Abstract
The effects of saturated fatty acids (SFAs) on cardiovascular disease (CVD) risk are modulated by the nutrients that replace them and their food matrices. Replacement of SFAs with polyunsaturated fatty acids has been associated with reduced CVD risk, although there is heterogeneity in both fatty acid categories. In contrast, replacement of SFAs with carbohydrates, particularly sugar, has been associated with no improvement or even a worsening of CVD risk, at least in part through effects on atherogenic dyslipidemia, a cluster of traits including small, dense low-density lipoprotein particles. The effects of dietary SFAs on insulin sensitivity, inflammation, vascular function, and thrombosis are less clear. There is growing evidence that SFAs in the context of dairy foods, particularly fermented dairy products, have neutral or inverse associations with CVD. Overall dietary patterns emphasizing vegetables, fish, nuts, and whole versus processed grains form the basis of heart-healthy eating and should supersede a focus on macronutrient composition.
Keywords: atherogenic dyslipidemia, lipids, diet, sugar, metabolism
INTRODUCTION
Despite improvements in its prevention and management, cardiovascular disease (CVD) continues to be the leading cause of morbidity and mortality in the United States, and reduction of low-density lipoprotein cholesterol (LDL-C) by reducing dietary saturated fatty acids (SFAs) remains a cornerstone of current recommendations for CVD prevention. However, recent studies have called into question the presumed relationship between dietary SFAs per se and CVD (29, 114, 159, 163), in part due to the need to consider the nutrients that replace SFAs (161) as well as effects that are dependent on the food source of SFAs (5). Whereas most epidemiological and clinical trial data support the replacement of SFAs with polyunsaturated fatty acids (PUFAs), particularly omega-3 (ω-3) fatty acids, for cardiovascular benefit, replacement of SFAs with dietary carbohydrates (CHOs) has been associated with no improvement or even a worsening of CVD risk (78, 115). This may be attributable at least in part to adverse effects on atherogenic dyslipidemia, a common trait characterized by elevated triglyceride (TG), reduced HDL cholesterol (HDL-C), and increased concentrations of small, dense LDL (sdLDL) particles (94, 95, 111, 113).
Atherogenic dyslipidemia is a central feature of metabolic syndrome and is particularly prevalent in individuals with abdominal adiposity and insulin resistance, in whom levels of cholesterol-depleted sdLDL can be increased disproportionately to total LDL-C. Notably, large prospective cohort studies have demonstrated significant and independent associations of sdLDL with increased CVD risk (72, 118, 126, 135, 172). The established effects of high CHO intake on atherogenic dyslipidemia, and recent studies linking high sugar intake with deleterious effects on cardiometabolic health and increased CVD and total mortality (169, 191), support the recommendation of the 2015 US Dietary Guidelines Advisory Committee (DGAC) to limit added sugars to a maximum of 10% of total energy (40).
The 2015 DGAC also retained the upper limit for SFA intake of 10% of energy, in contrast to the recently proposed limit of 5–6% of energy in the American Heart Association/American College of Cardiology (AHA/ACC) Dietary Guidelines (45), in part reflecting concern that more extreme SFA restriction may be accompanied by increased consumption of CHOs (75). The main premises for limiting dietary SFAs are (a) the prediction of a progressive reduction in CVD risk associated with greater reductions in LDL-C and (b) evidence for benefits on CVD risk of dietary patterns that include low SFA intake, i.e., Dietary Approaches to Stop Hypertension (DASH) and Mediterranean diets (3, 49). However, in the context of the current epidemics of obesity and insulin resistance, reduction of SFAs may be less important for the reduction of CVD risk in the population than limitation of dietary CHOs, in particular refined CHOs and sugar (27, 39, 53, 60, 80, 94, 100). Moreover, given evidence for variation in effects of different SFAs on CVD risk biomarkers, particularly lipids and lipoproteins (113), as well as the overall effects of the food matrices in which SFAs are consumed (36), blanket recommendations to reduce total SFAs may not be appropriate. Finally, the multifactorial nature of dietary patterns such as the DASH and Mediterranean diets preclude attribution of their cardiovascular benefits specifically to their content of SFAs. For example, it is not known how modifications of the DASH and Mediterranean diets that include higher SFAs might affect CVD risk.
We review here the evidence for effects of SFAs versus PUFAs versus CHOs on CVD risk factors and clinical outcomes, and show that these effects can be influenced by food sources as well as types of these macronutrients. Thus, although the study of individual dietary constituents has greatly advanced understanding of nutritional influences on CVD, we conclude that greater emphasis should be placed on whole foods and overall dietary patterns for achieving and maintaining cardiovascular health.
DIET AND CARDIOVASCULAR DISEASE RISK FACTORS
The effects of diet and macronutrient replacements on CVD risk are mediated by numerous physiological factors, including lipids and lipoproteins, inflammation, insulin sensitivity, blood pressure (BP), thrombosis, and vascular function. Although multifactorial algorithms have been developed that provide good statistical reliability for CVD risk prediction on a population basis (62), the variation and interplay of pathophysiologic factors presents a challenge for accurate risk assessment in individuals. Among modifiable CVD risk biomarkers, LDL-C and blood pressure have been the most strongly validated (110), and despite the complexity of individual risk assessment, they provide the major rationale for therapies aimed at reducing disease risk. As noted above, however, and described further below, reliance on identifying dietary macronutrient effects on LDL-C may obscure effects on LDL particles, especially sdLDL, that may have more direct and specific effects on the development and progression of CVD (93).
LIPIDS AND LIPOPROTEINS
Macronutrient-Influenced Lipoprotein Metabolism Pathways and Atherogenesis
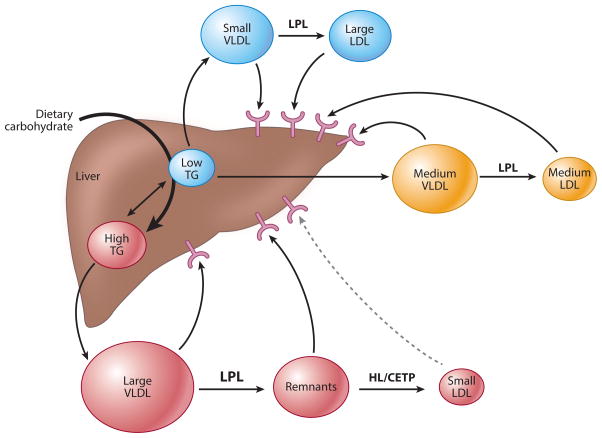
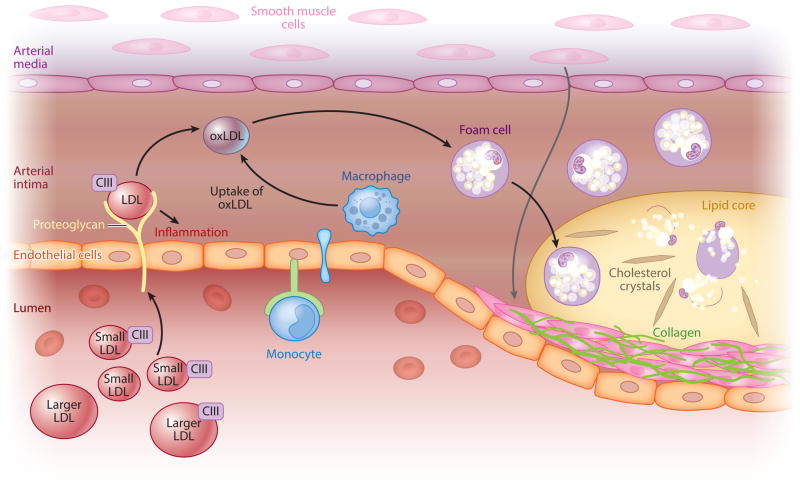
SFAs can suppress LDL receptor activity, resulting in reduced LDL clearance, and may also increase LDL production rate (18, 42). On the other hand, ω-6 PUFAs can increase LDL receptor activity and reduce LDL production rate (31, 154, 173). Dietary CHOs as well as excess calories have been shown to increase hepatic TG stores, thereby driving the secretion of large TG-enriched particles that undergo intravascular lipolysis and remodeling, ultimately giving rise to sdLDL (18) (Figure 1). This pathway is juxtaposed to other pathways of lipoprotein assembly and secretion that result in smaller very-low-density lipoprotein (VLDL) particles that are catabolized to larger LDL particles. Relative to larger LDL particles, sdLDL particles are less easily cleared from the circulation due to reduced receptor-mediated uptake and thus have a longer intravascular residence time (18). Additional mechanisms by which sdLDL may have relatively greater atherogenic potential than larger LDL include their higher affinity for binding to proteoglycans and increased susceptibility to oxidation, resulting in greater arterial retention and capacity to trigger inflammatory processes (Figure 2). Thus, dietary FAs and CHOs differentially affect lipoprotein metabolism pathways, resulting in plasma levels of LDL particles that vary in size, density, and atherogenic potential.
Figure 1.
Pathways of lipoprotein metabolism. Dietary carbohydrate increases hepatic TG that drives the secretion of very-low-density lipoproteins (VLDLs) that are larger and triglyceride (TG) enriched. These particles are rapidly lipolysed by lipoprotein lipase (LPL) to remnant lipoproteins that are then catabolized by hepatic lipase (HL) to small, dense low-density lipoprotein (LDL) particles that are less efficiently cleared from plasma, likely due to reduced LDL receptor affinity. Dietary saturated fat has been shown to preferentially increase plasma concentrations of larger LDL particles, likely by reducing their plasma clearance through suppression of LDL receptor activity, although increased hepatic secretion of their precursors may also play a role. Abbreviation: CETP, cholesteryl ester transfer protein.
Figure 2.
Low-density lipoprotein (LDL) particles and atherogenesis. In the initial steps of atherogenesis, LDL particles circulating in the blood infiltrate the endothelial layer of arteries and are bound by proteoglycans and become oxidized. This triggers inflammatory processes and foam cell formation by responding macrophages. These lipid-laden foam cells form the core of the atherosclerotic plaque and can amplify local inflammation and promote thrombosis. Apolipoprotein CIII (apoCIII), an exchangeable apoprotein whose concentrations vary on apoB-containing particles, has been shown to play a direct role in some of these processes. Small, dense LDL is considered more atherogenic due to its longer plasma residence time, higher apoCIII content, greater arterial retention, and increased susceptibility to oxidation, triggering inflammatory and thrombotic processes. Abbreviation: oxLDL, oxidized LDL.
Replacement of Saturated Fatty Acids with Carbohydrates
On average, dietary SFAs increase LDL-C concentrations by ~13 mg/dl when they replace 10% of energy as dietary CHOs (111). Specific SFAs have been shown to exert differing effects, such that in general there are progressive increases in LDL-C with diminishing chain length. Thus, the potency of the LDL-raising effects of individual SFAs are as follows: lauric acid (C12:0) > myristic acid (C14:0) > palmitic acid (C16:0). Lauric acid relative to other SFAs increases HDL-C significantly and thereby reduces the total cholesterol (TC):HDL-C ratio (113). In contrast, stearic acid (C18:0) generally has a neutral effect on lipid and lipoprotein profiles when replacing CHO (113).
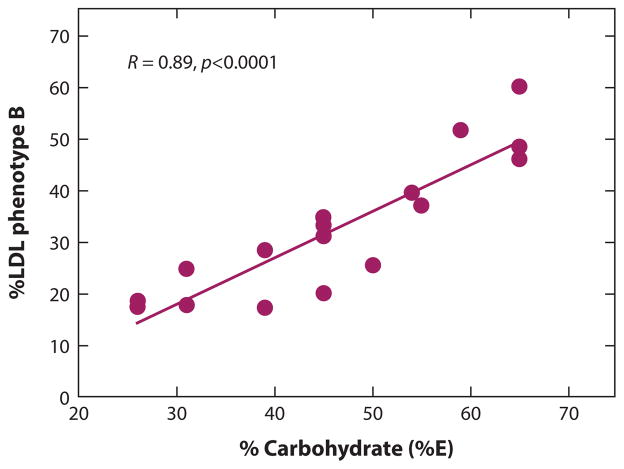
SdLDLs have been shown to be reduced with lower CHO intake, and this effect was found to be independent of high versus low dietary SFAs (15% versus ~8% of energy) when CHO intake was limited to 26% of energy (94). Further, the prevalence of LDL subclass pattern B, a categorical marker for atherogenic dyslipidemia defined by the predominance of sdLDL, has been linearly and positively associated with increasing concentrations of dietary CHOs in randomized controlled clinical trials (RCTs) (94) (Figure 3) due to effects of CHO that can occur in as few as three days (65). In contrast to CHOs, the main effect of dietary SFAs appears to be on larger LDL particles that are less strongly associated with CVD, as noted above; thus, SFA-induced increases in LDL-C may not signify an increase in CVD risk commensurate with that predicted from the relationship of LDL-C to CVD risk in the population (93).
Figure 3.
Dietary carbohydrate (CHO) and low-density lipoprotein (LDL) pattern B. Variation in dietary CHO is correlated with the prevalence of pattern B (R = 0.89; p < 0.0001) in metabolic feeding studies (92, 94, 105) (n = 833 men). Each data point is the summation of the response of at least 40 individuals to a dietary regimen that controlled for CHO and lasted three to six weeks. Abbreviation: E, energy.
Notably, the protein apolipoprotein CIII (apoCIII) represents a potential biomarker of the metabolic pathway leading to the production of sdLDL that likely contributes to the CVD risk of this pathway (93). ApoCIII has been found consistently to be associated with the risk of CVD likely because of combined effects of increased VLDL secretion (192), impaired VLDL and TG-rich lipoprotein remnant catabolism (57) related in part to its abundance relative to apoE (146), and proinflammatory activity (87). Further, apoCIII in apoB-containing particles is increased with high-CHO diets, with particular enrichment in very small LDL particles (Figure 2) (59, 156). Earlier kinetic studies showing increased apoCIII pool size, but no change in fractional catabolic rate, in men fed a high-CHO (68% of energy) compared to a low-CHO (39% of energy) diet (77) suggest that increased hepatic apoCIII production may be intimately related to the metabolic processes by which dietary CHO induces atherogenic dyslipidemia.
