). There is a linear relation between the number of events and the dilution factor. Also, the mean fluorescence intensity (MFI) of events did not change over the four orders of magnitude of the dilution series, and was on average 2679.6 ± 29 arbitrary fluorescence units, with a coefficient of variation (CV) of 5.07% (Fig. 1C).
Together, these results suggest that, in our analysis, the detected events correspond predominantly to individual particles [19]. Since antibodies may cause viral aggregation, we evaluated whether 2H2 Alexa Fluor 647 antibody or 3H5-1 Alexa Fluor 488 coupled MNPs cause aggregation. In both cases, the number of events detected by flow cytometry was inversely linearly dependent on dilution factors indicating the lack of a significant aggregation under our protocol (Fig.2A-C).
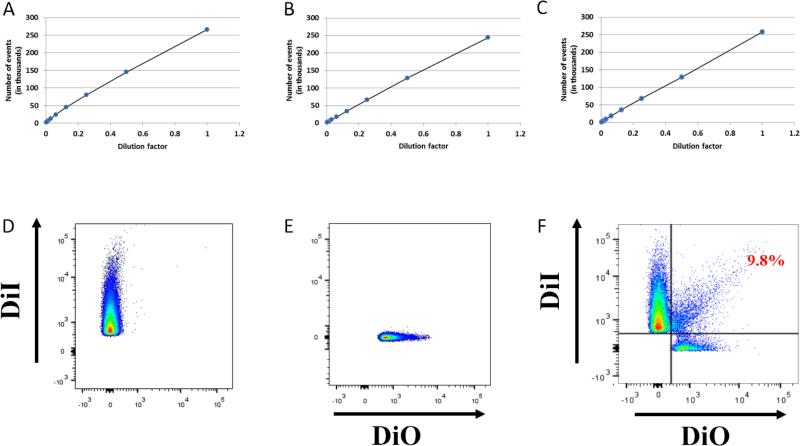
Figure 2. Antibodies against DENV do not induce virus aggregation.
DENV stock was labeled with DiI and treated or not with 2H2 antibodies or 3H5-1-MNPs. The viral preparation was then serially diluted two fold to 1:256. (A) DiI labeled DENV. (B) DiI DENV stained with 2H2 Alexa Fluor 647 antibody. (C) DiI DENV-captured by 3H5-1 Alexa Fluor 488 labeled MNPs. The events were acquired with HTS at a flow rate of 0.5 μl per second on the LSRII flow cytometer, set to be triggered by DiI fluorescence.
Single particle detection. Two preparations of DENV, one stained with DiI (D) and the other with DIO (E). The two preparations were mixed and the virions were captured by 3H5-1-MNPs and analyzed (F). Of the events recorded, approximately 10% appeared to be double positive. Thus, approximately 90% of events represent individual DENV virions.
Note that the number of events is inversely linearly dependent on the number of dilutions independently of the presence of the antibodies. Thus, the events correspond to single particles and not to aggregates.
Also to evaluate the level of aggregation we performed the following experiment: we divided our preparation in two parts and stained one with DiI and another with DiO, mixed these two fractions and performed our standard capture experiment. Aggregates, especially containing many particles, should be predominantly of two colors. This experiment (Fig.2 D-F) demonstrated that more than 90% of the flow events represent single captured virions.
Capture of virions on MNPs
We incubated 1×107 purified DiI-stained particles with 6.4×1011 15 nm MNPs coupled with 3H5-1 antibodies labeled with Zenon Alexa Fluor 488. The DENV-MNPs complexes were separated from unbound viral particles and from unbound antibodies on magnetic columns. We found that on average 98.35 ± 0.42% (n=8) of all DiI-stained particles for BHK-21 and 97.35 ± 0.46% (n=4) for LoVo were captured by 3H5-1 MNPs labeled with Alexa Fluor 488 and therefore constitute bona fide DENV particles (Fig.3). As control for the specificity of our capture, we used 15 nm MNPs coupled with Mouse IgG labeled with Alexa Fluor 488. We found that with these non-specific MNPs, we captured less than 0.5% of DENV that we captured with specific 3H5-1-MNPs (Fig.4) in similar conditions.
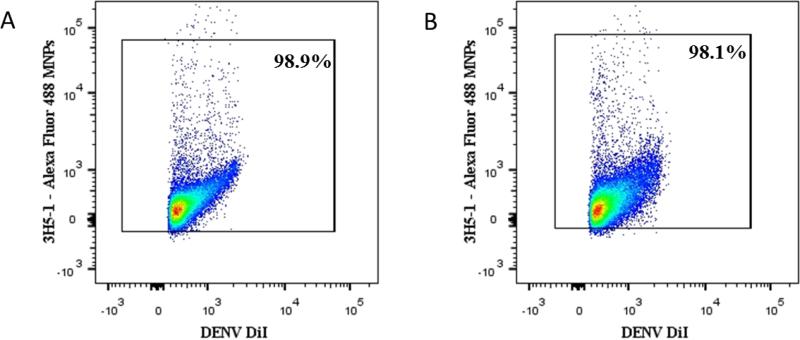
Figure 3. Detection of DENV virions from BHK-21 and LoVo cells.
