Abstract
A history of eating highly-palatable foods reduces physiological and emotional responses to stress. For instance, we have previously shown that limited sucrose intake (4 ml of 30% sucrose twice daily for 14 days) reduces hypothalamic-pituitary-adrenocortical (HPA) axis responses to stress. However, the neural mechanisms underlying stress relief by such ‘comfort’ foods are unclear, and could reveal an endogenous brain pathway for stress mitigation. As such, the present work assessed the expression of several proteins related to neuronal activation and/or plasticity in multiple stress- and reward-regulatory brain regions of rats after limited sucrose (vs. water control) intake. These data were then subjected to a series of statistical analyses, including Bayesian modeling, to identify the most likely neurocircuit mediating stress relief by sucrose. The analyses suggest that sucrose reduces HPA activation by dampening an excitatory basolateral amygdala - medial amygdala circuit, while also potentiating an inhibitory bed nucleus of the stria terminalis principle subdivision-mediated circuit, resulting in reduced HPA activation after stress. Collectively, the results support the hypothesis that sucrose limits stress responses via plastic changes to the structure and function of stress-regulatory neural circuits. The work also illustrates that advanced statistical methods are useful approaches to identify potentially novel and important underlying relationships in biological data sets.
Keywords: stress, comfort food, sucrose, synaptic plasticity, Bayesian modeling, reward
Introduction
Stress is often defined as either a real or potential threat to homeostasis or well-being (Ulrich-Lai and Herman 2009). When individuals encounter a stressor, the brain typically initiates physiological stress responses that include activation of the hypothalamic-pituitary-adrenocortical (HPA) axis (reviewed in (Ulrich-Lai and Herman 2009)). In this system, information regarding the presence of a stressor is processed through a complex network of brain regions, and ultimately impinges on hypophysiotropic neurons in the paraventricular nucleus of the hypothalamus (PVN). Once activated, these PVN neurons release factors (e.g., corticotropin releasing hormone (CRH) and vasopressin) from their terminals in the median eminence. These factors are then transported through the portal blood to the anterior pituitary where they stimulate the release of adrenocorticotropic hormone (ACTH) into systemic circulation. ACTH then acts on the adrenal cortex to initiate the production and release of glucocorticoid hormones (i.e., cortisol in humans and corticosterone in rats and mice). These glucocorticoid hormones exert numerous actions throughout the body, including mobilization of stored energy (Ulrich-Lai and Herman 2009, Ulrich-Lai and Ryan 2014). Such activation of the HPA axis is critical for survival during acute stress exposure. However, chronic or repeated HPA activation leads to excessive glucocorticoid exposure, which is linked to numerous debilitating side effects, such as increased risk for depression, cardiovascular disease and cognitive decline (Paykel et al. 1969, Girod and Brotman 2004, Hinkelmann et al. 2009, Toda and Nakanishi-Toda 2011, Ulrich-Lai and Ryan 2013). As such, individuals may develop strategies to limit HPA activation, particularly during times of chronic stress.
One strategy that many individuals adopt is the intake of highly-palatable ‘comfort’ foods. During times of stress, both humans (McCann et al. 1990, Oliver and Wardle 1999, Laugero et al. 2011, Groesz et al. 2012, Kim et al. 2013, Tryon et al. 2013a) and rodents (Pecoraro et al. 2004, Ulrich-Lai et al. 2007, Packard et al. 2014) preferentially consume highly-palatable foods that are generally high in sugars, other carbohydrates, and/or fats relative to more nutritious, less-tasty alternatives. Moreover, a history of eating these types of foods confers stress relief – it improves mood and sense of well-being and reduces glucocorticoid levels following stress in both humans (Markus et al. 2000, Gibson 2006, Macht and Mueller 2007, Tomiyama et al. 2011, Tryon et al. 2013b) and rodents (Shide and Blass 1989, Strack et al. 1997, Suchecki et al. 2003, Pecoraro et al. 2004, la Fleur et al. 2005, Ulrich-Lai et al. 2007, Ulrich-Lai et al. 2010). However, the mechanisms by which the intake of palatable foods dampens stress responses are largely unknown, and could reveal an important endogenous brain pathway for stress mitigation.
To explore the mechanisms by which palatable food intake dampens stress responses, we have developed a “snacking” paradigm, in which rats with ad libitum chow and water are given additional brief (30 minute (min)) twice-daily access to a small amount (4 ml) of 30% sucrose solution (or water as a control) for 14 days (d) (Ulrich-Lai et al. 2007). The rats rapidly learn to drink the sucrose, but not the water, with intakes approximating the maximum permitted (8 ml per day with ~9 calories/d) (Ulrich-Lai et al. 2007, Ulrich-Lai et al. 2010, Christiansen et al. 2011a, Ulrich-Lai et al. 2011b). This limited sucrose intake for 14 d decreases HPA responsivity, as evidenced by both reduced CRH mRNA expression in the PVN and blunted HPA axis hormonal responses to subsequent acute stressors (Ulrich-Lai et al. 2007, Ulrich-Lai et al. 2010, Christiansen et al. 2011a, Ulrich-Lai et al. 2011b). We have previously shown that this stress-dampening results primarily from the palatability/rewarding properties of the sucrose, depends on neuronal activity in the basolateral amygdala (BLA; a key brain reward region that also regulates stress responses), and is accompanied by synaptic plasticity in the BLA (Ulrich-Lai et al. 2010, Christiansen et al. 2011a, Ulrich-Lai et al. 2011b). However, the BLA does not have significant direct projections to the PVN (Berk and Finkelstein 1981, Tribollet and Dreifuss 1981, Ulrich-Lai et al. 2011a), suggesting that it is unlikely to work alone.
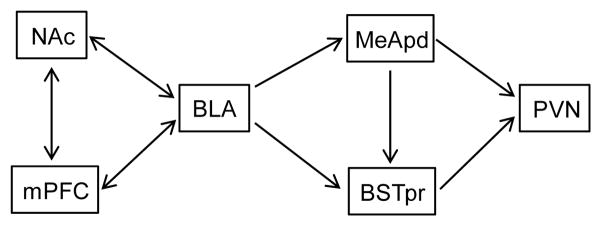
The purpose of the present work is to identify brain regions that are likely to work with the BLA to provide PVN inhibition following sucrose intake. We formed a hypothesized model of interaction of several reward- and stress-regulatory brain structures with the BLA (Figure 1). This model is based on known or hypothesized anatomical or functional connections between limbic reward circuits upstream of the BLA, and known or hypothesized BLA output to HPA axis effector neurons in the PVN. For example, the BLA receives extensive input from the medial prefrontal (prelimbic and infralimbic) cortex (mPFC) (Sesack et al. 1989, Takagishi and Chiba 1991, McDonald et al. 1996, Vertes 2004, Gabbott et al. 2005) and vice versa (Kita and Kitai 1990, McDonald 1991a, Bacon et al. 1996, Gabbott et al. 2006, Hoover and Vertes 2007). The mPFC is a critical brain reward region that is activated by palatable food intake (Mendoza et al. 2005, Petyko et al. 2009) and is thus well-positioned to communicate this information to the BLA. In addition, the nucleus accumbens (NAc) is a brain reward region that (1) is activated by palatable food intake (Park and Carr 1998, Grippo et al. 2004, Norgren et al. 2006), (2) receives input from both the BLA (Kita and Kitai 1990, McDonald 1991a, McDonald 1991b, Kirouac and Ganguly 1995) and mPFC (McDonald 1991a, Brog et al. 1993), and (3) could indirectly influence BLA activity (e.g., via intervening synapses in the lateral hypothalamus or ventral tegmental area) (Hosoya and Matsushita 1980, Heimer et al. 1991, Kirouac and Ganguly 1995, Usuda et al. 1998, Del-Fava et al. 2007, Schmitt et al. 2012). Furthermore, as the BLA itself does not project directly to the PVN (Berk and Finkelstein 1981, Tribollet and Dreifuss 1981, Ulrich-Lai et al. 2011a), it must communicate with the PVN via synapses in one or more intervening brain regions. One likely path for BLA information to reach the PVN is via BLA connections to stress-regulatory neurons located in other amygdalar subregions. Prior studies suggest that the posterodorsal subdivision of the medial amygdala (MeApd) is a primary amygdalar cell group controlling the HPA axis response to psychogenic stimuli, and may be the most likely intra-amygdalar target of HPA stress-related BLA output (Feldman et al. 1990, Feldman et al. 1994, Pitkanen et al. 1997, Prewitt and Herman 1998, Swanson and Petrovich 1998, Dayas et al. 1999, Solomon et al. 2010). Alternatively, the bed nucleus of the stria terminalis (BST) is downstream of the BLA (Weller and Smith 1982, Heimer et al. 1991, McDonald 1991b, Dong et al. 2001) and MeApd (Weller and Smith 1982, Canteras et al. 1995, Dong et al. 2001, Usunoff et al. 2009), projects to the PVN (Gu et al. 2003, Dong and Swanson 2004, Ulrich-Lai et al. 2011a), and plays a prominent role in HPA axis stress inhibition, particularly via its principle subnucleus (BSTpr) (Cullinan et al. 1993, Gray et al. 1993, Choi et al. 2007, Choi et al. 2008), suggesting that it may also contribute to PVN modulation by the BLA.
Fig. 1.
Schematic diagram of the hypothesized neural circuitry that mediates stress relief by sucrose.
To test this model, we used immunolabeling to assess expression of several proteins related to neuronal activation and/or synaptic plasticity in each of the brain regions hypothesized to play a role. These proteins included phosphorylated (S133) cAMP response element-binding protein (pCREB), phosphorylated (T286) calcium/calmodulin-dependent protein kinase IIα (pCamKII), and FosB/deltaFosB as markers of neuronal activation that are linked with synaptic plasticity (Silva et al. 1998, Nestler et al. 1999, Huang et al. 2000, Lisman et al. 2002, Colbran and Brown 2004, Rodrigues et al. 2004, Miyamoto 2006). In addition, synaptophysin (pre-synaptic terminals (Wiedenmann and Franke 1985, Tominaga-Yoshino et al. 2008)), gephyrin (post-synaptic inhibitory synapses (Kneussel and Betz 2000, Specht and Triller 2008, Bruneau et al. 2009)), and PSD95 (post-synaptic excitatory synapses (El-Husseini et al. 2000, Specht and Triller 2008, Bruneau et al. 2009)) were included as structural markers of synaptic plasticity. The resulting protein expression data were then used to perform a series of statistical modeling analyses, including Bayesian modeling, to determine if/how the brain regions are likely to work together to regulate stress responses, as well as how these relationships differ between rats with a history of limited sucrose (vs. water) intake.
