Abstract
Aims
Proprotein convertase subtilisin/kexin type 9 (PCSK9) is a secreted protein that enhances degradation of the LDL receptor. While agents that inhibit PCSK9 markedly reduce atherogenic lipoproteins and show great promise for event reduction, it is unknown whether plasma PCSK9 levels predict incident cardiovascular events.
Methods and results
In a nested case–control evaluation conducted in a prospective cohort of >28 000 initially healthy American women, we measured plasma concentrations of PCSK9 at baseline among 358 participants who subsequently developed major cardiovascular events (cases) and among 358 age, smoking, and hormone replacement therapy matched participants who remained free of disease during 17 years of follow-up (controls). Proprotein convertase subtilisin/kexin type 9 level was not significantly related to smoking status, hypertension, obesity, or a family history of premature cardiovascular disease but was positively associated with apolipoprotein B-100 (r = 0.20, P< 0.001), and triglycerides (r = 0.13, P = 0.004). No associations were observed between PCSK9 and apo A1, HDLC, lipoprotein(a), or high-sensitivity C-reactive protein. Despite modest positive association with atherogenic lipids, baseline levels of PCSK9 did not predict the first cardiovascular events; the odds ratios (ORs) for future vascular events for the lowest (referent) to highest baseline quartiles of PCSK9 were 1.0, 0.94, 0.98, and 1.15 (P-trend = 0.53). In contrast, the corresponding ORs for baseline apo B levels were 1.0, 1.14, 1.34, and 1.94 (P-trend = 0.002).
Conclusions
In a large-scale primary prevention cohort, plasma levels of PCSK9 measured at baseline did not predict future cardiovascular events.
Keywords: PCSK9, Epidemiology, Apolipoprotein B, Prevention, Myocardial infarction
Introduction
In 2003, Abifabel and colleagues identified gain-of-function mutations in proprotein convertase subtilisin/kexin type 9 (PCSK9) as a novel cause of autosomal dominant hypercholesterolaemia that associates with premature coronary heart disease.1 Soon thereafter, sequence variation in PCSK9 resulting in loss-of-function mutation was found to be associated with very low LDL cholesterol levels and protective against coronary artery disease.2,3 Subsequent work has shown PCSK9 to be highly expressed in the liver where it binds to the LDL receptor and promotes internalization and degradation of the LDL receptor in hepatic lysosomes.4,5 By slowing this degradation process, inhibitors of PCSK9 promote recycling of the LDL receptor, increasing its functional half-life and reducing circulating levels of apo B containing lipoprotein particles. Monoclonal antibodies that functionally inhibit PCSK9 have already proved capable of markedly reducing LDL cholesterol both as monotherapy6 and as adjuncts to statin treatment,7 and are now under investigation in multiple large-scale event reduction trials.8 Substantive LDL reduction is also seen with PCSK9 inhibitors when used in settings of heterozygous and homozygous familial hyperlipidaemia as long as there is at least partial LDL receptor function.9,10
Despite these data, whether plasma PCSK9 levels predict incident cardiovascular events is unknown since prior studies evaluating this question11,12 have been conducted largely among secondary prevention patients who are likely to be taking statin therapy. This is a potential source of confounding as statins increase circulating levels of PCSK9.13 To address this issue, we conducted a nested case–control study in which we measured plasma levels of PCSK9 as well as standard lipids, apolipoprotein B-100 (apo B), apolipoprotein A-1 (apo A), lipoprotein(a) (Lp(a)), and high-sensitivity C-reactive protein (hsCRP) at baseline in a prospective cohort of initially healthy American women who were followed over a 17-year period for incident vascular events.
Methods
Study cohort, endpoint ascertainment, and evaluation of proprotein convertase subtilisin/kexin type 9 levels
We investigated the relationship of baseline plasma PCSK9 levels as a predictor of future cardiovascular events in a nested case–control study conducted among initially healthy participants in the prospective Women's Health Study (WHS).14 In brief, 28 263 women aged 45 and over who were free of reported cardiovascular disease provided baseline plasma samples, which were collected in EDTA and stored in liquid nitrogen until the time of analysis. Once enrolled, all study participants were prospectively followed over an average period of 17 years for the occurrence of the first ever cardiovascular events (myocardial infarction, thromboembolic stroke, or cardiovascular death). The end point of myocardial infarction was confirmed if symptoms of ischaemia were present and if the event was associated with diagnostic changes in cardiac enzyme levels or if there were diagnostic electrocardiographic changes. The diagnosis of thromboembolic stroke was confirmed if the patient had a new neurological deficit of >24-h duration that was not coded by the WHS neurologic endpoint committee as having a primary haemorrhagic origin; computed tomography or magnetic resonance scanning was available for almost all cases. Deaths from coronary heart disease were confirmed by record review, death certificates, autopsy reports, and information provided by family members.
