Abstract
Constructs intended for bone tissue engineering (TE) are influenced by the initial cell seeding density. Therefore, the objective of this study was to determine the effect of bone marrow stromal stem cells (BMSCs) density loaded onto copolymer scaffolds on bone regeneration. BMSCs were harvested from rat's bone marrow and cultured in media with or without osteogenic supplements. Cells were seeded onto poly(l‐lactide‐co‐ε‐caprolactone) [poly(LLA‐co‐CL)] scaffolds at two different densities: low density (1 × 106 cells/scaffold) or high density (2 × 106 cells/scaffold) using spinner modified flasks and examined after 1 and 3 weeks. Initial attachment and spread of BMSC onto the scaffolds was recorded by scanning electron microscopy. Cell proliferation was assessed by DNA quantification and cell differentiation by quantitative real‐time reverse transcriptase‐polymerized chain reaction analysis (qRT‐PCR). Five‐millimeter rat calvarial defects (24 defects in 12 rats) were implanted with scaffolds seeded with either low or high density expanded with or without osteogenic supplements. Osteogenic supplements significantly increased cell proliferation (p < 0.001). Scaffolds seeded at high cell density exhibited higher mRNA expressions of Runx2 p = 0.001, Col1 p = 0.001, BMP2 p < 0.001, BSP p < 0.001, and OC p = 0.013. More bone was formed in response to high cell seeding density (p = 0.023) and high seeding density with osteogenic medium (p = 0.038). Poly (LLA‐co‐CL) scaffolds could be appropriate candidates for bone TE. The optimal number of cells to be loaded onto scaffolds is critical for promoting Extracellular matrix synthesis and bone formation. Cell seeding density and osteogenic supplements may have a synergistic effect on the induction of new bone. © 2015 Wiley Periodicals, Inc. J Biomed Mater Res Part A: 103A: 3649–3658, 2015.
Keywords: bone marrow stromal cells, polymer scaffolds, cell seeding density, osteogenic supplements, bone regeneration
INTRODUCTION
Aliphatic polyesters such as poly(lactide), poly(lactide‐co‐glycolide), and poly(ε‐caprolactone) and their synthesized copolymers are the most common synthetic biodegradable polymers used as scaffolding in bone tissue engineering (TE). By copolymerization of ε‐caprolactone with different lactones, the physical and mechanical properties of the polyesters can be tailored, extending the range of applications of scaffolds.1 Poly(l‐lactide‐co‐ε‐caprolactone) [poly(LLA‐co‐CL)] possesses appropriate mechanical and physical properties. Not only the degradation rate but also the shape of the scaffolds can readily be modified.2, 3, 4 Moreover, animal studies have confirmed that endothelial microvascular networks can be created in porous scaffolds of 3D copolymer and sustained after implantation.5
In developing TE constructs which may influence the features and functionality of the engineered tissues, cell seeding density is a critical factor. The optimal seeding density of a scaffold depends on the scaffold biomaterial, the structure of the scaffold, and the seeding technique.6, 7 The influence of cell seeding density on TE constructs has been studied in cardiac tissue, cartilage, and bone.8, 9, 10 In bone TE, cell seeding density influences cell proliferation, distribution, differentiation, extracellular matrix (ECM) synthesis, and tissue formation.11, 12, 13, 14, 15, 16 It has been reported that bone marrow stromal cells (BMSCs), cultured at density of 6.83 × 105 cells/cm2 in three‐dimensional (3D) poly(dl‐lactic‐co‐glycolic acid) scaffolds, exhibited rapid proliferation over the first 7 days.17 Increasing the number of BMSCs from 3.54 × 104 to 3.54 × 105 cells/cm2 promoted osteogenic expression in titanium mesh.18 Similarly, Zhou et al. demonstrated that the total number of cells loaded onto polycaprolactone\tricalciumphosphate scaffolds significantly influenced the production of ALP and osteocalcin.19 In rabbit segmental bone defect it was shown that a density of 1.5 × 106 cells/scaffold stimulated bone deposition after 2 weeks.20 However, another study reported that an increase in cell seeding density from 1 to 6 × 106 cells/mL did not enhance bone formation, but promoted more homogenous cell distribution throughout the constructs.21 Further, in vivo studies on cartilage and bone formation have failed to demonstrate any significant effects of high cell seeding density in 3D porous scaffolds.9, 22 The inconclusive results indicate the need for further evaluation of the in vitro and in vivo effects of cell seeding density.
BMSCs have been widely used and investigated because they can be expanded in vitro and differentiated into a variety of cell types such as adipocytes, chondrocytes, myoblasts, and osteoblasts, by supplementing the cell culture medium with specific growth and differentiation factors.23, 24, 25 Osteogenic differentiation of BMSCs can be induced by the introduction of supplements such as ascorbic acid, dexamethasone, and β‐glycerophosphate into the culture medium.26, 27 It has been reported that preculture of BMSCs in osteogenic medium for a short period may promote osteogenesis.28 On the other hand, a published study demonstrated that osteogenetic activity is significantly higher in non‐preculture of BMSCs.29 These contradictory findings indicate that the in vivo effect of osteogenic medium needs to be further addressed.
The main objective of this study was to assess the osteogenic potential of a tissue‐engineered construct of BMSCs and poly(LLA‐co‐CL) scaffolds in vitro and in vivo, using the critical size defect model. A further objective was to determine the effect of low and high seeding density of BMSCs, cultured with and without osteogenic supplements, on cell proliferation and differentiation and on bone formation. The synergistic effect of seeding density and osteogenic supplements was also studied.
MATERIALS AND METHODS
Preparation of polymer scaffolds
Copolymer poly(LLA‐co‐CL) material was synthesized as previously described.30 In brief, monomer, initiator, and catalysts were weighed inside a glove box and bulk polymerized at 110°C for 72 h, then precipitated three times in cold hexane and methanol. Porous scaffolds were produced from the copolymer using a solvent‐casting‐particulate‐leaching method. The pore size was >90 μm and the porosity 90%. After leaching of salt particles, the scaffolds were dried and sterilized in an inert atmosphere using electron beam radiation at a dose of 2.5 Mrad from a pulsed electron accelerator (Mikrotron, Acceleratorteknik, Stockholm, Sweden) at 6.5 MeV.
