Abstract
Rotavirus (RV) causes significant morbidity and mortality in children worldwide. The intestinal microbiota plays an important role in modulating host-pathogen interactions, but little is known about the impact of commonly used probiotics on human RV (HRV) infection. In this study, we compared the immunomodulatory effects of Gram-positive [Lactobacillus rhamnosus strain GG (LGG)] and Gram-negative [Escherichia coli Nissle (EcN)] probiotic bacteria on virulent human rotavirus (VirHRV) infection and immunity using neonatal gnotobiotic (Gn) piglets. Gn piglets were colonized with EcN, LGG, EcN+LGG or uncolonized and challenged with VirHRV. Mean peak virus shedding titers and mean cumulative fecal scores were significantly lower in EcN-colonized compared to LGG-colonized or uncolonized piglets. Reduced viral shedding titers were correlated with significantly reduced small intestinal HRV IgA antibody responses in EcN-colonized compared to uncolonized piglets post-VirHRV challenge. However the total IgA levels post-VirHRV challenge in the intestine and pre-VirHRV challenge in serum were significantly higher in EcN-colonized than in LGG-colonized piglets. In vitro treatment of mononuclear cells (MNCs) with these probiotics demonstrated that EcN, but not LGG, induced IL-6, IL-10, and IgA, with the latter partially dependent on IL-10. However, addition of exogenous recombinant porcine IL-10 + IL-6 to MNCs co-cultured with LGG significantly enhanced IgA responses. The greater effectiveness of EcN in moderating HRV infection, may also be explained by the binding of EcN, but not LGG to Wa HRV particles or HRV 2/4/6 virus-like particles (VLP) but not 2/6 VLP. Results suggest that EcN and LGG differentially modulate RV infection and B cell responses.
Keywords: Probiotics, lactobacilli, E. coli Nissle, human rotavirus, antibody responses, children
Introduction
Rotavirus (RV) is a leading cause of diarrhea. It causes an estimated 480, 000 deaths in children under five years of age in developing countries (1). The efficacy of the available RV vaccines is low in developing countries compared to developed countries (2). Many factors, such as malnutrition, micronutrient deficiencies, and breastfeeding (3–5) are implicated in the lower efficacy of enteric vaccines in impoverished countries. In addition to the aforementioned factors, recent studies have also shown a role for the intestinal microbiota in modulating enteric viral infections and oral vaccine responses (6, 7). Ablation of the intestinal microbiota reduced the severity of RV infection and modulated RV induced adaptive immunity in mice (8). A higher abundance of Clostridiales, Enterobacteriales, and Pseudomonadales was associated with poor oral poliovirus vaccine responses in infants, whereas higher bifidobacteria-abundance was positively correlated with greater oral poliovirus vaccine-specific T cell- and antibody-responses (9). Previous studies also showed a direct role of commensals in enhancing enteric viral infections, including poliovirus (10) and mouse mammary tumor virus (11) infections. Thus, the composition of the microbiota or certain members of commensal microbial communities play a significant role in modulating viral infections and host immunity to pathogens and vaccines.
Probiotics are increasingly utilized to enhance oral vaccine responses and to treat some enteric infections (12), as well as various inflammatory diseases of the GI tract in children (13). Among probiotics, Gram–positive (G+) probiotics such as Lactobacillus spp or Bifidobacteria spp have been administrated in randomized human clinical trials (14, 15) and experimental studies (16–19) to reduce the severity of RV induced diarrhea. Among G+ probiotics, Lactobacillus rhamnosus GG (LGG) has been extensively investigated for its beneficial health effects such as shortening the duration of HRV diarrhea and enhancing HRV specific immune responses in children (15, 20). However, mechanisms of action of LGG on HRV infection and whether LGG has any superior probiotic effects on HRV infection and immunity compared to a G− probiotic such as EcN are largely unknown. G+ and G− probiotics/commensals differ in microbe-associated molecular patterns, cell wall constituents, which may differentially influence neonatal immune maturation and susceptibility to HRV infections. Additionally, E. coli is one of the first species to colonize newborn babies (21). EcN is widely used to treat inflammatory disorders such as ulcerative colitis in humans (22). Beneficial effects of EcN are mediated through enhancing intestinal barrier function (23) and moderating inflammatory disorders (24). Further, similar to other probiotics, EcN has antimicrobial- and immunomodulatory-properties, such as inhibition of pathogenic bacterial invasion of epithelial cells (25), induction of beta-defensin in epithelial cells (26) and modulation of T cell proliferation (27). However, the role of EcN in the maturation of antibody responses, EcN direct effects on HRV pathogenesis and comparative effects of G+ and G− probiotics on HRV infection and immunity are unknown.
Gn piglets are an ideal model to delineate the direct beneficial effects of probiotics on enteric viral infections and virus-induced B cell responses. For instance, Gn piglets are susceptible to HRV diarrhea (28). Furthermore, piglets receive no antibodies in utero because of the epitheliochorial placenta of the sow, eliminating the maternal antibody influence on neonatal immune responses (29). Additionally, fetal as well as newborn piglets have a functional, although immature, immune system and they are capable of mounting immune responses to environmental antigens, commensal microorganisms and pathogens (30, 31). In this study, we compared the effects of G− EcN and G+ LGG probiotics on VirHRV infection and B cell responses in the Gn piglet model.
Materials and Methods
Probiotics
For colonization of the piglets with the probiotics, LGG ATCC 53103 (American Type Culture Collection, Manassas, VA) was prepared as previously described (17). EcN (Kindly supplied by DrUlrich Sonnenborn, Department of Biological Research, Ardeypharm GmbH, Herdecke, Germany) was streaked onto LB agar plates and incubated overnight at 37° C. Subsequently, a single colony from the streaked plate was picked and inoculated into six ml of LB broth. After overnight incubation, the number of EcN colony forming units (CFU) in the suspension was estimated by spectrophotometry at 600 nm based on comparison with predetermined standard growth curve.
Animals
All animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) at the Ohio State University. Near-term sows (Landrace × Yorkshire × Duroc crossbred) were purchased from The Ohio State University swine center facility. Cesarean-derived Gn piglets from the sows were maintained in sterile isolators as previously described (32). Prior to probiotic colonization, sterility was verified by aerobic and anaerobic culturing of rectal swabs collected from the piglets. Subsequently these piglets were assigned to one of the following four groups: 1) EcN colonized (EcN), 2) LGG colonized (LGG), 3) EcN and LGG dual-colonized (EcN+LGG) and 4) uncolonized piglets. For mono-colonization of probiotics, six-day-old piglets were colonized with 105 CFUs per pig and for dual-colonization of EcN and LGG probiotics, piglets were colonized with 1:1 ratio of EcN and LGG at a total of 105 CFUs per pig. Rectal swabs were collected weekly to assess the colonization status of each probiotics in the piglets as previously described (33). Total viable counts of LGG were determined using deMan, Rogosa and Sharpe (MRS) agar and EcN were enumerated after plating on LB agar. Subsequently all piglets were challenged with VirHRV [1×106 fluorescent focus units (FFU)]. Post-VirHRV challenge, rectal swabs were collected to assess HRV shedding. Fecal scores were recorded as previously described (34) and the mean cumulative fecal score was calculated as [( daily fecal scores from postchallenge days (PCD) 1 to 7]/n to assess the severity of diarrhea (35). Serum samples were collected to assess the impact of probiotics and VirHRV challenge on total immunoglobulin responses. All piglets were euthanized at post bacterial colonization day (PBCD) 36/post-HRV challenge day (PCD) 21 and blood, duodenum, ileum, and spleen were collected to isolate mononuclear cells (MNCs) as previously described (17) (Fig.1A). To determine the intestinal antibody responses, small intestinal contents were collected and protease inhibitors were incorporated.