For the current analysis, we limited the WHS cohort to those women not taking statin therapy, and then constructed a nested case–control study within the cohort in which baseline samples were obtained from 358 study participants who subsequently developed a confirmed cardiovascular end point during follow-up (cases). For each of these case women, baseline plasma samples were also obtained from a woman who was randomly selected from the pool of remaining study participants not taking statin therapy who did not develop cardiovascular events during follow-up (controls). Case and control women were matched on the basis of age (±2 years), smoking status (former, current, never), and use of hormone replacement therapy (HRT). No women with a history of heterozygous or homozygous familial hypercholesterolemia were included in the study.
Baseline plasma samples obtained in the fasting state from each case (N = 358) and control (N = 358) participant which had been stored since study initiation in liquid nitrogen were thawed and assayed for total plasma PCSK9 in a core laboratory certified by the NHLBI/CDC Lipid Standardization program. Proprotein convertase subtilisin/kexin type 9 levels were measured using a commercial assay (Quantikine Human Proprotein Convertase 9/PCSK9 Immunoassay, R&D Systems).
Matched plasma specimens were analysed in duplicate, with the position of the case specimen varied at random to reduce systematic bias and minimize inter-assay variability. To evaluate the PCSK9 assay, an initial pilot study was conducted in which 10 plasma samples were selected at random from other participants within the WHS cohort that were obtained, shipped, stored, and processed in an identical manner to those that would be used in the main study. These 10 samples were then split and sent as blinded slit samples to the reference lab in order to calculate coefficients of variation for PCSK9.
In addition to PCSK9, levels of standard lipids, apo B, apo A-I, Lp(a), and hsCRP were measured in the same central core laboratory facility as previously described.15–17
Statistical analysis
Medians and proportions for baseline risk factors were computed for case and control participants and compared using the Wilcoxon signed rank test or McNemar's test. The Wilcoxon rank sum test was used to compare plasma PCSK9 values in risk factor subgroups. Pearson correlation coefficients were used to evaluate for any evidence of relationship between plasma PCSK9 and the additional measured plasma biomarkers.
Tests for trend were used to evaluate evidence of association between increasing levels of PCSK9 and the first ever cardiovascular events after dividing the study population into quartiles on the basis of the distribution of control values. In these analyses, risk estimates were obtained with conditional logistic regression models that accounted for the matching variables of age, smoking status, and hormone replacement therapy. Additional logistic regression models were used that also adjusted for potential confounding variables (apo B, HDL cholesterol, systolic blood pressure, body mass index, diabetes, parental history of myocardial infarction before age 60 years).
To provide internal controls and a comparator for the magnitude of risk associated with PCSK9 levels, we then repeated this process for three plasma biomarkers with known relationship to future vascular events in this cohort, apo B, the total to HDL cholesterol ratio (TC:HDLC), and hsCRP. A subgroup analysis was additionally performed among those women not taking any lipid-lowering agents at study entry.
All probability values are two tailed and all confidence intervals computed at the 95% level.
Results
Baseline clinical characteristics and proprotein convertase subtilisin/kexin type 9 assay performance
Case and control women were matched on age (median 63 years), smoking status (21% current smokers), and a history of hormone replacement therapy (43.7%). As expected, initially healthy women who subsequently developed cardiovascular events (cases) were more likely at baseline to have a history of hypertension, obesity, diabetes, or hyperlipidaemia when compared with women who remained free of reported disease (controls). As anticipated, case women also had higher baseline levels of apo B, LDLC, ratios of total to HDLC, and hsCRP, and lower baseline levels of HDLC (Table 1).
Table 1.
