Abstract
The neocortex is the site of origin of several forms of acquired epilepsy. Here we provide a brief review of experimental models that were recently developed to study neocortical epileptogenesis as well as some major results obtained with these methods. Most of neocortical seizures appear to be nocturnal and it is known that neuronal activities reveal high levels of synchrony during slow-wave sleep. Therefore, we start the review with a description of mechanisms of neuronal synchronization and major forms of synchronized normal and pathological activities. Then, we describe three experimental models of seizures and epileptogenesis: ketamine-xylazine anesthesia as feline seizure triggered factor, cortical undercut as cortical penetrating wound model and neocortical kindling. Besides specific technical details describing these models we also provide major features of pathological brain activities recorded during epileptogenesis and seizures. The most common feature of all models of neocortical epileptogenesis is the increased duration of network silent states that up-regulates neuronal excitability and eventually leads to epilepsy.
Keywords: Epilepsy, epileptogenesis, seizure, trauma, kindling, undercut
1. Introduction
The term epilepsy is used to define over 40 different types of neurological pathologies resulting from different etiologies. They usually are all characterized by the occurrence of unprovoked recurrent seizures, which consists in a period of abnormal (paroxysmal) brain electrical activity. Epilepsy is either genetically determined or acquired (secondary). The causes of acquired epilepsies are multiple (stroke, cortical trauma, brain tumor, infections,…) and the recurring seizures are typically primarily focal (Timofeev et al., 2014). The common feature of acquired epilepsies is neuronal death leading to deafferentation. Brain activities could be roughly described using simple words such as activity, silence, excitation, and inhibition. During normal brain activities there is a balance between activity and silence and also between excitation and inhibition. If for any reason the balance is shifted, some homeostatic plasticity occurs to reestablish the balance. Deafferentation increases overall silence in the involved neuronal network. This triggers up-regulation of neuronal excitability. If deafferentation is large it would lead to uncontrolled up-regulation of neuronal excitability and epilepsy. A period between initial insult and development of epilepsy is called epileptogenesis and its exact mechanisms remain largely unknown, therefore, there is no effective treatment of epileptogenesis. Over the past several years, our studies aim to understand the network mechanisms of epileptogenesis in order to develop effective treatment of epileptogenesis and therefore, to prevent the development of epilepsy.
1.1 Neuronal synchronization
Neuronal synchronization is achieved via different mechanisms: chemical synaptic transmission, electrical synaptic transmission, ephaptic and non-specific interactions such as alterations in the extracellular ionic balance (reviewed in (Timofeev et al., 2012)). Chemical transmission occurs when an action potential is fired by the presynaptic neuron, invading the nerve terminal, allowing calcium to enter in the synaptic terminal, which will trigger the release of synaptic vesicle containing neurotransmitter, and finally causing depolarization or hyperpolarization of the postsynaptic neuron (Eccles, 1964; Katz and Miledi, 1968).
Electrical synapses use a spike-independent mechanism of communication between neurons connected via gap junctions allowing a direct flux of ions between them (Carlen et al., 2000; Dermietzel and Spray, 1993; Perez Velazquez and Carlen, 2000). When cells are connected by gap junctions, any change in the membrane potential in one cell will trigger a current flow in the other one leading to corresponding changes. In the neocortex, GABA-releasing interneurons (Galarreta and Hestrin, 1999; Gibson et al., 1999) and glial cells (Mugnaini, 1986) are interconnected by gap junctions. However as gap junctions have relatively high resistances, they act as low pass filters (Galarreta and Hestrin, 2001), therefore they are best situated to transmit to coupled neurons low frequency oscillations, but not fast processes, like action potentials.
Ephaptic interactions refer to influences produced by local electric fields. The extracellular currents produced by the activity of neurons constituting the local field potential might directly influence the electrical properties of surrounding neurons (Frohlich and McCormick, 2010; Jefferys, 1995). Although these influences are relatively weak, they might affect the neuronal excitability by exerting a global influence and may provide a significant impact when the network is already quite synchronized such as during slow-wave sleep or during a seizure. Normal neuronal activity leads to the opening of different ionic channels and produce short-term changes in the extracellular ionic composition. The most affected ion concentrations are for those that have a lower extracellular concentration such as calcium and potassium. For example, during a normal slow oscillation, the dominant type of activity observed during slow-wave sleep and under some anesthetics (see section 2), the extracellular calcium concentration varies from 1.2 mM during silent states to 1.0 mM during active states (Crochet et al., 2005; Massimini and Amzica, 2001), this can strongly impact the chemical synaptic transmission (Crochet et al., 2005). On the contrary, low calcium concentration promotes the opening of hemichannels (Thimm et al., 2005), which increases the electrical coupling. The changes in the extracellular milieu are even more dramatic during seizures as extracellular potassium concentration can reach up to 7–18 mM (Amzica et al., 2002; Moody et al., 1974; Prince et al., 1973) and the extracellular calcium concentration can decrease to as low as 0.4–0.7 mM (Amzica et al., 2002; Heinemann et al., 1977; Pumain and Heinemann, 1981; Pumain et al., 1983). These changes dramatically reduce the synaptic excitability and almost abolish any synaptic response (Seigneur and Timofeev, 2011). With the same levels of neuronal activity, the extent of changes in extracellular ionic concentrations should depend on extracellular space. The brain extracellular space during wake is smaller than during sleep or anesthesia (Xie et al., 2013) therefore, synchronous neuronal activity during wake should produce major changes in local ionic concentration.
At least two types of neuronal synchronization can be considered, local synchronization, which mediates generation of the local field potential and rely on all types of transmission, and long-range synchronization which is measured using distant recording electrodes and mainly relies on chemical synaptic transmission.
1.2 Disbalance in the excitation/inhibition ratio leading to seizures
During normal brain activities, the central nervous system maintains the homeostasis between the excitation and the inhibition. Under anesthesia they are balanced (Haider et al., 2006; Shu et al., 2003), while during natural states of vigilance the inhibitory activities dominate (Haider et al., 2013; Rudolph et al., 2007). Epileptiform activities are the result of a shift in the balance of excitation and inhibition (Dichter and Ayala, 1987; Galarreta and Hestrin, 1998; Nelson and Turrigiano, 1998; Tasker and Dudek, 1991; Timofeev and Steriade, 2004). The most known procedure to induce epileptiform discharges consists in blocking inhibition by using GABAergic antagonist (Bicuculline, penicillin, …), however a disbalance in the other direction: decreased excitation (or increased inhibition) as epileptogenic factor was much less investigated. The model of trauma-induced epilepsy that will be described in the main body of this review is a model in which the excitation is decreased as multiple glutamatergic thalamocortical fibers are severed by the undercut (Topolnik et al., 2003b). The undercut is a procedure in which the white matter is cut underneath a given cortical area using a custom-made knife (see section 4.1). The model of ketamine-xylazine anesthesia leading to paroxysmal activities in cats could also be considered as a model in which the excitation is decreased, as ketamine is an NMDA receptor antagonist. The third model that will be described in this review is kindling which could result from a disbalance toward excitation through hebbian synaptic plasticity. These three models will be discussed in more details in the following sections.
1.3 Focal vs generalized seizures
In the case of genetically determined epilepsies, the altered genes are usually present in every cells of the body therefore increasing the probability of generating generalized seizures. In acquired epilepsies, seizures are primarily focal but may become secondarily generalized (Timofeev et al., 2014). Focal seizures are also sometimes called partial seizures and might be further subdivided into simple or complex partial seizures depending whether the consciousness is impaired or not, respectively. Generalized seizures include absence (also called petit-mal seizures), myoclonic, clonic, tonic, tonic-clonic (also called grand-mal seizures), and atonic seizures.
1.4 Spike-wave, polyspike wave, and fast runs
Neocortical epilepsy is generated cortically, is focal and is mainly nocturnal (Timofeev, 2011). These seizures are usually composed of spike-wave/polyspike-wave (SW/PSW) electrographic discharges at 1.0–2.5 Hz and fast runs (runs of fast EEG spikes) at 7–16 Hz, which develop without discontinuity from the slow oscillation (< 1 Hz) (Boucetta et al., 2008; Steriade and Amzica, 1994; Steriade et al., 1998a; Steriade and Contreras, 1995; Timofeev, 2010; Timofeev et al., 2014; Timofeev et al., 2002).
Just prior to the onset of seizure, ripples which are waxing-and-waning very fast oscillations (80–200 Hz) can be observed at the focus (Grenier et al., 2001, 2003). The use of a gap junction blocker such as halothane anesthetic gas prevents the occurrence of ripples and stops neocortical seizures, suggesting that electrical gap junctions play a critical role in the generation of these rhythms. Following the appearance of ripples, the SW/PSW complexes occur at 1–2.5 Hz and they are often accompanied by fast runs (7–16 Hz) (Timofeev and Steriade, 2004).
1.4.1 Mechanisms of spike-wave complexes
The cortically generated seizures that arise without any discontinuity from the slow oscillation are accompanied by the generation of large amplitude field potentials SW/PSW complexes, which suggests the presence of enhanced local synchrony (Timofeev and Steriade, 2004). The precise measurements during SW/PSW complexes suggested that long-range synchrony recorded on a wave-by-wave basis is rather loose (Boucetta et al., 2008; Derchansky et al., 2006; Meeren et al., 2002; Timofeev and Steriade, 2004). During the SW complexes, the correlation of field potentials varies between 0.3 to 0.8 and each cycle would propagate at a speed of 100 mm/s in vivo and 10 mm/s in vitro (McCormick and Contreras, 2001; Steriade, 2003).
During the spike component, cortical neurons are depolarized and fire action potentials whereas during the wave component cortical neurons are hyperpolarized and silent (Timofeev, 2010). Just prior to and during the initial segment of a seizure, neocortical neurons firing increases and then it decreases toward the end of the seizure (Bazhenov et al., 2004). Any intense neuronal firing will lead to an increase in the extracellular potassium concentration and a decrease in the extracellular calcium concentration (Heinemann et al., 1977), which will change neuronal excitability (Boucetta et al., 2013; Seigneur and Timofeev, 2011) and would be an important factor in the generation of paroxysmal activities (Somjen, 2002). For example, during the spike component, fast-spiking presumed inhibitory interneurons fire high-frequency action potential trains and due to the high extracellular potassium concentration during seizure (Heinemann et al., 1977), the reversal potential of chloride-dependent IPSPs becomes depolarized (Cohen et al., 2002; Payne et al., 2003; Timofeev et al., 2002).
The strong depolarization will lead to an activation of high-threshold intrinsic currents such as the high-threshold calcium and the persistent sodium currents which will further contribute to the neuronal depolarization (Timofeev, 2010; Timofeev et al., 2004; Timofeev and Steriade, 2004). However, the increased neuronal activity will lead to an increase in the intracellular calcium and sodium concentrations, which in turn will activate both sodium- and calcium-activated potassium currents that will hyperpolarize neurons and contribute to the wave component of spike-wave complexes (Timofeev, 2010; Timofeev et al., 2004; Timofeev and Steriade, 2004). This hyperpolarization then contribute to the activation of hyperpolarization-activated depolarizing current (Ih) in a subset of neurons that will lead them to depolarize up to the firing threshold and initiate the next paroxysmal cycle (Timofeev, 2010; Timofeev et al., 2004; Timofeev and Steriade, 2004). Due to the dramatic decrease in the extracellular calcium concentration during paroxysmal activities (Heinemann et al., 1977; Pumain et al., 1983), chemical synaptic transmissions are strongly impaired but electrical synaptic transmission via gap-junction increases contributing to the local network synchronization (Jefferys, 1995).
1.4.2 Mechanisms of fast runs
Fast runs are also referred to runs of fast EEG spikes that occur at 7–16 Hz and accompany most of neocortical SW/PSW complexes. They last about 5 seconds on average but the range of their duration can vary from 1 to 30 seconds (Boucetta et al., 2008). The onset and offset of fast runs occur almost simultaneously in different recording sites, however during fast runs the different recording sites (even closely located) behave in a quasi-independent manner with low or even asynchronous patterns of coherence between them. Four different types of synchronization could be observed during fast runs, (1) synchronous and in phase, this pattern was observed in approximately 20% of cases; (2) synchronous with phase shift, this pattern was the most commonly observed (about 50% of cases); (3) patchy consisting in repeated phase/phase shift transitions, this pattern was observed in about 10% of cases; (4) non-synchronous (occurring in about 20% of cases), with slightly different frequencies in different recording sites or without the oscillatory activity in one of the recorded sites (Boucetta et al., 2008). Runs of fast EEG spikes likely originate in neocortex. This conclusion is based on two facts: (a) thalamocortical neurons do not show oscillatory activities during cortical fast runs and (b) fast runs can be easily triggered in isolated neocortical slabs (Timofeev et al., 1998). The firing of fast-spiking neurons (presumed-interneurons) is strongly reduced when not completely abolished during fast runs suggesting that inhibitory interneurons do not play any significant role in the generation of fast runs (Boucetta et al., 2008; Timofeev et al., 2002; Timofeev and Steriade, 2004). However, bursting neurons, both intrinsically-bursting and fast-rhythmic bursting neurons fire more action potentials than any other type of neurons during fast runs suggesting they might play an important role in the generation of these EEG spikes (Boucetta et al., 2008). The intrinsically-bursting neurons appear more suited to play a major role in the generation of these fast runs as upon depolarization, they generate burst of action potentials recurring at 5–15 Hz (Agmon and Connors, 1989; Connors and Gutnick, 1990) similar to the frequency of fast runs (7–16 Hz), while fast-rhythmic bursting neurons display bursts recurring at 20–50 Hz, but mainly at 30–40 Hz (Gray and McCormick, 1996; Steriade et al., 1998b). Intrinsically-bursting neurons were also shown to be depolarized and to fire earlier than other neuronal types during fast runs (Boucetta et al., 2008).
2. The slow oscillation (< 1 Hz)
In humans, sleep occupies about a third of their life and is composed of rapid-eye-movements (REM) sleep (also called paradoxical sleep) and of non-REM sleep. The non-REM sleep is then subdivided into three stages and the stage 3 (S3) is often referred to as slow-wave sleep. The hallmark of this sleep stage is the presence of large amplitude slow waves in the field potential (Blake and Gerard, 1937) and in cortical neurons (Steriade et al., 1993a, b; Steriade et al., 2001; Timofeev et al., 2001). Slow-wave sleep is characterized by a high network synchrony as virtually all cortical neurons are simultaneously active during network active state (with some delay) and virtually all cortical neurons enter the silent state simultaneously (Chauvette et al., 2010; Chen et al., 2012; Sheroziya and Timofeev, 2014; Volgushev et al., 2006). The slow oscillation has a cortical origin as it can be recorded in cortical slices (Sanchez-Vives et al., 2008; Sanchez-Vives and McCormick, 2000; Sanchez-Vives et al., 2007), in isolated cortical preparations in vivo (Timofeev et al., 2000) and in decorticated animals the thalamus does not display slow oscillation (Timofeev and Steriade, 1996). However the thalamus is likely playing a role of modulator of the slow oscillation (Crunelli and Hughes, 2010; David et al., 2013; Hughes et al., 2002; Lemieux et al., 2014). Layer 5 neurons appear to be critical in the onset of active states and for the maintenance of this rhythm (Beltramo et al., 2013; Chauvette et al., 2010; Crunelli and Hughes, 2010; Hughes and Crunelli, 2013; Sanchez-Vives and McCormick, 2000; Wester and Contreras, 2012).
