Abstract
Background
Fluid attenuated inversion recovery (FLAIR) vascular hyperintensity (FVH) is a novel radiographic marker detected in acute ischemic stroke (AIS) patients, which is linked to slow blood flow and potentially salvageable brain tissue. Poor leptomeningeal collateral status in AIS patients with proximal artery occlusion (PAO) is associated with larger final infarct and worse clinical outcomes, which are also affected by severity of white matter hyperintensity (WMH). We sought to evaluate FVH utility as a marker of acute collateral vessel status and its association with WMH burden in AIS patients.
Methods
Consecutive AIS patients with PAO on baseline CT angiography (CTA) were retrospectively selected from a prospectively-derived database. FVH was graded by its location, degree, and score on admission MRI obtained immediately after intravenous tissue plasminogen activator (IV tPA) administration. Leptomeningeal collateral flow grade was ranked on admission CTA. WMH volume (WMHV) was assessed using a validated volumetric protocol. Relationship between FVH, collateral flow grade, and WMHV were analyzed.
Results
Among 39 patients (mean age 70.5±12.7 years; 56% women, mean National Institutes of Health Stroke Scale (NIHSS) score 17.2 (±4.4)), median WMHV was 6.0 cm3. FVH score and collateral flow grade were significantly correlated (Spearman's ρ=0.41, p= 0.009). In a univariate regression model, FVH degree was inversely associated with WMHV (β=−0.33 p=0.04).
Conclusions
FVH score detected on acute MRI can be used as a surrogate of collateral flow grade in AIS patients. FVH degree is inversely associated with WMHV, possibly signifying diffuse disease of cerebral vasculature in patients with severe leukoaraiosis.
Keywords: FLAIR Vascular Hyperintensity, Acute Ischemic Stroke, White Matter Disease, Leukoaraiosis, Collateral
INTRODUCTION
Arterial hyperintensity seen with acute stroke is a common but under-recognized phenomena observed on conventional MR imaging. FVH is a circular or serpentine brightenings in brain parenchyma or cortical surface bordering the subarachnoid space.1 The appearance of FVH in stroke patients was first described 10 years ago.2,3 FVH has been observed in both large vessel steno-occlusive disease due to atherosclerosis and other vasculopathies such as Moyamoya disease. FVH is best characterized in the setting of AIS and is noted it to be present in 10% of MRI studies in this population.1,4
FVH is an emergent radiographic marker, which likely represents disordered or sluggish blood flow, often distal to arterial occlusion or stenosis. Slow flow and stasis cause a high signal on FLAIR in contrast to the normal flow void phenomenon of arteries. Although “proximal vessel sign” in the middle cerebral artery (MCA) territory most often represents thrombus, distal FVH likely represents slow blood flow.1
Poor leptomeningeal collateral status in AIS patients with PAO is associated with larger final infarct size and worse clinical outcomes, which are also affected by severity of leukoaraiosis, or WMH detected on FLAIR MRI in patients with stroke.5 Furthermore, impaired cerebral perfusion due to large vessel atherosclerosis may affect the severity of leukoaraiosis6. In patients with AIS, severity of leukoaraiosis is associated with infarct growth, poor functional outcomes, and increased risk of recurrent stroke.5,7,8
In this study, we hypothesized that FVH reflects the acute collateral vessel status, which is poor in AIS patients with PAO and severe WMH, as a manifestation of diffusely diseased cerebral vasculature. We sought to evaluate the utility of FVH ipsilateral to the site of PAO as a surrogate marker of acute collateral vessel status and its association with WMH burden in AIS patients.
METHODS
Study population
We retrospectively selected patients from a prospective institutional cohort of consecutive AIS patients over 18 years of age with PAO confirmed by head and neck CTA completed immediately upon admission to our institution between 2009-2011.9,10 AIS was defined as acute clinical vascular syndrome with evidence of cerebral infarction on diffusion weighted imaging (DWI). Based on the institutional acute stroke imaging protocol, IV tPA bolus is administered in the CT scanner, followed immediately by CTA and brain MRI. Median time from patient arrival to the hospital and IV tPA administration was 45-48 minutes; median time from hospital arrival to CT scan was 19-27 minutes; and median time from IV tPA to MRI was 30 minutes for this subset.