Of note, fasting plasma concentrations of SFAs are influenced by endogenous and exogenous pathways, and dietary CHOs appear to be more important determinants of plasma SFAs than dietary SFAs (55, 56, 71). A recent hypocaloric feeding study in 16 patients with metabolic syndrome showed no effects of increased dietary SFAs on plasma SFAs in the context of a very-low-CHO diet (177). In contrast, progressive decreases in dietary SFAs with concomitant stepwise increases in dietary CHOs were associated with incremental increases in palmitoleic acid in plasma TG and cholesteryl esters (177). Both increased plasma SFAs and palmitoleic acid, a desaturation product of SFAs formed during de novo lipogenesis, have been associated with adverse cardiometabolic profiles (177). These findings underscore the significant contribution of dietary CHOs to plasma biomarkers of CVD risk.
Carbohydrate quality
The effects of CHO quality on lipid and lipoprotein profiles have been extensively investigated. A meta-analysis of RCTs showed a positive relationship between dietary sugars and plasma TG, TC, and LDL-C, independent of effects on body weight (169). An analysis of National Health and Nutrition Examination Survey (NHANES) data also showed positive and inverse associations of added sugars with TG and HDL-C, respectively (183). The adverse effects of high sugar intake may be attributable in part to their fructose component, which has been shown to promote dyslipidemia, decrease insulin sensitivity, and increase visceral adiposity (167, 168). Of note, the effects of fructose appear to be most reproducible at high levels of consumption (>100 g/day) and in overweight or obese individuals (180), and may result in part from increased total energy consumption (157).
The quality of dietary CHOs has also been expressed in terms of their ability to raise blood glucose, in comparison to white bread or glucose, a characteristic referred to as the glycemic index (GI), or glycemic load (GL), which is the product of GI and CHO content. However, in the recently completed OmniCarb study, the largest clinical trial (n = 163) conducted to date to test effects of high- versus low-GI diets in the context of moderate- and low-CHO diets, increases in TG and decreases in HDL-C concentrations were attributed to higher total CHO rather than higher GI (148). GL was positively associated with plasma TG (P = 0.008) and inversely associated with HDL-C (P = 0.004) in 878 postmenopausal participants in the Women’s Health Initiative Observational Study (155), in overall agreement with an earlier meta-analysis of 32 RCTs that showed a 10% reduction in plasma TG for a 30 to 100 g eq./day reduction in GL (102). Taken together, the above observations are consistent with the notion that the CHO content of the diet, as reflected by the GL as well as sugar content, contributes to a greater extent to features of atherogenic dyslipidemia than does the GI.
A limited number of human trials have compared the lipid responses to rapidly digested versus resistant starches, with inconsistent findings. When contributing 19–24% of daily energy, diets high in amylose, a resistant starch, modestly reduced plasma TG and LDL-C (13, 14), but such effects were not observed when amylose contributed ≤10% of daily energy (69, 82, 130), suggesting that improvements occur only at high intake levels. How starch quality affects more detailed lipid and lipoprotein parameters of atherogenic dyslipidemia remains to be established.
Replacement of Saturated Fatty Acids with Other Fats or Protein
Replacement of SFAs with monounsaturated fatty acids (MUFAs) or ω-6 PUFAs decreases total, LDL, and HDL-C (111) and decreases the TC:HDL ratio (113). In the DELTA study, replacing 7% kcal from SFAs with MUFAs versus CHOs led to similar reductions in LDL-C, a lesser reduction in HDL-C, and lack of increase in TG (17). Similarly, greater reductions in TC and TG concentrations and thus improvements in lipid profiles were observed with MUFAs versus CHOs as a substitution for SFAs at 10% of total energy in the OmniHeart study (3). Of note, replacement of SFAs by MUFAs in experimental animal models has been shown to increase the cholesteryl oleate content of secreted LDL, a property associated with increased atherogenicity (37). With regard to effects of dietary protein, OmniHeart showed an even greater improvement in lipid profiles with protein versus MUFA replacement of CHOs (3) as well as significant reductions in atherogenic apoCIII-containing LDL and VLDL (59). Although these results suggest that protein may lower TG levels independent of the effect of reducing carbohydrate content, several feeding studies have not shown effects of dietary protein on lipid and lipoprotein profiles (27, 94).
Improvements in serum lipids and lipoproteins, including changes in the TC:HDL-C ratio, are slightly greater with PUFA relative to MUFA replacement of SFAs (111, 113). Of note, the effects of SFAs on lipids and lipoproteins may be modulated by PUFA availability, such that LDL-C is only increased by SFAs if PUFA intake is below ~5% of energy (68, 186). In a metabolic study, no effects of SFAs (22% versus 10% total energy) derived from coconut oil were observed on LDL-C, TC, or apoB when the polyunsaturated fat:saturated fat (P:S) ratio was kept constant at ~0.15 (125). In contrast, higher P:S ratios, i.e., 1.9 versus 0.14, in the context of diets with 38% of energy from fat, were associated with significantly lower ratios of LDL to HDL (125). These data suggest that the proportions of fatty acids may be more important in modulating CVD risk than the absolute amount of SFAs and PUFAs per se (125). The ω-3 fatty acids, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), have been shown to be effective in reducing plasma TG concentrations (11). This effect may be mediated in part by reducing hepatic TG synthesis and secretion of VLDL particles and by increasing TG catabolism through up-regulation of relevant enzymes such as lipoprotein lipase (11).
Replacement of SFAs with trans fatty acids (TFAs) increases LDL-C, decreases HDL-C, and increases the ratio of TC to HDL-C (113), effects that may be more pronounced in women than in men (26). On the other hand, the replacement of TFAs from partially hydrogenated vegetable oils with SFAs, MUFAs, or PUFAs has been associated with decreased TG, Lp(a), and ratios of TC to HDL-C and apoB to apoAI (122). On the basis of effects on lipoprotein profiles and C-reactive protein (CRP), reductions in coronary heart disease (CHD) risk were estimated at −2.7%, −11.9%, and −16.6%, respectively, when 20%, 35%, and 45% TFAs from partially hydrogenated vegetable oils were replaced with SFAs from butter (122), although changes in CRP cannot be inferred to yield CVD benefit. It has been suggested that ruminant sources of TFAs, in particular, conjugated linoleic acid (CLA), may exert different biological effects compared to industrial sources of TFAs. However, a summary review of intervention trials addressing this showed that TFAs from industrial hydrogenation (n = 29 studies), ruminant sources (n = 6 studies), and CLA (n = 17 studies) all increased the LDL:HDL ratio to a similar extent (22).
Postprandial Lipemia
The postprandial accumulation of lipoproteins and their remnants is increasingly recognized as contributing to CVD risk, an association that may be more robust in women than in men (reviewed in 21). The atherogenicity of these particles may be due in part to their high cholesterol ester content and the ability of small remnant particles to penetrate the arterial intima (reviewed in 16). The postprandial response to a fat-containing meal can be modulated both by the composition of the habitual diet consumed chronically and by the nutritional content of the challenge meal consumed acutely (16, 97). Replacing 7% kcal from SFAs with CHOs in the background diet resulted in a modest increase in fasting TG but had no effect on postprandial TGs (17), consistent with a shorter-term study using more extreme variations in fat and CHO intake in the background diets (34). In contrast, consuming fructose versus glucose over 10 weeks increased postprandial TGs by increasing hepatic TG synthesis and de novo lipogenesis (168). Furthermore, the addition of fructose versus glucose to a challenge meal high in SFAs resulted in a higher and delayed TG peak in Sf (flotation rate) >400 lipoproteins, and a slower return of TG to fasting levels in Sf 20–400 lipoproteins (28). In a recent study in which monosaccharides were fed alone or in combination with a high-SFA meal, the simultaneous intake of fructose and fat versus fat alone resulted in greater incremental areas under the 0- to 6-hour curve for plasma TG, apoB48, and remnant lipoprotein-TG and cholesterol, as well as significantly higher rises and delayed postprandial peaks in all the aforementioned analytes (149).
In a study designed to test the effect of dietary fat saturation on the magnitude and duration of the postprandial response (15), background diets and challenge meals high in SFAs or ω-6 PUFAs resulted in similar postprandial responses of TG and apoB48, a marker of intestinal triglyceride-rich lipoproteins. In contrast, consuming a background diet high in SFAs, but not ω-6 PUFAs, resulted in the prolonged postprandial accumulation of triglyceride-rich lipoprotein-apoC, -apoE, and -apoB100, a marker of liver-derived triglyceride-rich lipoprotein, consistent with the down-regulation of receptor-mediated uptake of VLDL remnants with high-SFA diets (16). A study of the acute effects of fatty acid chain length showed enhanced and prolonged TG responses with increasing chain length, and a ~30% greater area under the curve for TG with a challenge meal high in stearic acid (C18:0) compared to palmitic acid (C16:0) or lauric + myristic acid (C14:0 + C12:0) test meals (86). In an acute study of the effect of TG structure on postprandial lipid responses (150), increasing the proportion of palmitic acid at the sn2 position of dietary TG was associated with a blunted increase in plasma TG up to 3 hours after the meal, which translated into ~30% lower incremental area under the curve for TG after test meals high in lard or interesterified palm olein (70.5% and 39.1% C16:0 at sn2, respectively) compared to test meals high in either palm olein or high-oleic sunflower oil (9.2% and 0.6% C16:0 at sn2, respectively).
Differential Effects of Food Sources of SFAs
The major food sources of SFAs are dairy products and red meat, as well as tropical oils, such as palm and coconut oil. Recent evidence suggests that SFA effects on lipids and lipoproteins are modulated by the food source within which they are consumed (5). Observational studies based on self-reported intake suggest that consumption of dairy products is associated with improvements in car-diometabolic factors that may impact CVD risk (reviewed in 4). Studies utilizing plasma biomarkers of dairy intake have confirmed these associations (4, 54). Dairy products have various components that are candidates for improving cardiometabolic health, including conjugated linoleic acid (CLA), vitamin D (a result of fortification), calcium, magnesium, potassium, and whey protein. Relative to butter and/or nondairy-derived SFAs, several studies have shown that full-fat cheese results in lower TC, LDL-C, and/or LDL-C:HDL-C even after consideration of differences in SFA content (20, 70, 128, 170). In a small study comparing semiskim (1.5% fat) milk and full-fat cheese consumption in diets with comparable macronutrient and dairy calcium content, both diets reduced TC and LDL-C but not HDL-C compared to a low-calcium control diet with similar SFAs, consistent with evidence that dairy calcium may be a factor affecting blood lipids (103).
With regard to meat intake, a meta-analysis of RCTs showed no effect on TC, LDL-C, TG, and HDL-C of replacing beef with poultry or fish (104). Of note, most of the studies included in this meta-analysis evaluated lean meats in the context of lower-SFA diets. In contrast, when consumed in diets with increased SFAs derived from dairy products (15% versus 8% of energy), very high intake of lean beef resulted in significant increases in LDL and apoB, primarily due to increases in small and medium LDL particles (105). These results contrast with those of studies in which protein was derived from mixed sources (94), suggesting a possible atherogenic interaction between beef and SFAs. Of note, high meat intake has also been shown to influence the intestinal microbiome, resulting in the production of carnitine-derived metabolites that have been associated with CVD risk independent of the SFA content of the diet (91).
Palm oil is obtained from palm fruits and contains ~50% palmitic acid, 40% oleic acid, and 10% linoleic acid. Palm oil has become a popular substitute for partially hydrogenated vegetable oils due to its semisolid nature and stability compared to other vegetable oils. A recent meta-analysis (51) showed that compared to diets rich in stearic acid, MUFAs, or PUFAs, palm oil raised TC, LDL-C, and HDL-C as well as apoB and apoAI. When compared to diets with high content of myristic and/or lauric acid, palm oil was associated with lower TC, HDL-C, and apoAI and no significant differences in TG, TC:HDL-C, and VLDL-C.
Coconut oil contains >90% SFAs primarily in the form of lauric acid (C12:0), which is the most potent LDL-C- and HDL-C-raising SFA. Isoenergetic replacement of CHO for lauric acid is predicted to reduce the TC:HDL-C ratio but also to raise apoB compared to other SFAs (113). In one RCT, compared to an energy equivalent of butter, coconut oil resulted in lower TC and LDL-C, but both the butter and coconut oil diets raised TC and LDL-C compared to safflower oil (32). In a community-based intervention study from the same investigators, coconut oil reduced LDL-C and apoB compared to butter-based diets (33). These limited data raise the possibility that coconut oil may not increase LDL-C as much as predicted by its high content of lauric acid.
Modulation of Lipid Responses to Diet by Weight Loss and Other Factors
The effects on CVD risk factors of diets varying in macronutrient composition are significantly modulated by concomitant weight loss. Weight loss improves all features of cardiometabolic syndrome, including the major lipid and lipoprotein indicators of CVD risk, i.e., total and small, dense LDL; TG; apoB; and TC:HDL-C ratio (94, 162). Importantly, improvements in lipid and lipoprotein profiles observed with weight loss have been shown to be more significant in the context of high- versus low-CHO diets (54% versus 26% of energy) (94), suggesting a convergence of the effects of reducing adiposity and CHO intake on common pathways that affect atherogenic dyslipidemia.
Weight loss diets restricted in CHO and high in SFAs and protein have raised concerns, in part due to unfavorable effects on LDL-C (increase or no reduction) relative to lower-fat, higher-carbohydrate diets (131). However, such diets have also been associated with greater reductions in TG and increases or maintenance of HDL-C, with overall unclear consequences for CVD risk. Of note, in contrast to low-CHO diets that are usually animal based, a plant-based low-carbohydrate diet (26% of energy) was associated with greater reductions in LDL-C and the TC:HDL and apoB:apoAI ratios compared with a high-CHO, low-fat weight loss diet (83).
Very low-CHO diets, i.e., less than 50 g/day, have been shown to be at least as effective as (131), and at times more effective (12, 23, 61) than, high-CHO, low-fat diets for weight reduction for up to two years of follow-up. The greater weight loss achieved on very low-CHO diets may in part be related to the increased satiety provided by fat and/or protein (185), with a decrease in total calories consumed (184), and/or a lesser reduction in resting and total energy expenditure (44). Major caveats of very low-CHO diets are related to their sustainability over the long term and their unknown effects on CVD events.
