Abstract
Dendritic cells (DCs) initiate immune responses in barrier tissues including lung and skin. Conventional DC subsets, CD11b− (cDC1s) or CD11b+ (cDC2s), arise via distinct networks of transcription factors involving IRF4 and IRF8 and are specialized for unique functional responses. Using mice in which a conditional Irf4 or Irf8 allele is deleted in CD11c+ cells, we determined if IRF4 or IRF8 deficiency beginning in CD11c+ cDC precursors (pre-cDCs) changed the homeostasis of mature DCs or pre-DCs in the lung, dermis and spleen. CD11c-cre-Irf4−/− mice selectively lacked a lung-resident CD11chiCD11b+SIRPα+CD24+ DC subset, but not other lung CD11b+ DCs or alveolar macrophages. Numbers of CD11b+CD4+ splenic DCs, but not CD11b+ dermal DCs, were reduced, indicating cDC2s in the lung and dermis develop via different pathways. Irf4 deficiency did not alter numbers of cDC1s. CD11c-cre-Irf8−/− mice lacked lung-resident CD103+ DCs and splenic CD8α+ DCs, yet harbored increased IRF4-dependent DCs. This correlated with a reduced number of Irf8−/− pre-cDCs, which contained elevated IRF4, suggesting that Irf8 deficiency diverts pre-cDC fate. Analyses of Irf4 and Irf8 haploinsufficient mice showed that while one Irf4 allele was sufficient for lung cDC2 development, two functional Irf8 alleles were required for differentiation of lung cDC1s. Thus, IRF8 and IRF4 act in pre-cDCs to direct the terminal differentiation of cDC1 and cDC2 subsets in the lung and spleen. These data suggest that variation in IRF4 or IRF8 levels resulting from genetic polymorphisms or environmental cues will govern tissue DC numbers and therefore regulate the magnitude of DC functional responses.
Introduction
Lung resident DCs are essential regulators of innate and adaptive immune responses to respiratory pathogens and also promote chronic inflammatory diseases such as asthma (1, 2). Tissue DC subsets, including those in the lung and dermis, are specialized for particular types of functional responses, and their development is governed by specific networks of transcription factors, such as IRF4 and IRF8, expressed in DC progenitors (3–5). Mature DCs continue to express these transcription factors, which specify gene expression programs that direct their functional responses. IRF4-expressing DCs are important for the DC-driven polarization of TH17 responses in the intestine and lung (6, 7), for the induction of TH2 responses in lung allergy and skin parasite models (8–10) and for attenuation of TH1 responses (11). In turn, IRF8-expressing DCs are often most important for TH1 and CD8+ T cell responses, although the role of specific DC subsets is context dependent (12–16). In human blood, CD1c+ DCs preferentially express IRF4, while CD141+ DCs preferentially express IRF8 (6, 17). Thus, investigation of the role of IRF4 and IRF8 in the differentiation and homeostasis of DC subsets will help us to understand human inflammatory responses in peripheral tissues.
In mice, conventional DCs (cDCs) in lymphoid organs and nonlymphoid tissues are broadly classified as CD11b− (cDC1s) or CD11b+ (cDC2s) (18). The CD11b− DCs express CD8α+ in the spleen and CD103+ in nonlymphoid tissues and require IRF8 and BATF3 for their terminal differentiation (19). CD11c+CD11b+MHCII+ cells are heterogeneous in nonlymphoid tissues, and the definition of specific DC subsets has only recently been clarified by approaches to separate true CD11b+CD24+ cDC2s from macrophages and monocyte-derived DCs (20–22). Because of this diversity, it has been more difficult to discern the transcription factor networks required for terminal differentiation of the cDC2s and other CD11c+CD11b+ subsets.
IRF4 is required for differentiation of splenic CD11b+CD4+ cDCs (23, 24). However, the role of IRF4 in the development of tissue cDC2s remained unclear. We used mice globally deficient for Irf4 to show that IRF4 is not required for development or skin residence of CD11b+ dermal DCs, but does promote migration of dermal CD11b+ DCs to cutaneous LNs (25). More recent work, with mice bearing a conditional Irf4 allele and a CD11c-cre construct that directs Cre activity in CD11cint pre-cDCs and CD11chi mature DCs (CD11c-cre-Irf4 −/− mice), showed that numbers of CD11b+CD24+ lung DCs were reduced but not completely ablated by Irf4 deficiency; this was correlated with increased apoptosis in the remaining DCs suggesting an effect on DC survival (6). A similar conclusion was reached for CD103+CD11b+ DCs in the small intestinal lamina propria (SI-LP) in these mice (6, 7). In contrast, mice bearing a conditional Irf4 allele and a CD11c-cre construct that apparently directs Cre activity only in CD11chi cells showed no reduction in CD11b+CD24+ lung DCs (8). Taken together, these studies suggested that Irf4 deletion beginning at the CD11cint pre-cDC stage diminished in vivo survival (and therefore numbers) of CD11b+ lung DCs, while Irf4 deletion only in mature CD11chi DCs did not impact their numbers.
Despite these advances, it remained unclear whether IRF4 and IRF8 must act in immediate cDC precursors (pre-cDCs) to promote DC terminal differentiation and/or survival (19). Irf8 mRNA is present in common DC progenitors (CDPs) and pre-cDCs, while Irf4 mRNA is present in pre-cDCs but not CDPs [reviewed in (3)]. Global Irf8 deficiency leads to defects in the formation of the CDP and all splenic DC subsets (26), while global Irf4 deficiency did not apparently affect CDPs but did abolish splenic CD11b+CD4+ cDCs, suggesting effects on pre-cDCs (23, 24). Pre-cDCs (defined as [CD19, CD3, NK1.1-negative] CD11cint MHCII− SIRPαint Flt3+) with the potential to develop into cDCs in lymphoid and nonlymphoid organs have been identified in bone marrow (BM), lymphoid organs and nonlymphoid tissues including lungs (27–29). The pre-cDC population was initially divided based on low or high CD24 expression into precursors pre-committed to either the CD11b+ or the CD11b− pathways (27). Recent reports used CD24 and other markers to subdivide the pre-cDC population into discrete precursors for the cDC1s and cDC2s that preferentially express Irf8 and Batf3 mRNA or Irf4 mRNA, respectively (30, 31). Irf8-deficient mice lacked pre-cDC1s (30). However, the effects of Irf4 and Irf8 deficiency or haploinsufficiency beginning in pre-cDCs on pre-cDC numbers and their expression of IRF4 and IRF8 proteins in vivo have not been well characterized.
Herein, we have reexamined the effect of Irf4 deficiency on DC differentiation in CD11c-cre-Irf4 −/− and +/− mice in which Cre activity is present in pre-cDCs and mature DCs. Similar analyses of CD11c-restricted Irf8 deficiency were done using CD11c-cre-Irf8 −/− and +/− mice. We determined the effect of Irf4 and Irf8 gene dosage on numbers of pre-cDCs and DC subsets and their relative expression of IRF4 and IRF8 proteins. Taken together our data show that changes in IRF4 or IRF8 levels beginning in pre-cDCs have profound effects on relative numbers of the cDC1 and cDC2 subsets in the lung and spleen in homeostasis. Thus, variation in IRF4 or IRF8 levels resulting from environmental stimuli or genetic polymorphisms may regulate tissue DC numbers, and therefore modulate the magnitude of DC functional responses in inflammation. Indeed, polymorphisms in human IRF4 genes impart susceptibility to melanoma and lymphocytic leukemia, while polymorphisms in IRF8 genes impart susceptibility to systemic lupus erythematosus or lead to deficient antimycobacterial immunity secondary to the absence of select DC subsets (32–36).
Materials and Methods
Mice
Mice (purchased from The Jackson Laboratory) bearing a conditional allele of Irf4 (B6.129S1-Irf4<tm1Rdf>/J) (37) were bred to mice bearing Cre recombinase driven by the CD11c promoter (B6.Cg-Tg(Itgax-cre)1-1Reiz/J) (38) and then interbred to yield CD11c-cre-Irf4 −/−, +/− or +/+ mice. Mice used as “wild-type” were either Cre+ but bearing two wild-type alleles of Irf4, or Cre− and bearing either wild-type or conditional Irf4 alleles; we did not note any differences between these two groups. As noted in a prior report, the Cre activity may randomly act in CD11c− cells in this line of CD11c-Cre mice, leading to mice that have the conditional allele deleted in many tissues (7). Therefore, we screened for mice bearing a global deletion of Irf4 using PCR primers (for: CAGGATGTTGCCGTCCTCCTTG and rev: CCTGCAGCCAATAAGCTTATAAC), and excluded those mice from this study. Similarly, mice bearing a conditional allele of Irf8 (B6(Cg)-Irf8<tm1.1Hm>/J) (39) were purchased from the Jackson Laboratory, bred to B6.Cg-Tg(Itgax-cre)1-1Reiz/J mice and then interbred to produce CD11c-cre-Irf8 −/−, +/− or +/+ mice. Since a global deletion of Irf8 favors development of neutrophils at the expense of DCs (26), we excluded CD11c-cre-Irf8 mice from analysis that had an enlarged spleen or BM compartment, or high numbers of Ly-6G+ cells (identified by mAb Gr-1) in blood; we were unable to develop a PCR assay for this screening in the CD11c-cre-Irf8 mice. Littermate mice of each genotype and of both sexes were analyzed at 6–10 weeks of age, and we did not find a sex difference in any parameter analyzed. The OMRF IACUC approved the studies.
Isolation of cells from tissues
Lungs were perfused with PBS prior to digestion. Lung lobes were digested for 60 min with collagenase type D (2 mg/ml) and DNAse I (0.2 mg/ml) (both from Roche) in 10 mM HEPES-NaOH pH 7.4, 150 mM NaCl, 5 mM KCl, 1 mM MgCl2, 1.8 mM CaCl2. Spleens were digested to a single cell suspension with collagenase type D (1 mg/ml) and DNAse I (0.1 mg/ml) in Ca2+ and Mg2+-containing Hank’s Balanced Salt Solution (HBSS) at 37°C for 30 min. Bone marrow (BM) cells were isolated as described (40). Red blood cells in tissues were lysed using RBC lysis buffer (BD Biosciences). In some experiments to analyze pre-cDCs, CD11c+ cells in spleen and BM were enriched using a murine CD11c positive selection kit (Stem Cell Technologies).
Flow cytometry
After isolation from tissue, cells were immediately processed for flow cytometry by pre-incubating with anti-CD16/32, and labeling with optimally titered mAbs (obtained from BD Biosciences, eBioscience or Biolegend) in FACS buffer (PBS, 5% newborn calf serum, 0.1% sodium azide). To identify lung DC and macrophage populations, cells were stained with a lymphocyte marker cocktail (mAbs specific for CD19, CD3, B220 and NK1.1 linked to a common fluorochrome) to gate out lymphocytes, in conjunction with various combinations of fluorochrome-labeled mAbs specific for CD11c, MHCII, Siglec F, CD64, CD11b, CD103, SIRPα, CD24 and CD14. Splenic DCs were defined using a combination of mAbs specific for CD11c, CD8α, CD11b, CD4, and MHCII. Pre-cDCs (Lin− CD11c+ MHCII− SIRPαlo Flt3+) in BM or spleen were identified using a Lineage cocktail (mAbs specific for CD19, CD3, B220, NK1.1, Ter119 linked to a common fluorochrome) to gate out mature cells, in conjunction with mAbs specific for CD11c, MHCII, SIRPα and Flt3. After surface marker staining, intracellular staining with fluorochrome conjugated mAbs specific for IRF4(PE) and IRF8(APC) was done using a Foxp3 buffer kit (all from eBioscience). Intracellular staining for activated capase 3 (mAb from BD Biosciences) was done in conjunction with live/dead fixable Aqua stain (Invitrogen). The data were collected on an LSRII instrument (BD Biosciences) and analyzed with FlowJo (TreeStar) software.