Virions produced either by BHK-1 or LoVo cells were incubated with Alexa Fluor 488 3H5-1- MNPs, separated on magnetic columns and analyzed in a LSRII flow cytometer using the same settings. (A) DiI-labeled DENV from BHK-21 captured by fluorescent 3H5-1- MNPs (B) DiI-labeled DENV from LoVo cells captured by fluorescent 3H5-1- MNPs.
Presented is one typical experiment out of four.
Note that in both preparations we capture with virus-specific antibodies between 98 and 99% of DiI-labeled particles.
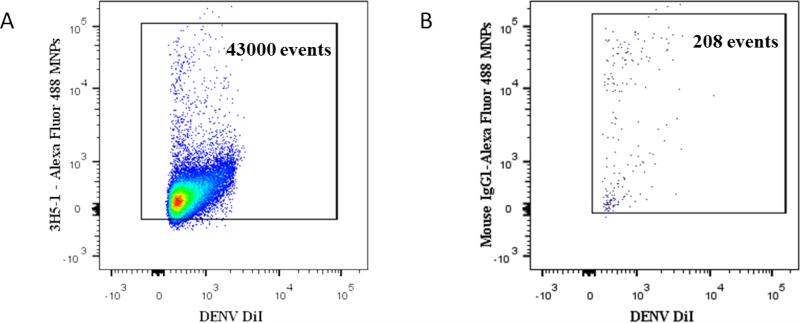
Figure 4. Specificity of capture of DENV with 3H5-1-MNPs.
DiI-stained DENV preparation was divided in two parts and virions in the first half were captured via E protein with Alexa Fluor 488 labeled 3H5-1-MNPs; virions in the second half of the preparation were incubated with non-specific Alexa Fluor 488-labeled Mouse IgG1-MNPs. Both preparations were separated in magnetic field and acquired using the same settings on LSRII flow cytometer. (A) A bivariate plot of flow analysis of virions. Specific DENV capture (43000 events correspond to 98.5% of virions). (B). Non-specific capture.
A typical experiment out of three.
Note that non-specific MNPs capture less than 0.5% of the virions captured with specific MNPs.
The efficiency of capture was evaluated also with real time PCR (RT-PCR). While in the input preparation there where approximately 1×107 DENV RNA copies/ml, in the flow-through fraction there were less than 4×104 DENV RNA copies/ml, thus with our method we capture more than 98% of viruses.
Characterization of virion maturity with flow virometry
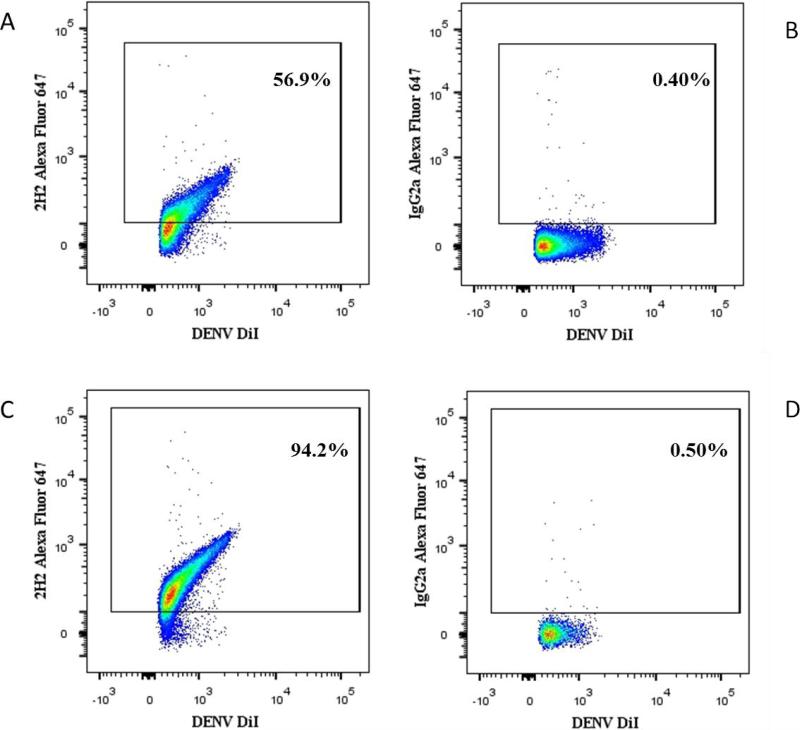
DENV virions in viral suspension were stained with DiI, incubated with Alexa Fluor 647-labeled 2H2 anti-prM antibodies (and their respective isotype controls) and captured with Zenon Alexa Fluor 488-labeled 3H5-1-MNPs. DENV-MNPs complexes were purified on magnetic column and analyzed with the flow cytometer. In the viral population produced by BHK-21 cells, on average 48.16 ± 5.35% (n=8) of DENV virions (DiI+/3H5-1+) were positive for the presence of prM as evaluated with the anti-prM antibody (Fig.5A). In viral population produced by LoVo cells, the size of this fraction was larger with prM-positive virions representing 84.5 ± 3.4% (n=4) of all captured virions (Fig.5C). The difference between mature and immature particles produced by BHK-21 and LoVo cells is significant with p=0.0005. Respectively 51.8 ± 5.3% (n=8) and 15.5 ± 3.4% (n=4) (p=0.0005) of the captured DENV were prM negative and thus can be classified as fully mature virions. The specificity of this staining protocol was confirmed by using isotype control antibodies (Fig.5B, D).