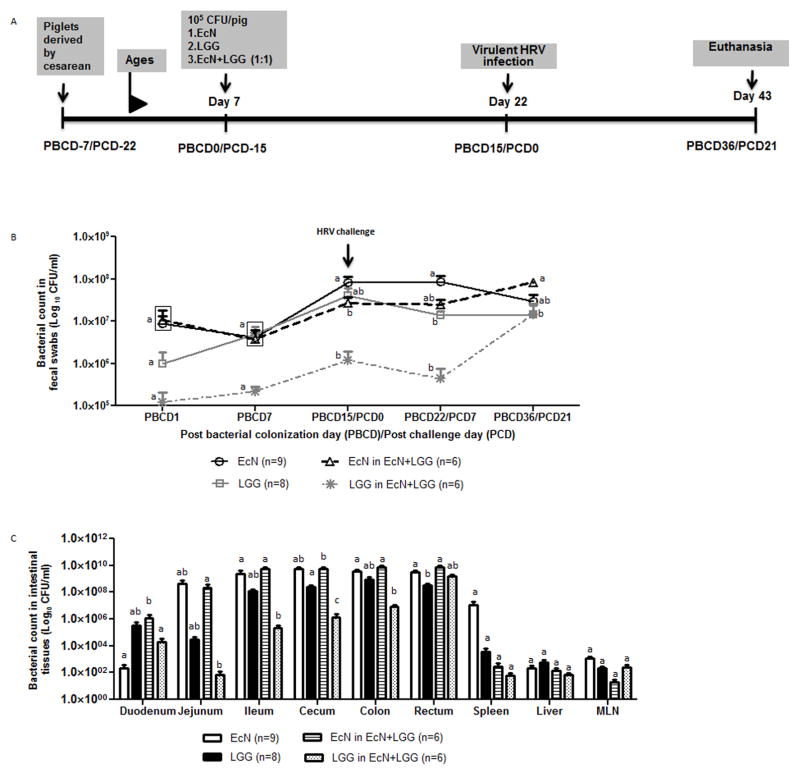
Figure 1.
Schematic diagram of the experimental design showing time points for probiotic colonization, VirHRV Wa challenge, and euthanasia. (B) Fecal probiotic bacterial shedding from probiotic colonized piglets. Different alphabetical letters indicate significant differences (p <0.05) at the same time point in fecal probiotics counts among treatment groups, whereas the same letters indicate no significant difference. The values that are statistically similar are in the same box. (C) Probiotic colonization levels at different segments of the intestinal tract. Different alphabetical letters indicate significant differences (p <0.05) at the same tissue in probiotics counts among treatment groups, whereas the same letters indicate no significant difference.
Virus shedding, ELISA, ELISPOT, and flow cytometry analysis
A cell culture immunofluorescence (CCIF) assay was used to detect shedding of HRV in rectal swab fluids as previously described (34). Antibody secreting cells (ASCs) in duodenal mononuclear cells (MNCs) were determined as previously described (36). HRV-specific antibody responses and total immunoglobulin levels were determined by ELISA (37). Flow cytometry staining to identify CD79β+CD21−CD2− B cells was performed as previously described (18, 38).
Bacterial specific antibody responses were determined as described previously with some modifications (39, 40). EcN and LGG were prepared as described previously for probiotic colonization. Wells of a 96-well ELISA plate were coated with 100 μL of 1 × 107 bacteria diluted in 0.05 M bicarbonate buffer and incubated at 4°C overnight. After overnight incubation, bacteria were fixed to the plates by adding 100 μL of 80% acetone per well for 10 minutes. Plates were washed twice with PBS-0.05% Tween-20, then 200 μL of a 4% nonfat milk solution was added to each well and incubated at 37°C for 2 h. Following plate washing, diluted serum samples were added and incubated at 37°C for 1 h. Plates were washed five times and HRP conjugated Goat anti pig IgA antibody ((0.3 μg/ml)) (AbD Serotec), or HRP conjugated Goat anti pig IgG antibody (Cat# 04-14-02,KPL) was added and incubated at 37°C for 1 h. Lastly, after washing, tetramethylbenzidine substrate (KPL) was added to each well and the reaction was stopped with 1 M Phosphoric Acid.
Probiotics effect on the binding of HRV to epithelial cells
Effects of probiotics on HRV binding to the human colonic epithelial (Caco-2) cells were assessed as previously described using a cell-virus binding assay with modifications (41). Briefly, Caco-2 cells were maintained in Dulbecco's Modified Eagle Medium: Nutrient Mixture F-12 (DMEM/F-12) media (Thermo Fisher Scientific) containing 15% fetal bovine serum at 37° C in the presence of 5% CO2. Confluent Caco-2 cell monolayers in 6 well cell culture plates (1×106 cells) were washed once with minimum essential media (MEM). Subsequently, cells were incubated with individual probiotic bacteria at a cell: bacteria ratio of 1:100 at 37° C for 4 h with gentle rocking. In the presence of bacteria, cells were cooled on ice for 10 minutes. Subsequently, HRV, at a multiplicity of infection 3.0, was added to the cells-bacterial mixture and then incubated at 4° C for 1 h with gentle rocking. After this incubation, cells were washed twice with cold MEM. After adding cold MEM, cells were subjected to two rounds of freeze-thaw to release bound virus (42) and concentrations of recovered virus in collected samples were determined by ELISA. Probiotics adhesion to Caco-2 cells was performed as previously described (43). Briefly,cells were incubated with individual probiotic bacteria at a cell: bacteria ratio of 1:10 at 37° C for 1.30 and 4 h. After washing three times, cells were detached from wells by treating with 0.05% trypsin – EDTA (Thermo Fisher Scientific Inc) for 20 min. Subsequently, serially diluted suspensions were plated on agar plates and bacteria adhered to the Caco-2 cells were quantified.
Assessing binding between probiotics and HRV or HRV VLP by flow cytometry
Direct binding of each probiotic to HRV was assessed by flow cytometry under in vitro conditions. Probiotic bacteria were first stained with green fluorescent nucleic acid dye SYTO 9 at 5 μM concentration (Life Technologies) (44). Subsequently, stained bacteria were incubated with either semi-purified HRV (45) or Alexa fluor 647 (Life Technologies) conjugated human rotavirus like particles (VLP) (46) at 37° C for 1.5 h. Subsequently, bacteria were washed thrice using sterile PBS to remove unbound virus and VLP. To detect bacterial bound HRV, bacteria were incubated with Alexa fluor 647 conjugated anti-RV monoclonal antibody (Clone RG23B9C5H11) or isotype control at 4° C for 45 min. After washing, bacteria were acquired by BD Accuri C6 flow cytometer and data were analyzed using the instrument software.
In vitro stimulation of MNCs with EcN and LGG
Splenic and ileal MNCs were cultured with EcN and LGG at 1:10 ratio (MNC: bacteria) for 24 hours as previously described (18). Culture supernatants were collected to quantify IL-6 and IL-10 cytokines as previously described (17). To assess the effects of IL-6 and IL-10 on IgA responses, splenic MNCs were cocultured with heat-killed EcN and LGG in the presence or absence of blocking antibodies to IL-6 (10μg/ml, R&D Systems) and IL-10 (10μg/ml, R&D Systems) or recombinant porcine IL-6 (10μg/ml, R&D Systems) and IL-10 (10μg/ml, R&D Systems) cytokines. After 7 days, culture supernatants were collected to quantify total IgA levels by ELISA.
Statistical analysis
Mean days to onset of virus shedding, mean duration of virus shedding, average peak virus shedding titer and mean duration of diarrhea, log transformed antibody titers, total immunoglobulin levels and ASC were analyzed by Kruskal–Wallis rank sum test. Mean cumulative fecal scores were analyzed by the area under the curve as previously described (17). Statistical analyzes were performed by using GraphPad Prism 5 software (Graph Pad Software, Inc., CA, USA).
Results
Probiotic colonization
Colonization of EcN, LGG, and EcN+LGG in the Gn piglets was determined by quantifying CFUs in fecal samples weekly and in different sections of the GI tract at the end of the study (PBCD36/PCD21). Except at PBCD22/PCD7, no significant differences in fecal bacterial CFUs were observed between EcN and LGG piglets (Fig. 1B). In EcN+LGG piglets, fecal LGG counts were consistently lower compared to EcN CFU counts which could be due to interference of EcN on the colonization of LGG in the EcN+LGG dually colonized piglets. Relative comparisons of LGG and EcN colonization in EcN+LGG piglets revealed a significant reduction in LGG compared to EcN in the duodenum, jejunum, ileum, cecum, and colon. In the probiotic colonized piglets, VirHRV infection had no significant effect on fecal bacterial CFU counts at all post-VirHRV challenge times assessed as compared to pre-VirHRV challenge (PBCD15/PCD0). Enumeration of each probiotic in different sections of the GI tract revealed that LGG and EcN colonized all sections of the GI tract of each mono-colonized piglet examined at PBCD36/PCD21 (Fig. 1C). Translocation of probiotics to the mesenteric lymph nodes, spleen and liver was observed in all the probiotic colonized piglets, but no septicemia or clinical signs of bacteremia were observed in the piglets.
