Abstract
Jamaica Bay is a major inlet opening to the Atlantic Ocean. It was abundant with oysters until early 1900's. Over-harvesting, pressure from predators, parasitic invasion and declining water quality often are cited as causes. Despite actions to arrest and reverse the pollution, oysters are not reestablished. We are studying factors relating to the rehabitation of Crassostrea virginica in Jamaica Bay to determine if the water quality and environmental conditions are suitable for their survival. Oysters placed in Jamaica Bay grew well when housed in protective containers and growth was influenced by placement near the sediment as compared to the surface. Oysters placed 1 foot above the sediment grew larger that those suspended 1 foot below the surface. Water temperature, pH, turbidity, salinity, conductivity, chlorophyll-a and dissolved O2 were taken to compare water quality at each site. To study growth and survival in a more natural condition, oyster seed and adults were placed just off the bottom in unprotected containers and photographed. After 1 year they are growing and surviving well and there has been evidence of reproduction. Thus far there are no serious signs of predation by crabs or starfish. The study shows that Jamaica Bay water quality is suitable for oyster growth under the various conditions of our experiments.
INTRODUCTION
Jamaica Bay is a 26 square mile estuarial embayment situated between southern Brooklyn and Queens that communicates with Lower New York Bay and the Atlantic Ocean via Rockaway Inlet. Much of the bay is incorporated within the Jamaica Bay Unit of Gateway National Recreation Area, a unit of the National Park Service (NPS). The bay is home to the Jamaica Bay Wildlife Refuge, the only wildlife refuge managed by the NPS, encompassing 9,155 acres of diverse habitats including salt marsh, upland field and woods, fresh and brackish water ponds and an open expanse of bay and islands. Described as “an oasis of nature surrounded by urban development,” Jamaica Bay provides a sanctuary for the protection of wildlife and other natural resources.1 The bay is also a critical component of a larger watershed that drains naturally or via storm sewers, on the seaward-sloping outwash plain south of the harbor hill terminal moraine2, and most of the uplands around the bay, including much of the Rockaway barrier beach, are dominated by urban, residential, commercial, and industrial development. Over the years, dredging, filling, and some major developments like the construction of Floyd Bennett Field and John F. Kennedy Airport have disturbed the bay. About 12,000 of the original 16,000 acres of wetlands have been filled in, mostly around the perimeter of the bay and extensive areas have been dredged for navigation channels or to provide fill for the airports and other construction projects2.
At one time, wild stocks of the Eastern Oyster, Crassostrea virginica, also known as the American Oyster, were found all along the Atlantic and Gulf coasts of North America and for centuries, supported subsistence fishing by Native American and early European colonists3. Historically, C. virginica, flourished in Jamaica Bay and the NY/NJ Harbor area as either self-sustaining or farmed populations4. Jamaica Bay’s oyster industry observed a steady decline in production after its peak in the early 1900’s. Lack of adequate supply of seed oysters, over-harvesting by commercial fishermen, increased pressure from natural predators, parasitic invasion, changing hydrographic patterns, siltation, and a decline in water quality are all cited as possible causes for the decline. The growing urbanization and local industrialization of the area caused severe pollution problems for the bay. Discharges of inadequately treated sewage were poisoning oysters, clams and ultimately people, and by 1921 the U.S. Department of Agriculture had closed shellfish lands in Jamaica Bay altogether. Jamaica Bay was not the only area that suffered a serious loss of oyster beds. During the same time there were declines in estuarine shellfish populations along the entire east coast of the United States and other important oyster fisheries, including that of Chesapeake Bay, also started to collapsed5. Today, very few if any wild oysters are found in Jamaica Bay, and the dramatic loss of this historic oyster bed has permanently altered the structure and function of the bay’s benthic ecosystem.
Since the 1970’s, there have been some major government initiatives aimed at protecting and improving the health of Jamaica Bay. In 1972 federal legislation incorporated much of the bay within Gateway National Recreation Area, creating the Jamaica Bay National Wildlife Refuge, and establishing that management of the bay would be guided by NPS policies regarding both resources and use of the area1. In 1992, the New York State Department of State designated Jamaica Bay as a “significant coastal habitat”6. In 1993, the New York City Department of City Planning designated Jamaica Bay as “one of three special natural waterfront areas”7 and the New York City Department of Environmental Protection completed a comprehensive watershed management plan for the bay to better protect and restore its habitats and improve its water quality8. Other efforts included the construction of sewage treatment plants with improved treatment procedures, and better enforcement of environmental regulations to help deter further pollution. As a result of these initiatives, many marine organisms that had been in decline are now thriving in the bay. However, the once abundant C. virginica has yet to show any signs of reestablishing itself to Jamaica Bay.
According to a Reconnaissance Study by the U.S. Army Corps of Engineers9, Jamaica Bay still exhibits poor water quality. There are contaminated sediments in the area causing adverse effects on benthic organisms and bioaccumulation further up the food chain. Loadings of nutrients and organic matter into the bay from sewage treatment plants and runoff have resulted in phytoplankton blooms and high suspended solid concentrations which, in turn, have increased water turbidity and decreased bottom dissolved oxygen concentrations2. The mean depth of the bay has been increased by dredging from 3 to 13 feet, with some areas dredged deeper than 60 feet, further contributing to hypoxic and anoxic conditions in these poorly flushed areas9. A 2004 report by the New York City Department of Environmental Protection states that throughout the Harbor, Jamaica Bay has suffered the most significant decline in water clarity with high levels of algae growth in specific regions of Jamaica Bay largely responsible for this decline10.