Figure 5. Maturation state of DENV virions.
DiI labeled DENV produced either in BHK-21 or in LoVo cells were stained for prM protein with Alexa Fluor 647-labeled 2H2 or with its isotype control IgG2a, then captured with Alexa Fluor 488 3H5-1-MNPs (A) DENV produced in BHK-21 cells, stained with specific 2H2 antibody. (B) Isotype control to A. (C) DENV produced in LoVo cells, stained with specific 2H2 antibody. (D) Isotype control to C.
A representative experiment out of four.
Note, a higher incidence of prM-positive virions produced in furin-deficient LoVo cells.
Discussion
DENV carries on its surface 180 copies of the envelope (E) glycoprotein responsible for cell attachment and fusion to the plasma membrane and 180 copies of the structural membrane (M) protein [20]. Viral maturation involves the cleavage of the prM precursor into M protein and pr peptide. In this work we investigated the antigenic composition of individual virions to reveal their maturation state by quantifying mature viral particles (virions that do not carry prM) and not fully mature (or completely immature) virions (carrying prM). Towards this goal we used flow virometry originally developed for the analysis of individual HIV virions [1] and extracellular vesicles (EVs) [21].
We collected DENV virions from supernatant of infected BHK-21 and LoVo cells and labeled them with a fluorescent lipidic dye DiI. The latter was separated from stained particles in a discontinuous density gradient. As a result, we obtained a preparation of fluorescent membrane particles released by DENV infected cells. To identify DENV virions among other membrane particles, we captured them with fluorescently labeled Zenon Alexa Fluor 488 3H5-1-MNPs, specific for the E protein of DENV. Practically all membrane particles isolated in our virus purification protocol based on Optiprep gradient carried E protein and thus, within our definition, represent DENV particles. In a direct flow analysis of this preparation it would be difficult to distinguish virions from antibodies (or their aggregates) by size or by fluorescence making impossible to attribute any detected fluorescent signal to labeled viruses or to free floating antibodies occupying the cytometer interrogation chamber. Therefore, it was critical to separate them physically before the flow analysis. Towards this goal, we run the preparation in magnetic column. Such a separation removes free antibody almost entirely [21]. Moreover, even if a small amount of fluorescent antibodies still contaminates the final preparation, it would not interfere with the analysis as DENV are revealed by two labels, DiI envelope label and the label of the capturing MNPs.
Next, we optimized the capture efficiency by testing various MNPs to virions ratios. In agreement with the capture of HIV-1 [1] we found that a ratio of MNP:DENV of ~105 was optimal and at this ratio we capture about 98% of virions. The excess of MNPs was similar to the excess of fluorescent antibodies for staining cells. The efficiency of capture was confirmed by a real-time PCR assay: there was less than 0.5% of DENV RNA in the “flow through” fraction, demonstrating that all the DENV genomes were captured.
After the development of the efficient capture, we had to prove that (i) this process was specific and, importantly, (ii) that we monitored individual virions (otherwise our assay would be another bulk analysis at which individual characteristics of virions are lost).
The specificity of DENV capture was evident from the comparison of the number of complexes captured with MNPs coupled to specific 3H5-1 antibodies with those captured with MNPs coupled to non-specific isotype control MNPs.
Although the viral preparation was filtered through 0.22μm filter, smaller aggregates could potentially contaminate our preparations. To test that the flow events correspond to single particles rather than to these aggregates, we investigated whether there was a linear correlation between the dilution factor and the number of events. We found a strong linear correlation between the number of events and dilutions over the range of 2048 fold dilutions. Also, the MFI of DENV DiI population did not change over the four logs of the sample dilution series. These were two criteria for the lack of aggregation (or swarms) of DENV in our preparation [18, 19]. However, if the aggregates were stable, their numbers upon dilution would still follow a linear relation with the dilution factor.
Therefore, we performed additional experiments to evaluate the number of aggregates in our captured preparation by mixing DENV stained with two different colors and capturing their mixture with anti-3H5-1 MNPs. We found that fewer than 10% of events were multicolor aggregates. This data are in agreement with the aggregation data of HIV [1].
Staining with lipidic dyes alone would not distinguish viruses from exosomes and impurities, additional staining is needed to identify DENV particles. Here, we defined a particle to be a DENV virion if and only if it is stained by DiI and is positive for 3H5-1 antibodies against E protein (Fig. 3). Of course, microvesicles that have captured viral proteins may also be included into our analysis. However such vesicles are essentially not different from defective or non-infectious viruses.
