Abstract
The looming food insecurity demands the utilization of nutrient-rich residues from food industries as value-added products. Whey, a dairy industry waste has been characterized to be excellent nourishment with an array of bioactive components. Whey protein comprises 20 % of total milk protein and it is rich in branched and essential amino acids, functional peptides, antioxidants and immunoglobulins. It confers benefits against a wide range of metabolic diseases such as cardiovascular complications, hypertension, obesity, diabetes, cancer and phenylketonuria. The protein has been validated to boost recovery from resistance exercise-injuries, stimulate gut physiology and protect skin against detrimental radiations. Apart from health invigoration, whey protein has proved its suitability as fat replacer and emulsifier. Further, its edible and antimicrobial packaging potential renders its highly desirable in food as well as pharmaceutical sectors. Considering the enormous nutraceutical worth of whey protein, this review emphasizes on its established and emerging biological roles. Present and future scopes in food processing and dietary supplement formulation are discussed. Associated hurdles are identified and how technical advancement might augment its applications are explored. This review is expected to provide valuable insight on whey protein-fortified functional foods, associated technical hurdles and scopes of improvement.
Keywords: Whey protein, Anticancer, Anti-diabetic, Anti-inflammatory, Cardio-protective
Introduction
Protein is the most satiating macronutrient and protein-rich diets are known to exert beneficial effects on body composition and metabolism (Bertenshaw et al. 2008; Chou et al. 2012). Increasing numbers of studies are reporting that the protein fraction in diet is insufficient (for available sources are scarce and expensive). Another study corroborates that protein deficiency is one of the major public health concerns, especially in developing countries (Gomes et al. 2009). In this scenario, prospecting for supplemental sources of dietary protein is of paramount importance. In the face of depleting resources and burgeoning population, repurposing of unconventional food ingredients by innovative technology can establish sustainability (Malik 2007). A slew of obscure and once-vilified agro-industrial by-products such as cereal brans (Pavlovich-Abril et al. 2012), soy pulp (Katayama and Wilson 2008), fruit pomace (Bhushan et al. 2008), seafood wastes (Ben Rebah and Miled 2012), and whey (Sousa et al. 2012) are soaring in popularity as nutrient-dense food components. Exploitation of bioactive ingredients is universally being encouraged (Baiano 2014). This review focuses on food and pharmaceutical relevance of whey proteins.
Whey (the liquid left after milk curdling) was deemed a waste by the dairy industry for decades. The effluent caused major disposal issues, due to its high organic matter and resultant high biological oxygen demand (Ahn et al. 2000). Fortunately, the potential of whey as a resource is being recognized now. Whey has been quantified to contain 15–20 % of total milk proteins and that is too big an amount to let go (Yalçin 2006). With discovery of the high protein content, whey has consolidated its position in food sector. Now, whey protein is valued more than egg, casein and soy protein, for its high nutritional quality and fast absorption (Sindayikengera and Xia 2006). Whey protein is globular with main components as β-lactoglobulins (35–65 %) and α-lactalbulins (12–25 %). The minor ingredients include immunoglobulins (8 %), serum albumins (5 %) and lactoferrin (1 %) (Smithers et al. 1996). Whey protein is a rich source of branched-chain amino acids (leucine, isoleucine, and valine), essential amino acids (cysteine) and peptides as well (Hulmi et al. 2010). Leucine is abundant (50–75 % higher than other protein sources) and it plays key role in the regulation of skeletal muscle protein synthesis (Chen et al. 2014). Whey protein is rich in sulfhydryl amino acid cysteine, a precursor of glutathione, the non-enzymatic thiol antioxidant obtained from diet (Bell 2000). Glutathione plays key role in reducing oxidative stress, regulating cellular processes, imbalance of which can trigger diseases (Trachootham et al. 2008). Lactoperoxidase enzymes, glycomacropeptides (12 %) and lactose are other important components of whey (Abdel-Salam and Effat 2010). Glycomacropeptide is a casein-derived whey peptide, released by rennet during the manufacture of cheese (Neelima et al. 2013). This peptide has many proven benefits such as satiating effect and phenylketonuria management potential. Based on their concentration and attributes, whey proteins are marketed in various forms such as whey protein concentrate (has fat and lactose along with proteins (29–89 %)), whey protein isolate (90 % protein) and whey protein hydrolysate (partially digested for ease of metabolism and hypo-allergenicity) (Barth and Behnke 1997). A broad range of functionality has been assigned to whey protein and its derivatives, such as reduction of oxidative stress, promotion of muscle growth and lean body mass, appetite suppression, hypoglycemia, cardiovascular risk mitigation, phenylketonuria management and protection from ultraviolet (UV) radiation (Sousa et al. 2012). Further, its role in food processing such as emulsifier, texturizer, fat-replacer, encapsulating agent, delivery vehicle and antimicrobial film are being recognized (Hu et al. 2003; Fernández-Pan et al. 2012). Current time has witnessed a deluge of publications on nutraceutical applications of whey proteins. The aim of this review is to keep track of the seminal findings in this field.
Implications of whey proteins
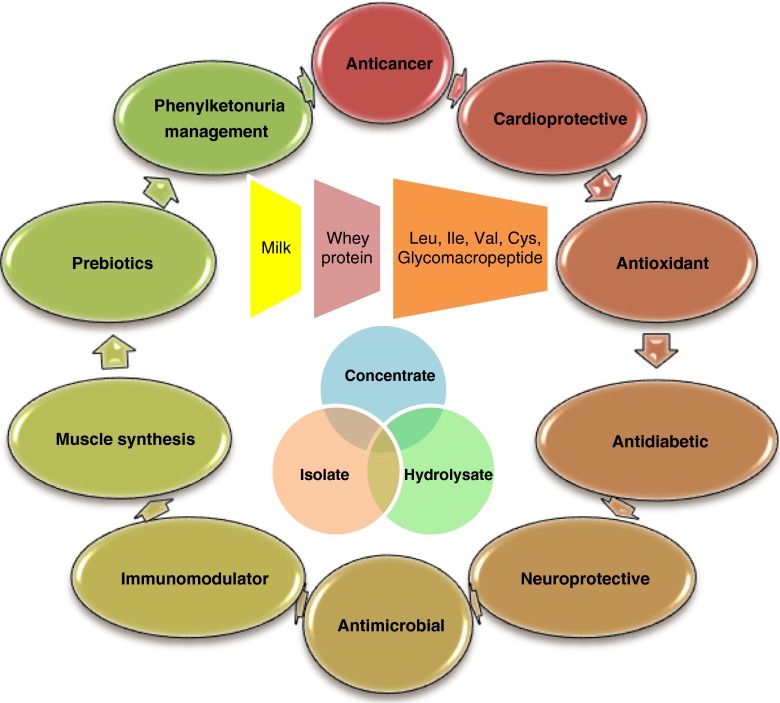
Whey protein in its various derivative forms (concentrate, isolate and hydrolysate) has been verified to encompass diverse physiological properties. Convincing number of therapeutic and food additive roles have been identified in the past decade and potential functions are surfacing regularly. The sections below embody the recent key studies and their profound results. Figure 1 illustrates the whey protein components and the validated health benefits.
Fig. 1.