A meta-analysis of short-term dietary trials (12.1 ± 9.3 weeks) found that in the context of lower fat intake (<30% of energy), higher-protein diets (~30% of energy) were moderately more advantageous for cardiometabolic health than lower-protein (~18% of energy) diets, with greater reductions in body weight, fat mass, and TG and mitigation of reductions in fat-free mass and resting energy expenditure (189). However, over the longer term, i.e., at least one year, the effect of higher protein on cardiometabolic health has been reported to be neutral (152) or relatively small (30). In the DIOGENES (Diet, Obesity, and Genes) randomized dietary intervention study (n = 1,209) conducted over 26 weeks and notable for its achievement of good adherence based on objective biomarkers, diets higher in protein (~22% versus ~17% E) and lower in glycemic index were associated with improved weight loss and maintenance in the context of ~30% E from fat (98).
Several significant studies have provided evidence that adherence to dietary regimens is more important than dietary macronutrient composition in determining weight loss. In the POUNDS LOST study, which randomly assigned 811 individuals to one of four diets varying in fat, protein, or CHO composition, clinically significant weight loss, i.e., 7% of initial body weight, was achieved irrespective of the macronutrient composition of the diet (147), with satiety, hunger, and satisfaction similar across all diet groups. Similarly, a meta-analysis of 48 RCTs showed that any low-CHO or low-fat diet was more effective for weight loss than no prescribed diet (8 kg at 6 months and ~6 to 7 kg at 12 months), with behavior counseling as a significant modulator of diet effectiveness (85). These studies point to compliance to diets as the key factor in successful weight loss and weight loss maintenance regimens, thereby providing individuals with multiple options for successful weight management. It should be noted, however, as exemplified in the POUNDS LOST study (6), that weight loss trials aimed at testing effects of differences in macronutrient composition on weight loss efficacy generally have failed to achieve the intended differences over the course of time, and hence it is difficult to draw conclusions as to the effects that might be observed with more substantial variation in macronutrient composition.
Responsiveness to diet can be further modulated by baseline metabolic phenotypes, including LDL-C, such that reductions in dietary SFAs result in greater reductions in LDL-C in persons with higher initial LDL-C concentrations (38). In contrast, weaker effects of dietary SFAs on LDL-C have been observed with obesity (27) and insulin resistance (100), and in women versus men (182). Genetic effects on dietary responsiveness have also been reported, although the variance explained by genetics is relatively small. The genes encoding the apoE4 isoform have been found to be most consistently predictive of a greater LDL-C response to diet (43, 160), whereas associations of intakes of PUFAs with lipids have been reported to vary with polymorphisms in apoAI, PPAR-α, apoA5, and 5 lipoxygenase (134).
SATURATED FATTY ACID EFFECTS ON OTHER BIOMARKERS OF CARDIOVASCULAR DISEASE RISK
Inflammation
In vitro and animal studies have demonstrated that SFAs can induce cellular inflammation and accelerate atherosclerosis through toll-like receptor activation (24), and both ω-3 PUFAs (25) and oleic acid (67) have been shown to counter this effect. However, there is limited information regarding the effects of dietary fatty acids on inflammatory processes in humans. The inflammatory markers interleukin (IL)-6 and E-selectin, but not CRP, were found to be lower after a diet high in oleic acid compared to diets in which 8% of energy as oleic acid was exchanged for comparable quantities of either stearic acid or a combination of lauric, myristic, and palmitic acids (9). However in another study, after five weeks of consumption of diets containing 20% energy as palmitic acid–rich palm olein, lauric and myristic acid–rich coconut oil, or oleic acid–rich olive oil, there were no differences in plasma levels of tumor necrosis factor (TNF)-α, IL-1β, IL-6, IL-8, CRP, or interferon-γ(178). Similarly, in the context of a very low-CHO diet (12% E), diets with high SFAs or PUFAs were not associated with changes in inflammatory markers (55). Of interest, low-versus high-GI diets resulted in reduced CRP in the context of weight maintenance after acute weight loss (63).
There is a transient postprandial inflammatory response to all meals, which may contribute to the development of atherosclerosis over time. Meals high in SFAs have been shown to elicit a modest but significantly greater postprandial inflammatory response as measured by circulating markers of inflammation in comparison to ω-6 PUFA–rich meals (109), MUFA-rich meals (141), or CHO-rich meals (127). A high-SFA meal has been shown to reduce the ability of HDL to inhibit expression of intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 6 hours postmeal, while HDL collected after a high-PUFA meal inhibited expression to a greater extent than in fasting plasma (129). Others have shown no differences in circulating inflammatory markers following meals with a high versus low P:S ratio in lean men (136), or between meals with either cream, olive oil, or canola oil in lean and obese women (106). Moreover, after four weeks of a SFA-rich Western diet, a MUFA-rich Mediterranean diet, or higher-CHO diet supplemented with ω-3 fatty acid, no differences were detected for plasma TNF-α or IL-6 after a breakfast with fat composition similar to each experimental diet (84). However, compared to the ω-3 acid–rich diet, the high-SFA diet induced a greater increase in mRNA of TNF-α and IL-6 in peripheral blood mononuclear cells (84). The inconsistent results may be due to differences in study population or meal composition.
In contrast, the replacement of TFAs with SFAs, MUFAs, or PUFAs has been consistently associated with reductions in inflammatory markers such as CRP (122). Thus, the evidence linking SFAs to increased inflammatory responses is limited compared to the evidence for adverse effects of TFAs.
Blood Pressure and Vascular Function
A dietary intervention study in healthy subjects showed decreases in systolic and diastolic blood pressure with diets high in MUFAs, whereas diets high in SFAs resulted in no changes in BP (140). Supplementation with ω-3 fatty acids did not lower BP in this study (140), although a more recent meta-analysis provides evidence that EPA + DHA supplementation effectively lowers BP, particularly in untreated hypertensives (117). Effective BP-lowering dietary patterns include the DASH and Mediterranean diets, which both emphasize multiple dietary modifications together with limitation of SFA intake, thereby precluding attribution of beneficial effects specifically to the SFA component of these diets (2, 142).
There is inconsistent evidence for effects of SFAs on vascular function as assessed by flow-mediated dilation (88, 139, 151). A summary of epidemiological, intervention, and meal test studies concluded that low-fat diets may be beneficial relative to diets high in SFAs or when MUFAs replace SFAs (174). This is in contrast to an earlier review evaluating effects of replacing SFAs with MUFAs, PUFAs, TFAs, or CHOs, which reported no improvements in endothelial function or arterial stiffness in seven of nine randomized controlled trials for any of the aforementioned macronutrient replacement scenarios (115).
Insulin Sensitivity
Despite human observational studies reporting positive associations between SFA intake and hyperinsulinemia, independent of body fat, the results from prospective cohort studies have been mixed and have not shown an independent relationship of SFAs with incident type 2 diabetes mellitus (T2DM) (115). Replacing SFAs with MUFAs in generally healthy subjects did not affect (27, 81, 171) or had modest effects (175) on insulin sensitivity or glucose metabolism. Similarly, no differences in insulin sensitivity were found when replacing SFAs with CHOs or ω-3 fatty acids (81, 171). In the EPIC-Interact case cohort study, plasma concentrations of odd-chain SFAs (C15:0 and C17:0), which reflect consumption of dairy fats, were inversely associated with T2DM (54), in line with epidemiological data showing a similar relationship, in particular, with low-fat fermented dairy products (132, 164). In contrast, even-chain SFAs (C14:0, C16:0, C18:0), which are influenced by both endogenous metabolism and dietary CHO, were positively associated with T2DM (54). Finally, there have been reports of associations of transpalmitoleic acid, C16:1n7, as a biomarker of dairy fat intake, with improved cardiometabolic profiles and decreased risk of T2DM (121). These studies suggest heterogeneous effects of specific SFAs on T2DM risk but provide growing evidence for protective effects of dairy intake, as reflected by plasma concentrations of specific SFAs.
DIET AND CARDIOVASCULAR DISEASE EVENTS
RCTs can, in principle, provide the strongest evidence for dietary effects on CVD outcomes. However, it is important to note that RCTs aimed at establishing relationships between nutrients and CVD risk are modulated by context, i.e., the replacement nutrient and/or food source (161), and are also limited by the challenges of long-term dietary adherence.
Associations of specific dietary components with CVD events have been primarily evaluated in epidemiological studies, of which prospective cohort studies provide the strongest design. Although such studies offer the statistical power to adjust for covariates and thereby enable evaluation of the effects of specific nutrients, there are caveats, including the inability to define cause-effect relationships given known and unknown confounding variables, the reliance on dietary assessment tools with limited accuracy, the assumption that diets remain the same over the long term, and/or selective reporting by study participants (161).
Replacement of SFAs with CHOs
Dietary SFAs were originally linked to increased CVD risk in the Seven Countries Study (89), an association subsequently supported by some epidemiological studies but not others (reviewed in 160). Several recent meta-analyses have not demonstrated an association of dietary SFAs with CHD (29, 114, 159, 163), likely because lower intakes of SFAs were accompanied by compensatory increases in CHOs (75). Of note, although inverse associations of dietary SFAs with stroke incidence have been reported, particularly in Japanese populations where SFA intake is lower and stroke prevalence is higher (160, 190), a 2010 summary analysis of studies showed no overall significant association of SFAs with stroke (159).
In support of the concept of lack of cardiovascular benefit when SFAs are replaced by CHOs, an analysis that pooled epidemiological data from 344,596 persons from 11 American and European cohorts showed a worsening or no benefit for CVD risk when CHOs replaced SFAs (HR for coronary events: 1.07; 95% CI: 1.01, 1.14; HR for coronary death: 0.96; 95% CI: 0.82, 1.13) (79). In a separate analysis by Mozaffarian et al. (124), estimated effects on CVD risk of replacing SFAs with CHOs based on changes in the TC:HDL-C ratio were neutral (RR: 1.01; 95% CI: 0.98, 1.04).
The Women’s Health Initiative, a RCT conducted in nearly 50,000 postmenopausal women, showed no difference in CVD risk (RR: 0.98; 95% CI: 0.88, 1.09) in the intervention versus control group with diets that were reduced in SFAs, higher in CHOs, and lower in PUFAs (74). Of note, the Women’s Health Initiative was not originally designed to evaluate CVD outcomes and reduced LDL-C only modestly (2.7 mg/dl), making interpretation of its results unclear.
Epidemiological studies evaluating CHO quality have demonstrated associations of high sugar intake with CVD. In the Nurses’ Health Study (n = 88,520 women; n = 3,105 incident CHD cases), a significant increase in incident CHD was observed across categories of sugar-sweetened beverage consumption even after accounting for other unhealthful dietary and lifestyle factors (p for trend <0.001; RR = 1.35; 95% CI: 1.07, 1.69 for women consuming two or more servings per day) (58). An analysis of NHANES data recently showed that intake of added sugars between 10% and 24.9% of total calories was associated with a 30% increased risk for CVD mortality relative to consumption of <10% of total calories from sugar (191). For persons consuming > 25% of energy from sugar, the RR of mortality was 2.75 (95% CI: 1.40, 5.42).
A prospective cohort study in 53,633 men and women (1,943 incident cases) found that the substitution of SFAs with low- and moderate-GI CHOs was not associated with risk of myocardial infarction, whereas the substitution of SFAs by high-GI CHOs was associated with an increased risk (HR per 5% increment of energy from CHO: 1.33; 95% CI: 1.08, 1.64) (78). A subsequent meta-analysis of eight prospective studies (n = 220,050 participants; 4,826 incident cases) (41) that did not include the aforementioned study (78) showed GI and GL to be significantly associated with CHD in women but not in men (41). In line with data from RCTs evaluating effects of GI versus GL on lipids (102), GL was more strongly associated with CVD events than was GI [RR = 1.69 (1.32, 2.16) versus 1.26 (1.12, 1.43)] in women (41). It is possible that the preferential associations in women are related to the known greater CVD risk conferred by higher TG (7) or diabetes (144) in women versus men, but additional research in this area is necessary. Also of interest is an apparently more pronounced association of both high-GI and high-GL diets with CVD in overweight and obese patients (41), concordant with similarly more adverse associations of GL with dyslipidemia in this population (102). These data contrast with the reduced response to dietary SFA in obese individuals (27) and support the concept that in the context of the current epidemics of obesity and insulin resistance, dietary CHOs, particularly refined and processed CHOs, may exert more deleterious effects on CVD than SFAs.
Replacement of SFAs with Other Fats
RCTs
Early clinical trials, including the Finnish Mental Hospital Study, the Los Angeles County Veterans Study, and the Oslo Diet-Heart Study, showed reduced CVD events with diets that replaced SFAs with PUFAs (reviewed in 160). Of note, in these trials the SFA content of the control diet was at least 9%, and the PUFA content of the diets was relatively high (>11% E). Therefore, it is possible that the CVD benefit may have been due to specific effects of PUFAs versus adverse effects of SFAs. Alternatively, improvements in CVD could have been related to the ratio of PUFAs to SFAs, which has been reported to be more significantly associated with CVD than SFAs alone, such that improvements in CVD were observed with a P:S ratio greater than 0.49 (76). Calculated P:S ratios from early intervention trials showing benefit of reduced SFAs on CVD were in the range of 1.25 to 2.4 (159).