Statistical analyses
Significant differences between values measured in wild-type and mutant mice were determined using an unpaired t test (if two genotypes compared), or a one-way ANOVA with Tukey post tests (if three genotypes evaluated) in Prism 6 software as indicated in figure legends. Differences were considered significant when p<0.05.
Results
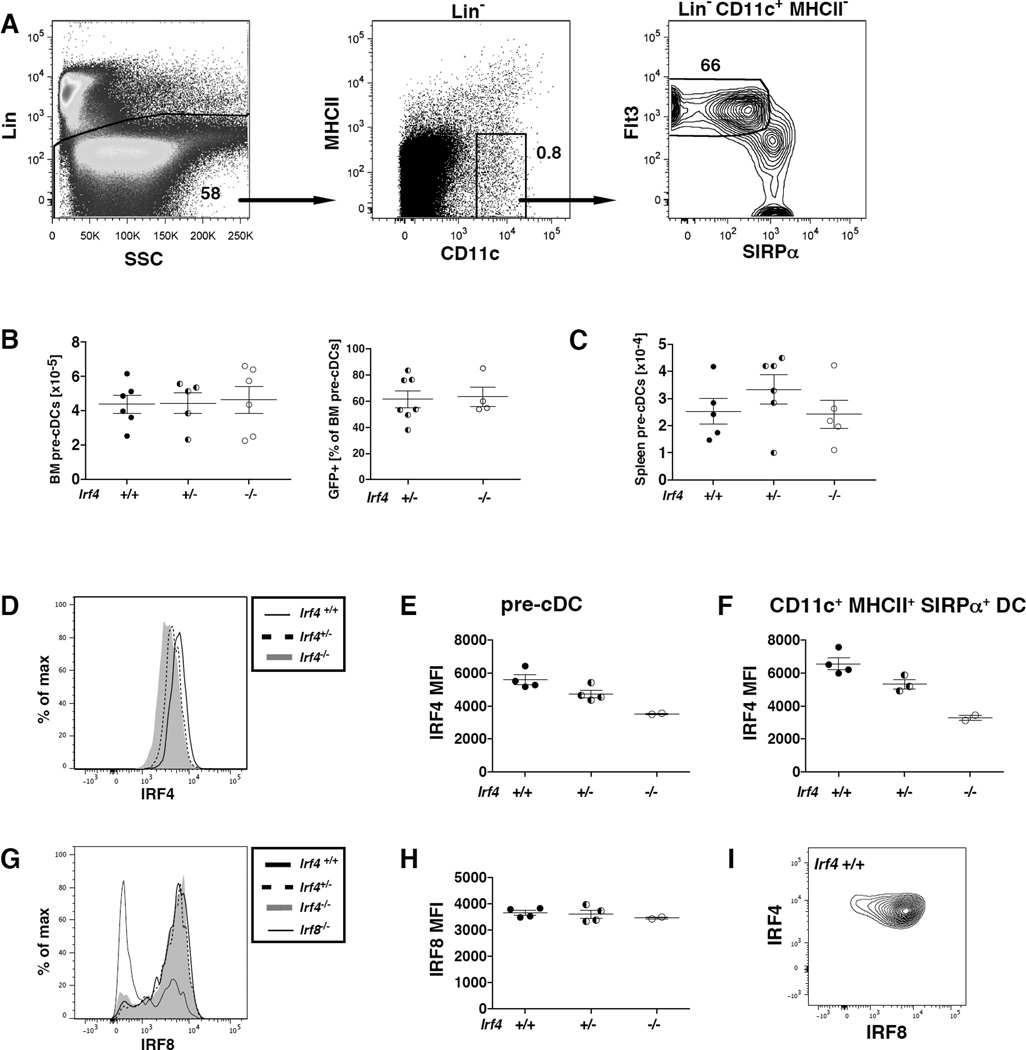
To evaluate the role of IRF4 in the terminal differentiation of tissue and lymphoid organ DCs, we bred mice with a conditional Irf4 allele to mice bearing Cre recombinase driven by the CD11c promoter. The CD11c-cre-Irf4 mice bear 0, 1 or 2 copies of Irf4 in CD11c+ cells, and are hereafter designated as CD11c-cre-Irf4 −/−, +/− or +/+, respectively. CD11c+ cells include pre-cDCs, mature DCs, NK cells and some macrophage populations, including alveolar macrophages. The CD11c-specific Irf4 deletion did not significantly alter numbers of CD11c− cell types as the +/− and −/− mice had normal numbers of lung (CD45+), spleen and bone marrow cells (Fig. S1). In these mice, the deletion of Irf4 places an Egfp minigene in frame, resulting in GFP expression in CD11c+ cells that deleted the Irf4 gene in +/− and −/− mice; wild-type mice do not express any GFP. Based on GFP expression, ~100% of CD11chi DCs have deleted the Irf4 gene (Fig. 1G, S1). However, a subset (~30%) of CD11cint cells is not clearly GFP+, and thus may not have deleted the Irf4 gene (Fig. S1, S2D). Backgating of GFP+ and GFP− CD11chi cells in the lung shows that all CD11chi MHCII+ cells are GFP+, while only a subset of CD11cint cells are GFP+ (Fig. S1C,D). GFP− CD11chi cells are primarily alveolar macrophages.
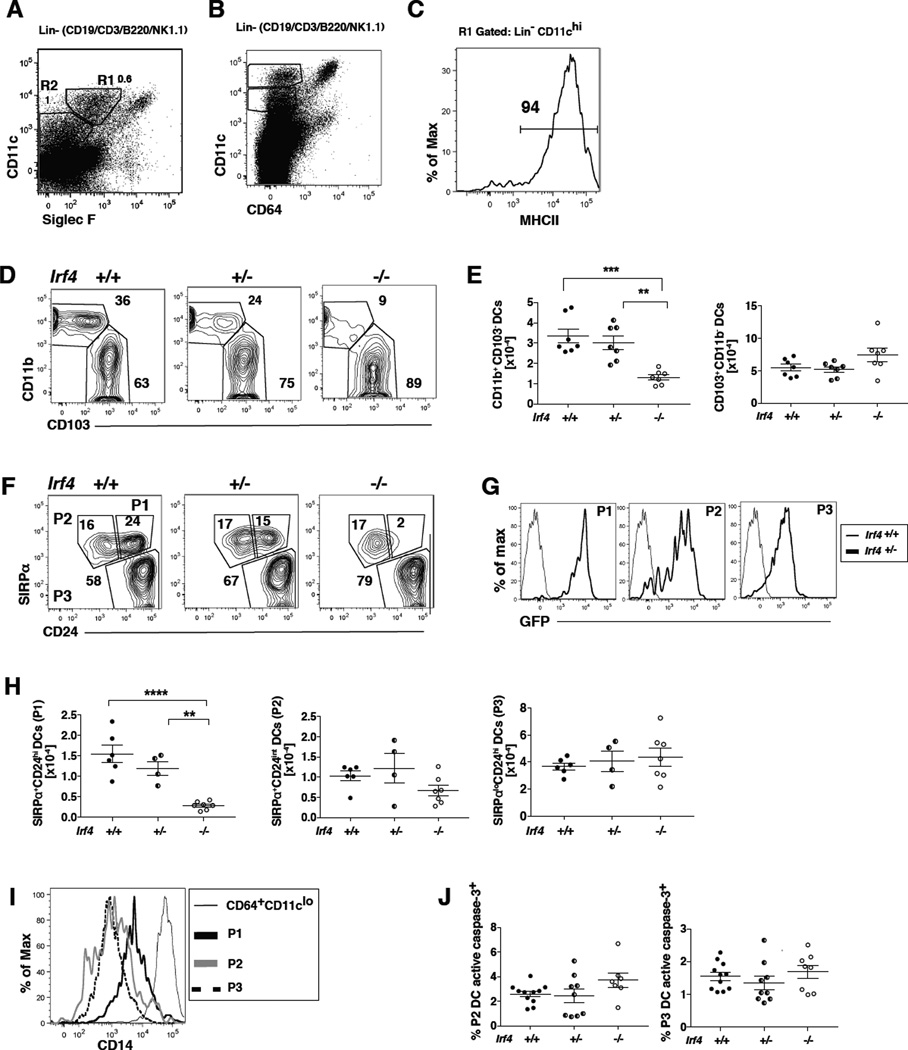
Fig. 1. IRF4 expression in CD11c+ cells is required for development of a CD11chiMHCIIhiCD11b+CD24hiSIRPα+ DC subset in the lung.
(A–C) Definition of DC populations in CD11c-cre-Irf4 +/+ mice. (A) CD11c+ myeloid cells displaying distinct levels of SiglecF are identified in the Lineage-negative (CD19−CD3−B220− NK1.1−) fraction of lung cells. The R1 gate defines CD11chiSiglecFlo DCs that are (B) CD64− and (C) MHCIIhi. The R2 gate defines CD11cintSiglecF− cells, some of which are (B) CD64− and MHCII+ (see Fig. S2). The numbers within these plots from +/+ mice indicate the percentage of cells within each gate. (D) CD11chiMHCIIhi cells in gate R1 are divided into CD11b+ and CD103+ subsets; a comparison of +/+, +/− and −/− mice is shown. (E) The total numbers of CD11b+ and CD103+ DC subsets in multiple +/+, +/− and −/− mice are compiled; shown are values for individual mice, n=7 per genotype. (F) CD11chiMHCIIhi cells in gate R1 are divided into P1, P2 and P3 subsets based on SIRPα and CD24; a comparison of +/+, +/− and −/− mice is shown. (G) The CD11chiMHCIIhi DC subsets SIRPα+CD24hi (P1), SIRPα+CD24int (P2) and SIRPαloCD24hi (P3) are GFP+ in +/− mice. (H) The total numbers of P1, P2 and P3 DC subsets in multiple +/+, +/− and −/− mice are compiled, n=4–7 per genotype. (I) DCs within the CD11chi P1, P2 and P3 subsets display lower levels of CD14 than the CD11clo CD64hi macrophages. (J) Shown is the percentage of DCs in P2 and P3 subsets that contain activated caspase-3 in +/+, +/− and −/− mice, n=8–11 per genotype. The significance of the data in panels E, H and J was evaluated using a one-way ANOVA with a Tukey multiple comparisons test; **p<0.01, ***p<0.001, ****p<0.0001.
To also evaluate the role of IRF8 in the terminal differentiation of lung tissue DCs, we bred mice bearing a conditional Irf8 allele to the CD11c-cre mice; these mice do not bear a Gfp reporter. Total numbers of bone marrow cells, lung (CD45+) cells and splenocytes were not altered in CD11c-cre-Irf8 +/− and −/− mice (Fig. S1).
Through comparisons of CD11c-cre-Irf4 and CD11c-cre-Irf8 −/−, +/− and +/+ mice, we determined if a reduction or absence of Irf4 and Irf8 gene expression in pre-cDCs and cDCs regulates the numbers and IRF4 or IRF8 expression of DCs and pre-cDCs in lymphoid organs and lung tissue during homeostasis.