Materials and Methods
Animals
To conserve on animal usage, brain samples analyzed in this study were from adult male Long-Evans rats used in an experiment reported by Ulrich-Lai, et al. (Ulrich-Lai et al. 2010). This manuscript reports immunolabeling data for pCREB, pCamKII and synaptophysin in the BLA following a history of sucrose vs. water intake (Ulrich-Lai et al. 2010). The present work greatly extends upon these protein expression data by expanding these analyses to include additional brain regions (PVN, ventral (prelimbic and infralimbic) medial prefrontal cortex (mPFC), nucleus accumbens (NAc), posterodorsal subregion of the medial amygdala (MeApd), and principle subdivision of the bed nucleus of the stria terminal (BSTpr)), as well as performing immunolabeling for additional proteins (gephyrin, PSD95, FosB/deltaFosB) in these brain samples.
In brief, adult male Long-Evans rats (~200–250 g body weight at start of experiments) were supplied by Harlan Labs (Indianapolis, IN). Rats were single-housed on a 12 hour-12 hour light cycle (lights typically on at 06:00 hour) in a temperature and humidity-controlled housing facility with ad libitum access to normal chow (LM-485, Harlan Teklad) and water. Upon arrival, rats acclimated to the housing facilities for at least 1 week prior to random assignment of treatment group and experiment onset. All procedures were approved by the University of Cincinnati Animal Care and Use Committee and are compliant with the United States National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals.
Sucrose paradigm
Rats (n = 10–11 per group) with free access to water and normal chow were given additional twice daily (at approximately 09:30 and 15:30 h) brief (up to 30 min) access to a second drink bottle containing 4 ml of sucrose (30% in water; Sigma-Aldrich Co., St. Louis, MO) or water (controls). In this paradigm, rats readily learn to drink sucrose in amounts approaching the maximum allowed and reduce their chow intake isocalorically to compensate for the sucrose calories (Ulrich-Lai et al. 2007, Ulrich-Lai et al. 2010, Christiansen et al. 2011a, Ulrich-Lai et al. 2011b). As a result, this limited sucrose intake does not affect body weight gain, nor overall adiposity (Ulrich-Lai et al. 2007, Ulrich-Lai et al. 2010, Christiansen et al. 2011a, Ulrich-Lai et al. 2011b).
On the morning following the cessation of the drink paradigm (day 15), rats were anesthetized with pentobarbital and transcardially perfused with saline and 4% paraformaldehyde. Collection of brain tissue at this time point (e.g., ~18 hours after last sucrose/water presentation) minimizes acute effects of drink presentation on protein expression, ensuring that protein levels primarily represent more enduring changes in expression following chronic sucrose intake. For example, FosB is a transiently-induced transcription factor, whereas deltaFosB is the stable truncated FosB isoform that is linked with plasticity (Nestler et al. 1999). FosB induction returns to baseline by this time point, suggesting that these studies predominantly assessed the longer-lasting deltaFosB protein (despite the fact that pan-FosB primary antibodies cannot distinguish between the two forms) (Perrotti et al. 2004, Wallace et al. 2008). After perfusion, brains were removed and post-fixed for ~16 h at room temperature and then stored in sucrose (30% in phosphate-buffered saline) at 4° C. Brains were sectioned (25 μm) on a microtome and the sections were stored in cryoprotectant (0.1 M PBS, 30% sucrose, 1% polyvinylpyrrolidone, 30% ethylene glycol) at −20° C.
Immunolabeling
Immunolabeling was performed as described in (Ulrich-Lai et al. 2010). Brain sections were immunolabeled using rabbit primary antisera directed against pCREB (1:500, 06-519, Millipore, Billerica, MA), pCamKII (1:100, ab5683, Abcam, Cambridge, MA), synaptophysin (1:300, 18-0130, Invitrogen, Carlsbad, CA), gephyrin (1:200, AB5725, Millipore), and FosB/deltaFosB (1:300, sc7203, Santa Cruz Biotechnology, Dallas, TX), and using mouse primary antisera directed against PSD95 (1:150, MAB-1598, Millipore), in a standard immunolabeling protocol (Flak et al. 2009). Immunolabeling was not present when primary antibodies were omitted. pCREB and FosB/deltaFosB immunolabeling were detected by use of biotin-conjugated goat anti-rabbit secondary antibody (Vector Laboratories, Burlingame, CA) followed by incubation with avidin-biotin-peroxidase (Vectastain ABC Solution, Vector Laboratories, Burlingame, CA) and reaction with 3,3′-diaminobenzidine. Synaptophysin, pCamKII and gephyrin immunolabeling were detected by use of cy3-conjugated donkey anti-rabbit secondary antibody (Jackson ImmunoResearch Laboratories Inc., West Grove, PA). PSD95 immunolabeling was detected by use of cy3-conjugated donkey anti-mouse secondary antibody (Jackson ImmunoResearch Laboratories Inc., West Grove, PA). Brain immunolabeling is commonly performed for all of these proteins. The patterns of immunolabeling observed in the present study are similar to that previously reported for each of these proteins (Simburger et al. 2000, Flak et al. 2009, Ulrich-Lai et al. 2010, Christiansen et al. 2011a, Seese et al. 2013, Egan and Ulrich-Lai 2015).
Microscopy and image analysis
Image analysis was performed as described in (Ulrich-Lai et al. 2010). Anatomical regions of interest were identified using the Swanson (Swanson 1998) and Paxinos and Watson (Paxinos and Watson 1998) rat brain atlases. pCREB and FosB/deltaFosB immunolabeling were each imaged using brightfield light microscopy (Zeiss Imager.Z1 microscope with Apotome, AxioCam camera, AxioVision Rel. 4.6 software; Carl Zeiss SMT Inc., Peabody, MA) and immunopositive cells were counted using Scion Image software (Scion Corp., Frederick, MA). pCamKII, synaptophysin, gephyrin and PSD95 immunolabeling were imaged using fluorescent microscopy (same imaging system as above). pCamKII-positive cells were manually counted. For synaptophysin, gephyrin and PSD95, the percent of area occupied was determined for sets of projections compiled from an Apotome z-stack image (~20 images of 0.5 μm thickness) for each side of the brain; these values were then averaged to obtain the “% occupied” for each rat. All analyses were performed by personnel unaware of group assignments.
Imputation of missing values
Occasionally we were unable to obtain a measurement for a particular protein in a brain region of a particular rat (e.g., tissue section was missing, folded, ripped, faulty immunolabeling, etc.) (Ulrich-Lai et al. 2010). As the statistical methods employed require a complete data set, these missing values were imputed by multidimensional linear regression, as suggested in Gelman and Hill (Gelman and Hill 2006), prior to completing the statistical analyses. More specifically, in order to impute missing values for a particular protein (say for example – synaptophysin), a multidimensional linear regression is performed using only the data from rats that are not missing any other values. The y-axis of this regression is the synaptophysin value for each rat, while the other proteins (e.g., pCamKII, pCREB, PSD95, gephyrin, FosB/deltaFosB) are each plotted on a distinct x-axis for each particular protein (i.e., x-axes in 5 dimensions). Then, for each rat with a missing synaptophysin value, the values of that rat’s other proteins (pCamKII, pCREB, PSD95, gephyrin, FosB/deltaFosB) are placed in this multidimensional linear regression and the corresponding value of the y-axis (synaptophysin) is predicted.
Imputed data values are indicated on Supplementary Table 1. The average number of imputed values for a given brain region is 8% (range of 3.6–14.3%). Likewise, the average number of imputed values for a given protein is 8% (range of 2.3–15.1%). These percentages of missing values are within the acceptable limits for accurate imputation using the multidimensional linear regression approach (Gelman and Hill 2006).
Correlation analysis
Correlation analysis is a simple and straightforward way to examine the pairwise dependence between brain regions. Correlation analysis calculates the coefficient of correlation, which is a measure of linear association between two random quantities. Correlation analyses were performed among the brain regions of interest for both pCREB and synaptophysin proteins. The correlations were calculated using the rcorr function of the Hmisc library in R version 3.0.1. The rcorr function provided the matrix of correlations as well as the asymptotic p-values.
Bayesian network analysis
In order to consider the contributions of all 6 proteins in all of the brain regions at once, we next performed an exploratory Bayesian network analysis. Bayesian network analysis is a graphical model that models the joint distribution for a set of variables (Bottcher and Dethlefsen 2003). The Bayesian network was estimated using R 2.15.1 with deal package version 1.2–35. The deal package uses a greedy search strategy with random restarts to screen all possible Bayesian networks, and then the ‘best fit’ network with the highest possible network scores was identified for the water- and sucrose-fed rats alone, and together (Bottcher and Dethlefsen 2003). More specifically, in this procedure all of the data is initially placed into an ‘empty’ network, which does not assume any connections among the various brain regions or their expressed proteins. The greedy search algorithm then adds a single connection between one specific protein/brain region and another specific protein/brain region, and the network score of this new network is calculated. The network score represents the extent to which the model’s theoretical distribution overlaps with the actual distribution of the data. The greedy search then continues to try all possible combinations of connectivity (i.e., among all the different proteins across all of the different brain regions) and the network score of each is calculated. Ultimately the ‘best fit’ model is determined as the model that gives the highest network score – this model is the one whose theoretical distribution has the highest likelihood of explaining the actual distribution of the data. As such, the Bayesian network analysis was performed in an exploratory manner that did not assume any prior information on the structure of the network. In summary, the resulting Bayesian network provides an overall (though very rough) picture of the likely underlying relationships among all 6 proteins in all 6 brain regions.