3. The ketamine-xylazine anesthesia model
Ketamine-xylazine anesthesia is often used as a model to study slow-wave sleep as it reproduces the main characteristics on the natural slow oscillation (Chauvette et al., 2011; Chauvette et al., 2012; Chauvette et al., 2007; Contreras and Steriade, 1995; Sharma et al., 2010), however some differences exist such as an increased synchrony and correlation between different cortical regions, an increased rhythmicity, and an increased amplitude of the slow oscillation compared to slow-wave sleep (Chauvette et al., 2011). Using this anesthesia as initial anesthetic in cats and using ketamine only as supplemental doses was shown to induce spontaneous paroxysmal activities in about 20–30% of cats (Boucetta et al., 2008; Steriade and Contreras, 1995). However using ketamine-xylazine for both the initial anesthesia and the recall doses led to electrographic seizures in about 75% of cats (Boucetta et al., 2008). Ketamine was also reported to induce seizures in dogs (Adami et al., 2013) and rhesus macaque (Christe et al., 2013). However ketamine is used to stop refractory status epilepticus in humans (Synowiec et al., 2013).
As mentioned earlier, neocortical seizures are mainly nocturnal and they develop without discontinuity from the slow oscillation. Seizures occur much more often under ketamine-xylazine anesthesia for which the slow oscillation is much more synchronized and more rhythmic than during natural slow-wave sleep. The amplitude of spike-wave components further increases as compared to the slow oscillation under ketamine-xylazine anesthesia suggesting an enhanced local synchrony. The high incidence of SW/PSW seizures occurring under ketamine-xylazine anesthesia was hypothesized to be due to the highly synchronized activity in the corticothalamic system produced by this anesthetic (Timofeev and Steriade, 2004). Thus the higher synchrony observed under ketamine-xylazine anesthesia might be the reason for the high propensity of this type of anesthetic to induce seizures.
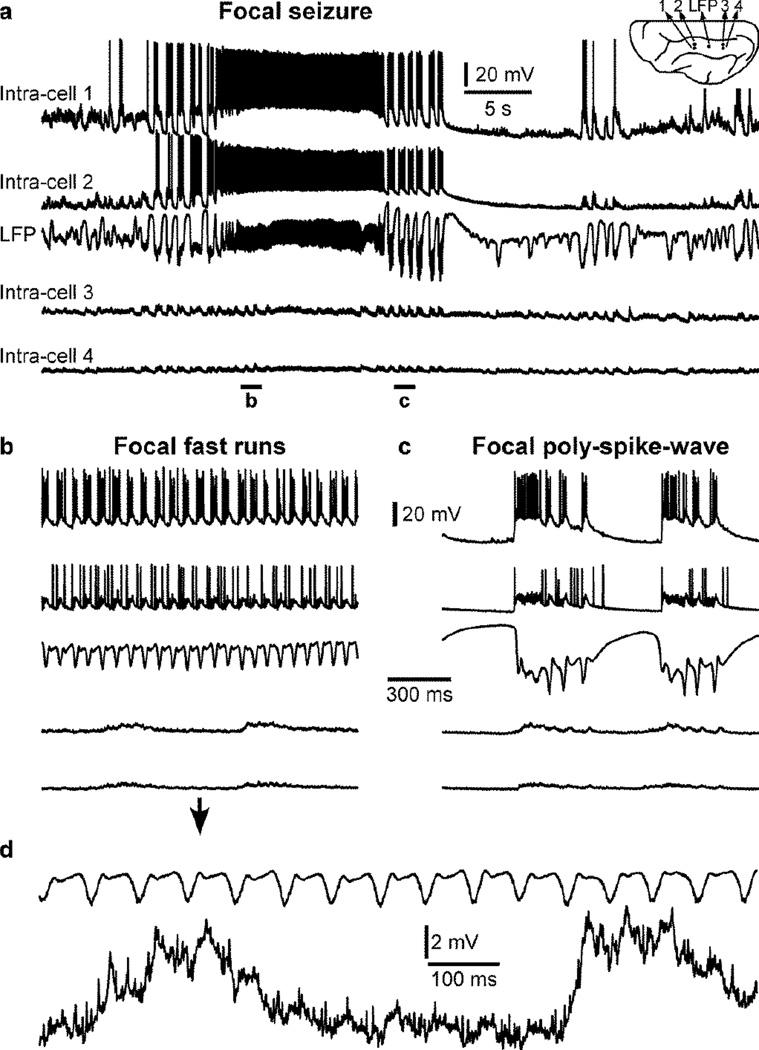
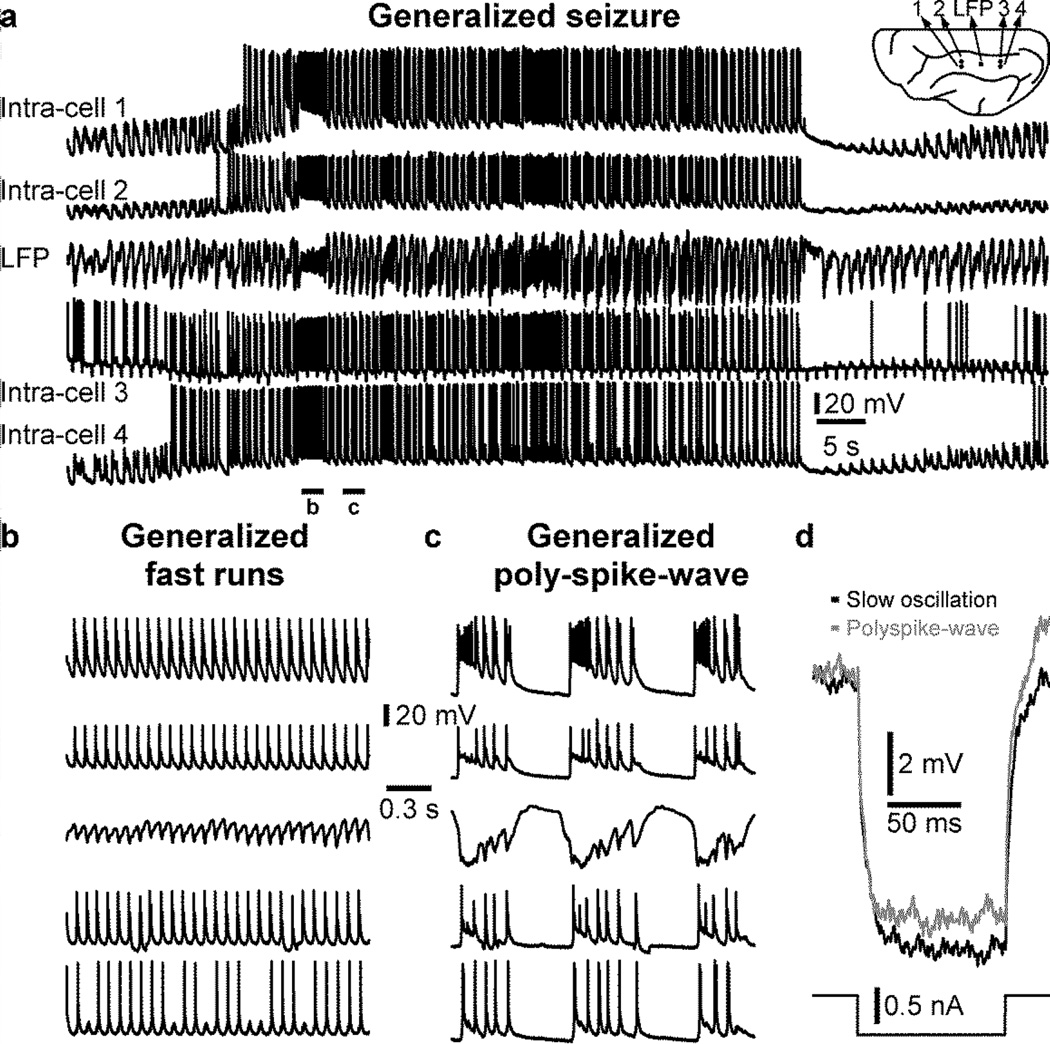
These seizures can be focal or generalized. Focal seizures can be recorded on a limited area, but not in more distant recordings (Fig. 1a). Both SW/PSW electrographic discharges and fast runs were recorded in the anterior and middle part of the suprasylvian gyrus of a cat anesthetized with ketamine-xylazine and the seizure was not recorded in electrodes located in the posterior suprasylvian gyrus (Fig. 1b,c). However, these intracellular recordings from the posterior part revealed rhythmic EPSPs with the frequency of fast runs recorded in the anterior part of the gyrus (Fig. 1d). Therefore distant locations received excitatory inputs from the seizure focus but these EPSPs were not strong enough to entrain the distant location into seizure, which could have developed into a generalized seizure. In another example, using the same recording setup, a generalized seizure was recorded (Fig. 2a). As it is often the case for seizures recorded under ketamine-xylazine anesthesia, the seizure was composed of SW/PSW and fast runs (Fig. 2b, c). As observed before (Nita et al., 2008c; Steriade et al., 1998a; Timofeev et al., 2002), we found that membrane potential deflection induced by a negative current pulse was smaller during the silent phase of the PSW component as compared to silent state during the normal slow oscillation (Fig. 2, intra-cell 3), suggesting a reduced neuronal input resistance during seizures.
Figure 1. Focal seizure recorded under ketamine-xylazine anesthesia.
a. Simultaneous quadruple intracellular and one local field potential (LFP) recordings performed in the suprasylvian gyrus of a cat anesthetized with ketamine-xylazine. The position of each recording location is indicated in the inset. Note that the seizure is recorded in the two anterior intracellular recordings as well as in the LFP recording, but not in the two most posterior intracellular recordings. The seizure is composed of fast runs (b) and polyspike-wave (c) that remain located in the anterior region of the suprasylvian gyrus. d. Zoom-in on the LFP and one posterior intracellular recording shown in (b) showing that posterior recordings receive EPSPs with the frequency of anterior fast runs but they are not contributing to the seizure.
Figure 2. Generalized seizure recorded under ketamine-xylazine anesthesia.
a. Simultaneous quadruple intracellular and one local field potential (LFP) recordings performed in the suprasylvian gyrus of a cat anesthetized with ketamine-xylazine. The position of each recording location is indicated in the inset. Note that the seizure is recorded in all electrodes. The seizure is composed of fast runs (b) and polyspike-wave (c) that are recorded in all electrodes. d. Voltage deflection induced by a 0.5 nA negative current pulse (100 ms) in intracellular recording #3 during silent state of the normal slow oscillation (black) and during the silent phase of polyspike-wave seizure (grey).
3.1 Effects of the anesthetic
Ketamine is an N-methyl-D-aspartic acid (NMDA) blocker (MacDonald et al., 1991; Sinner and Graf, 2008). Activation of NMDA receptors can contribute to the generation of paroxysmal activities although their activation is not essential (Barkai et al., 1994; Traub et al., 1996). Thus ketamine should reduce the occurrence of seizure, but the use of NMDA blocker at low concentrations could elicit epileptiform discharges (Gorji and Speckmann, 2001; Midzyanovskaya et al., 2004). Xylazine is an agonist of alpha-2 adrenoreceptors which are heavily present in the neocortex (Hedler et al., 1981; Nicholas et al., 1993) and at low doses it would favor the occurrence of seizures (Joy et al., 1983). The higher incidence of seizures (75% vs 20–30%) in animals receiving both ketamine and xylazine in recall doses as compared to animals receiving only ketamine as recall doses (Boucetta et al., 2008) suggests a leading role of xylazine in the generation of these seizures.
4. The trauma-induced epilepsy model
Traumatic brain injury (TBI) is a major risk factor for the development of epilepsy (Annegers et al., 1998; Feeney and Walker, 1979; Temkin et al., 1995). Immediate seizures (less than 24 hr after TBI) and early seizures (less than a week after TBI) are risk factors for the development of later epilepsy (Temkin, 2003). A study revealed that adult patients with moderate to severe head injury had a risk of about 86% to have recurrent seizure two years after TBI (Haltiner et al., 1997). However, a population-based USA study on patients with TBI for 3 years revealed the cumulative incidence of posttraumatic epilepsy was 4.4 per 100 persons for mild TBI, 7.6 per 100 persons for moderate TBI, and 13.6 per 100 persons for severe TBI (Ferguson et al., 2010). Cortical trauma with penetrating wound leads to paroxysmal activities in about 80% of patient within 24 hours and these seizures normally stop within 48 hours (Dinner, 1993; Kollevold, 1976). Therefore, it appears that the main determinant of the risk for post-traumatic epilepsy is the original severity of the TBI (Christensen, 2012). The trauma-induced epileptogenesis is an ensemble of latent processes that leads to the development of epilepsy following the initial insult and its mechanisms are largely unknown.
Post-war epidemiological studies revealed high incidences of recurring seizures in patients 10–20 years after penetrating cranial wounds ranging from 34 to 38% for world war I, world war II, and Korea war, about 53% for Vietnam war (Salazar et al., 1985), and about 75% for Iraq-Iran war (Eftekhar et al., 2009). The higher rate of epilepsy developed following brain trauma in more recent wars was hypothesized to be due to a longer follow-up and to the advancement in modern medicine which saves patients with so severe injuries that they would probably have died in previous wars (Kazemi et al., 2012).
Trauma-induced epilepsy is poorly controlled by the currently available medication. Most subjects with posttraumatic epilepsy receive anticonvulsants for their entire life (Raymont et al., 2010). Early administration of anticonvulsant medication decreases the percentage of early posttraumatic seizures but does not prevent chronic epilepsy (Chang and Lowenstein, 2003; D’Ambrosio and Perucca, 2004; Eftekhar et al., 2009; Kazemi et al., 2012; Temkin et al., 1999; Temkin et al., 1990).
4.1 Cortical undercut as a model of trauma-induced epileptogenesis
In the vast majority of brain-penetrating wounds, both the cortex and the white matter are damaged. The overall hypothesis of our studies is that neuronal and white matter damage induce some deafferentation that up-regulates neuronal excitability in damaged and surrounding areas, which then become an epileptogenic factor. We used a model of partial cortical deafferentation in two different species, namely cats and mice. In both preparations the idea is to do very limited damage to the cortex itself, to transect the axons (the white matter) underneath the cortex, and to study the mechanisms leading to the development of epilepsy, therefore the epileptogenesis mechanisms.
4.1.1 Cortical undercut technique in cats
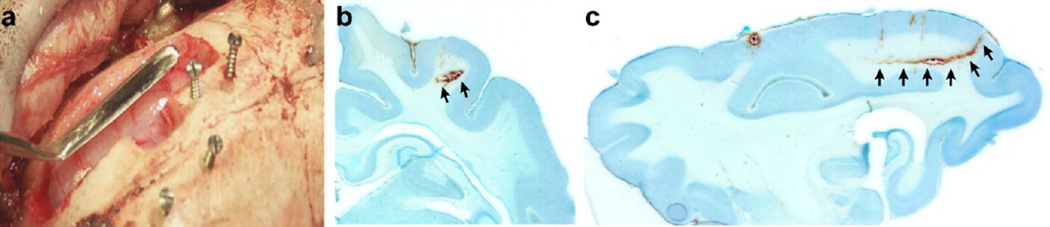
We used the cortical undercut as a model of trauma-induced epileptogenesis, and for both practical and ethical reasons, we mainly performed the cortical undercut in the associative cortical regions (areas 7 and 21) of the cats. The surgery was performed in sterile conditions under general anesthesia and approved by the animal care committee of Université Laval and in accordance with the guidelines published in the Canadian Council on Animal Care and in the National Institutes of Health guide for the care and use of laboratory animals. The details regarding the premedication content, the anesthetic used, the surgical monitoring, and the general surgical procedures can be found in our previous publications (Nita et al., 2006; Nita and Timofeev, 2007),as below we provide only a detailed procedure on how to perform the undercut itself. To perform the white matter cut underneath the cortex, a relatively large craniotomy (approximate coordinate: lateral 6 to 12 mm, anteroposterior (AP) −10 to +10 mm) was performed to expose most of the suprasylvian gyrus. A small dural opening was performed using a sterile needle in the very posterior part of the suprasylvian gyrus (around AP: - 8), where the brain curvature would allow an entry that is perpendicular to the surface at the entry point and that, when the custom-designed knife was advanced rostrally, it would be parallel to the suprasylvian gyrus (Fig. 3a). Attention was paid to avoid any major blood vessel. The knife was then entered from that opening and once slightly entered, the angle of the blade was adjusted for a rostral penetration that was parallel to the gyrus surface (Fig. 3c). The knife entry was made to cut the white matter at approximately 3–4 mm below the cortical surface and was then advanced rostrally for about 13–15 mm (Fig. 3b, c). The total length of the blade of our custom-designed undercut device was 19 mm. Using this technique, the anterior part of the suprasylvian gyrus remains relatively intact having a preserved intracortical connectivity and both descending and ascending fibers being only partially damaged. However, the posterior part of the suprasylvian gyrus has its white matter nearly completely transected. This procedure therefore produced conditions of partial cortical deafferentation. Following this undercut procedure, the dura was protected using quick drying silicone elastomer (Kwik-Sil, World Precision Instruments, Sarasota, FL, USA) that prevents the leakage of cerebrospinal fluid and tissue growth on the dura. Stainless steel screws were inserted in the intact skull for anchoring (Fig. 3a), and the bone removed during the procedure was reconstituted using dental acrylic glue.
