Abstract
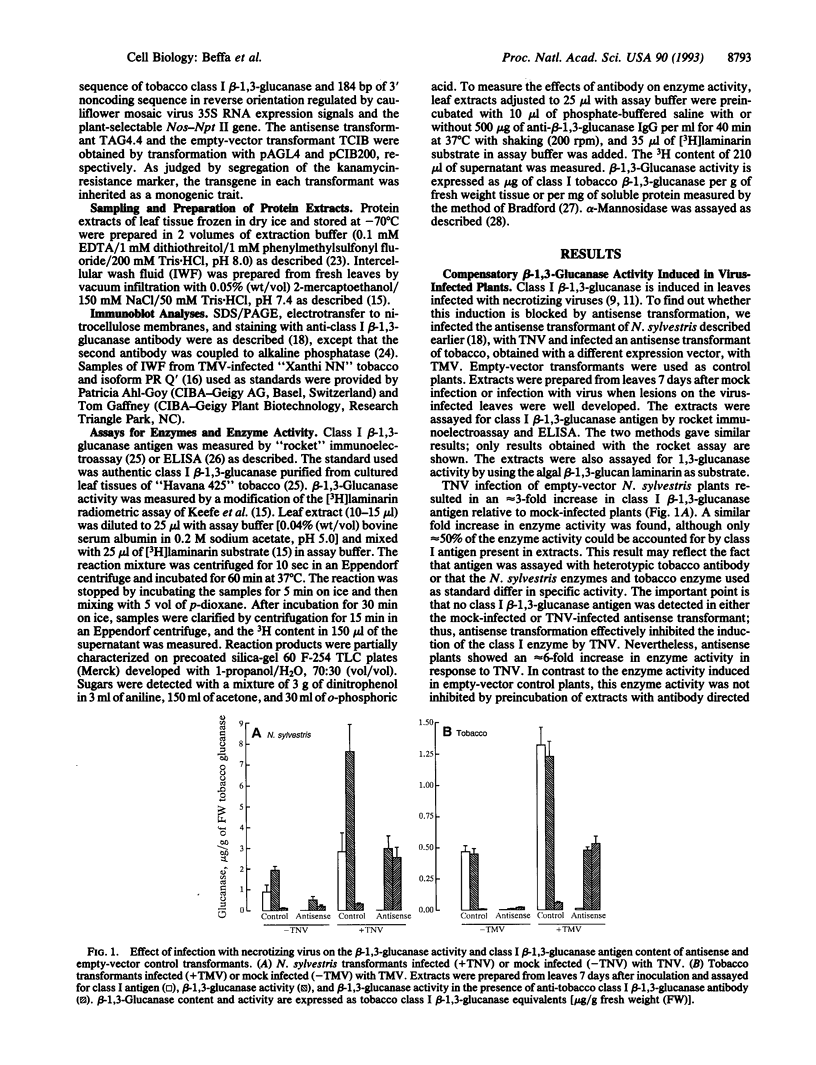
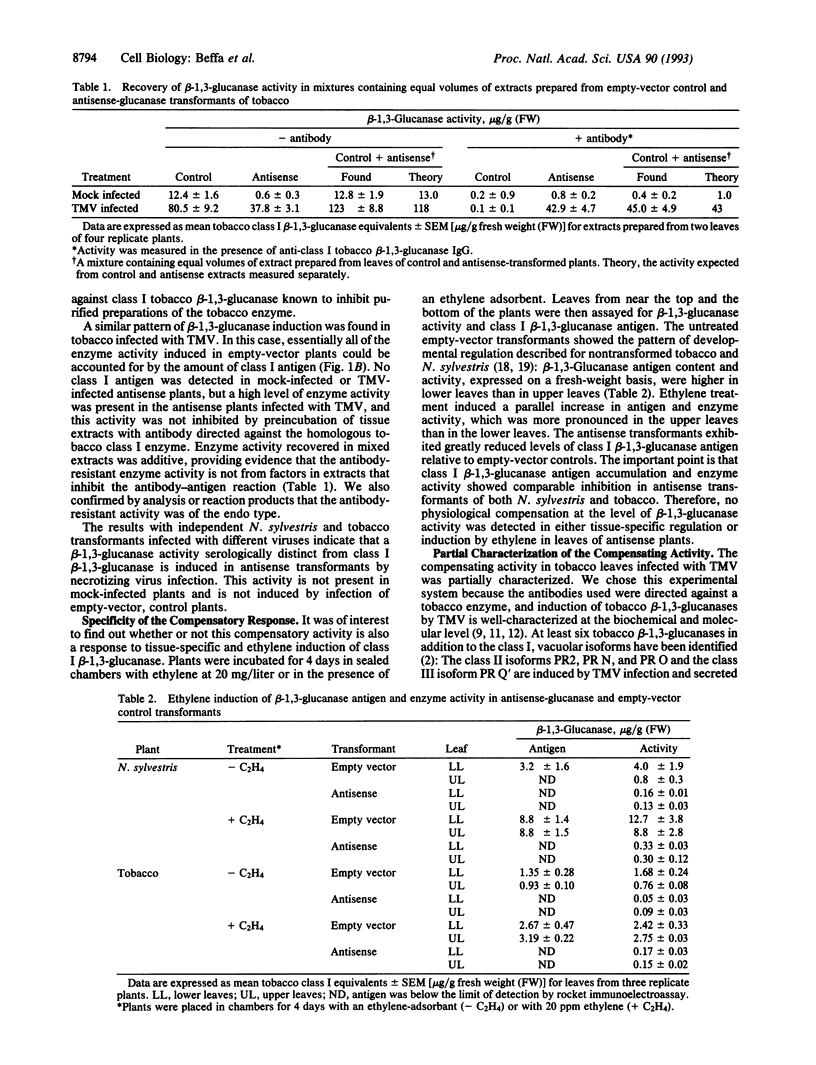
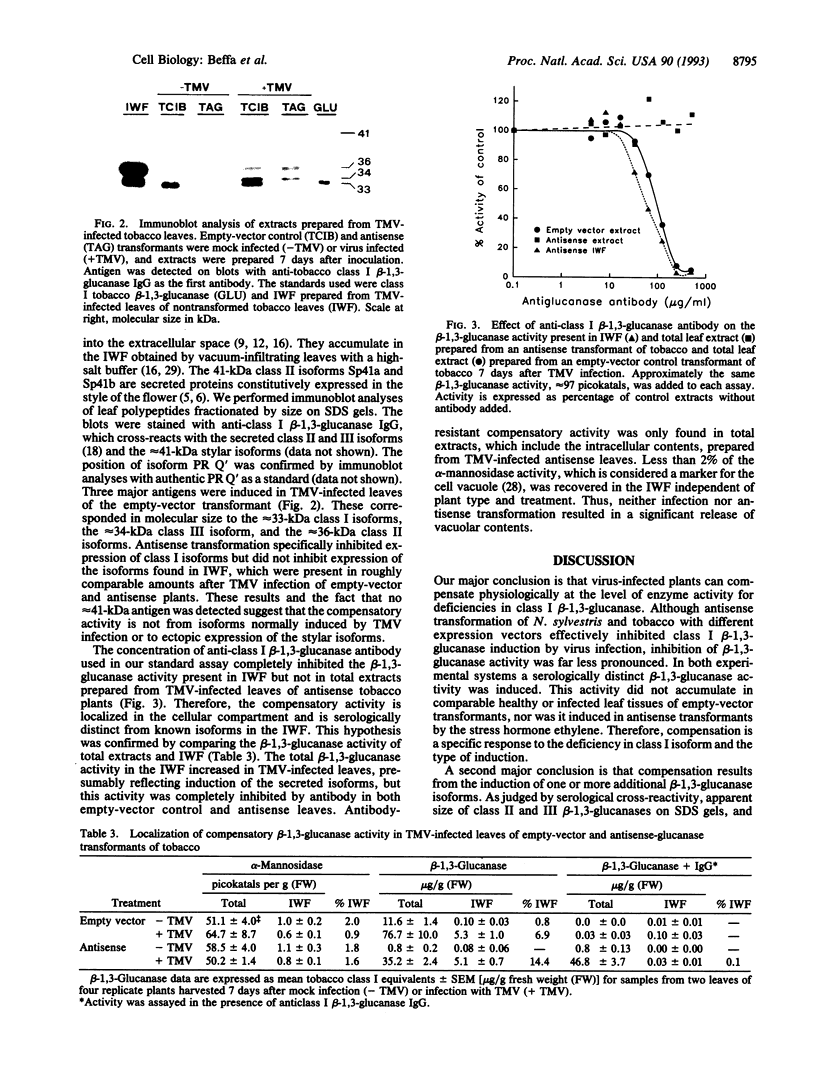
Plant class I glucan endo-1,3-beta-glucosidases (beta-1,3-glucanase; 1,3-beta-D-glucan glucanohydrolase, EC 3.2.1.39) have been implicated in development and defense against pathogen attack. Nevertheless, beta-1,3-glucanase deficiencies generated by antisense transformation of Nicotiana sylvestris and tobacco have little biological effect. We report here that another beta-1,3-glucanase activity is induced in these deficient mutants after infection with necrotizing viruses. Induction of class I beta-1,3-glucanase was markedly inhibited in leaves of N. sylvestris and tobacco antisense transformants infected with tobacco necrosis virus and tobacco mosaic virus, respectively. A serologically distinct beta-1,3-glucanase activity was present in the infected antisense transformants but was absent in both healthy and infected control plants and in antisense transformants treated with the stress hormone ethylene. Immunoblot analyses, localization studies, and measurements of antibody specificity indicate that this compensatory beta-1,3-glucanase activity is an intracellular enzyme different from known tobacco beta-1,3-glucanases. Therefore, plants can compensate for a deficiency in enzyme activity by producing a functionally equivalent replacement--i.e., "ersatz"--protein or proteins. The fact that compensation for beta-1,3-glucanase activity occurs in response to infection argues strongly for an important role of these enzymes in pathogenesis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boller T., Kende H. Hydrolytic enzymes in the central vacuole of plant cells. Plant Physiol. 1979 Jun;63(6):1123–1132. doi: 10.1104/pp.63.6.