A meta-analysis of RCTs by Mozaffarian et al. (124) estimated a 10% reduction in CVD risk for every 5% SFAs replaced with PUFAs, which corresponds to the reduction in CVD risk that would be predicted by the expected changes in lipids (111). Similarly, Hooper and colleagues’ (73) 2012 updated meta-analysis of RCTs evaluating reductions or modifications of dietary fats showed a reduction in cardiovascular events by 14%, with subgroup analyses suggesting these effects to be specific to studies of fat modification (versus reduction), of at least two years’ duration, and in men, but not women. The cardiovascular benefit of PUFA replacement of SFAs was recently challenged in two meta-analyses of RCTs that included different component studies and showed either no benefit (29) or a trend toward increased CVD risk (138). However, the validity of inclusion of reevaluated and potentially confounded data from the Sydney Diet Heart Study in these more recent analyses (29, 138) has been questioned, given that the PUFA supplementation arm included margarine with high trans fat (101, 187). Exclusion of the Sydney Diet Heart Study in the meta-analysis by Chowdhury et al. (29) yielded an RR estimate of 0.81 (0.68–0.98) based on seven clinical trials, thus supporting a cardiovascular benefit of replacing SFAs with PUFAs. However, Ramsden et al. (138) have raised a significant caveat to the Finnish Mental Hospital and Oslo Diet-Heart studies (included among the seven aforementioned trials), namely, that they allowed for the continued consumption of trans fats in the form of margarines in the control arms of the studies, thus confounding comparisons with the high-PUFA diets.
Prospective cohort studies
A systematic review and meta-analysis of 13 published and unpublished prospective cohort studies (n = 310,602 individuals and 12,479 total CHD events) indicated that substitution of 5% SFAs with the ω-6 PUFA linoleic acid was associated with a 9% lower risk of CHD events (RR = 0.91; 95% CI: 0.87, 0.96) and a 13% lower risk of CHD deaths (RR = 0.87; 95% CI: 0.82, 0.94) (50). The lack of benefit associated with increased ω-6 PUFA intake reported by Chowdhury et al. (29) was likely due to the inability to include relevant data sets that were included in the Jakobsen et al. pooling study (79) and Farvid et al. meta-analysis (50). These findings are particularly important in light of concerns that have been raised regarding possible proatherogenic effects of ω-6 PUFAs, i.e., through their promotion of inflammation (158), competition with ω-3 PUFAs (137), and possible role in cancer risk and progression (8). Although there is some evidence suggesting that conversion of linoleic acid to arachidonic acid induces a proinflammatory eicosanoid response (158), dietary intake of linoleic acid has not been closely associated with circulating or tissue concentrations of arachidonic acid at levels currently consumed (52). Furthermore, increased circulating concentrations of arachidonic acid have been associated with no changes in (188) or improved CVD risk in prospective cohort studies and RCTs (29). It has been suggested that high PUFA consumption may also drive the oxidation of LDL, which contributes to the process of atherosclerosis (96). Although clinical trial data in humans supporting adverse effects of dietary PUFA-induced oxidation products on CVD endpoints are lacking, studies in nonhuman primates have demonstrated beneficial effects on LDL cholesteryl oleate content and the extent of atherosclerosis in primates consuming ω-6 PUFA compared to the SFA- or MUFA-fed groups (37).
It has also been suggested that the benefits of PUFA interventions are specific to those that increased both ω-3 PUFAs as well as ω-6 PUFAs rather than ω-6 PUFAs only (137). Indeed, there is consistent epidemiological evidence for the association of dietary total long-chain ω-3 PUFAs, derived primarily from fatty fish, with reduced CVD risk (29, 119, 179). Most recently, the meta-analysis by Chowdhury et al. (29) estimated a reduced CVD risk of 0.87 (0.78–0.97) based on 16 prospective cohort studies with 422,786 participants and 9,089 events. Circulating concentrations of long-chain ω-3 fatty acids, including EPA and DHA, were also associated with reduced CVD [RR = 0.75 (0.62–0.89)] based on 13 studies with 20,809 participants and 4,073 events (29). The increased consumption of these ω-3 PUFAs can be relatively accurately assessed via blood biomarkers since they cannot be produced endogenously (10, 48). In contrast, ω-3 fatty acid supplementation has not been found to reduce CVD risk in recent trials (143). The lack of benefit may have been related to the populations evaluated, which were at high risk for or had existing CVD, were being treated with statins, and/or consumed high amounts of ω-3 fatty acids in their typical diets. Overall, these data suggest that there is cardiovascular benefit of high fish consumption as manifest by plasma biomarkers, and leave open the possibility that supplementation with EPA and/or DHA might have benefit in the primary versus secondary prevention of CVD (107).
In contrast to replacement of SFAs with PUFAs, replacement of SFAs with TFAs has been associated with increased risk of CVD (123). Data from prospective cohort studies indicates that each 2% energy replacement of TFAs with SFAs was associated with a 17% (95% CI: 7%–25%) reduction in CHD (122).
Food Sources of SFAs
Various food sources of SFAs may differentially affect CVD risk (5). In the MESA (Multi-Ethnic Study of Atherosclerosis) study, intake of dairy products as a source of SFAs was inversely associated with CVD whereas intake of red meats was associated with increased CVD after adjustment for demographics, lifestyle, and dietary confounders [n = 5,209 with 316 cases; HR for +5 grams SFA per day: 0.79 (95% CI: 0.68, 0.92) and 1.26 (95% CI: 1.02, 1.54) for dairy and meat, respectively] (36). These data are in line with several earlier meta-analyses of cohort studies that showed neutral or inverse associations of milk or dairy intake with CVD (46, 47, 165). In a prospective case control study, proportions of milk fat biomarkers, i.e., C15:0 and C17:0, in plasma phospholipids were higher in controls than in cases and were inversely associated with risk of myocardial infarction in women (181). A slightly increased total and ischemic heart disease–specific mortality with increased butter and dairy fat consumption has been reported specifically in women (64), suggesting differential responses to diet in women versus men. Notably, a modest inverse association of fermented full-fat dairy products with total mortality has also been observed (64), in line with beneficial effects of fermented dairy products on cardiometabolic risk factors (4).
The increased CVD risk observed with red meat intake as a source of SFA may be a function of other components of red meat, such as heme iron (36). Replacement of red meat with other major dietary protein sources, i.e., dairy, poultry, fish, or nuts, was associated with a 13% to 30% reduction in CHD risk in the Nurses’ Health Study (19). A meta-analysis that included 17 prospective cohort and 3 case control studies indicated that adverse effects on CVD were specific to processed versus unprocessed meats (116). Thus, some aspect of the processing of meat versus the meat itself—e.g., preservation with sodium, nitrites, and phosphates, or cooking or frying methods—may promote CVD. A more recent meta-analysis of meats in relation to CVD and total mortality showed processed meat to be associated with both total and CVD mortality, whereas red meat consumption was associated only with CVD mortality (1). However, the risk estimates used were not adjusted for dietary patterns, and there was adjustment for food groups in only a subset of the studies (1). Thus, it is possible that the observed associations were due to residual confounding related to dietary and lifestyle patterns. Of note, a meta-analysis of eight prospective cohort studies conducted in Asia, where dietary intake of fish and seafood is higher and intake of red meat and poultry is lower compared to Western countries, showed no associations of total meat intake with cause-specific mortalities and an inverse association of red meat with CVD in Asian men (99). Meat intake in Asia may not be high enough to confer CVD risk, or alternatively, meat consumption may contribute less substantially to CVD risk in Asians than do other factors, such as socioeconomic status, physical activity, and adiposity.
Dietary Patterns
Evidence for cardioprotective dietary effects from epidemiological studies and randomized clinical trials is strongest for a Mediterranean diet pattern (35, 114, 153, 166). Although various definitions exist, Mediterranean diets are generally characterized by high intakes of fruits, nuts, vegetables, whole-grain cereals, and olive oil; with moderate consumption of fish, poultry, and wine; and low intake of dairy, red meats, and sweets. The PREDIMED (PREvención con DIeta MEDiterránea) study (n = 7,447) was a parallel group, multicenter primary prevention trial of high-risk individuals initially free of CVD (49). Two arms of the three-arm study were assigned to a Mediterranean diet with either extra virgin olive oil or nut supplementation. Relative to the low-fat control arm, both the olive oil supplementation and nut groups had a significantly decreased risk of coronary events, an effect driven by a significantly reduced incidence of stroke [RR: 0.61 (0.44–0.86)] (49). Of note, a recent prospective cohort study in Finland showed increased CVD risk when SFAs were replaced with MUFAs, suggesting that this macronutrient replacement scenario may not be the cardioprotective component of the Mediterranean diet (176), in line with previous data conducted in African green monkeys that showed no benefit of replacing SFAs with MUFAs on coronary artery atherosclerosis (145).
Evidence for heart-healthy benefits of the DASH dietary pattern—which emphasizes fruits, vegetables, and low-fat dairy products; includes whole grains, poultry, fish, and nuts; and is lower in red meat, sweets, and sugar-sweetened beverages—stems from clinical trials demonstrating the effectiveness of the diet on reducing blood pressure (3), with observational data suggesting associations with lower risk of CVD (52). These data are in line with other observational data showing improved CVD morbidity and mortality with vegetarian versus meat-eating patterns in both Western and Asian populations (120, 133). In the Nurses’ Health Study, lower-carbohydrate diets with vegetable versus animal sources of protein showed protection from ischemic heart disease in those consuming high vegetable sources of protein (66). Notably, whereas a higher GL was strongly associated with CVD in this cohort, consumption of protein and fat from animal sources in the context of lower-carbohydrate diets was not associated with a worsening of CVD risk (66). Neutral associations with CVD risk of more Westernized diets rich in red and processed meat, refined grains, alcohol, and whole-dairy products were also recently observed in an a posteriori analysis of the PREDIMED study (108).
In summary, most epidemiological studies evaluating food sources of SFAs suggest neutral or beneficial effects of dairy foods on CVD, whereas intake of meat, and particularly processed meats, has been associated with neutral or increased risk of CVD, associations that may be independent of the SFA content of the foods. On the other hand, vegetarian dietary patterns have generally been associated with reduced CVD risk. It has been suggested that this is related to higher intake of PUFAs, fiber, and micronutrients as well as reduced GL (75), although other unknown or unmeasured lifestyle and dietary variables may be responsible. Both epidemiological and clinical trial data support the cardiovascular benefits of dietary patterns such as the Mediterranean and DASH diets that include vegetables, fruits, nuts, fish, and poultry and minimize sweets and red meats; however, the role of SFAs in such multifactorial diet patterns is unclear.
CONCLUSIONS
Replacing SFAs with PUFAs has been associated with cardiovascular benefit in the majority of metabolic, epidemiological, and clinical trial data, but study design caveats including residual confounding in epidemiological studies and changes in multiple dietary variables in RCTs should be considered when weighing the evidence for specific nutrient effects. Epidemiological studies support a beneficial association of ω-3 fatty acids with CVD; however, clinical trial studies to date have not consistently confirmed this. In contrast, the replacement of SFAs with TFAs has been associated with adverse CVD risk factors and outcomes, whereas the replacement of SFAs with CHOs has not been associated with benefit and may be associated with increased CVD risk. These effects are likely multifactorial, including effects on atherogenic lipoproteins, particularly remnants and sdLDL particles. A particular concern with regard to the growing population of individuals with excess adiposity and insulin resistance is that they may be particularly sensitive to the adverse lipoprotein effects of refined and processed CHOs while being concomitantly resistant to LDL-C-reducing effects of reduced SFAs. The effects of various SFA replacement scenarios on CVD risk factors other than lipids and lipoproteins are ambiguous, with the strongest evidence for proinflammatory effects derived from cellular and animal studies. Importantly, accumulating evidence indicates that food sources of SFAs can vary in their associations with CVD risk independent of their SFA content. This is likely due to components within foods other than SFAs that may singly or synergistically affect the development and progression of CVD. Therefore, the SFA content of foods is not necessarily a useful criterion on which to base food choices. Overall dietary patterns that emphasize vegetables, fish, nuts, and whole versus processed grains are the mainstays of heart-healthy eating. Whether SFAs need to be reduced in the context of such dietary patterns is not established.
SUMMARY POINTS.
Restriction of SFAs has been advised based on their LDL-cholesterol-raising ability and the established relationship between LDL cholesterol and CVD, but recent analyses suggest a lack of association between dietary SFAs per se with CVD.
The effects of SFAs on CVD risk factors and CVD clinical endpoints are intrinsically modulated by the nutrients that replace them, such that replacement by PUFAs has been associated with CVD benefit, whereas replacement with CHOs has been associated with no improvement or a worsening in CVD risk.
Dietary carbohydrates and SFAs affect different components of lipoprotein metabolic pathways, with differential production of LDL particles of varying size and quality, and associations with CVD risk.
The cardiometabolic effects of SFAs are modulated by the foods in which they are consumed, due in part to the predominance of different SFAs in various foods and likely also to the presence of other bioactive components. SFA effects can also be influenced by traits such as adiposity, gender, insulin resistance, and baseline lipid profiles.
Overall dietary patterns are more relevant to cardiovascular health than individual nutrients, and the focus of dietary guidance should be food based, with consideration for individual preferences and lifestyles.
FUTURE ISSUES.
It has not been determined whether there is an interaction between increased SFAs and carbohydrates on CVD risk.
Subsets of the population may be particularly susceptible to effects of dietary macronutrients on lipid profiles. Identifying and tailoring diet and lifestyle programs to these individuals may improve overall cardiometabolic health.
Identification of improved biomarkers of pathophysiological processes affecting CVD could help guide individualized dietary approaches for reducing CVD risk.
Footnotes
DISCLOSURE STATEMENT
R.M. Krauss has received research grants from the Dairy Research Institute, the Almond Board of California, and the Dr. Robert C. and Veronica Atkins Foundation. P.W. Siri-Tarino, S. Chiu, and N. Bergeron are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
Contributor Information
Patty W. Siri-Tarino, Email: psiri@chori.org.
Ronald M. Krauss, Email: rkrauss@chori.org.