In this work we stained DiI-labeled DENV for prM with 2H2 antibodies, captured them with MNPs coupled to anti-E antibodies 3H5-1, and isolated MNP-captured stained virions in a magnetic field. 3H5-1 is maturation insensitive since it binds on the positions 383 – 385 of the DENV E protein, which is present on the surface of all the viral particles independently of their maturation state [22]. In contrast, 2H2 is an antibody that does not bind to mature M protein since it recognizes specifically prM protein [12]. For the capture not to interfere with staining, we first incubated virions with 2H2 antibodies and then capture virions with 3H5-1 MNPs. Although a small reduction in binding of 3H5-1 in presence of 2H2 was reported [23], with our protocol we captured around 98% of virions.
Finally, to test whether our technique revealed the state of maturation of DENV we compared two DENV preparations: one produced in BHK-1 cells and another in LoVo cells. The latter are furin-deficient and thus should produce more immature virions as furin is required for prM cleavage and therefore for maturation process [24]. Our analysis revealed that ~ 55% of the E-carrying virions produced in BHK-21 cells and ~ 15% produced in LoVo cells do not carry detectable amount of prM. The remaining ~ 45% of DENV virions produced in BHK-21 and ~ 85% produced in LoVo cells carry prM and thus represent a mixture of immature and partially mature (mosaic) virions. The existence of mosaic flaviviruses has been documented in earlier electron microscopy studies that revealed co-existing smooth and spiky elements on viral surface [14, 26].
These conclusions were based on experiments with preparations of DENV produced by different cell cultures. The number of DENV positive for 2H2 in viral preparations produced by different cell cultures varied more than that positive for 3H5-1. However, for both antibodies the results were reproducible with CV ~ 1% for 3H5-1, and 31.4% and 8% for 2H2 positive DENV produced respectively by BHK-21 and LoVo cells.
Why do furin- deficient cells still produce mature virions? Since furin belongs to a family of the subtilisin-like serine endoproteases it is conceivable that different members of this family overlap in their substrate specificities and intracellular localizations. Some proteases of this family including C5/PC6 and PC7/PC8 (both known, as furin, to reside in the trans-Golgi network) are expressed in the furin-deficient LoVo cells [25]. We hypothesize that these or other furin-like proteases are responsible for a residual low efficiency cleavage of prM observed.
Although, in the case of DENV it is difficult to evaluate the absolute sensitivity of our measurements, in the case of HIV-1 with flow virometry using similarly labeled antibodies against HIV surface protein gp120 it was possible to reveal at least three of these proteins.
While with our technology it is possible to evaluate the difference in maturity state of different preparations of virions, this technology has several limitations. First, highly specific antibodies against viral surface antigens are needed. Second, the antigens should be accessible to the antibodies on the intact viral particles. Third, different antibodies should not interfere with each other in binding to the antigens. Future development may show other limitations of this technology not revealed in the present study.
In summary, our analysis of antigens on individual virions showed that our DENV preparation contains some fully mature particles lacking prM and a larger fraction of prM-containing virions. Contributions of fully immature and mosaic virions to infection are yet to be fully clarified. Fully immature virions produced by furin-negative cells were reported to be completely non-infectious [12]. However, anti-prM antibodies (which facilitate viral attachment to the cell surface) facilitate infection by fully immature DENV particles [27] and prM-containing virions have been found to infect immature dendritic cells, probably through DC-SIGN [28]. Future development of DENV flow virometry may reveal how many M proteins on an individual virion render it to be infectious for particular cells.
Flow virometry earlier applied to the analysis of single HIV virions and used here for analysis of single DENV virions seems to be a universal method for analysis of heterogeneity of different viruses that allows quantification of virions carrying particular surface proteins. Their physical separation will allow us to correlate the antigenic composition of virions with their biological functions.
Methods
Isolation of DENV 2 from supernatants of BHK-21 and LoVo cells
DENV 2 (New Guinea C strain) was purchased from ATCC (Manassas, VA) and then produced in our laboratory in BHK-21 and LoVo cells at 80% of confluence in T75 flasks. These cells were grown in 20 ml of DMEM supplemented with 10% FBS. For the infection 0.01% of Pluronic F-127 (Invitrogen, Life Technologies, Grand Island, NY) and 25 mM Tricine (Boston BioProducts, Ashland, MA) were added to the medium adjusted at pH 7.8 since DENV is a pH dependent virus. The cells were washed with PBS then inoculated with virus in 3 ml of medium at MOI of 0.5 and the flask was rocked to distribute the inoculum, followed by an incubation for 10 minutes at room temperature to maximize viral absorption. Then, 17 ml of medium were added to the flask, which was then incubated at 37°C, 5% CO2, 80% relative humidity. After 2 days, the supernatants were collected and filtered with a 0.22 μm syringe filter, pre-wetted with PBS 0.2% BSA. The obtained viral stocks was kept at 4°C (for not more than 3 months) and used for flow virometry.