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bulcke M. V., Bauw G., Castresana C., Van Montagu M., Vandekerckhove J. Characterization of vacuolar and extracellular beta(1,3)-glucanases of tobacco: Evidence for a strictly compartmentalized plant defense system. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2673–2677. doi: 10.1073/pnas.86.8.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNardo S., Voelkel K. A., Sternglanz R., Reynolds A. E., Wright A. Escherichia coli DNA topoisomerase I mutants have compensatory mutations in DNA gyrase genes. Cell. 1982 Nov;31(1):43–51. doi: 10.1016/0092-8674(82)90403-2. [DOI] [PubMed] [Google Scholar]
- Gottlob-McHugh S. G., Sangwan R. S., Blakeley S. D., Vanlerberghe G. C., Ko K., Turpin D. H., Plaxton W. C., Miki B. L., Dennis D. T. Normal growth of transgenic tobacco plants in the absence of cytosolic pyruvate kinase. Plant Physiol. 1992 Oct;100(2):820–825. doi: 10.1104/pp.100.2.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyner A. L., Herrup K., Auerbach B. A., Davis C. A., Rossant J. Subtle cerebellar phenotype in mice homozygous for a targeted deletion of the En-2 homeobox. Science. 1991 Mar 8;251(4998):1239–1243. doi: 10.1126/science.1672471. [DOI] [PubMed] [Google Scholar]
- Kauffmann S., Legrand M., Geoffroy P., Fritig B. Biological function of ;pathogenesis-related' proteins: four PR proteins of tobacco have 1,3-beta-glucanase activity. EMBO J. 1987 Nov;6(11):3209–3212. doi: 10.1002/j.1460-2075.1987.tb02637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsley D. M., Rinchik E. M., Russell L. B., Ottiger H. P., Sutcliffe J. G., Copeland N. G., Jenkins N. A. Genetic ablation of a mouse gene expressed specifically in brain. EMBO J. 1990 Feb;9(2):395–399. doi: 10.1002/j.1460-2075.1990.tb08123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotan T., Ori N., Fluhr R. Pathogenesis-related proteins are developmentally regulated in tobacco flowers. Plant Cell. 1989 Sep;1(9):881–887. doi: 10.1105/tpc.1.9.