LITERATURE CITED
- 1.Abete I, Romaguera D, Vieira AR, Lopez de Munain A, Norat T. Association between total, processed, red and white meat consumption and all-cause, CVD and IHD mortality: a meta-analysis of cohort studies. Br J Nutr. 2014;112:762–75. doi: 10.1017/S000711451400124X. [DOI] [PubMed] [Google Scholar]
- 2.Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1997;336:1117–24. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 3.Appel LJ, Sacks FM, Carey VJ, Obarzanek E, Swain JF, et al. Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids: results of the OmniHeart randomized trial. JAMA. 2005;294:2455–64. doi: 10.1001/jama.294.19.2455. [DOI] [PubMed] [Google Scholar]
- 4.Astrup A. Yogurt and dairy product consumption to prevent cardiometabolic diseases: epidemiologic and experimental studies. Am J Clin Nutr. 2014;99:1235–42S. doi: 10.3945/ajcn.113.073015. [DOI] [PubMed] [Google Scholar]
- 5.Astrup A, Dyerberg J, Elwood P, Hermansen K, Hu FB, et al. The role of reducing intakes of saturated fat in the prevention of cardiovascular disease: Where does the evidence stand in 2010? Am J Clin Nutr. 2011;93:684–88. doi: 10.3945/ajcn.110.004622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Astrup A, Pedersen SD. Is a protein calorie better for weight control? Am J Clin Nutr. 2012;95:535–36. doi: 10.3945/ajcn.111.031625. [DOI] [PubMed] [Google Scholar]
- 7.Austin MA, Hokanson JE, Edwards KL. Hypertriglyceridemia as a cardiovascular risk factor. Am J Cardiol. 1998;81:7–12B. doi: 10.1016/s0002-9149(98)00031-9. [DOI] [PubMed] [Google Scholar]
- 8.Azrad M, Turgeon C, Demark-Wahnefried W. Current evidence linking polyunsaturated fatty acids with cancer risk and progression. Front Oncol. 2013;3:224. doi: 10.3389/fonc.2013.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baer DJ, Judd JT, Clevidence BA, Tracy RP. Dietary fatty acids affect plasma markers of inflammation in healthy men fed controlled diets: a randomized crossover study. Am J Clin Nutr. 2004;79:969–73. doi: 10.1093/ajcn/79.6.969. [DOI] [PubMed] [Google Scholar]
- 10.Baylin A, Campos H. The use of fatty acid biomarkers to reflect dietary intake. Curr Opin Lipidol. 2006;17:22–27. doi: 10.1097/01.mol.0000199814.46720.83. [DOI] [PubMed] [Google Scholar]
- 11.Bays HE, Tighe AP, Sadovsky R, Davidson MH. Prescription omega-3 fatty acids and their lipid effects: physiologic mechanisms of action and clinical implications. Expert Rev Cardiovasc Ther. 2008;6:391–409. doi: 10.1586/14779072.6.3.391. [DOI] [PubMed] [Google Scholar]
- 12.Bazzano LA, Hu T, Reynolds K, Yao L, Bunol C, et al. Effects of low-carbohydrate and low-fat diets: a randomized trial. Ann Intern Med. 2014;161:309–18. doi: 10.7326/M14-0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Behall KM, Howe JC. Effect of long-term consumption of amylose versus amylopectin starch on metabolic variables in human subjects. Am J Clin Nutr. 1995;61:334–40. doi: 10.1093/ajcn/61.2.334. [DOI] [PubMed] [Google Scholar]
- 14.Behall KM, Scholfield DJ, Yuhaniak I, Canary J. Diets containing high amylose versus amylopectin starch: effects on metabolic variables in human subjects. Am J Clin Nutr. 1989;49:337–44. doi: 10.1093/ajcn/49.2.337. [DOI] [PubMed] [Google Scholar]
- 15.Bergeron N, Havel RJ. Influence of diets rich in saturated and omega-6 polyunsaturated fatty acids on the postprandial responses of apolipoproteins B-48, B-100, E, and lipids in triglyceride-rich lipoproteins. Arterioscler Thromb Vasc Biol. 1995;15:2111–21. doi: 10.1161/01.atv.15.12.2111. [DOI] [PubMed] [Google Scholar]
- 16.Bergeron N, Havel RJ. Assessment of postprandial lipemia: nutritional influences. Curr Opin Lipidol. 1997;8:43–52. doi: 10.1097/00041433-199702000-00010. [DOI] [PubMed] [Google Scholar]
- 17.Berglund L, Lefevre M, Ginsberg HN, Kris-Etherton PM, Elmer PJ, et al. Comparison of monounsaturated fat with carbohydrates as a replacement for saturated fat in subjects with a high metabolic risk profile: studies in the fasting and postprandial states. Am J Clin Nutr. 2007;86:1611–20. doi: 10.1093/ajcn/86.5.1611. [DOI] [PubMed] [Google Scholar]
- 18.Berneis KK, Krauss RM. Metabolic origins and clinical significance of LDL heterogeneity. J Lipid Res. 2002;43:1363–79. doi: 10.1194/jlr.r200004-jlr200. [DOI] [PubMed] [Google Scholar]
- 19.Bernstein AM, Sun Q, Hu FB, Stampfer MJ, Manson JE, Willett WC. Major dietary protein sources and risk of coronary heart disease in women. Circulation. 2010;122:876–83. doi: 10.1161/CIRCULATIONAHA.109.915165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Biong AS, Muller H, Seljeflot I, Veierod MB, Pedersen JI. A comparison of the effects of cheese and butter on serum lipids, haemostatic variables and homocysteine. Br J Nutr. 2004;92:791–97. doi: 10.1079/bjn20041257. [DOI] [PubMed] [Google Scholar]
- 21.Boren J, Matikainen N, Adiels M, Taskinen MR. Postprandial hypertriglyceridemia as a coronary risk factor. Clin Chim Acta. 2014;431:131–42. doi: 10.1016/j.cca.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 22.Brouwer IA, Wanders AJ, Katan MB. Effect of animal and industrial trans fatty acids on HDL and LDL cholesterol levels in humans—a quantitative review. PLOS ONE. 2010;5:e9434. doi: 10.1371/journal.pone.0009434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bueno NB, de Melo IS, de Oliveira SL, da Rocha Ataide T. Very-low-carbohydrate ketogenic diet v. low-fat diet for long-term weight loss: a meta-analysis of randomised controlled trials. Br J Nutr. 2013;110:1178–87. doi: 10.1017/S0007114513000548. [DOI] [PubMed] [Google Scholar]
- 24.Chait A, Kim F. Saturated fatty acids and inflammation: Who pays the toll? Arterioscler Thromb Vasc Biol. 2010;30:692–93. doi: 10.1161/ATVBAHA.110.203984. [DOI] [PubMed] [Google Scholar]
- 25.Chang CL, Torrejon C, Jung UJ, Graf K, Deckelbaum RJ. Incremental replacement of saturated fats by n-3 fatty acids in high-fat, high-cholesterol diets reduces elevated plasma lipid levels and arterial lipoprotein lipase, macrophages and atherosclerosis in LDLR−/− mice. Atherosclerosis. 2014;234:401–9. doi: 10.1016/j.atherosclerosis.2014.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chardigny JM, Destaillats F, Malpuech-Brugere C, Moulin J, Bauman DE, et al. Do trans fatty acids from industrially produced sources and from natural sources have the same effect on cardiovascular disease risk factors in healthy subjects? Results of the Trans Fatty Acids Collaboration (TRANSFACT) study. Am J Clin Nutr. 2008;87:558–66. doi: 10.1093/ajcn/87.3.558. [DOI] [PubMed] [Google Scholar]
- 27.Chiu S, Williams PT, Dawson T, Bergman RN, Stefanovski D, et al. Diets high in protein or saturated fat do not affect insulin sensitivity or plasma concentrations of lipids and lipoproteins in overweight and obese adults. J Nutr. 2014;144:1753–59. doi: 10.3945/jn.114.197624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chong MF, Fielding BA, Frayn KN. Mechanisms for the acute effect of fructose on postprandial lipemia. Am J Clin Nutr. 2007;85:1511–20. doi: 10.1093/ajcn/85.6.1511. [DOI] [PubMed] [Google Scholar]
- 29.Chowdhury R, Warnakula S, Kunutsor S, Crowe F, Ward HA, et al. Association of dietary, circulating, and supplement fatty acids with coronary risk: a systematic review and meta-analysis. Ann Intern Med. 2014;160:398–406. doi: 10.7326/M13-1788. [DOI] [PubMed] [Google Scholar]
- 30.Clifton PM, Condo D, Keogh JB. Long term weight maintenance after advice to consume low carbohydrate, higher protein diets—a systematic review and meta analysis. Nutr Metab Cardiovasc Dis. 2014;24:224–35. doi: 10.1016/j.numecd.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 31.Cortese C, Levy Y, Janus ED, Turner PR, Rao SN, et al. Modes of action of lipid-lowering diets in man: studies of apolipoprotein B kinetics in relation to fat consumption and dietary fatty acid composition. Eur J Clin Invest. 1983;13:79–85. doi: 10.1111/j.1365-2362.1983.tb00068.x. [DOI] [PubMed] [Google Scholar]
- 32.Cox C, Mann J, Sutherland W, Chisholm A, Skeaff M. Effects of coconut oil, butter, and safflower oil on lipids and lipoproteins in persons with moderately elevated cholesterol levels. J Lipid Res. 1995;36:1787–95. [PubMed] [Google Scholar]
- 33.Cox C, Sutherland W, Mann J, de Jong S, Chisholm A, Skeaff M. Effects of dietary coconut oil, butter and safflower oil on plasma lipids, lipoproteins and lathosterol levels. Eur J Clin Nutr. 1998;52:650–54. doi: 10.1038/sj.ejcn.1600621. [DOI] [PubMed] [Google Scholar]
- 34.Culling KS, Neil HA, Gilbert M, Frayn KN. Effects of short-term low- and high-carbohydrate diets on postprandial metabolism in non-diabetic and diabetic subjects. Nutr Metab Cardiovasc Dis. 2009;19:345–51. doi: 10.1016/j.numecd.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 35.de Lorgeril M, Salen P, Martin JL, Monjaud I, Delaye J, Mamelle N. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: final report of the Lyon Diet Heart Study. Circulation. 1999;99:779–85. doi: 10.1161/01.cir.99.6.779. [DOI] [PubMed] [Google Scholar]
- 36.de Oliveira Otto MC, Mozaffarian D, Kromhout D, Bertoni AG, Sibley CT, et al. Dietary intake of saturated fat by food source and incident cardiovascular disease: the Multi-Ethnic Study of Atherosclerosis. Am J Clin Nutr. 2012;96:397–404. doi: 10.3945/ajcn.112.037770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Degirolamo C, Shelness GS, Rudel LL. LDL cholesteryl oleate as a predictor for atherosclerosis: evidence from human and animal studies on dietary fat. J Lipid Res. 2009;50(Suppl):S434–39. doi: 10.1194/jlr.R800076-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Denke MA. Review of human studies evaluating individual dietary responsiveness in patients with hypercholesterolemia. Am J Clin Nutr. 1995;62:471–77S. doi: 10.1093/ajcn/62.2.471S. [DOI] [PubMed] [Google Scholar]
- 39.Denke MA, Adams-Huet B, Nguyen AT. Individual cholesterol variation in response to a margarine- or butter-based diet: a study in families. JAMA. 2000;284:2740–47. doi: 10.1001/jama.284.21.2740. [DOI] [PubMed] [Google Scholar]
- 40.Diet. Guidel. Advis. Comm. Scientific Report of the 2015 Dietary Guidelines Advisory Committee. Washington, DC: US Dep. Health Human Serv; 2015. http://www.health.gov/dietaryguidelines/2015-scientific-report/ [Google Scholar]
- 41.Dong JY, Zhang YH, Wang P, Qin LQ. Meta-analysis of dietary glycemic load and glycemic index in relation to risk of coronary heart disease. Am J Cardiol. 2012;109:1608–13. doi: 10.1016/j.amjcard.2012.01.385. [DOI] [PubMed] [Google Scholar]
- 42.Dreon DM, Fernstrom HA, Campos H, Blanche P, Williams PT, Krauss RM. Change in dietary saturated fat intake is correlated with change in mass of large low-density-lipoprotein particles in men. Am J Clin Nutr. 1998;67:828–36. doi: 10.1093/ajcn/67.5.828. [DOI] [PubMed] [Google Scholar]
- 43.Dreon DM, Fernstrom HA, Miller B, Krauss RM. Apolipoprotein E isoform phenotype and LDL subclass response to a reduced-fat diet. Arterioscler Thromb Vasc Biol. 1995;15:105–11. doi: 10.1161/01.atv.15.1.105. [DOI] [PubMed] [Google Scholar]
- 44.Ebbeling CB, Swain JF, Feldman HA, Wong WW, Hachey DL, et al. Effects of dietary composition on energy expenditure during weight-loss maintenance. JAMA. 2012;307:2627–34. doi: 10.1001/jama.2012.6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eckel RH, Jakicic JM, Ard JD, de Jesus JM, Houston Miller N, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2960–84. doi: 10.1016/j.jacc.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 46.Elwood PC, Pickering JE, Givens DI, Gallacher JE. The consumption of milk and dairy foods and the incidence of vascular disease and diabetes: an overview of the evidence. Lipids. 2010;45:925–39. doi: 10.1007/s11745-010-3412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Elwood PC, Pickering JE, Hughes J, Fehily AM, Ness AR. Milk drinking, ischaemic heart disease and ischaemic stroke II. Evidence from cohort studies. Eur J Clin Nutr. 2004;58:718–24. doi: 10.1038/sj.ejcn.1601869. [DOI] [PubMed] [Google Scholar]
- 48.Erkkila A, de Mello VD, Riserus U, Laaksonen DE. Dietary fatty acids and cardiovascular disease: an epidemiological approach. Prog Lipid Res. 2008;47:172–87. doi: 10.1016/j.plipres.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 49.Estruch R, Ros E, Salas-Salvado J, Covas MI, Corella D, et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med. 2013;368:1279–90. doi: 10.1056/NEJMc1806491. [DOI] [PubMed] [Google Scholar]