Confirmatory pathway analysis
A confirmatory pathway analysis was performed in order to test whether the a priori hypothesized model of sucrose’s effects (Figure 1) was supported by the protein expression data. Confirmatory pathway analysis requires the field of study to have a substantial amount of prior knowledge, which is then used to form an a priori model. The a priori model includes assumptions that various components are causally related to each other in a particular direction (i.e., information flows in a particular direction between two particular brain regions). The analysis then uses regression models to test whether the data support (or confirm) these assumed directional relationships.
More specifically, in the first step of this procedure, a principle component analysis was performed using the princomp function in the R software version 2.15.1. Second, a regression analysis of the first principle component was performed using PROC CALIS in SAS 9.3. The NAc was omitted from the pathway analysis since no significant relationships were detected between the NAc and the other brain regions, and since inclusion of the NAc made the model no longer estimable. Among all estimable models, the one with the highest Chi-square goodness of fit and lowest RMSEA (root mean square error of approximation) was selected as the ‘best fit’. This process determined the strength of correlation for each relationship designated in the hypothesized model, where statistically significant (p<0.05) correlation coefficients are suggestive of direct, causal relationships between the relevant brain regions. In this manner, each relationship in the hypothesized model was tested to determine which are likely based on the protein expression data.
Exploratory factor analysis
Factor analysis was performed to help understand the functional processes that underlie the altered protein expression within each brain region. The primary purpose of factor analysis is to reduce the number of factors that must be considered at one time. For example, normally simultaneous consideration of 6 different proteins requires 6 dimensions. However, factor analysis takes these many proteins and explains their relationships in just a couple of factors/dimensions. Moreover, when used in an exploratory way it can reveal the latent variables underlying a set of proteins in a given brain region. In other words, this analysis describes the covariance among the various proteins in terms of a few underlying, but unobservable, quantities called factors. This includes the ability to identify underlying factors that are not yet known in the field, thus revealing new and previously unknown relationships between the proteins.
In the present study, exploratory factor analysis considered one brain region at a time and looked at the relationships between all the measured proteins within the region. This analysis was performed for the PVN, BSTpr, MeApd and BLA of water- vs. sucrose-fed rats using the factanal function in R software 2.15.1. The ‘best fit’ factor loadings were identified using the maximum likelihood approach (Johnson and Wichern 2007). The varimax criterion was used to preferentially load data variability onto the first 1–2 factors (Johnson and Wichern 2007). The first two factors, which represent 44–69% of the data variability, were identified (p<0.05 by Chi-square test that 2 factors represent sufficient variance for each brain region). The individual proteins that contributed to each factor were identified as those that had factor loadings of ≥ 0.6 or ≤ −0.6. In this manner, factor analysis revealed the combinations of key proteins that reflect the predominant activity in a particular brain region of water- vs. sucrose-fed rats.
Results
To test the hypothesized model (Figure 1) of stress-relief by sucrose, we used immunolabeling to assess the expression of proteins related to neuronal activation and plasticity (pCREB, pCamKII, FosB/deltaFosB, synaptophysin, gephyrin, and PSD95) in the brain regions of interest (BLA, PVN, BSTpr, MeApd, mPFC, and NAc). These protein expression data are summarized in Supplemental Table 1. Using these data, we then performed a series of statistical modeling approaches to determine whether the hypothesized model was the most likely mechanistic representation for stress relief following sucrose intake.
Correlations
pCREB
We first performed simple correlations on the amount of pCREB immunolabeling between the various brain regions. This was done as an exploratory analysis to indicate whether these brain regions are likely to be working together across all of the animals, or specifically in either the sucrose-fed or water-control rats. pCREB was selected for analysis since it is an intracellular mediator that is activated by numerous signaling pathways, and is a general marker of neuronal activation that is linked with synaptic plasticity (Silva et al. 1998, Huang et al. 2000, Miyamoto 2006).
When all of the rats were analyzed together (regardless of drink history, Figure 2A), PVN pCREB was correlated positively with pCREB in the MeApd (p=0.027) and negatively with pCREB in the BSTpr (p=0.048). This is consistent with known stress-excitatory actions of the MeA (Feldman et al. 1990, Feldman et al. 1994, Dayas et al. 1999, Solomon et al. 2010) and stress-inhibitory actions of the BSTpr (Cullinan et al. 1993, Gray et al. 1993, Choi et al. 2007, Choi et al. 2008). Among water control rats specifically (Figure 2B), BLA pCREB was positively associated with pCREB in the mPFC (p=0.040), MeApd (p=0.034), and PVN (p=0.008), while MeApd pCREB was also positively associated with PVN pCREB (p=0.015). This supports the hypothesized model in that (1) mPFC activation is linked with BLA activation, (2) BLA activation is linked with MeApd activation, and (3) both BLA and MeApd activation are linked with PVN activation. This excitatory network likely promotes stress activation during stress in control rats. In contrast, after sucrose intake (Figure 2C), the excitatory correlations present in the control rats are lost (all p>0.05). Instead BSTpr pCREB becomes negatively associated with PVN pCREB (p=0.018) after sucrose, consistent with both the hypothesized model and the known stress-inhibitory actions of the BSTpr (Cullinan et al. 1993, Gray et al. 1993, Choi et al. 2007, Choi et al. 2008). This suggests that sucrose may act to recruit BSTpr-mediated stress inhibition while limiting the contribution of mPFC-, BLA-, and MeApd-mediated stress excitation.
Fig. 2.
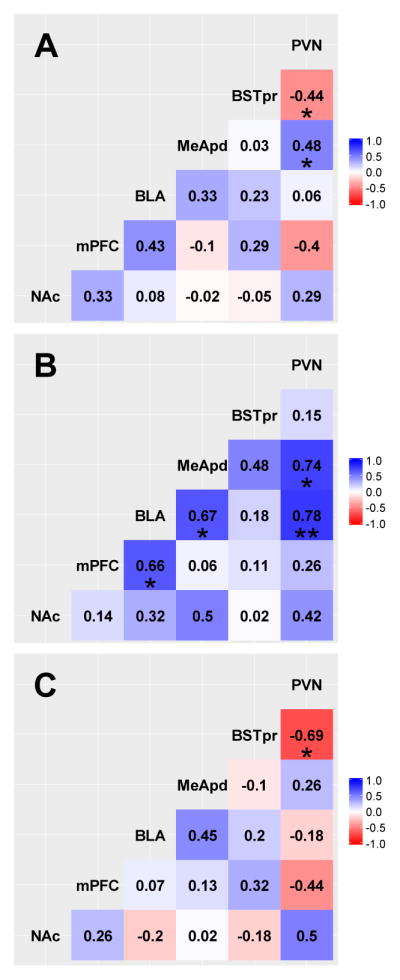
Correlation analysis of the pCREB immunolabeling among the various brain regions in all rats (A), water control rats only (B), and sucrose-fed rats only (C). Numbers denote correlation coefficients. *p<0.05, **p<0.01.
Synaptophysin
Correlational analyses were also performed for synaptophysin immunolabeling in the brain regions of interest since changes in synaptophysin are indicative of changes in overall pre-synaptic innervation density (i.e., structural plasticity). When all rats are analyzed together (Figure 3A), BSTpr synaptophysin was negatively associated with PVN synaptophysin (p=0.001). Among water control rats (Figure 3B), there were no significant correlations for synaptophysin (all p>0.05). However, in sucrose-fed rats (Figure 3C) BLA synaptophysin was positively associated with BSTpr synaptophysin (p=0.024), and BSTpr synaptophysin was negatively associated with PVN synaptophysin (p=0.014). This suggests that sucrose induces structural plasticity in the BLA, BSTpr and PVN that is linked to each other, consistent with key aspects of the hypothesized model.
Fig. 3.
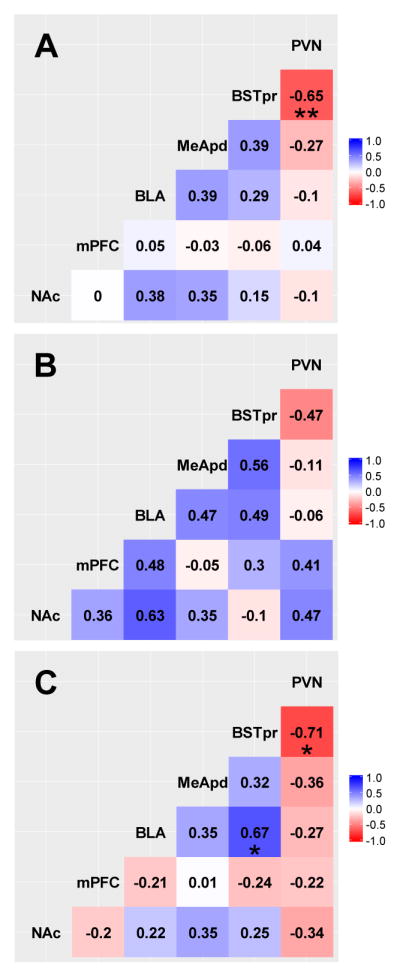
Correlation analysis of the synaptophysin immunolabeling among the various brain regions in all rats (A), water control rats only (B), and sucrose-fed rats only (C). Numbers denote correlation coefficients. *p<0.05, **p<0.01.
Notably, correlation analyses for pCREB and synaptophysin did not reveal any associations between the NAc and any of the other brain regions regardless of drink type. This does not support the inclusion of the NAc in the hypothesized model, and suggests that the NAc is less likely to work with the other structures to mediate sucrose stress relief. In contrast, correlation analyses for pCREB and synaptophysin indicate that the PFC, BLA, MeApd, BSTpr and PVN are likely to work together to regulate stress responses, and that sucrose may act by modifying these relationships.