Cell isolation
Bone marrow stromal cells (BMSCs) were isolated from the femurs of two donor Lewis rats and maintained by a modification of a method previously described.31 The animals were housed under uniform conditions for at least 1 week before the experiment, then euthanized by an overdose of carbon dioxide (CO2) inhalation. The femurs were retrieved, cleaned, and washed three times for 5 min in phosphate‐buffered saline (PBS) supplemented with 3% penicillin–streptomycin (PS). The metaphyseal ends of the femurs were cut off, and the marrow cavity was flushed with minimum essential medium (αMEM, Invitrogen™, Carlsbad, CA) supplemented with 1% PS and 15% fetal bovine serum (FBS) into a sterile falcon tube. The cells were centrifuged and resuspended in fresh α‐MEM medium containing 15% FBS and plated in culture flasks (NUNC A/S, Roskilde, Denmark). The medium was changed the next day, with fresh αMEM medium containing 1% PS and 10% FBS. Cells were cultured in αMEM 1% AB and 10% FBS until they reached 80% confluence, after which they were passaged. Passages 3–5 were used for the in vitro studies and passages 3 and 4 for the in vivo studies. Half of the cells were cultured in αMEM only, supplemented with 1% PS and 10% FBS. For the other half, the culture medium was supplemented with osteogenic factors [100 nM dexamethasone (dex), 10 mMb glycerophosphate, and 0.05 mM ascorbic acid]11, 32 7 days before the experiments.
The study was approved by the Norwegian Animal Research Authority and conducted according to the European Convention for the Protection of Vertebrates Used for Scientific Purposes (local approval number 20124903).
Scanning electron microscopy
The poly(LLA‐co‐CL) scaffolds with BMSCs seeded at different densities were examined under scanning electron microscopy (SEM) to determine cell adhesion and spreading. After 7 and 21 days of culture, samples were prepared for SEM as follows; first, the medium was replaced with 2.5% glutaraldehyde in α‐MEM without serum and fixed for 30 min at room temperature. Second, samples were fixed in 2.5% glutaraldehyde in 0.1M sodium cacodylate pH 7.2 with 0.1M sucrose for 30 min at room temperature. The samples were then treated with 1% osmium tetroxide in distilled water for 1 h, followed by dehydration through a graded series of ethanol solutions (70, 80, 95, and 100%), critical‐point‐dried (using CO2 as transitional fluid and the specimens mounted on aluminum holders), and sputter‐coated with a 10 nm conducting layer of gold platinum. Finally, the samples were examined by SEM (Jeol JSM 7400F, Tokyo, Japan) using a voltage of 10 kV.
DNA quantification of cell proliferation
DNA quantification was carried out as described previously, with some modifications,33 using reagents from the MasterPure™ Complete DNA and RNA Purification Kit (Epicentre® Biotechnologies, Madison, WI). The amount and purity of DNA per scaffold (n = 4 scaffolds for each group and time point) were measured by optical densitometry at 260 and 280 nm, using a Nanodrop ND 1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE).
Real‐time reverse transcription‐polymerase chain reaction analysis
RNA isolation and RT‐PCR were performed as described previously.32 Briefly, total RNA was collected from cells grown onto the scaffolds (n = 4 scaffolds for each group and time point) using an isolation kit (E.Z.N.AVR, Omega Bio‐Tek, Norcross, GA) according to the manufacturer's protocol. RNA purity and quantification were determined by spectrophotometry (NanoDrop Spectrophotometer, NanoDrop Technologies). Real‐time reverse transcription‐polymerase (RT‐PCR) was conducted under standard enzyme and cycling conditions on a StepOne™ real‐time PCR system, using TaqManVR gene expression assays (Applied Biosystems™, Carlsbad, CA): runt‐related transcription factor 2 (Runx2), collagen type I (Col I), alkaline phosphatase (ALP), bone morphogenetic protein 2 (BMP2), bone sialoprotein (BSP), osteocalcin (OC), and glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH). The data were analyzed using a comparative Ct method by StepOne. Expression levels of the genes were normalized to the Housekeeper index with GAPDH serving as the endogenous control.
Graft preparation
Poly (LLA‐co‐CL) scaffolds were placed at the bottom of wells in 96‐well plates, prewet with the culture media, and incubated at 37°C and 5% CO2 overnight. The following morning, BMSCs were trypsinized from the culture flasks and seeded on the top of each scaffold, at low density (1 × 106 cells/scaffold) or high density (2 × 106 cells/scaffold). An orbital shaker (EppendorfVR, Hamburg, Germany) was applied to facilitate the distribution of the cells from the surface of the scaffold into the pores.6 The cell/scaffold grafts were incubated for 3 h for cell attachment and then transferred either to rat calvarial bone defects (n = 12 rats) for 8 weeks or to four separate spinner flasks (Wheaton Science, Millville, NJ).32 The spinner flasks were placed on a magnetic stirrer (Stem Stirrer, UK) and the side arm caps kept loose. The grafts were separated by spacers made of silicone tubes and cultured in a CO2 incubator for 3 weeks.
Surgical procedure and implantation
Twelve male Lewis rats (2.5 months old, weight: 300–350 g) were kept in the animal facility for 1 week to acclimatize to diet, water, and housing, under a 12 h/12 h light/dark cycle. The rats were anesthetized with isofluorane (Isoba vetVR, Schering Plough, Kenilworth, NJ) in combination with NO2 and O2, using a custom‐made mask. The surgical site was shaved and scrubbed with 70% alcohol. Using sterile instruments and an aseptic technique, a 2‐cm anteroposterior cranial skin incision was made along the midline. The subcutaneous tissue, musculature, and periosteum were dissected and reflected to expose the calvaria. A full‐thickness defect (5 mm in diameter) was created in the central area of each parietal bone, using a saline‐cooled trephine drill to prevent overheating of the bone margins and to remove the bone debris. The dura mater was left undisturbed. Twenty‐four defects were implanted with disc‐shaped scaffolds of poly(LLA‐co‐CL), 5 mm in diameter × 1.5 mm height, seeded with high or low cell density using two cell culture environment: in medium with or without osteogenic supplements. Accordingly, the scaffolds were classified into four different groups:
Six defects implanted with scaffolds seeded with cells in low density without osteogenic supplements (LD‐OM).
Six defects implanted with scaffolds seeded with cells in high density without osteogenic supplements (HD‐OM).
Six defects implanted with scaffolds seeded with cells in low density with osteogenic supplements (LD + OM).