Labeling with DiI and purification of DENV 2 from supernatants of BHK-21 and LoVo cells
For the labeling of DENV with the lipophilic tracer DiI (Invitrogen, Life Technologies), the viral preparations were incubated with 0.01% Pluronic F-127 and 1μM DiI in the dark for 30 minutes at room temperature. For the Optiprep (Sigma, St. Louis, MO) discontinuous gradient, a 10× buffer was prepared with 20% NaCl 5 M + 20% Tricine 1 M (pH 7.8) + 0.01% Pluronic F-127 + 60% deionized water. Then four gradient solutions, 10%, 20%, 25% and 35% of Optiprep in deionized water were prepared using 10% buffer 10×. The solutions were mixed and vortexed. After incubation, the solutions were added in two 4 ml Seton open top tubes (Thermo Scientific, Asheville, NC) to form a discontinuous gradient starting from 35% to 10%. 1 ml of virus (~ 108 viral particles/ml) with 10% buffer 10× and 5% Optiprep solution was overlaid in each gradient tube. The tubes were centrifuged at 240,000g for 1.5 h at 4°C with no brake. After centrifugation the DENV fraction between 20% and 25% Optiprep layers was recovered in an eppendorf tube. The biggest viral aggregates were removed with a 0.22 μm filter (Millipore, Waltham, MA) with centrifugation of 2,000g for 5 minutes at 4°C. The viral particles were visualized on FACS as DiI positive events thresholding on DiI fluorescence.
Preparation of Alexa Fluor 647 2H2 detection antibody and its isotype control
Labeling of monoclonal antibodies was performed according to the Invitrogen protocol. 1 M solution of sodium bicarbonate at pH ~8.3 was prepared adding 1ml of deionized water to 84 mg of sodium bicarbonate powder. Then 10 μl of this solution were added in a vial of 100 μl (1 mg/ml) of IgG2a 2H2 monoclonal antibody (Millipore) against prM protein and mouse anti-human IgG2a isotype control (Biolegend, San Diego, CA). Then 2H2 and its isotype control were added in two different Alexa Fluor 647 carboxylic acid vials (Invitrogen, Life Technologies), mixed well and incubated in rotation for 1h at room temperature in the dark. During the incubation two Zeba spin desalting columns (Thermo Scientific) were washed with PBS two times to remove the storage solution. Then 2H2 Alexa Fluor 647-labeled and isotype control Alexa Fluor 647-labeled antibodies were added to the column and were centrifuged at 2,000g for 3 minutes. The antibodies were recovered in the flow through and stored at 4°C.
Next, we calculated the number of fluorophores per antibody molecule (the F:P, fluorochrome/protein ratio) as described in the Invitrogen Antibody Labeling Kit by measuring the adsorbance of the labeled antibodies on a Nanodrop 1000 (Thermo Scientific). On average there were 6.5 fluorophores of Alexa Fluor 647 per molecule of 2H2.
Preparation of 3H5-1 15 nm MNPs
1 mg of 15 nm carboxyl-terminated magnetic iron oxide nanoparticles (Ocean NanoTech, Springdale, AZ) was coupled to IgG1 mouse monoclonal antibodies against DENV-2 (New Guinea C) E protein (Millipore) following the Ocean Nanotech protocol for conjugation. Briefly, 200 μl of MNPs (1 mg) were aliquoted in an eppendorf tube with 100 μl Activation Buffer and 100 μl EDAC/NHS solution for 10 minutes at room temperature with continuous mixing. After activation, 400 μl Coupling Buffer were added, mixed well, and immediately followed by the addition of 1mg of the purified 3H5-1 antibody (Biovest International Inc., Minneapolis, MN). After 2 hours coupling with continuous mixing at room temperature the reaction was stopped by adding 10 μl of Quenching Buffer and transferred to a 12×75 mm FACS tube. After an overnight incubation in a SuperMAG-01 magnetic separator (Ocean NanoTech) at 4°C the MNPs were washed with Washing Buffer and left again in SuperMAG-01 magnetic separator overnight at 4°C and another wash was performed the day after. Finally, the 3H5-1 coupled MNPs were resuspended in 2 ml of Storage Buffer and stored at 4°C.
Titration of MNPs on DENV DiI particles
To optimize viral capture, we performed this procedure at different MNP/virus ratios. We mixed 3.5×105 viral particles, as calculated based on real time PCR (RT-PCR) of viral genomes with MNPs at concentrations ranging from 3.7 × 105 to 3.7 × 1013 particles/ml in buffer (PBS + 2% mouse serum + 0.5% BSA + 2 mM EDTA) followed by purification of DENV-MNPs complexes on a magnetic column. After two washes, the column was detached from the magnet and the DENV-MNPs complexes were eluted, analyzed, and quantified by flow virometry using the DiI fluorescence as a trigger parameter. To minimize the variability in sample acquisition and to quantify the number of events in our preparation we acquired a fixed volume (160 μl) of eluted samples in duplicate using the High Throughput Sampler (HTS) at a flow rate of 0.5 μl per second on the LSRII flow cytometer. This analysis showed that the efficiency of capture dropped as a function of the dilution of the MNPs. When the MNPs stock was diluted more than 105 fold, which corresponds to a MNPs/DENV ratio <0.98, DENV recovery dropped below 1% of the input virus. At higher MNPs/DENV ratios, DENV recovery increased and was between 98 and 99% at a ratio of 9.8×104. For all experiments described hereafter we maintained this MNPs/DENV ratio.