Baseline clinical characteristics of study participants
| Controls (N = 358) | Cases (N = 358) | P-value | |
|---|---|---|---|
| Age (years) | 63 (58, 68) | 63 (58, 68) | Matching variable |
| Smoking status (%) | |||
| Current | 20.9 | 20.9 | Matching variable |
| Past | 37.2 | 37.2 | |
| Never | 41.9 | 41.9 | |
| HRT use (%) | 43.7 | 43.7 | Matching variable |
| Body mass index (kg/m2) | 25.0 (22.3, 28.9) | 26.2 (22.7, 29.3) | 0.005 |
| Diabetes (%) | 2.8 | 14.0 | <0.0001 |
| HbA1c (%) | 5.1 (4.9, 5.3) | 5.1 (4.9, 5.4) | 0.01 |
| Hypertension (%) | 35.2 | 54.3 | <0.0001 |
| Hyperlipidaemia (%) | 39.7 | 41.3 | <0.001 |
| Family history (%) | 9.7 | 15.6 | 0.04 |
| Triglycerides (mg/dL) | 130 (93, 180) | 157 (104, 240) | <0.0001 |
| Total cholesterol (mg/dL) | 219 (192, 241) | 222 (199, 250) | 0.04 |
| HDL cholesterol (mg/dL) | 51 (43, 63) | 48 (40, 58) | 0.0004 |
| Apolipoprotein A1 (mg/dL) | 150 (134, 168) | 147 (130, 169) | 0.17 |
| Apolipoprotein B-100 (mg/dL) | 108 (90, 128) | 115 (93, 138) | <0.0001 |
| Lipoprotein(a) (mg/dL) | 9.9 (4.4, 36.0) | 12.3 (5, 49) | 0.12 |
| hsCRP (mg/L) | 2.3 (1.2, 4.6) | 3.5 (1.7, 6.5) | <0.0001 |
| PCSK9 (ng/mL) | 299.7 (252.9–358.8) | 304.4 (252.9–365.9) | 0.94 |
Values are proportions or medians (inter-quartile range). P-value is for the comparison of women who remained free of cardiovascular disease during follow-up (controls) when compared with women who developed incident myocardial infarction, stroke, or cardiovascular death (cases). Case and control participants were matched on age, smoking status, and use of hormone replacement therapy.
HRT, hormone replacement therapy; HbA1c, haemoglobin A1c; hsCRP, high-sensitivity C-reactive protein; PCSK9, proprotein convertase subtilisin/kexin type 9.
Blinded repeat analysis of PCSK9 for the 10 split-samples used in the pilot study showed a correlation in excess of 0.98 and a coefficient of variation <5%.
Determinants of plasma proprotein convertase subtilisin/kexin type 9 levels
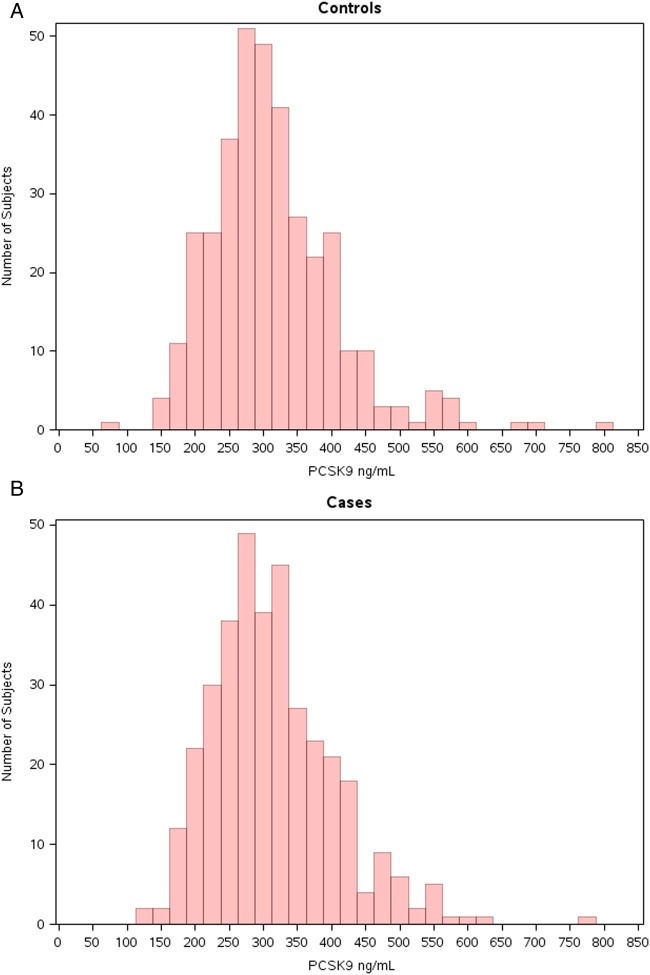
The distribution of PCSK9 levels for the control women is shown in Figure 1A. Among controls as well as among cases, non-lipid cardiovascular risk factors did not substantively impact upon plasma PCSK9 levels; the Pearson correlation coefficients between PCSK9 and age (r = 0.044, P = 0.23), body mass index (r = 0.078, P = 0.04), systolic blood pressure (r = 0.044, P = 0.25), and diastolic blood pressure (r = 0.014, P = 0.72) were all quite small, and no differences in PCSK9 levels were observed between smokers and non-smokers (319 vs. 312 ng/dL, P = 0.39), those with and without hypertension (317 vs. 311 ng/dL, P = 0.35), HRT users and non-users (310 vs. 317 ng/dL, P = 0.37), or those with and without a family history of premature atherosclerosis (315 vs. 314 ng/dL, P = 0.96).