All patients were assessed on admission by a vascular neurologist, and clinical characteristics were recorded prospectively including NIHSS score, demographics, past medical history, details of clinical presentation, laboratory, and neuroimaging characteristics. All patients in this study received IV tPA within three hours from symptom onset (or last seen well) at the standard dose of 0.9mg/kg. There was no evidence of intracerebral hemorrhage on the initial imaging (CT or MRI) in this patient cohort.
At our institution, de-identified data for all AIS patients are entered in the quality improvement database, and our institutional review board (IRB) has waived the need for informed consent and approved the analysis of this database.
Neuroimaging analysis
Single phase, bolus triggered CTA of head and neck was performed using a helical scanner (High-Speed Advantage; General Electric (GE) Medical Systems, Milwaukee, WI) as part of emergency AIS evaluation. Collateral circulation grade was assigned in accordance to a 5-point scale,11 based on comparison of collateral flow in the infarcted MCA territory with the contralateral territory: (0 – no collaterals in greater than 50% of an M2 territory, 1- decreased collaterals in greater than 50% of M2 territory, 2- decreased collaterals in less than 50% of M2 territory, 3 – equal, 4 - greater collateral circulation on the infarcted side). A trained investigator (D.V.G.) blinded to clinical data and WMHV measurements assigned collateral grades.
MRI was performed using 1.5 T scanners (GE Signa, GE Medical Systems, Milwaukee, WI). We used MRIcro software (University of Nottingham School of Psychology, Nottingham, UK; www.mricro.com) to convert scans from digital imaging and communications in medicine (DICOM) into Analyze format and for computer-assisted determination of WMHV.12-14 Analysis of WMHV was performed on the T2 FLAIR axial sequences using previously described semi-automated method.13-15 All WMHV measurements were performed by readers blinded to clinical data and the results of CTA and FVH assessment.
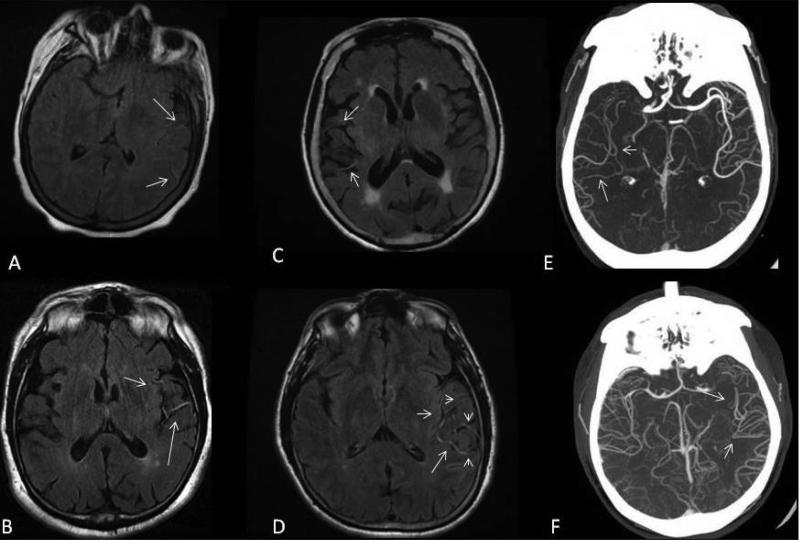
FVH ipsilateral to the site of PAO and acute infarction was assessed on admission FLAIR MRI obtained immediately after IV tPA administration. FVH was graded by its location (0-none, 1-proximal, 2-distal, and 3 – both); degree (0-none, 1-subtle, 2-prominent, and 3-both); and its score (0-none, 1- less than four hyperintense vessels, and 2-four or more hyperintense vessels) (Figure 1). FVH assessment was performed by an experienced reader (H.H.K.) blinded to CTA and WMHV measurements. Inter-rater reliability of the FVH rating method has been tested by two independent readers from different institutions (N.S.R. and M.E.O.), who completed standardized training, with intra-class correlation coefficient (ICC) of 0.74.
Figure 1.
Assessment of FVH using qualitative (FVH degree 1, panel A vs. FVH degree 2, panel B) and quantitative (FVH score 1, panel C vs. FVH score 2, panel D) measurements. Examples of CTA collateral flow patterns (matching FVH score 1, panel E vs. FVH score 2, panel F) are included.