Oyster beds are important to an estuary ecosystem. They provide an environment for a variety of invertebrates, fish and benthic algae; increase habitat structure and faunal diversity; and may reduce turbidity and hypoxia by reducing suspended silt and phytoplankton populations11. The filtering action of oysters can significantly alter the phytoplankton assemblage in an embayment12,13 and dense populations of suspension-feeding shellfish have been shown to have a significant positive impact on basin-wide water quality and phytoplankton dynamics14, 15, 16, 17.
Since efforts to arrest and reverse the pollution problems in the bay has not resulted in an improvement in water quality nor the return of C. virginica to its natural habitat, studies aimed at determining the growth and survival of transplanted oysters, may be the first important step in assessing the feasibility of a future oyster restoration program in Jamaica Bay. In July 2001 our lab initiated a one-year study to monitor the growth and survival of C. virginica seed transplanted in protected surface floats to two ecologically different locations in Jamaica Bay. After just a six-week exposure in the bay, both sites showed substantial oyster growth (up to 110%) and excellent seed survival (over 85%)18. By the end of the one-year period, these oysters had attained a nearly 400% average increase in shell height along the anterio-posterior axis and survival remained over 80%19. While our lab has since used many of the original 2001 seed for various other physiology/biochemical studies, a sampling of 150 of these adults are being maintained in protected floats to follow their long term growth and survival.
To further test the growth and survival of oyster seed under more natural but perhaps stressed conditions, this study was designed to (1) compare the growth and survival of C. virginica when oyster seed was positioned in protected containers one foot off the sediment versus protected floats suspended one foot below the surface in Jamaica Bay; and (2) monitor the growth and survival of oyster seed and adults when placed in an undisturbed and unprotected container at the bottom of the bay.
MATERIALS AND METHODS
In June 2002, four modified Taylor Floats20 of approximately 3’ × 4’ were constructed using PVC tubing and 1/4” mesh nylon screening. Each float was designed to hold up to three 1/8” mesh nylon boxes in which oyster seed were to be placed. Each float had 1/4” nylon mesh lids to keep out predators. Oyster seed of approximately 20 mm anterio-posterial (height) shell lengths and 5 mm shell hinge width were obtained from Frank M. Flower & Sons, Inc. Oyster Nursery in Oyster Bay, NY. 150 oyster seeds were distributed among the 3 nylon boxes in each float. Two floats were positioned 1 foot below the water surface at two ecologically different sites in Jamaica Bay, the Gateway National Park Marine Station (GNPMS) at Fort Tilden, and the Kingsborough Community College Marina (KBCCM) site in Brooklyn’s Sheepshead Bay, a large cove of Jamaica Bay. The two other floats that were designed to sink and position the oyster seed 1 foot off the bay bottom were each placed at the same sites. Over the next 13 months, water quality was monitored and oyster seed growth and survival were measured under each condition. Surface and bottom bay water was collected using a Van Dorn Bottle. Salinity, pH, dissolved oxygen, specific gravity, and temperature were measured with a Horiba Water Quality Testing Instrument. Chlorophyll-a levels were measured spectrophotmetrically using an Aquamate Spectrophotometer and according to the trichromic method of the American Public Health Association21. Both surface and bottom floats were inspected and cleaned of any fouling, biweekly in the summer and monthly in the winter. At those times, the shell lengths (height) and hinge width of each oyster were measured with calipers. After 2 months, the bottom floats were deemed clumsy/difficult to work and were replaced with commercially constructed hanging nets, suspended one foot off the bottom, for the rest of the experiment.
In July 2004, a second set of 300 oyster seed (20 mm average height) and two dozen adult oysters (75 mm average height) were placed directly in an uncovered sunken float and left undisturbed at the bay bottom at KBCCM. Oyster growth and survival were monitored through the summer 2004 and again in summer 2005 using an Atlantis AUW 5600 underwater color camera connected to a monitor. Pictures were captured on a HP 3660 PDA with a Fly Jacket Video Capture card.