- 50.Farvid MS, Ding M, Pan A, Sun Q, Chiuve SE, et al. Dietary linoleic acid and risk of coronary heart disease: a systematic review and meta-analysis of prospective cohort studies. Circulation. 2014;130:1568–78. doi: 10.1161/CIRCULATIONAHA.114.010236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fattore E, Bosetti C, Brighenti F, Agostoni C, Fattore G. Palm oil and blood lipid-related markers of cardiovascular disease: a systematic review and meta-analysis of dietary intervention trials. Am J Clin Nutr. 2014;99:1331–50. doi: 10.3945/ajcn.113.081190. [DOI] [PubMed] [Google Scholar]
- 52.Flock MR, Fleming JA, Kris-Etherton PM. Macronutrient replacement options for saturated fat: effects on cardiovascular health. Curr Opin Lipidol. 2014;25:67–74. doi: 10.1097/MOL.0000000000000039. [DOI] [PubMed] [Google Scholar]
- 53.Flock MR, Green MH, Kris-Etherton PM. Effects of adiposity on plasma lipid response to reductions in dietary saturated fatty acids and cholesterol. Adv Nutr. 2011;2:261–74. doi: 10.3945/an.111.000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Forouhi NG, Koulman A, Sharp SJ, Imamura F, Kroger J, et al. Differences in the prospective association between individual plasma phospholipid saturated fatty acids and incident type 2 diabetes: the EPIC-InterAct case-cohort study. Lancet Diabetes Endocrinol. 2014;2:810–18. doi: 10.1016/S2213-8587(14)70146-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Forsythe CE, Phinney SD, Feinman RD, Volk BM, Freidenreich D, et al. Limited effect of dietary saturated fat on plasma saturated fat in the context of a low carbohydrate diet. Lipids. 2010;45:947–62. doi: 10.1007/s11745-010-3467-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Forsythe CE, Phinney SD, Fernandez ML, Quann EE, Wood RJ, et al. Comparison of low fat and low carbohydrate diets on circulating fatty acids composition and markers of inflammation. Lipids. 2008;43:65–77. doi: 10.1007/s11745-007-3132-7. [DOI] [PubMed] [Google Scholar]
- 57.Fredenrich A. Role of apolipoprotein CIII in triglyceride-rich lipoprotein metabolism. Diabetes Metab. 1998;24:490–95. [PubMed] [Google Scholar]
- 58.Fung TT, Malik V, Rexrode KM, Manson JE, Willett WC, Hu FB. Sweetened beverage consumption and risk of coronary heart disease in women. Am J Clin Nutr. 2009;89:1037–42. doi: 10.3945/ajcn.2008.27140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Furtado JD, Campos H, Appel LJ, Miller ER, Laranjo N, et al. Effect of protein, unsaturated fat, and carbohydrate intakes on plasma apolipoprotein B and VLDL and LDL containing apolipoprotein C-III: results from the OmniHeart Trial. Am J Clin Nutr. 2008;87:1623–30. doi: 10.1093/ajcn/87.6.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gardner CD. Tailoring dietary approaches for weight loss. Int J Obes Suppl. 2012;2:S11–15. doi: 10.1038/ijosup.2012.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gardner CD, Kiazand A, Alhassan S, Kim S, Stafford RS, et al. Comparison of the Atkins, Zone, Ornish, and LEARN diets for change in weight and related risk factors among overweight premenopausal women. The A TO Z Weight Loss Study: a randomized trial. JAMA. 2007;297:969–77. doi: 10.1001/jama.297.9.969. [DOI] [PubMed] [Google Scholar]
- 62.Goff DC, Jr, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Sr, et al. 2013 ACC/AHA Guideline on the Assessment of Cardiovascular Risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2935–59. doi: 10.1016/j.jacc.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gogebakan O, Kohl A, Osterhoff MA, van Baak MA, Jebb SA, et al. Effects of weight loss and long-term weight maintenance with diets varying in protein and glycemic index on cardiovascular risk factors. The Diet, Obesity, and Genes (DiOGenes) study: a randomized, controlled trial. Circulation. 2011;124:2829–38. doi: 10.1161/CIRCULATIONAHA.111.033274. [DOI] [PubMed] [Google Scholar]
- 64.Goldbohm RA, Chorus AM, Galindo Garre F, Schouten LJ, van den Brandt PA. Dairy consumption and 10-y total and cardiovascular mortality: a prospective cohort study in the Netherlands. Am J Clin Nutr. 2011;93:615–27. doi: 10.3945/ajcn.110.000430. [DOI] [PubMed] [Google Scholar]
- 65.Guay V, Lamarche B, Charest A, Tremblay AJ, Couture P. Effect of short-term low- and high-fat diets on low-density lipoprotein particle size in normolipidemic subjects. Metabolism. 2012;61:76–83. doi: 10.1016/j.metabol.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 66.Halton TL, Willett WC, Liu S, Manson JE, Albert CM, et al. Low-carbohydrate-diet score and the risk of coronary heart disease in women. N Engl J Med. 2006;355:1991–2002. doi: 10.1056/NEJMoa055317. [DOI] [PubMed] [Google Scholar]
- 67.Harvey KA, Walker CL, Xu Z, Whitley P, Pavlina TM, et al. Oleic acid inhibits stearic acid-induced inhibition of cell growth and pro-inflammatory responses in human aortic endothelial cells. J Lipid Res. 2010;51:3470–80. doi: 10.1194/jlr.M010371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hayes KC, Khosla P, Hajri T, Pronczuk A. Saturated fatty acids and LDL receptor modulation in humans and monkeys. Prostaglandins Leukot Essent Fatty Acids. 1997;57:411–18. doi: 10.1016/s0952-3278(97)90420-8. [DOI] [PubMed] [Google Scholar]
- 69.Heijnen ML, van Amelsvoort JM, Deurenberg P, Beynen AC. Neither raw nor retrograded resistant starch lowers fasting serum cholesterol concentrations in healthy normolipidemic subjects. Am J Clin Nutr. 1996;64:312–18. doi: 10.1093/ajcn/64.3.312. [DOI] [PubMed] [Google Scholar]
- 70.Hjerpsted J, Leedo E, Tholstrup T. Cheese intake in large amounts lowers LDL-cholesterol concentrations compared with butter intake of equal fat content. Am J Clin Nutr. 2011;94:1479–84. doi: 10.3945/ajcn.111.022426. [DOI] [PubMed] [Google Scholar]
- 71.Hodson L, Skeaff CM, Fielding BA. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog Lipid Res. 2008;47:348–80. doi: 10.1016/j.plipres.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 72.Hoogeveen RC, Gaubatz JW, Sun W, Dodge RC, Crosby JR, et al. Small dense low-density lipoprotein-cholesterol concentrations predict risk for coronary heart disease: the Atherosclerosis Risk In Communities (ARIC) study. Arterioscler Thromb Vasc Biol. 2014;34:1069–77. doi: 10.1161/ATVBAHA.114.303284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hooper L, Summerbell CD, Thompson R, Sills D, Roberts FG, et al. Reduced or modified dietary fat for preventing cardiovascular disease. Cochrane Database Syst Rev. 2012;5:CD002137. doi: 10.1002/14651858.CD002137.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Howard BV, Van Horn L, Hsia J, Manson JE, Stefanick ML, et al. Low-fat dietary pattern and risk of cardiovascular disease: the Women’s Health Initiative Randomized Controlled Dietary Modification Trial. JAMA. 2006;295:655–66. doi: 10.1001/jama.295.6.655. [DOI] [PubMed] [Google Scholar]
- 75.Hu FB. Are refined carbohydrates worse than saturated fat? Am J Clin Nutr. 2010;91:1541–42. doi: 10.3945/ajcn.2010.29622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hu FB, Stampfer MJ, Manson JE, Ascherio A, Colditz GA, et al. Dietary saturated fats and their food sources in relation to the risk of coronary heart disease in women. Am J Clin Nutr. 1999;70:1001–8. doi: 10.1093/ajcn/70.6.1001. [DOI] [PubMed] [Google Scholar]
- 77.Huff MW, Nestel PJ. Metabolism of apolipoproteins CII, CIII1, CIII2 and VLDL-B in human subjects consuming high carbohydrate diets. Metabolism. 1982;31:493–98. doi: 10.1016/0026-0495(82)90240-2. [DOI] [PubMed] [Google Scholar]
- 78.Jakobsen MU, Dethlefsen C, Joensen AM, Stegger J, Tjonneland A, et al. Intake of carbohydrates compared with intake of saturated fatty acids and risk of myocardial infarction: importance of the glycemic index. Am J Clin Nutr. 2010;91:1764–68. doi: 10.3945/ajcn.2009.29099. [DOI] [PubMed] [Google Scholar]
- 79.Jakobsen MU, O’Reilly EJ, Heitmann BL, Pereira MA, Balter K, et al. Major types of dietary fat and risk of coronary heart disease: a pooled analysis of 11 cohort studies. Am J Clin Nutr. 2009;89:1425–32. doi: 10.3945/ajcn.2008.27124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jansen S, Lopez-Miranda J, Salas J, Castro P, Paniagua JA, et al. Plasma lipid response to hypolipidemic diets in young healthy non-obese men varies with body mass index. J Nutr. 1998;128:1144–49. doi: 10.1093/jn/128.7.1144. [DOI] [PubMed] [Google Scholar]
- 81.Jebb SA, Lovegrove JA, Griffin BA, Frost GS, Moore CS, et al. Effect of changing the amount and type of fat and carbohydrate on insulin sensitivity and cardiovascular risk: the RISCK (Reading, Imperial, Surrey, Cambridge, and Kings) trial. Am J Clin Nutr. 2010;92:748–58. doi: 10.3945/ajcn.2009.29096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jenkins DJ, Vuksan V, Kendall CW, Wursch P, Jeffcoat R, et al. Physiological effects of resistant starches on fecal bulk, short chain fatty acids, blood lipids and glycemic index. J Am Coll Nutr. 1998;17:609–16. doi: 10.1080/07315724.1998.10718810. [DOI] [PubMed] [Google Scholar]
- 83.Jenkins DJ, Wong JM, Kendall CW, Esfahani A, Ng VW, et al. The effect of a plant-based low-carbohydrate (“Eco-Atkins”) diet on body weight and blood lipid concentrations in hyperlipidemic subjects. Arch Intern Med. 2009;169:1046–54. doi: 10.1001/archinternmed.2009.115. [DOI] [PubMed] [Google Scholar]
- 84.Jimenéz-Gómez Y, López-Miranda J, Blanco-Colio LM, Marin C, Pérez-Martinez P, et al. Olive oil and walnut breakfasts reduce the postprandial inflammatory response in mononuclear cells compared with a butter breakfast in healthy men. Atherosclerosis. 2009;204:e70–76. doi: 10.1016/j.atherosclerosis.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 85.Johnston BC, Kanters S, Bandayrel K, Wu P, Naji F, et al. Comparison of weight loss among named diet programs in overweight and obese adults: a meta-analysis. JAMA. 2014;312:923–33. doi: 10.1001/jama.2014.10397. [DOI] [PubMed] [Google Scholar]
- 86.Karupaiah T, Tan CH, Chinna K, Sundram K. The chain length of dietary saturated fatty acids affects human postprandial lipemia. J Am Coll Nutr. 2011;30:511–21. doi: 10.1080/07315724.2011.10719997. [DOI] [PubMed] [Google Scholar]
- 87.Kawakami A, Aikawa M, Alcaide P, Luscinskas FW, Libby P, Sacks FM. Apolipoprotein CIII induces expression of vascular cell adhesion molecule-1 in vascular endothelial cells and increases adhesion of monocytic cells. Circulation. 2006;114:681–87. doi: 10.1161/CIRCULATIONAHA.106.622514. [DOI] [PubMed] [Google Scholar]
- 88.Keogh JB, Grieger JA, Noakes M, Clifton PM. Flow-mediated dilatation is impaired by a high-saturated fat diet but not by a high-carbohydrate diet. Arterioscler Thromb Vasc Biol. 2005;25:1274–79. doi: 10.1161/01.ATV.0000163185.28245.a1. [DOI] [PubMed] [Google Scholar]
- 89.Keys A, Aravanis C, Blackburn HW, Van Buchem FS, Buzina R, et al. Epidemiological studies related to coronary heart disease: characteristics of men aged 40–59 in seven countries. Acta Med Scand Suppl. 1966;460:1–392. [PubMed] [Google Scholar]
- 90.Khosla P, Hayes KC. Saturated fat and lipemia: importance of study design and triglyceride structure. Am J Clin Nutr. 2012;96:216–18. doi: 10.3945/ajcn.112.037234. author reply 218–19. [DOI] [PubMed] [Google Scholar]
- 91.Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19:576–85. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Krauss RM. Dietary and genetic probes of atherogenic dyslipidemia. Arterioscler Thromb Vasc Biol. 2005;25:2265–72. doi: 10.1161/01.ATV.0000186365.73973.f0. [DOI] [PubMed] [Google Scholar]
- 93.Krauss RM. All low-density lipoprotein particles are not created equal. Arterioscler Thromb Vasc Biol. 2014;34:959–61. doi: 10.1161/ATVBAHA.114.303458. [DOI] [PubMed] [Google Scholar]
- 94.Krauss RM, Blanche PJ, Rawlings RS, Fernstrom HS, Williams PT. Separate effects of reduced carbohydrate intake and weight loss on atherogenic dyslipidemia. Am J Clin Nutr. 2006;83:1025–31. doi: 10.1093/ajcn/83.5.1025. quiz 1205. [DOI] [PubMed] [Google Scholar]
- 95.Krauss RM, Siri PW. Metabolic abnormalities: triglyceride and low-density lipoprotein. Endocrinol Metab Clin North Am. 2004;33:405–15. doi: 10.1016/j.ecl.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 96.Kummerow FA. Interaction between sphingomyelin and oxysterols contributes to atherosclerosis and sudden death. Am J Cardiovasc Dis. 2013;3:17–26. [PMC free article] [PubMed] [Google Scholar]
- 97.Lairon D, Defoort C. Effects of nutrients on postprandial lipemia. Curr Vasc Pharmacol. 2011;9:309–12. doi: 10.2174/157016111795495576. [DOI] [PubMed] [Google Scholar]