Figure 3. Undercut in cats.
a. Picture of the undercut tool used to perform undercut in cats. The craniotomy was performed above the supasylvian gyrus and stainless steel screws were used as anchor for the dental acrylic to be applied. Coronal (b) and sagittal (c) sections of an undercut with Nissl staining. Arrows indicate the undercut location. Note that the knife was entered using the natural curvature of the brain doing minimal damage to the cortex, but cutting severely the underlying white matter.
Within the first 1–2 hours following the undercut the local field potential activity was generally depressed in the undercut region, then, the amplitude of slow waves in the undercut cortex was increased. Acute paroxysmal activities with an onset around the undercut region developed in 40 % of cats and lasted for several hours (Topolnik et al., 2003b). Both intrinsic and synaptic neuronal excitability was decreased within the undercut cortex and it was significantly increased in areas surrounding the undercut cortex providing neuronal substrate for seizure generation (Topolnik et al., 2003a).
The undercut procedure led to the development of recurrent seizures in chronic stages (1–5 weeks from the undercut) in more than 90 % of cats anesthetized with ketamine/xylazine (Nita et al., 2006) and in about 65% of behaving cats (Nita et al., 2007). Moreover, the undercut cortex often displays slow waves, an oscillation normally present only during non-REM sleep, in all states of vigilance (Nita et al., 2007; Timofeev et al., 2013).
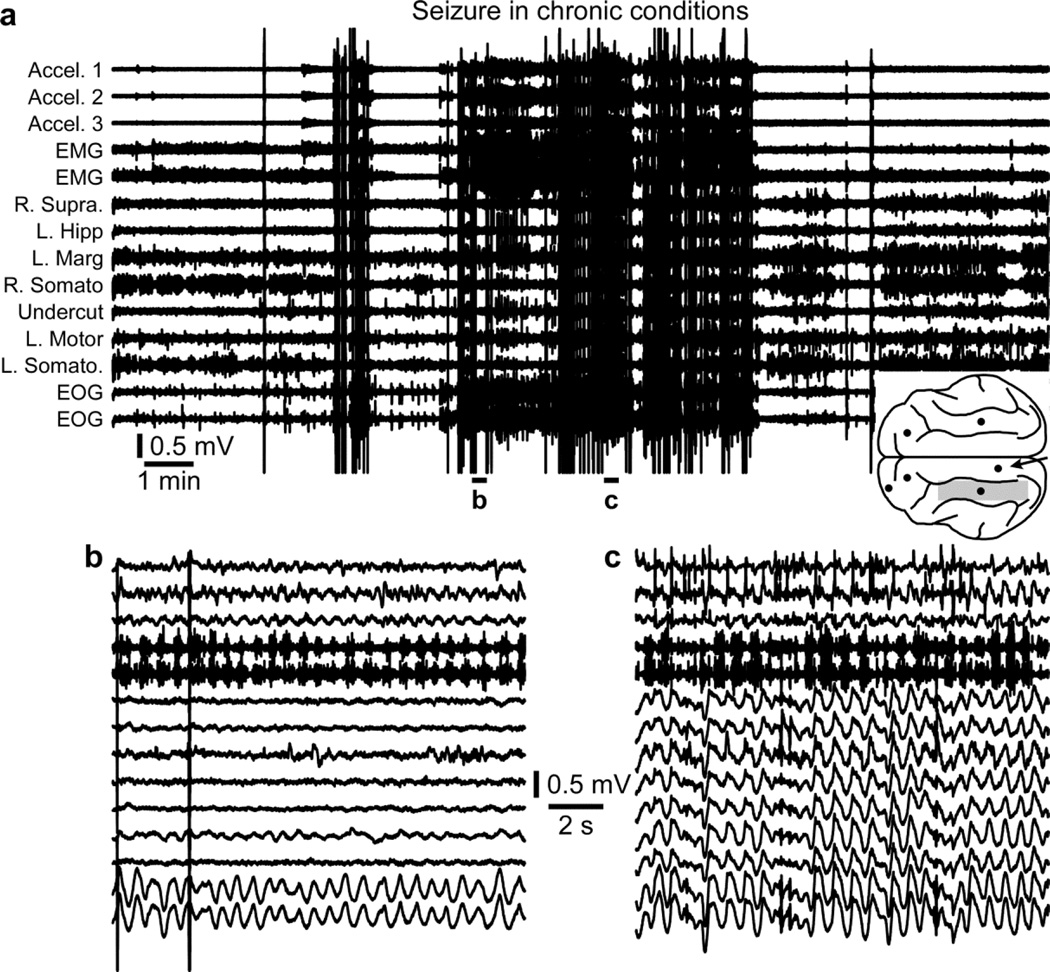
Paroxysmal activities appear first in electrodes surrounding the undercut, but not within the undercut itself, and they typically start about one week after the undercut (Nita et al., 2006; Nita and Timofeev, 2007; Nita et al., 2007). More and more areas start to be implicated in the paroxysmal activities with time passing, and 1.5–4 months post-undercut, cats start to display generalized (and behavioral) seizures (Nita and Timofeev, 2007; Nita et al., 2007) (Fig. 4a). These seizures can be generalized or primarily focal (Fig. 4b) and secondarily generalized (Fig. 4c).
Figure 4. Seizure in chronic conditions.
a. Electrographic recordings of a seizure in chronic conditions. The location of recording electrodes and of the undercut region (shaded area) are indicated in the inset. b. Recordings from the accelerometer, EMG, and EOG shows rhythmic activities although none of the local field potential electrodes captured this rhythmic activity suggesting that the seizure was primarily focal. c. All recording electrodes display activities of the generalized seizure. b and c are enlarged segments from a as indicated.
4.1.2 Cortical undercut technique in mice
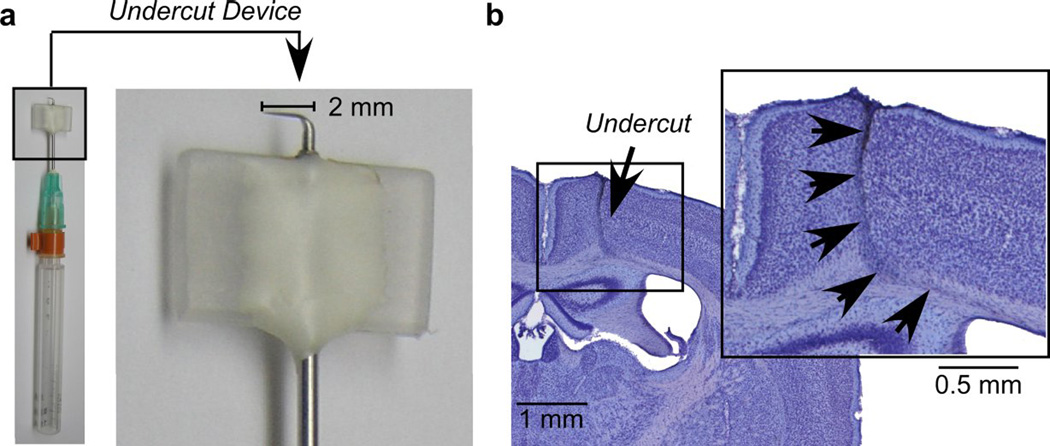
We recently started to use a technique of trauma-induced epileptogenesis in mice using a custom-designed device similar to the one developed recently (Xiong et al., 2011). Briefly, the device consisted in a small needle (25G, 1 1/2") inserted in a bigger needle (21G, 1") in which the smaller needle was folded at 90 degrees to form an “L” and was used as a knife (Fig 5a). The length of the needles was adjusted so that no vertical movement could be done independently, but that the knife could be rotated. Then an acrylic rectangle (for more stability on the skull) was glued to the bigger needle at about 1.2 mm from the knife (corresponding to the depth of the white matter in mice measured from the bone surface). The length of the blade was about 2 mm (Fig. 5a).
Figure 5. Undercut in mice.
a. Pictures of the undercut tool used to perform undercut in mice. b. Coronal section of an undercut with Nissl staining. The inset shows an expanded image of the undercut. Arrows indicate the undercut location. Note that the knife was entered perpendicularly to the brain surface, inserted up to the white matter, the handles was then raised by 90 degrees and rotated by 90–135 degrees doing minimal damage to the cortex, but cutting severely the underlying white matter without damaging the hippocampus.
The surgery was performed in sterile conditions under isoflurane (1–2 %) anesthesia and approved by the animal care committee of Université Laval. A small craniotomy was performed above the somatosensory cortex (approximate coordinate AP −1.0 mm to −2.0 mm, lateral 0.5 mm to 1.5 mm). Contrary to the cats, the size of the craniotomy is crucial in mice if one wants to perform histology at the end of the experiment. Both the cortex and the white matter being much thinner than in cats and due to the proximity of the hippocampus in mice, which is at a depth of approximately 1.2 mm, the undercut has to be performed at a depth of 1–1.2 mm from the cortical surface. The cortex above the undercut then becomes very fragile and could easily detach from the rest of the brain due to the presence of fibrin or other reparation molecules when the experimenter wants to collect the brain post-mortem. Thus the craniotomy has to be drilled as small as possible to allow seeing whether big blood vessels are present or not and to allow the experimenter to enter the needle. The tip of the blade was then entered through the dura perpendicular to the cortical surface, the handle of device being parallel to the cortical surface and the acrylic rectangle being on top of the handle. Once the folded part of the needle had entered completely in the brain, the device handle was then raised at 90 degrees until the acrylic rectangle would touch the skull, making the blade parallel to the cortical surface at a depth of 1–1.2 mm in the white matter. The blade was then rotated by 90–135 degrees depending on the extent of deafferentation desired and the undercut was performed at about 1–1.2 mm from the surface (Fig. 5b). The blade was then rotated back to its original orientation; the handle was lowered to 0 degree (parallel to the skull) making the blade perpendicular to the cortical surface. Finally the blade was extracted from the brain. Therefore this procedure produced conditions of partial cortical deafferentation in the mouse somatosensory cortex and following this undercut procedure, the dura was protected using Kwik-Sil (World Precision Instruments, Sarasota, FL, USA) and the skull was reconstituted using dental acrylic glue.
4.2 Trauma-induced epileptogenesis
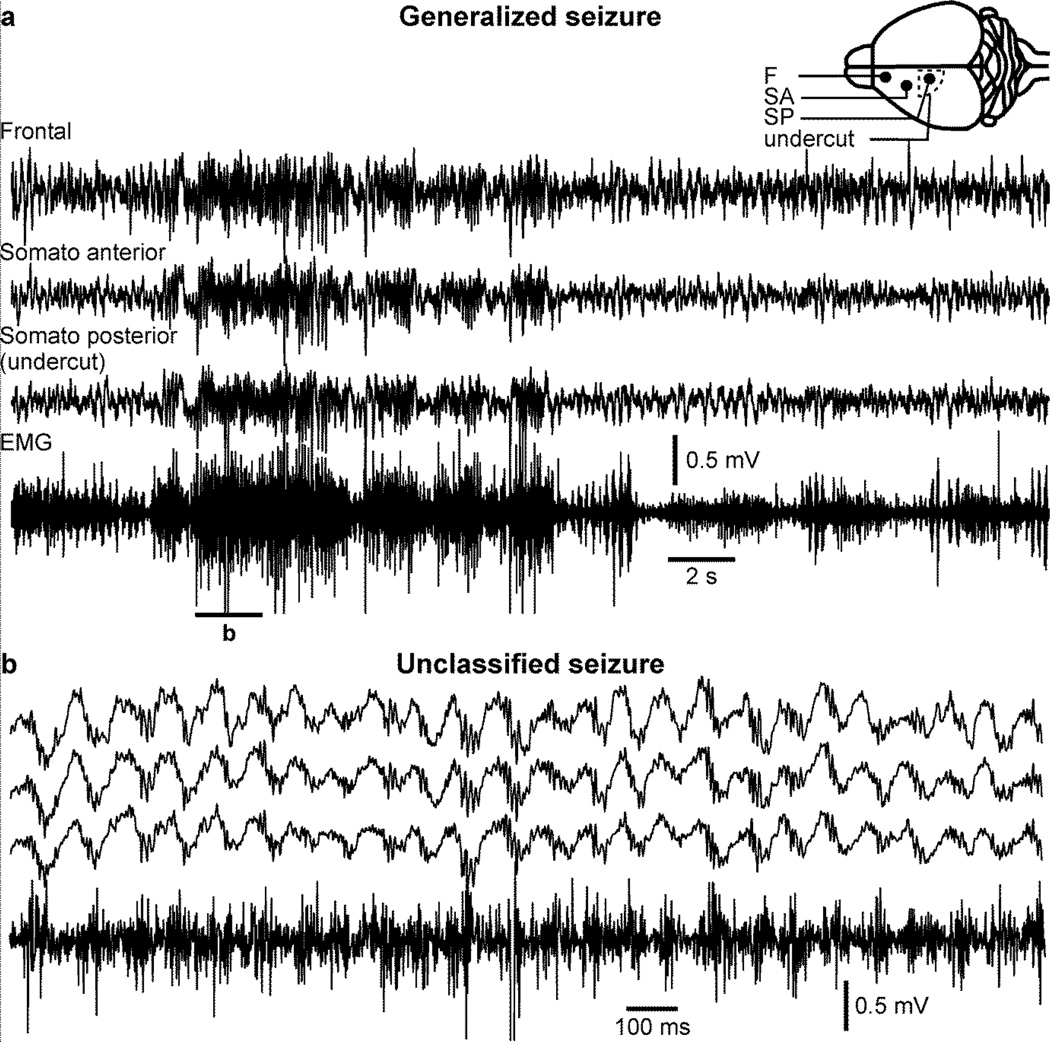
The immediate reaction following the brain trauma is a dramatic reduction in the LFP amplitude in areas with the cortical undercut (Topolnik et al., 2003b). With the technological advancement, we were able to use wireless transmission in chronic conditions and to start recordings immediately after the end of anesthesia which was not possible before (Grand et al., 2013). We showed that these early seizures following the undercut procedure were not ketamine/xylazine-dependent as paroxysmal activities were observed in non-anesthetized cats few hours after the undercut procedures (Timofeev et al., 2013). As for the cats, acute seizures can be observed shortly after the undercut procedure in mice. In figure 6, we show an acute seizure lasting about 12 seconds (Fig. 6a) with a frequency of about 2 Hz (Fig. 6b) recorded four hours after the undercut procedure in a mouse anesthetized with ketamine-xylazine. As for cats, these seizures are not dependent on ketamine-xylazine anesthesia as they can also be recorded in unanesthetized mice (Fig. 7). These seizures can be focal (Fig. 7a) or generalized (not shown). That particular example shows paroxysmal activities in the frontal cortex electrode, but not within the undercut and was characterized by a low muscle tone (Fig. 7b).