Six defects implanted with scaffolds seeded with cells in high density with osteogenic supplements (HD + OM).
The periosteum and skin were repositioned and stabilized with sutures (Vicryl Plus 4‐0). Topical antibiotic Bacimycine (Bacitracin ointment) was applied to the wound to prevent postoperative infection. All animals were given an intramuscular dose of Buprenorphine (Temgesic® 0.3 mg\kg) as an analgesic and allowed to recover. The status of the surgical wound, food intake, activity, and signs of infection were monitored daily. After 8 weeks, the animals were sacrificed by inhalation of CO2 and the calvarial defects with surrounding bone and soft tissue were harvested for subsequent evaluation.
X‐ray micro‐computed tomography
For quantitative evaluation of new bone formation in the rat calvarial defects at 8 weeks, micro‐computed tomography (μCT) scans were taken using the SkyScan1172VR microfocus X‐ray system (SkyScanVR, Kontich, Belgium) with the CTAn 1.8VR and NRECON RECONSTRUCTIONVR CT software (SkyScanVR), as previously described.34 A 0.5‐mm aluminum filter was used to optimize the images. Source voltage and current were set at 50 kV and 200 μA, respectively. After operating CTAn 1.8VR to each reconstructed BMP files, bone volume (BV), tissue volume (TV), and bone volume/tissue volume (BV/TV) values were obtained.
Histology
Specimens for histological examination were fixed with 4% paraformaldehyde (Merck, White House Station, NJ) and decalcified for 4 weeks, using 10% ethylenediaminetetraacetic acid (EDTA) in 0.1M Tris buffer and 7.5% polyvinylpyrrolidone (PVP) (Merck). The specimens were then washed in PBS, embedded in paraffin, and serially sectioned using a microtome (HM 325, Thermo Scientific). The sections, 4–6 μm thick, were mounted on glass slides, deparaffinized, hydrated by the application of xylene and alcohol in series, and stained with Masson's Trichrome (MT).
Statistical analysis
Sixteen scaffolds were available for the statistical analyses. From each scaffold four measures were taken: two at day 7 and 2 at day 21. Twelve rats were included in the in vivo analysis. To provide more accurate data of the hierarchical structure of the outcome variables a multilevel modeling analysis was applied. For the PCR statistical analyses, reference values were first calculated for the low seeding densities without osteogenic medium, for day 7 and day 21, respectively. This was done for all the expression measures. A random effect model with each particular gene as the random factor (to control for the two repeated measures for each gene) was applied. The reference value was defined as the predicted mean from these models. ΔCt values for each gene were thereafter calculated as the difference between the gene measures and the reference values. The ΔΔCt values for all the expressions were then analyzed in linear models using robust variance estimates to control for the repeated measures for each particular gene. Mean values, standard deviations, and 95% confidence intervals were estimated from these models. For low seeding densities without osteogenic medium the mean values are by definition “0.” For DNA and the μCT the measured values were used directly in the analyses.
The effects were tested hierarchically. First the main effects of seeding density, osteogenic medium, and days were tested. Thereafter, a model including the first‐order interaction was performed (densities*medium, medium*days, density*days), and then a model including the second‐order interaction (densities*medium*days). The μCT observations were measured at only one time point. This analytic approach will correspond to performing repeated measures analyses of variance. The statistical package StataIC version 13 was used to analyze the data. The p‐values less than 0.05 were considered statistically significant.
RESULTS
SEM analysis
Scaffolds with low and high cell seeding densities preincubated in different media demonstrated good cellular attachment at day 7 and day 21. The cells appeared to be flattened and well spread, covering the surface of the scaffolds and migrating into the inner pores of the scaffolds (Fig. 1).
Figure 1.
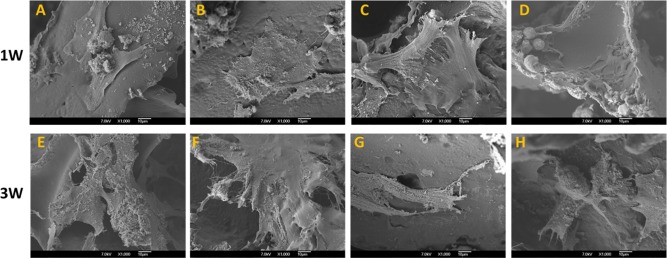
SEM images of scaffolds 1 week (1W) (A–D) and 3 weeks (3W) (E–H) after seeding with cultured BMSCs. A and E: Low cell seeding density with cells preincubated with osteogenic medium. B and F: High cell seeding density with cells preincubated with osteogenic medium. C and G: Low cell seeding density with cells preincubated without osteogenic medium. D and H: High cell seeding density with cells preincubated without osteogenic medium. Although seeded at different densities and preincubated with and without osteogenic medium, all cells appear to be flattened and well spread on the scaffolds. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Cell proliferation
Osteogenic medium and incubation time showed a significant overall positive effect on the quantity of DNA (p = 0.001), whereas cell seeding density showed no overall effect (p = 0.32). There was a significant relationship between osteogenic medium and incubation time (p = 0.001). The pairwise comparison at day 7 showed a significant stimulating effect of high cell seeding density with osteogenic medium on the amount of DNA compared with low cell seeding density with osteogenic medium (p = 0.039). Similarly, high cell seeding density with osteogenic medium significantly stimulated the amount of DNA compared with high cell seeding density without osteogenic medium (p < 0.001). For the pairwise comparison at day 21, significantly higher amounts of DNA were detected for high cell seeding density with osteogenic medium than for high cell seeding density without osteogenic medium (p < 0.001) (Fig. 2).
Figure 2.
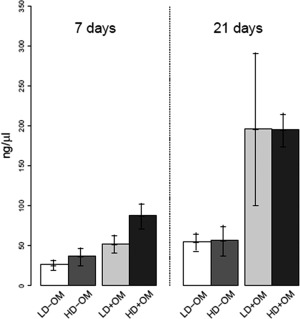
Total DNA quantification of cultured cell/scaffold constructs (n = 4 for each group and time point). The data are presented as means ± 95% confidence intervals. The results indicate continued proliferation of BMSCs for up to 3 weeks (p = 0.001) and a positive effect of osteogenic supplements on cell proliferation (p < 0.001).