IRF4 expression in CD11c+ cells is required for development of a CD11chi MHCIIhi CD11b+ CD24hi SIRPα+ DC subset in the lung
To determine if IRF4 were required for the development or survival of specific lung DC subsets, we analyzed the CD11chiSiglecFloCD64−MHCIIhi population in perfused lungs (Fig. 1A–C). Combined with a lineage gate to exclude CD19+, CD3+ and NK1.1+ cells, this marker combination also facilitates exclusion of CD11chiSiglecFhiCD64+ alveolar macrophages, and helps to clearly delineate CD11chi (R1 gate) vs. CD11cint (R2 gate) cells. Once gated, the CD11chiMHCIIhi cells in gate R1 were divided into CD11b+ and CD103+ fractions (Fig. 1D). CD11c-cre-Irf4 −/− mice showed a significant reduction of the percentage and number of CD11chi MHCIIhi CD11b+ DCs (Fig. 1D–E). In an alternate marker scheme, the CD11chi MHCIIhi DCs were divided into 3 subsets based on expression of CD24 and SIRPα (CD172a) (Fig. 1F). Previous reports showed that the CD103+ DCs are CD24hiSIRPαlo, while CD11b+ DCs are CD24intSIRPαhi. We identified two populations of SIRPα+ DCs, differing in the level of expression of CD24. Notably, Irf4 deficiency results in a near absolute reduction in the SIRPα+CD24hi DCs (designated the P1 population), while the numbers of the SIRPα+CD24int cells (P2 population) and the SIRPαloCD24hi cells (P3 population; also CD103+) are present in normal numbers (Fig. 1F,H). The P1, P2 and P3 populations express high levels of GFP, reporting the deletion of Irf4 (Fig. 1G). Since a fraction of CD11b+ tissue DCs is derived from monocytes, and thus would not be reduced by CD11c-Cre activity (41), it is possible that the CD11chiMHCII+ P2 cells represent this monocyte-derived fraction. However, the P2 cells do not express high levels of CD14 or CD64, relative to the CD11cloCD64+ macrophage population (Fig. 1B,I).
CD11c-cre-Irf4 +/− heterozygotes showed a trend to reduced percentages and numbers of the CD11b+SIRPα+CD24hi population relative to +/+ mice, but this reduction did not reach statistical significance (Fig. 1H). This suggests that one copy of the Irf4 gene leads to decreased levels of IRF4, which is nearly sufficient for complete lung cDC2 development.
Thus, separation of the CD11chiMHCIIhiSIRPα+ DCs into CD24hi and CD24int cells revealed that only one of two distinct DC subsets within the CD11b+ SIRPα+ population is dependent upon Irf4 for development. Prior reports of the CD11c-cre-Irf4 −/− mice showed a reduction, but not absence, of the numbers of CD11b+ lung resident DCs and CD11b+CD103+ DCs in the SI-LP (6, 7). Based on this reduction and other data showing increased apoptosis of the CD11b+ subset, it was suggested that Irf4 was not required for development of the CD11b+ subset, but rather important for regulation of its survival. To address DC survival in view of our finding that the CD11c-cre-Irf4 −/− mice do lack the SIRPα+CD24hi (P1) DC subset, we determined activated caspase 3 levels in the SIRPα+CD24int (P2) subset directly ex vivo. In CD11c-cre-Irf4 −/− mice, the P2 subset did not show a significant difference in the low fraction of cells bearing activated caspase 3 (Fig. 1J), suggesting that these cells are not undergoing higher levels of apoptosis than in +/+ mice. Taken together, these data show that Irf4 is required for the development of the P1 DC subset, without an effect on the numbers or survival of the P2 and P3 DC subsets.
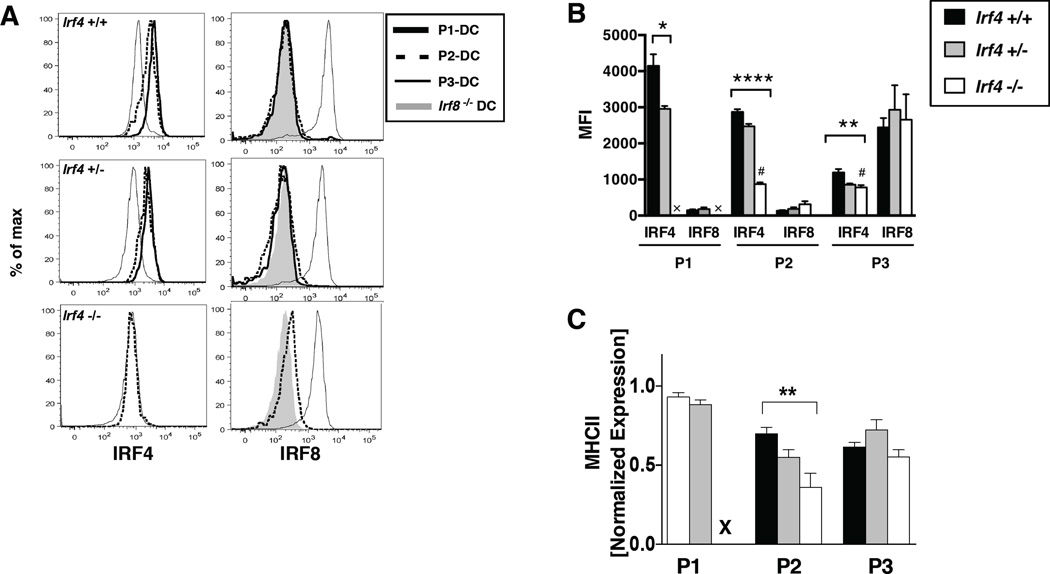
cDC subsets differ in expression of IRF4 and IRF8 and those cDCs that develop in CD11c-cre-Irf4 −/− mice do not alter their expression of IRF8
We next determined IRF4 and IRF8 protein levels in these distinct lung DC subsets using specific Abs for intracellular flow cytometry. The P1 DCs harbored the highest level of IRF4 and no IRF8, while P3 DCs expressed very low levels of IRF4 and high levels of IRF8 (Fig. 2A–B). The P2 population also expressed IRF4 and very little IRF8. Analyses of CD11c-cre-Irf4 +/− mice showed that a single copy of the Irf4 gene led to a reduced level of IRF4 protein, ~75% of the level in +/+ mice, in the P1 subset (Fig. 2B). In CD11c-cre-Irf4 −/− and +/− mice, the IRF8 protein levels in the P2 and P3 subsets were not different from +/+ mice (Fig. 2A–B). Thus, a single copy of the Irf4 gene leads to a reduced amount of IRF4 protein in DCs, yet Irf4 deficiency or haploinsufficiency did not alter IRF8 expression in the P2 and P3 DC subsets. Furthermore, although the P2 DCs express IRF4, Irf4 deficiency did not alter their numbers.
Fig. 2. cDC subsets in CD11c-cre-Irf4 mice differ in expression of IRF4 and IRF8 while heterozygotes show reduced levels of protein.
(A) In CD11c-cre-Irf4 mice, lung cDCs were subdivided into P1, P2 and P3 subsets as in Fig. 1F, and intracellular IRF4 and IRF8 levels were determined using flow cytometry. For each subset in +/+, +/− and −/− mice, the binding of anti-IRF4 (left panels) and anti-IRF8 (right panels) is shown. The CD11c-cre-Irf4 −/− and CD11c-cre-Irf8 −/− mice were used to determine the nonspecific level of anti-IRF4 and anti-IRF8 Ab binding, respectively, to DCs. (B) The mean fluorescence intensity (MFI) of anti-IRF4 and anti-IRF8 binding is shown for each cDC subset (P1, P2, P3) in CD11c-cre-Irf4 +/+, +/− and −/− mice. The # indicates the nonspecific binding of the anti-IRF4 Ab on Irf4 −/− cells. X indicates that the P1 subset is absent in the −/− mouse. (C) The relative MHCII expression (normalized MFI) on the P1, P2 and P3 subsets present in CD11c-cre-Irf4 +/+, +/− and −/− mice is shown. X indicates that the P1 subset is absent in the −/− mouse. For panels B and C, the significance of the data was evaluated using an unpaired t test (P1) or a one-way ANOVA (P2, P3); *p<0.05, **p<0.01, ****p<0.0001, n=4 per genotype.
IRF4 was reported to regulate CIITA, a transcription factor that promotes expression of MHCII (23). Despite the reduction in IRF4 in the P1 subset of +/− mice, the MHCII level was not reduced (Fig. 2C). In the P2 subset, IRF4 deficiency, but not hemizygosity, led to decreased MHCII expression. This suggests that the amount of IRF4 in +/− DCs is sufficient for induction of CIITA. MHCII levels in the P3 subset were not affected by loss of IRF4.
Deletion of Irf4 in CD11c+ cells does not alter numbers of CD11cint DCs or macrophage subsets in the lung
We also determined if IRF4 were required for the development of the CD11cintSiglecFlo CD64−MHCIIhi subset, which appears to represent a distinct population of DCs (R2 gate in Fig. 1A; S2A). This population is CD11b+, with a SIRPα+CD24int phenotype similar to the CD11chi P2 population described above (Fig. S2B), but only ~50% of the cells in +/− mice express GFP (Fig. S2D), suggesting that not all cells bear a deletion of Irf4. Numbers of this CD11cintCD11b+MHCIIhi population do not differ among CD11c-cre-Irf4 −/−, +/− and +/+ mice, even when gating only upon the GFP+ population in +/− and −/− mice (Fig. S2C–D). The CD11cintCD11b+MHCIIhi DCs express low levels of IRF4 and IRF8 (Fig. S2E). CD11chiSiglecFhiCD64+ alveolar macrophages also will have deleted Irf4; however, numbers of these cells did not differ among CD11c-cre-Irf4 −/−, +/− and +/+ mice (Fig. S2F–G). This is consistent with low expression of Irf4 in alveolar macrophages (immgen.org).