EcN colonization ameliorates the severity of HRV infection
The effects of LGG, EcN and LGG+EcN colonization on VirHRV infection were assessed. Fecal virus shedding was confirmed for all VirHRV challenged pigs. However, mean peak virus shedding titers and mean cumulative fecal scores were significantly lower in EcN compared to LGG-colonized or uncolonized piglets (Table 1). Further, LGG colonized piglets had similar mean peak virus shedding titers as in the uncolonized piglets. Further, EcN+LGG co-colonized piglets had three-fold lower average peak virus shedding titers and significantly reduced cumulative mean fecal scores compared to uncolonized piglets. Additionally, the mean duration of diarrhea was 3.4 days shorter in EcN+LGG colonized compared to uncolonized piglets.
Table 1.
Summary of HRV shedding and diarrhea scores in the four different treatment groups
| Treatment | N | Virus shedding#
|
Diarrhea*
|
||||
|---|---|---|---|---|---|---|---|
| % shed | Mean duration days† | Avg. peak titer shed† (FFU/ml) | % with diarrhea | Mean duration days† | Mean cumulative fecal score†@ | ||
| EcN+VirHRV | 9 | 100 | 3.6 | 0.95 × 104 a | 66 | 1.5a | 8.2a |
| LGG+VirHRV | 8 | 100 | 4.5 | 6.8 × 104 b | 100 | 3.6ab | 11.0bc |
| EcN+LGG+VirHRV | 7 | 100 | 2.8 | 1.9 × 104 ab | 71 | 1.4a | 8.3ac |
| Control+VirHRV | 5 | 100 | 4.2 | 7.0 × 104 b | 100 | 4.8b | 12.6b |
Determined by cell culture immunofluorescence assay.
Pigs with fecal score >1 were considered diarrheic. Fecal consistency was scored as follows: 0, normal; 1, pasty/semiliquid; and 2, liquid.
Means with different letters in the same column differ significantly (determined by Kruskal–Wallis rank sum test, P≤ 0.05).
AUC indicates diarrhea severity.
Probiotics colonization modulated intestinal HRV IgA antibody responses
The effects of selected probiotics on HRV-specific antibody responses were assessed. Consistent with decreased fecal HRV shedding and diarrhea, EcN+VirHRV piglets had significantly lower small intestinal IgA HRV antibody titers (Fig. 2A) and duodenal IgA and IgG HRV ASC (Fig. 2B, 2C) as compared to uncolonized piglets at PBCD36/PCD21. The reduced HRV-specific antibody titers were correlated with the reduced virus shedding titers (data not shown). Regardless of similar mean peak virus shedding titers between LGG− and uncolonized-piglets, intestinal HRV specific IgA and IgG antibody responses were significantly lower in LGG-colonized compared to uncolonized-piglets post-VirHRV challenge (Fig 2). Intestinal virus-specific IgA antibody responses were comparable between the EcN+LGG+VirHRV and uncolonized+VirHRV groups regardless of the reduced virus shedding titers and a significant reduction in mean fecal scores in the former group compared to the later one.
Figure 2.
EcN and LGG probiotic colonization significantly modulated the small intestinal HRV specific antibody responses at the post-VirHRV challenge. (A) Geometric mean titers (GMT) of HRV specific-IgA antibody titers in intestinal contents and (B, C) HRV-specific IgA IgG ASCs responses in duodenum of Gn pigs challenged with or without probiotic colonization at post-VirHRV challenge (PBCD36/PCD21) (One-way ANOVA followed by Duncan’s multiple range test on log10 transferred titers, * p < 0.05). Error bars indicate SEM. PBCD-Post-bacterial colonization day. PCD-Post-VirHRV challenge day.
EcN colonization significantly enhanced total immunoglobulin responses compared to LGG colonized piglets
The impact of probiotic colonization and VirHRV infection on intestinal B cell responses was assessed. Small intestinal total IgA levels (Fig. 3A) and total IgA secreting cells (Fig. 3B) were significantly higher in EcN piglets compared to LGG colonized piglets post-VirHRV challenge. Further, the comparable intestinal total IgA responses between EcN±LGG+VirHRV and VirHRV groups also indicate the immunostimulatory effects of HRV on the B cell responses (Fig. 3A, 3B).
Figure 3.
EcN enhanced small intestinal total IgA responses at PBCD36/PCD21. (A) Total IgA levels in the small intestinal contents and (B) total IgA immunoglobulin secreting cell responses in the duodenum of the Gn pigs challenged with or without probiotic colonization at post-HRV challenge (PBCD36/PCD21) (one-way ANOVA followed by Duncan’s multiple range test on log10 transferred titers, * p < 0.05). Error bars indicate SEM. PBCD-Post-bacterial colonization day. PCD-Post-VirHRV challenge day.
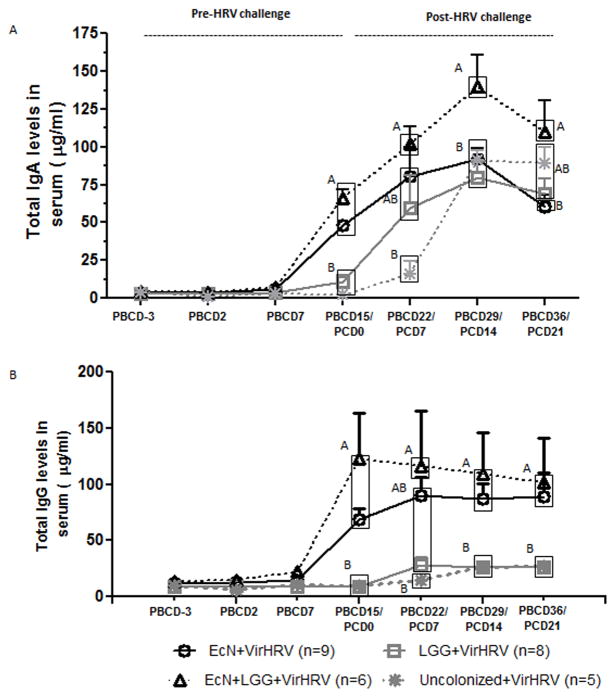
The effect of EcN and LGG monocolonization on serum total IgA and IgG responses was also assessed. No differences in total serum IgA or IgG levels were observed in any group at PBCD7 (Fig. 4A, 4B). However, the EcN and EcN+LGG piglets had significantly higher total serum IgA (Fig. 4A) and IgG (Fig. 4B) levels compared to uncolonized or LGG monocolonized piglets at PBCD15/PCD0. Further, significantly higher total IgA responses were also observed in EcN+LGG piglets compared to the other three groups at PBCD29/PCD14. This indicates a positive interaction among EcN, LGG, and VirHRV in inducing total serum IgA responses. Further, induction of EcN-, but not LGG-specific IgA antibody responses may also have partially contributed to the higher total IgA antibody responses in EcN and EcN+LGG piglets (Supplementary fig 1). Additionally, significant activation of B cells by EcN was also evident in our study in which higher frequencies of antibody forming B cells (CD79β+CD21−CD2− B cells) was observed in EcN and EcN+LGG compared to LGG alone treated MNCs in vitro (Supplementary fig 2). Notably, total IgG responses were similar between LGG and uncolonized piglets at pre- and post-VirHRV challenge time points. This suggests that the LGG colonization alone was not sufficient to induce markedly increased systemic total IgG responses. In uncolonized piglets, VirHRV challenge increased total IgA responses, but not total IgG responses, as evident by higher IgA responses at various post-VirHRV challenge days compared to pre-VirHRV challenge (PBCD15/PCD0).
Figure 4.