RT‐PCR
Runx2 expression exhibited significant overall upregulation in relation to high cell seeding density (p = 0.001) and significant overall downregulation in relation to osteogenic medium (p = 0.005). There were significant interactions between high cell seeding density and incubation time (p = 0.042) and between high cell seeding density and osteogenic medium (p = 0.046). The pairwise comparison at day 7 revealed a significantly higher expression of Runx2 for scaffolds with high cell seeding density with osteogenic medium compared with low cell seeding density with osteogenic medium (p = 0.042). By day 21, Runx2 expression had increased significantly for high cell seeding density without osteogenic medium compared with low cell seeding density without osteogenic medium (p = 0.032). Moreover, from day 7 to day 21, Runx2 expression was significantly upregulated in scaffolds with high cell seeding density without osteogenic medium (p = 0.029) [Fig. 3(A)].
Figure 3.
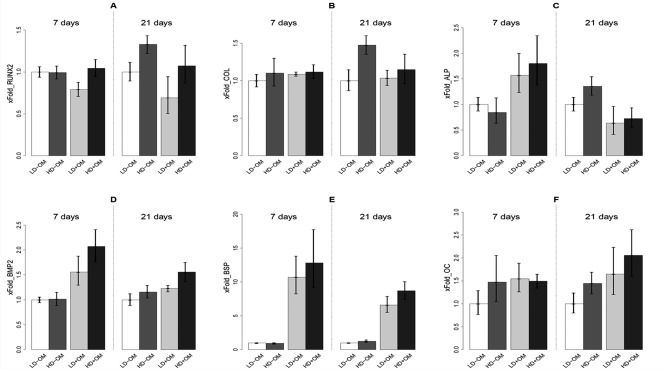
mRNA expression of (A) Runx2, (B) Col 1, (C) ALP, (D) BMP2, (E) BSP, and (F) OC by qRT‐PCR, presented as x‐fold changes relative to the expression of the mean of the calibrator sample LD‐OM. A: Runx2 expression is downregulated by osteogenic medium (p = 0.005) and upregulated by high cell seeding density (p = 0.001). B: Col1 expression is upregulated by high cell seeding density (p = 0.001). C: ALP expression, disclosing a significant relationship between high cell density, osteogenic medium, and number of days (p = 0.026). D: BMP2 expression is upregulated by osteogenic medium (p < 0.001) and high cell seeding density (p = 0.003). E: BSP expression is upregulated by osteogenic medium (p < 0.001) and high cell seeding density (p = 0.033). F: OC expression is upregulated by osteogenic medium (p = 0.002) and high cell seeding density (p = 0.013). The data are presented as means ± 95% confidence intervals.
Col1 expression disclosed a significant overall upregulation effect of high cell seeding density (p = 0.009). There were also significant interactions between high cell density and incubation time (p = 0.009) and osteogenic medium and incubation time (p = 0.019). Pairwise comparison at day 21 showed significantly higher expression of Col1 for scaffolds with high cell seeding density without osteogenic medium than for low cell seeding density without osteogenic medium (p = 0.011) [Fig. 3(B)].
ALP expression was not overall significantly affected by high cell seeding density (p = 0.38) or osteogenic medium (p = 0.69). Significant relationships were disclosed between osteogenic medium and incubation time (p < 0.001) and among high cell seeding density, osteogenic medium, and incubation time (p = 0.026). The pairwise comparison at day 7 showed significant upregulation of ALP associated with high cell seeding density with osteogenic medium compared with low cell seeding density with osteogenic medium (p = 0.020). Similarly, high cell seeding density with osteogenic medium showed significant upregulation of ALP compared with high cell seeding density without osteogenic medium (p = 0.05). By day 21, ALP levels from scaffolds with high cell seeding density without osteogenic medium showed significant upregulation compared with scaffolds with high cell seeding density and osteogenic medium (p = 0.021) [Fig. 3(C)].
BMP‐2 expression was significantly overall upregulated in scaffolds with high cell seeding density (p = 0.003) and osteogenic medium (p < 0.001). Significant relationships were disclosed between high cell seeding density and osteogenic medium (p = 0.047) and between osteogenic medium and incubation time (p = 0.001). The pairwise comparison at day 7 showed significant upregulation of BMP‐2 in low cell seeding density with osteogenic medium compared with low cell seeding density without osteogenic medium (p = 0.013). In addition, expression of BMP‐2 in high cell seeding density with osteogenic medium was significantly upregulated compared with high cell seeding density without osteogenic medium (p < 0.001) [Fig. 3(D)].
BSP expression showed significant overall upregulation in scaffolds with high cell seeding density (p = 0.033) and osteogenic medium (p < 0.001). In addition, the interaction between osteogenic medium and incubation time was significant (p < 0.001). Pairwise comparison at day 7 and day 21 showed significant upregulation of BSP in low cell seeding density with osteogenic medium compared with low cell seeding density without osteogenic medium (p < 0.001) and significant upregulation of BSP in high cell seeding density with osteogenic medium compared with high cell seeding density without osteogenic medium (p < 0.001) [Fig. 3(E)].
OC expression exhibited a significant overall upregulation effect of high cell seeding density (p = 0.013) and osteogenic medium (p = 0.002) [Fig. 3(F)].
μCT
Bone formation in the calvarial defects was evaluated at 8 weeks. In defects implanted with constructs seeded with cells cultured at low density in nonosteogenic medium, healing was 14.27% (95% CI: 10.66, 17.88); the corresponding rate for cells cultured at high density in nonosteogenic medium was 14.46% (95% CI: 10.40, 18.51) (p = 0.99). Healing of defects treated with cells preincubated in osteogenic medium was 13.43% (95% CI: 7.26, 19.61) for cells cultured at low density and 21.71% (95% CI: 14.63, 28.79) for those cultured at high density (p = 0.023). There was a significant interaction effect between high cell density and osteogenic medium (p = 0.038) (Fig. 4).
Figure 4.