RESULTS
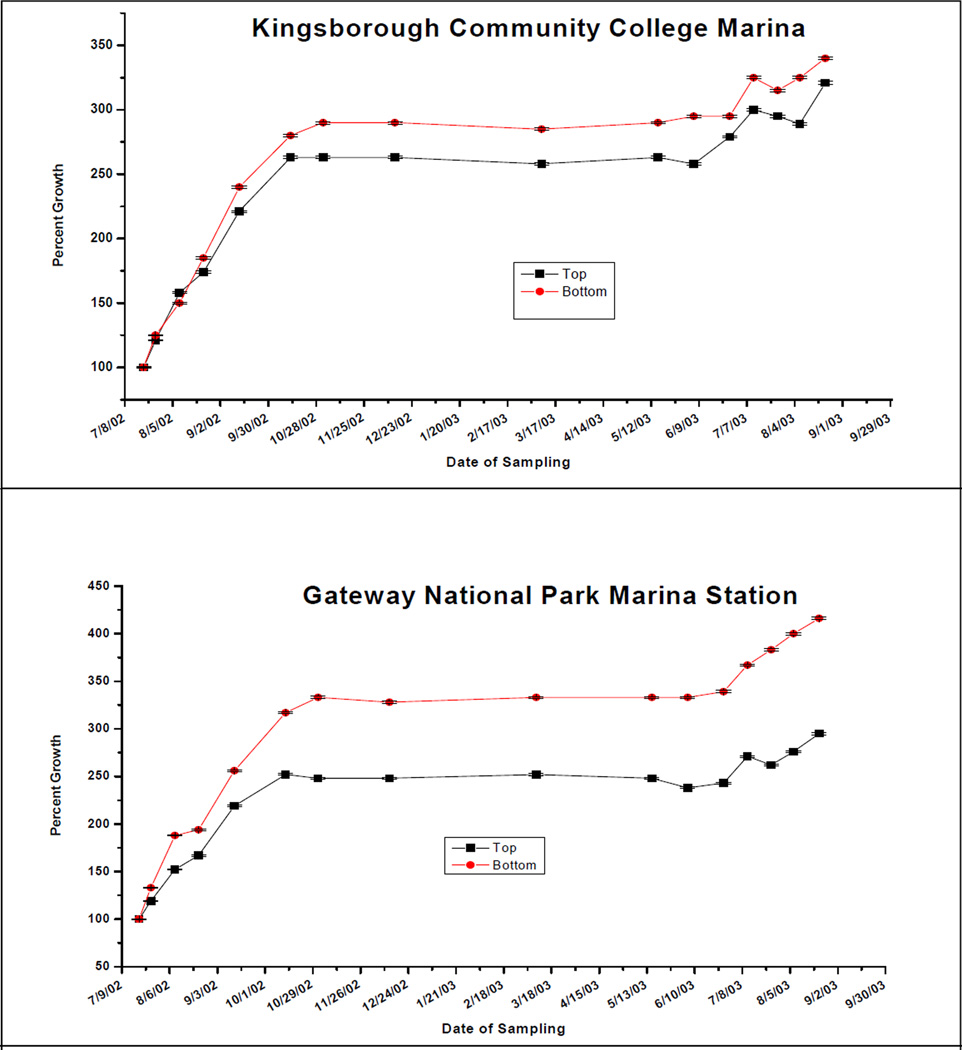
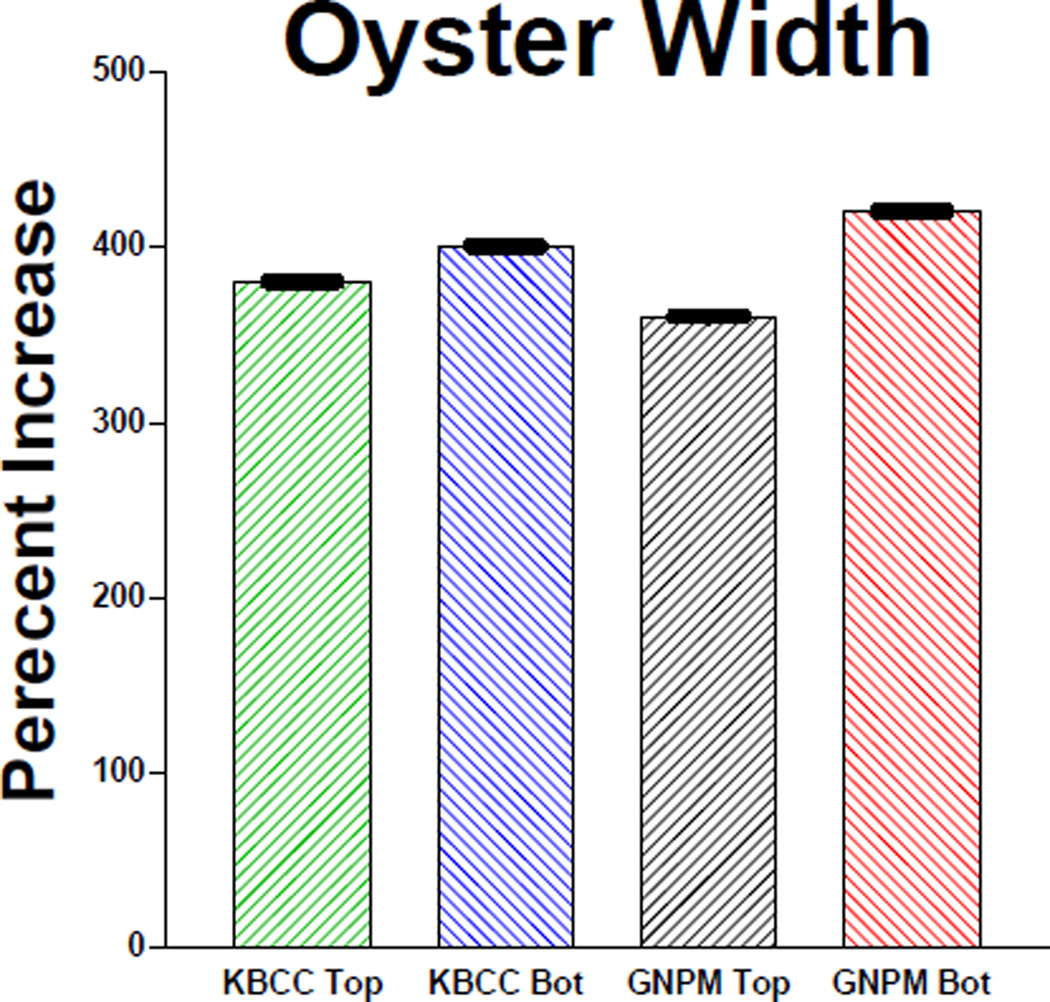
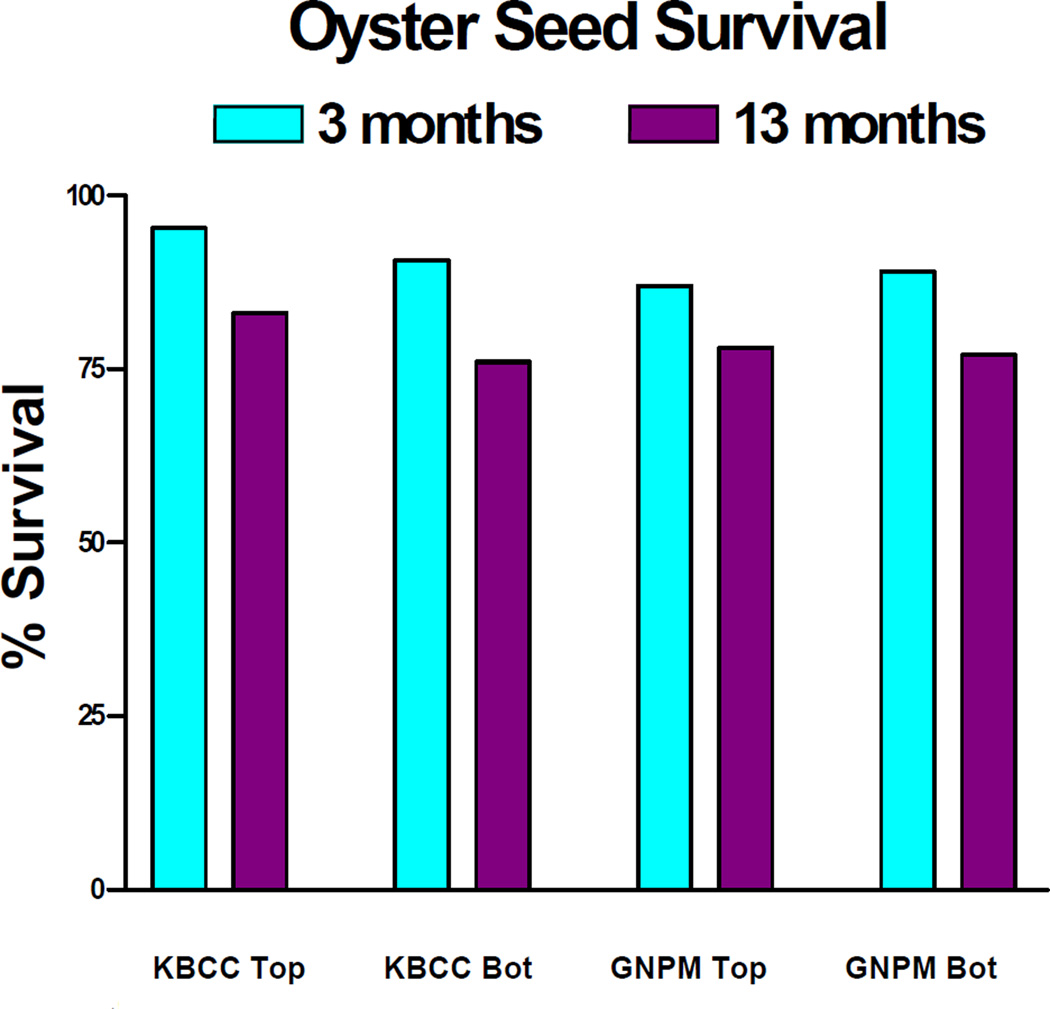
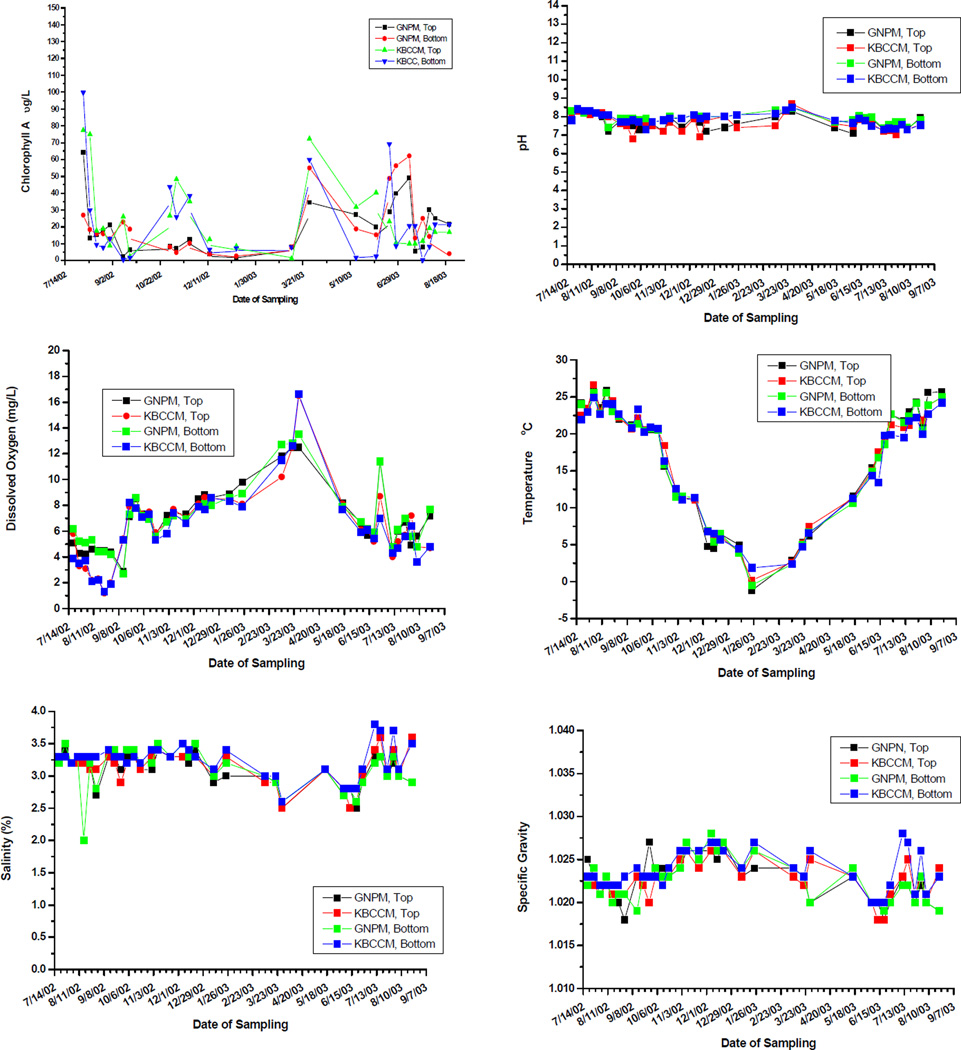
Growth of C. virginica to harvestable size (about 75mm) can take from one to three years, depending on temperature, water salinity and food supply22. Our results indicate that top and bottom C. virginica seed at both locations grew at rates comparable to or better than what has been reported for comparable clean sites. Figure 1 shows the growth of shell height along the anterio-posterial axis of top and bottom positioned oysters over 2 growing seasons (July 2002 to September 2003) at GNPMS and KBCCM. At both sites bottom positioned oysters demonstrated faster growth rates than top positioned oysters. This difference in height growth rates between bottom and top was more significant at the GNPMS site. After 3 months, the rates of bottom seed height growth compared to top seed, were 50% greater at the GNPMS site, but only 18% greater at the KBCCM site. By the end of the 13 month period, bottom seed height growth had exceeded top seed height growth by 60% at the GNPMS site but by only 9% at the KBCCM site. A similar pattern was found when oyster seed hinge width was measured. Figure 2 shows that by the end of the second growing season, width growth