Statistical analysis
All continuous numerical variables are expressed as mean ± standard deviation (SD), with the exception of NIHSS, WMHV and DWI volume which are expressed as median and interquartile range (IQR). WMHV was normalized for linear regression analysis using natural log transformation. Spearman's rank correlation was used to assess the association between FVH score and collateral flow grade. Univariate linear regression analysis was used to analyze the relationship between FVH degree and natural logarithm transformed white matter hyperintensity volume (lnWMHV). P-value<0.05 was considered significant for all analyses. All statistical analyses were performed using SAS software (SAS 9.1, SAS Institute, Cary, NC).
RESULTS
A total of 39 consecutive PAO patients with admission head and neck CTA and brain MRI suitable for WMH volumetric analysis were included in the study. Baseline characteristics are shown in Table 1. Mean age was 70.5±12.7 years, 56% were women. Hypertension (HTN) was the most prevalent risk factor (72%), followed by hyperlipidemia (41%). Median NIHSS score was 16 (IQR 14-20), median WMHV was 6.0 cm3 (IQR 3.4-9.6 cm3) and median DWI volume was 17.7 cm3 (IQR 9.9-26.0 cm3).
Table 1.
Baseline characteristics and MRI findings of 39 acute ischemic stroke patients with proximal artery occlusion.
| Clinical Characteristics | |
|---|---|
| Age, years, mean ± SD | 70.5 ± 12.7 |
| Female, n (%) | 22 (56.41) |
| Hypertension, n (%) | 28 (71.79) |
| Diabetes mellitus, n (%) | 6 (19.35) |
| Hyperlipidemia, n (%) | 16 (41.03) |
| Coronary artery disease, n (%) | 9 (23.08) |
| Atrial fibrillation, n (%) | 11 (28.01) |
| Current smoking, n (%) | 11 (28.01) |
| WMHV, cm3, median (IQR) | 6 (3.4 – 9.6) |
| lnWMHV, mean ± SD | 8.13±7.65 |
| NIHSS, median (IQR) | 16 (14-20) |
| DWIv, cm3, median (IQR) | 17.7 (9.9-26) |
Abbreviations: DWI - diffusion weighted imaging, lnWMHV - natural logarithm transformed white matter hyperintensity volume, WMH - white matter hyperintensity, WMHV - white matter hyperintensity volume
There were 7 subjects (17.9%) with collateral grade 0; 10 subjects (25.6%) with collateral grade 1; 8 subjects (20.5%) with collateral grade 2; 10 subjects (25.6%) with collateral grade 3; and 4 subjects (10.3%) collateral grade 4. Level of large vessel occlusion in this cohort was as follows: 36 patients with the middle cerebral artery, M1 segment occlusion; 2 with the middle cerebral artery, M2 segment occlusion; 1 with the extracranial carotid artery occlusion.
Results of association testing between FVH measurements and collateral flow grade, as well as WMHV are presented in Tables 2 and 3, respectively. FVH score and collateral flow grade were significantly correlated (Spearman's rank correlation ρ=0.41, p= 0.009). There was an inverse relationship between the FVH score and DWI volume (Spearman's rank correlation ρ=−0.03), albeit not statistically significant (p= 0.87). In a univariate regression model, FVH degree was inversely associated with WMHV (β=−0.33, p=0.04).
Table 2.
Association between FVH measurements and collateral flow grade in 39 acute ischemic stroke patients with proximal artery occlusion.
| Grade of Collateral flow Spearman Rank, ρ | P value | |
|---|---|---|
| FVH degree | 0.18 | 0.26 |
| FVH score | 0.41 | 0.009* |
| FVH location | 0.27 | 0.08 |
Abbreviations: FVH- FLAIR vascular hyperintensity
statistically significant
Table 3.