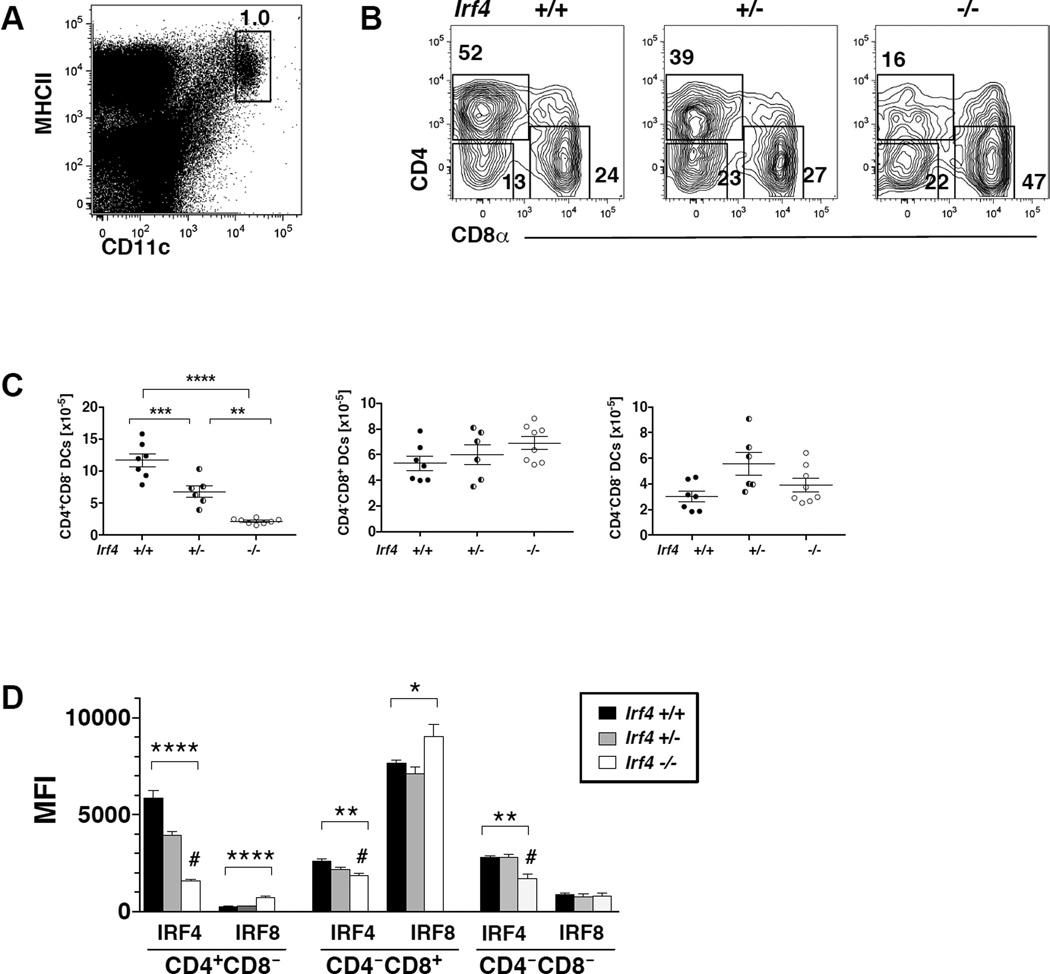
IRF4 expression in CD11c+ cells is required for development of splenic CD11b+CD4+DCs
Consistent with previous reports in globally Irf4 deficient mice, in CD11c-cre-Irf4 −/− and +/− mice, we observed an Irf4 dose-dependent reduction in the percentages and numbers of splenic CD4+CD8− DCs; these DCs are also CD11b+ (Fig. 3A–C). Numbers of the CD4−CD8− DCs and the IRF8-dependent CD4−CD8+ DCs were not altered by Irf4 deficiency. These data show that a reduction of IRF4 activity beginning at the pre-cDCs stage preferentially reduces the development of splenic CD11b+CD4+CD8− DCs. Consistent with this finding, in +/+ mice, the CD4+CD8− DCs express IRF4 but little IRF8, while the CD4−CD8+ DCs express IRF8 but not IRF4 (Fig. 3D). CD4−CD8− DCs express IRF4 but little IRF8, but their numbers and expression of IRF8 were not altered by Irf4 deficiency. Levels of IRF4 protein in CD4+CD8− DCs in CD11c-cre-Irf4 +/− mice were reduced. In −/− mice, the levels of IRF8 were reproducibly increased in CD4+CD8− and CD4−CD8+ DCs, suggesting that Irf4 deficiency increases expression of IRF8 in the DCs that develop (Fig. 3D).
Fig. 3. IRF4 expression in CD11c+ cells is required for development of splenic CD11b+CD4+DCs.
(A) Definition of CD11chiMHCIIhi cDCs in the spleen of CD11c-cre-Irf4 +/+ mice. (B) CD11chiMHCIIhi cells are divided into CD4+CD8−, CD4−CD8+ and CD4−CD8− DC subsets; a comparison of +/+, +/− and −/− mice is shown. (C) The total numbers of CD4+CD8−, CD4−CD8+ and CD4−CD8− DC subsets in multiple +/+, +/− and −/− mice are compiled; shown are values for individual mice, n=6–8 per genotype. The significance of the data was evaluated using a one-way ANOVA with a Tukey multiple comparisons test; **p<0.01, ***p<0.001, ****p<0.0001. (D) The mean fluorescence intensity (MFI) of anti-IRF4 and anti-IRF8 binding is shown for the CD4+CD8−, CD4−CD8+ and CD4−CD8− DC subsets in +/+, +/− and −/− mice. The # indicates the background binding of the anti-IRF4 Ab on Irf4−/− cells. The binding of anti-IRF8 to Irf8−/− splenic DCs was a MFI of 195. The significance of the data was evaluated using a one-way ANOVA; *p<0.05, **p<0.01, ****p<0.0001, n=4 per genotype.
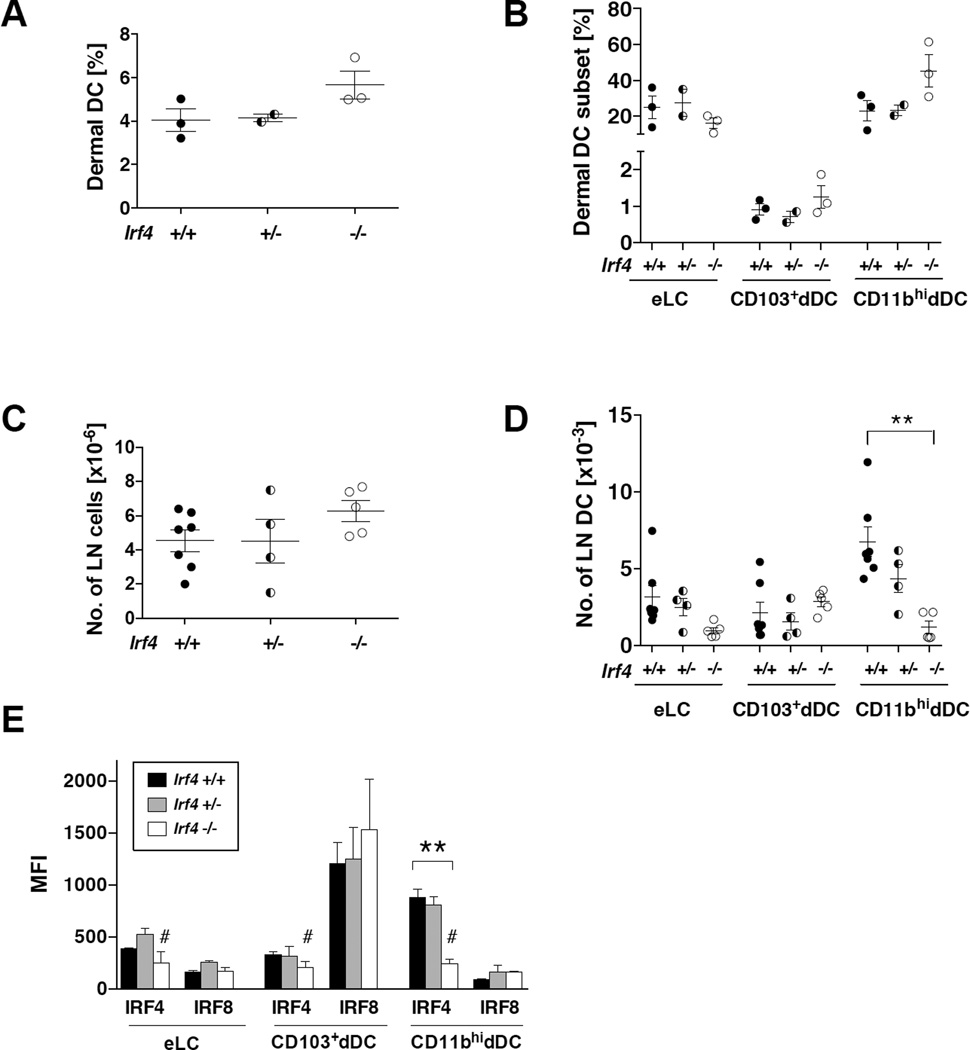
Irf4 deficiency in CD11c+ cells does not affect skin DC development but reduces migration of CD11b+ dermal DCs to local draining lymph nodes
We previously published that CD11b+ dermal (dDCs) and epidermal (eLCs) DCs developed normally and were present in the skin of globally Irf4-deficient mice (25). However, these Irf4−/− CD11b+ DC subsets failed to migrate to LN in homeostasis and inflammation, consistent with the failure to upregulate CCR7. To determine if CD11c-specific Irf4 deficiency led to the same phenotype, we analyzed skin and cutaneous LN DC populations, gating as in Bajaña et al (25). The percentages of total DCs and DC subsets in the dermis were similar in CD11c-cre-Irf4 −/−, +/− and +/+ mice, and tended toward being increased in the −/− mice as if they had accumulated (Fig. 4A–B). Although the total number of cells in the cutaneous lymph node cells was not different, we detected an Irf4 gene dosage-dependent reduction in numbers of CD11b+ dermal DCs in the cutaneous LN of CD11c-cre-Irf4 −/− and +/− mice (Fig. 4C–D). However, unlike the global Irf4−/− mice, the numbers of migrating eLC were not significantly reduced in the CD11c-cre-Irf4 −/− and +/− mice (Fig. 4D). Expression of GFP, indicating Irf4 deletion, was equivalent in the three skin DC subsets (Fig. S1). As in the lung, CD11b+ dDCs expressed high levels of IRF4 and little IRF8, while CD103+ dDCs expressed high levels of IRF8 and little IRF4 (Fig. 4E). eLCs expressed low levels of both IRF4 and IRF8. Taken together, these data show that the IRF4-expressing tissue resident CD11b+ DC populations in the lung and dermis show a differential dependence on IRF4 for their development and tissue residence.
Fig. 4. Irf4 deficiency in CD11c+ cells does not affect skin DC development but reduces migration of CD11b+ dermal DCs to local draining lymph nodes.
(A–B) The percentages of total dermal DCs and distinct DC subsets (eLC, CD103+, CD11bhi) in the skin of multiple CD11c-cre-Irf4 +/+, +/− and −/− mice, n=2–3. (C–D) Numbers of LN cells and migratory DC subsets in CD11c-cre-Irf4 +/+, +/− and −/− mice, n=4–7. (E) The MFI of anti-IRF4 and anti-IRF8 binding is shown for the eLC, CD103+ and CD11bhi DC subsets in +/+, +/− and −/− mice. The # indicates the background binding of the anti-IRF4 Ab on Irf4−/− cells. The binding of anti-IRF8 to Irf8−/− CD11bhi DCs was a MFI of 102. The significance of the data was evaluated with a one-way ANOVA with a Tukey’s multiple comparisons test, **p<0.01.
IRF4 deficiency in CD11c+ cells does not alter numbers or IRF8 expression of pre-cDCs in bone marrow or spleen
The immediate precursors of conventional DCs are CD11cint pre-cDCs, defined as lineage-negative CD11c+MHCII−SIRPαloFlt3+ (Fig. 5A). In CD11c-cre-Irf4 −/− and +/− mice, in which GFP expression can be used to monitor Irf4 deletion, 60–85% of the pre-cDCs were GFP+ (Fig. 5B). Numbers of total and GFP+ pre-cDCs in the bone marrow (BM) and spleen did not differ in CD11c-cre-Irf4 −/−, +/− and +/+ mice (Fig. 5B–C), suggesting that Irf4 deficiency does not affect pre-cDC numbers. Intracellular staining with IRF4- and IRF8-specific mAbs showed that BM pre-cDCs in +/+ mice express fairly uniform levels of IRF4 and IRF8; a small percentage of pre-cDCs are IRF8−, but their IRF4 expression level is unchanged (Fig. 5D,I). Thus, the pre-cDCs cannot be divided into cells expressing variable levels of IRF4. In pre-cDCs of +/− mice, IRF4 was present at a reduced level relative to +/+ mice (Fig. 5D–E). These IRF4 levels were similar to levels on mature cDCs in BM of the same +/+ and +/− mice (Fig. 5F). Despite the incomplete Irf4 deletion predicted by the GFP expression, we did not identify two levels of anti-IRF4 binding in −/− pre-cDCs that would be indicative of IRF4 expression stemming from intact Irf4 genes vs. nonspecific binding of the mAb. Indeed, a similar level of nonspecific binding of the anti-IRF4 mAb to −/− mature BM cDCs, which were ~88% GFP+, was observed (Fig. 5F). We could not directly correlate IRF4 expression with GFP (indicating Irf4 deficiency) in pre-cDCs, since GFP leaks out of cells permeabilized for intracellular staining. IRF8 levels on pre-cDCs did not change in CD11c-cre-Irf4 +/− or −/− mice (Fig. 5G–H). Binding of the anti-IRF8 mAb to pre-cDCs of Irf8-deficient mice was used to show background levels of mAb binding, and this revealed that not all pre-cDCs in the Irf8-deficient mice have deleted Irf8 (Fig. 5G,7D).