Figure 1.
Distribution of baseline proprotein convertase subtilisin/kexin type 9 levels in the Women's Health Study for controls (A) and cases (B).
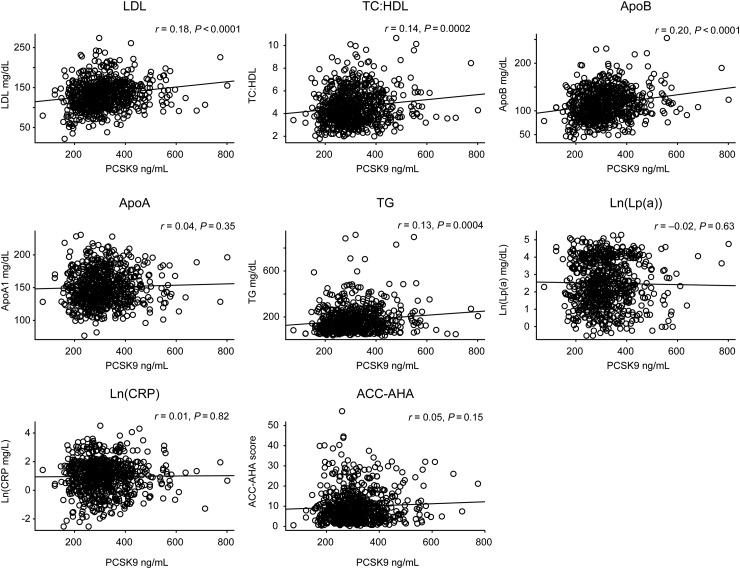
In contrast, as would be anticipated given PCSK9 function, plasma PCSK9 levels correlated modestly with apo B-100 (r = 0.20, P < 0.001), LDL cholesterol (r = 0.18, P < 0.001), non-HDL cholesterol (r = 0.21, P < 0.001), and the total to HDL cholesterol ratio (r = 0.14, P = 0.0002). A modest but statistically significant positive correlation with plasma triglycerides was also observed (r = 0.13, P = 0.004) (Figure 2).
Figure 2.
Correlation plots between plasma proprotein convertase subtilisin/kexin type 9 levels and apo-A, apo-B, LDL-C, the TC:HDLC ratio, ln[Lp(a)], ln[triglycerides], ln[hsCRP], and estimated 10-year risk using the 2013 ACC-AHA risk calculator.
No significant relationships were observed between PCSK9 levels and ln(hsCRP) (r = 0.009, P = 0.82), HDL cholesterol (r = 0.003, P = 0.94), apo A1 (r = 0.035, P = 0.35), ln(Lp(a)) (r = 0.018, P = 0.63), or calculated 10-year risk estimates using the 2013 ACC/AHA risk calculator.
Proprotein convertase subtilisin/kexin type 9 levels and incident cardiovascular events
The distribution of PCSK9 levels at baseline among case women (Figure 1B) was quite similar to that of controls (Figure 1A). The median (interquartile range) for PCSK9 in the cases was 304.4 (252.9–365.9) ng/mL which was not significantly different than that observed in the controls (299.7 [252.9–358.8], P = 0.94).
Table 2 presents odds ratios (ORs) for the first ever cardiovascular events according to increasing quartiles of PCSK9 at study entry. In conditional logistic regression analyses matched for age, smoking, and HRT use, the OR's from lowest (referent) to highest baseline quartile of PCSK9 for future vascular events were 1.0, 0.94, 0.98, and 1.15 (P-trend = 0.5). In multivariable adjustment that additionally controlled for Apo B levels, these OR's were 1.0, 0.98, 0.91, and 0.96, respectively, indicating no confounding effects by this factor.
Table 2.