Serial dilutions of the virus alone, with antibodies and with MNPs
Serial two-fold dilutions of the DENV DiI produced in BHK-21 cells were performed to show that the viral count by flow cytometry is inversely linearly dependent on the viral dilutions. The serial dilutions were from 1:2 to 1:2048 folds. Samples were run using a HTS at a flow rate of 0.5 μl per second that was set to acquire 160 μl of each sample using a LSRII flow cytometer with a threshold set at 600 fluorescence units on the viral fluorescence channel. Next, to show that antibodies do not cause aggregation in our system we treated or not DENV with 60 μl of 3H5-1-MNPs or 5 μg of 2H2 Alexa Fluor 647 antibodies and all viral preparations were serially diluted two fold from 1:2 to 1:256. The events were acquired with the same settings on HTS at a flow rate of 0.5 μl per second on the LSRII flow cytometer, set to be triggered by DiI fluorescence.
Capture with MNPs and detection of DENV DiI produced in BHK-21 and LoVo cells
In order to visualize the 3H5-1MNPs complexes, 5 μl of a Zenon Alexa Fluor 488 mouse IgG1 (Life Technologies) were added to the mixture and incubated 30 minutes at room temperature in continuous mixing. 1×107 (60 μl) viral particles were incubated with 5μg of Alexa Fluor 647 labeled 2H2 antibody against prM protein and 1×107 with 5 μg of Alexa Fluor 647 labeled IgG2a isotype control for 30 minutes at room temperature. After 30 minutes the 3H5-1-MNPs complexes were washed to remove free floating Zenon Alexa Fluor 488 Fab fragments in 100K filters (Millipore). Then, in the tubes with labeled virus with specific antibody and its isotype control were added 9.6×1011 (60 μl) of 15nm 3H5-1-MNPs. After 30 minutes of incubation at 37°C with continuous mixing, the DENV-MNPs complexes with bound antibodies were separated from unbound reactants on MS MACS magnetic columns (Miltenyi Biotech, Auburn, CA) mounted on a high field MS MACS magnet (Miltenyi Biotech). The columns were washed 3 times with the washing buffer (PBS + 0.5% BSA + 1mM EDTA) and the complexes were eluted off the magnet in 400μl of PBS and fixed with 1% paraformaldehyde in PBS. These purified complexes and their corresponding flow through fractions were analyzed with a LSRII (BD Biosciences, San Jose, CA) flow cytometer equipped with a blue laser with a power of 200mw and the blue filters are B515: 505DCLP 515/20; B660: 555DCLP 670/14; B710: 685LPXR 710/50 HQ. The violet laser (55mw) has V-450: 450/50; V510: 505DCLP 521E; V-560: 555DCLP 560/40; V-605: 595DCLP 605/40; V-655: 635DCLP 655/20; V-690: 675 DCLP 695/40. The UV laser (25mW) UV-450: 450/50. The red laser (50mW) R670: 675/20; R710: 690DCLP 710/40; R780: 735DCLP 780/60. The green laser (150mW) G585: 575/26; G610: 600LPXR 610/20; G675: 655DCLP 660/40 HQ; G695: 690LPXR 710/50 HQ; G800: 740LPXR 780/40 HQ. The configuration we used was 3B-6V-1UV-3R-5G. The voltages used to run our samples were FSC 288, SSC 205, G585 500, B515 450, R670 520. The background level of fluorescence was evaluated with 0.1 μm filtered PBS and the threshold was set up to the lowest fluorescence channel that did not generate a G585 signal with this solution. Data were acquired using Diva 6.3 and were analyzed with FlowJo software v9.4.9 (Treestar Software, Ashland, OR). Using quantum MESF (Molecules of Equivalent Soluble Fluorochrome) beads (Bangs Laboratories, Inc., Fishers, IN) and the manufacturer protocol, we evaluated the threshold for the fluorescent measurement for the setting of our LSR II, which for Alexa Fluor 488 3H5 antibodies constituted 66 fluorophores (approximatively 10 antibodies with a F:P ratio of 6.5-7.5).
Extraction and quantification of DENV RNA by RT-PCR
A 200 μl aliquot of the MNPs complexes with DENV produced in BHK-21 and LoVo cells and flow through fractions were subjected to nucleic acid extraction using a NucliSENS easyMAG 2.0 instrument (BioMérieux, Durham, NC). RT-PCR was performed with a qScript One-Step Fast MGB qRT-PCR kit (Quanta BioSciences, Gaithersburg, MD) using a one-step assay with the following primer set: DENV 2 NGC (forward): TATGCTGAAACGCGAGAGAAA, DENV 2 NGC (reverse): CTGCAGCATTCCAAGTGAGA, and the probe: FAM- CCG CGT GTC GAC TG TAC AAC AGC -MGB. Amplifications were carried out on a BioRad CFX96 Touch Thermocycler according to the following cycling parameters: 7.5 minutes at 48°C, 30 seconds at 95°C, followed by 45 cycles of 3 seconds at 95°C and 25 seconds at 60°C.