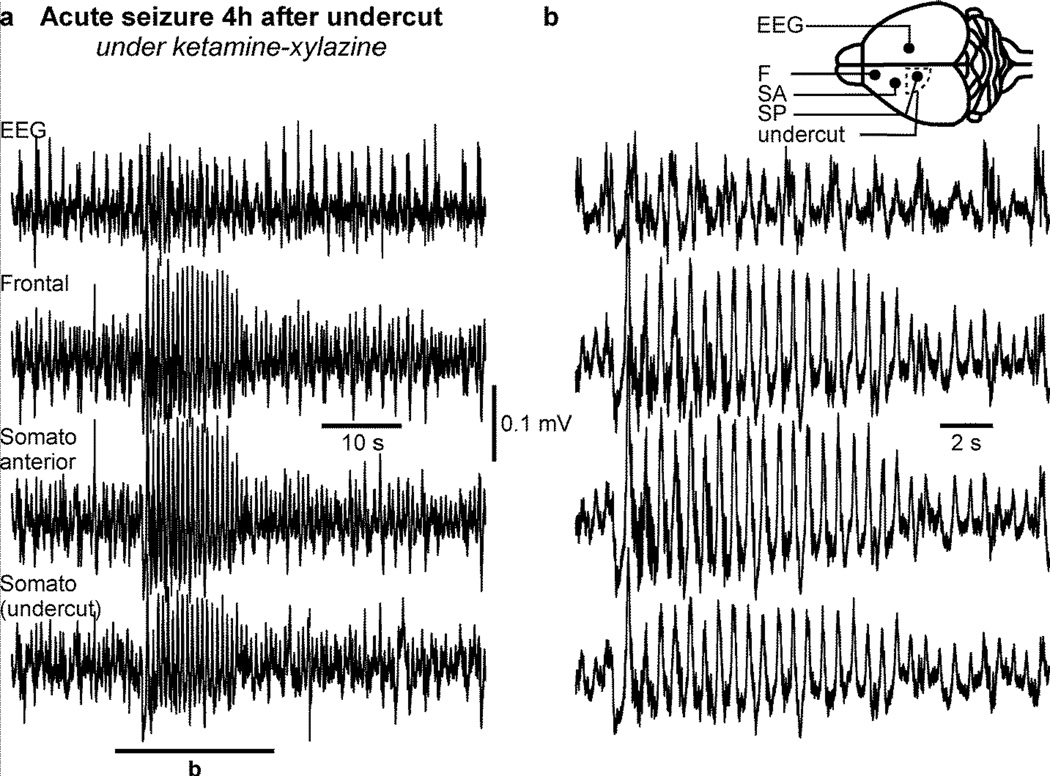
Figure 6. Acute seizure in ketamine-xylazine anesthetized mice following cortical undercut.
a. Electrographic recordings of an acute seizure in a ketamine-xylazine anesthetized mouse occuring four hours post-undercut. b. Enlarged segment from a as indicated. The electrodes location (filled circles) and the undercut location (dotted line) are indicated in the inset. Note that this seizure was recorded on all electrodes located in the left hemisphere but not in the right hemisphere.
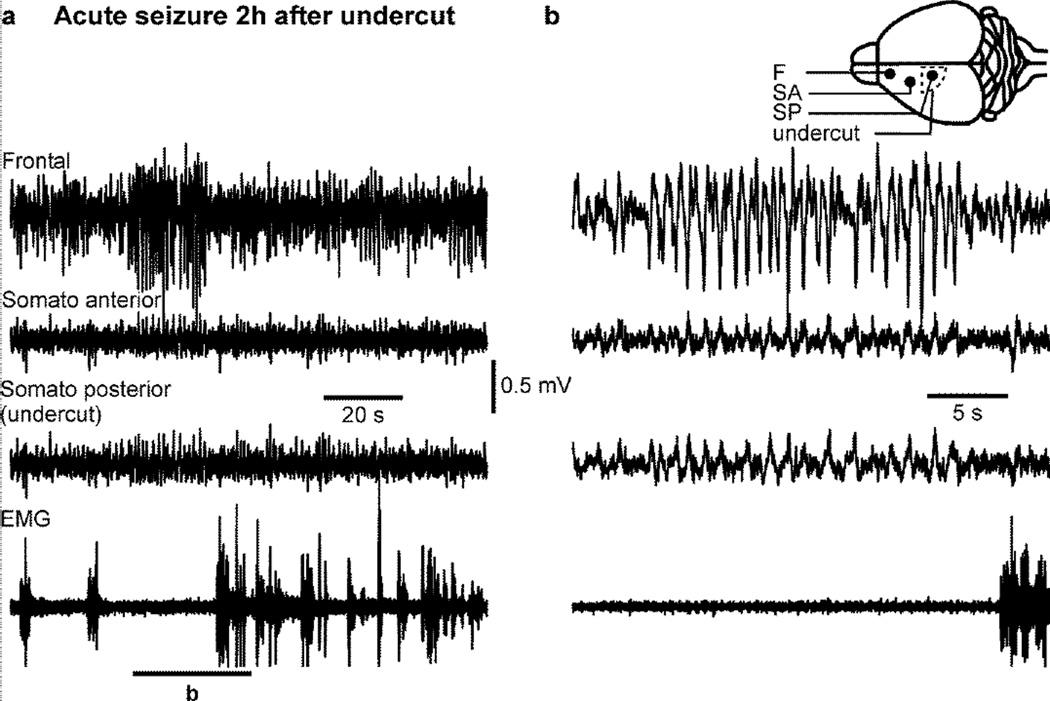
Figure 7. Acute seizure in unanesthetized mice following cortical undercut.
a. Electrographic recordings of an acute seizure in an unanesthetized mouse occuring two hours post-undercut. b. Enlarged segment from a as indicated. Note that this seizure was not associated with any increased muscle activity. The electrodes location (filled circles) and the undercut location (dotted line) are indicated in the inset. Note that the highest amplitude of paroxysmal activities is located in the frontal electrode.
As we mentioned above in cat, a week to few weeks after the undercut procedure, some paroxysmal activities start to appear in cortical region surrounding the undercut region, but never within the undercut region itself (Nita et al., 2007). About one month following the undercut procedure, paroxysmal activity started to be observed in progressively more cortical regions, but still not within the undercut region (Nita et al., 2007). After 1.5–4 months, the seizures became progressively generalized implicating also the undercut region (Nita et al., 2007; Timofeev et al., 2013). Behavioral seizures also started to appear with generalized seizures in about 65% of cats (Nita et al., 2007). Seizures were sometimes observed during waking state, were mainly present during slow-wave sleep, and were totally absent during REM sleep (Nita et al., 2007). The activity within the undercut region was also modified as slow waves, typical of slow-wave sleep, could be recorded during slow-wave sleep, quiet wakefulness, and even during REM sleep (Nita et al., 2007; Timofeev et al., 2010).
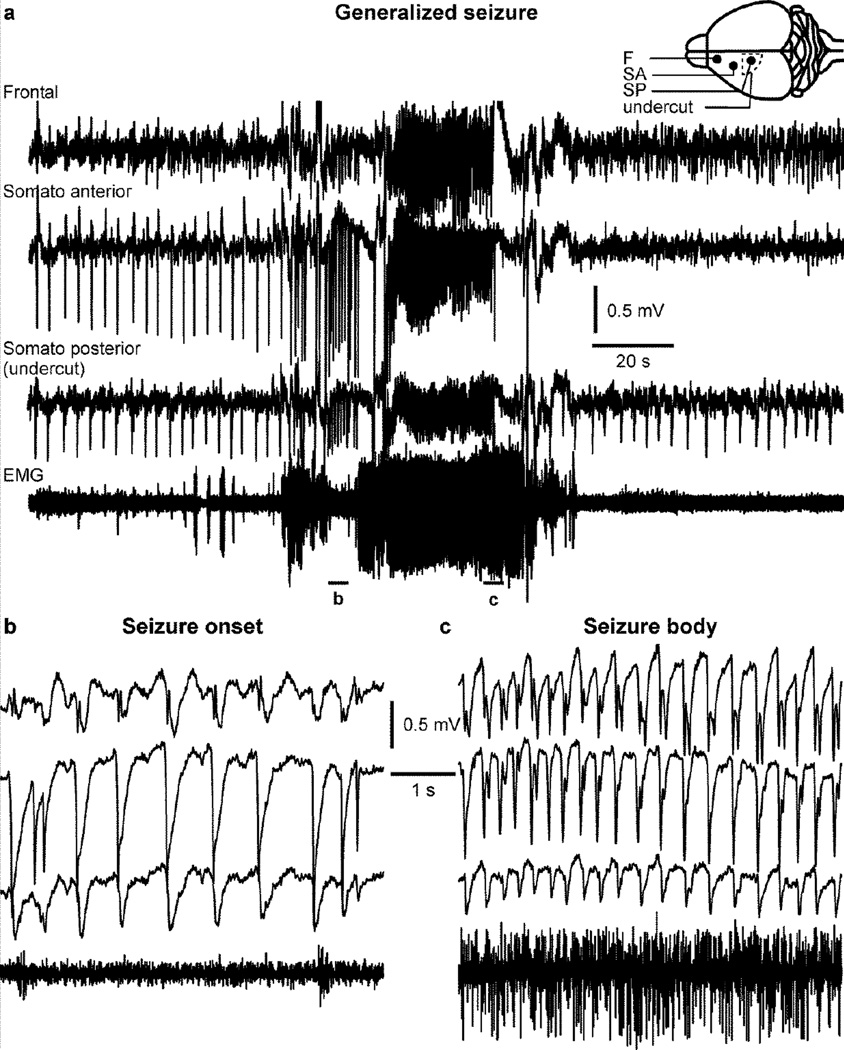
Interestingly, when mice started to display generalized (and behavioral) seizures in chronic stages (Fig. 8a), the leading electrode for paroxysmal wave was usually the one being just outside the undercut (Fig. 8b, see also Fig. 2.2 from (Timofeev et al., 2014)). In this particular example, the beginning of the seizure was characterized by a low muscle tone (Fig. 8b), while the body of the seizure was accompanied by an increased muscle tone (Fig. 8c). Another example of generalized seizure shows a higher-frequency seizure of about 12 Hz (Fig. 9b), which is also accompanied by an increased muscle tone (Fig. 9a).
Figure 8. Generalized seizure in a mouse with undercut in chronic conditions.
a. Electrographic recordings of a generalized seizure in a mouse with an undercut in the somatosensory cortex. The inset shows a scheme of the electrodes (filled circles) and the undercut location (dotted line). b. Enlarged segment from a showing the beginning of the seizure with a frequency slightly above 1 Hz, and without any particular muscle activity. c. The seizure evolved to reach 3–4 Hz and was accompanied by an increased muscle tone. Note that the seizure was recorded in all electrodes.
Figure 9. Generalized unclassified high-frequency seizure in a mouse with undercut in chronic conditions.
a. Electrographic recordings of a generalized seizure in a mouse with an undercut in the somatosensory cortex. The inset shows a scheme of the electrodes (filled circles) and the undercut location (dotted line). b. Enlarged segment from a showing an unclassified high-frequency seizure. Note that the seizure was recorded in all electrodes.
4.3 Structural changes in trauma-induced epileptogenesis
The undercut procedure leads to some functional and structural changes. The procedure implicating a transection of axons, some neurons will degenerate due to the axonal damage while some others might survive via axonal sprouting. The neuronal deaths might affect the thickness and the lamination of the cortex, and principal neurons and interneurons might be affected differently as only the axons of projecting pyramidal neurons are severed. However previous studies suggested that some interneurons might be sensitive to epilepsy. Chandelier interneurons, an aspiny interneuron distinguished by its axon terminals forming a vertical row of boutons that resemble a candlestick (Szentagothai and Arbib, 1974), are a major source of pyramidal axon initial segment inhibition and each chandelier cell axon gives off hundreds of terminals (DeFelipe et al., 1985; Freund et al., 1983). This type of interneurons was found to be particularly affected by epilepsy (DeFelipe, 1999).
4.3.1 Neuronal degeneration
Although the axons of interneurons remained intact during the undercut procedure, they appear to be especially sensitive to this procedure. A very significant reduction in the number of GABAergic interneurons was found in both superficial and deep cortical layers (Avramescu et al., 2009). Accordingly, neurons located at the edge of the undercut were shown to have a severe loss of inhibitory synapses (Ribak and Reiffenstein, 1982), which might be the cause for this region to be more prone to seizure generation. A major loss of excitatory neurons was also observed in deep cortical layers (>1.5 mm, mainly layer VI) while only a moderate loss was observed for superficial layers (Avramescu et al., 2009). The effect of these cell deaths was a significant reduction of about a fourth of the cortical thickness, the presence of delamination, and a change in the orientation of deeper neurons (Avramescu et al., 2009; Kuśmierczak et al., 2015). The survival of layer V neurons might appear surprising because their extracortically projecting axons were strongly transected by the undercut, however these neurons have a strong intracortical connectivity (Markram et al., 1997), which certainly help their survival. One of the consequences of this delamination was a laminar shift in the generator of active states of the slow oscillation with layer II/III neurons leading most of cycles as compared to layer V neurons in normal conditions (Avramescu, 2008).
4.3.2 Axonal sprouting
The undercut preparation was also used in rats and guinea pigs, and recordings from neocortical slices collected few weeks after the undercut procedure showed the presence of epileptiform activities in cortical slices maintained in vitro (Hoffman et al., 1994; Prince and Tseng, 1993; Salin et al., 1995). A number of studies suggest the presence of local axonal sprouting. Neurons within this model of undercut were shown to have a significant increase in the number of presumed synaptic boutons, axon collaterals, and in the total length of their axons; especially for large layer V pyramidal neurons (Salin et al., 1995). Using glutamate uncaging experiments, it was shown that layer V pyramidal neurons and interneurons from the undercut had an increased number of sites from which a synaptic event could be evoked (Jin et al., 2011; Jin et al., 2006). The study on pyramidal layer V neurons also revealed that averaged EPSCs were of smaller amplitude as compared to control cortex (Jin et al., 2006). Both pyramidal neurons and fast-spiking interneurons from a slice with cortical undercut were shown to have reduced IPSCs and it was suggested that interlaminar inhibition efficacy was reduced in the undercut cortex (Jin et al., 2011). In vivo studies also revealed an increased connection probability within the undercut cortex starting two weeks after the undercut procedure suggesting that axonal sprouting had occurred (Avramescu and Timofeev, 2008). Recently we demonstrated the presence of long-range intracortical axonal sprouting in the undercut model of epileptogenesis. The sprouting occurs mainly from relatively intact regions of the undercut towards more damaged ones and this leads to a reorientation of processes that becomes more horizontal within the undercut as compared to the intact cortex (Kuśmierczak et al., 2015). The axonal sprouting is strongly correlated to the presence of synchronous neuronal activities (Carmichael and Chesselet, 2002). This might lead the undercut region to a pathological loop in which some synchronous activities leads to axonal sprouting, axonal sprouting leads to an increased network synchronization leading to paroxysmal activities, and the synchrony of paroxysmal activities reinforcing the axonal sprouting.
4.4 Homeostatic plasticity in trauma-induced epileptogenesis
Brain excitability is maintained at a normal level via homeostatic mechanisms. Pioneering studies on homeostatic plasticity were performed in cell cultures for which an imposed change in activity was compensated by a change in the opposite direction. For example, silencing a cortical cell culture using TTX led to an upregulation of synaptic excitability and increased mEPSCs amplitude, while increasing activity using bicuculline down-regulated the excitatory synaptic efficacy and decreased mEPSCs amplitude (Murthy et al., 2001; Turrigiano, 1999; Turrigiano et al., 1998; Watt et al., 2000). Interestingly, the blockage of activity reduced the amplitude of mIPSCs, which corresponds to the opposite direction of changes occurring in excitatory currents (Kilman et al., 2002). The intrinsic activity is also regulated through homeostatic mechanisms as a chronic blockage of activity leads to enhanced sodium currents and decreased potassium currents, which results in an enhanced responsiveness of pyramidal neurons to current injections (Desai et al., 1999).