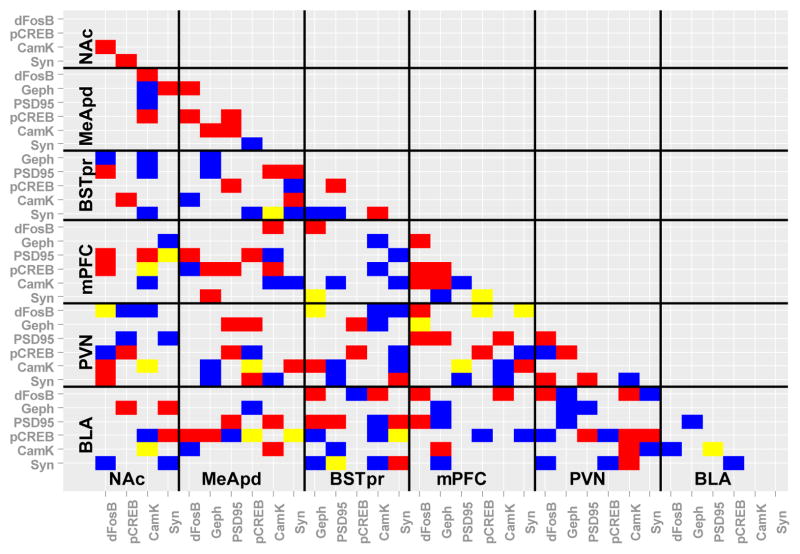
Bayesian network analysis
The simple correlations employed above reveal the probable pairwise functional connections among the 6 brain regions of interest when considering the expression of a single protein-- either pCREB or synaptophysin. In order to consider the contributions of all 6 proteins in all of the brain regions at once, we next performed an exploratory Bayesian network analysis. All possible Bayesian networks were screened and the one that ‘best-fit’ the data was identified for the water control vs. sucrose-fed rats.
Exploratory Bayesian network analysis revealed numerous proteins whose expression was likely to be linked with other proteins, either within or across brain regions (Figure 4). In control rats, the BLA, MeApd and mPFC each had numerous protein expression associations with the BSTpr, while the BSTpr, BLA and NAc each had numerous associations with the PVN. This suggests that the synaptic structure and/or function of these brain regions are likely to be linked, with the BSTpr forming a potential gateway for limbic forebrain information to reach the PVN. After sucrose intake, the number of associated proteins between the mPFC–BSTpr and BSTpr–PVN are reduced. Moreover, associations between the BLA–MeApd, PFC–MeApd and MeApd–PVN are increased. This suggests that sucrose may act, at least in part, by changing the relative contribution of the MeApd and BSTpr to stress-regulatory circuits.
Fig. 4.
Exploratory Bayesian network analysis considered the contributions of all 6 measured proteins in all of the brain regions at once. Predicted significant relationships between proteins within and across brain regions are shown in blue for water control rats, in red for sucrose-fed rats, and in yellow for those predicted in both water control and sucrose-fed rats. dFosB= FosB/deltaFosB, Geph= gephyrin, CamK= CamKIIa, Syn= synaptophysin.
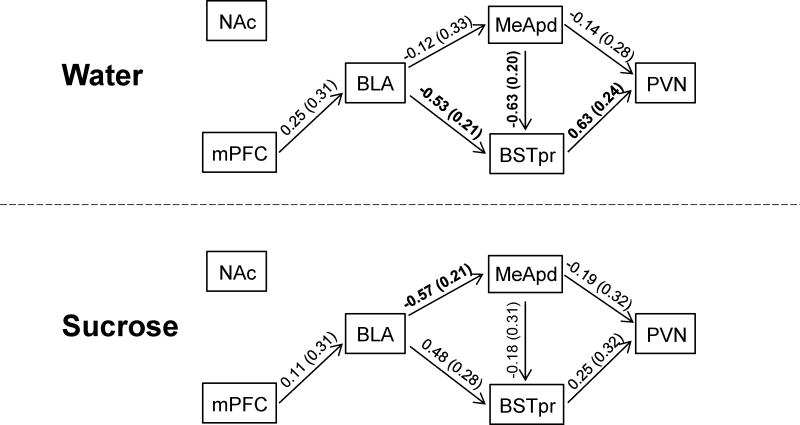
Confirmatory pathway analysis
A confirmatory pathway analysis (Figure 5) was performed in order to test whether the a priori hypothesized model of sucrose’s effects (Figure 1) was supported by the protein expression data. Among water control rats, pathway analysis revealed relationships (i.e., significant regression coefficients) between BLA-BSTpr, MeApd-BSTpr, and BSTpr-PVN. This suggests that the BSTpr is functionally linked with the BLA, MeApd and PVN, and may be an important route for information to reach the PVN in control rats. In contrast, in sucrose-fed rats the regression coefficient between the BLA-MeApd is significant, while others are not. This suggests that sucrose may act to increase BLA regulation of the MeApd, while also decreasing regulation of the PVN by the BSTpr and MeApd.
Fig. 5.
Confirmatory pathway analysis on the first principle component tested the extent to which the a priori hypothesized model was supported by the protein expression data in both water control (top) and sucrose-fed (bottom) rats. Connecting lines are labeled with regression coefficients (standard error in parenthesis); statistically significant regression coefficients (p<0.05) are shown in bold.
Factor analysis
Lastly, we performed a factor analysis for each of the brain regions implicated by the pathway analysis (i.e., PVN, BSTpr, MeApd, and BLA) (Table 1). This analysis determined which proteins/markers: (1) were the primary contributors to the overall variance in each of the brain regions (i.e., any marker that loaded onto either factors 1 or 2), and (2) had variances that correlated with each other (i.e., markers that loaded together on a single factor). In addition, this was performed for rats that had a history of limited sucrose vs. water controls to determine whether sucrose intake altered this analysis.
Table 1.
Factor analysis was performed for the protein expression data from the PVN, BSTpr, MeApd and BLA of water control vs. sucrose-fed rats. The first two factors were identified; when combined these factors represent 44–69% of the data variability. Factor loadings of ≥ 0.6 (or ≤ −0.6) are listed; these reached criterion for classification as contributing to the respective factor (factor loadings that failed to reach criterion are not shown).
| Brain region | Protein | Water | Sucrose | ||
|---|---|---|---|---|---|
| Factor 1 | Factor 2 | Factor 1 | Factor 2 | ||
| PVN | Synaptophysin | 0.98 | 0.99 | ||
| pCamKII | |||||
| pCREB | 0.95 | −0.78 | |||
| PSD95 | |||||
| Gephyrin | 1 | ||||
| FosB/deltaFosB | 0.76 | 0.75 | |||
| BSTpr | Synaptophysin | 0.85 | |||
| pCamKII | 0.99 | ||||
| pCREB | 0.96 | ||||
| PSD95 | −0.75 | 1 | |||
| Gephyrin | 0.86 | ||||
| MeApd | Synaptophysin | 0.85 | |||
| pCamKII | 0.69 | ||||
| pCREB | −0.93 | ||||
| PSD95 | 0.9 | ||||
| Gephyrin | 0.86 | ||||
| FosB/deltaFosB | 0.85 | ||||
| BLA | Synaptophysin | ||||
| pCamKII | 0.83 | −0.7 | |||
| pCREB | |||||
| PSD95 | 0.94 | 0.67 | −0.74 | ||
| Gephyrin | 0.87 | ||||
| FosB/deltaFosB | 0.68 | ||||
PVN
For water control rats, factor 1 explains 29% of the variance with positive loading for pCREB and FosB/deltaFosB expression, while factor 2 explains 27% of the variance and has positive loading for synaptophysin (Table 1). This suggests that in control rats, the variance of PVN protein expression is governed primarily by the pCREB/FosB pathway, and secondarily by the overall density of presynaptic innervation. In sucrose-fed rats, factor 1 explains 31% of the variance with positive loading for synaptophysin and FosB/deltaFosB expression, while factor 2 explains 30% of the variance and has positive loading for gephyrin and negative for pCREB (Table 1). This suggests that a history of intermittent sucrose drink biases PVN protein expression towards FosB/synaptophysin plasticity, as well as increased gephyrin (and reduced pCREB).
BSTpr
For water control rats, factor 1 explains 42% of the variance with positive loading for gephyrin and synaptophysin and negative loading for PSD95. Factor 2 explains 27% of the variance and has positive loading for pCREB (Table 1). This suggests that without sucrose drink, the variance of BSTpr protein expression is primarily attributed to the relative amount of inhibitory (vs. excitatory) synaptic plasticity, and secondarily to activation of pCREB intracellular signaling. In contrast, in sucrose-fed rats, factor 1 explains 32% of the variance with positive loading for pCamKII, while factor 2 explains 30% of the variance and has positive loading for PSD95 (Table 1). This suggests that sucrose drink biases BSTpr protein expression towards pCamKII-associated changes in PSD95/excitatory synapses, while placing comparatively less importance on the gephyrin/inhibitory synapses.
MeApd
For water control rats (Table 1), factor 1 explains 32% of the variance with positive loading for synaptophysin and negative loading for pCREB. Factor 2 explains 23% of the variance and has positive loading for FosB/deltaFosB. This suggests that without sucrose drink, the variance of MeApd protein expression is primarily attributed to pCREB-related innervation density, and secondarily to activation of FosB/deltaFosB intracellular signaling. In contrast, in sucrose-fed rats, factor 1 explains 31% of the variance with positive loading for pCamKII and PSD95, while factor 2 explains 23% of the variance and has positive loading for gephyrin (Table 1). This suggests that sucrose drink biases MeApd protein expression primarily towards pCamKII-associated changes in PSD95/excitatory synapses, and secondarily towards gephyrin/inhibitory synapses.
BLA
For water control rats (Table 1), factor 1 explains 38% of the variance with positive loading for gephyrin and PSD95. Factor 2 explains 21% of the variance and has positive loading for pCamKII. This suggests that without sucrose drink, the variance of BLA protein expression is primarily attributed to an association between PSD95/excitatory and gephyrin/inhibitory synapses, and secondarily to activation of pCamKII intracellular signaling. In contrast, in sucrose-fed rats (Table 1), factor 1 explains 25% of the variance with positive loading for PSD95 and negative loading for pCamKII. Factor 2 explains 19% of the variance and has positive loading for FosB/deltaFosB and negative loading for PSD95. This suggests that sucrose drink biases BLA protein expression primarily towards pCamKII-associated changes in PSD95/excitatory synapses, and secondarily towards FosB/deltaFosB-associated changes in PSD95/excitatory synapses.