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauch F., Mauch-Mani B., Boller T. Antifungal Hydrolases in Pea Tissue : II. Inhibition of Fungal Growth by Combinations of Chitinase and beta-1,3-Glucanase. Plant Physiol. 1988 Nov;88(3):936–942. doi: 10.1104/pp.88.3.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mierendorf R. C., Percy C., Young R. A. Gene isolation by screening lambda gt11 libraries with antibodies. Methods Enzymol. 1987;152:458–469. doi: 10.1016/0076-6879(87)52054-7. [DOI] [PubMed] [Google Scholar]
- Neuhaus J. M., Ahl-Goy P., Hinz U., Flores S., Meins F., Jr High-level expression of a tobacco chitinase gene in Nicotiana sylvestris. Susceptibility of transgenic plants to Cercospora nicotianae infection. Plant Mol Biol. 1991 Jan;16(1):141–151. doi: 10.1007/BF00017924. [DOI] [PubMed] [Google Scholar]
- Neuhaus J. M., Flores S., Keefe D., Ahl-Goy P., Meins F., Jr The function of vacuolar beta-1,3-glucanase investigated by antisense transformation. Susceptibility of transgenic Nicotiana sylvestris plants to Cercospora nicotianae infection. Plant Mol Biol. 1992 Aug;19(5):803–813. doi: 10.1007/BF00027076. [DOI] [PubMed] [Google Scholar]
- O'Neill A. E., Reid K., Garberi J. C., Karl M., Flaherty L. Extensive deletions in the Q region of the mouse major histocompatibility complex. Immunogenetics. 1986;24(6):368–373. doi: 10.1007/BF00377954. [DOI] [PubMed] [Google Scholar]
- Ori N., Sessa G., Lotan T., Himmelhoch S., Fluhr R. A major stylar matrix polypeptide (sp41) is a member of the pathogenesis-related proteins superclass. EMBO J. 1990 Nov;9(11):3429–3436. doi: 10.1002/j.1460-2075.1990.tb07550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne G., Ward E., Gaffney T., Goy P. A., Moyer M., Harper A., Meins F., Jr, Ryals J. Evidence for a third structural class of beta-1,3-glucanase in tobacco. Plant Mol Biol. 1990 Dec;15(6):797–808. doi: 10.1007/BF00039420. [DOI] [PubMed] [Google Scholar]