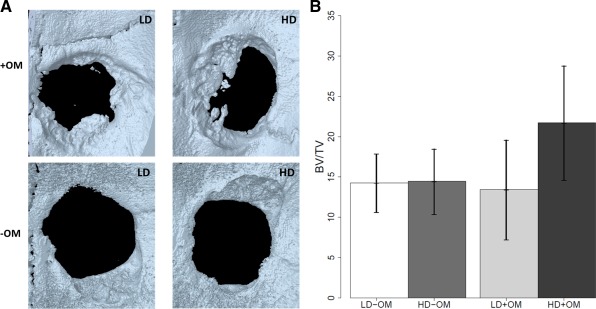
Bone formation in critical‐size rat calvarial defects. A: Three‐dimensionally reconstructed high‐resolution μCT image of defects implanted with cells/scaffolds after 8 weeks of healing. Note the new bone formed in the four groups; with osteogenic medium (+OM) or without osteogenic medium (−OM) with different densities of cell seeding (low density (LD) or high density (HD)). B: Quantification of percentage of area and volume of bone regeneration in calvarial defects after 8 weeks of healing. Implantation of scaffolds containing cells seeded at high density and cultured with osteogenic medium exhibit a significant percentage of bone volume (p = 0.038). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Histology
Various levels of osteoid‐like tissue formation are illustrated in Figure 5. Compared with the other groups, more bone‐like tissue formed in the group implanted with constructs containing cells cultured in osteogenic medium at high cell seeding density.
Figure 5.
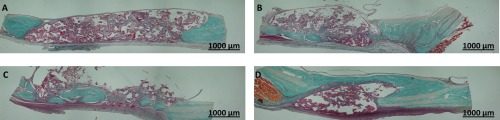
Representative sections of Masson's trichrome staining through calvarial defects at 8 weeks (×10). A: Section of a defect implanted with a scaffold containing cells cultured without osteogenic medium and seeded at low density, showing fibrous connective tissue and collagen. B: Defect implanted with a scaffold containing cells cultured without osteogenic medium and seeded at high density, showing osteoid‐like tissue (green areas). C: Defect implanted with a scaffold containing cells cultured with osteogenic medium and seeded at low density, showing osteoid‐like tissue. D: Defect implanted with a scaffold containing cells cultured with osteogenic medium and seeded at high density, showing formation of a bridge of bone‐like tissue. Scale bar = 1000 μm. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
DISCUSSION
The objectives of this study were to determine the initial biological responses, the osteogenic potential, and the induction of new bone in response to implanted poly(LLA‐co‐CL) scaffolds seeded with two different densities of BMSCs. The effects of cell seeding density and pretreatment of BMSCs with osteogenic supplements on cell proliferation, differentiation, and bone formation were also assessed. Moreover, a potential synergistic effect of these factors was evaluated.
In the in vitro experiments, the cells were cultured under dynamic cell culture conditions, using spinner‐modified flasks. Previous studies show that the shear stress induced by spinner flasks regulates cellular physiological activity through stimulation of mechano‐transduction pathways and promotes in vitro cell proliferation and differentiation.33, 35, 36 Further, the critical size cranial defect model is well established for evaluating orthotopic implantation. However, calvarial bone has a relatively poor blood supply and relative lack of bone marrow, that is, conditions less than ideal for bone formation.37
Interactions of BMSCs with their microenvironment play an important role in their morphogenesis and differentiation. An important component of the cell microenvironment is the surrounding matrix, which includes several biophysical and chemical signals. These signals are recognized, integrated, and processed by the cells to determine the behavior and function of the engineered tissues. It has been shown that fibronectin, the extracellular protein present in serum and plasma, is a major mediator of BMSC adhesion to polymeric scaffolds.38 Thus, by controlling physical and chemical characteristics of poly(LLA‐co‐CL) scaffolds, such as solubility, degradation behavior, chemical composition, crystallinity, and hydrophilicity, it is possible to regulate cell survival, migration, proliferation, and differentiation during the regeneration process.2, 4, 30, 39
The number of cells capable of attaching to scaffolds depends on the porosity, mean pore size, and surface area. The porosity of poly(LLA‐co‐CL) scaffolds used in the current experiments is about 85%, providing a large surface area for cellular attachment and proliferation, conducive to uniform cell distribution.40, 41, 42 In a previous study, co‐culturing BMSCs with endothelial cells at a density of 5 × 105 cells/scaffold resulted in low bone induction.32 Hence, the number of cells was increased in this work. We hypothesized that a large scaffold surface area containing more attached cells would further stimulate bone formation.
Cell signaling can result either from direct cell−cell communication or from secreted signaling molecules. With high cell density, cell–cell communication and paracrine signaling increase. Direct cell–cell communication via gap junctions [i.e., gap junction intercellular communication (GJIC)] is an important element promoting growth and differentiation in various tissues.43 GJIC is mediated by connexins. In particular, connexin 43 (Cx43) plays an important role in regulating signal transmission among different bone cells. Increased cell proliferation has been observed as a result of connexin 43 stimulation.44 In this study, the in vitro data generated at day 7 showed that high density seeding of the copolymer scaffolds led to increased cell proliferation. This stimulation is probably caused by GJIC activity. At day 21, however, there was no correlation between cell proliferation and cell seeding density, suggesting that maturation level had been reached. A logarithmic relationship has been demonstrated before between cell density and bone formation.13 The optimal cell density above which bone in‐growth did not change was identified, that is, increasing the cell numbers above this level did not stimulate more bone formation. This indicates that the direct cell–cell communication through optimal cell seeding density and soluble osteogenic factors might act as synergistic modulators in promoting bone formation.
In animal studies, mesenchymal stem cells (MSCs) have been reported to induce osteogenesis and have been used extensively for regeneration of bone defects.29, 45, 46 The optimal protocol for expanding MSCs in medium containing osteogenic supplements may depend on the tissues from which the MSCs were isolated; cells of different origin may have inherited different degrees of osteogenicity.29, 47, 48 Osteoprogenitor cells can differentiate in the presence or absence of osteogenic supplements.49 At least two classes of osteoblast progenitor cells could be defined: those differentiating in the absence of osteogenic supplements and those requiring the supplements to differentiate. In the absence, few cells differentiate and these are only detectable after cell numbers are increased.49 This hypothesis is supported by the in vitro data of the present work which demonstrate that after 21 days in the absence of osteogenic supplements, an increase in cell density upregulates the expression of osteogenic markers Runx 2, COL1, and ALP. Accordingly, the osteogenic differentiation of BMSCs might depend on the cell density, culture condition, and time. However, when MSCs are fully differentiated, their pluripotency and immunosuppressibility may also decrease and this may impair osteogenicity and bone formation.50
Osteogenic differentiation of BMSCs proceeds in three stages: early or commitment to osteogenic differentiation, matrix synthesis, and then the final stage, mineralization.51 In the osteoblastic differentiation model, cells proliferate rapidly from 7 to 14 days and then start to secrete ECM proteins and produce early differentiation markers such as ALP, which is produced from day 7.52 Thereafter, as the cells mature, proliferation decreases over time. In in vitro data of this study, continued proliferation of BMSCs indicates that the cells were still at the early maturation stage when the experiments were conducted. mRNA expression of ALP was first upregulated at day 7 and then downregulated at day 21. ALP expression is controlled by BMP2 through the Wnt/LRP5 signaling cascade.53 BMP2 is known to participate in the regulation of cell growth and differentiation, along with the induction of osteogenic progenitor cells in bone defect sites during the healing process. In the group with high cell seeding density and osteogenic supplements, there was a general decline of BMP2 expression at day 21, suggesting that the BMSCs had entered a maturation stage of differentiation.