Discussion
Stress relief by limited sucrose intake includes a dampening of the HPA axis response to stress that is accompanied by synaptic plasticity in the BLA and is BLA-dependent (Ulrich-Lai et al. 2007, Ulrich-Lai et al. 2010, Christiansen et al. 2011a, Ulrich-Lai et al. 2011b). As the BLA does not have significant direct projections to the PVN (Berk and Finkelstein 1981, Tribollet and Dreifuss 1981, Ulrich-Lai et al. 2011a), information from the BLA must reach the PVN indirectly via synapses in one or more intervening brain regions. In order to identify this circuitry, we used immunolabeling to assess the expression of six proteins related to neuronal activation and synaptic plasticity in the BLA, PVN, and several other brain regions known to be anatomically and functionally linked with the BLA and/or PVN. These data were then subjected to a series of statistical analyses (correlations, Bayesian modeling, path analysis, factor analysis) in order to uncover the most likely set of brain regions (and proteins) that govern neuronal function in rats with a history of limited sucrose (vs. water) intake.
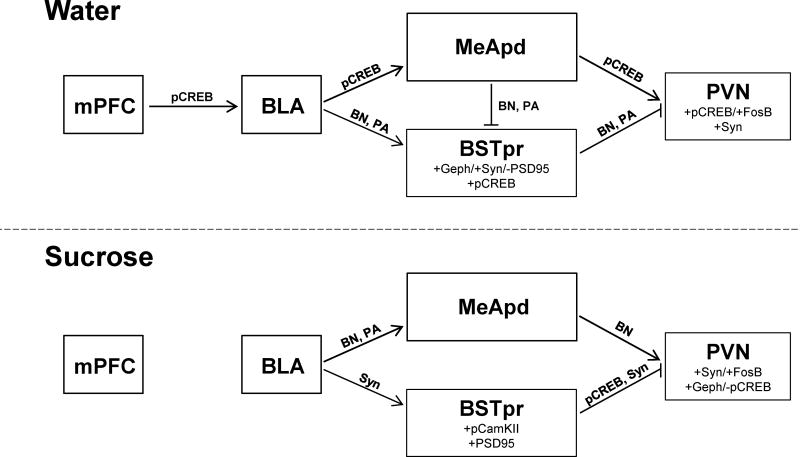
Implicated circuitry in water controls
Among the water control group, statistical analyses implicate two concurrent paths for stress modulation by the BLA (Figure 6). The first is an excitatory path dominated by the BLA, MeApd, and PVN. For instance, pairwise correlations on the water control group show that the expression of the neuronal activity marker pCREB is positively linked between the BLA-MeApd, BLA-mPFC, BLA-PVN, and MeApd-PVN. When taken with the established stress-excitatory roles of the BLA (Coover et al. 1973, Shekhar et al. 2003, Bhatnagar et al. 2004) and MeA (Feldman et al. 1990, Feldman et al. 1994, Dayas et al. 1999, Solomon et al. 2010), this suggests that BLA, mPFC, MeApd and PVN likely form a network that promotes stress activation in control rats. This idea is further supported by the numerous protein associations that were identified between the BLA-PVN in the Bayesian model, as well as the PVN factor analysis that showed positive loadings for pCREB, FosB/deltaFosB, and synaptophysin, consistent with PVN activation.
Fig. 6.
Schematic summarizing the principle neurocircuitry that was identified by statistical analyses of activity- and plasticity-related protein expression in water control (top) and sucrose-fed (bottom) rats. Lines connect brain regions with identified associations and are labeled with the primary analyses that that revealed these associations (pCREB= pCREB correlation; Syn= Synaptophysin correlation; BN= Bayesian network analysis; PA= pathway analysis). The identified associations may either be direct (single synapse) or indirect (multiple synapses). Note that lines with arrows denote presumed excitatory influences, while blunted lines denote presumed inhibitory influences (based largely on relationships established in literature). Factor analysis results are also shown for the BSTpr and PVN; proteins loading on factor 1 are listed above those loading on factor 2, with Geph= gephyrin and FosB= FosB/deltaFosB.
The second implicated route for BLA modulation is a BSTpr-mediated inhibitory pathway that is constrained in water control rats, thereby permitting robust HPA activation (Figure 6). For example, Bayesian modeling revealed numerous protein associations between the BSTpr and the BLA, MeApd and mPFC, and between the PVN and the BSTpr. Similarly, the confirmatory pathway analysis on the hypothesized model (Figure 1) also supports associations between the BSTpr and the BLA, MeApd and PVN. Taken together, these analyses suggest that BSTpr is a potential gateway for information from other structures to reach the PVN, a role that has been previously ascribed to the BSTpr (Dong and Swanson 2004, Ulrich-Lai and Herman 2009). Furthermore, factor analysis on BSTpr variance identified that the primary factor has positive loadings for gephyrin and synaptophysin and negative loading for PSD95, possibly suggesting a relatively greater inhibitory/excitatory balance in the BSTpr of controls. As the BSTpr is known to be stress-inhibitory (Cullinan et al. 1993, Gray et al. 1993, Choi et al. 2007, Choi et al. 2008), this suggests that BSTpr activity may be constrained in controls, thereby limiting BSTpr-mediated inhibition of the PVN and allowing HPA activation. Thus in the control group, the BLA may mediate PVN activation via a combination of increased MeApd-mediated excitation and constrained BSTpr-mediated inhibition.
Altered circuitry following limited sucrose intake
Importantly, these relationships are markedly altered by a history of limited sucrose intake, with sucrose appearing to potentiate HPA inhibition by the BSTpr, while possibly limiting HPA activation by the BLA-MeApd-PVN excitatory network (Figure 6). For instance, after sucrose pCREB expression in the BSTpr and PVN become negatively correlated. Moreover, synaptophysin becomes positively correlated between the BLA and BSTpr, and negatively correlated between the BSTpr and PVN. Similarly, both the Bayesian network and pathway analyses showed altered associations between the BSTpr and PVN following sucrose, and pathway analysis shows a loss of the relationship between the MeApd and the BSTpr. As GABAergic projections from the MeApd are poised to otherwise inhibit BSTpr activity (Swanson 2000, Dong et al. 2001), this collectively suggests that sucrose may act, at least in part, via decreasing MeApd-mediated inhibition of BSTpr, thereby permitting greater PVN inhibition by the BSTpr. Factor analysis on the BSTpr reveals positive loadings for pCamKII and PSD95, and we have previously shown that glutamic acid decarboxylase (enzyme that synthesizes GABA) mRNA expression is altered in the BSTpr after sucrose (Christiansen et al. 2011b), suggesting the possibility of greater excitation of this PVN-projecting region. Factor analysis on the PVN after sucrose reveals a new positive loading for gephyrin that is accompanied by a negative loading for pCREB. As sucrose also reduces CRH mRNA expression in the PVN (Ulrich-Lai et al. 2007), collectively these results are suggestive of greater PVN inhibition following sucrose.
Notably, enhanced PVN inhibition could also occur via attenuated activation by the identified stress-excitatory network comprising the BLA, MeApd and PVN, and some of the present data support this possibility. For example, after sucrose positive pCREB correlations between the BLA and the MeApd, mPFC and PVN are all lost, and several protein associations between these structures are altered in the Bayesian network analysis. Taken as a whole, these analyses suggest that sucrose dampens PVN and HPA axis activation by both limiting BLA-MeApd-PVN excitation and enhancing BSTpr-PVN inhibition.
Of note, the present work did not support the inclusion of the NAc in the stress-regulatory circuitry of either water or sucrose rats. Given the primary role of the NAc in reward processing (Park and Carr 1998, Grippo et al. 2004, Norgren et al. 2006), we had hypothesized that it would be involved, particularly in rats with a history of consuming the highly-palatable sucrose drink. This unexpected finding may suggest that the NAc is primarily involved in mediating sucrose reward and promoting sucrose intake, but that it does not also play a role in mediating the subsequent HPA-dampening. Additional work will be required to more explicitly test this proposition.
Role of synaptic plasticity
Stress-dampening by limited sucrose (vs. water) intake is accompanied by multiple indices of synaptic plasticity in the BLA (Ulrich-Lai et al. 2010, Christiansen et al. 2011a), suggesting that such plasticity may be a general mechanism for sucrose-mediated stress relief. The present work focused on measuring the expression of several markers that are either directly or indirectly associated with synaptic plasticity. Synaptophysin labels pre-synaptic nerve terminals (Wiedenmann and Franke 1985, Tominaga-Yoshino et al. 2008) and provides an overall index of innervation density. Gephyrin labels the post-synaptic side of inhibitory synapses (Kneussel and Betz 2000, Specht and Triller 2008, Bruneau et al. 2009), and PSD95 labels the post-synaptic side of excitatory synapses (El-Husseini et al. 2000, Specht and Triller 2008, Bruneau et al. 2009), providing indices of the overall density of inhibitory and excitatory synapses respectively. The other proteins analyzed are intracellular signaling molecules that are general markers of neuronal activation that are also linked with mediating synaptic plasticity. CamKII is a kinase that is activated by phosphorylation to become pCamKII; pCamKII phosphorylates and activates downstream targets, including CREB, and is linked with synaptic plasticity (Lisman et al. 2002, Colbran and Brown 2004, Rodrigues et al. 2004). CREB is activated by kinase-mediated (e.g., CamKII, protein kinase A, Map kinase, etc.) phosphorylation to become pCREB; pCREB is induced in neurons by multiple types of stimulation and is extensively linked with plasticity, learning and memory (Silva et al. 1998, Huang et al. 2000, Miyamoto 2006). FosB is a second messenger whose expression is induced transiently by neuronal activation, while deltaFosB is a stable isoform of FosB that exhibits sustained expression following repeated or chronic neuronal activation and is linked with synaptic plasticity (Nestler et al. 1999, Perrotti et al. 2004, Wallace et al. 2008). As the present results are based on the expression of these plasticity-related proteins, it suggests that sucrose-mediated alterations in the stress-regulatory circuitry are likely underpinned by plasticity-related alterations in neuronal structure and function.
Comparing the merits of each type of statistical analysis
The present work employed a variety of statistical analyses that varied greatly in complexity and scope. Each method has strengths and limitations:
Correlation analysis explores the degree of association between two sets of data. It is a simple, straightforward test that is commonly included in commercial statistical analysis software. However, it can only consider a very limited scope of data at once (e.g., comparing a single protein across 2 brain regions) and cannot assess the directionality of their relationship.