Constituents and applications of whey protein
Antioxidant, anti-inflammation and hepato-protection
Inflammatory or oxidative stress begets cystic fibrosis, pneumonia, diabetes, cancer, atherosclerosis, myocardial infarction, aging and a host of other degenerative diseases (Essick and Sam 2010). As precursor of the antioxidant glutathione, whey protein holds eminese in nullifying the adverse effects of the stressors. Hyperbaric treatment of whey protein accelerated the release of bioactive peptides, raised intracellular glutathione level and abated the in vitro generation of interleukin IL-8 (this cytokine is believed to mediate pathogenesis of respiratory tract diseases) (Piccolomini et al. 2012). It was observed that one month dietary supplementation with pressurized whey (20 g/day) in cystic fibrosis patients significantly reduced serum C-reactive protein (CRP level is the metric of inflammation in body) level (Lands et al. 2010). Alcalase-hydrolysed whey protein was screened for anti-oxidative peptides. Two fragments, P4 and P4c (a pentapeptide Val-His-Leu-Lys-Pro) demonstrated significant protection of human lung fibroblast MRC-5 cells from H2O2 abuse (Kong et al. 2012). The anti-inflammatory effect of a new enteral diet (tube feeding) MHN-02, rich in antioxidants and whey peptide was evaluated in rats (Takayanagi et al. 2011). The survival rates of the rodents receiving the MHN-02 diet and the control diet were 90 and 55 %, respectively. In the MHN-02 diet group, levels of serum liver enzymes (alanine transaminase and aspartate transaminase) and serum cytokines (IL-1β, IL-6, TNF-α) were significantly lower than in the control group. The oxidative-attenuation potential was inferred from the lowered level of the above oxidative stress biomarkers. Superoxide dismutase activity (conversion of superoxide radical to H2O2 and O2) in the MHN-02 diet group was higher and pathological lesions were lower. It was suggested that supplementation of enteral diets enriched with whey peptide and antioxidants might protect against hepatitis. The evidence supporting the role of whey protein in augmenting glutathione synthesis in neurons and alleviating neurodegenerative maladies was reviewed (Ross et al. 2012). The antioxidant and anti-inflammatory effects of pressurized whey protein isolate and native hydrolysate in human epithelial colorectal adenocarcinoma Caco-2 cells exposed to H2O2 were compared (Piccolomini et al. 2012). Both the pressurized and native hydrolysate inhibited IL-8 secretion and reactive oxygen species (ROS) generation while increasing ferric ion reducing antioxidant power (FRAP) in a dose-dependent manner. The inhibition of IL-8 secretion and ROS generation was significantly greater for pressurized whey protein isolate compared to the native hydrolysate. The results suggested that whey protein isolate hydrolysates can alleviate inflammation and oxidative stress in intestinal cells exposed to oxidative injury, which is further enhanced by their hyperbaric treatment. Peptide mixture derived from the neutrase hydrolysis of cheese whey protein exhibited strong angiotensin-converting enzyme (ACE) inhibition activity. As ACE regulates multiple biological processes and often implicated with cardiovascular and renal complications (Eliseeva 2001), its inhibition by the whey peptides holds relevance. The principal functional peptides were below 1 kDa and accounted for 38 % of the initial protein content in the hydrolysate (Estévez et al. 2012). The effects of whey protein intake on heat shock protein HSP70 (known to confer greater cellular tolerance against stressors) expression were studied in rats. Test animals were fed with different protein diets for 3 weeks. Treadmill running was used as the source of stress. Significant HSP70 expression in the soleus, gastrocnemius and lungs of the whey protein hydrolysate-fed rats was observed compared to whey protein or casein-fed rats. Protein carbonyls, biomarkers of oxidative stress were lower in the group that consumed whey protein hydrolysate. From the result, it was inferred that the consumption of whey protein hydrolysate enhances HSP70 expression (De Moura et al. 2013). An extension of this study indicated that the whey protein hydrolysate is caoable of augmenting cell survival factors such as HSP90 and vascular endothelial growth factor (VEGF) (Moura et al. 2014). The ameliorating effect of whey protein on oxidative stress in rats was studied. The test animals subjected to iron overload were given placebo or whey protein. After 6 week, the iron overload group showed a reduction in radical scavenging capacity with a concomitant increase in lipid peroxidation. On the other hand, the concentration of blood glutathione level was significantly higher in the iron overload supplemented with whey group compared to only iron overload group. Iron is a known genotoxic agent, capable of DNA aberration and consequent carcinogenesis (Knöbel et al. 2007). Whey protein reversed the iron overload-induced DNA damage in leukocytes and colonocytes (Kim et al. 2013). As the detrimental effect of iron is ROS-mediated (Park et al. 2007), whey protein is assumed to combat them. The pathogen Pseudomonas aeruginosa is capable of infecting multiple organs and system in human. Lungs colonization by the pathogen results in chills, fever, cough and dyspnea (difficulty in breathing) (Lyczak et al. 2002). The feasibility of using pressurized whey protein for lowering the risk of pulmonary infection by this pathogen was investigated (Kishta et al. 2013). Decreased level of inflammatory response, oxidative stress, and lung damage in the pressurized whey-fed mice was observed. This outcome corroborated previous reports that whey protein subjected to hyperbaric treatment has superior biological attributes. Shielding the airway proteins from oxidation and stimulating leukocytes to kill the pathogens was derived to be the underlying mechanism. The antioxidant effect of whey protein hydrolysate against paracetamol-induced hepato-nephrotoxicity was evaluated in mice. The animals on paracetamol were administered with the hydrolysate (intraperitoneally 4 mg/kg or orally 8 mg/kg) for 4 days followed by euthanizing. Analysis of the liver homogenate showed increased level of antioxidant enzymes (catalase, superoxide dismutase, and glutathione peroxidase) and lowered thiobarbituric acid reactive substances (TBARS). The hydrolysate lowered the concentration of oxidative biomarkers alkaline phosphatase, glutathione pyruvate transaminase and creatinine and restored the normal level of blood urea nitrogen in the sera of mice subjected to paracetamol abuse (Athira et al. 2013). The in vitro free radical scavenging activity of sheep whey protein was determined. Results showed that the protein efficiently scavenged 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid (ABTS) and hydroxyl radicals, increasing glutathione level (Kerasioti et al. 2014). Chymotrypsin-generated whey protein hydrolysate had better DPPH-scavenging and ferrous-chelating capacity than whey protein isolate. The former proved to be an effective anti-fatigue agent in mice model (Liu et al. 2014a). Longer swimming time and haematological parameters (higher level of glucose, free fatty acids, glycogen, superoxide dismutase and glutathione peroxidase and lower concentration of lactate) led to this conclusion.
Anticancer
Several studies have suggested that whey protein may confer benefits on cancer patients. Further it has been demonstrated that the hydrolysis of the protein might improve the anticancer efficacy. Rat with colon cancer, when fed with whey protein hydrolysate developed significantly less macroscopic and microscopic tumours compared to the group, fed with untreated whey protein (Attaallah et al. 2012). Anticancer effect of whey protein was investigated using melanoma B16F10 cells as the model. Caspase-3 expression increased significantly in the whey protein isolate-containing media (Castro et al. 2009). The role of Caspase-3 in mediating apoptotic cell death has been well-documented (Takata et al. 2001). A 48-year-old Caucasian female with recurrent cervical cancer was administered with whey protein (10 g thrice daily) and a weekly intra-muscular injection of testosterone enanthate before and during the standard-of-care (SOC) chemotherapy. As a result of the combination therapy, improvement of lean body mass, physical activity, and overall quality of life was observed (Dillon et al. 2012). The protective effect of whey protein hydrolysate against oxidative damage on rat pheochromocytoma PC12 cells was studied. At a dose of 100–400 μg hydrolysate/ml, the viable cells increased by 20–30 % compared to those incubated in H2O2, suggesting antioxidant potential of the former (Zhang et al. 2012).
Immunomodulation
Whey protein concentrates enhance innate mucosal immunity during early life and have a protective role in some immune disorders (Pérez-Cano et al. 2007). The incidence of atopic dermatitis (a chronic skin disease characterized by swollen, scaly and itchy rashes) is increasing worldwide, infants being a major vulnerable group. A meta-analysis of systematic review revealed that incidence of atopic dermatitis was considerably lower among infants in the partially hydrolyzed whey-based formula group compared to the bovine milk group (Alexander et al. 2010). The finding suggested that whey-based formula might protect infants from atopic dermatitis. The effect of whey protein concentrate on blood parameters, plasma cytokine profiles, immune cell proliferation and migration was investigated in mice model (Badr et al. 2012b). The plasma levels of IL-1α, IL-1β, IL-10 and TNF-α and the levels of ROS and cholesterol were significantly lowered in the whey protein-treated group compared to the control group. In the treated group, levels of IL-2, IL-4, IL-7, IL-8 and glutathione concentration significantly improved, also the ability of lymphocytes, monocytes and macrophages to proliferate in response to stimulation with different antigens increased. Cytokine CC chemokine ligand-21 (CCL21) and CXC chemokine ligand-12 (CXCL12) attracts and tethers immune cells towards them. It was observed that in vitro migration of B cells, T cells and dendritic cells towards the two cytokines significantly increased in whey protein-treated group compared to the control (Badr et al. 2012b). Psoriasis is chronic autoimmune disease causing thick skin, dry scales and red patches. It was investigated whether bioactive whey protein isolate can increase glutathione levels and resultantly combat the severity of systemic inflammation due to psoriasis. The intake of 20 g/day whey protein isolate improved the conditions of the patients (Prussick et al. 2013).
Cardioprotective and hypotensive
Whey protein intake reduces cardiovascular disease (ischemic stroke) risk, but precise role of their peptides in regulation of vascular endothelial function has not been adequately investigated. Whey-derived extract (NOP-47) ingestion increased impaired brachial artery flow-mediated dilation (improved endothelial function). Postprandial plasma amino acids level increased. The improvement in arterial dilation was found to be independent of the circulating vasoactive compounds such as nitric oxide, prostacyclin and endothelium-derived hyperpolarizing factor. It was inferred that cardiovascular risk might be alleviated using rapid-absorbable extracts derived from whey (Ballard et al. 2013). The effects of whey protein supplementation and resistance training on antioxidant status and cardiovascular risk factors were examined in overweight young men (Sheikholeslami Vatani and Ahmadi Kani Golzar 2012). Findings suggested the synergistic effect of resistance training and whey consumption as manifested in higher total antioxidant capacity, glutathione and HDL levels.
Gut function and prebiotic
Gut dysfunction (delayed gastric emptying, abnormal motility patterns, and weak intestinal barrier) is a serious issue in critically-ill patients. It was suggested that whey protein fortification might impart inflammation and improve tolerance towards enteral nutrition (Abrahão 2012). In order to exert its therapeutic property, lactic acid bacteria and yeast need to be viable. Ensuring their high survival rate in the inhospitable gastrointestinal environment poses a critical challenge. Further, storage period tends to inhibit the probiotic strains. Whey protein gels have shown efficacy in protecting the microbes against the adverse conditions. The efficacy of encapsulating Lactobacillus rhamnosus CRL 1505 in whey protein and pectin to improve the survival rate in low pH and bile milieu was evaluated (Gerez et al. 2012). Based on results, pectin beads with a whey protein layer could be used as probiotic carrier in acidic functional foods. A novel peptide Gly-Tyr with a specific calcium-binding capacity was isolated from whey protein hydrolysate. The chelating mechanism of the peptide was investigated and the major binding sites were found to be the oxygen atom of the carbonyl group and nitrogen of the amino or imino group (Zhao et al. 2014). This peptide might boost calcium absorption in the gastrointestinal tract and developing the peptide-calcium chelate as a supplement for enhanced calcium bioavailability seems promising. The role of whey protein in conferring stability to probiotics and prebiotics was investigated. Lactobacillus acidophilus and Bifidobacterium were viable in yogurt beverages stabilized with high-methoxyl pectin and whey protein concentrate (Walsh et al. 2014).