EcN±LGG colonization significantly increased serum total IgA and IgG responses pre-VirHRV challenge (PBCD15/PCD0). (A, B) Serum total IgA and IgG levels were measured by ELISA at indicated time points. Different alphabetical letters indicate significant differences (P<0.05) at the same time point in total immunoglobulin levels among treatment groups, whereas the same letters indicate no significant difference. The values that are statistically similar are in the same box. (one-way ANOVA followed by Duncan’s multiple range test, p < 0.05). Error bars indicate SEM. PBCD-Post-bacterial colonization day. PCD-Post-VirHRV challenge day.
EcN±LGG, but not LGG alone induced IL-6, IL-10 and IgA in MNCs in vitro
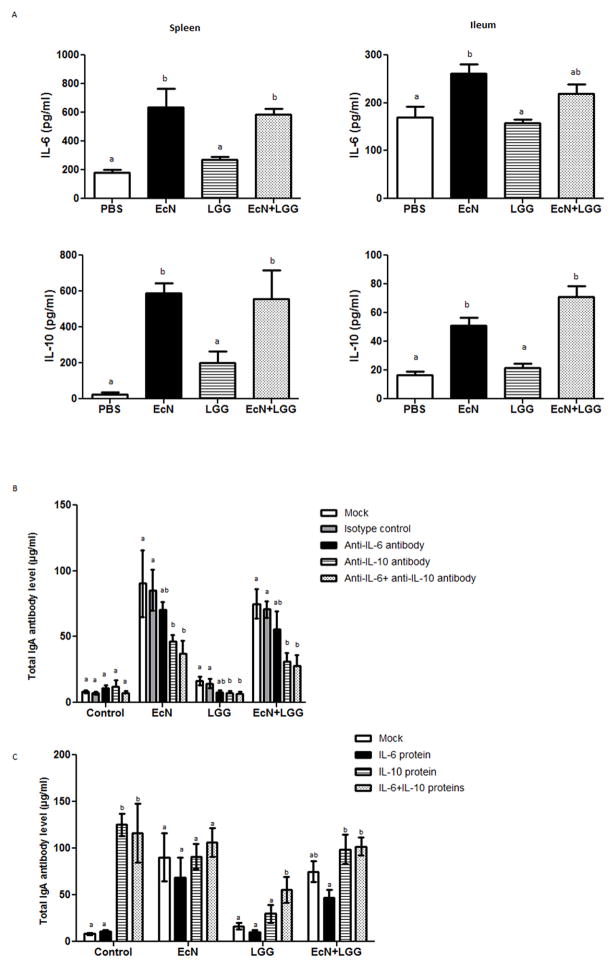
EcN or EcN+LGG, but not LGG colonization significantly enhanced systemic as well as intestinal total IgA responses pre-VirHRV challenge. This might be due to the EcN probiotic dependent stimulation of IgA-inducing cytokines such as IL-6 and IL-10 as we assessed by co-culturing MNCs with the live probiotics. Significantly higher IL-6 and IL-10 levels were observed in EcN and EcN+LGG compared to LGG treated MNCs (Fig. 5A).
Figure 5.
(A) Effect of EcN and LGG on IL-6 and IL-10 cytokine responses in splenic and ileal MNCS under in vitro condition. Splenic- and Ileal- MNCs from control Gn piglets were co-cultured with indicated probiotics for 24 h at 37° C, and supernatants were collected for IL-6 and IL-10 determination by ELISA. . Different alphabetical letters above the bars indicate significant differences (p <0.05) among treatment groups, whereas the same letters indicate no significant difference. (B) Blocking antibodies to IL-6 and IL-10 were added to MNC+probiotic co-culture to determine their effects on total IgA responses. (C) Effect of recombinant porcine IL-6 and IL-10 on total IgA responses in MNCs co-cultured with the probiotics. Different alphabetical letters above the bars indicate significant differences (P<0.05) among treatment groups within each probiotic treatment, whereas the same letters indicate no significant difference. Results are mean ± SEM (n=4, One-way ANOVA, * p < 0.05).
Treatment of MNCs with probiotics for 7 days revealed that total IgA responses were higher in culture supernatants from EcN±LGG compared to LGG alone treated MNCs (Fig 5B). Significantly higher levels of total IgA were observed in culture supernatants from EcN treated MNCs compared to that of LGG treated MNCs. The addition of porcine IL-10 antibody, but not porcine IL-6 antibody, significantly reduced total IgA levels (Fig. 5B). Thus, it appears that IL-10 plays a significant role in the EcN-induced IgA responses. We also examined whether the addition of recombinant porcine IL-10, IL-6, and IL-10+IL-6 in MNC co-cultured with LGG had any enhancing effect on total IgA responses. The addition of IL-10 and IL-6+IL-10 cytokines induced higher, and significantly higher total IgA levels, respectively, in the cytokine treated MNC+LGG co-cultures compared to LGG alone treated MNCs (Fig. 5C).
EcN, but not LGG reduced attachment of HRV to Caco-2 epithelial cells in vitro
The observed variable effects of probiotics on virus shedding and diarrhea severity might be caused by a difference in their interference with virus attachment to epithelial cells. Pre-incubation of Caco-2 epithelial cells with EcN, but not with LGG, significantly reduced (approximately 50% reduction) cellular attachment of HRV (Fig.6A). Further, a significantly higher percentage of EcN binds to Caco-2 cells compared with a negligible percentage of LGG that binds to Caco-2 cells (Fig. 6B). These results suggest that EcN potentially directly interferes with HRV attachment to target (epithelial) cells, subsequently resulting in lower virus shedding and diarrhea.
Figure 6.
(A) Confluent Caco-2 cell monolayers in 6 well cell culture plates were incubated with individual probiotic bacteria at 37° C for 4 h. In the presence of bacteria, HRV at a multiplicity of infection 3.0, was added to the cells-bacterial mixture and then incubated at 4° C for 1 h. After washing, cells were subjected to two rounds of freeze-thaw to release bound virus and concentrations of virus in collected samples were determined by ELISA. Results are expressed as mean percentage virus bound to probiotic treated Caco-2 cells relative to the no-probiotic treated control cells (n=four independent experiments, One-way ANOVA, * p < 0.05). (B) Caco-2 cell monolayers were incubated with individual probiotic bacteria at 37° C for 1.30 h and 4 h. Subsequently, percent bacteria adhered to cells were quantified (n=two independent experiments, t test, * p < 0.05). (C) Analysis of Wa HRV binding to EcN and LGG. SYTO 9 stained probiotic bacteria were incubated with semi-purified HRV. After washing, bacterial bound HRV was detected by incubating with Alexa fluor 647 (Life Technologies) conjugated RV mAb (Clone RG23B9C5H11). The percent of virus bound to bacteria was assessed by flow cytometry. (D) Determination of rotavirus VLP 2/6/4 and VLP2/6 binding to EcN and LGG probiotics. SYTO 9 stained probiotic bacteria were incubated with Alexa fluor 647 conjugated (Life Technologies) human rotavirus like particles (VLP). After washing, the percent of VLP bound to bacteria was assessed by flow cytometry. Results are mean ± SEM (n=4, t test, * p < 0.05).
Human rotavirus or HRV VLP bind to EcN, but not to LGG
The reduced virus shedding and the significant reduction in fecal scores in EcN compared to LGG piglets may potentially be caused by direct binding of EcN to HRV. Analysis of the interaction between HRV and the probiotic bacteria revealed that a significantly higher percentage of EcN binds to HRV Wa strain compared with a negligible percentage of LGG that binds to HRV (Fig. 6C). Similarly, only EcN, but not LGG, binds to HRV VLP 2/4/6 particles. In comparison, the inner capsid VLP (VLP2/6) binding to EcN and LGG was very low or undetectable (Fig. 6D). Thus, VP4 of HRV might be mediating the interaction between Wa HRV and EcN.
Discussion
The role of intestinal commensals in modulating viral infections has been increasingly recognized in recent studies (7). In this study, we compared anti-infectious and immunomodulatory effects of G+, G− probiotics, and their combination on RV infection and immunity. A significant protective effect against HRV infection was observed in EcN colonized, compared with LGG colonized or uncolonized piglets. EcN+LGG colonization resulted in three fold reduction in fecal virus shedding and induced marked B cell responses in the Gn piglets.