The exploratory factor analysis is also relatively simple and is often included in statistical analysis software. This analysis can consider a somewhat more complex data set at one time (e.g., all 6 proteins within a single brain region). Factor analysis expresses the relationship between these proteins in just a few factors, and can be used to uncover underlying latent variables. However, it should be noted that the results of factor analysis can be extremely difficult to interpret and translate into biological meaning, particularly since the underlying latent variables may represent concepts or entities that are not yet known to the field of study.
The confirmatory pathway analysis requires substantial a priori knowledge, which is used to predict the presence and directionality of relationships in a network with multiple components. The protein data for each brain region is first simplified by principle component analysis. Then a regression analysis is performed using the first principle component to assess whether the predicted network relationships are confirmed by the data. Pathway analyses are of intermediate complexity and are somewhat more difficult to perform, but can consider a broader scope of data (e.g., considering all brain regions at once where each region is represented by the first principle component of its protein expression).
Lastly, exploratory Bayesian network analysis can simultaneously consider the full scope of the data (e.g., comparing all individual proteins in all brain regions in a single analysis). It is an advanced statistical approach that is mathematically complicated and requires sophisticated statistical knowledge, programming skills, and extensive computer processing. However, it is also a powerful technique that can be used to predict relationships within large and complex data sets, and does not require testing of an a priori model based on large amounts of prior knowledge.
Thus, each of these statistical methods has its own advantages and disadvantages that should be considered when selecting, performing and interpreting each of these analyses.
Implications of statistical approach
Biological experiments necessarily have relatively small ‘n’s due to the desire to limit the number of experimental subjects (e.g., ethical concerns that constrain rodent use), as well as practical limitations in data acquisition and analysis, and this limited ‘n’ is an enormous challenge for conventional statistical analyses. The present series of statistical analyses demonstrate the ability of advanced statistical approaches (e.g., Bayesian modeling) to identify key relationships in a typical biological data set (Needham et al. 2007, Gelman et al. 2013). This is a powerful approach that can be applied to a wide-variety of biological data in order to identify potential underlying mechanisms. Modeling of connectivity provides insight for empirical studies aimed at elucidating circuit mechanisms underlying physiology and behavior.
Summary
The present work assessed the expression of several activity- and plasticity-related proteins in multiple brain regions that are anatomically and functionally linked with the BLA and/or PVN. These data were then subjected to a series of statistical analyses (correlations, Bayesian modeling, pathway analysis and factor analysis) in order to identify the most likely neurocircuitry mediating the BLA-dependent stress-dampening that occurs following a history of limited sucrose intake. When taken as a whole, the analyses identified two likely pathways that promote PVN activation (and subsequent HPA axis stress responses) in water controls. These paths include activation of a BLA/MeApd-mediated excitatory pathway and inhibition of a BSTpr-mediated inhibitory pathway. Sucrose intake altered these relationships, appearing to limit BLA/MeApd-mediated excitation, while possibly also promoting BSTpr-mediated inhibition, thereby contributing to diminished HPA axis responses following sucrose intake. This work supports the hypothesis that sucrose limits stress responses via plastic changes to the structure and function of stress-regulatory neural circuits. Future work can now address how the relationships among members of these circuits are altered by prior (acute or chronic) stress experience. Importantly, the work illustrates the utility of advanced statistical methods for identification of novel underlying relationships in routine biological data sets.
Supplementary Material
Acknowledgments
The authors thank Amanda Jones, Ben Packard and Kenneth Jones for their expert technical assistance. The work was support by U.S. National Institutes of Health grants R01 DK091425 (YMU), K01 DK078906 (YMU), and R03 DK089018 (YMU).
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- Bacon SJ, Headlam AJ, Gabbott PL, Smith AD. Amygdala input to medial prefrontal cortex (mPFC) in the rat: a light and electron microscope study. Brain Res. 1996;720(1–2):211–219. doi: 10.1016/0006-8993(96)00155-2. [DOI] [PubMed] [Google Scholar]
- Berk ML, Finkelstein JA. Afferent projections to the preoptic area and hypothalamic regions in the rat brain. Neuroscience. 1981;6(8):1601–1624. doi: 10.1016/0306-4522(81)90227-x. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Vining C, Denski K. Regulation of chronic stress-induced changes in hypothalamic-pituitary-adrenal activity by the basolateral amygdala. Ann N Y Acad Sci. 2004;1032:315–319. doi: 10.1196/annals.1314.050. [DOI] [PubMed] [Google Scholar]
- Bottcher SG, Dethlefsen C. DEAL: A package for learning Bayesian networks. J Statistical Software. 2003;8:200–203. [Google Scholar]
- Brog JS, Salyapongse A, Deutch AY, Zahm DS. The patterns of afferent innervation of the core and shell in the “accumbens” part of the rat ventral striatum: immunohistochemical detection of retrogradely transported fluoro-gold. J Comp Neurol. 1993;338(2):255–278. doi: 10.1002/cne.903380209. [DOI] [PubMed] [Google Scholar]
- Bruneau EG, Esteban JA, Akaaboune M. Receptor-associated proteins and synaptic plasticity. FASEB J. 2009;23(3):679–688. doi: 10.1096/fj.08-107946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canteras NS, Simerly RB, Swanson LW. Organization of projections from the medial nucleus of the amygdala: a PHAL study in the rat. J Comp Neurol. 1995;360(2):213–245. doi: 10.1002/cne.903600203. [DOI] [PubMed] [Google Scholar]
- Choi DC, Furay AR, Evanson NK, Ostrander MM, Ulrich-Lai YM, Herman JP. Bed nucleus of the stria terminalis subregions differentially regulate hypothalamic-pituitary-adrenal axis activity: implications for the integration of limbic inputs. J Neurosci. 2007;27(8):2025–2034. doi: 10.1523/JNEUROSCI.4301-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DC, Furay AR, Evanson NK, Ulrich-Lai YM, Nguyen MM, Ostrander MM, Herman JP. The role of the posterior medial bed nucleus of the stria terminalis in modulating hypothalamic-pituitary-adrenocortical axis responsiveness to acute and chronic stress. Psychoneuroendocrinology. 2008;33(5):659–669. doi: 10.1016/j.psyneuen.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen AM, Dekloet AD, Ulrich-Lai YM, Herman JP. “Snacking” causes long term attenuation of HPA axis stress responses and enhancement of brain FosB/deltaFosB expression in rats. Physiol Behav. 2011a;103(1):111–116. doi: 10.1016/j.physbeh.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen AM, Herman JP, Ulrich-Lai YM. Regulatory interactions of stress and reward on rat forebrain opioidergic and GABAergic circuitry. Stress. 2011b;14(2):205–215. doi: 10.3109/10253890.2010.531331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbran RJ, Brown AM. Calcium/calmodulin-dependent protein kinase II and synaptic plasticity. Curr Opin Neurobiol. 2004;14(3):318–327. doi: 10.1016/j.conb.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Coover G, Ursin H, Levine S. Corticosterone and avoidance in rats with basolateral amygdala lesions. J Comp Physiol Psychol. 1973;85(1):111–122. doi: 10.1037/h0034858. [DOI] [PubMed] [Google Scholar]
- Cullinan WE, Herman JP, Watson SJ. Ventral subicular interaction with the hypothalamic paraventricular nucleus: evidence for a relay in the bed nucleus of the stria terminalis. J Comp Neurol. 1993;332(1):1–20. doi: 10.1002/cne.903320102. [DOI] [PubMed] [Google Scholar]
- Dayas CV, Buller KM, Day TA. Neuroendocrine responses to an emotional stressor: evidence for involvement of the medial but not the central amygdala. Eur J Neurosci. 1999;11(7):2312–2322. doi: 10.1046/j.1460-9568.1999.00645.x. [DOI] [PubMed] [Google Scholar]
- Del-Fava F, Hasue RH, Ferreira JG, Shammah-Lagnado SJ. Efferent connections of the rostral linear nucleus of the ventral tegmental area in the rat. Neuroscience. 2007;145(3):1059–1076. doi: 10.1016/j.neuroscience.2006.12.039. [DOI] [PubMed] [Google Scholar]
- Dong HW, Petrovich GD, Swanson LW. Topography of projections from amygdala to bed nuclei of the stria terminalis. Brain Res Brain Res Rev. 2001;38(1–2):192–246. doi: 10.1016/s0165-0173(01)00079-0. [DOI] [PubMed] [Google Scholar]
- Dong HW, Swanson LW. Projections from bed nuclei of the stria terminalis, posterior division: implications for cerebral hemisphere regulation of defensive and reproductive behaviors. J Comp Neurol. 2004;471(4):396–433. doi: 10.1002/cne.20002. [DOI] [PubMed] [Google Scholar]
- Egan AE, Ulrich-Lai YM. Activation of physiological stress responses by a natural reward: Novel vs. repeated sucrose intake. Physiol Behav. 2015 doi: 10.1016/j.physbeh.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Husseini AE, Schnell E, Chetkovich DM, Nicoll RA, Bredt DS. PSD-95 involvement in maturation of excitatory synapses. Science. 2000;290(5495):1364–1368. [PubMed] [Google Scholar]