Age, smoking, and hormone replacement therapy use adjusted odds ratios for incident vascular events according to increasing baseline quartiles of proprotein convertase subtilisin/kexin type 9 level
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P-trend | |
|---|---|---|---|---|---|
| PCSK9 (range, ng/mL) | (<252) | (252–299) | (300–358) | (>358) | |
| RR* | 1.00 | 0.94 | 0.98 | 1.15 | 0.52 |
| 95% CI | Referent | 0.61–1.45 | 0.64–1.49 | 0.74–1.79 | |
| P | Referent | 0.78 | 0.93 | 0.53 | |
| Apo B-100 (range, mg/dL) | (<90) | (90–108) | (109–127) | (>127) | |
| RR* | 1.00 | 1.14 | 1.34 | 1.94 | 0.002 |
| 95% CI | Referent | 0.73–1.77 | 0.85–2.10 | 1.26–3.01 | |
| P | Referent | 0.57 | 0.20 | 0.003 | |
| TC:HDLC (range) | (<3.45) | (3.45–4.20) | (4.21–5.18) | (>5.18) | |
| RR* | 1.00 | 0.97 | 1.09 | 1.85 | 0.002 |
| 95% CI | Referent | 0.61–1.53 | 0.69–1.73 | 1.19–2.84 | |
| P | Referent | 0.88 | 0.69 | 0.006 | |
| hsCRP (range, mg/L) | (<1.17) | (1.17–2.34) | (2.354.58) | (>4.58) | |
| RR* | 1.00 | 0.87 | 1.49 | 2.10 | 0.00004 |
| 95% CI | Referent | 0.55–1.35 | 0.95–2.34 | 1.39–3.17 | |
| P | Referent | 0.53 | 0.08 | 0.0004 |
For comparison, comparable analyses are also shown for increasing baseline quartiles of apo B-100, the total to HDL cholesterol ratio (TC:HDLC), and for hsCRP.
PCSK9, proprotein convertase subtilisin/kexin type 9; Apo B-100, apolipoprotein B-100; TC:HDLC, total to HDL cholesterol ratio; hsCRP, high-sensitivity C-reactive protein.
For comparison, the corresponding OR's for baseline Apo B levels were 1.0, 1.14, 1.34, and 1.94 (P-trend = 0.002), the corresponding OR's for the total to HDL cholesterol ratio were 1.0, 0.97, 1.09, and 1.85 (P-trend = 0.002), and the corresponding OR's for hsCRP were 1.0, 0.87, 1.50, and 2.11 (P-trend < 0.0001; Table 2). Thus, our study design was capable of demonstrating expected relationships between known risk markers and subsequent vascular events in this cohort, despite being measured only once at baseline (Figure 3). None of these latter relationships were substantially altered by adjustment for additional traditional risk factors.
Figure 3.
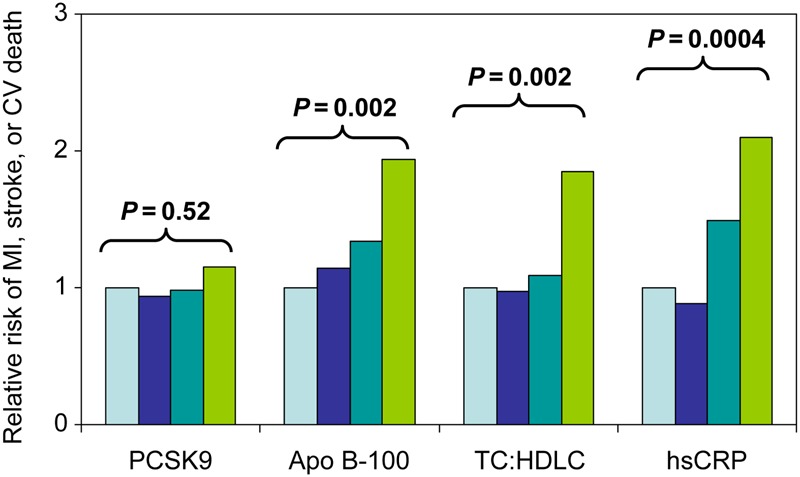
Odds ratios for future cardiovascular events according to baseline levels of proprotein convertase subtilisin/kexin type 9, apo B, the total to HDL cholesterol ratio, and high-sensitivity C-reactive protein. Data are shown for increasing quartiles of each biomarker from conditional logistic regression analyses adjusted for the matching variables of age, smoking, and HRT use.
Similar null findings for PCSK9 were observed in a subgroup analysis limited to those not taking any lipid-lowering agents throughout the follow-up period; in this analysis inclusive of 329 cases, the OR's from lowest (referent) to highest baseline quartile of PCSK9 for future vascular events were 1.0, 0.99, 1.01, and 1.02 (P-trend = 0.91).
Discussion
In this prospective primary prevention cohort of apparently healthy women, we found little evidence that plasma PCSK9 levels are influenced by a wide variety of traditional cardiovascular risk factors including age, smoking status, obesity, blood pressure, HDLC, apo A1, Lp(a), hsCRP, or estimated 10-year risk. Moreover, while plasma PCSK9 levels correlated modestly with circulating levels of apo B, LDLC, and triglycerides, PCSK9 levels measured at baseline did not predict future vascular event rates. In contrast, as anticipated, baseline levels of apo B, the TC:HDLC ratio, and hsCRP were strongly associated with future vascular risk.