Univariate predictors of lnWMHV in 39 acute ischemic stroke patients with proximal artery occlusion.
| ln(WMHV), β estimate | P value | |
|---|---|---|
| FVH degree | −0.33 | 0.04* |
| FVH score | 0.07 | 0.84 |
| FVH location | 0.11 | 0.68 |
Abbreviations: FVH- FLAIR vascular hyperintensity
statistically significant
DISCUSSION
In this feasibility, proof-of-principle study, we show that AIS patients with high collateral flow grade present with higher FVH score, whereas the patients with relatively low collateral flow grade show lower FVH score. Furthermore, the degree of FVH is inversely associated with WMHV. Therefore, these data support our hypothesis that FVH reflects the acute collateral vessel status, and may possibly serve as a novel marker of an overall burden of cerebrovascular disease based on its association with WMH burden in AIS patients.
FVH has been previously categorized by its appearance (dot sign, linear hyperintensity, serpentine hyperintensity)16, location (proximal vs. distal FVH)16 and score (low vs. high FVH).17,18 As previously described, FVH score reflects the number of leptomeningeal collateral vessels recruited during the acute disruption of blood flow in the middle cerebral artery.19 Thus, the greater the FVH score, the more collateral vessels appear on FLAIR MRI as detectable hyperintensities. On the other hand, FVH degree is a scale that reflects the extent (i.e., quality) of the collateral vessel recruitment. Therefore, subtle appearance of hyperintensities on MRI is presumed to reflect a less effective recruitment of the collateral vessels; whereas prominent FVH appearance may represent a more significant collateral vessel involvement. The extent of collateral response could be driven by quality of the cerebral blood flow, which in turn has been linked to the extent of vasoreactivity and an overall burden of small cerebral microangiopathy including leukoaraiosis.20
Evidence for FVH representing impaired hemodynamics and retrograde collateral blood flow includes its presence in acute stroke due to large vessel stenosis or occlusion.1 Arterial hyperintensity represents an early sign of infarction and frequently observed at early stage of infarction21. According to Noguchi et al,19 angiography showed markedly slow retrograde filling of the insular, opercular, and cortical branches of the affected MCA from leptomeningeal anastomoses in a case of hyperacute infarct with arterial hyperintensity of the insular and opercular branches of the affected MCA. Arterial hyperintensity may provide a clue to the early detection of impending infarction.21
Previous studies showed that high FVH score was associated with larger territorial infarctions18. Our data suggest that FVH score - but not FVH degree or location - was correlated with collateral vessel grade. We posit that since FVH score is thought to reflect the actual count of collateral vessels recruited during acute ischemia to prevent infarct enlargement, it correlates with the grade of collaterals seen on CTA. In our dataset, the relationship between FVH score and acute infarct volume was, indeed, inverse; however, lack of statistical significance in this analysis is likely to reflect insufficient power within this sample size to differentiate singular effect of collateral circulation on the acute infarct volume. In fact, the final infarct volume is determined by a multitude of factors in addition to the collateral vessel grade, and these factors should be accounted for in the complex models of neuroimaging stroke outcomes. Nevertheless, the association between FVH score and CTA-based collateral vessel grade, as seen in our study, is most likely explained by the fact that both represent a numerical account of the individual vessels recruited during the acute response, not otherwise captured by the FVH degree or location metrics.
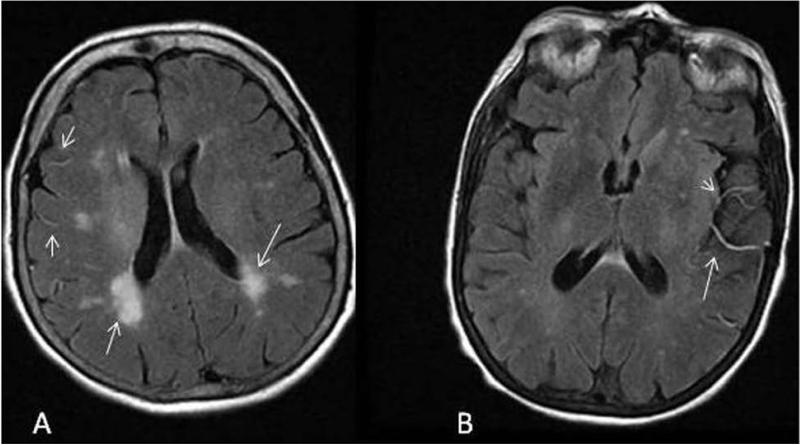
In addition, our data shows that FVH degree - but not FVH score or location - is inversely associated with severity of leukoaraiosis measured as WMHV on FLAIR MRI, which most likely signifies the overall quality of collateral vessel response during acute ischemia. In AIS patients, a larger volume of WMH is thought to reflect greater burden of total cerebrovascular disease, including the involvement of leptomeningeal collaterals that are affected by the same vascular risk factors (i.e., hypertension, hyperlipidemia, diabetes mellitus etc) as the native small cerebral vasculature.5 During acute ischemia, the diseased leptomeningeal collateral vessels may not have the same capacity to be effectively recruited due to poor vasoreactivity20 thus, resulting in a diminished response seen as “subtle” FVH on acute FLAIR MRI in subjects with severe WMH (Figure 2).