Fig. 5. IRF4 deficiency in CD11c+ cells does not alter numbers or IRF8 expression of pre-cDCs in bone marrow or spleen.
(A) Definition of pre-cDCs in the bone marrow of CD11c-cre-Irf4 +/+ mice as lineage-negative (Lin−, CD19−CD3−NK1.1−Ter119−B220−) CD11c+MHCII−SIRPαloFlt3+. (B–C) The numbers of total pre-cDCs and GFP+ pre-cDCs in the bone marrow and spleen in multiple +/+, +/− and −/− mice are compiled, n=4–6 per genotype. (D) IRF4 protein levels were determined in +/+, +/− and −/− BM pre-cDCs by intracellular staining, and (E) the anti-IRF4 MFI values were compiled from multiple mice. (F) IRF4 protein levels were determined in CD11c+MHCII+SIRPα+ cDCs in BM of +/+, +/− and −/− mice by intracellular staining, and the anti-IRF4 MFI values were compiled from multiple mice. (G) IRF8 protein levels were determined in +/+, +/− and −/− pre-cDCs by intracellular staining, and (H) the anti-IRF8 MFI values were compiled from multiple mice. Binding of the anti-IRF8 mAb to pre-cDCs of Irf8-deficient mice was used to show background levels of mAb binding, and this revealed that not all pre-cDCs in the Irf8-deficient mice have deleted Irf8 (see Fig. 7). (I) Binding of anti-IRF4 and anti-IRF8 mAbs to pre-cDCs in +/+ mice.
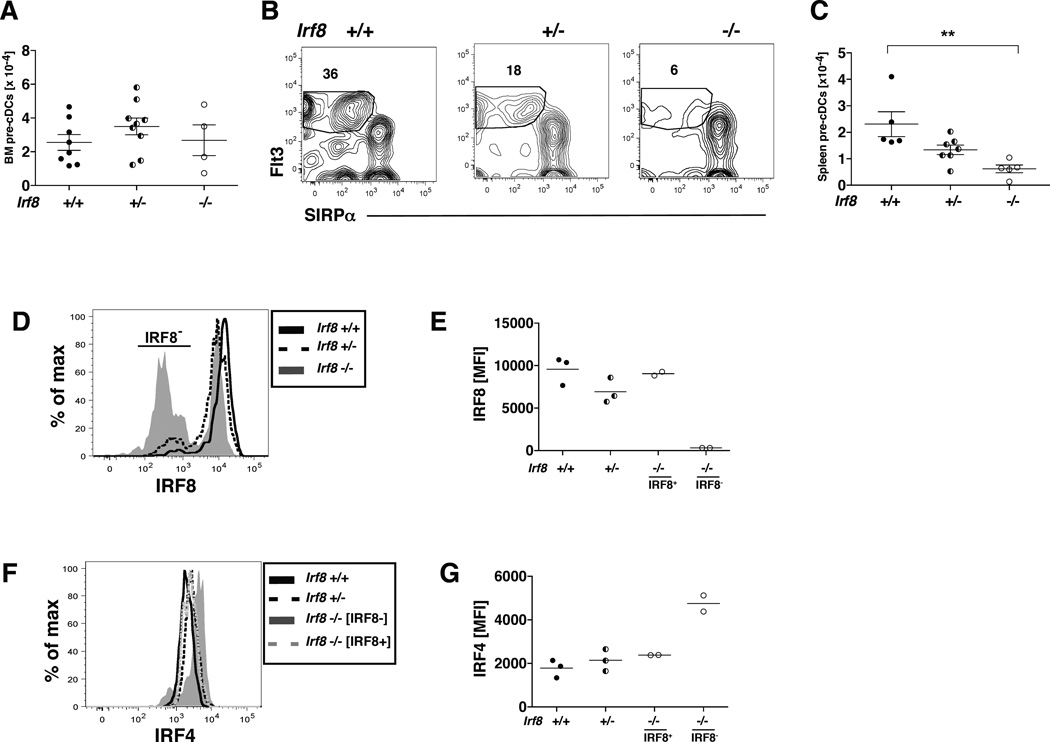
Fig. 7. IRF8 deficiency in CD11c+ cells leads to decreased numbers of splenic pre-cDCs expressing elevated levels of IRF4.
(A) The numbers of pre-cDCs in the bone marrow of multiple CD11c-cre-Irf8 +/+, +/− and −/− mice are compiled, n=4–9 per genotype. (B) Shown is the gating of SIRPα−Flt3+ pre-cDCs within the lineage-negative (CD19−CD3−NK1.1−Ter119−B220−) CD11c+MHCII− fraction of splenocytes in CD11c-cre-Irf8 +/+, +/− and −/− mice. (C) The numbers of pre-cDCs in the spleens of multiple CD11c-cre-Irf8 +/+, +/− and −/− mice are compiled, n=5–7 per genotype. (D) IRF8 protein levels were determined in +/+, +/− and −/− pre-cDCs by intracellular staining. Binding of the anti-IRF8 mAb to pre-cDCs of Irf8-deficient mice revealed that not all pre-cDCs in the Irf8-deficient mice have deleted Irf8. (E) The anti-IRF8 MFI values were compiled from multiple mice (n=2–3). In −/− mice, the IRF8 MFI is reported separately for the IRF8+ and IRF8− populations. (F) IRF4 protein levels were determined in +/+, +/− and −/− pre-cDCs by intracellular staining, and (G) the anti-IRF4 MFI values were compiled from multiple mice (n=2–3). In −/− mice, the IRF4 MFI is reported separately for the IRF8+ and IRF8− populations.
Taken together, these data show that Irf4 deficiency in CD11c+ cells (as judged by GFP expression) did not alter numbers of GFP+ pre-cDCs or their expression of IRF8. This is consistent with the absence of an effect of Irf4 deficiency on numbers of lung and splenic IRF8-dependent DCs, and suggests that IRF4 is not needed for maintenance of normal numbers of pre-cDCs. Furthermore our data using this intracellular staining approach did not reveal a relationship between levels of IRF4 and IRF8 in individual pre-cDCs in the BM.
IRF8 deficiency in CD11c+ cells alters numbers of both IRF8- and IRF4-dependent lung DCs
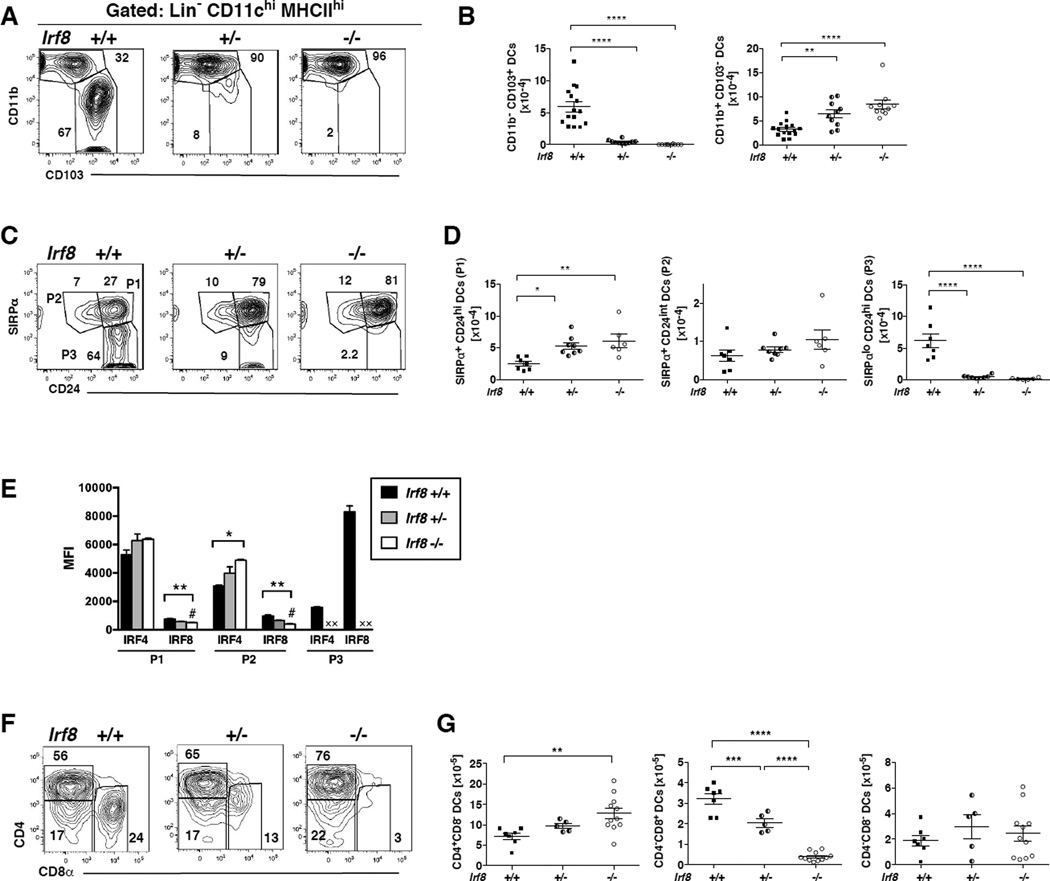
In CD11c-cre-Irf8 −/−, +/− and +/+ mice, we analyzed the CD11chiSiglecFloCD64−MHCIIhi lung DC population (gated as in Fig. 1). Unexpectedly, the CD103+ DC subset was absent in both CD11c-cre-Irf8 −/− and +/− mice (Fig. 6A,B). The numbers of CD11b+ DCs increased in both CD11c-cre-Irf8 −/− and +/− mice (Fig. 6A,B). The same results were obtained when CD11chiMHCIIhi DCs were defined by CD24 and SIRPα: numbers of SIRPαloCD24hi (P3) DCs were reduced while SIRPα+CD24hi (P1) DCs were increased in both CD11c-cre-Irf8 −/− and +/− mice (Fig. 6C,D). Numbers of SIRPα+CD24int (P2) DCs did not change in the CD11c-cre-Irf8 −/− and +/− mice (Fig. 6D). Thus, one copy of the Irf8 gene is insufficient to promote lung CD11chiMHCIIhiCD103+ DC development, and 0 or 1 copies of Irf8 leads to increased differentiation of CD11b+SIRPα+CD24hi DCs. Interestingly, in CD11c-cre-Irf8 −/− and +/− mice, IRF4 protein levels were increased in the P2 but not the P1 DC subset (Fig. 6E), indicating that the increased numbers of the IRF4-dependent P1 DCs is not due to their significantly increased IRF4 levels in mature DCs.
Fig. 6. IRF8 deficiency in CD11c+ cells alters numbers of both IRF8- and IRF4-dependent lung and spleen DCs.