Taken together, these data suggest that, unlike traditional risk markers, plasma PCSK9 levels do not appear to be linked to the underlying extent of atherosclerosis nor to any specific propensity toward future vascular risk. Thus, from an epidemiologic perspective, these data suggest that regulatory factors influencing PCSK9 blood concentration are largely, if not fully, independent of non-LDL factors that track with underlying vascular risk.
Prior data addressing epidemiologic factors that associate with plasma PCSK9 levels are limited. However, our data are consistent with those from the Dallas Heart Study18 as well as a Canadian cross-sectional study19 in which the correlation coefficients between non-lipid risk factors and PCSK9 were also of small magnitude. Our null data for 10-year risk scores is further consistent with Dallas Heart Study data indicating no relationship of PCSK9 levels to underlying coronary artery calcium score. All of these studies, as well as earlier work in diabetic patients20 and in men,21 are consistent with the finding that the primary plasma correlates of PCSK9 are LDL cholesterol and apo B containing lipoproteins, both reflecting the known function of PCSK9. In our data and in the studies cited above, modest but significant associations between PCSK9 levels and plasma triglycerides were additionally observed. This is of interest and may require further investigation as there is currently little evidence that PCSK9 circulates in association with VLDL. However, recent data suggest that circulating PCSK9 regulates VLDL receptor protein and triglyceride accumulation in visceral adipose tissues.22
We are unaware of prior data evaluating PCSK9 levels as a risk predictor in primary prevention or among individuals not on lipid-lowering therapy. These issues are relevant as statin therapy increases circulating PCSK9 levels13 and thus studies of those taking statins could be confounded. In one study of stable coronary artery disease patients on statin treatment, circulating PCSK9 levels modestly associated with vascular risk, but this effect was attenuated and no longer significant after adjustment for triglycerides.12 A second analysis of PCSK9 levels that reported a stronger positive association with vascular risk in statin-treated patients has recently been retracted by the authors.11
From a pathophysiologic perspective, our finding that PCSK9 levels are minimally related to usual non-lipid risk factors is consistent with laboratory data indicating that PCSK9 is regulated primarily at the level of transcription by sterol regulatory element binding proteins (SREBPs), predominantly SREBP-2 which is critical to cholesterol metabolism.23 Static levels of plasma PCSK9 are a result of the balance of production and clearance, both of which are directly influenced by SREBP-2. The majority of circulating PCSK9 is derived from the liver24 and its secretion is largely dependent on transcriptional activation via SREBP-2. The one known major route of PCSK9 clearance is via hepatic LDL receptors; however, in contrast to PCSK9 production, additional uncharacterized factors also influence clearance of PCSK9 from blood.25 In addition, a portion of PCSK9 travels with LDL particles.26 It is not known how LDL-bound PCSK9 is regulated nor if this portion of PCSK9 is active or cleared at the same rate as unbound PCSK9. Nevertheless, the lack of relationship between PCSK9 and traditional non-lipid risk factors is also consistent with the observations in man that PCSK9 does not appear to have any specific function other than promoting LDL receptor degradation.
The assay for PCSK9 used in our study is for total circulating PCSK9. We thus cannot exclude the theoretical possibility that an assay designed to measure active or unbound PCSK9 might have different correlations with vascular risk factors or be a stronger predictor of subsequent risk. Observed PCSK9 levels in our study are very similar to those expected in fresh plasma; these data suggest that our collection and storage procedures, which include freezing in liquid nitrogen at −170°C are sufficient to preserve the integrity of PCSK9. We measured PCSK9 only once in our study and thus cannot exclude the possibility that variation over time might lead to a differential outcome. However, such variation has not been noted in other cohorts and our data for Apo B, the TC:HDL cholesterol ratio, and CRP indicate that our study design was adequate to demonstrate expected relationships between established biomarkers of risk despite being measured at a single time. Strengths of our study include its prospective nested case:control design in which plasma samples for all study participants were obtained at study entry, many years before the occurrence of the first ever vascular events. Such a study design considerably reduces potential biases inherent in cross-sectional or retrospective studies where plasma sampling is done after rather than before the onset of disease. Further, by matching case and control participants by age, smoking, and HRT use, and by adjusting in multivariable analysis for additional factors like Apo B, it is unlikely that confounding effects due to any of these variables could explain our results.