EcN colonization alone or co-colonization of EcN with LGG reduced fecal HRV shedding and the severity of diarrhea post-VirHRV challenge. The significant reduction in the intestinal and serum HRV specific antibody responses in EcN colonized piglets compared to control piglets was consistent with the reduction in fecal HRV shedding titers and diarrhea in the EcN group. The EcN-induced partial protection against HRV infection likely accounts for the significantly lower HRV specific intestinal IgA antibody in the EcN piglets compared to the uncolonized group post-VirHRV challenge. A potential mechanism that might underlie EcN induced partial protection against VirHRV infection is through the direct interference by EcN on HRV infection or indirectly through modulating host immunity. In support of the first possibility, our finding of HRV or VLP2/4/6 (but not VLP2/6) binding to EcN, but not to LGG, suggests that the partial protection conferred by EcN may be mediated by direct interaction between HRV and EcN. This observation is further supported by our in vitro virus-epithelial cell binding assay in which a significant reduction in virus attachment to epithelial cells was observed in EcN compared to LGG treated cells. Further, EcN had a higher capacity for adhesion to epithelial cells than LGG which coincided with the EcN induced reduction in virus attachment to Caco-2 cells in vitro. Rotavirus capsid is composed of three concentric layers and among the viral proteins, VP2 is present in the internal core layer, VP6 is part of the intermediate layer and VP4 is present in the form of spikes in the external (VP7) layer (47). Bacteria or bacterial derived ligands have been shown to interact with viruses and the interaction modulated viral pathogenesis (10, 11, 48). Further, binding of EcN with VLP2/4/6, but not with VLP2/6 suggests that EcN mainly interacts with VP4, the major viral cell attachment protein of HRV (49). Additionally, EcN may enhance innate immunity which may also confer protective effects against VirHRV infection. A recent study showed that administration of flagellin, a TLR5 ligand derived from flagella of bacteria, prevented RV infection via activation of innate immunity in a mouse model (50). Similar to the effect of EcN on HRV infection, EcN also inhibited the invasion of an intestinal cell line by several bacterial pathogens such as Salmonella enterica serovar Typhimurium, Yersinia enterocolitica, Shigella flexneri, Legionella pneumophila and Listeria monocytogenes (25). Apart from the effects on pathogens, EcN also has anti-inflammatory properties as observed in treatment of T cells with EcN which resulted in decreased secretion of inflammatory cytokines such as TNF-α, and MCP-1 (51). Additionally, our findings of reduced HRV diarrhea severity was also consistent with human clinical trials in which EcN treatment markedly reduced symptoms of infectious diarrhea in children and young infants (52, 53). Collectively, we showed that EcN had a significant protective effect on HRV shedding and diarrhea severity.
EcN and EcN+LGG colonization significantly increased serum total immunoglobulin responses in the Gn piglets pre-VirHRV challenge. Serum total IgA and IgG levels remained unchanged in uncolonized piglets pre-VirHRV challenge which indicates a requirement of the intestinal microbes (including enteric viruses) for induction of B cell responses. The enhanced B cell responses in EcN colonized piglets might be due to either direct activation the B cells by EcN or through stimulating antigen presenting cells. Similar to our study, colonization of benign commensal E. coli (G58-1) alone in Gn piglets significantly enhanced serum total immunoglobulin levels (54). In another study, mono-association of piglets with E.coli O83 resulted in increased frequency of dendritic cells and T cells in the small intestinal lamina propria (55). Lipopolysaccharide (LPS) of G− bacteria are a well-known B cell mitogen (56) and LPS also induces B cell maturation via the TLR4 signaling pathway (57). Further, EcN supplementation significantly enhanced systemic total IgM and cellular proliferative responses in preterm infants (40). Although both EcN and EcN+LGG piglets had statistically similar mean peak virus shedding titers, HRV specific IgA antibody responses were higher in EcN+LGG compared to EcN alone colonized piglets. Potential synergistic immunostimulatory effects of microbe-associated molecular patterns from these two probiotics through multiple TLRs (58) and two-fold higher virus shedding titers and might have contributed to the higher HRV specific IgA responses in the EcN+LGG compared to EcN alone colonized piglets. Thus, EcN has an immunostimulatory effect on both the systemic and intestinal immune systems.
LGG colonization had minimal effects on induction of total immunoglobulin responses in comparison to EcN. Specifically, serum total IgA and IgG levels were comparable between LGG colonized and uncolonized piglets at PBCD15/PCD0 (Pre-VirHRV challenge). A similar trend was also observed for the intestinal HRV-specific antibody responses post-VirHRV challenge. Considering the comparable virus shedding titers between the LGG and uncolonized piglets, one would expect similar or higher small intestinal HRV-specific IgA antibody responses in LGG colonized piglets compared to uncolonized piglets. Rather, we observed significantly lower HRV specific intestinal- and total IgA responses in LGG compared with uncolonized piglets post-VirHRV challenge. The mechanisms for lower induction of antibody responses in LGG colonized piglets is unclear, but our in vitro studies indicated that LGG, but not EcN, failed to induce cytokines such as IL-10 and IL-6 which are well-known inducers of IgA antibody responses (59–61). This observation is further supported by the significantly enhanced total IgA responses after addition of porcine IL-6 and IL-10 cytokines to the MNC-LGG co-cultures. These findings are consistent with a previous study in which G+ lactobacilli bacteria failed to induce IL-6 and IL-10 cytokines, but E.coli induced higher levels of these cytokines in dendritic cells (62). Similarly, Hessle et al (63) observed that various G+ commensal bacteria are less potent inducers of IL-10 compared to that of G− commensal bacteria. In an earlier study we also observed a significant reduction in ileal IL-10 cytokine secreting cell numbers in Lactobacillus acidophilus and Lactobacillus reuteri dual-colonized piglets compared to uncolonized piglets post-VirHRV infection (64). A previous study also showed that treatment of T cells with EcN conditioned media upregulated expression of IL-10 (27). Thus, it appears that LGG alone may be a less potent inducer of IgA responses in comparison to EcN. Several earlier studies (14, 15) reported beneficial effects of LGG on RV infection and RV vaccine-induced immunity. One potential explanation for the discordance of present results from the previous studies (using conventional animal models, or human subjects) is that LGG exert its beneficial effects through interaction with other members of the intestinal microbiota. In support this possibility, a recent study showed that LGG supplementation stabilized VirHRV-induced changes in the gut microbiota in Gn piglets transplanted with infant fecal microbiota (65). Further, LGG supplementation significantly enhanced frequencies of CD4+IFNγ+ T cells but had no effect on HRV specific antibody responses in human microbiota colonized, HRV-vaccinated piglets compared to non-colonized, HRV vaccinated Gn piglets (66). Apart from the effects on microbiota composition, LGG supplementation significantly altered functionality of resident intestinal commensals in human (67). Thus, we speculate that LGG might promote enhanced cellular or innate immunity in the presence of complex microbiota which might have contributed the beneficial effects observed for LGG on HRV infections in children (15). In the absence of a complex microbiota in our Gn piglets, LGG may have only minimal beneficial effects on RV infection and immunity.
The differences in adherence properties of LGG and EcN in the intestinal microenvironment might also have contributed to the observed differential effects on immune responses. Our in vitro probiotics adherence assay to epithelial cells also suggests that there might be a variation in colonization properties of the probiotics which might have resulted in the observed differential immunomodulatory effects. We observed consistently reduced levels of LGG in EcN+LGG co-colonized compared to LGG alone colonized piglets. Similarly, EcN introduction in the Gn mice colonized with E. coli K-12, Lactobacillus johnsonii, and Bifidobacterium longum resulted in complete elimination of E. coli K-12 and L. johnsonii (68). The mechanism mediating this EcN dominance over LGG is not clear. One potential explanation might be that factors such as the ability of EcN to produce microcin and also the presence of several different iron uptake systems (69) in its genome might have conferred a competitive edge to EcN over LGG in EcN+LGG co-colonized piglets. Our study has implications for the use of probiotics to treat RV infections to effectively manage an active HRV outbreak. We envisage that supplementation of EcN probiotic might be a cost effective therapy to ameliorate the severity of RV infections. One caveat of this study is use of the Gn piglets instead of conventional animals to determine beneficial effects of the probiotics on RV infection and immunity. Although our approach is advantageous in terms of eliminating the confounding effects of the intestinal microbiota and defining the effects of individual or combined probiotics on HRV infections, these findings have to be further validated using conventionalized animals such as Gn piglets colonized with human fecal microbiota. Further, our study indicates that EcN directly interacts with HRV and it remains to be determined whether EcN has a positive or negative effect on responses to HRV vaccine. In summary, compared to the G+ LGG probiotic, the G− EcN had greater beneficial effects in ameliorating VirHRV infection and promoting the development of the neonatal intestinal and systemic antibody responses.