(A) CD11chiMHCIIhi DCs (gate R1) in the lungs of CD11c-cre-Irf8 mice were gated as in Fig. 1A and divided into CD11b+ and CD103+ subsets; a comparison of +/+, +/− and −/− mice is shown. (B) The total numbers of CD11b+ and CD103+ DC subsets in multiple +/+, +/− and −/− mice are compiled; shown are values for individual mice, n=10–15 per genotype. (C) Lung CD11chiMHCIIhi cells in gate R1 (see Fig. 1A) are divided into subsets based on SIRPα and CD24; a comparison of +/+, +/− and −/− mice is shown. (D) The total numbers of SIRPα+CD24hi (P1), SIRPα+CD24int (P2) and SIRPαloCD24hi (P3) DC subsets in multiple +/+, +/− and −/− mice are compiled, n=7–8 per genotype. For panels B and D, the significance of the data was evaluated using a one-way ANOVA with a Tukey’s multiple comparisons test; *p<0.05, **p<0.01, ****p<0.0001. (E) The mean fluorescence intensity (MFI) of anti-IRF4 and anti-IRF8 binding is shown for each lung cDC subset (P1, P2, P3) in CD11c-cre-Irf8 +/+, +/− and −/− mice. The # indicates the nonspecific binding of the anti-IRF8 Ab to Irf8 −/− cells. X indicates that the P3 subset is absent in the +/− and −/− mice. The significance of the data was evaluated using a one-way ANOVA (P1, P2); *p<0.05, **p<0.01, n=3 per genotype. (F) Splenic CD11chiMHCIIhi cells (gated as in Fig. 3A) are divided into CD4+ and CD8α+ subsets; a comparison of +/+, +/− and −/− mice is shown. (G) The total numbers of CD4+ and CD8α+ splenic DC subsets in multiple +/+, +/− and −/− mice are compiled; shown are values for individual mice, n=5–11 per genotype.
CD11c-cre-Irf8 −/− and +/− mice did not show differences in the numbers of lung CD11cintSiglecFloCD64−MHCIIhi DCs (Fig. S2I), yet the Irf8 −/− DCs expressed elevated levels of IRF4 (Fig. S2J), as observed for the CD11chi P2 population (Fig. 6E). Numbers of CD11chiSiglecFhiCD64+ alveolar macrophages also were unaffected by Irf8 deficiency (Fig. S2K). However, in CD11c-cre-Irf8 −/− mice, the alveolar macrophages displayed reduced levels of CD64 (FcγRI); this was not observed on alveolar macrophages in CD11c-cre-Irf4 −/− mice (Fig. S2H,L). This suggests that the Irf8 −/− macrophages may be functionally impaired.
IRF8 deficiency in CD11c+ cells alters both IRF8 and IRF4-dependent splenic DC differentiation
Consistent with previous reports in globally Irf8-deficient or mutant mice (42), in CD11c-cre-Irf8 −/− and +/− mice, we observed a reduction in the percentages and numbers of splenic CD4−CD8+ (CD11b−) DCs (Fig. 6F–G). These data show that a reduction of IRF8 activity beginning at the pre-cDC stage decreases the development of splenic CD4−CD8+ DCs. The CD11c-cre-Irf8 −/− mice also showed a significant increase in the numbers of CD4+CD8− (CD11b+) DCs (Fig. 6G). This increment in CD4+CD8− DCs was not in proportion to the reduction in CD4−CD8+ DCs, but suggests that the absence of IRF8 expression potentiates the development of IRF4-dependent DCs, as we observed in the lung. Numbers of CD4−CD8− (CD11b+) DCs were not affected by IRF8 deficiency (Fig. 6G).
IRF8 deficiency in CD11c+ cells leads to decreased numbers of splenic pre-cDCs expressing elevated levels of IRF4
While numbers of pre-cDCs in the BM of CD11c-cre-Irf8 −/−, +/− and +/+ mice were similar (Fig. 7A), the +/− and −/− mice harbored an IRF8-dose dependent decrease in the numbers of pre-cDCs in the spleen (Fig. 7B–C). Intracellular staining with IRF4- and IRF8-specific mAbs showed that BM pre-cDCs in +/+ mice expressed uniform levels of IRF4 and IRF8, which did not permit identification of pre-cDC subsets (Fig. 7D–G). In the −/− mice, not all of the pre-cDCs appeared to have deleted the Irf8 gene as approximately half of the cells expressed IRF8 (Fig. 7D). Notably, gating on the IRF8− pre-cDCs in the BM of −/− mice revealed an increase in IRF4 expression relative to the IRF8+ pre-cDCs (Fig. 7D–G). Taken together, these data show that IRF8 deficiency reduces numbers of splenic pre-cDCs, and the remaining pre-cDCs express elevated IRF4 compared to +/+ pre-cDCs. This elevated IRF4 in pre-cDCs likely explains the increased development of IRF4-dependent CD11b+ DCs in the lung and spleens of CD11c-cre-Irf8 −/− mice (Fig. 6).
Discussion
Through studies of CD11c-cre-Irf4 −/− and +/− mice, we determined if IRF4 deficiency or haploinsufficiency in CD11c+ cells altered the numbers of pre-cDCs and specific DC subsets in the lung, dermis and spleen. Herein, we have shown that CD11c-Cre-Irf4 −/− mice selectively lacked a lung-resident CD11b+CD11chiSIRPα+CD24hiMHCIIhi DC subset, while other lung CD11b+ CD64− (CD11chi or CD11cint) DC subsets and CD11chi alveolar macrophages were present in normal numbers. Consistent with prior studies of globally Irf4 −/− mice, the CD11c-Cre-Irf4 −/− and +/− mice also showed a gene dose-dependent reduction in splenic CD4+CD11b+ DCs, but no defect in numbers of CD11b+ dermal DCs. Measurement of IRF4 and IRF8 protein levels revealed that IRF4 is expressed at the highest levels in CD11b+ DCs and less so in Irf8-dependent DCs in the spleen and lung, while IRF8 is expressed almost exclusively in Irf8-dependent DCs. A reduced amount of IRF4 protein was present in +/− CD11b+CD11chi DCs, but this amount was ~75% of the amount in +/+ DCs, consistent with near normal numbers of the IRF4-dependent DCs in +/− mice. IRF4 reduction or absence led to minimal changes in IRF8 expression. Irf4 deficiency (as judged by GFP expression) did not alter numbers of pre-cDCs in the bone marrow and spleen, or pre-cDC expression of IRF8; this was consistent with normal numbers of IRF8-dependent DCs in the CD11c-cre-Irf4 −/− and +/− mice.
Similar experiments were done with CD11c-cre-Irf8 −/− and +/− mice, in which Irf8 is deleted in CD11c+ cells. CD11c-cre-Irf8 −/− and +/− mice both lacked lung-resident CD103+ DCs, indicating that a single copy of Irf8 leads to insufficient amounts of IRF8 required to promote their development. In contrast, the spleens of CD11c-cre-Irf8 −/− and +/− mice showed an Irf8 dose-dependent reduction in CD4−CD8+ DCs. Irf8 deficiency led to a reduced number of splenic pre-cDCs with increased IRF4 expression relative to +/+ mice, which correlated with increased numbers of IRF4-dependent DCs in the spleen and lung. Taken together, these data from CD11c-cre-Irf8 −/− and CD11c-cre-Irf4 −/− mice show that IRF4 and IRF8 are critically required in pre-cDCs to direct the terminal differentiation of select cDC subsets in the lung and spleen.
Adoptive transfer and lineage tracing studies have shown that lung resident CD11b+ DCs arise from both pre-cDCs and monocytes in homeostasis (28, 29, 41, 43, 44). The CD11b+ population is heterogeneous, and these initial studies did not clarify if distinct subsets of lung resident CD11b+MHCIIhi DCs were derived from different precursors. Here we have subdivided the CD11b+MHCIIhi DCs to show that the CD11chiSIRPα+CD24hi subset is uniquely dependent on IRF4 expression in CD11c+ cells, while two other CD64− subsets, CD11chiSIRPα+CD24int and CD11cintSIRPα+CD24int, are not. This suggests that the Irf4-dependent lung CD11b+ DCs arise from CD11c+ pre-cDCs, while the Irf4-independent CD11b+ DCs arise from CD11c− monocytes. However, it is formally possible that Irf4-dependent CD11b+ DCs also arise from monocytes, and are deficient in these mice because IRF4 is required at a later CD11c+ stage of the monocyte to DC differentiation pathway. This latter possibility is supported by studies showing that a significant proportion of lung CD11b+ DCs originate from monocytes (41, 43).
A prior report using the same CD11c-cre-Irf4 −/− mice showed that numbers of lung resident CD11b+ tissue DCs were reduced but not absent (6). Based on evidence for increased apoptosis of these CD11b+ DCs, the conclusion was made that IRF4 was required in mature CD11b+ DCs for their survival, but not their development from pre-cDCs. In contrast, our more detailed separation of the CD11chi CD11b+ tissue DC subset in the lung revealed that in fact one CD11b+ DC subset (SIRPα+CD24hi) is absent, while a second CD11b+ DC subset (SIRPα+CD24int) is present in normal numbers. Furthermore, levels of activated caspase 3 in the remaining CD11b+ DCs were low in CD11c-cre-Irf4 −/− mice, suggesting that their survival was not impaired. Thus, we favor the interpretation that IRF4 is selectively required for the terminal differentiation of the CD11chiSIRPα+CD24hi subset of lung CD11b+ DCs from pre-cDCs.
Our finding is consistent with a recent report that a developmental requirement for KLF4 subdivides IRF4-expressing CD11b+ DCs. KLF4 is required for the differentiation of lung CD11chiSIRPα+CD24hi DCs that promote Th2 responses but not CD11chiSIRPα+CD24int DCs that promote Th17 responses (10). This may help to explain observations in CD11c-cre-Irf4 −/− mice that IRF4-dependent DCs are required for both Th2 and Th17 responses. The deficit of Th17 responses upon Aspergillus fumigatus challenge of CD11c-cre-Irf4 −/− mice (6) is likely due to IRF4 deficiency in the CD11chiSIRPα+CD24int DCs, which are present in the lungs but may lack capacity to produce IL-23 or migrate to LNs.
It is surprising that Irf4 deficiency restricted to CD11c+ cells led to differential effects on CD11c+CD11b+MHCIIhi DCs in the lung and the dermis. While a subset of CD11b+ lung DCs expresses and depends on IRF4 expression as outlined above, our data show that numbers of CD11b+ DCs in the dermis were not reduced by this same Irf4 deficiency. In fact, numbers of dermal CD11b+ DCs were increased in CD11c-cre-Irf4 −/− mice, consistent with their inability to migrate to LN, as we and others observed previously (11, 25). While both pre-cDCs and monocytes were reported to give rise to lung and dermal CD11b+ DCs, it is notable that lung CD11b+ DCs were found to be much more dependent on Flt3L (29). Thus, our data suggest that the Flt3L-driven IRF4-dependent pathway for pre-cDC development into CD11b+ DCs proceeds to a greater extent in the lung than in the dermis. Development of splenic CD4+CD11b+ DCs also was significantly impaired with Irf4 deficiency restricted to CD11c+ cells, consistent with prior work showing that most splenic DCs develop from pre-cDCs (45). The distinct developmental pathways of CD11b+ DCs in different peripheral tissues may arise due to differences in homeostatic tissue microenvironments, such as levels of Flt3L, M-CSF or GM-CSF directing pre-cDC-derived vs. monocyte-derived DC differentiation, or the production of chemokines that preferentially attract monocytes or pre-cDCs.
Recent reports demonstrated the subdivision of the pre-cDC population into discrete precursors for cDC1s and cDC2s (30, 31). Pre-cDC1s preferentially express Irf8 and Batf3 mRNA while pre-cDC2s preferentially express Irf4 mRNA. Our own attempts to subdivide the pre-cDCs into discrete populations based on relative IRF4 and IRF8 protein expression were unsuccessful. This could be due to incomplete deletion of the Irf4 or Irf8 genes since use of the GFP reporter showed that the CD11c-cre did not act in all CD11cint pre-cDCs in the CD11c-cre-Irf4 −/− mice (Fig. 5B). Alternately, Grajales-Reyes et al reported that pre-cDC2s do express IRF8 directly ex vivo, and the IRF8 is lost as the pre-cDC2s differentiate in vitro (30).