Whey protein isolate and alginate microparticles proved suitable as oral delivery systems for probiotic yeast Saccharomyces boulardii. The study conducted using simulated gastric and intestinal fluid demonstrated survival rate of 40 % for the encapsulated yeast compared to 10 % for free yeast cells (Hébrard et al. 2010). The effect of whey protein on the survival of S. boulardii within spray-dried microcapsules was investigated further. It was concluded that the early crust formation at a given sample pH and temperature during spray drying improves the viability of S. boulardii within microcapsules (Duongthingoc et al. 2013).
Obesity management
Dietary adjustment has been shown to fight obesity and in this regard, whey protein is considered helpful. A 1-week trial with mice fed ad libitum with high-fat diet containing generous amount of whey protein, or supplemented with leucine was performed. It was inferred that the ameliorating effects of the protein-rich diet on metabolic disorders are precisely due to modulation of satiety mediated by liver lipogenesis attenuation (Freudenberg et al. 2013). A 12-week study showed that whey protein concentrate preloads conducted 30 min prior to the ad libitum main meal exerts stronger beneficial effects than that of soy protein isolate on appetite, calorie intake, anthropometry (body mass index and waist circumference), and body composition (body fat mass and lean muscle) of obese men (Tahavorgar et al. 2014). Understanding the modulation of anorectic and orexigenic hormone levels by whey proteins might provide further insight in this context.
Anti-diabetic
Diabetes remains a major public health issue of epidemic stature that begets many complications such as loss of vision, angiopathy, reduced blood flow leading to tissue hypoxia and ulcers with difficult healing (Casqueiro et al. 2012). Type-2 diabetes is treated both by controlled diet and hypoglycaemic drugs. Whey protein have been demonstrated to reduce serum glucose level in healthy individuals, maintain muscle mass, boost the release of satiety hormones (cholecystokinin, leptin, and glucagon like-peptide 1 (GLP-1)) and lower the secretion of hunger hormone ghrelin (Sousa et al. 2012). It was shown that cysteine, a key component of whey protein could be used as an ancillary therapy in glycaemia and vascular inflammation control in the diabetics (Jain 2012). The effects of whey proteins on the recuperation of diabetic wounds in an induced type I diabetic mouse model was investigated (Badr et al. 2012a). Compared with untreated diabetic mice, supplementation with whey proteins significantly accelerated the closure of diabetic lesions by restricting the access of inflammatory cytokines by maintaining normal IL-10, TNF-α, IL-1β and IL-6 levels. Further, the supplementation modulated the expression of the chemokines MIP-1α, MIP-2, KC, CX3CL1 and TGF-β in wound tissue compared to untreated diabetic mice. The insulin secreting effect of whey protein was investigated and increased serum levels of leucine, isoleucine, valine, lysine, threonine was inferred as the core mechanism (Salehi et al. 2012). The action of whey protein on insulin secretion from isolated mouse Langerhans islets was studied. Whey protein fractions (whey isolate and whey hydrolysate) added to a fat-rich meal lowered postprandial triglyceride responses in type 2 diabetic subjects. Both components provoked a higher insulin response (Mortensen et al. 2012). A hydrolyzed whey protein-based supplement was fed to rats for 30 days. It resulted in a higher leucine level followed by increased insulin level (Toedebusch et al. 2012). It was reviewed that whey protein is metabolized in gut as peptides and amino acids which stimulate gut hormones (cholecystokinin, peptide tyrosine tyrosine (PYY)) and incretin hormones (gastric inhibitory peptide and GLP-1) that further induce insulin secretion from the pancreatic β-cells. It was further reported that the peptides might be inhibiting dipeptidyl peptidase-4 (DPP-4) (an inhibitor of incretins) in the proximal gut, preventing incretin degradation (Jakubowicz and Froy 2013). The mechanism of whey protein action on the reduction of postprandial spikes in glucose was evaluated through a randomized, crossover study. During the study spanning 230 min, whey protein resulted in lowered plasma glucose, insulin and C-peptide level but elevated GLP-1 and PYY level than the glucose preloads. Results suggested that pre-meal consumption of whey protein lowers post-meal glycaemia by both insulin-dependent and insulin-independent mechanisms (Akhavan et al. 2014). The effect of whey protein and its hydrolysate-leucine supplementation in non-obese, insulin-resistant rat model was studied. Results showed that both forms of protein can improve insulin resistance (Tong et al. 2014).
Resistance exercise and muscle synthesis
Resistance exercise, eccentric (muscle lengthening), isometric (non-lengthening) and concentric (shortening) contractions cause skeletal muscle damage and generate inflammatory markers (muscle proteins in blood) (Morton et al. 2009). Anabolic interventions with protein hydrolysates and amino acid supplements have been evidenced to expedite the repair. Leucine-derived metabolite β-hydroxy-β-methylbutyrate ingestion has proved beneficial in recovery from the soreness. Resistance exercise (weight-lifting) elevates oxidation products in plasma, perturbs leukocyte redistribution and leukocyte functionality (Freidenreich and Volek 2012). Whey protein isolate nanoparticles were prepared using ethanol desolvation and their capacity to incorporate ZnCl2 was analysed. The amount of zinc incorporated in the particle suspensions was within the range of daily zinc requirements for healthy adults. Also, the nanoparticles remained stable after 30 days of storage at 22 °C (Gülseren et al. 2012). Cell surface glucose transporter 4 (GLUT 4) is the major glucose transporter isoform expressed in skeletal muscle that determines the rate of muscle glucose transport in the cell membrane, in response to insulin and muscle contraction. Whey protein hydrolysate was evaluated for its ability to translocate GLUT 4 and accumulate them in the membrane thereby augmenting glucose trapping by skeletal muscle. The amino acid l-isoleucine and the peptide l-leucyl-l-isoleucine in the hydrolysate contributed the most (Morato et al. 2013). The effect of whey supplementation in comparison to casein diet, on the recovery of muscle functional properties such as contractility, extensibility, elasticity and excitability was investigated in rats. The whey protein diet promoted a faster recovery from injury sustained due to isometric as well as concentric exercise in comparison to the casein diet (Martin et al. 2013). The effect of the supplementation of a beverage with varying doses of leucine or a mixture of branched chain amino acids on myofibrillar protein synthesis after resistance exercise was assessed. Results showed that low-protein (6.25 g) beverage can be as effective as a high-protein dose (25 g) at stimulating myofibrillar protein synthesis rates when supplemented with a high (5 g) leucine content (Churchward-Venne et al. 2014). As leucine comprises 10 % of the total whey amino acid, the latter appears important for augmenting muscle hypertrophy. Health parameters, performance and body composition effects produced by 12 week intake of hydrolysed whey protein were compared in players. Intervention with the hydrolysed whey protein resulted in significant reduction in the muscle damage markers (creatine kinase and lactate dehydrogenase) (Lollo et al. 2014). Muscle mass growth by daily consumption of whey protein was compared with that of soy protein, using a randomized study on subjects undergoing resistance exercise (Volek et al. 2013). Lean body mass gains were significantly high in whey protein than soy protein group and the remarkable response was correlated with the elevated levels of leucine and faster absorption.
Phenylketonuria management
Phenylketonuria is a genetic disorder (mutation in the phenylalanine hydroxylase gene) causing excessive accumulation of phenylalanine (Williams et al. 2008). Failure to use phenylalanine leads to many health complications. Key management strategy includes low phenylalanine diet. It was reported that whey protein glycomacropeptide with low phenylalanine content is suitable for incorporation into the diets of these patients (van Calcar and Ney 2012). A study on phenylketonuria mice showed that glycomacropeptide diet significantly attenuated the adverse effects assoacited with this disease. Energy expenditure, food intake, systemic inflammation markers and plasma level of phenylalanine were lowered more efficiently than that of casein diet (it has high phenylalanine content). Moreover, total fat mass and the respiratory exchange ratio were significantly lower in phenylketonuria mice fed with glycomacropeptide (Solverson et al. 2012). Osteopenia (skeletal fragility due to reduced mineral content of bones) is a complication associated with phenylketonuria (Demirdas et al. 2015). It was reported that glycomacropeptide diet ameliorated this condition. Bone health improved and higher radial bone growth was observed (Ney et al. 2014). The above studies indicate that glycomacropeptide is a better alternative to the regular synthetic amino acids, for it improves the taste and satiety of diet leading to better patient compliance (Strisciuglio and Concolino 2014).
Skin protectant
Photoaging or dermatoheliosis is the adverse changes in skin caused by long-term exposure to UV radiations (Lowe et al. 1995). The effects of consuming whey peptides (200 and 400 mg/kg, twice daily) on chronic UV-B radiation-induced skin alterations (thickness, suppleness and wrinkle formation) were investigated in mice models. The peptides ameliorated photoaging by inhibiting the increase in dermal stiffness, wrinkling and melanin granule formation. The peptides lowered the expression of matrix metalloproteinase (MMP-2 and pro-MMP-9) and VEGF. Also, they averted increase in the number of apoptotic, Ki-67-positive and 8-hydroxy-2′-deoxyguanosine (8-OHdG)-positive cells induced by chronic UV-B irradiation. The peptides prevented type IV collagen (most abundant structural basement membrane component of tissue) degradation, angiogenesis, proliferation and DNA damage caused by irradiation (Kimura et al. 2014). Scavenging of free radicals might be the corrective mechanism.