Supplementary Material
Acknowledgments
Funding source: This work was supported by a grant from the NIAID at NIH (grant # R01 A1099451 to LJS) and federal funds appropriated to the Ohio Agricultural Research and Development Center (OARDC) of The Ohio State University.
We gratefully acknowledge the technical assistance of Dr. Juliette Hanson, R. Wood, J. Ogg, Thavamathi Annamalai and Kyle T. Scheuer.
Abbreviations used in this article
- HRV
human rotavirus
- VirHRV
virulent human rotavirus, AttHRV, attenuated human rotavirus
- Gn
gnotobiotic
- MNCs
mononuclear cells
- RV
rotavirus
- LGG
Lactobacillus rhamnosus strain GG
- EcN
Escherichia coli Nissle
- VLP
virus-like particles
- GI
gastrointestinal
- CCIF
cell-culture immunofluorescence
- FFU
fluorescent-forming unit
- G+
Gram-positive
- G-
Gram-negative
- PCD
postchallenge day
- PBCD
post bacterial colonization day
- MRS
deMan, Rogosa and Sharpe
- ASCs
antibody secreting cells
References
- 1.Tate JE, Burton AH, Boschi-Pinto C, Steele AD, Duque J, Parashar UD. 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. The Lancet infectious diseases. 2012;12:136–141. doi: 10.1016/S1473-3099(11)70253-5. [DOI] [PubMed] [Google Scholar]
- 2.Viboud C, Lopman BA, Pitzer VE, Sarkar R, Gladstone B, Patel M, Glasser J, Gambhir M, Atchison C, Grenfell BT. Understanding reduced rotavirus vaccine efficacy in low socio-economic settings. PloS one. 2012;7:Article ID e41720, 41727. doi: 10.1371/journal.pone.0041720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferreira RB, Antunes LCM, Finlay BB. Should the human microbiome be considered when developing vaccines? PLoS pathogens. 2010;6:e1001190. doi: 10.1371/journal.ppat.1001190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qadri F, Bhuiyan TR, Sack DA, Svennerholm AM. Immune responses and protection in children in developing countries induced by oral vaccines. Vaccine. 2013;31:452–460. doi: 10.1016/j.vaccine.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 5.Valdez Y, Brown EM, Finlay BB. Influence of the microbiota on vaccine effectiveness. Trends in immunology. 2014;35:526–537. doi: 10.1016/j.it.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 6.Wilks J, Golovkina T. Influence of microbiota on viral infections. PLoS Pathog. 2012;8:e1002681. doi: 10.1371/journal.ppat.1002681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robinson CM, Pfeiffer JK. Viruses and the Microbiota. Annual review of virology. 2014;1:55–69. doi: 10.1146/annurev-virology-031413-085550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uchiyama R, Chassaing B, Zhang B, Gewirtz AT. Antibiotic treatment suppresses rotavirus infection and enhances specific humoral immunity. The Journal of infectious diseases. 2014;210:171–182. doi: 10.1093/infdis/jiu037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huda MN, Lewis Z, Kalanetra KM, Rashid M, Ahmad SM, Raqib R, Qadri F, Underwood MA, Mills DA, Stephensen CB. Stool microbiota and vaccine responses of infants. Pediatrics. 2014;134:e362–372. doi: 10.1542/peds.2013-3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuss SK, Best GT, Etheredge CA, Pruijssers AJ, Frierson JM, Hooper LV, Dermody TS, Pfeiffer JK. Intestinal microbiota promote enteric virus replication and systemic pathogenesis. Science. 2011;334:249–252. doi: 10.1126/science.1211057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kane M, Case LK, Kopaskie K, Kozlova A, MacDearmid C, Chervonsky AV, Golovkina TV. Successful transmission of a retrovirus depends on the commensal microbiota. Science. 2011;334:245–249. doi: 10.1126/science.1210718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Szajewska H, Mrukowicz JZ. Probiotics in the treatment and prevention of acute infectious diarrhea in infants and children: a systematic review of published randomized, double-blind, placebo-controlled trials. Journal of pediatric gastroenterology and nutrition. 2001;33(Suppl 2):S17–25. doi: 10.1097/00005176-200110002-00004. [DOI] [PubMed] [Google Scholar]
- 13.Sanders ME, Guarner F, Guerrant R, Holt PR, Quigley EM, Sartor RB, Sherman PM, Mayer EA. An update on the use and investigation of probiotics in health and disease. Gut. 2013;62:787–796. doi: 10.1136/gutjnl-2012-302504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Majamaa H, Isolauri E, Saxelin M, Vesikari T. Lactic acid bacteria in the treatment of acute rotavirus gastroenteritis. Journal of pediatric gastroenterology and nutrition. 1995;20:333–338. doi: 10.1097/00005176-199504000-00012. [DOI] [PubMed] [Google Scholar]
- 15.Kaila M, Isolauri E, Soppi E, Virtanen E, Laine S, Arvilommi H. Enhancement of the circulating antibody secreting cell response in human diarrhea by a human Lactobacillus strain. Pediatric research. 1992;32:141–144. doi: 10.1203/00006450-199208000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Zhang W, Azevedo MS, Wen K, Gonzalez A, Saif LJ, Li G, Yousef AE, Yuan L. Probiotic Lactobacillus acidophilus enhances the immunogenicity of an oral rotavirus vaccine in gnotobiotic pigs. Vaccine. 2008;26:3655–3661. doi: 10.1016/j.vaccine.2008.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chattha KS, Vlasova AN, Kandasamy S, Rajashekara G, Saif LJ. Divergent immunomodulating effects of probiotics on T cell responses to oral attenuated human rotavirus vaccine and virulent human rotavirus infection in a neonatal gnotobiotic piglet disease model. Journal of immunology. 2013;191:2446–2456. doi: 10.4049/jimmunol.1300678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kandasamy S, Chattha KS, Vlasova AN, Rajashekara G, Saif LJ. Lactobacilli and Bifidobacteria enhance mucosal B cell responses and differentially modulate systemic antibody responses to an oral human rotavirus vaccine in a neonatal gnotobiotic pig disease model. Gut microbes. 2014;5:639–651. doi: 10.4161/19490976.2014.969972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vlasova AN, Chattha KS, Kandasamy S, Liu Z, Esseili M, Shao L, Rajashekara G, Saif LJ. Lactobacilli and bifidobacteria promote immune homeostasis by modulating innate immune responses to human rotavirus in neonatal gnotobiotic pigs. PLoS One. 2013;8:e76962. doi: 10.1371/journal.pone.0076962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guandalini S, Pensabene L, Zikri MA, Dias JA, Casali LG, Hoekstra H, Kolacek S, Massar K, Micetic-Turk D, Papadopoulou A, de Sousa JS, Sandhu B, Szajewska H, Weizman Z. Lactobacillus GG administered in oral rehydration solution to children with acute diarrhea: a multicenter European trial. Journal of pediatric gastroenterology and nutrition. 2000;30:54–60. doi: 10.1097/00005176-200001000-00018. [DOI] [PubMed] [Google Scholar]
- 21.Nowrouzian F, Hesselmar B, Saalman R, Strannegard IL, Aberg N, Wold AE, Adlerberth I. Escherichia coli in infants' intestinal microflora: colonization rate, strain turnover, and virulence gene carriage. Pediatric research. 2003;54:8–14. doi: 10.1203/01.PDR.0000069843.20655.EE. [DOI] [PubMed] [Google Scholar]
- 22.Kruis W, Fric P, Pokrotnieks J, Lukas M, Fixa B, Kascak M, Kamm MA, Weismueller J, Beglinger C, Stolte M, Wolff C, Schulze J. Maintaining remission of ulcerative colitis with the probiotic Escherichia coli Nissle 1917 is as effective as with standard mesalazine. Gut. 2004;53:1617–1623. doi: 10.1136/gut.2003.037747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hering NA, Richter JF, Fromm A, Wieser A, Hartmann S, Gunzel D, Bucker R, Fromm M, Schulzke JD, Troeger H. TcpC protein from E. coli Nissle improves epithelial barrier function involving PKCzeta and ERK1/2 signaling in HT-29/B6 cells. Mucosal immunology. 2014;7:369–378. doi: 10.1038/mi.2013.55. [DOI] [PubMed] [Google Scholar]