- 98.Larsen TM, Dalskov SM, van Baak M, Jebb SA, Papadaki A, et al. Diets with high or low protein content and glycemic index for weight-loss maintenance. N Engl J Med. 2010;363:2102–13. doi: 10.1056/NEJMoa1007137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lee JE, McLerran DF, Rolland B, Chen Y, Grant EJ, et al. Meat intake and cause-specific mortality: a pooled analysis of Asian prospective cohort studies. Am J Clin Nutr. 2013;98:1032–41. doi: 10.3945/ajcn.113.062638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lefevre M, Champagne CM, Tulley RT, Rood JC, Most MM. Individual variability in cardiovascular disease risk factor responses to low-fat and low-saturated-fat diets in men: body mass index, adiposity, and insulin resistance predict changes in LDL cholesterol. Am J Clin Nutr. 2005;82:957–63. doi: 10.1093/ajcn/82.5.957. quiz 1145–46. [DOI] [PubMed] [Google Scholar]
- 101.Liebman BF, Katan MB, Jacobson MF. Association of dietary, circulating, and supplement fatty acids with coronary risk. Ann Intern Med. 2014;161:454–55. doi: 10.7326/L14-5018-4. [DOI] [PubMed] [Google Scholar]
- 102.Livesey G, Taylor R, Hulshof T, Howlett J. Glycemic response and health—a systematic review and meta-analysis: relations between dietary glycemic properties and health outcomes. Am J Clin Nutr. 2008;87:258–68S. doi: 10.1093/ajcn/87.1.258S. [DOI] [PubMed] [Google Scholar]
- 103.Lorenzen JK, Astrup A. Dairy calcium intake modifies responsiveness of fat metabolism and blood lipids to a high-fat diet. Br J Nutr. 2011;105:1823–31. doi: 10.1017/S0007114510005581. [DOI] [PubMed] [Google Scholar]
- 104.Maki KC, Van Elswyk ME, Alexander DD, Rains TM, Sohn EL, McNeill S. A meta-analysis of randomized controlled trials that compare the lipid effects of beef versus poultry and/or fish consumption. J Clin Lipidol. 2012;6:352–61. doi: 10.1016/j.jacl.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 105.Mangravite LM, Chiu S, Wojnoonski K, Rawlings RS, Bergeron N, Krauss RM. Changes in atherogenic dyslipidemia induced by carbohydrate restriction in men are dependent on dietary protein source. J Nutr. 2011;141:2180–85. doi: 10.3945/jn.111.139477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Manning PJ, Sutherland WH, McGrath MM, de Jong SA, Walker RJ, Williams MJ. Postprandial cytokine concentrations and meal composition in obese and lean women. Obesity (Silver Spring) 2008;16:2046–52. doi: 10.1038/oby.2008.334. [DOI] [PubMed] [Google Scholar]
- 107.Manson JE, Bassuk SS, Lee IM, Cook NR, Albert MA, et al. The VITamin D and OmegA-3 TriaL (VITAL): rationale and design of a large randomized controlled trial of vitamin D and marine omega-3 fatty acid supplements for the primary prevention of cancer and cardiovascular disease. Contemp Clin Trials. 2012;33:159–71. doi: 10.1016/j.cct.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Martínez-González MA, Zazpe I, Razquin C, Sánchez-Tainta A, Corella D, et al. Empirically-derived food patterns and the risk of total mortality and cardiovascular events in the PREDIMED study. Clin Nutr. 2014 doi: 10.1016/j.clnu.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 109.Masson CJ, Mensink RP. Exchanging saturated fatty acids for (n-6) polyunsaturated fatty acids in a mixed meal may decrease postprandial lipemia and markers of inflammation and endothelial activity in overweight men. J Nutr. 2011;141:816–21. doi: 10.3945/jn.110.136432. [DOI] [PubMed] [Google Scholar]
- 110.Inst. Med (IOM) Evaluation of Biomarkers and Surrogate Endpoints in Chronic Disease. Washington, DC: Natl. Acad. Press; 2010. [PubMed] [Google Scholar]
- 111.Mensink RP, Katan MB. Effect of dietary fatty acids on serum lipids and lipoproteins. A meta-analysis of 27 trials. Arterioscler Thromb. 1992;12:911–19. doi: 10.1161/01.atv.12.8.911. [DOI] [PubMed] [Google Scholar]
- 112.Mensink RP, Zock PL, Katan MB, Hornstra G. Effect of dietary cis and trans fatty acids on serum lipoprotein[a] levels in humans. J Lipid Res. 1992;33:1493–501. [PubMed] [Google Scholar]
- 113.Mensink RP, Zock PL, Kester AD, Katan MB. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am J Clin Nutr. 2003;77:1146–55. doi: 10.1093/ajcn/77.5.1146. [DOI] [PubMed] [Google Scholar]
- 114.Mente A, de Koning L, Shannon HS, Anand SS. A systematic review of the evidence supporting a causal link between dietary factors and coronary heart disease. Arch Intern Med. 2009;169:659–69. doi: 10.1001/archinternmed.2009.38. [DOI] [PubMed] [Google Scholar]
- 115.Micha R, Mozaffarian D. Saturated fat and cardiometabolic risk factors, coronary heart disease, stroke, and diabetes: a fresh look at the evidence. Lipids. 2010;45:893–905. doi: 10.1007/s11745-010-3393-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Micha R, Wallace SK, Mozaffarian D. Red and processed meat consumption and risk of incident coronary heart disease, stroke, and diabetes mellitus: a systematic review and meta-analysis. Circulation. 2010;121:2271–83. doi: 10.1161/CIRCULATIONAHA.109.924977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Miller PE, Van Elswyk M, Alexander DD. Long-chain omega-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid and blood pressure: a meta-analysis of randomized controlled trials. Am J Hypertens. 2014;27:885–96. doi: 10.1093/ajh/hpu024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mora S, Caulfied MP, Wohlgemuth J, Chen Z, Superko HR, Glynn RJ, Ridker PM, Krauss RM. Atherogenic lipoprotein subclasses determined by ion mobility analysis and first cardiovascular events after random allocation to high-intensity statin therapy or placebo: the JUPITER Trial. 2015 doi: 10.1161/CIRCULATIONAHA.115.016857. Manuscript submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mozaffarian D. Fish and n-3 fatty acids for the prevention of fatal coronary heart disease and sudden cardiac death. Am J Clin Nutr. 2008;87:1991–96S. doi: 10.1093/ajcn/87.6.1991S. [DOI] [PubMed] [Google Scholar]
- 120.Mozaffarian D, Appel LJ, Van Horn L. Components of a cardioprotective diet: new insights. Circulation. 2011;123:2870–91. doi: 10.1161/CIRCULATIONAHA.110.968735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mozaffarian D, Cao H, King IB, Lemaitre RN, Song X, et al. Trans-palmitoleic acid, metabolic risk factors, and new-onset diabetes in U.S. adults: a cohort study. Ann Intern Med. 2010;153:790–99. doi: 10.1059/0003-4819-153-12-201012210-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mozaffarian D, Clarke R. Quantitative effects on cardiovascular risk factors and coronary heart disease risk of replacing partially hydrogenated vegetable oils with other fats and oils. Eur J Clin Nutr. 2009;63(Suppl 2):S22–33. doi: 10.1038/sj.ejcn.1602976. [DOI] [PubMed] [Google Scholar]
- 123.Mozaffarian D, Katan MB, Ascherio A, Stampfer MJ, Willett WC. Trans fatty acids and cardiovascular disease. N Engl J Med. 2006;354:1601–13. doi: 10.1056/NEJMra054035. [DOI] [PubMed] [Google Scholar]
- 124.Mozaffarian D, Micha R, Wallace S. Effects on coronary heart disease of increasing polyunsaturated fat in place of saturated fat: a systematic review and meta-analysis of randomized controlled trials. PLOS Med. 2010;7:e1000252. doi: 10.1371/journal.pmed.1000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Muller H, Lindman AS, Brantsaeter AL, Pedersen JI. The serum LDL/HDL cholesterol ratio is influenced more favorably by exchanging saturated with unsaturated fat than by reducing saturated fat in the diet of women. J Nutr. 2003;133:78–83. doi: 10.1093/jn/133.1.78. [DOI] [PubMed] [Google Scholar]
- 126.Musunuru K, Orho-Melander M, Caulfield MP, Li S, Salameh WA, et al. Ion mobility analysis of lipoprotein subfractions identifies three independent axes of cardiovascular risk. Arterioscler Thromb Vasc Biol. 2009;29:1975–80. doi: 10.1161/ATVBAHA.109.190405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nappo F, Esposito K, Cioffi M, Giugliano G, Molinari AM, et al. Postprandial endothelial activation in healthy subjects and in type 2 diabetic patients: role of fat and carbohydrate meals. J Am Coll Cardiol. 2002;39:1145–50. doi: 10.1016/s0735-1097(02)01741-2. [DOI] [PubMed] [Google Scholar]
- 128.Nestel PJ, Chronopulos A, Cehun M. Dairy fat in cheese raises LDL cholesterol less than that in butter in mildly hypercholesterolaemic subjects. Eur J Clin Nutr. 2005;59:1059–63. doi: 10.1038/sj.ejcn.1602211. [DOI] [PubMed] [Google Scholar]
- 129.Nicholls SJ, Lundman P, Harmer JA, Cutri B, Griffiths KA, et al. Consumption of saturated fat impairs the anti-inflammatory properties of high-density lipoproteins and endothelial function. J Am Coll Cardiol. 2006;48:715–20. doi: 10.1016/j.jacc.2006.04.080. [DOI] [PubMed] [Google Scholar]
- 130.Noakes M, Clifton PM, Nestel PJ, Le Leu R, McIntosh G. Effect of high-amylose starch and oat bran on metabolic variables and bowel function in subjects with hypertriglyceridemia. Am J Clin Nutr. 1996;64:944–51. doi: 10.1093/ajcn/64.6.944. [DOI] [PubMed] [Google Scholar]
- 131.Nordmann AJ, Nordmann A, Briel M, Keller U, Yancy WS, Jr, et al. Effects of low-carbohydrate versus low-fat diets on weight loss and cardiovascular risk factors: a meta-analysis of randomized controlled trials. Arch Intern Med. 2006;166:285–93. doi: 10.1001/archinte.166.3.285. [DOI] [PubMed] [Google Scholar]
- 132.O’Connor LM, Lentjes MA, Luben RN, Khaw KT, Wareham NJ, Forouhi NG. Dietary dairy product intake and incident type 2 diabetes: a prospective study using dietary data from a 7-day food diary. Diabetologia. 2014;57:909–17. doi: 10.1007/s00125-014-3176-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Odegaard AO, Koh WP, Yuan JM, Gross MD, Pereira MA. Dietary patterns and mortality in a Chinese population. Am J Clin Nutr. 2014;100:877–83. doi: 10.3945/ajcn.114.086124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ordovas JM. Genetic interactions with diet influence the risk of cardiovascular disease. Am J Clin Nutr. 2006;83:443–46S. doi: 10.1093/ajcn/83.2.443S. [DOI] [PubMed] [Google Scholar]
- 135.Parish S, Offer A, Clarke R, Hopewell JC, Hill MR, et al. Lipids and lipoproteins and risk of different vascular events in the MRC/BHF Heart Protection Study. Circulation. 2012;125:2469–78. doi: 10.1161/CIRCULATIONAHA.111.073684. [DOI] [PubMed] [Google Scholar]
- 136.Poppitt SD, Keogh GF, Lithander FE, Wang Y, Mulvey TB, et al. Postprandial response of adiponectin, interleukin-6, tumor necrosis factor-α, and C-reactive protein to a high-fat dietary load. Nutrition. 2008;24:322–29. doi: 10.1016/j.nut.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 137.Ramsden CE, Hibbeln JR, Majchrzak SF, Davis JM. n-6 fatty acid-specific and mixed polyunsaturate dietary interventions have different effects on CHD risk: a meta-analysis of randomised controlled trials. Br J Nutr. 2010;104:1586–600. doi: 10.1017/S0007114510004010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ramsden CE, Zamora D, Leelarthaepin B, Majchrzak-Hong SF, Faurot KR, et al. Use of dietary linoleic acid for secondary prevention of coronary heart disease and death: evaluation of recovered data from the Sydney Diet Heart Study and updated meta-analysis. BMJ. 2013;346:e8707. doi: 10.1136/bmj.e8707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ras RT, Streppel MT, Draijer R, Zock PL. Flow-mediated dilation and cardiovascular risk prediction: a systematic review with meta-analysis. Int J Cardiol. 2013;168:344–51. doi: 10.1016/j.ijcard.2012.09.047. [DOI] [PubMed] [Google Scholar]
- 140.Rasmussen BM, Vessby B, Uusitupa M, Berglund L, Pedersen E, et al. Effects of dietary saturated, monounsaturated, and n-3 fatty acids on blood pressure in healthy subjects. Am J Clin Nutr. 2006;83:221–26. doi: 10.1093/ajcn/83.2.221. [DOI] [PubMed] [Google Scholar]
- 141.Raz O, Steinvil A, Berliner S, Rosenzweig T, Justo D, Shapira I. The effect of two iso-caloric meals containing equal amounts of fats with a different fat composition on the inflammatory and metabolic markers in apparently healthy volunteers. J Inflamm (Lond) 2013;10:3. doi: 10.1186/1476-9255-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rees K, Hartley L, Flowers N, Clarke A, Hooper L, et al. “Mediterranean” dietary pattern for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013;8:CD009825. doi: 10.1002/14651858.CD009825.pub2. [DOI] [PubMed] [Google Scholar]
- 143.Rizos EC, Ntzani EE, Bika E, Kostapanos MS, Elisaf MS. Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: a systematic review and meta-analysis. JAMA. 2012;308:1024–33. doi: 10.1001/2012.jama.11374. [DOI] [PubMed] [Google Scholar]
- 144.Roche MM, Wang PP. Sex differences in all-cause and cardiovascular mortality, hospitalization for individuals with and without diabetes, and patients with diabetes diagnosed early and late. Diabetes Care. 2013;36:2582–90. doi: 10.2337/dc12-1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rudel LL, Parks JS, Sawyer JK. Compared with dietary monounsaturated and saturated fat, polyun-saturated fat protects African green monkeys from coronary artery atherosclerosis. Arterioscler Thromb Vasc Biol. 1995;15:2101–10. doi: 10.1161/01.atv.15.12.2101. [DOI] [PubMed] [Google Scholar]