- Feldman S, Conforti N, Itzik A, Weidenfeld J. Differential effect of amygdaloid lesions on CRF-41, ACTH and corticosterone responses following neural stimuli. Brain Res. 1994;658(1–2):21–26. doi: 10.1016/s0006-8993(09)90005-1. [DOI] [PubMed] [Google Scholar]
- Feldman S, Conforti N, Saphier D. The preoptic area and bed nucleus of the stria terminalis are involved in the effects of the amygdala on adrenocortical secretion. Neuroscience. 1990;37(3):775–779. doi: 10.1016/0306-4522(90)90107-f. [DOI] [PubMed] [Google Scholar]
- Flak JN, Ostrander MM, Tasker JG, Herman JP. Chronic stress-induced neurotransmitter plasticity in the PVN. J Comp Neurol. 2009;517(2):156–165. doi: 10.1002/cne.22142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbott PL, Warner TA, Busby SJ. Amygdala input monosynaptically innervates parvalbumin immunoreactive local circuit neurons in rat medial prefrontal cortex. Neuroscience. 2006;139(3):1039–1048. doi: 10.1016/j.neuroscience.2006.01.026. [DOI] [PubMed] [Google Scholar]
- Gabbott PL, Warner TA, Jays PR, Salway P, Busby SJ. Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. J Comp Neurol. 2005;492(2):145–177. doi: 10.1002/cne.20738. [DOI] [PubMed] [Google Scholar]
- Gelman A, Carlin J, Stern H, Dunson D, Vehtari A, Rubin D. Bayesian data analysis. Chapman and Hall/CRC; 2013. [Google Scholar]
- Gelman A, Hill J. Data analysis using regression and multilevel/hierarchical models. Cambridge University Press; 2006. [Google Scholar]
- Gibson EL. Emotional influences on food choice: Sensory, physiological and psychological pathways. Physiol Behav. 2006;89(1):53–61. doi: 10.1016/j.physbeh.2006.01.024. [DOI] [PubMed] [Google Scholar]
- Girod JP, Brotman DJ. Does altered glucocorticoid homeostasis increase cardiovascular risk? Cardiovasc Res. 2004;64(2):217–226. doi: 10.1016/j.cardiores.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Gray TS, Piechowski RA, Yracheta JM, Rittenhouse PA, Bethea CL, Van de Kar LD. Ibotenic acid lesions in the bed nucleus of the stria terminalis attenuate conditioned stress-induced increases in prolactin, ACTH and corticosterone. Neuroendocrinology. 1993;57(3):517–524. doi: 10.1159/000126400. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Na ES, Johnson RF, Beltz TG, Johnson AK. Sucrose ingestion elicits reduced Fos expression in the nucleus accumbens of anhedonic rats. Brain Res. 2004;1019(1–2):259–264. doi: 10.1016/j.brainres.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Groesz LM, McCoy S, Carl J, Saslow L, Stewart J, Adler N, Laraia B, Epel E. What is eating you? Stress and the drive to eat. Appetite. 2012;58(2):717–721. doi: 10.1016/j.appet.2011.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu G, Cornea A, Simerly RB. Sexual differentiation of projections from the principal nucleus of the bed nuclei of the stria terminalis. J Comp Neurol. 2003;460(4):542–562. doi: 10.1002/cne.10677. [DOI] [PubMed] [Google Scholar]
- Heimer L, Zahm DS, Churchill L, Kalivas PW, Wohltmann C. Specificity in the projection patterns of accumbal core and shell in the rat. Neuroscience. 1991;41(1):89–125. doi: 10.1016/0306-4522(91)90202-y. [DOI] [PubMed] [Google Scholar]
- Hinkelmann K, Moritz S, Botzenhardt J, Riedesel K, Wiedemann K, Kellner M, Otte C. Cognitive impairment in major depression: association with salivary cortisol. Biol Psychiatry. 2009;66(9):879–885. doi: 10.1016/j.biopsych.2009.06.023. [DOI] [PubMed] [Google Scholar]
- Hoover WB, Vertes RP. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct. 2007;212(2):149–179. doi: 10.1007/s00429-007-0150-4. [DOI] [PubMed] [Google Scholar]
- Hosoya Y, Matsushita M. Cells of origin of the descending afferents to the lateral hypothalamic area in the rat, studied with the horseradish peroxidase method. Neurosci Lett. 1980;18(3):231–236. doi: 10.1016/0304-3940(80)90290-6. [DOI] [PubMed] [Google Scholar]
- Huang YY, Martin KC, Kandel ER. Both protein kinase A and mitogen-activated protein kinase are required in the amygdala for the macromolecular synthesis-dependent late phase of long-term potentiation. J Neurosci. 2000;20(17):6317–6325. doi: 10.1523/JNEUROSCI.20-17-06317.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RA, Wichern DW. Applied multivariate statistical analysis. Pearson Prentice Hall; Upper Saddle River, NJ: 2007. [Google Scholar]
- Kim Y, Yang HY, Kim AJ, Lim Y. Academic stress levels were positively associated with sweet food consumption among Korean high-school students. Nutrition. 2013;29(1):213–218. doi: 10.1016/j.nut.2012.08.005. [DOI] [PubMed] [Google Scholar]
- Kirouac GJ, Ganguly PK. Topographical organization in the nucleus accumbens of afferents from the basolateral amygdala and efferents to the lateral hypothalamus. Neuroscience. 1995;67(3):625–630. doi: 10.1016/0306-4522(95)00013-9. [DOI] [PubMed] [Google Scholar]
- Kita H, Kitai ST. Amygdaloid projections to the frontal cortex and the striatum in the rat. J Comp Neurol. 1990;298(1):40–49. doi: 10.1002/cne.902980104. [DOI] [PubMed] [Google Scholar]
- Kneussel M, Betz H. Receptors, gephyrin and gephyrin-associated proteins: novel insights into the assembly of inhibitory postsynaptic membrane specializations. J Physiol. 2000;525(Pt 1):1–9. doi: 10.1111/j.1469-7793.2000.t01-4-00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- la Fleur SE, Houshyar H, Roy M, Dallman MF. Choice of lard, but not total lard calories, damps adrenocorticotropin responses to restraint. Endocrinology. 2005;146(5):2193–2199. doi: 10.1210/en.2004-1603. [DOI] [PubMed] [Google Scholar]
- Laugero KD, Falcon LM, Tucker KL. Relationship between perceived stress and dietary and activity patterns in older adults participating in the Boston Puerto Rican Health Study. Appetite. 2011;56(1):194–204. doi: 10.1016/j.appet.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J, Schulman H, Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat Rev Neurosci. 2002;3(3):175–190. doi: 10.1038/nrn753. [DOI] [PubMed] [Google Scholar]
- Macht M, Mueller J. Immediate effects of chocolate on experimentally induced mood states. Appetite. 2007;49(3):667–674. doi: 10.1016/j.appet.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Markus R, Panhuysen G, Tuiten A, Koppeschaar H. Effects of food on cortisol and mood in vulnerable subjects under controllable and uncontrollable stress. Physiol Behav. 2000;70(3–4):333–342. doi: 10.1016/s0031-9384(00)00265-1. [DOI] [PubMed] [Google Scholar]
- McCann BS, Warnick GR, Knopp RH. Changes in plasma lipids and dietary intake accompanying shifts in perceived workload and stress. Psychosom Med. 1990;52(1):97–108. doi: 10.1097/00006842-199001000-00008. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Organization of amygdaloid projections to the prefrontal cortex and associated striatum in the rat. Neuroscience. 1991a;44(1):1–14. doi: 10.1016/0306-4522(91)90247-l. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Topographical organization of amygdaloid projections to the caudatoputamen, nucleus accumbens, and related striatal-like areas of the rat brain. Neuroscience. 1991b;44(1):15–33. doi: 10.1016/0306-4522(91)90248-m. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F, Guo L. Projections of the medial and lateral prefrontal cortices to the amygdala: a Phaseolus vulgaris leucoagglutinin study in the rat. Neuroscience. 1996;71(1):55–75. doi: 10.1016/0306-4522(95)00417-3. [DOI] [PubMed] [Google Scholar]
- Mendoza J, Angeles-Castellanos M, Escobar C. Entrainment by a palatable meal induces food-anticipatory activity and c-Fos expression in reward-related areas of the brain. Neuroscience. 2005;133(1):293–303. doi: 10.1016/j.neuroscience.2005.01.064. [DOI] [PubMed] [Google Scholar]
- Miyamoto E. Molecular mechanism of neuronal plasticity: induction and maintenance of long-term potentiation in the hippocampus. J Pharmacol Sci. 2006;100(5):433–442. doi: 10.1254/jphs.cpj06007x. [DOI] [PubMed] [Google Scholar]
- Needham CJ, Bradford JR, Bulpitt AJ, Westhead DR. A primer on learning in Bayesian networks for computational biology. PLoS Comput Biol. 2007;3(8):e129. doi: 10.1371/journal.pcbi.0030129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Kelz MB, Chen J. DeltaFosB: a molecular mediator of long-term neural and behavioral plasticity. Brain Res. 1999;835(1):10–17. doi: 10.1016/s0006-8993(98)01191-3. [DOI] [PubMed] [Google Scholar]
- Norgren R, Hajnal A, Mungarndee SS. Gustatory reward and the nucleus accumbens. Physiol Behav. 2006 doi: 10.1016/j.physbeh.2006.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver G, Wardle J. Perceived effects of stress on food choice. Physiol Behav. 1999;66(3):511–515. doi: 10.1016/s0031-9384(98)00322-9. [DOI] [PubMed] [Google Scholar]
- Packard AE, Ghosal S, Herman JP, Woods SC, Ulrich-Lai YM. Chronic variable stress improves glucose tolerance in rats with sucrose-induced prediabetes. Psychoneuroendocrinology. 2014;47:178–188. doi: 10.1016/j.psyneuen.2014.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park TH, Carr KD. Neuroanatomical patterns of fos-like immunoreactivity induced by a palatable meal and meal-paired environment in saline- and naltrexone-treated rats. Brain Res. 1998;805(1–2):169–180. doi: 10.1016/s0006-8993(98)00719-7. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; New York: 1998. [DOI] [PubMed] [Google Scholar]
- Paykel ES, Myers JK, Dienelt MN, Klerman GL, Lindenthal JJ, Pepper MP. Life events and depression. A controlled study. Arch Gen Psychiatry. 1969;21(6):753–760. doi: 10.1001/archpsyc.1969.01740240113014. [DOI] [PubMed] [Google Scholar]