rates of bottom oysters at GMPMS and KBCCM were 20% and 7% faster respectively, than their top oyster counterparts. Overall, bottom-positioned oysters at the GNPMS, which grew an average of 420% in height and 410% in hinge width over the 13 month period of the study, showed the best growth rate. Figure 3 shows very good oyster seed survival for top and bottom positioned oysters at both the 3 and 13 month periods. Under the protected conditions of our experiment, there were no significant differences in survival rates for top or bottom position oysters at either location. At 3 months survival was at least 87%, and by the end of the 13 month period oyster seed survival was at least 75%. Figure 4 shows an analysis of the top and bottom water quality at each site between July 2002 and August 2003. There were insignificant differences among the parameters monitored between the two sites or between the top and bottom. The only parameter that correlated significantly with oyster seed growth was water temperature with higher growth rates occurring during warmer periods. No growth correlations were seen with the other parameters measured.
Figure 1.
a,b: 150 oyster seed were place in nylon containers and positioned in Taylor Floats either floating at the surface or just off the bottom. Their height were measure as indicated. The results of the bottom oysters are significantly different from those at the top.
Figure 2.
Measurements of oyster widths at the two sites. N = 150 oysters at each site. The differences in widths between the top and bottom oysters are significantly different.
Figure 3.
Measurement of oyster survival. N = 150 oysters at each site. There were no significant differences between survival rates at either site or between the tops and bottoms.
Figure 4.
Analysis of water samples taken at the top or bottom at the Kingsborough Marina and Gateway National Park Marina.
For Figure 5 an underwater camera was used to film oyster seed and adults that were placed in an uncovered sunken float, left undisturbed at the bay bottom at KBCCM. Photographs indicate minimal siltation or fouling and no evidence of crab or starfish predation. After 1 year, seed grew and the filmed oysters appeared to be in good shape with no signs of mass predation or mortality. While performing maintenance on the floats containing the remaining cohort of the original 2001 oysters, we fortuitously found eight new seed oysters adhering to the inside of a nylon oyster bag, along with 2 seed clams (Figure 6). Two of the seed oysters were alive, 1 had recently died and the others had died at least a few days or weeks earlier based upon tissue degradation.