We investigated the effect of Irf4 and Irf8 deficiency beginning in the pre-cDCs, thus bypassing an effect on CDPs. Irf4 deficiency (as judged by GFP expression) did not apparently change numbers of total pre-cDCs or their expression of IRF8, consistent with unchanged numbers of IRF8-dependent DCs in the lung and spleen. Thus, IRF4 is apparently not required for maintenance of pre-cDC2s, although we cannot rule this out definitively since we were unable to assess IRF4 expression and GFP indicating Irf4 deletion at the same time using intracellular flow cytometry. Furthermore, the absence of IRF4 does not significantly alter IRF8 expression nor divert pre-cDC2s into the cDC1 lineage. In contrast, Irf8 deficiency led to normal numbers of BM pre-cDCs expressing higher levels of IRF4 and a reduction in splenic pre-cDC numbers, which correlated with increased numbers of IRF4-dependent lung and splenic DCs. This suggests that IRF8 is required for maintenance of a pre-cDC1 population in the spleen, and that the absence of IRF8 allows greater expression of IRF4 in the remaining pre-cDC2s. Indeed, in Batf3−/− mice lacking appropriate IRF8 autoactivation, the pre-cDC1s did not commit to the CD8+ DC lineage, and were diverted into the CD4+CD11b+ lineage (30). More studies are needed to determine how Irf8 deficiency might modulate the expression or activity of IRF4 in pre-cDC subsets. Our data show that IRF4 protein levels in pre-cDCs are low, which is consistent with low Irf4 mRNA levels reported for pre-cDC2s (30). Thus, the substantial increase in IRF4 expression in the absence of IRF8 is notable and suggests that IRF8 may act to limit IRF4. IRF4 activity also may increase in the absence of IRF8, since IRF4 and IRF8 partner with common transcription factors such as PU.1 or BATF (46).
We also investigated the effect of Irf4 or Irf8 haploinsufficiency on cDC development. A single copy of Irf4 in CD11c+ cells led to reduced IRF4 protein levels in lung and spleen cDCs; this correlated with reduced numbers of CD4+ splenic DCs but not lung CD11b+ DCs, suggesting that a single copy of Irf4 produces sufficient amount of IRF4 protein to drive DC differentiation. In contrast, a single copy of Irf8 in CD11c+ cells did not support development of lung CD103+ DCs, while numbers of CD8+ cDCs in the spleen were reduced but not absent. This suggests that development of the lung resident CD103+ cDCs is critically dependent on threshold levels of IRF8. Indeed, the amount of IRF8 produced from a single copy of Irf8 was reported to be significantly less than 50%, consistent with the requirement for IRF8 to autoregulate its own transcription to maintain cDC1 fate (30). BATF3 is needed for this autoactivation of IRF8 (30), and levels of Batf3 mRNA were reported to be lower in lung CD103+ DCs than spleen CD8+ DCs (13). This lesser amount of BATF3 likely decreases the amount of IRF8 produced from a single copy of Irf8, leading to deficient development of lung CD103+ DCs in Irf8 +/− mice.
In future studies, we can expect to learn more about how variable levels of transcription factors regulate immune responses, as an increasing number of reports describe gene dosage effects of regulatory factors involved in DC biology (47). Indeed, polymorphisms in human IRF8 have been linked to autoimmune diseases such as systemic lupus erythematosus (32), while IRF4 variants predispose to nevi and melanoma and have been linked to lymphocytic leukemia (33, 48). Although we lack information about how most of these polymorphisms alter protein levels of IRF4 or IRF8 in DCs or other immune cells, one mutation in IRF8 leads to CD1c+ DC deficiency and defects in antimycobacterial immunity (36). Identified polymorphisms in the human IRF4 promoter change transcription factor binding and gene expression levels (34, 35). Interestingly, Irf4 RNA levels also are increased or decreased by physiological cues such as prostaglandins and estrogens (40, 49). Thus, consistent with our data obtained from these murine models during homeostasis, variable IRF4 or IRF8 expression resulting from genetic polymorphisms or environmental stimuli may govern tissue DC numbers, and therefore regulate the magnitude of DC functional responses during inflammation.
Supplementary Material
Acknowledgements
We thank Dr. José Alberola-Ila for helpful discussions and Mike Jones for assistance with the animal colony.
Abbreviations
- AM
alveolar macrophage
- BM
bone marrow
- CDP
common DC progenitor
- cDC1 and cDC2
conventional DC type 1 or 2
- DC
dendritic cell
- IRF4 and IRF8
interferon regulatory factor 4 and 8
- pre-cDC
precursor of cDCs
Footnotes
Supported by NIH HL119501
References
- 1.Braciale TJ, Sun J, Kim TS. Regulating the adaptive immune response to respiratory virus infection. Nat Rev Immunol. 2012;12:295–305. doi: 10.1038/nri3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lambrecht BN, Hammad H. Lung dendritic cells in respiratory viral infection and asthma: from protection to immunopathology. Annu Rev Immunol. 2012;30:243–270. doi: 10.1146/annurev-immunol-020711-075021. [DOI] [PubMed] [Google Scholar]
- 3.Satpathy AT, Wu X, Albring JC, Murphy KM. Re(de)fining the dendritic cell lineage. Nat Immunol. 2012;13:1145–1154. doi: 10.1038/ni.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belz GT, Nutt SL. Transcriptional programming of the dendritic cell network. Nat Rev Immunol. 2012;12:101–113. doi: 10.1038/nri3149. [DOI] [PubMed] [Google Scholar]
- 5.Miller JC, Brown BD, Shay T, Gautier EL, Jojic V, Cohain A, Pandey G, Leboeuf M, Elpek KG, Helft J, Hashimoto D, Chow A, Price J, Greter M, Bogunovic M, Bellemare-Pelletier A, Frenette PS, Randolph GJ, Turley SJ, Merad M. Deciphering the transcriptional network of the dendritic cell lineage. Nat Immunol. 2012;13:888–899. doi: 10.1038/ni.2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schlitzer A, McGovern N, Teo P, Zelante T, Atarashi K, Low D, Ho AW, See P, Shin A, Wasan PS, Hoeffel G, Malleret B, Heiseke A, Chew S, Jardine L, Purvis HA, Hilkens CM, Tam J, Poidinger M, Stanley ER, Krug AB, Renia L, Sivasankar B, Ng LG, Collin M, Ricciardi-Castagnoli P, Honda K, Haniffa M, Ginhoux F. IRF4 transcription factor-dependent CD11b+ dendritic cells in human and mouse control mucosal IL-17 cytokine responses. Immunity. 2013;38:970–983. doi: 10.1016/j.immuni.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Persson EK, Uronen-Hansson H, Semmrich M, Rivollier A, Hagerbrand K, Marsal J, Gudjonsson S, Hakansson U, Reizis B, Kotarsky K, Agace WW. IRF4 transcription-factor-dependent CD103(+)CD11b(+) dendritic cells drive mucosal T helper 17 cell differentiation. Immunity. 2013;38:958–969. doi: 10.1016/j.immuni.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 8.Williams JW, Tjota MY, Clay BS, Vander Lugt B, Bandukwala HS, Hrusch CL, Decker DC, Blaine KM, Fixsen BR, Singh H, Sciammas R, Sperling AI. Transcription factor IRF4 drives dendritic cells to promote Th2 differentiation. Nat Commun. 2013;4:2990. doi: 10.1038/ncomms3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao Y, Nish SA, Jiang R, Hou L, Licona-Limon P, Weinstein JS, Zhao H, Medzhitov R. Control of T Helper 2 Responses by Transcription Factor IRF4-Dependent Dendritic Cells. Immunity. 2013;39:722–732. doi: 10.1016/j.immuni.2013.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tussiwand R, Everts B, Grajales-Reyes GE, Kretzer NM, Iwata A, Bagaitkar J, Wu X, Wong R, Anderson DA, Murphy TL, Pearce EJ, Murphy KM. Klf4 expression in conventional dendritic cells is required for T helper 2 cell responses. Immunity. 2015;42:916–928. doi: 10.1016/j.immuni.2015.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akbari M, Honma K, Kimura D, Miyakoda M, Kimura K, Matsuyama T, Yui K. IRF4 in dendritic cells inhibits IL-12 production and controls Th1 immune responses against Leishmania major. J Immunol. 2014;192:2271–2279. doi: 10.4049/jimmunol.1301914. [DOI] [PubMed] [Google Scholar]
- 12.Kim TS, Braciale TJ. Respiratory dendritic cell subsets differ in their capacity to support the induction of virus-specific cytotoxic CD8+ T cell responses. PLoS One. 2009;4:e4204. doi: 10.1371/journal.pone.0004204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edelson BT, Wumesh KC, Juang R, Kohyama M, Benoit LA, Klekotka PA, Moon C, Albring JC, Ise W, Michael DG, Bhattacharya D, Stappenbeck TS, Holtzman MJ, Sung SS, Murphy TL, Hildner K, Murphy KM. Peripheral CD103+ dendritic cells form a unified subset developmentally related to CD8alpha+ conventional dendritic cells. J Exp Med. 2010;207:823–836. doi: 10.1084/jem.20091627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Helft J, Manicassamy B, Guermonprez P, Hashimoto D, Silvin A, Agudo J, Brown BD, Schmolke M, Miller JC, Leboeuf M, Murphy KM, García-Sastre A, Merad M. Cross-presenting CD103+ dendritic cells are protected from influenza virus infection. J Clin Invest. 2012;122:4037–4047. doi: 10.1172/JCI60659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu CI, Becker C, Wang Y, Marches F, Helft J, Leboeuf M, Anguiano E, Pourpe S, Goller K, Pascual V, Banchereau J, Merad M, Palucka K. Human CD1c+ dendritic cells drive the differentiation of CD103+ CD8+ mucosal effector T cells via the cytokine TGF-β. Immunity. 2013;38:818–830. doi: 10.1016/j.immuni.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ballesteros-Tato A, Leon B, Lund FE, Randall TD. Temporal changes in dendritic cell subsets, cross-priming and costimulation via CD70 control CD8(+) T cell responses to influenza. Nat Immunol. 2010;11:216–224. doi: 10.1038/ni.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haniffa M, Shin A, Bigley V, McGovern N, Teo P, See P, Wasan PS, Wang XN, Malinarich F, Malleret B, Larbi A, Tan P, Zhao H, Poidinger M, Pagan S, Cookson S, Dickinson R, Dimmick I, Jarrett RF, Renia L, Tam J, Song C, Connolly J, Chan JK, Gehring A, Bertoletti A, Collin M, Ginhoux F. Human tissues contain CD141hi cross-presenting dendritic cells with functional homology to mouse CD103+ nonlymphoid dendritic cells. Immunity. 2012;37:60–73. doi: 10.1016/j.immuni.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Helft J, Ginhoux F, Bogunovic M, Merad M. Origin and functional heterogeneity of non-lymphoid tissue dendritic cells in mice. Immunol Rev. 2010;234:55–75. doi: 10.1111/j.0105-2896.2009.00885.x. [DOI] [PubMed] [Google Scholar]
- 19.Murphy KM. Transcriptional control of dendritic cell development. Adv Immunol. 2013;120:239–267. doi: 10.1016/B978-0-12-417028-5.00009-0. [DOI] [PubMed] [Google Scholar]