Figure 2.
Collateral vascular response and severity of leukoaraiosis. (panel A: subtle FVH degree in the presence of severe WMH; panel B: prominent FVH is evident in AIS subject with mild WMH). The extent of leptomeningeal collateral vessel recruitment as measured by FVH degree is inversely associated with WMHV.
In our study, unlike other imaging signs of vessel occlusion such as gradient-echo susceptibility or CT hyperattenuation within a vessel lumen, FVH indicates the status of leptomeningial collateral perfusion available to the vulnerable brain tissue, rather than directly visualizing thrombus. Thus, FVH may prove to be more clinically valuable than the two other vessel signs as a diagnostic, as well as a clinical decision-making tool regarding the potential benefits of recanalization, or weather tissue salvage can be expected. Given that the latest endovascular clinical trials have used collateral status as inclusion criteria,22 FVH may be useful for detecting the collateral status in the future studies and developing practice guidelines.
The strength of our study is in its innovative approach to testing a biological hypothesis linking the extent of cerebrovascular pathology related to an overall burden of vascular risk factors and the quality of collateral vascular response in acute cerebral ischemia. Our findings of the inverse relationship between the pre-existing burden of WMH and FVH degree demonstrate - for the first time - that diffuse small cerebral vessel pathology contributing to severity of leukoaraiosis may also in part be responsible for poor collateral flow response during AIS. Common vascular risk factors such as hypertension, hyperlipidemia, diabetes, and tobacco use could contribute to progression of WMHV, while also contributing to collateral vessel pathology and increasing risk of stroke.
Secondly, we have established that FVH score is strongly correlated with the CTA-based collateral vessel grade in a uniform subset of AIS subjects presenting with signs and symptoms of a territorial stroke and undergoing an MRI immediately post IV thrombolysis for validation of acute infarct and assessment of FVH.
Other strengths of our study include systematic AIS subject ascertainment, as well as PAO diagnosis using the hyperacute CTA, which demonstrate an advantage as compared to other non-invasive assessment methods for large vessel stenosis .23,24 Furthermore, we used a validated, highly accurate semi-automated protocol of WMHV assessment,6 and an exhaustive analysis of the FVH extent using all previously reported methods in order to determine feasibility of such assessment.
Limitations of this study are mainly related to its retrospective study design, as well as its relatively small sample size, which has most likely limited our statistical power to demonstrate additional associations in this analysis. In order to minimize the inter-patient variability, we specifically selected AIS patients, who received IV tPA prior to MRI imaging; consequently, this analysis was not positioned to examine the effect of tPA on FVH status. Another limitation is that we had no follow-up vessel imaging to assess recanalization status following IV tPA bolus, nor we had clinical data to identify “rapid responders” within our cohort. Rapid recanalization, as manifested clinically or radiographically might have significant effect on the status of collateral circulation and consequently, the measures of FVH.25 Future prospective studies properly powered to assess the effect of revascularization on markers of collateral circulation will provide additional data, fueled by the insights of the current investigation. Similarly, our hypothesis-driven, proof-of-principle study of novel MRI-based cerebrovascular biomarkers might lead the way for future investigations to validate and extend our initial findings.
CONCLUSION
This pilot study suggests that the presence of high score FVH is associated with increased grade of collateral flow in AIS patients with PAO. The extent of FVH, especially if subtle, may help identifying patients with greater burden of WMH. Our data demonstrate that the prognostic value of FVH still needs to be better elucidated; although, this pilot study may lead a line of investigation that could ultimately inform clinicians regarding patient selection with favorable collateral blood flow, who are likely to benefit from aggressive revascularization therapies.26
Acknowledgements
Funding:
This work is supported by the NIH--NINDS (K23NS064052 and R01NS082285-01A1, N.S.R) and the TUBITAK (1059B191400176, H.H.K.) awards.