Figure 5.
Underwater photos of seed (right) and adult (left) oysters placed unprotected on the bottom of the Kingsborough Marina site. Markers = 25 mm.
Figure 6.
Picture of oysters which reproduced in the wild along with 2 clams.
DISCUSSION
This study shows that oyster seed survived and grew very well under the conditions of our experiments. Top and bottom positioned oyster seed, which were protected from predators but still subject to pollutants in the sediment and waters of Jamaica Bay, had very good survival and excellent growth rates. At both sites, oysters positioned one foot above the bottom grew at a statistically significant greater rate than top-positioned oysters. Considering the high turbidity, contaminated sediment, and overall poorly judged benthic environment of Jamaica Bay, these findings were unexpected. Furthermore our results tend to contradict current aquaculture recommendations that stress the use of water column suspension techniques, a method that has become an integral part of the C. virginica industry. Water column suspension technique, also referred to as “off-bottom” culture is the major method used to cultivate oysters all around the world and was designed to minimize bottom predators, provide easier access for harvesting oysters, and maximize the use of a three-dimensional space for cultivation23. In Japan, one of the largest oyster producers, cultivation methods include rafts, lantern nets, and longlines. In Australia, another large oyster-producer, sticks and trays are used. In France and England, oysters are cultivated on off-bottom posts, in mesh nets, and on longlines. In 1962, Shaw reported that seed oysters suspended from log rafts in the waters of Chatham, Massachusetts grew almost twice as fast and had six times greater survival than bottom grown oysters24. Ruesink et al.25 showed that bottom-placed C. gigas has a slower growth rate than those grown on PVC poles in the water column. In the water column, silt loading is reduced and presumably algal concentration is increased and these reasons are attributed to the observed faster growth in oysters in suspended culture rather than those kept on the bottom.
Many explanations can account for the differences seen in the top/bottom growth rates of our experiments. Oysters do best in areas where salinities are from 10 to 30 parts per thousand (ppt), water flow is adequate to bring food, sediment does not smother oysters, and oxygen concentrations remain greater than 3 ppm22. Our analysis of water quality parameters correlates well with what has been reported by others. Measurements taken by the United States Fish and Wildlife Service2 in Jamaica Bay indicate average yearly ranges for temperature of 1 to 26°C, salinity of 20.5 to 26 ppt (2.05 to 2.6 %) dissolved oxygen of 3.5 to 18.5 milligrams/liter, and pH of 6.8 to 9. Algae growth is reported to be on the increase throughout the New York/New Jersey Harbor, possibly due to an increase in nutrients, especially nitrogen, into the Harbor and this trend is most noted as a major problem in Jamaica Bay. Through the late 1980s Jamaica Bay had summer chlorophyll-a averages in the 10–20 µg/l range; since 1992, values have ranged from 30–50 µg/l10. While our salinity values were higher than what has been reported by the USFWS, this can be explained by the fact that both sites at located near Rockaway Inlet and would therefore be more expected to have salinities closer to that of ocean water (35 ppt). While standard water quality parameters showed no significant difference between the GNPMS and the KBCCM sites or between top and bottom (fig. 4), correlating oyster growth to water quality is complicated and not straightforward.
The fact that our bottom-positioned oysters had faster growth rates might be site specific. Debris and organic pollutants floating on the bay surface may be a factor, as might the more constant stress of surface wave movements at both marina sites. While measurements of chlorophyll-a in surface/bottom water showed no consistent variation, the type and quality of microalgae available to the two groups of oysters might be different. Alternatively, our bottom-dwelling oysters may not have done as well if they weren’t periodically cleaned of silt and other fouling, or if they had been positioned directly and left undisturbed on the soft sediment of the bay. These are some of the factors that will be considered in our future studies of oyster growth.
Oysters provide a habitat for both commensal and competing organisms. The boring sponge, barnacles, tunicates, clam worms, and several species of algae were found living on both top and bottom positioned oysters with no major detrimental effects. Occasional small mud crabs were found with our protected oysters, and they were removed during float/cage maintenance. When not cultivated in protected environments, oysters are also subject to a variety of natural large predators. Depending upon salinity, oyster drills, rock crabs, knobbed whelks, channeled whelks and starfish can all prey on oysters, but none of these predators were found with our protected oysters. Photos of the oysters, which were positioned at the bottom in sunken floats without protection from large predators, also appeared to be surviving and growing well without signs of serious predation.
The severity of impacts by disease and parasites on oyster populations is thought to be related to water quality; higher salinity, high temperatures, and nutrient loading appear to make oysters more susceptible to disease. Protistan parasites, in particular Perkinsus marinus (Dermo disease) and Haplosporidium nelsoni (MSX disease) are currently considered to be major threats on the longterm survival of C. virginica26. While neither organism is harmful to humans, nor a health threat to humans who ingest infected shellfish, these parasites can chronically weaken and eventually kill C. virginica over a period of years26, 27. Both parasites thrive in salinities above 15 ppt and exhibit lowered virulence at lower salinities28. This may become a major problem for our transplanted oysters since both sites have higher than average bay salinity. We will be monitoring the infection rates of both these organisms as part of our long-term study on growth and survival of C. virginica in Jamaica Bay.