Emerging ameliorative roles
Apart from the above therapeutic roles, whey proteins are proving effective in management of several other health issues. An in vitro study indicated that the whey protein stimulates proliferation and differentiation of osteoblasts, thus it might have therapeutic role in osteoporosis (Xu 2009). Periodontal disease is a common chronic infection in adults (Loesche and Grossman 2001). Consumption of diet rich in Ca, casein and whey protein had positive correlation with periodontitis prevention (Adegboye et al. 2015). Oral supplementation of whey protein concentrate reduced the hepatitis C virus (HCV) burden in a clinical trial. Anti-inflammation, augmentation of hepatic and phagocytic functionality was discerned to be the underlying mechanism (Elattar et al. 2010). Discovery of other prospective roles constitutes an exciting avenue.
Food additives
Whey protein has found favor in food applications owing to its gelation, thermal stability, foaming and emulsification properties. This protein is used for food quality improvement in multifarious ways such as sensorial enhancement and texture promotion. Several studies have reported the structural and nutritional impact of whey protein such as yoghurt, beverage, pasta, energy bars and bakery preparation. This study demonstrated the ability of non-heated whey protein-high methoxyl pectin complex to act as fat-replacer and texturing agent in reduced-fat yoghurt (Krzeminski et al. 2014). The composite-amended skim milk formulations imparted a texture akin to whole-fat yoghurt. This study investigated the characteristic of emulsions stabilized by whey protein and reported good stability. Droplets merger generated high molecular weight protein aggregates which reduced the emulsifying capacity. High-pressure (20–100 MPa) homogenization and a greater number of passes resulted in more stable emulsions (Kuhn and Cunha 2012). The influence of milk protein-based ingredients on microstructure of probiotic yogurt (amended with a commercial starter culture and Bifidobacterium lactis Bb12) during a 28 day refrigeration period was studied (Akalın et al. 2012). Fortification with sodium calcium caseinate (2 %) improved the firmness, adhesiveness and viscosity of yoghurt. However, whey protein concentrate (2 %) enhanced water-holding capacity more than the caseinate. Whey protein concentrate-fortified yoghurt had thicker texture and lower syneresis (collection of whey on yoghurt surface) issues. A date bar fortified with whey protein and another plant protein was prepared for school-going children. The nutritional profile of the product was optimized with response surface methodology (RSM) and 6.05 % whey protein concentrate proved to be ideal for the purpose (Nadeem et al. 2012). Pearl millet supplemented with barley flour, whey protein concentrate and carboxy methyl cellulose and water was used to make pasta (Yadav et al. 2014). Consistent research might result in many whey-protein based food products.
Encapsulation, edible coating, active packaging, delivery systems
A range of food, additives, essential oils and vitamins can be encapsulated, packaged in whey protein gel for stability augmentation and rancidity reduction. Sustaining the accessibility of iron in fortified foods often poses bottlenecks. To handle this issue, the cold-set gelling ability of whey protein isolate was explored. In this pursuit, iron was entrapped in presence of ascorbate using cold-set gelation (Martin and de Jong 2012). TNO Intestinal Model (TIM) was studied to optimize the ratio of iron and ascorbate. The cold-set gelation of whey protein provoked by iron and ascorbate proved fruitful in boosting the recovery and in vitro bio-accessibility of iron (increased from 10 to 80 %). Gel strengthening effect of ascorbate was attributed to the superior encapsulation efficiency and controlled release of iron. Another study compared the encapsulation competency of the desolvated suspension of whey protein isolate-nanoparticle with or without methoxyl pectin (Gülseren et al. 2012). The suspension with pectin resisted homogenization and rendered stability. Also, this complex demonstrated higher interfacial pressures during storage at pH 3, compared to the whey protein isolate-nanoparticle suspension without pectin, suggesting their candidacy as surfactants. Essential oils extracted from botanical sources are abundant in phenolic acids and flavonoids. They possess multiple pharmacological benefits but are prone to instability. Encapsulation is deemed to be a solution in this regard. The feasibility of microencapsulating cardamom essential oil in whey protein isolate was explored. At 30 % concentration whey protein isolate microcapsules retained the essential oil optimally throughout storage at the studied temperatures. Also, whey protein isolate microcapsules had smooth texture, regular contours and spherical shapes (Mehyar et al. 2014). Appreciable encapsulation of folic acid was achieved using whey protein concentrate as matrix. The encapsulation was traced down to the favourable interactions between the protein and folic facilitating incorporation of the vitamin. Empirical results showed that whey protein concentrate serve better than the commercial resistant starch for folic acid stability (Pérez-Masiá et al. 2015). This study assessed the oxygen-scavenging potential of an edible whey protein isolate film impregnated with ascorbic acid. The film with good tensile strength reduced oxygen permeability which might be used to extend the shelf life of a wide variety of oxygen-sensitive products by eliminating headspace oxygen as well as oxygen diffusion through the barrier (Janjarasskul et al. 2011). Nuts are healthy snacks but have poor shelf life. Edible coating have shown efficacy in delaying the onset of rancidity in them. Implication of whey protein isolate in reducing oxidation and rancidity of walnuts and pine nuts were studied (Mehyar et al. 2012). When used along with pea starch and carnauba wax, it generated a non-homogenous film. The nuts coated with this film had better sensory characteristics that untreated nuts, when stored at 25 °C for 12 days. Active packaging involves additives which provide benefits beyond basic barrier properties. Antimicrobial packaging (the use of edible films to release antimicrobial constituents) is a form of active packaging. Antimicrobial properties of whey protein isolate films containing spice (oregano, rosemary, garlic etc.) essential oils are desirable for this objective. Whey protein was evaluated to reinforce oxygen barrier properties of commercial compostable plastic film. The coating not only upgraded the barricade but also bestowed fast biodegradability (Cinelli et al. 2014). Vitamin D fortification of cheese is anticipated to enhance its nutritional value. However, the long ripening period tends to degrade the vitamin. This study was conducted to increase the retention of vitamin D3 in Cheddar cheese by incorporating it as an oil-in-water emulsion to obtain a fortification limit of 280 IU/serving. When an emulsifier made of sodium caseinate, calcium caseinate and whey protein was used about 74–78 % vitamin holding was reported (Tippetts et al. 2012). Stable nanoemulsions are sought after for food and pharmaceutical applications. Several studies have shown that food proteins can be developed as emulsifiers. In this study nanoemulsions were stabilized by whey protein isolates as verified under various ionic strengths and thermal treatments which maintained stability during the storage period. The result suggested that it may not be necessary to use a polysaccharide such as carrageenan, chitosan, xanthan, gum arabic or alginate as a second layer for the preparation of nanoemulsions when a relatively high protein concentration was used as the emulsifier (Li et al. 2014).
Technical advancement
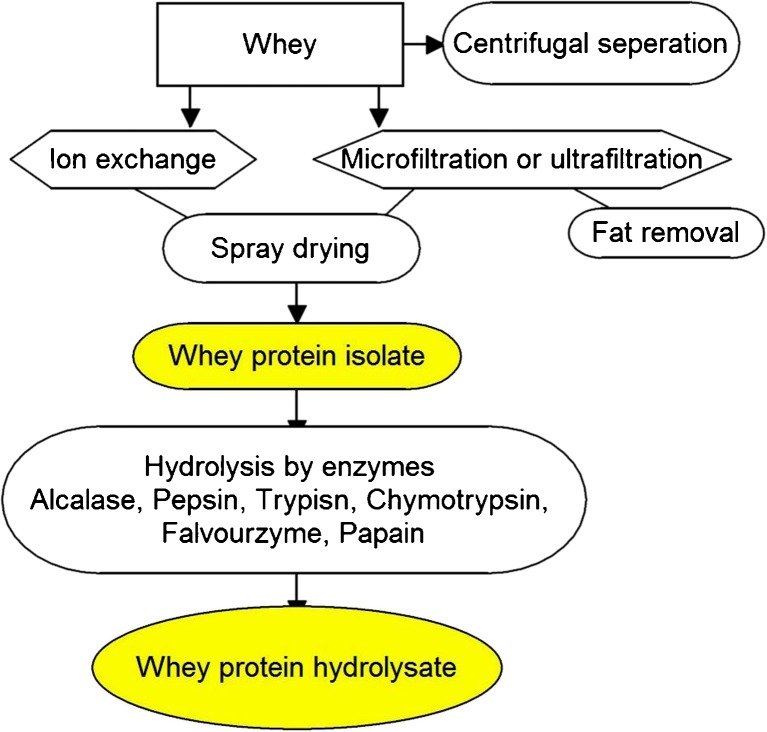
Whey proteins are extracted from whey by membrane filtration followed by spray drying. Figure 2 shows the crucial steps in generation of various whey protein products. The concentrate does not undergo rigorous filtration steps, so they contain some fats and lactose. However, the isolate is manufactured using ultra-filtration, spray drying and evaporation. Ultra-filtration is an ideal technology for preparing whey concentrate. Assorted enzymes are used to hydrolyze whey protein concentrate to reduce its antigenic fractions and increase the peptides content. Alcalase, flavourzyme, trypsin, chymotrypsin or papain are recruited to degrade major antigenic fractions (β-lactoglobulin) in the concentrate (Liu et al. 2014b; Zhang et al. 2012). Cutting-edge techniques such as laser diffraction spectroscopy, rheology, tribology, scanning electron microscopy have facilitated characterization of whey components. Supercritical carbon dioxide was developed to fractionate α-lactalbumin and β-lactoglobulin from whey protein isolates. At 5.5–34 MPa and 60–65 °C, solubilised supercritical carbon dioxide decreased solution pH and induced the formation and precipitation of α-lactalbumin aggregates (Bonnaillie and Tomasula 2012). Combination of membrane technology is expected to increase cheese yield by incorporation of the whey proteins. Flavor plays a critical role in extensive use of whey protein ingredients. Off-flavours in the protein negatively influence consumer acceptance. Cheddar cheese whey is yellow colored due to the additive pigment annatto (benign, orange-coloured dye from plant origin). Bleaching to generate colourless whey is important. Currently, hydrogen peroxide and benzoyl peroxide are utilized for bleaching whey before spray drying to produce neutral-coloured whey protein, which often gives off-flavour. Wide usage of whey protein requires a bland-tasting final product (Croissant et al. 2009). Lipid oxidation products (hexanal, heptanal, octanal, and nonanal) are primary contributors to the unpleasant odor. Adequate understanding of flavor formation and strategy to minimize it seems important. A study suggested that the addition of antioxidant ascorbic acid to liquid whey before subsequent processing might be beneficial in suppressing the flavour of spray-dried whey protein (Liaw et al. 2010). Also, the acidification of liquid whey protein (to pH 3.5) before spray drying (at 5.5–6.5) decreased off-flavour of the dried whey protein concentrate. Based on the results, it was inferred that the foul odour is generated due to interaction of the protein with volatile compounds. At low pH, production of the aroma compounds such as hexanal, heptanal, nonanal, decanal, dimethyl disulfide, and dimethyl trisulfide were substantially attenuated (Park et al. 2014). Limiting the off-flavor production by reduced proteolysis of the why protein by above discussed methods can enhance consumer acceptance.