- 20.Jakubzick C, Gautier EL, Gibbings SL, Sojka DK, Schlitzer A, Johnson TE, Ivanov S, Duan Q, Bala S, Condon T, van Rooijen N, Grainger JR, Belkaid Y, Ma’ayan A, Riches DW, Yokoyama WM, Ginhoux F, Henson PM, Randolph GJ. Minimal differentiation of classical monocytes as they survey steady-state tissues and transport antigen to lymph nodes. Immunity. 2013;39:599–610. doi: 10.1016/j.immuni.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Plantinga M, Guilliams M, Vanheerswynghels M, Deswarte K, Branco-Madeira F, Toussaint W, Vanhoutte L, Neyt K, Killeen N, Malissen B, Hammad H, Lambrecht BN. Conventional and monocyte-derived CD11b(+) dendritic cells initiate and maintain T helper 2 cell-mediated immunity to house dust mite allergen. Immunity. 2013;38:322–335. doi: 10.1016/j.immuni.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 22.Nakano H, Moran TP, Nakano K, Gerrish KE, Bortner CD, Cook DN. Complement receptor C5aR1/CD88 and dipeptidyl peptidase-4/CD26 define distinct hematopoietic lineages of dendritic cells. J Immunol. 2015;194:3808–3819. doi: 10.4049/jimmunol.1402195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tamura T, Tailor P, Yamaoka K, Kong HJ, Tsujimura H, O’Shea JJ, Singh H, Ozato K. IFN regulatory factor-4 and -8 govern dendritic cell subset development and their functional diversity. J Immunol. 2005;174:2573–2581. doi: 10.4049/jimmunol.174.5.2573. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki S, Honma K, Matsuyama T, Suzuki K, Toriyama K, Akitoyo I, Yamamoto K, Suematsu T, Nakamura M, Yui K, Kumatori A. Critical roles of interferon regulatory factor 4 in CD11bhighCD8alpha- dendritic cell development. Proc Natl Acad Sci U S A. 2004;101:8981–8986. doi: 10.1073/pnas.0402139101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bajana S, Roach K, Turner S, Paul J, Kovats S. IRF4 Promotes Cutaneous Dendritic Cell Migration to Lymph Nodes during Homeostasis and Inflammation. J Immunol. 2012;189:3368–3377. doi: 10.4049/jimmunol.1102613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Becker AM, Michael DG, Satpathy AT, Sciammas R, Singh H, Bhattacharya D. IRF-8 extinguishes neutrophil production and promotes dendritic cell lineage commitment in both myeloid and lymphoid mouse progenitors. Blood. 2012;119:2003–2012. doi: 10.1182/blood-2011-06-364976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naik SH, Metcalf D, van Nieuwenhuijze A, Wicks I, Wu L, O’Keeffe M, Shortman K. Intrasplenic steady-state dendritic cell precursors that are distinct from monocytes. Nat Immunol. 2006;7:663–671. doi: 10.1038/ni1340. [DOI] [PubMed] [Google Scholar]
- 28.Liu K, Victora GD, Schwickert TA, Guermonprez P, Meredith MM, Yao K, Chu FF, Randolph GJ, Rudensky AY, Nussenzweig M. In vivo analysis of dendritic cell development and homeostasis. Science. 2009;324:392–397. doi: 10.1126/science.1170540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ginhoux F, Liu K, Helft J, Bogunovic M, Greter M, Hashimoto D, Price J, Yin N, Bromberg J, Lira SA, Stanley ER, Nussenzweig M, Merad M. The origin and development of nonlymphoid tissue CD103+ DCs. J Exp Med. 2009;206:3115–3130. doi: 10.1084/jem.20091756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grajales-Reyes GE, Iwata A, Albring J, Wu X, Tussiwand R, Kc W, Kretzer NM, Briseño CG, Durai V, Bagadia P, Haldar M, Schönheit J, Rosenbauer F, Murphy TL, Murphy KM. Batf3 maintains autoactivation of Irf8 for commitment of a CD8α(+) conventional DC clonogenic progenitor. Nat Immunol. 2015;16:708–717. doi: 10.1038/ni.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schlitzer A, Sivakamasundari V, Chen J, Sumatoh HR, Schreuder J, Lum J, Malleret B, Zhang S, Larbi A, Zolezzi F, Renia L, Poidinger M, Naik S, Newell EW, Robson P, Ginhoux F. Identification of cDC1- and cDC2-committed DC progenitors reveals early lineage priming at the common DC progenitor stage in the bone marrow. Nat Immunol. 2015;16:718–728. doi: 10.1038/ni.3200. [DOI] [PubMed] [Google Scholar]
- 32.Lessard CJ, Adrianto I, Ice JA, Wiley GB, Kelly JA, Glenn SB, Adler AJ, Li H, Rasmussen A, Williams AH, Ziegler J, Comeau ME, Marion M, Wakeland BE, Liang C, Ramos PS, Grundahl KM, Gallant CJ, Alarcón-Riquelme ME, Alarcón GS, Anaya JM, Bae SC, Boackle SA, Brown EE, Chang DM, Cho SK, Criswell LA, Edberg JC, Freedman BI, Gilkeson GS, Jacob CO, James JA, Kamen DL, Kimberly RP, Kim JH, Martin J, Merrill JT, Niewold TB, Park SY, Petri MA, Pons-Estel BA, Ramsey-Goldman R, Reveille JD, Scofield RH, Song YW, Stevens AM, Tsao BP, Vila LM, Vyse TJ, Yu CY, Guthridge JM, Kaufman KM, Harley JB, Wakeland EK, Langefeld CD, Gaffney PM, Montgomery CG, Moser KL, BIOLUPUS N, GENLES N. Identification of IRF8, TMEM39A, and IKZF3-ZPBP2 as susceptibility loci for systemic lupus erythematosus in a large-scale multiracial replication study. Am J Hum Genet. 2012;90:648–660. doi: 10.1016/j.ajhg.2012.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duffy DL, Iles MM, Glass D, Zhu G, Barrett JH, Höiom V, Zhao ZZ, Sturm RA, Soranzo N, Hammond C, Kvaskoff M, Whiteman DC, Mangino M, Hansson J, Newton-Bishop GenoMEL JA, Bataille V, Hayward NK, Martin NG, Bishop DT, Spector TD, Montgomery GW. IRF4 variants have age-specific effects on nevus count and predispose to melanoma. Am J Hum Genet. 2010;87:6–16. doi: 10.1016/j.ajhg.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Havelange V, Pekarsky Y, Nakamura T, Palamarchuk A, Alder H, Rassenti L, Kipps T, Croce CM. IRF4 mutations in chronic lymphocytic leukemia. Blood. 2011;118:2827–2829. doi: 10.1182/blood-2011-04-350579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Do TN, Ucisik-Akkaya E, Davis CF, Morrison BA, Dorak MT. An intronic polymorphism of IRF4 gene influences gene transcription in vitro and shows a risk association with childhood acute lymphoblastic leukemia in males. Biochim Biophys Acta. 2010;1802:292–300. doi: 10.1016/j.bbadis.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 36.Hambleton S, Salem S, Bustamante J, Bigley V, Boisson-Dupuis S, Azevedo J, Fortin A, Haniffa M, Ceron-Gutierrez L, Bacon CM, Menon G, Trouillet C, McDonald D, Carey P, Ginhoux F, Alsina L, Zumwalt TJ, Kong XF, Kumararatne D, Butler K, Hubeau M, Feinberg J, Al-Muhsen S, Cant A, Abel L, Chaussabel D, Doffinger R, Talesnik E, Grumach A, Duarte A, Abarca K, Moraes-Vasconcelos D, Burk D, Berghuis A, Geissmann F, Collin M, Casanova JL, Gros P. IRF8 mutations and human dendritic-cell immunodeficiency. N Engl J Med. 2011;365:127–138. doi: 10.1056/NEJMoa1100066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klein U, Casola S, Cattoretti G, Shen Q, Lia M, Mo T, Ludwig T, Rajewsky K, Dalla-Favera R. Transcription factor IRF4 controls plasma cell differentiation and class-switch recombination. Nat Immunol. 2006;7:773–782. doi: 10.1038/ni1357. [DOI] [PubMed] [Google Scholar]
- 38.Caton ML, Smith-Raska MR, Reizis B. Notch-RBP-J signaling controls the homeostasis of CD8- dendritic cells in the spleen. J Exp Med. 2007;204:1653–1664. doi: 10.1084/jem.20062648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feng J, Wang H, Shin DM, Masiuk M, Qi CF, Morse HC. IFN regulatory factor 8 restricts the size of the marginal zone and follicular B cell pools. J Immunol. 2011;186:1458–1466. doi: 10.4049/jimmunol.1001950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carreras E, Turner S, Frank MB, Knowlton N, Osban J, Centola M, Park CG, Simmons A, Alberola-Ila J, Kovats S. Estrogen receptor signaling promotes dendritic cell differentiation by increasing expression of the transcription factor IRF4. Blood. 2010;115:238–246. doi: 10.1182/blood-2009-08-236935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jakubzick C, Bogunovic M, Bonito AJ, Kuan EL, Merad M, Randolph GJ. Lymph-migrating, tissue-derived dendritic cells are minor constituents within steady-state lymph nodes. J Exp Med. 2008;205:2839–2850. doi: 10.1084/jem.20081430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schiavoni G, Mattei F, Sestili P, Borghi P, Venditti M, Morse HC, Belardelli F, Gabriele L. ICSBP is essential for the development of mouse type I interferon-producing cells and for the generation and activation of CD8alpha(+) dendritic cells. J Exp Med. 2002;196:1415–1425. doi: 10.1084/jem.20021263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jakubzick C, Tacke F, Ginhoux F, Wagers AJ, van Rooijen N, Mack M, Merad M, Randolph GJ. Blood monocyte subsets differentially give rise to CD103+ and CD103- pulmonary dendritic cell populations. J Immunol. 2008;180:3019–3027. doi: 10.4049/jimmunol.180.5.3019. [DOI] [PubMed] [Google Scholar]
- 44.Varol C, Landsman L, Fogg DK, Greenshtein L, Gildor B, Margalit R, Kalchenko V, Geissmann F, Jung S. Monocytes give rise to mucosal, but not splenic, conventional dendritic cells. J Exp Med. 2007;204:171–180. doi: 10.1084/jem.20061011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Waskow C, Liu K, Darrasse-Jeze G, Guermonprez P, Ginhoux F, Merad M, Shengelia T, Yao K, Nussenzweig M. The receptor tyrosine kinase Flt3 is required for dendritic cell development in peripheral lymphoid tissues. Nat Immunol. 2008;9:676–683. doi: 10.1038/ni.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murphy TL, Tussiwand R, Murphy KM. Specificity through cooperation: BATF-IRF interactions control immune-regulatory networks. Nat Rev Immunol. 2013;13:499–509. doi: 10.1038/nri3470. [DOI] [PubMed] [Google Scholar]
- 47.Dakic A, Wu L, Nutt SL. Is PU.1 a dosage-sensitive regulator of haemopoietic lineage commitment and leukaemogenesis? Trends Immunol. 2007;28:108–114. doi: 10.1016/j.it.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 48.Di Bernardo MC, Crowther-Swanepoel D, Broderick P, Webb E, Sellick G, Wild R, Sullivan K, Vijayakrishnan J, Wang Y, Pittman AM, Sunter NJ, Hall AG, Dyer MJ, Matutes E, Dearden C, Mainou-Fowler T, Jackson GH, Summerfield G, Harris RJ, Pettitt AR, Hillmen P, Allsup DJ, Bailey JR, Pratt G, Pepper C, Fegan C, Allan JM, Catovsky D, Houlston RS. A genome-wide association study identifies six susceptibility loci for chronic lymphocytic leukemia. Nat Genet. 2008;40:1204–1210. doi: 10.1038/ng.219. [DOI] [PubMed] [Google Scholar]
- 49.Valdez PA, Vithayathil PJ, Janelsins BM, Shaffer AL, Williamson PR, Datta SK. Prostaglandin E2 suppresses antifungal immunity by inhibiting interferon regulatory factor 4 function and interleukin-17 expression in T cells. Immunity. 2012;36:668–679. doi: 10.1016/j.immuni.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.