Historically, Jamaica Bay was a site of extensive oyster beds and shellfish culture leases that supported a significant oyster fishery in New York dating back to the 1800's. The Canarsie area in the northwest portion of the bay was a center of commercial and recreational fishing with a substantial shellfish industry for mainly oysters and hard clams that reached its peak in the early 20th century29. The decline in wild C. virginica stocks throughout the eastern seaboard of the United States3 has led to concerted efforts focused on oyster restoration30 in many areas including a major effort in Chesapeake Bay as well other efforts in Connecticut, Virginia, Massachusetts, Florida, and North and South Carolina. Locally, the NY/NJ BAYKEEPER® (Baykeeper) has been working since 1997 to restore oyster habitat at 3 sites in the Hudson-Raritan Estuary including Liberty Flats in the Upper New York Bay, Keyport Harbor in Raritan Bay, and the Oyster Point on the Navesink River31. However, no such restoration attempts have yet been made in Jamaica Bay.
Jamaica Bay is one of the most valuable natural resources within the New York City area. Its ecological significance has been recognized by city, state and federal agencies. However, the bay continues to be threatened by poor water quality, loss of upland and wetland buffer, and disturbance of habitat areas2. Attempts to reestablish oyster beds to Jamaica Bay may be one way to help preserve/improve the bay’s ecosystem. While modern sewage technology and regulatory efforts to arrest the further polluting of the bay are important, the resurgence of oysters will provide an added and natural means of improving Jamaica Bay water quality.
Acknowledgments
This work was supported by grants 0516051091 of the New York State Education Department, 1R25GM62003 of NIGMS, 0516041071 of the New York State Education Department, the CUNY Groundworks Program, and 66288-0035 of PSC-CUNY to G. Sarinsky, M.A. Carroll, E. Nduka and E.J. Catapane. We thank Frank M. Flower & Sons, Inc., Oyster Bay, NY for supplying oysters. We thank the following students for their dedicated work on the project: J. Attia, W. Barreiro, S. Edwards, J. Espinoza, A. Greene, L. Gumenik, C. King, K. Lett, J. Luxama, S. Persaud, I. Reid, R. Rodriguiz, M. Stewart-Walker, M. Tejeda and O. Urdanigo.
REFERENCES
- 1.National Park Service (NPS) US Department of the Interior, Gateway National Recreation Area - Jamaica Bay Unit. The Evolving Legacy of Jamaica Bay. 2003:21. [Google Scholar]
- 2.United States Fish and Wildlife Service (USFWS) Significant Habitats and Habitat Complexes of the New York Bight Watershed, Southern New England–New York Bight Coastal Ecosystems Program, Jamaica Bay and Breezy Point, Complex #16, November 1997. 1997 [Google Scholar]
- 3.MacKenzie CL Jr, Burrell VG Jr, Rosenfield A, Hobart WL, editors. The history, present condition, and future of the molluscan fisheries of North and Central America and Europe. Vol. 1. Seattle, WA: Atlantic and Gulf Coasts. US Dept. Commerce, NOAA Tech. Rep. NMFS 127; 1997. p. 234. [Google Scholar]
- 4.New York/New Jersey Harbor Estuary Program (NYNJHEP) Health of the Harbor - The first Comprehensive Look at the State of the NY/NJ Harbor Estuary. NY: Hudson River Foundation; 2004. p. 82. [Google Scholar]
- 5.MacKenzie CL. History of oystering in the United States and Canada, featuring the eight greatest oyster estuaries. Mar. Fish. Rev. 1996a;58:1–78. [Google Scholar]
- 6.New York State Department of State (NYSDOS), Division of Coastal Resources and Waterfront Revitalization. Significant coastal fish and wildlife habitats program. Jamaica Bay habitat narrative, Breezy Point habitat narrative. 1992 [Google Scholar]
- 7.New York City Department of City Planning (NYC Dept. City Planning) Plan for the Queens Waterfront, New York City comprehensive waterfront plan: Reach 17 – Jamaica Bay and Rockaway. 1993:125–179. NYC DCP 93-43. [Google Scholar]
- 8.New York City Department of Environmental Protection (NYCDEP) Jamaica Bay comprehensive watershed management plan. 1993:44. [Google Scholar]
- 9.United States Army Corps of Engineers (USACE) Section 905(b) WRDA 86 Preliminary Analysis. New York: 2000. Reconnaissance Study, Hudson-Raritan Estuary Environmental Restoration Study. [Google Scholar]
- 10.New York City Department of Environmental Protection (NYCDEP) New York Harbor Water Quality Report. 2004 Jul;:60. 2004. [Google Scholar]