Fig. 2.
Key steps in generation of various whey protein products
Quality improvement/combination with other supplement
Bitterness of whey protein hydrolysate is a detriment in its wider applications in food and pharmacy. Enzymatic hydrolysis leads to break down of α-lactalbumin, β-lactoglobulin, serum albumin and β-casein, liberating the bitter peptides. Different inhibitors capable of masking the unpleasant taste of whey protein hydrolysate were studied (Leksrisompong et al. 2012). Sucralose, fructose, sucrose, adenosine 5′ monophosphate (5’AMP), adenosine 5’monophosphate disodium, sodium acetate, monosodium glutamate, sodium gluconate and sodium chloride were found effective in concealing the bitterness. However, it is important to trace down the peptides compromising the taste and remove them. In this regard, four bitter peptides were identified in the hydrolysate by sensory-guided fractionation techniques (combination of ultra-filtration and chromatography). LC-TOF-MS/MS analysis identified the constituent amino acids in the peptides (Liu et al. 2014b). Supplementing whey protein concentrates with spirulina (blue-green freshwater algae rich in protein) improved antioxidant, radical scavenging and metal-chelating activities in dose-dependent manner, in both in vitro and in vivo studies. In rat models, CCl4-induced liver damage amelioration was more pronounced in the group who received the combination (Gad et al. 2011). Some instances of allergic conditions have been associated with whey protein. Children constitute the vulnerable group for cow milk protein allergy (CMPA), 2–7 % of them developing this intolerance (Solinas et al. 2010). Atopic dermatitis is a major manifestation of this allergy (Botteman and Detzel 2015). Respiratory allergies include asthma, rhinitis, wheezing, coughing, and laryngeal edema (Hochwallner et al. 2014). Gastrointestinal upsets like diarrhea, abdominal pain, nausea, vomiting are observed (Kattan et al. 2011). Also, the milk ingestion can provoke anaphylactic reactions in sensitive infants (Ameratunga and Woon 2010). It necessitates assessment of allergenicity risks prior to administration of whey protein-based supplements. Heat treatment is known to reduce the antigenicity of whey proteins. The effect of heat in moderating the antigenicity of α-lactalbumin and β-lactoglobulin in whey protein isolate was investigated through indirect competitive enzyme-linked immunosorbent assay (ELISA). Above 90 °C, the antigenicity of both proteins showed significant drop (Bu et al. 2009). Yet it is a predominant risk factor for infants. Enzymatic hydrolysis also reduces the allergenicity. As mentioned in the technical advancement section, an array of enzymes are used to decimate the antigenic fragments by hydrolysis (Zhang et al. 2012; Liu et al. 2014b). In vitro enzymatic hydrolysis of whey protein concentrates was performed using trypsin. Mice fed with the hydrolysate demonstrated increased IFN-γ secretion, which suggested that hydrolysis might be crucial in lowering allergenicity of whey protein concentrate (Duan et al. 2014). However, the hydrolysis often produces bitter peptides and destroys the biological function of the protein. Ultrafiltration is used to remove the large residual peptides causing the bitterness (Liu et al. 2014b). So, at present scenario, there is a trade-off between allergenity reduction and bitterness increment.
Conclusions
The findings above testify that whey is no more a mere by-product of dairy processing. Increasing number of nutritionists are endorsing whey protein as an excellent nutrient. An array of whey protein-enriched formula can be prepared for target group such as infants, cardiac-risk group, geriatrics, diabetics, phenylketonuria patients, and athletes. Sensorial improvement and allergenic reduction are areas to address for optimal exploitation. Whey protein is proving to be an immune-nutrient and its dietary intervention to tackle cancer could be a promising area of research. New strategies must be devised to expand the utility of this underutilized resource. Its usage in improvement of nutritional status and alleviation of metabolic syndromes ought to be intensified.
Acknowledgments
Conflict of interest
There is no conflict of interest in submission of this manuscript.
References
- Abdel-Salam AM, Effat LK. Preparation and evaluation of a novel therapeutic dairy-based drink for phenylketonuria. N Am J Med Sci. 2010;2:66–70. doi: 10.4297/najms.2010.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrahão V. Nourishing the dysfunctional gut and whey protein. Curr Opin Clin Nutr Metab Care. 2012;15:480–484. doi: 10.1097/MCO.0b013e328356b71e. [DOI] [PubMed] [Google Scholar]
- Adegboye AR, Boucher BJ, Kongstad J, et al (2015) Calcium, vitamin D, casein and whey protein intakes and periodontitis among Danish adults. Public Health Nutr:1–8. doi:10.1017/S1368980015001202 [DOI] [PMC free article] [PubMed]
- Ahn WS, Park SJ, Lee SY. Production of Poly(3-hydroxybutyrate) by fed-batch culture of recombinant Escherichia coli with a highly concentrated whey solution. Appl Environ Microbiol. 2000;66:3624–3627. doi: 10.1128/AEM.66.8.3624-3627.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akalın AS, Unal G, Dinkci N, Hayaloglu AA. Microstructural, textural, and sensory characteristics of probiotic yogurts fortified with sodium calcium caseinate or whey protein concentrate. J Dairy Sci. 2012;95:3617–3628. doi: 10.3168/jds.2011-5297. [DOI] [PubMed] [Google Scholar]
- Akhavan T, Luhovyy BL, Panahi S, et al. Mechanism of action of pre-meal consumption of whey protein on glycemic control in young adults. J Nutr Biochem. 2014;25:36–43. doi: 10.1016/j.jnutbio.2013.08.012. [DOI] [PubMed] [Google Scholar]
- Alexander DD, Schmitt DF, Tran NL, et al. Partially hydrolyzed 100 % whey protein infant formula and atopic dermatitis risk reduction: a systematic review of the literature. Nutr Rev. 2010;68:232–245. doi: 10.1111/j.1753-4887.2010.00281.x. [DOI] [PubMed] [Google Scholar]
- Ameratunga R, Woon S-T. Anaphylaxis to hyperallergenic functional foods. Allergy Asthma Clin Immunol. 2010;6:33. doi: 10.1186/1710-1492-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athira S, Mann B, Sharma R, Kumar R. Ameliorative potential of whey protein hydrolysate against paracetamol-induced oxidative stress. J Dairy Sci. 2013;96:1431–1437. doi: 10.3168/jds.2012-6080. [DOI] [PubMed] [Google Scholar]
- Attaallah W, Yilmaz AM, Erdoğan N, et al. Whey protein versus whey protein hydrolyzate for the protection of azoxymethane and dextran sodium sulfate induced colonic tumors in rats. Pathol Oncol Res. 2012;18:817–822. doi: 10.1007/s12253-012-9509-9. [DOI] [PubMed] [Google Scholar]
- Badr G, Badr BM, Mahmoud MH, et al. Treatment of diabetic mice with undenatured whey protein accelerates the wound healing process by enhancing the expression of MIP-1α, MIP-2, KC, CX3CL1 and TGF-β in wounded tissue. BMC Immunol. 2012;13:32. doi: 10.1186/1471-2172-13-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badr G, Ebaid H, Mohany M, Abuelsaad AS. Modulation of immune cell proliferation and chemotaxis towards CC chemokine ligand (CCL)-21 and CXC chemokine ligand (CXCL)-12 in undenatured whey protein-treated mice. J Nutr Biochem. 2012;23:1640–1646. doi: 10.1016/j.jnutbio.2011.11.006. [DOI] [PubMed] [Google Scholar]
- Baiano A. Recovery of biomolecules from food wastes - a review. Molecules. 2014;19:14821–14842. doi: 10.3390/molecules190914821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard KD, Kupchak BR, Volk BM, et al. Acute effects of ingestion of a novel whey-derived extract on vascular endothelial function in overweight, middle-aged men and women. Br J Nutr. 2013;109:882–893. doi: 10.1017/S0007114512002061. [DOI] [PubMed] [Google Scholar]
- Barth CA, Behnke U. Nutritional physiology of whey and whey components. Nahrung. 1997;41:2–12. doi: 10.1002/food.19970410103. [DOI] [PubMed] [Google Scholar]
- Bell SJ. Whey protein concentrates with and without immunoglobulins: a review. J Med Food. 2000;3:1–13. doi: 10.1089/jmf.2000.3.1. [DOI] [PubMed] [Google Scholar]