In the undercut model of epileptogenesis, a large number of axons is severed, and that creates conditions favorable for homeostatic up-regulation of neuronal excitability. A computational model of large-scale neuronal network suggested that cortical deafferentation triggered homeostatic up-regulation of neuronal excitability and that was a sufficient factor of epileptogenesis (Fröhlich et al., 2005; Frohlich et al., 2006; Houweling et al., 2005; Volman et al., 2011). In vivo experiments showed that following cortical trauma, neuronal intrinsic currents are also modified to increase the neuronal excitability. In the hours following the trauma (acute condition), the number of intrinsically-bursting neurons was shown to double within the undercut and in surrounding regions (Topolnik et al., 2003b). The membrane potential of neurons in the undercut area was also more hyperpolarized compared to intact cortex neurons and both the neuronal excitability and firing rate were strongly reduced within the undercut (Topolnik et al., 2003a). However in chronic conditions the changes were in opposite direction suggesting that some homeostatic processes occurred. From two weeks following the undercut, the input resistance of neurons was increased pointing to an increase in overall neuronal excitability (Avramescu and Timofeev, 2008). The intrinsic excitability of neurons within the undercut cortex measured as the instantaneous firing rate or as a number of action potentials elicited by a given current pulse was increased starting from two weeks post-undercut (Avramescu and Timofeev, 2008). As we mentioned above, cortical undercut is associated with overall reduction of the number of neurons. Therefore, the cortical network is unable to maintain long active states and thus the duration of silent states increases. In agreement with homeostatic principals, an increase in neuronal silence was associated with increase in the instantaneous firing rates of spontaneous active network states (Avramescu and Timofeev, 2008). Excitatory synaptic excitability was also affected by undercut. Starting from two weeks from the undercut, the amplitude of single axon EPSPs was increased and their coefficient of variation was decreased, but the properties of single axon IPSPSs were not affected by the undercut (Avramescu and Timofeev, 2008). We conclude that homeostatic up-regulation of synaptic excitability triggered by undercut was a sufficient factor of epileptogenesis (Timofeev, 2011; Timofeev et al., 2010). It appears that the extent of deafferentation is a factor in triggering epileptogenesis. We recently investigated a modulation of synaptic excitability in suprasylvian gyrus of cats, when the lateral posterior (LP) thalamic nucleus was inactivated (Lemieux et al., 2014). It is known that thalamocortical synapses account for about 6% of cortical synapses in a given cortical area (Ahmed et al., 1994). Our study showed that at least within first 36 hours from thalamic inactivation, the synaptic excitability in target cortical area was increased, but we did not detect any signs of paroxysmal activity (Lemieux et al., 2014). This study is in agreement with human data showing that only moderate and severe, but not mild cortical injury is an epileptogenic factor (Haltiner et al., 1997).
4.5 Age-dependency of trauma-induced epilepsy
We recently proposed that the development of trauma-induced epilepsy is age-dependent as we observed the appearance of epilepsy following a cortical trauma in about 65% of cats of unknown age (most of them likely adult) (Nita et al., 2006; Nita and Timofeev, 2007; Nita et al., 2007), but in none of adolescent cats (10–14 months old) (Timofeev et al., 2013). The 65% occurrence rate corresponds roughly to results coming from postwar epidemiological studies (Eftekhar et al., 2009; Salazar et al., 1985). In children the rate of late epilepsy following head trauma might be as low as 1% (Leviton and Cowan, 1981). In a more recent study, although the rate was much higher than 1% following TBI, it was shown that children are more prone to early seizures while adolescent and adult are more prone to late seizure (Asikainen et al., 1999). In a population-based study, it was shown that people of 65 year of age or more have a 2.5 time higher risk to have unprovoked seizure following head trauma than people under 65 years old (Annegers et al., 1998). Another population-based study performed in Denmark population revealed that the risk of developing epilepsy increased with age following both mild or severe brain injuries and was the highest among people older than 15 years at the time of the injury (Christensen et al., 2009). Based on these studies and on our previous data, we hypothesized that young animals are equipped with a controller of homeostatic up-regulation of activity that will control the extent of activity up-regulation and keep it at a normal level, while this controller would be absent or not function properly in adult animals leading to an uncontrolled upregulation of activity and finally to the development of epilepsy. Experiments to test this hypothesis remain to be done and it will likely be easier to perform them in mice, as they are easily available commercially at different ages.
5. The kindling model
Kindling is the relatively permanent increased propensity to convulsions that follows repeated electrical or chemical stimulation of certain areas of the brain even at intensities that are initially too low to produce any behavioral response or convulsions. Once established, the changes are so persistent that even the administration of a weak stimulus will elicit a seizure (Goddard, 1967; Goddard et al., 1969; Kandratavicius et al., 2014). Neocortical kindling is characterized by a higher threshold to elicit acute seizures, an unstable seizure development, and for its difficulty to induce a generalized convulsive seizure state (Majkowski et al., 1981; Okamoto, 1982). However, neocortical kindling has been described in the visual (Baba, 1982; Ono et al., 1981; Wada et al., 1989), somatosensory (Majkowski et al., 1981), motor (Fukushima et al., 1987), auditory (Valentine et al., 2004), and associative cortices of the cat (Nita et al., 2008a; Nita et al., 2008b). Kindling was also demonstrated in different species including frogs, mice, gerbils, rats, rabbits, cats, dogs, rhesus monkeys, and baboons (reviewed in (McNamara et al., 1980)). The first afterdischarges induced by the kindling procedure usually produce behavioral alterations that resemble partial seizures (class 1–3) (Racine, 1972a), but then evolve in secondarily generalized seizure (class 4–5) Racine, 1972b).
5.1 Inducing neocortical kindling
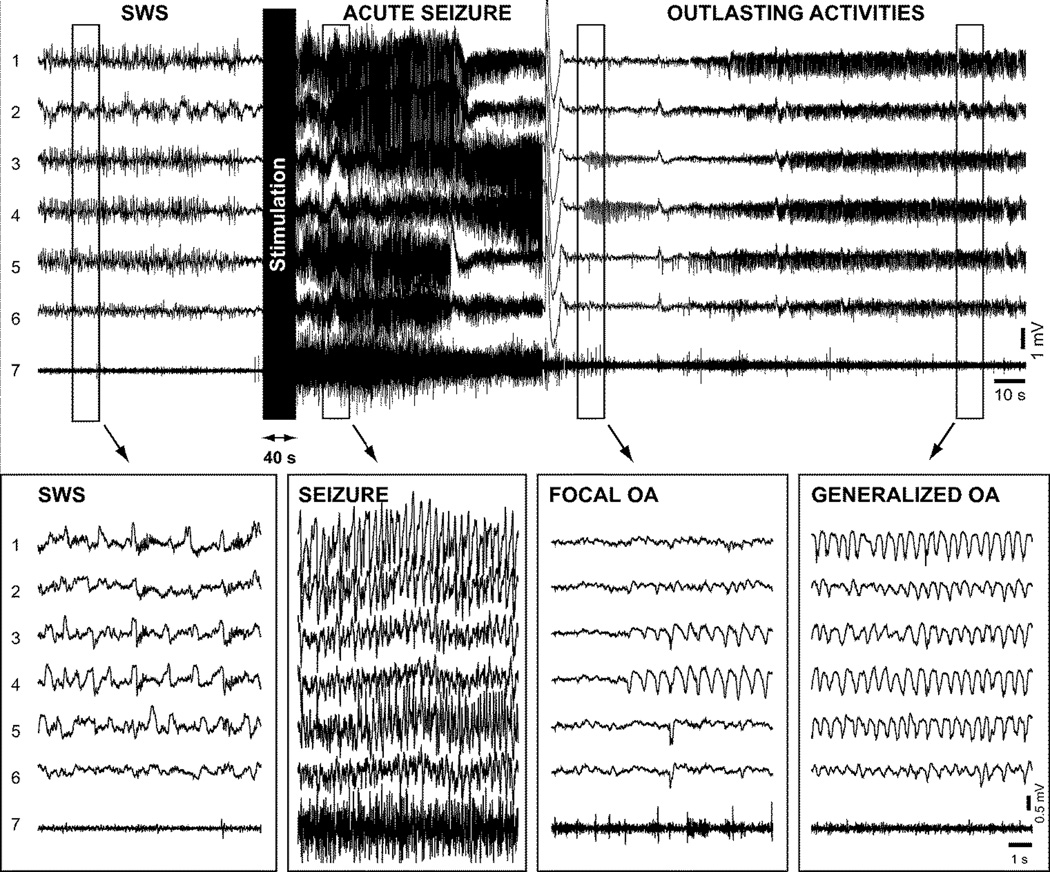
The standard procedure we used to induce kindling was to deliver 50 Hz rectangular pulse-train for 40–60 seconds at low current intensity (0.5 – 1.5 mA) through a coaxial bipolar macroelectrodes (FHC Inc., USA) 3–5 times a day (Nita et al., 2008a; Nita et al., 2008b). We found that these repetitive stimulations were not efficient to induce kindling or seizure activities when delivered during stable states of vigilance. However when this stimulation paradigm was delivered during the first minute of transition from slow-wave sleep to wake it was very efficient in inducing kindling (Fig. 10) (Nita et al., 2008a; Nita et al., 2008b). Seizures appeared after 5–7 days of kindling and consisted in spike-wave complexes with a frequency of 1–2 Hz followed by a postictal depression. These seizures were usually generalized and could last few minutes. Following the postictal depression, some rhythmic activities appeared with a frequency of about 1.5–2 Hz that we termed as "outlasting activities" (Fig. 10) (Nita et al., 2008a; Nita et al., 2008b) and will be further described in a section 5.3.
Figure 10. Generalization of outlasting activity discharges in a kindled cat (day 21).
Top panel, electrographic recording of a cat receiving kindling stimulation at transition from slow-wave sleep (SWS) to waking state. The recordings show chronologically slow-wave sleep (SWS), acute elicited seizure, focal and generalized outlasting activity (OA). Electrical stimuli were applied ~10 s after the onset of wake state. Depicted field-potential recordings were obtained from: 1. motor cortex (area 4 left); 2. motor cortex (area 4 right); 3. auditory cortex (area 22 right); 4. auditory cortex (area 22 left); 5. primary visual cortex (area 17 left); 6. primary visual cortex (area 17 right); and 7. EMG. Bottom panels show expanded recordings from the corresponding periods in the top panel. Reproduced with permission from (Nita et al., 2008b).
5.2 Structural changes
The progressive propensity to induce seizures or the lowering with time of the threshold to induce seizures that is present during kindling suggests that some structural changes may occur. However, a study on structural changes performed in rats receiving kindling procedure in the anterior cortical regions revealed no changes in the dendritic branching properties nor in the spine density in the implicated cortical region (Racine et al., 1975). Few years later it was found that, at least in pyramidal neurons from layer III of the frontal cortex, sex- and time-dependent changes could be observed following kindling procedure. These included short lasting (less than a month) changes: a decreased spine density, length, and branching for both apical and basal dendrites (Teskey et al., 1999). Kindling was also shown to modify the cortical motor map in rats by almost doubling the cortical area able to induce a forelimb movement and these changes were still present three weeks after the last kindling session (Teskey et al., 2002). Measured in the primary auditory cortex, both the mean firing rate and the bursts occurrence were reduced in kindled animals compared to sham control or normal animals (Valentine et al., 2004). The tonotopic map of primary auditory cortex was also substantially affected by kindling as a large extent of this area became tuned to similar frequencies as compared to a normal caudo-rostral frequency gradient in control animals and a higher degree of firing synchrony was observed in kindled cats (Valentine et al., 2004). Some neuronal loss was shown to occur following hippocampal kindling (Sayin et al., 2003), however whether such a phenomena occurs in neocortical kindling remains to be investigated. Thus, although some structural changes could be observed following neocortical kindling, these changes were not long-lasting and even if some functional changes have been observed in motor and primary auditory cortices, the structural changes underlying these functional changes remain to be demonstrated.
5.3 Outlasting activities
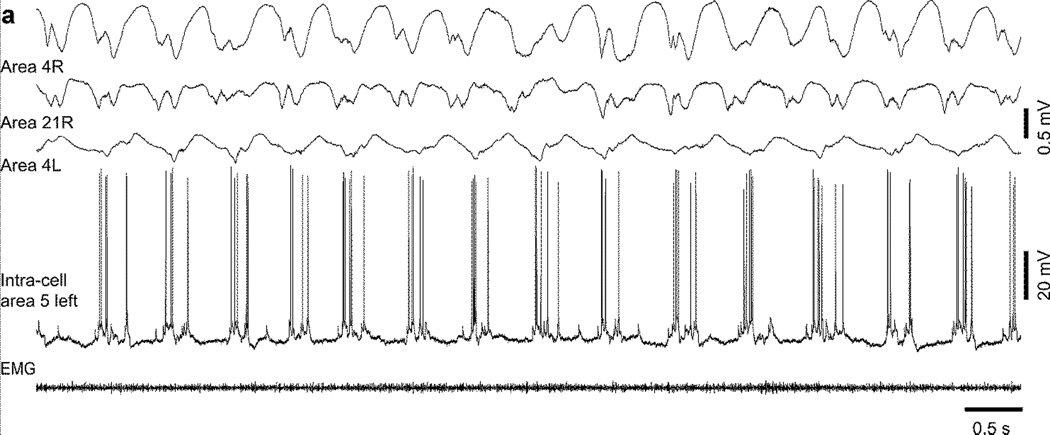
Outlasting activities refer to 1.5–2 Hz activities that follow acute seizures in the kindling model of epilepsy (Fig. 10). Simultaneous field potential recordings performed in the neocortex and the thalamus revealed that the neocortex is leading the activity during acute seizure while during outlasting activities, the thalamus leads the cortex suggesting that the thalamus plays a critical role in the emergence of these outlasting activities (Nita et al., 2008a). In addition, during outlasting activities, thalamic neurons regularly display bursts with the frequency of outlasting activities (Nita et al., 2008a). However, neither the stimulation of a cholinergic structure (pedonculo-pontine tegmentum (PPT)) nor the stimulation of a noradrenergic structure (locus coerulus) that are believed to depolarize thalamocortical cells and prevent low-threshold spike bursting (McCormick, 1992) were able to block completely the occurrence of outlasting activities although their stimulation decreased both the amplitude of outlasting activities and the burst occurrence in the thalamus (Nita et al., 2008a). Therefore, the thalamus plays a major role in the regulation of outlasting activities but this role is not exclusive. These activities could last up to two hours and were clearly seen during wake, they were slightly reduced during slow-wave sleep, and were completely abolished during REM sleep. As the kindling evolves, these outlasting activities also evolve being focal when they start to appear and becoming more and more generalized over the cortical surface as the daily stimulations progressed (Nita et al., 2008b). At least during waking state, neocortical neurons displayed active and silent state during outlasting activities although the silent states were not accompanied with a major hyperpolarization as would be the case during slow-wave sleep (Fig. 11) (Nita et al., 2008b). This likely occurred because the neuromodulatory brainstem systems were active, which blocked potassium channels that normally hyperpolarize cortical neurons during sleep silent states (Timofeev et al., 2001). Thus, the outlasting activities in the neocortical kindling model of epileptogenesis increase the overall duration of the network silence, like in the undercut model. This network silence should increase neuronal excitability. Although detailed investigations of neuronal excitability in vivo in kindling model of epileptogenesis are lacking, we previously have shown that cortical neurons displayed spike-doublets during activities of slow oscillation, which appear to be built on the summation of successive EPSPs and fast-prepotentials causing an increased dendritic excitation (Nita et al., 2008b). Therefore, these outlasting activities might play a major role in the epileptogenesis mechanisms following neocortical kindling.
Figure 11. Intracellular oscillations during outlasting activities (OA) in a kindled cat (day 10) during wake.
Intracellular recording during OA occurring during wake in area 5. Electrodes were placed in: 1. motor cortex (area 4 right); 2. posterior associative cortex (area 21 right); and 3. motor cortex (area 4 left). Modified with permission from (Nita et al., 2008b).