- 11.MacKenzie CL. Management of Natural Populations. In: Kennedy VS, Newell RIE, Eble AF, editors. The eastern oyster, Crassostrea virginica. College Park, MD: Maryland Sea Grant College; 1996b. pp. 707–721. [Google Scholar]
- 12.Newell RIE. Ecological changes in Chesapeake Bay: are they the result of overharvesting the American oyster, Crassostrea virginica? In: Lynch MP, Krome EC, editors. Understanding the Estuary: Advances in Chesapeake Bay Research. Gloucester Point, VA: Chesapeake Research Consortium, Publication 129 CBP/TRS 24/88; 1988. pp. 536–546. [Google Scholar]
- 13.Dame RF, Chrzanowski T, Bildstein K, Kjerfve B, McKeller H, Nelson D, Spurrier J, Stevenson S, Vernberg J, Zingmark R. The outwelling hypothesis and North Inlet, South Carolina. Mar. Ecol. Prog. Ser. 1986;33:217–229. [Google Scholar]
- 14.Cloern JE. Does the benthos control phytoplankton biomass in South San Francisco Bay? Mar. Ecol. Prog. Ser. 1982;9:191–202. [Google Scholar]
- 15.Cohen RRH, Dresler PV, Phillips EJP, Cory RL. The effect of the Asiatic clam, Corbicula fluminea, on phytoplankton of the Potamac River, Maryland. Limnol. Oceanogr. 1984;29:170–180. [Google Scholar]
- 16.Fréchette M, Butman CA, Geyer WR. The importance of boundary-layer flows in supplying phytoplankton to the benthic suspension feeder. Limnol. Oceanogr. 1989;34:19–36. [Google Scholar]
- 17.Dame RF. Ecology of Bivalves: An Ecosystem Approach. Boca Raton, FL: CRC Press; 1996. p. 254. [Google Scholar]
- 18.Edwards S, Nduka E, Barrerio W, Carroll MA, Catapane EJ, Sarinsky G. Rehabitation of the American Oyster, Crassostrea virginica in Jamaica Bay, New York; Proceedings of the 2001 Biomedical Research Conference for Minority Students; 2001. p. 109. (abstract) [Google Scholar]
- 19.Rodriguez R, Sarinsky G, Catapane EJ, Barreiro W, Carroll MA, Nduka E. A One Year Report on the Rehabitation of the American Oyster, Crassostrea virginica, in Jamaica Bay, NY. Annals of the ABRCMS Conference. 2002;2:375–376. (abstract) [Google Scholar]
- 20.Luckenbach MW, O’Beirn FX, Taylor J. An Introduction to Culturing Oysters in Virginia. Gloucester Point, VA: Virginia Institute of Marine Science Press; 1999a. p. 24. On-line at http://www.vims.edu/oystergarden/CulturingOysters.pdf. [Google Scholar]
- 21.American Public Health Association (APHA) American Water Works Association and Water Environment Federation, Standard Methods for the Examination of Water and Wastewater – 20th Edition. Method 10200 H.2.c. Chlorophyll a, b, c, trichromic method. 1998:10–20. [Google Scholar]
- 22.Wallace RK. Cultivating the Eastern Oyster, Crassostrea virginica. Southern Regional Aquaculture Center (SRAC); 2001. Aug, Publication No. 432, 2001. [Google Scholar]
- 23.Coastal Zone Management (CZM) Shellfish Bottom and Off-Bottom Culture in Massachusetts Aquaculture White Paper and Strategic Plan. Boston: Massachusetts Coastal Zone Management; 1995. p. 236. [Google Scholar]
- 24.Shaw WN. Raft culture of oysters in Massachusetts. U.S. Fish Wildl. Serv., Fish. Bull. 1962;61:481–495. [Google Scholar]
- 25.Ruesink JL, Roegner CC, Dumbauld BR, Newton J, Armstrong DA. Contributions of Coastal and Watershed Energy Sources to Secondary Production in a Northeastern Pacific Estuary. Estuaries. 2003;26(4B):1079–1093. [Google Scholar]
- 26.Ford SE, Tripp MR. Diseases and Defense Mechanisms. In: Kennedy VS, Newell RIE, Eble AF, editors. The Eastern Oyster Crassostrea virginica. College Park, Maryland: Maryland Sea Grant College; 1996. pp. 581–660. [Google Scholar]
- 27.Lenihan HS, Micheli F, Shelton SH, Peterson CH. The influence of multiple environmental stressors on susceptibility to parasites: an experimental determination with oysters. Limnology and Oceanography. 1999;44(3):910–924. [Google Scholar]
- 28.Ford SE. Avoiding the spread of disease in commercial culture of molluscs,with special reference to Perkinsus marinus (Dermo) and Haplosporidium nelsoni (MSX) J. Shellfish Res. 1992;11:539–546. [Google Scholar]
- 29.New York City Department of Environmental Protection (NYCDEP) Use and Standards Attainment Project Preliminary Waterbody /Watershed Characterization Report, Jamaica Bay. 2001 May;:8. 2001. [Google Scholar]
- 30.Luckenbach MW, Mann R, Wesson JA, editors. Oyster reef habitat restoration: a synopsis and synthesis of approaches. Gloucester Point, VA: Virginia Institute of Marine Science Press; 1999b. p. 366. [Google Scholar]
- 31.Baykeeper. The NY/NJ Baykeeper Oyster Restoration Program Annual Report. 2005:34. online at: http://www.nynjbaykeeper.org/sitefiles/anoysrep.pdf. [Google Scholar]