- 24.Rembacken BJ, Snelling AM, Hawkey PM, Chalmers DM, Axon AT. Non-pathogenic Escherichia coli versus mesalazine for the treatment of ulcerative colitis: a randomised trial. Lancet. 1999;354:635–639. doi: 10.1016/s0140-6736(98)06343-0. [DOI] [PubMed] [Google Scholar]
- 25.Altenhoefer A, Oswald S, Sonnenborn U, Enders C, Schulze J, Hacker J, Oelschlaeger TA. The probiotic Escherichia coli strain Nissle 1917 interferes with invasion of human intestinal epithelial cells by different enteroinvasive bacterial pathogens. FEMS immunology and medical microbiology. 2004;40:223–229. doi: 10.1016/S0928-8244(03)00368-7. [DOI] [PubMed] [Google Scholar]
- 26.Schlee M, Wehkamp J, Altenhoefer A, Oelschlaeger TA, Stange EF, Fellermann K. Induction of human beta-defensin 2 by the probiotic Escherichia coli Nissle 1917 is mediated through flagellin. Infection and immunity. 2007;75:2399–2407. doi: 10.1128/IAI.01563-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sturm A, Rilling K, Baumgart DC, Gargas K, Abou-Ghazale T, Raupach B, Eckert J, Schumann RR, Enders C, Sonnenborn U, Wiedenmann B, Dignass AU. Escherichia coli Nissle 1917 distinctively modulates T-cell cycling and expansion via toll-like receptor 2 signaling. Infection and immunity. 2005;73:1452–1465. doi: 10.1128/IAI.73.3.1452-1465.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saif L, Ward L, Yuan L, Rosen B, To T. The gnotobiotic piglet as a model for studies of disease pathogenesis and immunity to human rotaviruses. Springer; 1996. [DOI] [PubMed] [Google Scholar]
- 29.Butler J, Lager K, Splichal I, Francis D, Kacskovics I, Sinkora M, Wertz N, Sun J, Zhao Y, Brown W. The piglet as a model for B cell and immune system development. Veterinary immunology and immunopathology. 2009;128:147–170. doi: 10.1016/j.vetimm.2008.10.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Butler JE, Sun J, Weber P, Ford SP, Rehakova Z, Sinkora J, Lager K. Antibody repertoire development in fetal and neonatal piglets. IV. Switch recombination, primarily in fetal thymus, occurs independent of environmental antigen and is only weakly associated with repertoire diversification. The Journal of Immunology. 2001;167:3239–3249. doi: 10.4049/jimmunol.167.6.3239. [DOI] [PubMed] [Google Scholar]
- 31.Šinkora M, Šinkorova J, Butler JE. B cell development and VDJ rearrangement in the fetal pig. Veterinary immunology and immunopathology. 2002;87:341–346. doi: 10.1016/s0165-2427(02)00062-4. [DOI] [PubMed] [Google Scholar]
- 32.Meyer RC, Bohl EH, Kohler EM. Procurement and Maintenance of Germ-Free Seine for Microbiological Investigations. Applied microbiology. 1964;12:295–300. doi: 10.1128/am.12.4.295-300.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumar A, Vlasova AN, Liu Z, Chattha KS, Kandasamy S, Esseili M, Zhang X, Rajashekara G, Saif LJ. In vivo gut transcriptome responses to Lactobacillus rhamnosus GG and Lactobacillus acidophilus in neonatal gnotobiotic piglets. Gut microbes. 2014;5:152–164. doi: 10.4161/gmic.27877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ward LA, Rosen BI, Yuan L, Saif LJ. Pathogenesis of an attenuated and a virulent strain of group A human rotavirus in neonatal gnotobiotic pigs. The Journal of general virology. 1996;77(Pt 7):1431–1441. doi: 10.1099/0022-1317-77-7-1431. [DOI] [PubMed] [Google Scholar]
- 35.Yuan L, Kang SY, Ward LA, To TL, Saif LJ. Antibody-secreting cell responses and protective immunity assessed in gnotobiotic pigs inoculated orally or intramuscularly with inactivated human rotavirus. Journal of virology. 1998;72:330–338. doi: 10.1128/jvi.72.1.330-338.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yuan L, Ward LA, Rosen BI, To TL, Saif LJ. Systematic and intestinal antibody-secreting cell responses and correlates of protective immunity to human rotavirus in a gnotobiotic pig model of disease. Journal of virology. 1996;70:3075–3083. doi: 10.1128/jvi.70.5.3075-3083.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parreno V, Hodgins DC, de Arriba L, Kang SY, Yuan L, Ward LA, To TL, Saif LJ. Serum and intestinal isotype antibody responses to Wa human rotavirus in gnotobiotic pigs are modulated by maternal antibodies. The Journal of general virology. 1999;80( Pt 6):1417–1428. doi: 10.1099/0022-1317-80-6-1417. [DOI] [PubMed] [Google Scholar]
- 38.Sinkora M, Stepanova K, Butler JE, Francis D, Santiago-Mateo K, Potockova H, Karova K, Sinkorova J. Ileal Peyer's patches are not necessary for systemic B cell development and maintenance and do not contribute significantly to the overall B cell pool in swine. Journal of immunology. 2011;187:5150–5161. doi: 10.4049/jimmunol.1101879. [DOI] [PubMed] [Google Scholar]
- 39.Zhang W, Azevedo MS, Gonzalez AM, Saif LJ, Van Nguyen T, Wen K, Yousef AE, Yuan L. Influence of probiotic Lactobacilli colonization on neonatal B cell responses in a gnotobiotic pig model of human rotavirus infection and disease. Vet Immunol Immunopathol. 2008;122:175–181. doi: 10.1016/j.vetimm.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cukrowska B, LodInova-ZadnIkova R, Enders C, Sonnenborn U, Schulze J, Tlaskalova-Hogenova H. Specific proliferative and antibody responses of premature infants to intestinal colonization with nonpathogenic probiotic E. coli strain Nissle 1917. Scandinavian journal of immunology. 2002;55:204–209. doi: 10.1046/j.1365-3083.2002.01005.x. [DOI] [PubMed] [Google Scholar]
- 41.Graham KL, Halasz P, Tan Y, Hewish MJ, Takada Y, Mackow ER, Robinson MK, Coulson BS. Integrin-using rotaviruses bind alpha2beta1 integrin alpha2 I domain via VP4 DGE sequence and recognize alphaXbeta2 and alphaVbeta3 by using VP7 during cell entry. Journal of virology. 2003;77:9969–9978. doi: 10.1128/JVI.77.18.9969-9978.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hewish MJ, Takada Y, Coulson BS. Integrins α2β1 and α4β1 can mediate SA11 rotavirus attachment and entry into cells. Journal of virology. 2000;74:228–236. doi: 10.1128/jvi.74.1.228-236.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Letourneau J, Levesque C, Berthiaume F, Jacques M, Mourez M. In vitro assay of bacterial adhesion onto mammalian epithelial cells. Journal of visualized experiments : JoVE. 2011 doi: 10.3791/2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bunthof CJ, Abee T. Development of a flow cytometric method to analyze subpopulations of bacteria in probiotic products and dairy starters. Applied and environmental microbiology. 2002;68:2934–2942. doi: 10.1128/AEM.68.6.2934-2942.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yuan L, Geyer A, Saif LJ. Short-term immunoglobulin AB-cell memory resides in intestinal lymphoid tissues but not in bone marrow of gnotobiotic pigs inoculated with Wa human rotavirus. Immunology. 2001;103:188–198. doi: 10.1046/j.1365-2567.2001.01229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Iosef C, Van Nguyen T, Jeong K-i, Bengtsson K, Morein B, Kim Y, Chang K-O, Azevedo MS, Yuan L, Nielsen P. Systemic and intestinal antibody secreting cell responses and protection in gnotobiotic pigs immunized orally with attenuated Wa human rotavirus and Wa 2/6-rotavirus-like-particles associated with immunostimulating complexes. Vaccine. 2002;20:1741–1753. doi: 10.1016/s0264-410x(02)00031-2. [DOI] [PubMed] [Google Scholar]