6. Conclusions
We have described different approaches used to model neocortical seizures and epileptogenesis. Ketamine-xylazine anesthesia in cats leads to the occurrence of seizure in a large number of animals. These seizures can be focal or generalized and are usually composed of SW/PSW components, often accompanied with fast runs of EEG/LFP spikes. During these seizures, the extracellular milieu is modified by the increased neuronal activity in such a way that the chemical synaptic transmission is impaired. During fast runs, a very loose synchrony is observed in neighboring neurons very likely due to the very low extracellular calcium concentration available for chemical synaptic transmission. During the SW/PSW component, the LFPs and/or intracellular recordings reveal high amplitude transitions, higher than those recorded during normal slow-wave sleep or during the slow oscillation induced by anesthesia, which suggests a very high local synchrony among cortical neurons during these events. High local synchrony and loose long-range synchrony can be mediated by increased activity of gap junctions, ephaptic interactions or other non-chemical synaptic mechanisms. The slow oscillation recorded under anesthesia is also of higher amplitude as compared to slow-wave sleep, suggesting also a higher cortical synchrony under anesthesia. The duration of silent states in the condition of this anesthesia is longer than during slow-wave sleep. As most of cortical seizures are recorded during sleep or at transitions from slow-wave sleep to wake and because cats anesthetized with ketamine-xylazine anesthesia are prone to have seizures, it was suggested that more silence in the intact cortical network, more seizure prone cortex is.
We then described two models of epileptogenesis: an undercut model of trauma-induced epileptogenesis and a model of cortical kindling. We described in details our techniques to perform a cortical undercut in cats and in mice. As the procedure is to cut the white matter underlying a given cortical areas, the thalamus is very likely not implicated in the mechanisms of epileptogenesis following cortical undercut. This model of cortical undercut reproduces the main features of traumatic brain injuries leading to epilepsy in human patients. Acute seizures can be observed in the first 48 hours, but the recurrent seizures appear several weeks to month after the cortical undercut. Multiple mechanisms play a role in the penetrating wound induced epileptogenesis. (a) Deafferentation increases the overall silence in cortical network and thus up-regulates neuronal excitability. (b) There is a neuronal death that affects GABAergic cells more than excitatory neurons; therefore, the excitation/inhibition balance shifts toward excitation. (c) Axonal sprouting both long-range and local is another source of overexcitability of traumatized cortex.
The kindling model of epileptogenesis was shown to develop some activities in the range of 1.5–2 Hz, which were termed outlasting activities. This particular type of activities has a thalamic component as thalamocortical neurons revealed regular bursts of action potentials with the frequency of these outlasting activities. However, the role of the thalamus is not exclusive as neither PPT, nor locus coerulus stimulation was able to completely block this activity in the cortex. Outlasting activities are composed of alternating periods of active and silent states and they are present during both slow-wave sleep and waking state for up to two hours: therefore, following neocortical kindling procedure, the overall silence of the cortical network increases.
Neuronal silence is a common feature in all three described models of neocortical epileptogenesis and seizures. We conclude that an increase in neuronal silence increases neuronal excitability and makes the neocortex more prone to epilepsy.
Highlights.
Epileptogenesis was studied in ketamine-xylazine anesthesia, trauma and kindling
The common feature of described models is an increased network silence.
Increased neuronal silence up-regulates neuronal excitability
Increased neuronal excitability makes cortex more prone to epilepsy.
Acknowledgments
In recent years, our work was supported by CIHR (MOP-325213, MOP-324941, MOP-37862 and MOP-67175), NIH-NINDS (1R01-NS060870 and 1R01-NS059740), NSERC (Grant 298475), and FRQ-S, FRQ-NT. We are thankful to Sergiu Ftomov for his excellent technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adami C, Spadavecchia C, Casoni D. Seizure activity occurring in two dogs after S-ketamine-induction. Schweiz Arch Tierheilkd. 2013;155:569–572. doi: 10.1024/0036-7281/a000513. [DOI] [PubMed] [Google Scholar]
- Agmon A, Connors BW. Repetitive burst-firing neurons in the deep layers of mouse somatosensory cortex. Neurosci Lett. 1989;99:137–141. doi: 10.1016/0304-3940(89)90278-4. [DOI] [PubMed] [Google Scholar]
- Ahmed B, Anderson JC, Douglas RJ, Martin KA, Nelson JC. Polyneuronal innervation of spiny stellate neurons in cat visual cortex. The Journal of comparative neurology. 1994;341:39–49. doi: 10.1002/cne.903410105. [DOI] [PubMed] [Google Scholar]
- Amzica F, Massimini M, Manfridi A. Spatial buffering during slow and paroxysmal sleep oscillations in cortical networks of glial cells in vivo. J Neurosci. 2002;22:1042–1053. doi: 10.1523/JNEUROSCI.22-03-01042.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annegers JF, Hauser WA, Coan SP, Rocca WA. A population-based study of seizures after traumatic brain injuries. N Engl J Med. 1998;338:20–24. doi: 10.1056/NEJM199801013380104. [DOI] [PubMed] [Google Scholar]
- Asikainen I, Kaste M, Sarna S. Early and late posttraumatic seizures in traumatic brain injury rehabilitation patients: brain injury factors causing late seizures and influence of seizures on long-term outcome. Epilepsia. 1999;40:584–589. doi: 10.1111/j.1528-1157.1999.tb05560.x. [DOI] [PubMed] [Google Scholar]
- Avramescu S. Département d’anatomie et physiologie. Quebec: Université Laval; 2008. Cellular and homeostatic network mechanisms of posttraumatic epilepsy; p. 253. [Google Scholar]
- Avramescu S, Nita DA, Timofeev I. Neocortical post-traumatic epileptogenesis is associated with loss of GABAergic neurons. Journal of neurotrauma. 2009;26:799–812. doi: 10.1089/neu.2008.0739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avramescu S, Timofeev I. Synaptic strength modulation following cortical trauma: a role in epileptogenesis. J Neurosci. 2008;28:6760–6772. doi: 10.1523/JNEUROSCI.0643-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba H. Facilitatory effects of intermittent photic stimulation on visual cortical kindling. Epilepsia. 1982;23:663–670. doi: 10.1111/j.1528-1157.1982.tb05082.x. [DOI] [PubMed] [Google Scholar]
- Barkai E, Grossman Y, Gutnick MJ. Long-term changes in neocortical activity after chemical kindling with systemic pentylenetetrazole: an in vitro study. Journal of neurophysiology. 1994;72:72–83. doi: 10.1152/jn.1994.72.1.72. [DOI] [PubMed] [Google Scholar]
- Bazhenov M, Timofeev I, Steriade M, Sejnowski TJ. Potassium model for slow (2–3 Hz) in vivo neocortical paroxysmal oscillations. J Neurophysiol. 2004;92:1116–1132. doi: 10.1152/jn.00529.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltramo R, D’Urso G, Dal Maschio M, Farisello P, Bovetti S, Clovis Y, Lassi G, Tucci V, De Pietri Tonelli D, Fellin T. Layer-specific excitatory circuits differentially control recurrent network dynamics in the neocortex. Nature neuroscience. 2013;16:227–234. doi: 10.1038/nn.3306. [DOI] [PubMed] [Google Scholar]
- Blake H, Gerard RW. Brain potentials during sleep. Am J Physiol. 1937;119:692–703. [Google Scholar]
- Boucetta S, Chauvette S, Bazhenov M, Timofeev I. Focal generation of paroxysmal fast runs during electrographic seizures. Epilepsia. 2008;49:1925–1940. doi: 10.1111/j.1528-1167.2008.01707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucetta S, Crochet S, Chauvette S, Seigneur J, Timofeev I. Extracellular Ca2+ fluctuations in vivo affect afterhyperpolarization potential and modify firing patterns of neocortical neurons. Experimental Neurology. 2013;245:5–14. doi: 10.1016/j.expneurol.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlen PL, Skinner F, Zhang L, Naus C, Kushnir M, Perez Velazquez JL. The role of gap junctions in seizures. Brain Res Brain Res Rev. 2000;32:235–241. doi: 10.1016/s0165-0173(99)00084-3. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Chesselet MF. Synchronous neuronal activity is a signal for axonal sprouting after cortical lesions in the adult. J Neurosci. 2002;22:6062–6070. doi: 10.1523/JNEUROSCI.22-14-06062.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang BS, Lowenstein DH. Practice parameter: antiepileptic drug prophylaxis in severe traumatic brain injury: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2003;60:10–16. doi: 10.1212/01.wnl.0000031432.05543.14. [DOI] [PubMed] [Google Scholar]
- Chauvette S, Crochet S, Volgushev M, Timofeev I. Properties of slow oscillation during slow-wave sleep and anesthesia in cats. J Neurosci. 2011;31:14998–15008. doi: 10.1523/JNEUROSCI.2339-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauvette S, Seigneur J, Timofeev I. Sleep Oscillations in the Thalamocortical System Induce Long-Term Neuronal Plasticity. Neuron. 2012;75:1105–1113. doi: 10.1016/j.neuron.2012.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauvette S, Volgushev M, Mukovski M, Timofeev I. Local origin and long-range synchrony of active state in neocortex during slow oscillation. In: Timofeev I, editor. Mechanisms of spontaneous active states in the neocortex. Kerala, India: Research Signpost; 2007. pp. 73–92. [Google Scholar]
- Chauvette S, Volgushev M, Timofeev I. Origin of active states in local neocortical networks during slow sleep oscillation. Cereb Cortex. 2010;20:2660–2674. doi: 10.1093/cercor/bhq009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J-Y, Chauvette S, Skorheim S, Timofeev I, Bazhenov M. Interneuron-mediated inhibition synchronizes neuronal activity during slow oscillation. The Journal of physiology. 2012;590:3987–4010. doi: 10.1113/jphysiol.2012.227462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christe KL, Lee UJ, Lemoy M-J, Havton LA. Generalized Seizure Activity in an Adult Rhesus Macaque (Macaca mulatta) during Ketamine Anesthesia and Urodynamic Studies. Comparative Medicine. 2013;63:445–447. [PMC free article] [PubMed] [Google Scholar]
- Christensen J. Traumatic brain injury: risks of epilepsy and implications for medicolegal assessment. Suppl 4. Vol. 53. Epilepsia: 2012. pp. 43–47. [DOI] [PubMed] [Google Scholar]
- Christensen J, Pedersen MG, Pedersen CB, Sidenius P, Olsen J, Vestergaard M. Long-term risk of epilepsy after traumatic brain injury in children and young adults: a population-based cohort study. Lancet. 2009;373:1105–1110. doi: 10.1016/S0140-6736(09)60214-2. [DOI] [PubMed] [Google Scholar]
- Cohen I, Navarro V, Clemenceau S, Baulac M, Miles R. On the origin of interictal activity in human temporal lobe epilepsy in vitro. Science. 2002;298:1418–1421. doi: 10.1126/science.1076510. [DOI] [PubMed] [Google Scholar]
- Connors BW, Gutnick MJ. Intrinsic firing patterns of diverse neocortical neurons. Trends Neurosci. 1990;13:99–104. doi: 10.1016/0166-2236(90)90185-d. [DOI] [PubMed] [Google Scholar]
- Contreras D, Steriade M. Cellular basis of EEG slow rhythms: a study of dynamic corticothalamic relationships. J Neurosci. 1995;15:604–622. doi: 10.1523/JNEUROSCI.15-01-00604.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crochet S, Chauvette S, Boucetta S, Timofeev I. Modulation of synaptic transmission in neocortex by network activities. The European journal of neuroscience. 2005;21:1030–1044. doi: 10.1111/j.1460-9568.2005.03932.x. [DOI] [PubMed] [Google Scholar]
- Crunelli V, Hughes SW. The slow (<1 Hz) rhythm of non-REM sleep: a dialogue between three cardinal oscillators. Nature neuroscience. 2010;13:9–17. doi: 10.1038/nn.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Ambrosio R, Perucca E. Epilepsy after head injury. Curr Opin Neurol. 2004;17:731–735. doi: 10.1097/00019052-200412000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David F, Schmiedt JT, Taylor HL, Orban G, Di Giovanni G, Uebele VN, Renger JJ, Lambert RgC, Leresche N, Crunelli V. Essential thalamic contribution to slow waves of natural sleep. J Neurosci. 2013;33:19599–19610. doi: 10.1523/JNEUROSCI.3169-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFelipe J. Chandelier cells and epilepsy. Brain. 1999;122(Pt 10):1807–1822. doi: 10.1093/brain/122.10.1807. [DOI] [PubMed] [Google Scholar]
- DeFelipe J, Hendry SH, Jones EG, Schmechel D. Variability in the terminations of GABAergic chandelier cell axons on initial segments of pyramidal cell axons in the monkey sensory-motor cortex. The Journal of comparative neurology. 1985;231:364–384. doi: 10.1002/cne.902310307. [DOI] [PubMed] [Google Scholar]
- Derchansky M, Rokni D, Rick JT, Wennberg R, Bardakjian BL, Zhang L, Yarom Y, Carlen PL. Bidirectional multisite seizure propagation in the intact isolated hippocampus: the multifocality of the seizure “focus”. Neurobiol Dis. 2006;23:312–328. doi: 10.1016/j.nbd.2006.03.014. [DOI] [PubMed] [Google Scholar]
- Dermietzel R, Spray DC. Gap junctions in the brain: where, what type, how many and why? Trends Neurosci. 1993;16:186–192. doi: 10.1016/0166-2236(93)90151-b. [DOI] [PubMed] [Google Scholar]
- Desai NS, Rutherford LC, Turrigiano GG. Plasticity in the intrinsic excitability of cortical pyramidal neurons. Nature neuroscience. 1999;2:515–520. doi: 10.1038/9165. [DOI] [PubMed] [Google Scholar]
- Dichter MA, Ayala GF. Science. Vol. 237. New York, N.Y: 1987. Cellular mechanisms of epilepsy: a status report; pp. 157–164. [DOI] [PubMed] [Google Scholar]
- Dinner D. Posttraumatic epilepsy. In: EW, editor. The Treatment of Epilepsy: Principles. Philadelphia: Lea & Fibinger; 1993. pp. 654–658. [Google Scholar]
- Eccles JC. The physiology of synapses. Berlin: Springer; 1964. [Google Scholar]
- Eftekhar B, Sahraian MA, Nouralishahi B, Khaji A, Vahabi Z, Ghodsi M, Araghizadeh H, Soroush MR, Esmaeili SK, Masoumi M. Prognostic factors in the persistence of posttraumatic epilepsy after penetrating head injuries sustained in war. J Neurosurg. 2009;110:319–326. doi: 10.3171/2008.4.17519. [DOI] [PubMed] [Google Scholar]
- Feeney DM, Walker AE. The prediction of posttraumatic epilepsy. A mathematical approach. Arch Neurol. 1979;36:8–12. doi: 10.1001/archneur.1979.00500370038005. [DOI] [PubMed] [Google Scholar]