- Ben Rebah F, Miled N. Fish processing wastes for microbial enzyme production: a review. 3. Biotech. 2012;3:255–265. doi: 10.1007/s13205-012-0099-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertenshaw EJ, Lluch A, Yeomans MR. Satiating effects of protein but not carbohydrate consumed in a between-meal beverage context. Physiol Behav. 2008;93:427–436. doi: 10.1016/j.physbeh.2007.09.014. [DOI] [PubMed] [Google Scholar]
- Bhushan S, Kalia K, Sharma M, et al. Processing of apple pomace for bioactive molecules. Crit Rev Biotechnol. 2008;28:285–296. doi: 10.1080/07388550802368895. [DOI] [PubMed] [Google Scholar]
- Bonnaillie LM, Tomasula PM. Fractionation of whey protein isolate with supercritical carbon dioxide to produce enriched α-lactalbumin and β-lactoglobulin food ingredients. J Agric Food Chem. 2012;60:5257–5266. doi: 10.1021/jf3011036. [DOI] [PubMed] [Google Scholar]
- Botteman M, Detzel P. Cost-effectiveness of partially hydrolyzed whey protein formula in the primary prevention of atopic dermatitis in high-risk urban infants in southeast Asia. Ann Nutr Metab. 2015;66(Suppl 1):26–32. doi: 10.1159/000370222. [DOI] [PubMed] [Google Scholar]
- Bu G, Luo Y, Zheng Z, Zheng H. Effect of heat treatment on the antigenicity of bovine α-lactalbumin and β-lactoglobulin in whey protein isolate. Food Agric Immunol. 2009;20:195–206. doi: 10.1080/09540100903026116. [DOI] [Google Scholar]
- Casqueiro J, Casqueiro J, Alves C. Infections in patients with diabetes mellitus: a review of pathogenesis. Indian J Endocrinol Metab. 2012;16(Suppl 1):S27–S36. doi: 10.4103/2230-8210.94253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro GA, Maria DA, Bouhallab S, Sgarbieri VC. In vitro impact of a whey protein isolate (WPI) and collagen hydrolysates (CHs) on B16F10 melanoma cells proliferation. J Dermatol Sci. 2009;56:51–57. doi: 10.1016/j.jdermsci.2009.06.016. [DOI] [PubMed] [Google Scholar]
- Chen W-C, Huang W-C, Chiu C-C, et al. Whey protein improves exercise performance and biochemical profiles in trained mice. Med Sci Sports Exerc. 2014;46:1517–1524. doi: 10.1249/MSS.0000000000000272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou CJ, Affolter M, Kussmann M. A nutrigenomics view of protein intake: macronutrient, bioactive peptides, and protein turnover. Prog Mol Biol Transl Sci. 2012;108:51–74. doi: 10.1016/B978-0-12-398397-8.00003-4. [DOI] [PubMed] [Google Scholar]
- Churchward-Venne TA, Breen L, Di Donato DM, et al. Leucine supplementation of a low-protein mixed macronutrient beverage enhances myofibrillar protein synthesis in young men: a double-blind, randomized trial. Am J Clin Nutr. 2014;99:276–286. doi: 10.3945/ajcn.113.068775. [DOI] [PubMed] [Google Scholar]
- Cinelli P, Schmid M, Bugnicourt E, et al. Whey protein layer applied on biodegradable packaging film to improve barrier properties while maintaining biodegradability. Polym Degrad Stab. 2014;108:151–157. doi: 10.1016/j.polymdegradstab.2014.07.007. [DOI] [Google Scholar]
- Croissant AE, Kang EJ, Campbell RE, et al. The effect of bleaching agent on the flavor of liquid whey and whey protein concentrate. J Dairy Sci. 2009;92:5917–5927. doi: 10.3168/jds.2009-2535. [DOI] [PubMed] [Google Scholar]
- De Moura CS, Lollo PCB, Morato PN, et al. Whey protein hydrolysate enhances the exercise-induced heat shock protein (HSP70) response in rats. Food Chem. 2013;136:1350–1357. doi: 10.1016/j.foodchem.2012.09.070. [DOI] [PubMed] [Google Scholar]
- Demirdas S, Coakley KE, Bisschop PH, et al. Bone health in phenylketonuria: a systematic review and meta-analysis. Orphanet J Rare Dis. 2015;10:17. doi: 10.1186/s13023-015-0232-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon EL, Basra G, Horstman AM, et al. Cancer cachexia and anabolic interventions: a case report. J Cachex Sarcopenia Muscle. 2012;3:253–263. doi: 10.1007/s13539-012-0066-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan C, Yang L, Li A, et al. Effects of enzymatic hydrolysis on the allergenicity of whey protein concentrates. Iran J Allergy Asthma Immunol. 2014;13:231–239. [PubMed] [Google Scholar]
- Duongthingoc D, George P, Katopo L, et al. Effect of whey protein agglomeration on spray dried microcapsules containing Saccharomyces boulardii. Food Chem. 2013;141:1782–1788. doi: 10.1016/j.foodchem.2013.04.093. [DOI] [PubMed] [Google Scholar]
- Elattar G, Saleh Z, El-Shebini S, et al. The use of whey protein concentrate in management of chronic hepatitis C virus - a pilot study. Arch Med Sci. 2010;6:748–755. doi: 10.5114/aoms.2010.17091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliseeva IE (2001) [Angiotensin-converting enzyme and its physiological role]. Vopr medit͡sinskoĭ khimii 47:43–54 [PubMed]
- Essick EE, Sam F. Oxidative stress and autophagy in cardiac disease, neurological disorders, aging and cancer. Oxidative Med Cell Longev. 2010;3:168–177. doi: 10.4161/oxim.3.3.12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estévez N, Fuciños P, Sobrosa AC, et al. Modeling the angiotensin-converting enzyme inhibitory activity of peptide mixtures obtained from cheese whey hydrolysates using concentration-response curves. Biotechnol Prog. 2012;28:1197–1206. doi: 10.1002/btpr.1587. [DOI] [PubMed] [Google Scholar]
- Fernández-Pan I, Royo M, Ignacio Maté J. Antimicrobial activity of whey protein isolate edible films with essential oils against food spoilers and foodborne pathogens. J Food Sci. 2012;77:M383–M390. doi: 10.1111/j.1750-3841.2012.02752.x. [DOI] [PubMed] [Google Scholar]
- Freidenreich DJ, Volek JS. Immune responses to resistance exercise. Exerc Immunol Rev. 2012;18:8–41. [PubMed] [Google Scholar]
- Freudenberg A, Petzke KJ, Klaus S. Dietary L-leucine and L-alanine supplementation have similar acute effects in the prevention of high-fat diet-induced obesity. Amino Acids. 2013;44:519–528. doi: 10.1007/s00726-012-1363-2. [DOI] [PubMed] [Google Scholar]
- Gad AS, Khadrawy YA, El-Nekeety AA, et al. Antioxidant activity and hepatoprotective effects of whey protein and spirulina in rats. Nutrition. 2011;27:582–589. doi: 10.1016/j.nut.2010.04.002. [DOI] [PubMed] [Google Scholar]
- Gerez CL, Font de Valdez G, Gigante ML, Grosso CRF. Whey protein coating bead improves the survival of the probiotic Lactobacillus rhamnosus CRL 1505 to low pH. Lett Appl Microbiol. 2012;54:552–556. doi: 10.1111/j.1472-765X.2012.03247.x. [DOI] [PubMed] [Google Scholar]
- Gomes SP, Nyengaard JR, Misawa R, et al. Atrophy and neuron loss: effects of a protein-deficient diet on sympathetic neurons. J Neurosci Res. 2009;87:3568–3575. doi: 10.1002/jnr.22167. [DOI] [PubMed] [Google Scholar]
- Gülseren I, Fang Y, Corredig M. Complexation of high methoxyl pectin with ethanol desolvated whey protein nanoparticles: physico-chemical properties and encapsulation behaviour. Food Funct. 2012;3:859–866. doi: 10.1039/c2fo10235h. [DOI] [PubMed] [Google Scholar]
- Hébrard G, Hoffart V, Beyssac E, et al. Coated whey protein/alginate microparticles as oral controlled delivery systems for probiotic yeast. J Microencapsul. 2010;27:292–302. doi: 10.3109/02652040903134529. [DOI] [PubMed] [Google Scholar]
- Hochwallner H, Schulmeister U, Swoboda I, et al. Cow’s milk allergy: from allergens to new forms of diagnosis, therapy and prevention. Methods. 2014;66:22–33. doi: 10.1016/j.ymeth.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M, McClements DJ, Decker EA. Impact of whey protein emulsifiers on the oxidative stability of salmon oil-in-water emulsions. J Agric Food Chem. 2003;51:1435–1439. doi: 10.1021/jf0203794. [DOI] [PubMed] [Google Scholar]
- Hulmi JJ, Lockwood CM, Stout JR. Effect of protein/essential amino acids and resistance training on skeletal muscle hypertrophy: a case for whey protein. Nutr Metab (Lond) 2010;7:51. doi: 10.1186/1743-7075-7-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain SK. L-cysteine supplementation as an adjuvant therapy for type-2 diabetes. Can J Physiol Pharmacol. 2012;90:1061–1064. doi: 10.1139/y2012-087. [DOI] [PubMed] [Google Scholar]