When assays for PCSK9 first became available, it was hoped that they might have clinical application both for the detection of vascular risk and for the monitoring of therapies designed to inhibit PCSK9 itself.19 While emerging monoclonal antibodies that target PCSK9 have shown remarkable efficacy for cholesterol reduction and there is considerable promise that PCSK9 inhibitors will prove effective for event reduction,8 these agents are easily monitored by standard assays for direct LDL cholesterol. As such, the current neutral data suggest that the clinical use of PCSK9 plasma levels as a surrogate biomarker may be limited.
Authors’ contributions
P.R., L.R.: performed statistical analysis. P.R., N.R.: handled funding and supervision. P.R., G.B., N.R.: acquired the data. P.R.: conceived and designed the research. P.R.: drafted the manuscript. L.R.: made critical revision of the manuscript for key intellectual content.
Funding
Supported by CA047988, HL043851, HL080467, HL099355, HL061795, HL107192, and HL108630 from the National Institutes of Health, Bethesda, MD, USA.
Conflict of interest: P.M.R. has received investigator initiated research grant support for Pfizer, AstraZeneca, Amgen, and Novartis and is listed as a co-inventor on patents held by the Brigham and Women's Hospital that relate to the use of inflammatory biomarkers in cardiovascular disease and diabetes that have been licensed to AstraZeneca and Siemens. The remaining authors report no additional conflicts.
Acknowledgement
The authors thank Dr Jay Horton for reading and commenting on the content of this manuscript.
References
- 1. Abifadel M, Varret M, Rabes JP, Allard D, Ouguerram K, Devillers M, Cruaud C, Benjannet S, Wickham L, Erlich D, Derre A, Villeger L, Farnier M, Beucler I, Bruckert E, Chambaz J, Chanu B, Lecerf JM, Luc G, Moulin P, Weissenbach J, Prat A, Krempf M, Junien C, Seidah NG, Boileau C. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet 2003;34:154–156. [DOI] [PubMed] [Google Scholar]
- 2. Cohen J, Pertsemlidis A, Kotowski IK, Graham R, Garcia CK, Hobbs HH. Low LDL cholesterol in individuals of African descent resulting from frequent nonsense mutations in PCSK9. Nat Genet 2005;37:161–165. [DOI] [PubMed] [Google Scholar]
- 3. Cohen JC, Boerwinkle E, Mosley TH Jr, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med 2006;354:1264–1272. [DOI] [PubMed] [Google Scholar]
- 4. Horton JD, Cohen JC, Hobbs HH. Molecular biology of PCSK9: its role in LDL metabolism. Trends Biochem Sci 2007;32:71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Seidah NG, Prat A. The biology and therapeutic targeting of the proprotein convertases. Nat Rev Drug Disc 2012;11:367–383. [DOI] [PubMed] [Google Scholar]
- 6. Koren MJ, Scott R, Kim JB, Knusel B, Liu T, Lei L, Bolognese M, Wasserman SM. Efficacy, safety, and tolerability of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 as monotherapy in patients with hypercholesterolaemia (MENDEL): a randomised, double-blind, placebo-controlled, phase 2 study. Lancet 2012;380:1995–2006. [DOI] [PubMed] [Google Scholar]
- 7. Stein EA, Mellis S, Yancopoulos GD, Stahl N, Logan D, Smith WB, Lisbon E, Gutierrez M, Webb C, Wu R, Du Y, Kranz T, Gasparino E, Swergold GD. Effect of a monoclonal antibody to PCSK9 on LDL cholesterol. N Engl J Med 2012;366:1108–1118. [DOI] [PubMed] [Google Scholar]
- 8. Ridker PM. LDL cholesterol: controversies and future therapeutic directions. Lancet 2014;384:607–617. [DOI] [PubMed] [Google Scholar]
- 9. Stein EA, Honarpour N, Wasserman SM, Xu F, Scott R, Raal FJ. Effect of the proprotein convertase subtilisin/kexin 9 monoclonal antibody, AMG 145, in homozygous familial hypercholesterolemia. Circulation 2013;128:2113–2120. [DOI] [PubMed] [Google Scholar]
- 10. Lambert G, Chatelais M, Petrides F, Passard M, Thedrez A, Rye KA, Schwahn U, Gusarova V, Blom DJ, Sasiela W, Marais AD Normalization of low-density lipoprotein receptor expression in receptor defective homozygous familial hypercholesterolemia by inhibition of PCSK9 with alirocumab. J Am Coll Cardiol 2014;64:2299–2300. [DOI] [PubMed] [Google Scholar]