Statistical analysis
Descriptive statistics were calculated with Excel. The results are presented as means ± standard errors of the mean (SEM), and n, the number of replicates, is indicated. CV, the coefficient of variation, was calculated by expressing the standard deviation as % of the mean, group comparisons were performed with the Student t test.
Highlights.
Antigens on single Dengue virions (DENV) were detected with flow virometry
Maturity of virions produced in BHK-21 and furin-deficient LoVo cells were compared
Immature and partially mature virions predominate in LoVo cells-produced DENV
Acknowledgements
We are grateful to Dr. S. Whitehead for providing the stock of DENV. We thank Dr. Kamran Melikov for helpful discussions and Dr. Barry Alpher for correcting the English style of the text. This work was supported by the NICHD Intramural Program.
References
- 1.Arakelyan A, Fitzgerald W, Margolis L, Grivel JC. Nanoparticle-based flow virometry for the analysis of individual virions. The Journal of Clinical Investigation. 2013;123(9):3716–3727. doi: 10.1172/JCI67042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roederer M, Keele BF, Schmidt SD, Mason RD, Welles HC, Fischer W, Labranche C, Foulds KE, Louder MK, Yang ZY, Todd JP, Buzby AP, Mach LV, Shen L, Seaton KE, Ward BM, Bailer RT, Gottardo R, Gu W, Ferrari G, Alam SM, Denny TN, Montefiori DC, Tomaras GD, Korber BT, Nason MC, Seder RA, Koup RA, Letvin NL, Rao SS, Nabel GJ, Mascola JR. Immunological and virological mechanisms of vaccine-mediated protection against SIV and HIV. Nature. 2014;505(7484):502–8. doi: 10.1038/nature12893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.St John AL, Abraham SN, Gubler DJ. Barriers to preclinical investigations of anti-dengue immunity and dengue pathogenesis. Nature Reviews. Microbiology. 2013;11(6):420–426. doi: 10.1038/nrmicro3030. [DOI] [PubMed] [Google Scholar]
- 4.Kuhn RJ, Zhang W, Rossmann MG, Pletnev SV, Corver J, Lenches E, Jones CT, Mukhopadhyay S, Chipman PR, Strauss EG, Baker TS, Strauss JH. Structure of dengue virus: implications for flavivirus organization, maturation, and fusion. Cell. 2002;108(5):717–725. doi: 10.1016/s0092-8674(02)00660-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mukhopadhyay S, Kuhn RJ, Rossmann MG. A structural perspective of the flavivirus life cycle. Nature Reviews. Microbiology. 2005;3(1):13–22. doi: 10.1038/nrmicro1067. [DOI] [PubMed] [Google Scholar]
- 6.Lindenbach BD, Rice CM. Molecular biology of flaviviruses. Advances in Virus Research. 2003;59:23–61. doi: 10.1016/s0065-3527(03)59002-9. [DOI] [PubMed] [Google Scholar]
- 7.Kielian M, Rey FA. Virus membrane-fusion proteins: more than one way to make a hairpin. Nature reviews. Microbiology. 2006;4(1):67–76. doi: 10.1038/nrmicro1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mackenzie JM, Westaway EG. Assembly and maturation of the flavivirus Kunjin virus appear to occur in the rough endoplasmic reticulum and along the secretory pathway, respectively. Journal of Virology. 2001;75(22):10787–10799. doi: 10.1128/JVI.75.22.10787-10799.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murray JM, Aaskov JG, Wright PJ. Processing of the dengue virus type 2 proteins prM and C-prM. The Journal of General Virology. 1993;74(Pt 2):175–182. doi: 10.1099/0022-1317-74-2-175. [DOI] [PubMed] [Google Scholar]
- 10.Randolph VB, Winkler G, Stollar V. Acidotropic amines inhibit proteolytic processing of flavivirus prM protein. Virology. 1990;174(2):450–458. doi: 10.1016/0042-6822(90)90099-d. [DOI] [PubMed] [Google Scholar]
- 11.Yu IM, Zhang W, Holdaway HA, Li L, Kostyuchenko VA, Chipman PR, Kuhn RJ, Rossmann MG, Chen J. Structure of the immature dengue virus at low pH primes proteolytic maturation. Science. 2008;319(5871):1834–1837. doi: 10.1126/science.1153264. [DOI] [PubMed] [Google Scholar]
- 12.Zybert IA, van der Ende-Metselaar H, Wilschut J, Smit JM. Functional importance of dengue virus maturation: infectious properties of immature virions. The Journal of General Virology. 2008;89(Pt 12):3047–3051. doi: 10.1099/vir.0.2008/002535-0. [DOI] [PubMed] [Google Scholar]