- Jakubowicz D, Froy O. Biochemical and metabolic mechanisms by which dietary whey protein may combat obesity and type 2 diabetes. J Nutr Biochem. 2013;24:1–5. doi: 10.1016/j.jnutbio.2012.07.008. [DOI] [PubMed] [Google Scholar]
- Janjarasskul T, Tananuwong K, Krochta JM. Whey protein film with oxygen scavenging function by incorporation of ascorbic acid. J Food Sci. 2011;76:E561–E568. doi: 10.1111/j.1750-3841.2011.02409.x. [DOI] [PubMed] [Google Scholar]
- Katayama M, Wilson LA. Utilization of okara, a byproduct from soymilk production, through the development of soy-based snack food. J Food Sci. 2008;73:S152–S157. doi: 10.1111/j.1750-3841.2008.00662.x. [DOI] [PubMed] [Google Scholar]
- Kattan JD, Cocco RR, Järvinen KM. Milk and soy allergy. Pediatr Clin N Am. 2011;58:407–426. doi: 10.1016/j.pcl.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerasioti E, Stagos D, Priftis A, et al. Antioxidant effects of whey protein on muscle C2C12 cells. Food Chem. 2014;155:271–278. doi: 10.1016/j.foodchem.2014.01.066. [DOI] [PubMed] [Google Scholar]
- Kim J, Paik H-D, Yoon Y-C, Park E. Whey protein inhibits iron overload-induced oxidative stress in rats. J Nutr Sci Vitaminol (Tokyo) 2013;59:198–205. doi: 10.3177/jnsv.59.198. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Sumiyoshi M, Kobayashi T. Whey peptides prevent chronic ultraviolet B radiation-induced skin aging in melanin-possessing male hairless mice. J Nutr. 2014;144:27–32. doi: 10.3945/jn.113.180406. [DOI] [PubMed] [Google Scholar]
- Kishta OA, Iskandar M, Dauletbaev N, et al. Pressurized whey protein can limit bacterial burden and protein oxidation in Pseudomonas aeruginosa lung infection. Nutrition. 2013;29:918–924. doi: 10.1016/j.nut.2012.11.009. [DOI] [PubMed] [Google Scholar]
- Knöbel Y, Weise A, Glei M, et al. Ferric iron is genotoxic in non-transformed and preneoplastic human colon cells. Food Chem Toxicol. 2007;45:804–811. doi: 10.1016/j.fct.2006.10.028. [DOI] [PubMed] [Google Scholar]
- Kong B, Peng X, Xiong YL, Zhao X. Protection of lung fibroblast MRC-5 cells against hydrogen peroxide-induced oxidative damage by 0.1–2.8 kDa antioxidative peptides isolated from whey protein hydrolysate. Food Chem. 2012;135:540–547. doi: 10.1016/j.foodchem.2012.04.122. [DOI] [PubMed] [Google Scholar]
- Krzeminski A, Prell KA, Busch-Stockfisch M, et al. Whey protein–pectin complexes as new texturising elements in fat-reduced yoghurt systems. Int Dairy J. 2014;36:118–127. doi: 10.1016/j.idairyj.2014.01.018. [DOI] [Google Scholar]
- Kuhn KR, Cunha RL. Flaxseed oil – whey protein isolate emulsions: effect of high pressure homogenization. J Food Eng. 2012;111:449–457. doi: 10.1016/j.jfoodeng.2012.01.016. [DOI] [Google Scholar]
- Lands LC, Iskandar M, Beaudoin N, et al. Dietary supplementation with pressurized whey in patients with cystic fibrosis. J Med Food. 2010;13:77–82. doi: 10.1089/jmf.2008.0326. [DOI] [PubMed] [Google Scholar]
- Leksrisompong P, Gerard P, Lopetcharat K, Drake M. Bitter taste inhibiting agents for whey protein hydrolysate and whey protein hydrolysate beverages. J Food Sci. 2012;77:S282–S287. doi: 10.1111/j.1750-3841.2012.02800.x. [DOI] [PubMed] [Google Scholar]
- Li M, Ma Y, Cui J. Whey-protein-stabilized nanoemulsions as a potential delivery system for water-insoluble curcumin. LWT Food Sci Technol. 2014;59:49–58. doi: 10.1016/j.lwt.2014.04.054. [DOI] [Google Scholar]
- Liaw IW, Eshpari H, Tong PS, Drake MA. The impact of antioxidant addition on flavor of cheddar and mozzarella whey and cheddar whey protein concentrate. J Food Sci. 2010;75:C559–C569. doi: 10.1111/j.1750-3841.2010.01695.x. [DOI] [PubMed] [Google Scholar]
- Liu J, Wang X, Zhao Z. Effect of whey protein hydrolysates with different molecular weight on fatigue induced by swimming exercise in mice. J Sci Food Agric. 2014;94:126–130. doi: 10.1002/jsfa.6220. [DOI] [PubMed] [Google Scholar]
- Liu X, Jiang D, Peterson DG. Identification of bitter peptides in whey protein hydrolysate. J Agric Food Chem. 2014;62:5719–5725. doi: 10.1021/jf4019728. [DOI] [PubMed] [Google Scholar]
- Loesche WJ, Grossman NS. Periodontal disease as a specific, albeit chronic, infection: diagnosis and treatment. Clin Microbiol Rev. 2001;14:727–752. doi: 10.1128/CMR.14.4.727-752.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lollo PCB, Amaya-Farfan J, Faria IC, et al. Hydrolysed whey protein reduces muscle damage markers in brazilian elite soccer players compared with whey protein and maltodextrin. A twelve-week in-championship intervention. Int Dairy J. 2014;34:19–24. doi: 10.1016/j.idairyj.2013.07.001. [DOI] [Google Scholar]
- Lowe NJ, Meyers DP, Wieder JM, et al. Low doses of repetitive ultraviolet a induce morphologic changes in human skin. J Investig Dermatol. 1995;105:739–743. doi: 10.1111/1523-1747.ep12325517. [DOI] [PubMed] [Google Scholar]
- Lyczak JB, Cannon CL, Pier GB. Lung infections associated with cystic fibrosis. Clin Microbiol Rev. 2002;15:194–222. doi: 10.1128/CMR.15.2.194-222.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik P. Value-added nutrition. Can J Cardiol. 2007;23:956. doi: 10.1016/S0828-282X(07)70867-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin AH, de Jong GAH. Enhancing the in vitro Fe(2+) bio-accessibility using ascorbate and cold-set whey protein gel particles. Dairy Sci Technol. 2012;92:133–149. doi: 10.1007/s13594-011-0055-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin V, Ratel S, Siracusa J, et al. Whey proteins are more efficient than casein in the recovery of muscle functional properties following a casting induced muscle atrophy. PLoS One. 2013;8:e75408. doi: 10.1371/journal.pone.0075408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehyar GF, Al-Isamil KM, Al-Ghizzawi HM, Holley RA. Stability of cardamom (Elettaria Cardamomum) essential oil in microcapsules made of whey protein isolate, guar gum, and carrageenan. J Food Sci. 2014 doi: 10.1111/1750-3841.12652. [DOI] [PubMed] [Google Scholar]
- Mehyar GF, Al-Ismail K, Han JH, Chee GW. Characterization of edible coatings consisting of pea starch, whey protein isolate, and Carnauba wax and their effects on oil rancidity and sensory properties of walnuts and pine nuts. J Food Sci. 2012;77:E52–E59. doi: 10.1111/j.1750-3841.2011.02559.x. [DOI] [PubMed] [Google Scholar]
- Morato PN, Lollo PC, Moura CS, Batista TM, Carneiro EM, Amaya-Farfan J (2013) A dipeptide and an amino acid; present in whey protein hydrolysate increase translocation of GLUT-4 to the plasma membrane in Wistar rats. Food Chem 139:853–859. doi:10.1016/j.foodchem.2012.12.062 [DOI] [PubMed]
- Mortensen LS, Holmer-Jensen J, Hartvigsen ML, et al. Effects of different fractions of whey protein on postprandial lipid and hormone responses in type 2 diabetes. Eur J Clin Nutr. 2012;66:799–805. doi: 10.1038/ejcn.2012.48. [DOI] [PubMed] [Google Scholar]
- Morton JP, Kayani AC, McArdle A, Drust B. The exercise-induced stress response of skeletal muscle, with specific emphasis on humans. Sports Med. 2009;39:643–662. doi: 10.2165/00007256-200939080-00003. [DOI] [PubMed] [Google Scholar]
- Moura CS, Lollo PCB, Morato PN, et al. Whey protein hydrolysate enhances HSP90 but does not alter HSP60 and HSP25 in skeletal muscle of rats. PLoS One. 2014;9:e83437. doi: 10.1371/journal.pone.0083437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeem M, Salim-ur-Rehman, Muhammad Anjum F, et al (2012) Development, characterization, and optimization of protein level in date bars using response surface methodology. ScientificWorldJournal 2012:518702. doi:10.1100/2012/518702 [DOI] [PMC free article] [PubMed]
- Neelima Sharma R, Rajput YS, Mann B. Chemical and functional properties of glycomacropeptide (GMP) and its role in the detection of cheese whey adulteration in milk: a review. Dairy Sci Technol. 2013;93:21–43. doi: 10.1007/s13594-012-0095-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ney DM, Blank RD, Hansen KE. Advances in the nutritional and pharmacological management of phenylketonuria. Curr Opin Clin Nutr Metab Care. 2014;17:61–68. doi: 10.1097/MCO.0000000000000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CW, Bastian E, Farkas B, Drake M. The effect of acidification of liquid whey protein concentrate on the flavor of spray-dried powder. J Dairy Sci. 2014;97:4043–4051. doi: 10.3168/jds.2013-7877. [DOI] [PubMed] [Google Scholar]