- 47.Trask SD, McDonald SM, Patton JT. Structural insights into the coupling of virion assembly and rotavirus replication. Nature reviews Microbiology. 2012;10:165–177. doi: 10.1038/nrmicro2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Robinson CM, Jesudhasan PR, Pfeiffer JK. Bacterial lipopolysaccharide binding enhances virion stability and promotes environmental fitness of an enteric virus. Cell host & microbe. 2014;15:36–46. doi: 10.1016/j.chom.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ludert JE, Feng N, Yu JH, Broome RL, Hoshino Y, Greenberg HB. Genetic mapping indicates that VP4 is the rotavirus cell attachment protein in vitro and in vivo. Journal of virology. 1996;70:487–493. doi: 10.1128/jvi.70.1.487-493.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang B, Chassaing B, Shi Z, Uchiyama R, Zhang Z, Denning TL, Crawford SE, Pruijssers AJ, Iskarpatyoti JA, Estes MK. Prevention and cure of rotavirus infection via TLR5/NLRC4–mediated production of IL-22 and IL-18. Science. 2014;346:861–865. doi: 10.1126/science.1256999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grabig A, Paclik D, Guzy C, Dankof A, Baumgart DC, Erckenbrecht J, Raupach B, Sonnenborn U, Eckert J, Schumann RR, Wiedenmann B, Dignass AU, Sturm A. Escherichia coli strain Nissle 1917 ameliorates experimental colitis via toll-like receptor 2- and toll-like receptor 4-dependent pathways. Infection and immunity. 2006;74:4075–4082. doi: 10.1128/IAI.01449-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Henker J, Laass M, Blokhin BM, Bolbot YK, Maydannik VG, Elze M, Wolff C, Schulze J. The probiotic Escherichia coli strain Nissle 1917 (EcN) stops acute diarrhoea in infants and toddlers. European journal of pediatrics. 2007;166:311–318. doi: 10.1007/s00431-007-0419-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Henker J, Laass MW, Blokhin BM, Maydannik VG, Bolbot YK, Elze M, Wolff C, Schreiner A, Schulze J. Probiotic Escherichia coli Nissle 1917 versus placebo for treating diarrhea of greater than 4 days duration in infants and toddlers. The Pediatric infectious disease journal. 2008;27:494–499. doi: 10.1097/INF.0b013e318169034c. [DOI] [PubMed] [Google Scholar]
- 54.Butler JE, Weber P, Sinkora M, Baker D, Schoenherr A, Mayer B, Francis D. Antibody repertoire development in fetal and neonatal piglets. VIII. Colonization is required for newborn piglets to make serum antibodies to T-dependent and type 2 T-independent antigens. The Journal of Immunology. 2002;169:6822–6830. doi: 10.4049/jimmunol.169.12.6822. [DOI] [PubMed] [Google Scholar]
- 55.Haverson K, Rehakova Z, Sinkora J, Sver L, Bailey M. Immune development in jejunal mucosa after colonization with selected commensal gut bacteria: a study in germ-free pigs. Vet Immunol Immunopathol. 2007;119:243–253. doi: 10.1016/j.vetimm.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 56.Wechsler-Reya RJ, Monroe JG. Lipopolysaccharide prevents apoptosis and induces responsiveness to antigen receptor cross-linking in immature B cells. Immunology. 1996;89:356–362. doi: 10.1046/j.1365-2567.1996.d01-749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hayashi EA, Akira S, Nobrega A. Role of TLR in B cell development: signaling through TLR4 promotes B cell maturation and is inhibited by TLR2. Journal of immunology. 2005;174:6639–6647. doi: 10.4049/jimmunol.174.11.6639. [DOI] [PubMed] [Google Scholar]
- 58.Pone EJ, Lou Z, Lam T, Greenberg ML, Wang R, Xu Z, Casali P. B cell TLR1/2, TLR4, TLR7 and TLR9 interact in induction of class switch DNA recombination: modulation by BCR and CD40, and relevance to T-independent antibody responses. Autoimmunity. 2015;48:1–12. doi: 10.3109/08916934.2014.993027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Burdin N, Van Kooten C, Galibert L, Abrams JS, Wijdenes J, Banchereau J, Rousset F. Endogenous IL-6 and IL-10 contribute to the differentiation of CD40-activated human B lymphocytes. Journal of immunology. 1995;154:2533–2544. [PubMed] [Google Scholar]
- 60.Rousset F, Garcia E, Defrance T, Peronne C, Vezzio N, Hsu DH, Kastelein R, Moore KW, Banchereau J. Interleukin 10 is a potent growth and differentiation factor for activated human B lymphocytes. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:1890–1893. doi: 10.1073/pnas.89.5.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ramsay AJ, Husband AJ, Ramshaw IA, Bao S, Matthaei KI, Koehler G, Kopf M. The role of interleukin-6 in mucosal IgA antibody responses in vivo. Science. 1994;264:561–563. doi: 10.1126/science.8160012. [DOI] [PubMed] [Google Scholar]
- 62.Karlsson H, Larsson P, Wold AE, Rudin A. Pattern of cytokine responses to gram-positive and gram-negative commensal bacteria is profoundly changed when monocytes differentiate into dendritic cells. Infection and immunity. 2004;72:2671–2678. doi: 10.1128/IAI.72.5.2671-2678.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hessle C, Andersson B, Wold AE. Gram-positive bacteria are potent inducers of monocytic interleukin-12 (IL-12) while gram-negative bacteria preferentially stimulate IL-10 production. Infection and immunity. 2000;68:3581–3586. doi: 10.1128/iai.68.6.3581-3586.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Azevedo MS, Zhang W, Wen K, Gonzalez AM, Saif LJ, Yousef AE, Yuan L. Lactobacillus acidophilus and Lactobacillus reuteri modulate cytokine responses in gnotobiotic pigs infected with human rotavirus. Beneficial microbes. 2012;3:33–42. doi: 10.3920/BM2011.0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang H, Wang H, Shepherd M, Wen K, Li G, Yang X, Kocher J, Giri-Rachman E, Dickerman A, Settlage R, Yuan L. Probiotics and virulent human rotavirus modulate the transplanted human gut microbiota in gnotobiotic pigs. Gut pathogens. 2014;6:39. doi: 10.1186/s13099-014-0039-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wen K, Tin C, Wang H, Yang X, Li G, Giri-Rachman E, Kocher J, Bui T, Clark-Deener S, Yuan L. Probiotic lactobacillus rhamnosus GG enhanced Th1 cellular immunity but did not affect antibody responses in a human gut microbiota transplanted neonatal gnotobiotic pig model. PloS one. 2014;9 doi: 10.1371/journal.pone.0094504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Eloe-Fadrosh EA, Brady A, Crabtree J, Drabek EF, Ma B, Mahurkar A, Ravel J, Haverkamp M, Fiorino AM, Botelho C, Andreyeva I, Hibberd PL, Fraser CM. Functional dynamics of the gut microbiome in elderly people during probiotic consumption. mBio. 2015;6 doi: 10.1128/mBio.00231-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Denou E, Rezzonico E, Panoff JM, Arigoni F, Brussow H. A Mesocosm of Lactobacillus johnsonii, Bifidobacterium longum, and Escherichia coli in the mouse gut. DNA and cell biology. 2009;28:413–422. doi: 10.1089/dna.2009.0873. [DOI] [PubMed] [Google Scholar]
- 69.Patzer SI, Baquero MR, Bravo D, Moreno F, Hantke K. The colicin G, H and X determinants encode microcins M and H47, which might utilize the catecholate siderophore receptors FepA, Cir, Fiu and IroN. Microbiology. 2003;149:2557–2570. doi: 10.1099/mic.0.26396-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.