- Pietrzak M., Shillito R. D., Hohn T., Potrykus I. Expression in plants of two bacterial antibiotic resistance genes after protoplast transformation with a new plant expression vector. Nucleic Acids Res. 1986 Jul 25;14(14):5857–5868. doi: 10.1093/nar/14.14.5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochazka M., Kozak U. C., Kozak L. P. A glycerol-3-phosphate dehydrogenase null mutant in BALB/cHeA mice. J Biol Chem. 1989 Mar 15;264(8):4679–4683. [PubMed] [Google Scholar]
- Shinshi H., Wenzler H., Neuhaus J. M., Felix G., Hofsteenge J., Meins F. Evidence for N- and C-terminal processing of a plant defense-related enzyme: Primary structure of tobacco prepro-beta-1,3-glucanase. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5541–5545. doi: 10.1073/pnas.85.15.5541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrash C., Bankier A. T., Barrell B. G., Sternglanz R. Cloning, characterization, and sequence of the yeast DNA topoisomerase I gene. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4374–4378. doi: 10.1073/pnas.82.13.4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward E. R., Payne G. B., Moyer M. B., Williams S. C., Dincher S. S., Sharkey K. C., Beck J. J., Taylor H. T., Ahl-Goy P., Meins F. Differential Regulation of beta-1,3-Glucanase Messenger RNAs in Response to Pathogen Infection. Plant Physiol. 1991 Jun;96(2):390–397. doi: 10.1104/pp.96.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worrall D., Hird D. L., Hodge R., Paul W., Draper J., Scott R. Premature dissolution of the microsporocyte callose wall causes male sterility in transgenic tobacco. Plant Cell. 1992 Jul;4(7):759–771. doi: 10.1105/tpc.4.7.759. [DOI] [PMC free article] [PubMed] [Google Scholar]