- Park E, Glei M, Knöbel Y, Pool-Zobel BL. Blood mononucleocytes are sensitive to the DNA damaging effects of iron overload–in vitro and ex vivo results with human and rat cells. Mutat Res. 2007;619:59–67. doi: 10.1016/j.mrfmmm.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Pavlovich-Abril A, Rouzaud-Sández O, Torres P, Robles-Sánchez RM. Cereal bran and wholegrain as a source of dietary fibre: technological and health aspects. Int J Food Sci Nutr. 2012;63:882–892. doi: 10.3109/09637486.2012.676030. [DOI] [PubMed] [Google Scholar]
- Pérez-Cano FJ, Marín-Gallén S, Castell M, et al. Bovine whey protein concentrate supplementation modulates maturation of immune system in suckling rats. Br J Nutr. 2007;98(Suppl 1):S80–S84. doi: 10.1017/S0007114507838074. [DOI] [PubMed] [Google Scholar]
- Pérez-Masiá R, López-Nicolás R, Periago MJ, et al. Encapsulation of folic acid in food hydrocolloids through nanospray drying and electrospraying for nutraceutical applications. Food Chem. 2015;168:124–133. doi: 10.1016/j.foodchem.2014.07.051. [DOI] [PubMed] [Google Scholar]
- Piccolomini AF, Iskandar MM, Lands LC, Kubow S. High hydrostatic pressure pre-treatment of whey proteins enhances whey protein hydrolysate inhibition of oxidative stress and IL-8 secretion in intestinal epithelial cells. Food Nutr Res. 2012 doi: 10.3402/fnr.v56i0.17549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prussick R, Prussick L, Gutman J. Psoriasis improvement in patients using glutathione-enhancing, nondenatured whey protein isolate: a pilot study. J Clin Aesthet Dermatol. 2013;6:23–26. [PMC free article] [PubMed] [Google Scholar]
- Ross EK, Gray JJ, Winter AN, Linseman DA. Immunocal® and preservation of glutathione as a novel neuroprotective strategy for degenerative disorders of the nervous system. Recent Pat CNS Drug Discov. 2012;7:230–235. doi: 10.2174/157488912803252014. [DOI] [PubMed] [Google Scholar]
- Salehi A, Gunnerud U, Muhammed SJ, et al. The insulinogenic effect of whey protein is partially mediated by a direct effect of amino acids and GIP on β-cells. Nutr Metab (Lond) 2012;9:48. doi: 10.1186/1743-7075-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikholeslami Vatani D, Ahmadi Kani Golzar F. Changes in antioxidant status and cardiovascular risk factors of overweight young men after six weeks supplementation of whey protein isolate and resistance training. Appetite. 2012;59:673–678. doi: 10.1016/j.appet.2012.08.005. [DOI] [PubMed] [Google Scholar]
- Sindayikengera S, Xia W. Nutritional evaluation of caseins and whey proteins and their hydrolysates from protamex. J Zhejiang Univ Sci B. 2006;7:90–98. doi: 10.1631/jzus.2006.B0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smithers GW, Ballard FJ, Copeland AD, et al. New opportunities from the isolation and utilization of whey proteins. J Dairy Sci. 1996;79:1454–1459. doi: 10.3168/jds.S0022-0302(96)76504-9. [DOI] [PubMed] [Google Scholar]
- Solinas C, Corpino M, Maccioni R, Pelosi U. Cow’s milk protein allergy. J Matern Fetal Neonatal Med. 2010;23(Suppl 3):76–79. doi: 10.3109/14767058.2010.512103. [DOI] [PubMed] [Google Scholar]
- Solverson P, Murali SG, Brinkman AS, et al. Glycomacropeptide, a low-phenylalanine protein isolated from cheese whey, supports growth and attenuates metabolic stress in the murine model of phenylketonuria. Am J Physiol Endocrinol Metab. 2012;302:E885–E895. doi: 10.1152/ajpendo.00647.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa GTD, Lira FS, Rosa JC, et al. Dietary whey protein lessens several risk factors for metabolic diseases: a review. Lipids Health Dis. 2012;11:67. doi: 10.1186/1476-511X-11-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strisciuglio P, Concolino D. New strategies for the treatment of phenylketonuria (PKU) Metabolites. 2014;4:1007–1017. doi: 10.3390/metabo4041007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahavorgar A, Vafa M, Shidfar F, et al. Whey protein preloads are more beneficial than soy protein preloads in regulating appetite, calorie intake, anthropometry, and body composition of overweight and obese men. Nutr Res. 2014 doi: 10.1016/j.nutres.2014.08.015. [DOI] [PubMed] [Google Scholar]
- Takata T, Tanaka F, Yamada T, et al. Clinical significance of caspase-3 expression in pathologic-stage I, nonsmall-cell lung cancer. Int J Cancer. 2001;96(Suppl):54–60. doi: 10.1002/ijc.10347. [DOI] [PubMed] [Google Scholar]
- Takayanagi T, Sasaki H, Kawashima A, et al. A new enteral diet, MHN-02, which contains abundant antioxidants and whey peptide, protects against carbon tetrachloride-induced hepatitis. JPEN J Parenter Enteral Nutr. 2011;35:516–522. doi: 10.1177/0148607110381599. [DOI] [PubMed] [Google Scholar]
- Tippetts M, Martini S, Brothersen C, McMahon DJ. Fortification of cheese with vitamin D3 using dairy protein emulsions as delivery systems. J Dairy Sci. 2012;95:4768–4774. doi: 10.3168/jds.2011-5134. [DOI] [PubMed] [Google Scholar]
- Toedebusch RG, Childs TE, Hamilton SR, et al. Postprandial leucine and insulin responses and toxicological effects of a novel whey protein hydrolysate-based supplement in rats. J Int Soc Sports Nutr. 2012;9:24. doi: 10.1186/1550-2783-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong X, Li W, Xu J-Y, et al. Effects of whey protein and leucine supplementation on insulin resistance in non-obese insulin-resistant model rats. Nutrition. 2014;30:1076–1080. doi: 10.1016/j.nut.2014.01.013. [DOI] [PubMed] [Google Scholar]
- Trachootham D, Lu W, Ogasawara MA, et al. Redox regulation of cell survival. Antioxid Redox Signal. 2008;10:1343–1374. doi: 10.1089/ars.2007.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Calcar SC, Ney DM. Food products made with glycomacropeptide, a low-phenylalanine whey protein, provide a new alternative to amino acid-based medical foods for nutrition management of phenylketonuria. J Acad Nutr Diet. 2012;112:1201–1210. doi: 10.1016/j.jand.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volek JS, Volk BM, Gómez AL, et al. Whey protein supplementation during resistance training augments lean body mass. J Am Coll Nutr. 2013;32:122–135. doi: 10.1080/07315724.2013.793580. [DOI] [PubMed] [Google Scholar]
- Walsh H, Cheng J, Guo M. Effects of carbonation on probiotic survivability, physicochemical, and sensory properties of milk-based symbiotic beverages. J Food Sci. 2014;79:M604–M613. doi: 10.1111/1750-3841.12381. [DOI] [PubMed] [Google Scholar]
- Williams RA, Mamotte CDS, Burnett JR. Phenylketonuria: an inborn error of phenylalanine metabolism. Clin Biochem Rev. 2008;29:31–41. [PMC free article] [PubMed] [Google Scholar]
- Xu R. Effect of whey protein on the proliferation and differentiation of osteoblasts. J Dairy Sci. 2009;92:3014–3018. doi: 10.3168/jds.2008-1702. [DOI] [PubMed] [Google Scholar]
- Yadav DN, Balasubramanian S, Kaur J, et al. Non-wheat pasta based on pearl millet flour containing barley and whey protein concentrate. J Food Sci Technol. 2014;51:2592–2599. doi: 10.1007/s13197-012-0772-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalçin AS. Emerging therapeutic potential of whey proteins and peptides. Curr Pharm Des. 2006;12:1637–1643. doi: 10.2174/138161206776843296. [DOI] [PubMed] [Google Scholar]
- Zhang Q-X, Ling Y-F, Sun Z, et al. Protective effect of whey protein hydrolysates against hydrogen peroxide-induced oxidative stress on PC12 cells. Biotechnol Lett. 2012;34:2001–2006. doi: 10.1007/s10529-012-1017-1. [DOI] [PubMed] [Google Scholar]
- Zhao L, Huang Q, Huang S, et al. Novel peptide with a specific calcium-binding capacity from whey protein hydrolysate and the possible chelating mode. J Agric Food Chem. 2014 doi: 10.1021/jf502412f. [DOI] [PubMed] [Google Scholar]