Footnotes
Disclosure:
No external funding source of the work and authors’ potential financial conflicts-of-interest.
REFERENCES
- 1.Azizyan A, Sanossian N, Mogensen MA, Liebeskind DS. Fluid-attenuated inversion recovery vascular hyperintensities: an important imaging marker for cerebrovascular disease. AJNR Am J Neuroradiol. 2011;32:1771–5. doi: 10.3174/ajnr.A2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cosnard G, Duprez T, Grandin C, Smith AM, Munier T, Peeters A. Fast FLAIR sequence for detecting major vascular abnormalities during the hyperacute phase of stroke: a comparison with MR angiography. Neuroradiology. 1999;41:342–6. doi: 10.1007/s002340050761. [DOI] [PubMed] [Google Scholar]
- 3.Kamran S, Bates V, Bakshi R, Wright P, Kinkel W, Miletich R. Significance of hyperintense vessels on FLAIR MRI in acute stroke. Neurology. 2000;55:265–9. doi: 10.1212/wnl.55.2.265. [DOI] [PubMed] [Google Scholar]
- 4.Sanossian N, Saver JL, Alger JR, et al. Angiography reveals that fluid-attenuated inversion recovery vascular hyperintensities are due to slow flow, not thrombus. AJNR Am J Neuroradiol. 2009;30:564–8. doi: 10.3174/ajnr.A1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giurgiutiu DV, Yoo AJ, Fitzpatrick K, et al. Severity of leukoaraiosis, leptomeningeal collaterals, and clinical outcomes after intra-arterial therapy in patients with acute ischemic stroke. J Neurointerv Surg. 2014 doi: 10.1136/neurintsurg-2013-011083. [DOI] [PubMed] [Google Scholar]
- 6.Chutinet A, Biffi A, Kanakis A, Fitzpatrick KM, Furie KL, Rost NS. Severity of leukoaraiosis in large vessel atherosclerotic disease. AJNR Am J Neuroradiol. 2012;33:1591–5. doi: 10.3174/ajnr.A3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ay H, Arsava EM, Rosand J, et al. Severity of leukoaraiosis and susceptibility to infarct growth in acute stroke. Stroke. 2008;39:1409–13. doi: 10.1161/STROKEAHA.107.501932. [DOI] [PubMed] [Google Scholar]
- 8.van Swieten JC, Kappelle LJ, Algra A, van Latum JC, Koudstaal PJ, van Gijn J. Hypodensity of the cerebral white matter in patients with transient ischemic attack or minor stroke: influence on the rate of subsequent stroke. Dutch TIA Trial Study Group. Ann Neurol. 1992;32:177–83. doi: 10.1002/ana.410320209. [DOI] [PubMed] [Google Scholar]
- 9.Rost NS, Smith EE, Nogueira RG, et al. Implementation of a patient selection protocol for intra-arterial therapy increases treatment rates in patients with acute ischemic stroke. J Neurointerv Surg. 2013;5(Suppl 1):i44–7. doi: 10.1136/neurintsurg-2011-010240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.LaBuzetta JN, Yoo AJ, Ali S, et al. Determinants of early outcomes in patients with acute ischemic stroke and proximal artery occlusion. J Stroke Cerebrovasc Dis. 2014;23:2527–32. doi: 10.1016/j.jstrokecerebrovasdis.2014.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Souza LC, Yoo AJ, Chaudhry ZA, et al. Malignant CTA collateral profile is highly specific for large admission DWI infarct core and poor outcome in acute stroke. AJNR Am J Neuroradiol. 2012;33:1331–6. doi: 10.3174/ajnr.A2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith EE, Gurol ME, Eng JA, et al. White matter lesions, cognition, and recurrent hemorrhage in lobar intracerebral hemorrhage. Neurology. 2004;63:1606–12. doi: 10.1212/01.wnl.0000142966.22886.20. [DOI] [PubMed] [Google Scholar]
- 13.Gurol ME, Irizarry MC, Smith EE, et al. Plasma beta-amyloid and white matter lesions in AD, MCI, and cerebral amyloid angiopathy. Neurology. 2006;66:23–9. doi: 10.1212/01.wnl.0000191403.95453.6a. [DOI] [PubMed] [Google Scholar]