- Ferguson PL, Smith GM, Wannamaker BB, Thurman DJ, Pickelsimer EE, Selassie AW. A population-based study of risk of epilepsy after hospitalization for traumatic brain injury. Epilepsia. 2010;51:891–898. doi: 10.1111/j.1528-1167.2009.02384.x. [DOI] [PubMed] [Google Scholar]
- Freund TF, Martin KA, Smith AD, Somogyi P. Glutamate decarboxylase-immunoreactive terminals of Golgi-impregnated axoaxonic cells and of presumed basket cells in synaptic contact with pyramidal neurons of the cat’s visual cortex. The Journal of comparative neurology. 1983;221:263–278. doi: 10.1002/cne.902210303. [DOI] [PubMed] [Google Scholar]
- Fröhlich F, Bazhenov M, Timofeev I, Sejnowski TJ. Maintenance and termination of neocortical oscillations by dynamic modulation of intrinsic and synaptic excitability. Thalamus & related systems. 2005;3:147–156. doi: 10.1017/S147292880700015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohlich F, Bazhenov M, Timofeev I, Steriade M, Sejnowski TJ. Slow state transitions of sustained neural oscillations by activity-dependent modulation of intrinsic excitability. J Neurosci. 2006;26:6153–6162. doi: 10.1523/JNEUROSCI.5509-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohlich F, McCormick DA. Endogenous Electric Fields May Guide Neocortical Network Activity. Neuron. 2010;67:129–143. doi: 10.1016/j.neuron.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima J, Kohsaka S, Fukushima K, Kato M. Motor cortical kindling in cats: a comparison of adult cats and kittens. Epilepsia. 1987;28:651–657. doi: 10.1111/j.1528-1157.1987.tb03696.x. [DOI] [PubMed] [Google Scholar]
- Galarreta M, Hestrin S. Electrical synapses between GABA-releasing interneurons. Nature reviews. 2001;2:425–433. doi: 10.1038/35077566. [DOI] [PubMed] [Google Scholar]
- Galarreta M, Hestrin S. Frequency-dependent synaptic depression and the balance of excitation and inhibition in the neocortex. Nature neuroscience. 1998;1:587–594. doi: 10.1038/2822. [DOI] [PubMed] [Google Scholar]
- Galarreta M, Hestrin S. A network of fast-spiking cells in the neocortex connected by electrical synapses. Nature. 1999;402:72–75. doi: 10.1038/47029. [DOI] [PubMed] [Google Scholar]
- Gibson JR, Beierlein M, Connors BW. Two networks of electrically coupled inhibitory neurons in neocortex. Nature. 1999;402:75–79. doi: 10.1038/47035. [DOI] [PubMed] [Google Scholar]
- Goddard GV. Development of epileptic seizures through brain stimulation at low intensity. Nature. 1967;214:1020–1021. doi: 10.1038/2141020a0. [DOI] [PubMed] [Google Scholar]
- Goddard GV, McIntyre DC, Leech CK. A permanent change in brain function resulting from daily electrical stimulation. Exp Neurol. 1969;25:295–330. doi: 10.1016/0014-4886(69)90128-9. [DOI] [PubMed] [Google Scholar]
- Gorji A, Speckmann EJ. Low concentration of DL-2-amino-5-phosphonovalerate induces epileptiform activity in guinea pig hippocampal slices. Epilepsia. 2001;42:1228–1230. doi: 10.1046/j.1528-1157.2001.01301.x. [DOI] [PubMed] [Google Scholar]
- Grand L, Ftomov S, Timofeev I. Long-term synchronized electrophysiological and behavioral wireless monitoring of freely moving animals. Journal of neuroscience methods. 2013;212:237–241. doi: 10.1016/j.jneumeth.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray CM, McCormick DA. Science. Vol. 274. New York, N.Y: 1996. Chattering cells: superficial pyramidal neurons contributing to the generation of synchronous oscillations in the visual cortex; pp. 109–113. [DOI] [PubMed] [Google Scholar]
- Grenier F, Timofeev I, Steriade M. Focal synchronization of ripples (80–200 Hz) in neocortex and their neuronal correlates. Journal of neurophysiology. 2001;86:1884–1898. doi: 10.1152/jn.2001.86.4.1884. [DOI] [PubMed] [Google Scholar]
- Grenier F, Timofeev I, Steriade M. Neocortical very fast oscillations (ripples, 80–200 Hz) during seizures: intracellular correlates. Journal of neurophysiology. 2003;89:841–852. doi: 10.1152/jn.00420.2002. [DOI] [PubMed] [Google Scholar]
- Haider B, Duque A, Hasenstaub AR, McCormick DA. Neocortical network activity in vivo is generated through a dynamic balance of excitation and inhibition. J Neurosci. 2006;26:4535–4545. doi: 10.1523/JNEUROSCI.5297-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider B, Hausser M, Carandini M. Inhibition dominates sensory responses in the awake cortex. Nature. 2013;493:97–100. doi: 10.1038/nature11665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haltiner AM, Temkin NR, Dikmen SS. Risk of seizure recurrence after the first late posttraumatic seizure. Arch Phys Med Rehabil. 1997;78:835–840. doi: 10.1016/s0003-9993(97)90196-9. [DOI] [PubMed] [Google Scholar]
- Hedler L, Stamm G, Weitzell R, Starke K. Functional characterization of central alpha-adrenoceptors by yohimbine diastereomers. European journal of pharmacology. 1981;70:43–52. doi: 10.1016/0014-2999(81)90430-1. [DOI] [PubMed] [Google Scholar]
- Heinemann U, Lux HD, Gutnick MJ. Extracellular free calcium and potassium during paroxsmal activity in the cerebral cortex of the cat. Exp Brain Res. 1977;27:237–243. doi: 10.1007/BF00235500. [DOI] [PubMed] [Google Scholar]
- Hoffman SN, Salin PA, Prince DA. Chronic neocortical epileptogenesis in vitro. Journal of neurophysiology. 1994;71:1762–1773. doi: 10.1152/jn.1994.71.5.1762. [DOI] [PubMed] [Google Scholar]
- Houweling AR, Bazhenov M, Timofeev I, Steriade M, Sejnowski TJ. Homeostatic synaptic plasticity can explain post-traumatic epileptogenesis in chronically isolated neocortex. Cereb Cortex. 2005;15:834–845. doi: 10.1093/cercor/bhh184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes S, Crunelli V. UP states rise from the depths. Nature neuroscience. 2013;16:115–117. doi: 10.1038/nn.3313. [DOI] [PubMed] [Google Scholar]
- Hughes SW, Cope DW, Blethyn KL, Crunelli V. Cellular mechanisms of the slow (<1 Hz) oscillation in thalamocortical neurons in vitro. Neuron. 2002;33:947–958. doi: 10.1016/s0896-6273(02)00623-2. [DOI] [PubMed] [Google Scholar]
- Jefferys JG. Nonsynaptic modulation of neuronal activity in the brain: electric currents and extracellular ions. Physiol Rev. 1995;75:689–723. doi: 10.1152/physrev.1995.75.4.689. [DOI] [PubMed] [Google Scholar]
- Jin X, Huguenard JR, Prince DA. Reorganization of inhibitory synaptic circuits in rodent chronically injured epileptogenic neocortex. Cereb Cortex. 2011;21:1094–1104. doi: 10.1093/cercor/bhq181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X, Prince DA, Huguenard JR. Enhanced excitatory synaptic connectivity in layer v pyramidal neurons of chronically injured epileptogenic neocortex in rats. J Neurosci. 2006;26:4891–4900. doi: 10.1523/JNEUROSCI.4361-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joy RM, Stark LG, Albertson TE. Dose-dependent proconvulsant and anticonvulsant actions of the alpha 2 adrenergic agonist, xylazine, on kindled seizures in the rat. Pharmacol Biochem Behav. 1983;19:345–350. doi: 10.1016/0091-3057(83)90063-1. [DOI] [PubMed] [Google Scholar]
- Kandratavicius L, Balista PA, Lopes-Aguiar C, Ruggiero RN, Umeoka EH, Garcia-Cairasco N, Bueno-Junior LS, Leite JP. Animal models of epilepsy: use and limitations. Neuropsychiatric Disease and Treatment. 2014;10:1693–1705. doi: 10.2147/NDT.S50371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B, Miledi R. The role of calcium in neuromuscular facilitation. The Journal of physiology. 1968;195:481–492. doi: 10.1113/jphysiol.1968.sp008469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazemi H, Hashemi-Fesharaki S, Razaghi S, Najafi M, Kolivand PH, Kovac S, Gorji A. Intractable epilepsy and craniocerebral trauma: Analysis of 163 patients with blunt and penetrating head injuries sustained in war. Injury. 2012;43:2132–2135. doi: 10.1016/j.injury.2012.06.007. [DOI] [PubMed] [Google Scholar]
- Kilman V, van Rossum MC, Turrigiano GG. Activity deprivation reduces miniature IPSC amplitude by decreasing the number of postsynaptic GABA(A) receptors clustered at neocortical synapses. J Neurosci. 2002;22:1328–1337. doi: 10.1523/JNEUROSCI.22-04-01328.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollevold T. Immediate and early cerebral seizures after head injuries. Part I. J Oslo City Hosp. 1976;26:99–114. [PubMed] [Google Scholar]
- Kuśmierczak M, Lajeunesse F, Grand L, Timofeev I. Changes in long-range connectivity and neuronal reorganization in partial cortical deafferentation model of epileptogenesis. Neuroscience. 2015;284:153–164. doi: 10.1016/j.neuroscience.2014.09.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemieux M, Chen J-Y, Lonjers P, Bazhenov M, Timofeev I. The impact of cortical deafferentation on the neocortical slow oscillation. J Neurosci. 2014;34:5689–5703. doi: 10.1523/JNEUROSCI.1156-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leviton A, Cowan LD. Methodological issues in the epidemiology of seizure disorders in children. Epidemiol Rev. 1981;3:67–89. doi: 10.1093/oxfordjournals.epirev.a036240. [DOI] [PubMed] [Google Scholar]
- MacDonald JF, Bartlett MC, Mody I, Pahapill P, Reynolds JN, Salter MW, Schneiderman JH, Pennefather PS. Actions of ketamine, phencyclidine and MK-801 on NMDA receptor currents in cultured mouse hippocampal neurones. The Journal of physiology. 1991;432:483–508. doi: 10.1113/jphysiol.1991.sp018396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majkowski J, Bilinska-Nigot B, Sobieszek A. Development of EEG epileptic activity and seizures during kindling in sensorimotor cortex in cats. Epilepsia. 1981;22:275–284. doi: 10.1111/j.1528-1157.1981.tb04110.x. [DOI] [PubMed] [Google Scholar]
- Markram H, Lubke J, Frotscher M, Roth A, Sakmann B. Physiology and anatomy of synaptic connections between thick tufted pyramidal neurones in the developing rat neocortex. The Journal of physiology. 1997;500(Pt 2):409–440. doi: 10.1113/jphysiol.1997.sp022031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massimini M, Amzica F. Extracellular calcium fluctuations and intracellular potentials in the cortex during the slow sleep oscillation. Journal of neurophysiology. 2001;85:1346–1350. doi: 10.1152/jn.2001.85.3.1346. [DOI] [PubMed] [Google Scholar]
- McCormick DA. Neurotransmitter actions in the thalamus and cerebral cortex and their role in neuromodulation of thalamocortical activity. Prog Neurobiol. 1992;39:337–388. doi: 10.1016/0301-0082(92)90012-4. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Contreras D. On the cellular and network bases of epileptic seizures. Annu Rev Physiol. 2001;63:815–846. doi: 10.1146/annurev.physiol.63.1.815. [DOI] [PubMed] [Google Scholar]
- McNamara JO, Byrne MC, Dasheiff RM, Fitz JG. The kindling model of epilepsy: a review. Prog Neurobiol. 1980;15:139–159. doi: 10.1016/0301-0082(80)90006-4. [DOI] [PubMed] [Google Scholar]
- Meeren HK, Pijn JP, Van Luijtelaar EL, Coenen AM, Lopes da Silva FH. Cortical focus drives widespread corticothalamic networks during spontaneous absence seizures in rats. J Neurosci. 2002;22:1480–1495. doi: 10.1523/JNEUROSCI.22-04-01480.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midzyanovskaya IS, Salonin DV, Bosnyakova DY, Kuznetsova GD, van Luijtelaar EL. The multiple effects of ketamine on electroencephalographic activity and behavior in WAG/Rij rats. Pharmacol Biochem Behav. 2004;79:83–91. doi: 10.1016/j.pbb.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Moody WJ, Futamachi KJ, Prince DA. Extracellular potassium activity during epileptogenesis. Exp Neurol. 1974;42:248–263. doi: 10.1016/0014-4886(74)90023-5. [DOI] [PubMed] [Google Scholar]
- Mugnaini E. Cell junctions of astrocytes, ependyma, and related cells in the mammalian central nervous system, with emphasis on the hypothesis of a generalized functional syncytium of supporting cells, Astrocytes. New York: Academic: New York; 1986. [Google Scholar]
- Murthy VN, Schikorski T, Stevens CF, Zhu Y. Inactivity produces increases in neurotransmitter release and synapse size. Neuron. 2001;32:673–682. doi: 10.1016/s0896-6273(01)00500-1. [DOI] [PubMed] [Google Scholar]
- Nelson SB, Turrigiano GG. Synaptic depression: a key player in the cortical balancing act. Nature neuroscience. 1998;1:539–541. doi: 10.1038/2775. [DOI] [PubMed] [Google Scholar]
- Nicholas AP, Pieribone V, Hokfelt T. Distributions of mRNAs for alpha-2 adrenergic receptor subtypes in rat brain: an in situ hybridization study. The Journal of comparative neurology. 1993;328:575–594. doi: 10.1002/cne.903280409. [DOI] [PubMed] [Google Scholar]
- Nita D, Cisse Y, Frohlich F, Timofeev I. Cortical and thalamic components of neocortical kindling-induced epileptogenesis in behaving cats. Exp Neurol. 2008a;211:518–528. doi: 10.1016/j.expneurol.2008.02.028. [DOI] [PubMed] [Google Scholar]
- Nita D, Cisse Y, Timofeev I. State-dependent slow outlasting activities following neocortical kindling in cats. Exp Neurol. 2008b;211:456–468. doi: 10.1016/j.expneurol.2008.02.010. [DOI] [PubMed] [Google Scholar]
- Nita D, Cissé Y, Timofeev I, Steriade M. Increased propensity to seizures after chronic cortical deafferentation in vivo. J Neurophysiol. 2006;95:902–913. doi: 10.1152/jn.00742.2005. [DOI] [PubMed] [Google Scholar]
- Nita D, Timofeev I. Incessant transitions between active and silent states in thalamocortical circuits lead to epilepsy. In: Timofeev I, editor. Mechanisms of spontaneous active states in the neocortex. Kerala: Research Signpost; 2007. pp. 135–168. [Google Scholar]
- Nita DA, Cisse Y, Timofeev I. EPSP depression following neocortical seizures in cat. Epilepsia. 2008c;49:705–709. doi: 10.1111/j.1528-1167.2007.01431.x. [DOI] [PubMed] [Google Scholar]
- Nita DA, Cisse Y, Timofeev I, Steriade M. Waking-sleep modulation of paroxysmal activities induced by partial cortical deafferentation. Cereb Cortex. 2007;17:272–283. doi: 10.1093/cercor/bhj145. [DOI] [PubMed] [Google Scholar]
- Okamoto M. [An experimental study of temporal cortical kindling in cats: the effects of midline-bisection on seizure generalization mechanism (author’s transl)] Seishin Shinkeigaku Zasshi. 1982;84:48–67. [PubMed] [Google Scholar]
- Ono K, Nakatsuka K, Baba H. Changes of visually evoked potential during seizure development in kindled cats. Int J Neurosci. 1981;12:53–58. doi: 10.3109/00207458108990672. [DOI] [PubMed] [Google Scholar]
- Payne JA, Rivera C, Voipio J, Kaila K. Cation-chloride co-transporters in neuronal communication, development and trauma. Trends Neurosci. 2003;26:199–206. doi: 10.1016/S0166-2236(03)00068-7. [DOI] [PubMed] [Google Scholar]
- Perez Velazquez JL, Carlen PL. Gap junctions, synchrony and seizures. Trends Neurosci. 2000;23:68–74. doi: 10.1016/s0166-2236(99)01497-6. [DOI] [PubMed] [Google Scholar]
- Prince DA, Lux HD, Neher E. Measurement of extracellular potassium activity in cat cortex. Brain research. 1973;50:489–495. doi: 10.1016/0006-8993(73)90758-0. [DOI] [PubMed] [Google Scholar]