In this work, cells cultured at high density with osteogenic supplements demonstrated an increase and upregulation of osteocalcin mRNA expression during the experimental period. Osteocalcin is a late, specific marker of osteoblast maturation.54 This is in agreement with a previous report showing that an increase in cell numbers in the presence of osteogenic supplements upregulated osteocalcin expression and maturation of osteoblasts.12 A correlation may exist between increased extracellular protein secretion and an increased number of mature osteoblasts, leading to promotion of bone formation. Our in vivo data confirmed his observation demonstrating more bone formation in response to an increase in the number of expanded and differentiated cells. Accordingly, the present results indicate that the number of mature osteoblast determined the rate of bone formation.
The in vivo findings demonstrate synergistic stimulation of cell seeding density and osteogenic supplements on bone formation. Dex is a synthetic glucocorticoid reported to be an essential requirement for osteoprogenitor cell differentiation of MSCs in vitro. The mechanism of action of dex on BMSCs can be through induced transcription of BSP by binding on a glucocorticoid response element in the promoter region of the BSP gene, which is associated with osteoblast differentiation.55 This was verified in vitro by BSP mRNA expression. BSP is an indicator of cellular maturation. On the other hand, osteogenic differentiation of BMSCs was clearly influenced by the initial seeding densities via cell–cell communication. Thus, both factors may accelerate osteoblastic differentiation, leading primarily to more mature osteoblasts and secondarily to more bone formation.
Although the in vivo data clearly confirm new bone formation, the μCT images and histology at 8 weeks did not show complete healing and bone regeneration. In bone TE, the transplanted scaffold should act as a temporary ECM substitute, stimulating cell attachment, proliferation, and differentiation, with subsequent bone in‐growth until finally being completely degraded and replaced by regenerated bone. Scaffold degradation should be adjusted appropriately to the rate of neobone formation,56 thus allowing the mechanical load on the scaffold to be transferred gradually to the regenerated tissue. Finally, when total tissue regeneration has been achieved, the scaffold should be completely degraded.
A previous in vivo study using similar scaffolds showed slow, gradual degradation of the poly(LLA‐co‐Cl) scaffolds within 91 days of the experimental period.4 The delayed degradation of the scaffolds might suggest a longer healing process in this experimental model.
CONCLUSIONS
The induction of new bone in a critical size defect indicates that poly(LLA‐co‐CL) scaffolds are appropriate candidates for constructs in bone TE. Bone regeneration might depend on cell–cell communication, which is an important element promoting growth and differentiation in various tissues. The appropriate number of cells to be loaded onto a specific scaffold is a critical, vital factor for promoting ECM synthesis and bone formation. This study demonstrates that increasing cell numbers seeded onto poly(LLA‐co‐Cl) scaffolds promote BMSCs differentiation and bone formation. Osteogenic supplements are key determinants of the ability of MSCs to induce new bone tissue formation. Thus, the synergistic effect of cell density and osteogenic supplements appears to be of major importance in bone formation.
ACKNOWLEDGMENT
The authors thank Dr. Joan Bevenius for language revision of the manuscript.
How to cite this article: Yassin MA, Leknes KN, Pedersen TO, Xing Z, Sun Y, Lie SA, Finne‐Wistrand A, Mustafa K. 2015. Cell seeding density is a critical determinant for copolymer scaffolds‐induced bone regeneration. J Biomed Mater Res Part A 2015:103A:3649–3658.
REFERENCES
- 1. Fernández J, Etxeberria A, Sarasua J‐R. Synthesis, structure and properties of poly(L‐lactide‐co‐caprolactone) statistical copolymers. J Mech Behav Biomed Mater 2012;9:100–112. [DOI] [PubMed] [Google Scholar]
- 2. Idris SB, Arvidson K, Plikk P, Ibrahim S, Finne‐Wistrand A, Albertsson AC, Bolstad AI, Mustafa K. Polyester copolymer scaffolds enhance expression of bone markers in osteoblast‐like cells. J Biomed Mater Res A 2010;94:631–639. [DOI] [PubMed] [Google Scholar]
- 3. Xue Y, Danmark S, Xing Z, Arvidson K, Albertsson AC, Hellem S, Finne‐Wistrand A, Mustafa K. Growth and differentiation of bone marrow stromal cells on biodegradable polymer scaffolds: An in vitro study. J Biomed Mater Res A 2010;95:1244–1251. [DOI] [PubMed] [Google Scholar]
- 4. Danmark S, Finne‐Wistrand A, Schander K, Hakkarainen M, Arvidson K, Mustafa K, Albertsson AC. In vitro and in vivo degradation profile of aliphatic polyesters subjected to electron beam sterilization. Acta Biomater 2011;7:2035–2046. [DOI] [PubMed] [Google Scholar]
- 5. Pedersen TO, Xing Z, Finne‐Wistrand A, Hellem S, Mustafa K. Hyperbaric oxygen stimulates vascularization and bone formation in rat calvarial defects. Int J Oral Maxillofac Surg 2013;42:907–914. [DOI] [PubMed] [Google Scholar]
- 6. Xing Z, Xue Y, Danmark S, Finne‐Wistrand A, Arvidson K, Hellem S, Yang ZQ, Mustafa K. Comparison of short‐run cell seeding methods for poly(L‐lactide‐co‐1,5‐dioxepan‐2‐one) scaffold intended for bone tissue engineering. Int J Artif Org 2011;34:432–441. [DOI] [PubMed] [Google Scholar]