- Pecoraro N, Reyes F, Gomez F, Bhargava A, Dallman MF. Chronic stress promotes palatable feeding, which reduces signs of stress: feedforward and feedback effects of chronic stress. Endocrinology. 2004;145(8):3754–3762. doi: 10.1210/en.2004-0305. [DOI] [PubMed] [Google Scholar]
- Perrotti LI, Hadeishi Y, Ulery PG, Barrot M, Monteggia L, Duman RS, Nestler EJ. Induction of deltaFosB in reward-related brain structures after chronic stress. J Neurosci. 2004;24(47):10594–10602. doi: 10.1523/JNEUROSCI.2542-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petyko Z, Toth A, Szabo I, Galosi R, Lenard L. Neuronal activity in rat medial prefrontal cortex during sucrose solution intake. Neuroreport. 2009;20(14):1235–1239. doi: 10.1097/WNR.0b013e32832fbf30. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Savander V, LeDoux JE. Organization of intra-amygdaloid circuitries in the rat: an emerging framework for understanding functions of the amygdala. Trends Neurosci. 1997;20(11):517–523. doi: 10.1016/s0166-2236(97)01125-9. [DOI] [PubMed] [Google Scholar]
- Prewitt CM, Herman JP. Anatomical interactions between the central amygdaloid nucleus and the hypothalamic paraventricular nucleus of the rat: a dual tract-tracing analysis. J Chem Neuroanat. 1998;15(3):173–185. doi: 10.1016/s0891-0618(98)00045-3. [DOI] [PubMed] [Google Scholar]
- Rodrigues SM, Farb CR, Bauer EP, LeDoux JE, Schafe GE. Pavlovian fear conditioning regulates Thr286 autophosphorylation of Ca2+/calmodulin-dependent protein kinase II at lateral amygdala synapses. J Neurosci. 2004;24(13):3281–3288. doi: 10.1523/JNEUROSCI.5303-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt O, Usunoff KG, Lazarov NE, Itzev DE, Eipert P, Rolfs A, Wree A. Orexinergic innervation of the extended amygdala and basal ganglia in the rat. Brain Struct Funct. 2012;217(2):233–256. doi: 10.1007/s00429-011-0343-8. [DOI] [PubMed] [Google Scholar]
- Seese RR, Chen LY, Cox CD, Schulz D, Babayan AH, Bunney WE, Henn FA, Gall CM, Lynch G. Synaptic abnormalities in the infralimbic cortex of a model of congenital depression. J Neurosci. 2013;33(33):13441–13448. doi: 10.1523/JNEUROSCI.2434-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR, Deutch AY, Roth RH, Bunney BS. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J Comp Neurol. 1989;290(2):213–242. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- Shekhar A, Sajdyk TJ, Gehlert DR, Rainnie DG. The amygdala, panic disorder, and cardiovascular responses. Ann N Y Acad Sci. 2003;985:308–325. doi: 10.1111/j.1749-6632.2003.tb07090.x. [DOI] [PubMed] [Google Scholar]
- Shide DJ, Blass EM. Opioidlike effects of intraoral infusions of corn oil and polycose on stress reactions in 10-day-old rats. Behav Neurosci. 1989;103(6):1168–1175. doi: 10.1037//0735-7044.103.6.1168. [DOI] [PubMed] [Google Scholar]
- Silva AJ, Kogan JH, Frankland PW, Kida S. CREB and memory. Annu Rev Neurosci. 1998;21:127–148. doi: 10.1146/annurev.neuro.21.1.127. [DOI] [PubMed] [Google Scholar]
- Simburger E, Plaschke M, Kirsch J, Nitsch R. Distribution of the receptor-anchoring protein gephyrin in the rat dentate gyrus and changes following entorhinal cortex lesion. Cereb Cortex. 2000;10(4):422–432. doi: 10.1093/cercor/10.4.422. [DOI] [PubMed] [Google Scholar]
- Solomon MB, Jones K, Packard BA, Herman JP. The medial amygdala modulates body weight but not neuroendocrine responses to chronic stress. J Neuroendocrinol. 2010;22(1):13–23. doi: 10.1111/j.1365-2826.2009.01933.x. [DOI] [PubMed] [Google Scholar]
- Specht CG, Triller A. The dynamics of synaptic scaffolds. Bioessays. 2008;30(11–12):1062–1074. doi: 10.1002/bies.20831. [DOI] [PubMed] [Google Scholar]
- Strack AM, Akana SF, Horsley CJ, Dallman MF. A hypercaloric load induces thermogenesis but inhibits stress responses in the SNS and HPA system. Am J Physiol. 1997;272(3 Pt 2):R840–848. doi: 10.1152/ajpregu.1997.272.3.R840. [DOI] [PubMed] [Google Scholar]
- Suchecki D, Antunes J, Tufik S. Palatable solutions during paradoxical sleep deprivation: reduction of hypothalamic-pituitary-adrenal axis activity and lack of effect on energy imbalance. J Neuroendocrinol. 2003;15(9):815–821. doi: 10.1046/j.1365-2826.2003.01067.x. [DOI] [PubMed] [Google Scholar]
- Swanson LW. Brain maps: structure of the rat brain. Elsevier; Amsterdam: 1998. [Google Scholar]
- Swanson LW. Cerebral hemisphere regulation of motivated behavior. Brain Res. 2000;886(1–2):113–164. doi: 10.1016/s0006-8993(00)02905-x. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Petrovich GD. What is the amygdala? Trends Neurosci. 1998;21(8):323–331. doi: 10.1016/s0166-2236(98)01265-x. [DOI] [PubMed] [Google Scholar]
- Takagishi M, Chiba T. Efferent projections of the infralimbic (area 25) region of the medial prefrontal cortex in the rat: an anterograde tracer PHA-L study. Brain Res. 1991;566(1–2):26–39. doi: 10.1016/0006-8993(91)91677-s. [DOI] [PubMed] [Google Scholar]
- Toda N, Nakanishi-Toda M. How mental stress affects endothelial function. Pflugers Arch. 2011;462(6):779–794. doi: 10.1007/s00424-011-1022-6. [DOI] [PubMed] [Google Scholar]
- Tominaga-Yoshino K, Urakubo T, Okada M, Matsuda H, Ogura A. Repetitive induction of late-phase LTP produces long-lasting synaptic enhancement accompanied by synaptogenesis in cultured hippocampal slices. Hippocampus. 2008;18(3):281–293. doi: 10.1002/hipo.20391. [DOI] [PubMed] [Google Scholar]
- Tomiyama AJ, Dallman MF, Epel ES. Comfort food is comforting to those most stressed: evidence of the chronic stress response network in high stress women. Psychoneuroendocrinology. 2011;36(10):1513–1519. doi: 10.1016/j.psyneuen.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tribollet E, Dreifuss JJ. Localization of neurones projecting to the hypothalamic paraventricular nucleus area of the rat: a horseradish peroxidase study. Neuroscience. 1981;6(7):1315–1328. doi: 10.1016/0306-4522(81)90190-1. [DOI] [PubMed] [Google Scholar]
- Tryon MS, Carter CS, Decant R, Laugero KD. Chronic stress exposure may affect the brain’s response to high calorie food cues and predispose to obesogenic eating habits. Physiol Behav. 2013a;120:233–242. doi: 10.1016/j.physbeh.2013.08.010. [DOI] [PubMed] [Google Scholar]
- Tryon MS, DeCant R, Laugero KD. Having your cake and eating it too: a habit of comfort food may link chronic social stress exposure and acute stress-induced cortisol hyporesponsiveness. Physiol Behav. 2013b;114–115:32–37. doi: 10.1016/j.physbeh.2013.02.018. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Christiansen AM, Ostrander MM, Jones AA, Jones KR, Choi DC, Krause EG, Evanson NK, Furay AR, Davis JF, Solomon MB, de Kloet AD, Tamashiro KL, Sakai RR, Seeley RJ, Woods SC, Herman JP. Pleasurable behaviors reduce stress via brain reward pathways. Proc Natl Acad Sci U S A. 2010;107(47):20529–20534. doi: 10.1073/pnas.1007740107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10(6):397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Jones KR, Ziegler DR, Cullinan WE, Herman JP. Forebrain origins of glutamatergic innervation to the rat paraventricular nucleus of the hypothalamus: differential inputs to the anterior versus posterior subregions. J Comp Neurol. 2011a;519(7):1301–1319. doi: 10.1002/cne.22571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Ostrander MM, Herman JP. HPA axis dampening by limited sucrose intake: reward frequency vs. caloric consumption. Physiol Behav. 2011b;103(1):104–110. doi: 10.1016/j.physbeh.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Ostrander MM, Thomas IM, Packard BA, Furay AR, Dolgas CM, Van Hooren DC, Figueiredo HF, Mueller NK, Choi DC, Herman JP. Daily limited access to sweetened drink attenuates hypothalamic-pituitary-adrenocortical axis stress responses. Endocrinology. 2007;148(4):1823–1834. doi: 10.1210/en.2006-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Ryan KK. PPARgamma and stress: implications for aging. Exp Gerontol. 2013;48(7):671–676. doi: 10.1016/j.exger.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Ryan KK. Neuroendocrine Circuits Governing Energy Balance and Stress Regulation: Functional Overlap and Therapeutic Implications. Cell Metab. 2014;19(6):910–925. doi: 10.1016/j.cmet.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usuda I, Tanaka K, Chiba T. Efferent projections of the nucleus accumbens in the rat with special reference to subdivision of the nucleus: biotinylated dextran amine study. Brain Res. 1998;797(1):73–93. doi: 10.1016/s0006-8993(98)00359-x. [DOI] [PubMed] [Google Scholar]
- Usunoff KG, Schmitt O, Itzev DE, Haas SJ, Lazarov NE, Rolfs A, Wree A. Efferent projections of the anterior and posterodorsal regions of the medial nucleus of the amygdala in the mouse. Cells Tissues Organs. 2009;190(5):256–285. doi: 10.1159/000209233. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51(1):32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Wallace DL, Vialou V, Rios L, Carle-Florence TL, Chakravarty S, Kumar A, Graham DL, Green TA, Kirk A, Iniguez SD, Perrotti LI, Barrot M, DiLeone RJ, Nestler EJ, Bolanos-Guzman CA. The influence of DeltaFosB in the nucleus accumbens on natural reward-related behavior. J Neurosci. 2008;28(41):10272–10277. doi: 10.1523/JNEUROSCI.1531-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller KL, Smith DA. Afferent connections to the bed nucleus of the stria terminalis. Brain Res. 1982;232(2):255–270. doi: 10.1016/0006-8993(82)90272-4. [DOI] [PubMed] [Google Scholar]
- Wiedenmann B, Franke WW. Identification and localization of synaptophysin, an integral membrane glycoprotein of Mr 38,000 characteristic of presynaptic vesicles. Cell. 1985;41(3):1017–1028. doi: 10.1016/s0092-8674(85)80082-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.