- 14.Chen YW, Gurol ME, Rosand J, et al. Progression of white matter lesions and hemorrhages in cerebral amyloid angiopathy. Neurology. 2006;67:83–7. doi: 10.1212/01.wnl.0000223613.57229.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nandigam RN, Chen YW, Gurol ME, Rosand J, Greenberg SM, Smith EE. Validation of intracranial area as a surrogate measure of intracranial volume when using clinical MRI. J Neuroimaging. 2007;17:74–7. doi: 10.1111/j.1552-6569.2006.00069.x. [DOI] [PubMed] [Google Scholar]
- 16.Perez de la Ossa N, Hernandez-Perez M, Domenech S, et al. Hyperintensity of distal vessels on FLAIR is associated with slow progression of the infarction in acute ischemic stroke. Cerebrovasc Dis. 2012;34:376–84. doi: 10.1159/000343658. [DOI] [PubMed] [Google Scholar]
- 17.Olindo S, Chausson N, Joux J, et al. Fluid-attenuated inversion recovery vascular hyperintensity: an early predictor of clinical outcome in proximal middle cerebral artery occlusion. Arch Neurol. 2012;69:1462–8. doi: 10.1001/archneurol.2012.1310. [DOI] [PubMed] [Google Scholar]
- 18.Hohenhaus M, Schmidt WU, Brunecker P, et al. FLAIR vascular hyperintensities in acute ICA and MCA infarction: a marker for mismatch and stroke severity? Cerebrovasc Dis. 2012;34:63–9. doi: 10.1159/000339012. [DOI] [PubMed] [Google Scholar]
- 19.Noguchi K, Ogawa T, Inugami A, et al. MRI of acute cerebral infarction: a comparison of FLAIR and T2-weighted fast spin-echo imaging. Neuroradiology. 1997;39:406–10. doi: 10.1007/s002340050433. [DOI] [PubMed] [Google Scholar]
- 20.Mandell DM, Han JS, Poublanc J, et al. Selective reduction of blood flow to white matter during hypercapnia corresponds with leukoaraiosis. Stroke. 2008;39:1993–8. doi: 10.1161/STROKEAHA.107.501692. [DOI] [PubMed] [Google Scholar]
- 21.Maeda M, Yamamoto T, Daimon S, Sakuma H, Takeda K. Arterial hyperintensity on fast fluid-attenuated inversion recovery images: a subtle finding for hyperacute stroke undetected by diffusion-weighted MR imaging. AJNR Am J Neuroradiol. 2001;22:632–6. [PMC free article] [PubMed] [Google Scholar]
- 22.Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372:1019–30. doi: 10.1056/NEJMoa1414905. [DOI] [PubMed] [Google Scholar]
- 23.Brott TG, Halperin JL, Abbara S, et al. 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American Stroke Association, American Association of Neuroscience Nurses, American Association of Neurological Surgeons, American College of Radiology, American Society of Neuroradiology, Congress of Neurological Surgeons, Society of Atherosclerosis Imaging and Prevention, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of NeuroInterventional Surgery, Society for Vascular Medicine, and Society for Vascular Surgery. Vasc Med. 2011;16:35–77. doi: 10.1177/1358863X11399328. [DOI] [PubMed] [Google Scholar]
- 24.Bash S, Villablanca JP, Jahan R, et al. Intracranial vascular stenosis and occlusive disease: evaluation with CT angiography, MR angiography, and digital subtraction angiography. AJNR Am J Neuroradiol. 2005;26:1012–21. [PMC free article] [PubMed] [Google Scholar]
- 25.Liu W, Yin Q, Yao L, et al. Decreased hyperintense vessels on FLAIR images after endovascular recanalization of symptomatic internal carotid artery occlusion. Eur J Radiol. 2012;81:1595–600. doi: 10.1016/j.ejrad.2011.04.026. [DOI] [PubMed] [Google Scholar]
- 26.Berkhemer OA, Fransen PS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372:11–20. doi: 10.1056/NEJMoa1411587. [DOI] [PubMed] [Google Scholar]