- 13.Guirakhoo F, Bolin RA, Roehrig JT. The Murray Valley encephalitis virus prM protein confers acid resistance to virus particles and alters the expression of epitopes within the R2 domain of E glycoprotein. Virology. 1992;191(2):921–31. doi: 10.1016/0042-6822(92)90267-S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, Kauffmann B, Chipman PR, Kuhn RJ, Rossmann MG. Structure of immature West Nile virus. Journal of Virology. 2007;81(11):6141–5. doi: 10.1128/JVI.00037-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zaitseva E, Yang ST, Melikov K, Pourmal S, Chernomordik LV. Dengue virus ensures its fusion in late endosomes using compartment-specific lipids. PLoS Pathogens. 2010;6(10):e1001131. doi: 10.1371/journal.ppat.1001131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henchal EA, Gentry MK, McCown JM, Brandt WE. Dengue virus specific and flavivirus group determinants identified with monoclonal antibodies by indirect immunofluorescence. The American Journal of Tropical Medicine and Hygiene. 1982;31(4):830–836. doi: 10.4269/ajtmh.1982.31.830. [DOI] [PubMed] [Google Scholar]
- 17.Gentry MK, Henchal EA, McCown JM, Brandt WE, Dalrymple JM. Identification of distinct antigenic determinants on dengue-2 virus using monoclonal antibodies. The American Journal of Tropical Medicine and Hygiene. 1982;31(3 Pt 1):548–555. doi: 10.4269/ajtmh.1982.31.548. [DOI] [PubMed] [Google Scholar]
- 18.van der Pol E, van Gemert MJ, Sturk A, Nieuwland R, van Leeuwen TG. Single vs. swarm detection of microparticles and exosomes by flow cytometry. Journal of Thrombosis and Haemostasis. 2012;10(5):919–930. doi: 10.1111/j.1538-7836.2012.04683.x. [DOI] [PubMed] [Google Scholar]
- 19.Nolan JP, Stoner SA. A trigger channel threshold artifact in nanoparticle analysis. Cytometry A. 2013;83(3):301–305. doi: 10.1002/cyto.a.22255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y, Corver J, Chipman PR, Zhang W, Pletnev SV, Sedlak D, Baker TS, Strauss JH, Kuhn RJ, Rossmann MG. Structures of immature flavivirus particles. The EMBO Journal. 2003;22(11):2604–2613. doi: 10.1093/emboj/cdg270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arakelyan A, Ivanova O, Vasilieva E, Grivel JC, Margolis L. Antigenic composition of single nano-sized extracellular blood vesicles. Nanomedicine. 2015;11(3):489–98. doi: 10.1016/j.nano.2014.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hiramatsu K, Tadano M, Men R, Lai CJ. Mutational Analysis of a Neutralization Epitope on the Dengue Type 2 Virus (DEN2) Envelope Protein: Monoclonal Antibody Resistant DEN2/DEN4 Chimeras Exhibit Reduced Mouse Neurovirulence. Virology. 1996;224(2):437–45. doi: 10.1006/viro.1996.0550. [DOI] [PubMed] [Google Scholar]
- 23.Henchal EA, McCown JM, Burke DS, Seguin MC, Brandt WE. Epitopic analysis of antigenic determinants on the surface of dengue-2 virions using monoclonal antibodies. The American Journal of Tropical Medicine and Hygiene. 1985;34(1):162–169. doi: 10.4269/ajtmh.1985.34.162. [DOI] [PubMed] [Google Scholar]
- 24.Stadler K, Allison SL, Schalich J, Heinz FX. Proteolytic activation of tick-borne encephalitis virus by furin. Journal of Virology. 1997;71(11):8475–81. doi: 10.1128/jvi.71.11.8475-8481.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bolt G, Pedersen IR. The role of subtilisin-like proprotein convertases for cleavage of the measles virus fusion glycoprotein in different cell types. Virology. 1998;252(2):387–98. doi: 10.1006/viro.1998.9464. [DOI] [PubMed] [Google Scholar]
- 26.Junjhon J, Edwards TJ, Utaipat U, Bowman VD, Holdaway HA, Zhang W, Keelapang P, Puttikhunt C, Perera R, Chipman PR, Kasinrerk W, Malasit P, Kuhn RJ, Sittisombut N. Influence of pr-M cleavage on the heterogeneity of extracellular dengue virus particles. Journal of Virology. 2010;84(16):8353–8358. doi: 10.1128/JVI.00696-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodenhuis-Zybert IA, van der Schaar HM, da Silva Voorham JM, van der Ende-Metselaar H, Lei HY, Wilschut J, Smit JM. Immature dengue virus: a veiled pathogen?. PLoS Pathogens. 2010;6(1):e1000718. doi: 10.1371/journal.ppat.1000718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richter MK, da Silva Voorham JM, Torres Pedraza S, Hoornweg TE, van de Pol DP, Rodenhuis-Zybert IA, Wilschut J, Smit JM. Immature dengue virus is infectious in human immature dendritic cells via interaction with the receptor molecule DC-SIGN. PLoS One. 2014;9(6):e98785. doi: 10.1371/journal.pone.0098785. [DOI] [PMC free article] [PubMed] [Google Scholar]