- 7. Chen Y, Bloemen V, Impens S, Moesen M, Luyten FP, Schrooten J. Characterization and optimization of cell seeding in scaffolds by factorial design: Quality by design approach for skeletal tissue engineering. Tissue Eng Part C Methods 2011;17:1211–1221. [DOI] [PubMed] [Google Scholar]
- 8. Dar A, Shachar M, Leor J, Cohen S. Optimization of cardiac cell seeding and distribution in 3D porous alginate scaffolds. Biotechnol Bioeng 2002;80:305–312. [DOI] [PubMed] [Google Scholar]
- 9. Hansen O, Foldager C, Christensen B, Everland H, Lind M. Increased chondrocyte seeding density has no positive effect on cartilage repair in an MPEG‐PLGA scaffold. Knee Surg Sports Traumatol Arthrosc 2013;21:485–493. [DOI] [PubMed] [Google Scholar]
- 10. Grayson WL, Bhumiratana S, Cannizzaro C, Chao PH, Lennon DP, Caplan AI, Vunjak‐Novakovic G. Effects of initial seeding density and fluid perfusion rate on formation of tissue‐engineered bone. Tissue Eng Part A 2008;14:1809–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhou H, Weir MD, Xu HH. Effect of cell seeding density on proliferation and osteodifferentiation of umbilical cord stem cells on calcium phosphate cement‐fiber scaffold. Tissue Eng Part A 2011;17(21–22):2603–2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kim K, Dean D, Mikos AG, Fisher JP. Effect of initial cell seeding density on early osteogenic signal expression of rat bone marrow stromal cells cultured on cross‐linked poly(propylene fumarate) disks. Biomacromolecules 2009;10:1810–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kruyt M, De Bruijn J, Rouwkema J, Van Bliterswijk C, Oner C, Verbout A, Dhert W. Analysis of the dynamics of bone formation, effect of cell seeding density, and potential of allogeneic cells in cell‐based bone tissue engineering in goats. Tissue Eng Part A 2008;14:1081–1088. [DOI] [PubMed] [Google Scholar]
- 14. Bitar M, Brown RA, Salih V, Kidane AG, Knowles JC, Nazhat SN. Effect of cell density on osteoblastic differentiation and matrix degradation of biomimetic dense collagen scaffolds. Biomacromolecules 2008;9:129–135. [DOI] [PubMed] [Google Scholar]
- 15. Goldstein AS. Effect of seeding osteoprogenitor cells as dense clusters on cell growth and differentiation. Tissue Eng 2001;7:817–827. [DOI] [PubMed] [Google Scholar]
- 16. Almarza AJ, Athanasiou KA. Effects of initial cell seeding density for the tissue engineering of the temporomandibular joint disc. Ann Biomed Eng 2005;33:943–950. [DOI] [PubMed] [Google Scholar]
- 17. Ishaug SL, Crane GM, Miller MJ, Yasko AW, Yaszemski MJ, Mikos AG. Bone formation by three‐dimensional stromal osteoblast culture in biodegradable polymer scaffolds. J Biomed Mater Res 1997;36:17–28. [DOI] [PubMed] [Google Scholar]
- 18. Vehof JW, de Ruijter AE, Spauwen PH, Jansen JA. Influence of rhBMP‐2 on rat bone marrow stromal cells cultured on titanium fiber mesh. Tissue Eng 2001;7:373–383. [DOI] [PubMed] [Google Scholar]
- 19. Zhou YF, Sae‐Lim V, Chou AM, Hutmacher DW, Lim TM. Does seeding density affect in vitro mineral nodules formation in novel composite scaffolds? J Biomed Mater Res A 2006;78:183–193. [DOI] [PubMed] [Google Scholar]
- 20. Rathbone CR, Guda T, Singleton BM, Oh DS, Appleford MR, Ong JL, Wenke JC. Effect of cell‐seeded hydroxyapatite scaffolds on rabbit radius bone regeneration. J Biomed Mater Res A 2014;102:1458–1466. [DOI] [PubMed] [Google Scholar]
- 21. Holy CE, Shoichet MS, Davies JE. Engineering three‐dimensional bone tissue in vitro using biodegradable scaffolds: Investigating initial cell‐seeding density and culture period. J Biomed Mater Res 2000;51:376–382. [DOI] [PubMed] [Google Scholar]
- 22. van Gaalen SM, de Bruijn JD, Wilson CE, van Blitterswijk CA, Verbout AJ, Alblas J, Dhert WJ. Relating cell proliferation to in vivo bone formation in porous ca/P scaffolds. J Biomed Mater Res A 2010;92:303–310. [DOI] [PubMed] [Google Scholar]
- 23. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science 1999;284:143–147. [DOI] [PubMed] [Google Scholar]
- 24. Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: Implications for cell‐based therapies. Tissue Eng 2001;7:211–228. [DOI] [PubMed] [Google Scholar]
- 25. Majumdar MK, Thiede MA, Mosca JD, Moorman M, Gerson SL. Phenotypic and functional comparison of cultures of marrow‐derived mesenchymal stem cells (MSCs) and stromal cells. J Cell Physiol 1998;176:57–66. [DOI] [PubMed] [Google Scholar]
- 26. Ohgushi H, Dohi Y, Katuda T, Tamai S, Tabata S, Suwa Y. In vitro bone formation by rat marrow cell culture. J Biomed Mater Res 1996;32:333–340. [DOI] [PubMed] [Google Scholar]
- 27. Coelho MJ, Fernandes MH. Human bone cell cultures in biocompatibility testing. Part II: Effect of ascorbic acid, β‐glycerophosphate and dexamethasone on osteoblastic differentiation. Biomaterials 2000;21:1095–1102. [DOI] [PubMed] [Google Scholar]
- 28. Castano‐Izquierdo H, Álvarez‐Barreto J, van den Dolder J, Jansen JA, Mikos AG, Sikavitsas VI. Pre‐culture period of mesenchymal stem cells in osteogenic media influences their in vivo bone forming potential. J Biomed Mater Res Part A 2007;82:129–138. [DOI] [PubMed] [Google Scholar]
- 29. Jo C, Yoon P, Kim H, Kang K, Yoon K. Comparative evaluation of in vivo osteogenic differentiation of fetal and adult mesenchymal stem cell in rat critical‐sized femoral defect model. Cell Tissue Res 2013;353:1–12. [DOI] [PubMed] [Google Scholar]