- Prince DA, Tseng GF. Epileptogenesis in chronically injured cortex: in vitro studies. Journal of neurophysiology. 1993;69:1276–1291. doi: 10.1152/jn.1993.69.4.1276. [DOI] [PubMed] [Google Scholar]
- Pumain R, Heinemann U. Extracellular calcium and potassium changes in mammalian neocortex. Adv Biochem Psychopharmacol. 1981;29:53–58. [PubMed] [Google Scholar]
- Pumain R, Kurcewicz I, Louvel J. Science. Vol. 222. New York, N.Y: 1983. Fast extracellular calcium transients: involvement in epileptic processes; pp. 177–179. [DOI] [PubMed] [Google Scholar]
- Racine R, Tuff L, Zaide J. Kindling, unit discharge patterns and neural plasticity. Can J Neurol Sci. 1975;2:395–405. doi: 10.1017/s0317167100020540. [DOI] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. I. After-discharge threshold. Electroencephalogr Clin Neurophysiol. 1972a;32:269–279. doi: 10.1016/0013-4694(72)90176-9. [DOI] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972b;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Raymont V, Salazar AM, Lipsky R, Goldman D, Tasick G, Grafman J. Correlates of posttraumatic epilepsy 35 years following combat brain injury. Neurology. 2010;75:224–229. doi: 10.1212/WNL.0b013e3181e8e6d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribak CE, Reiffenstein RJ. Selective inhibitory synapse loss in chronic cortical slabs: a morphological basis for epileptic susceptibility. Can J Physiol Pharmacol. 1982;60:864–870. doi: 10.1139/y82-122. [DOI] [PubMed] [Google Scholar]
- Rudolph M, Pospischil M, Timofeev I, Destexhe A. Inhibition determines membrane potential dynamics and controls action potential generation in awake and sleeping cat cortex. J Neurosci. 2007;27:5280–5290. doi: 10.1523/JNEUROSCI.4652-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar AM, Jabbari B, Vance SC, Grafman J, Amin D, Dillon JD. Epilepsy after penetrating head injury. I. Clinical correlates: a report of the Vietnam Head Injury Study. Neurology. 1985;35:1406–1414. doi: 10.1212/wnl.35.10.1406. [DOI] [PubMed] [Google Scholar]
- Salin P, Tseng GF, Hoffman S, Parada I, Prince DA. Axonal sprouting in layer V pyramidal neurons of chronically injured cerebral cortex. J Neurosci. 1995;15:8234–8245. doi: 10.1523/JNEUROSCI.15-12-08234.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Vives MV, Descalzo VF, Reig R, Figueroa NA, Compte A, Gallego R. Rhythmic spontaneous activity in the piriform cortex. Cereb Cortex. 2008;18:1179–1192. doi: 10.1093/cercor/bhm152. [DOI] [PubMed] [Google Scholar]
- Sanchez-Vives MV, McCormick DA. Cellular and network mechanisms of rhythmic recurrent activity in neocortex. Nature neuroscience. 2000;3:1027–1034. doi: 10.1038/79848. [DOI] [PubMed] [Google Scholar]
- Sanchez-Vives MV, Reig R, Winograd M, Descalzo VF. An active cortical network in vitro. In: Timofeev I, editor. Mechanisms of spontaneous actrive states in neocortex. Kerala, India: Research Signpost; 2007. pp. 23–44. [Google Scholar]
- Sayin U, Osting S, Hagen J, Rutecki P, Sutula T. Spontaneous seizures and loss of axo-axonic and axo-somatic inhibition induced by repeated brief seizures in kindled rats. J Neurosci. 2003;23:2759–2768. doi: 10.1523/JNEUROSCI.23-07-02759.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seigneur J, Timofeev I. Synaptic impairment induced by paroxysmal ionic conditions in neocortex. Epilepsia. 2011;52:132–139. doi: 10.1111/j.1528-1167.2010.02784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma AV, Wolansky T, Dickson CT. A Comparison of Sleeplike Slow Oscillations in the Hippocampus Under Ketamine and Urethane Anesthesia. Journal of neurophysiology. 2010;104:932–939. doi: 10.1152/jn.01065.2009. [DOI] [PubMed] [Google Scholar]
- Sheroziya M, Timofeev I. Global Intracellular Slow-Wave Dynamics of the Thalamocortical System. The Journal of Neuroscience. 2014;34:8875–8893. doi: 10.1523/JNEUROSCI.4460-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu Y, Hasenstaub A, McCormick DA. Turning on and off recurrent balanced cortical activity. Nature. 2003;423:288–293. doi: 10.1038/nature01616. [DOI] [PubMed] [Google Scholar]
- Sinner B, Graf BM. Ketamine. Handbook of experimental pharmacology. 2008:313–333. doi: 10.1007/978-3-540-74806-9_15. [DOI] [PubMed] [Google Scholar]
- Somjen GG. Ion regulation in the brain: implications for pathophysiology. Neuroscientist. 2002;8:254–267. doi: 10.1177/1073858402008003011. [DOI] [PubMed] [Google Scholar]
- Steriade M. Neuronal Substrates of Sleep and Epilepsy. Cambridge University Press; 2003. [Google Scholar]
- Steriade M, Amzica F. Dynamic coupling among neocortical neurons during evoked and spontaneous spike-wave seizure activity. Journal of neurophysiology. 1994;72:2051–2069. doi: 10.1152/jn.1994.72.5.2051. [DOI] [PubMed] [Google Scholar]
- Steriade M, Amzica F, Neckelmann D, Timofeev I. Spike-wave complexes and fast components of cortically generated seizures. II. Extra- and intracellular patterns. J Neurophysiol. 1998a;80:1456–1479. doi: 10.1152/jn.1998.80.3.1456. [DOI] [PubMed] [Google Scholar]
- Steriade M, Contreras D. Relations between cortical and thalamic cellular events during transition from sleep patterns to paroxysmal activity. J Neurosci. 1995;15:623–642. doi: 10.1523/JNEUROSCI.15-01-00623.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Nunez A, Amzica F. Intracellular analysis of relations between the slow (< 1 Hz) neocortical oscillation and other sleep rhythms of the electroencephalogram. J Neurosci. 1993a;13:3266–3283. doi: 10.1523/JNEUROSCI.13-08-03266.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Nunez A, Amzica F. A novel slow (< 1 Hz) oscillation of neocortical neurons in vivo: depolarizing and hyperpolarizing components. J Neurosci. 1993b;13:3252–3265. doi: 10.1523/JNEUROSCI.13-08-03252.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Timofeev I, Durmuller N, Grenier F. Dynamic properties of corticothalamic neurons and local cortical interneurons generating fast rhythmic (30–40 Hz) spike bursts. Journal of neurophysiology. 1998b;79:483–490. doi: 10.1152/jn.1998.79.1.483. [DOI] [PubMed] [Google Scholar]
- Steriade M, Timofeev I, Grenier F. Natural waking and sleep states: a view from inside neocortical neurons. Journal of neurophysiology. 2001;85:1969–1985. doi: 10.1152/jn.2001.85.5.1969. [DOI] [PubMed] [Google Scholar]
- Synowiec AS, Singh DS, Yenugadhati V, Valeriano JP, Schramke CJ, Kelly KM. Ketamine use in the treatment of refractory status epilepticus. Epilepsy Res. 2013;105:183–188. doi: 10.1016/j.eplepsyres.2013.01.007. [DOI] [PubMed] [Google Scholar]
- Szentagothai J, Arbib MA. Conceptual models of neural organization. Neurosci Res Program Bull. 1974;12:305–510. [PubMed] [Google Scholar]
- Tasker JG, Dudek FE. Electrophysiology of GABA-mediated synaptic transmission and possible roles in epilepsy. Neurochem Res. 1991;16:251–262. doi: 10.1007/BF00966088. [DOI] [PubMed] [Google Scholar]
- Temkin NR. Risk factors for posttraumatic seizures in adults. Epilepsia. 2003;44(Suppl 10):18–20. doi: 10.1046/j.1528-1157.44.s10.6.x. [DOI] [PubMed] [Google Scholar]
- Temkin NR, Dikmen SS, Anderson GD, Wilensky AJ, Holmes MD, Cohen W, Newell DW, Nelson P, Awan A, Winn HR. Valproate therapy for prevention of posttraumatic seizures: a randomized trial. J Neurosurg. 1999;91:593–600. doi: 10.3171/jns.1999.91.4.0593. [DOI] [PubMed] [Google Scholar]
- Temkin NR, Dikmen SS, Wilensky AJ, Keihm J, Chabal S, Winn HR. A randomized, double-blind study of phenytoin for the prevention of post-traumatic seizures. N Engl J Med. 1990;323:497–502. doi: 10.1056/NEJM199008233230801. [DOI] [PubMed] [Google Scholar]
- Temkin NR, Haglund MM, Winn HR. Causes, prevention, and treatment of post-traumatic epilepsy. New Horiz. 1995;3:518–522. [PubMed] [Google Scholar]
- Teskey GC, Hutchinson JE, Kolb B. Sex differences in cortical plasticity and behavior following anterior cortical kindling in rats. Cereb Cortex. 1999;9:675–682. doi: 10.1093/cercor/9.7.675. [DOI] [PubMed] [Google Scholar]
- Teskey GC, Monfils MH, VandenBerg PM, Kleim JA. Motor map expansion following repeated cortical and limbic seizures is related to synaptic potentiation. Cereb Cortex. 2002;12:98–105. doi: 10.1093/cercor/12.1.98. [DOI] [PubMed] [Google Scholar]
- Thimm J, Mechler A, Lin H, Rhee S, Lal R. Calcium-dependent open/closed conformations and interfacial energy maps of reconstituted hemichannels. J Biol Chem. 2005;280:10646–10654. doi: 10.1074/jbc.M412749200. [DOI] [PubMed] [Google Scholar]
- Timofeev I. Injury Induced Epileptogenesis: Contribution of Active Inhibition, Disfacilitation and Deafferentation to Seizure Induction in Thalamocortical System. In: Woodin MA, Maffei A, editors. Inhibitory Synaptic Plasticity. New York: Springer; 2011. pp. 107–122. [Google Scholar]
- Timofeev I. Pathophysiology of neocortical seizures. In: Panayiotopoulos CP, editor. The Atlas of Epilepsies. London: Springer-Verlag; 2010. pp. 203–212. [Google Scholar]
- Timofeev I, Bazhenov M, Avramescu S, Nita DA. Posttraumatic epilepsy: the roles of synaptic plasticity. Neuroscientist. 2010;16:19–27. doi: 10.1177/1073858409333545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timofeev I, Bazhenov M, Seigneur J, Sejnowski T. Neuronal Synchronization and Thalamocortical Rhythms in Sleep, Wake and Epilepsy. In: NoebelsL JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper’s Basic Mechanisms of the Epilepsies. 2012. [PubMed] [Google Scholar]
- Timofeev I, Chauvette S, Soltani S. Neocortical focus: experimental view. In: Jiruska P, Jefferys JG, de Curtis M, editors. International Review of Neurobiology: Modern Concepts of the Epileptic Focus. 2014. pp. 9–33. [DOI] [PubMed] [Google Scholar]
- Timofeev I, Grenier F, Bazhenov M, Sejnowski TJ, Steriade M. Origin of slow cortical oscillations in deafferented cortical slabs. Cereb Cortex. 2000;10:1185–1199. doi: 10.1093/cercor/10.12.1185. [DOI] [PubMed] [Google Scholar]
- Timofeev I, Grenier F, Steriade M. Contribution of intrinsic neuronal factors in the generation of cortically driven electrographic seizures. Journal of neurophysiology. 2004;92:1133–1143. doi: 10.1152/jn.00523.2003. [DOI] [PubMed] [Google Scholar]
- Timofeev I, Grenier F, Steriade M. Disfacilitation and active inhibition in the neocortex during the natural sleep-wake cycle: an intracellular study. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:1924–1929. doi: 10.1073/pnas.041430398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timofeev I, Grenier F, Steriade M. The role of chloride-dependent inhibition and the activity of fast-spiking neurons during cortical spike-wave seizures. Neuroscience. 2002;114:1115–1132. doi: 10.1016/s0306-4522(02)00300-7. [DOI] [PubMed] [Google Scholar]
- Timofeev I, Grenier F, Steriade M. Spike-wave complexes and fast components of cortically generated seizures. IV. aroxysmal fast runs in cortical and thalamic neurons. J Neurophysiol. 1998;80:1495–1513. doi: 10.1152/jn.1998.80.3.1495. [DOI] [PubMed] [Google Scholar]
- Timofeev I, Sejnowski TJ, Bazhenov M, Chauvette S, Grand LB. Age dependency of trauma-induced neocortical epileptogenesis. Frontiers in Cellular Neuroscience. 2013;7 doi: 10.3389/fncel.2013.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timofeev I, Steriade M. Low-frequency rhythms in the thalamus of intact-cortex and decorticated cats. Journal of neurophysiology. 1996;76:4152–4168. doi: 10.1152/jn.1996.76.6.4152. [DOI] [PubMed] [Google Scholar]
- Timofeev I, Steriade M. Neocortical seizures: initiation, development and cessation. Neuroscience. 2004;123:299–336. doi: 10.1016/j.neuroscience.2003.08.051. [DOI] [PubMed] [Google Scholar]
- Topolnik L, Steriade M, Timofeev I. Hyperexcitability of intact neurons underlies acute development of trauma-related electrographic seizures in cats in vivo. Eur J Neurosci. 2003a;18:486–496. doi: 10.1046/j.1460-9568.2003.02742.x. [DOI] [PubMed] [Google Scholar]
- Topolnik L, Steriade M, Timofeev I. Partial cortical deafferentation promotes development of paroxysmal activity. Cereb Cortex. 2003b;13:883–893. doi: 10.1093/cercor/13.8.883. [DOI] [PubMed] [Google Scholar]
- Traub RD, Borck C, Colling SB, Jefferys JG. On the structure of ictal events in vitro. Epilepsia. 1996;37:879–891. doi: 10.1111/j.1528-1157.1996.tb00042.x. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG. Homeostatic plasticity in neuronal networks: the more things change, the more they stay the same. Trends Neurosci. 1999;22:221–227. doi: 10.1016/s0166-2236(98)01341-1. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG, Leslie KR, Desai NS, Rutherford LC, Nelson SB. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature. 1998;391:892–896. doi: 10.1038/36103. [DOI] [PubMed] [Google Scholar]
- Valentine PA, Teskey GC, Eggermont JJ. Kindling changes burst firing, neural synchrony and tonotopic organization of cat primary auditory cortex. Cereb Cortex. 2004;14:827–839. doi: 10.1093/cercor/bhh041. [DOI] [PubMed] [Google Scholar]
- Volgushev M, Chauvette S, Mukovski M, Timofeev I. Precise long-range synchronization of activity and silence in neocortical neurons during slow-wave oscillations [corrected] J Neurosci. 2006;26:5665–5672. doi: 10.1523/JNEUROSCI.0279-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volman V, Sejnowski TJ, Bazhenov M. Topological basis of epileptogenesis in a model of severe cortical trauma. J Neurophysiol. 2011;106:1933–1942. doi: 10.1152/jn.00458.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada Y, Hasegawa H, Okuda H, Yoshida K, Yamaguchi N. Kindling of the visual cortex in cats: comparison with amygdaloid kindling. Jpn J Psychiatry Neurol. 1989;43:245–253. doi: 10.1111/j.1440-1819.1989.tb02576.x. [DOI] [PubMed] [Google Scholar]
- Watt AJ, van Rossum MC, MacLeod KM, Nelson SB, Turrigiano GG. Activity coregulates quantal AMPA and NMDA currents at neocortical synapses. Neuron. 2000;26:659–670. doi: 10.1016/s0896-6273(00)81202-7. [DOI] [PubMed] [Google Scholar]
- Wester JC, Contreras D. Columnar Interactions Determine Horizontal Propagation of Recurrent Network Activity in Neocortex. The Journal of Neuroscience. 2012;32:5454–5471. doi: 10.1523/JNEUROSCI.5006-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan MO, ÄôDonnell J, Christensen DJ, Nicholson C, Iliff JJ, Takano T, Deane R, Nedergaard M. Sleep Drives Metabolite Clearance from the Adult Brain. Science. 2013;342:373–377. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong W, Ping X, Gao J, Jin X. Preparing undercut model of posttraumatic epileptogenesis in rodents. J Vis Exp. 2011 doi: 10.3791/2840. [DOI] [PMC free article] [PubMed] [Google Scholar]