- 11. Huijgen R, Boekholdt SM, Arsenault BJ, Bao W, Davaine JM, Tabet F, Petrides F, Rye KA, DeMicco DA, Barter PJ, Kastelein JJP, Lambert G. Plasma PCSK9 levels and clinical outcomes in the TNT (treating to new targets) trial. J Am Coll Cardiol 2012;59:1778–1784(retracted). [DOI] [PubMed] [Google Scholar]
- 12. Werner C, Hoffmann MM, Winkler K, Bohm M, Laufs U. Risk prediction with proprotein convertase subtilisin/kexin type 9 (PCSK9) in patients with stable coronary disease on statin treatment. Vascul Pharmacol 2014;62:94–102. [DOI] [PubMed] [Google Scholar]
- 13. Welder G, Zineh I, Pacanowski MA, Troutt JS, Cao G, Konrad RJ. High-dose atorvastatin casuses a rapid sustained increasein human serum PCSK9 and disrupts its correlation with LDL cholesterol. J Lipid Res 2010;51:2714–2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ridker PM, Cook NR, Lee IM, Gordon D, Gaziano JM, Manson JE, Hennekens CH, Buring JE. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med 2005;352:1293–1304. [DOI] [PubMed] [Google Scholar]
- 15. Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med 2000;342:836–843. [DOI] [PubMed] [Google Scholar]
- 16. Suk Danik J, Rifai N, Buring JE, Ridker PM. Lipoprotein(a), measured with an assay independent of apolipoprotein(a) isoform size, and risk of future cardiovascular events among initially healthy women. JAMA 2006;296:1363–1370. [DOI] [PubMed] [Google Scholar]
- 17. Ridker PM, Stampfer MJ, Rifai N. Novel risk factors for systemic atherosclerosis: a comparison of C-reactive protein, fibrinogen, homocysteine, lipoprotein(a), and standard cholesterol screening as predictors of peripheral arterial disease. JAMA 2001;285:2481–2485. [DOI] [PubMed] [Google Scholar]
- 18. Lakoski SG, Lagace TA, Cohen JC, Horton JD, Hobbs HH. Genetic and metabolic determinants of plasma PCSK9 levels. J Clin Endocrin Metab 2009;94:2537–2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dubuc G, Tremblay M, Pare G, Jacques H, Hamelin J, Benjannet S, Boulet L, Genest J, Bernier L, Seidah NG, Davignon J. A new method for measurement of total plasma PCSK9: clinical applications. J Lipid Res 2010;51:140–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lambert G, Ancellin N, Charlton F, Comas D, Pilot J, Keech A, Patel S, Sullivan DR, Cohn JS, Rye KA, Barter PJ. Plasma PCSK9 concentrations correlate with LDL and total cholesterol in diabetic patients and are decreased by fenofibrate treatment. Clin Chem 2008;54:1038–1045. [DOI] [PubMed] [Google Scholar]
- 21. Mayne J, Raymond A, Chaplin A, Cousins M, Kaefer N, Gyamera-Acheampong C, Seidah HG, Mbikay M, Chretien M, Ooi TC. Plasma PCSK9 levels correlate with cholesterol in men but not women. Biochem Biophys Res Commun 2007;361:451–456. [DOI] [PubMed] [Google Scholar]
- 22. Roubstova A, Munkonda MN, Awan Z, Marcinkiewicz J, CHamberalnd A, Lazure C, Cianflone K, Seidah NG, Prat A. Circulating proprotein convertase subtilisin/kexin 9 (PCSK9) regulates VLDLR protein and triglyceride accumulation in visceral adipose tissue. Arterioscler Thromb Vasc Biol 2011;31:785–791. [DOI] [PubMed] [Google Scholar]
- 23. Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest 2002;109:1125–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zaid A, Roubstova A, Essalmani R, Marcinkiewicz J, Chamberland A, Hamelin J, Tremblay M, Jacques H, Jin W, Davignon J, Seidah NG, Prat A. Proprotein convertase subtilis/kexin type 9 (PCSK9) hepatocyte-specific low-density lipoprotein receptor degradation and critical role in mouse liver regeneration. Hepatology 2008;48 :646–654. [DOI] [PubMed] [Google Scholar]
- 25. Grefhorst A, McNutt MC, Lagace TA, Horton JD. Plasma PCSK9 preferentially reduces liver LDL receptors in mice. J Lipid Res 2008;49:1303–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kosenko T, Golder M, Leblond G, Weng W, Lagace TA. Low density lipoprotein binds to proprotein convertase subtilisin/kexin type-9 (PCSK9) in human plasma and inhibits PCSK9-mediated low density lipoprotein receptor degradation. J Biol Chem 2013;288:8279–8288. [DOI] [PMC free article] [PubMed] [Google Scholar]