- 146.Sacks FM. The crucial roles of apolipoproteins E and C-III in apoB lipoprotein metabolism in normolipidemia and hypertriglyceridemia. Curr Opin Lipidol. 2015;26:56–63. doi: 10.1097/MOL.0000000000000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sacks FM, Bray GA, Carey VJ, Smith SR, Ryan DH, et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med. 2009;360:859–73. doi: 10.1056/NEJMoa0804748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sacks FM, Carey VJ, Anderson CA, Miller ER, 3rd, Copeland T, et al. Effects of high versus low glycemic index of dietary carbohydrate on cardiovascular disease risk factors and insulin sensitivity: the OmniCarb Randomized Clinical Trial. JAMA. 2014;312:2531–41. doi: 10.1001/jama.2014.16658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Saito H, Kagaya M, Suzuki M, Yoshida A, Naito M. Simultaneous ingestion of fructose and fat exacerbates postprandial exogenous lipidemia in young healthy Japanese women. J Atheroscler Thromb. 2013;20:591–600. doi: 10.5551/jat.17301. [DOI] [PubMed] [Google Scholar]
- 150.Sanders TA, Filippou A, Berry SE, Baumgartner S, Mensink RP. Palmitic acid in the sn-2 position of triacylglycerols acutely influences postprandial lipid metabolism. Am J Clin Nutr. 2011;94:1433–41. doi: 10.3945/ajcn.111.017459. [DOI] [PubMed] [Google Scholar]
- 151.Sanders TA, Lewis FJ, Goff LM, Chowienczyk PJ, Group RS. SFAs do not impair endothelial function and arterial stiffness. Am J Clin Nutr. 2013;98:677–83. doi: 10.3945/ajcn.113.063644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Schwingshackl L, Hoffmann G. Long-term effects of low-fat diets either low or high in protein on cardiovascular and metabolic risk factors: a systematic review and meta-analysis. Nutr J. 2013;12:48. doi: 10.1186/1475-2891-12-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Serra-Majem L, Roman B, Estruch R. Scientific evidence of interventions using the Mediterranean diet: a systematic review. Nutr Rev. 2006;64:S27–47. doi: 10.1111/j.1753-4887.2006.tb00232.x. [DOI] [PubMed] [Google Scholar]
- 154.Shepherd J, Packard CJ, Grundy SM, Yeshurun D, Gotto AM, Jr, Taunton OD. Effects of saturated and polyunsaturated fat diets on the chemical composition and metabolism of low density lipoproteins in man. J Lipid Res. 1980;21:91–99. [PubMed] [Google Scholar]
- 155.Shikany JM, Tinker LF, Neuhouser ML, Ma Y, Patterson RE, et al. Association of glycemic load with cardiovascular disease risk factors: the Women’s Health Initiative Observational Study. Nutrition. 2010;26:641–47. doi: 10.1016/j.nut.2009.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Shin MJ, Krauss RM. Apolipoprotein CIII bound to apoB-containing lipoproteins is associated with small, dense LDL independent of plasma triglyceride levels in healthy men. Atherosclerosis. 2010;211:337–41. doi: 10.1016/j.atherosclerosis.2010.02.025. [DOI] [PubMed] [Google Scholar]
- 157.Sievenpiper JL, de Souza RJ, Mirrahimi A, Yu ME, Carleton AJ, et al. Effect of fructose on body weight in controlled feeding trials: a systematic review and meta-analysis. Ann Intern Med. 2012;156:291–304. doi: 10.7326/0003-4819-156-4-201202210-00007. [DOI] [PubMed] [Google Scholar]
- 158.Simopoulos AP. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp Biol Med (Maywood) 2008;233:674–88. doi: 10.3181/0711-MR-311. [DOI] [PubMed] [Google Scholar]
- 159.Siri-Tarino PW, Sun Q, Hu FB, Krauss RM. Meta-analysis of prospective cohort studies evaluating the association of saturated fat with cardiovascular disease. Am J Clin Nutr. 2010;91:535–46. doi: 10.3945/ajcn.2009.27725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Siri-Tarino PW, Sun Q, Hu FB, Krauss RM. Saturated fat, carbohydrate, and cardiovascular disease. Am J Clin Nutr. 2010;91:502–9. doi: 10.3945/ajcn.2008.26285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Siri-Tarino PW, Sun Q, Hu FB, Krauss RM. Saturated fatty acids and risk of coronary heart disease: modulation by replacement nutrients. Curr Atheroscler Rep. 2010;12:384–90. doi: 10.1007/s11883-010-0131-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Siri-Tarino PW, Williams PT, Fernstrom HS, Rawlings RS, Krauss RM. Reversal of small, dense LDL subclass phenotype by normalization of adiposity. Obesity (Silver Spring) 2009;17:1768–75. doi: 10.1038/oby.2009.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Skeaff CM, Miller J. Dietary fat and coronary heart disease: summary of evidence from prospective cohort and randomised controlled trials. Ann Nutr Metab. 2009;55:173–201. doi: 10.1159/000229002. [DOI] [PubMed] [Google Scholar]
- 164.Sluijs I, Forouhi NG, Beulens JW, van der Schouw YT, Agnoli C, et al. The amount and type of dairy product intake and incident type 2 diabetes: results from the EPIC-InterAct Study. Am J Clin Nutr. 2012;96:382–90. doi: 10.3945/ajcn.111.021907. [DOI] [PubMed] [Google Scholar]
- 165.Soedamah-Muthu SS, Ding EL, Al-Delaimy WK, Hu FB, Engberink MF, et al. Milk and dairy consumption and incidence of cardiovascular diseases and all-cause mortality: dose-response meta-analysis of prospective cohort studies. Am J Clin Nutr. 2011;93:158–71. doi: 10.3945/ajcn.2010.29866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sofi F, Abbate R, Gensini GF, Casini A. Accruing evidence on benefits of adherence to the Mediterranean diet on health: an updated systematic review and meta-analysis. Am J Clin Nutr. 2010;92:1189–96. doi: 10.3945/ajcn.2010.29673. [DOI] [PubMed] [Google Scholar]
- 167.Stanhope KL, Bremer AA, Medici V, Nakajima K, Ito Y, et al. Consumption of fructose and high fructose corn syrup increase postprandial triglycerides, LDL-cholesterol, and apolipoprotein-B in young men and women. J Clin Endocrinol Metab. 2011;96:E1596–605. doi: 10.1210/jc.2011-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Stanhope KL, Schwarz JM, Keim NL, Griffen SC, Bremer AA, et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest. 2009;119:1322–34. doi: 10.1172/JCI37385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Te Morenga LA, Howatson AJ, Jones RM, Mann J. Dietary sugars and cardiometabolic risk: systematic review and meta-analyses of randomized controlled trials of the effects on blood pressure and lipids. Am J Clin Nutr. 2014;100:165–79. doi: 10.3945/ajcn.113.081521. [DOI] [PubMed] [Google Scholar]
- 170.Tholstrup T, Hoy CE, Andersen LN, Christensen RD, Sandstrom B. Does fat in milk, butter and cheese affect blood lipids and cholesterol differently? J Am Coll Nutr. 2004;23:169–76. doi: 10.1080/07315724.2004.10719358. [DOI] [PubMed] [Google Scholar]
- 171.Tierney AC, McMonagle J, Shaw DI, Gulseth HL, Helal O, et al. Effects of dietary fat modification on insulin sensitivity and on other risk factors of the metabolic syndrome—LIPGENE: a European randomized dietary intervention study. Int J Obes (Lond) 2011;35:800–9. doi: 10.1038/ijo.2010.209. [DOI] [PubMed] [Google Scholar]
- 172.Tsai MY, Steffen BT, Guan W, McClelland RL, Warnick R, et al. New automated assay of small dense low-density lipoprotein cholesterol identifies risk of coronary heart disease: the Multi-Ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2014;34:196–201. doi: 10.1161/ATVBAHA.113.302401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Turner JD, Le NA, Brown WV. Effect of changing dietary fat saturation on low-density lipoprotein metabolism in man. Am J Physiol. 1981;241:E57–63. doi: 10.1152/ajpendo.1981.241.1.E57. [DOI] [PubMed] [Google Scholar]
- 174.Vafeiadou K, Weech M, Sharma V, Yaqoob P, Todd S, et al. A review of the evidence for the effects of total dietary fat, saturated, monounsaturated and n-6 polyunsaturated fatty acids on vascular function, endothelial progenitor cells and microparticles. Br J Nutr. 2012;107:303–24. doi: 10.1017/S0007114511004764. [DOI] [PubMed] [Google Scholar]
- 175.Vessby B, Uusitupa M, Hermansen K, Riccardi G, Rivellese AA, et al. Substituting dietary saturated for monounsaturated fat impairs insulin sensitivity in healthy men and women: the KANWU Study. Diabetologia. 2001;44:312–19. doi: 10.1007/s001250051620. [DOI] [PubMed] [Google Scholar]
- 176.Virtanen JK, Mursu J, Tuomainen T, Voutilainen S. Dietary fatty acids and risk of coronary heart diesase in men: the Kuopio Ischemic Heart Disease Risk Factor Study. Arterioscler Thromb Vasc Biol. 2014;34:2679–87. doi: 10.1161/ATVBAHA.114.304082. [DOI] [PubMed] [Google Scholar]
- 177.Volk BM, Kunces LJ, Freidenreich DJ, Kupchak BR, Saenz C, et al. Effects of step-wise increases in dietary carbohydrate on circulating saturated fatty acids and palmitoleic acid in adults with metabolic syndrome. PLOS ONE. 2014;9:e113605. doi: 10.1371/journal.pone.0113605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Voon PT, Ng TK, Lee VK, Nesaretnam K. Diets high in palmitic acid (16:0), lauric and myristic acids (12:0 + 14:0), or oleic acid (18:1) do not alter postprandial or fasting plasma homocysteine and inflammatory markers in healthy Malaysian adults. Am J Clin Nutr. 2011;94:1451–57. doi: 10.3945/ajcn.111.020107. [DOI] [PubMed] [Google Scholar]
- 179.Wang C, Harris WS, Chung M, Lichtenstein AH, Balk EM, et al. n-3 Fatty acids from fish or fish-oil supplements, but not alpha-linolenic acid, benefit cardiovascular disease outcomes in primary-and secondary-prevention studies: a systematic review. Am J Clin Nutr. 2006;84:5–17. doi: 10.1093/ajcn/84.1.5. [DOI] [PubMed] [Google Scholar]
- 180.Wang D, Sievenpiper JL, de Souza RJ, Cozma AI, Chiavaroli L, et al. Effect of fructose on postprandial triglycerides: a systematic review and meta-analysis of controlled feeding trials. Atherosclerosis. 2014;232:125–33. doi: 10.1016/j.atherosclerosis.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 181.Warensjo E, Jansson JH, Cederholm T, Boman K, Eliasson M, et al. Biomarkers of milk fat and the risk of myocardial infarction in men and women: a prospective, matched case-control study. Am J Clin Nutr. 2010;92:194–202. doi: 10.3945/ajcn.2009.29054. [DOI] [PubMed] [Google Scholar]
- 182.Weggemans RM, Zock PL, Urgert R, Katan MB. Differences between men and women in the response of serum cholesterol to dietary changes. Eur J Clin Invest. 1999;29:827–34. doi: 10.1046/j.1365-2362.1999.00524.x. [DOI] [PubMed] [Google Scholar]
- 183.Welsh JA, Sharma A, Abramson JL, Vaccarino V, Gillespie C, Vos MB. Caloric sweetener consumption and dyslipidemia among US adults. JAMA. 2010;303:1490–97. doi: 10.1001/jama.2010.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Westerterp-Plantenga MS, Nieuwenhuizen A, Tome D, Soenen S, Westerterp KR. Dietary protein, weight loss, and weight maintenance. Annu Rev Nutr. 2009;29:21–41. doi: 10.1146/annurev-nutr-080508-141056. [DOI] [PubMed] [Google Scholar]
- 185.Westman EC, Feinman RD, Mavropoulos JC, Vernon MC, Volek JS, et al. Low-carbohydrate nutrition and metabolism. Am J Clin Nutr. 2007;86:276–84. doi: 10.1093/ajcn/86.2.276. [DOI] [PubMed] [Google Scholar]
- 186.Wijendran V, Hayes KC. Dietary n-6 and n-3 fatty acid balance and cardiovascular health. Annu Rev Nutr. 2004;24:597–615. doi: 10.1146/annurev.nutr.24.012003.132106. [DOI] [PubMed] [Google Scholar]
- 187.Willett WC, Stampfer MJ, Sacks FM. Association of dietary, circulating, and supplement fatty acids with coronary risk. Ann Intern Med. 2014;161:453. doi: 10.7326/L14-5018. [DOI] [PubMed] [Google Scholar]
- 188.Wu JH, Lemaitre RN, King IB, Song X, Psaty BM, et al. Circulating omega-6 polyunsaturated fatty acids and total and cause-specific mortality: the Cardiovascular Health Study. Circulation. 2014;130:1245–53. doi: 10.1161/CIRCULATIONAHA.114.011590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wycherley TP, Moran LJ, Clifton PM, Noakes M, Brinkworth GD. Effects of energy-restricted high-protein, low-fat compared with standard-protein, low-fat diets: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2012;96:1281–98. doi: 10.3945/ajcn.112.044321. [DOI] [PubMed] [Google Scholar]
- 190.Yamagishi K, Iso H, Kokubo Y, Saito I, Yatsuya H, et al. Dietary intake of saturated fatty acids and incident stroke and coronary heart disease in Japanese communities: the JPHC Study. Eur Heart J. 2013;34:1225–32. doi: 10.1093/eurheartj/eht043. [DOI] [PubMed] [Google Scholar]
- 191.Yang Q, Zhang Z, Gregg EW, Flanders WD, Merritt R, Hu FB. Added sugar intake and cardiovascular diseases mortality among US adults. JAMA Intern Med. 2014;174:516–24. doi: 10.1001/jamainternmed.2013.13563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Yao Z, Wang Y. Apolipoprotein C-III and hepatic triglyceride-rich lipoprotein production. Curr Opin Lipidol. 2012;23:206–12. doi: 10.1097/MOL.0b013e328352dc70. [DOI] [PubMed] [Google Scholar]