- 30. Dånmark S, Finne‐Wistrand A, Wendel M, Arvidson K, Albertsson A‐C, Mustafa K. Osteogenic differentiation by rat bone marrow stromal cells on customized biodegradable polymer scaffolds. J Bioactive Compatible Polym 2010;25:207–223. [Google Scholar]
- 31. Maniatopoulos C, Sodek J, Melcher AH. Bone formation in vitro by stromal cells obtained from bone marrow of young adult rats. Cell Tissue Res 1988;254:317–330. [DOI] [PubMed] [Google Scholar]
- 32. Xing Z, Xue Y, Dånmark S, Schander K, Østvold S, Arvidson K, Hellem S, Finne‐Wistrand A, Albertsson A–C, Mustafa K. Effect of endothelial cells on bone regeneration using poly(L‐lactide‐co‐1,5‐dioxepan‐2‐one) scaffolds. J Biomed Mater Res A 2011;96:349–357. [DOI] [PubMed] [Google Scholar]
- 33. Stiehler M, Bünger C, Baatrup A, Lind M, Kassem M, Mygind T. Effect of dynamic 3‐D culture on proliferation, distribution, and osteogenic differentiation of human mesenchymal stem cells. J Biomed Mater Res A 2009;89:96–107. [DOI] [PubMed] [Google Scholar]
- 34. Kim J, Kim IS, Cho TH, Kim HC, Yoon SJ, Choi J, Park Y, Sun K, Hwang SJ. In vivo evaluation of MMP sensitive high‐molecular weight HA‐based hydrogels for bone tissue engineering. J Biomed Mater Res A 2010;95:673–681. [DOI] [PubMed] [Google Scholar]
- 35. Wang Y, Tang L, Wang J, Cai S. [The mechanotransduction mechanism of how osteoblasts respond to mechanical stimulation]. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi 2005;22:400–402. [PubMed] [Google Scholar]
- 36. Meinel L, Karageorgiou V, Fajardo R, Snyder B, Shinde‐Patil V, Zichner L, Kaplan D, Langer R, Vunjak‐Novakovic G. Bone tissue engineering using human mesenchymal stem cells: Effects of scaffold material and medium flow. Ann Biomed Eng 2004;32:112–122. [DOI] [PubMed] [Google Scholar]
- 37. Viateau V, Guillemin G, Quarto R, Petite H. Animal Models for Bone Tissue Engineering Purposes In: Conn PM, editor. Sourcebook of Models for Biomedical Research: Humana Press; 2008;725–736. [Google Scholar]
- 38. Dånmark S, Finne‐Wistrand A, Albertsson A‐C, Patarroyo M, Mustafa K. Integrin‐mediated adhesion of human mesenchymal stem cells to extracellular matrix proteins adsorbed to polymer surfaces. Biomed Mater 2012;7:035011. [DOI] [PubMed] [Google Scholar]
- 39. Xing Z, Pedersen TO, Wu X, Xue Y, Sun Y, Finne‐Wistrand A, Kloss FR, Waag T, Krueger A, Steinmuller‐Nethl D and others. Biological effects of functionalizing copolymer scaffolds with nanodiamond particles. Tissue Eng Part A 2013;19:1783–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. O'Brien FJ, Harley BA, Yannas IV, Gibson LJ. The effect of pore size on cell adhesion in collagen‐GAG scaffolds. Biomaterials 2005;26:433–441. [DOI] [PubMed] [Google Scholar]
- 41. Leong KF, Cheah CM, Chua CK. Solid freeform fabrication of three‐dimensional scaffolds for engineering replacement tissues and organs. Biomaterials 2003;24:2363–2378. [DOI] [PubMed] [Google Scholar]
- 42. Agrawal CM, Ray RB. Biodegradable polymeric scaffolds for musculoskeletal tissue engineering. J Biomed Mater Res 2001;55:141–150. [DOI] [PubMed] [Google Scholar]
- 43. Chang J‐C, Fujita S, Tonami H, Kato K, Iwata H, Hsu S‐h. Cell orientation and regulation of cell–cell communication in human mesenchymal stem cells on different patterns of electrospun fibers. Biomed Mater 2013;8:055002. [DOI] [PubMed] [Google Scholar]
- 44. Gramsch B, Gabriel HD, Wiemann M, Grümmer R, Winterhager E, Bingmann D, Schirrmacher K. Enhancement of connexin 43 expression increases proliferation and differentiation of an osteoblast‐like cell line. Exp Cell Res 2001;264:397–407. [DOI] [PubMed] [Google Scholar]
- 45. Levi B, James AW, Nelson ER, Vistnes D, Wu B, Lee M, Gupta A, Longaker MT. Human adipose derived stromal cells heal critical size mouse calvarial defects. PLoS One 2010;5:e11177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bruder SP, Kurth AA, Shea M, Hayes WC, Jaiswal N, Kadiyala S. Bone regeneration by implantation of purified, culture‐expanded human mesenchymal stem cells. J Orthopaed Res 1998;16:155–162. [DOI] [PubMed] [Google Scholar]
- 47. Peterson B, Zhang J, Iglesias R, Kabo M, Hedrick M, Benhaim P, Lieberman JR. Healing of critically sized femoral defects, using genetically modified mesenchymal stem cells from human adipose tissue. Tissue Eng 2005;11:120–129. [DOI] [PubMed] [Google Scholar]
- 48. Wright V, Peng H, Usas A, Young B, Gearhart B, Cummins J, Huard J. BMP4‐expressing muscle‐derived stem cells differentiate into osteogenic lineage and improve bone healing in immunocompetent mice. Mol Ther 2002;6:169–178. [DOI] [PubMed] [Google Scholar]
- 49. Aubin JE. Osteoprogenitor cell frequency in rat bone marrow stromal populations: Role for heterotypic cell‐cell interactions in osteoblast differentiation. J Cell Biochem 1999;72:396–410. [PubMed] [Google Scholar]
- 50. Prigozhina TB, Khitrin S, Elkin G, Eizik O, Morecki S, Slavin S. Mesenchymal stromal cells lose their immunosuppressive potential after allotransplantation. Exp Hematol 2008;36:1370–1376. [DOI] [PubMed] [Google Scholar]
- 51. Beck GR. Inorganic phosphate as a signaling molecule in osteoblast differentiation. J Cell Biochem 2003;90:234–243. [DOI] [PubMed] [Google Scholar]
- 52. Stein GS, Lian JB. Molecular mechanisms mediating proliferation/differentiation interrelationships during progressive development of the osteoblast phenotype. Endocr Rev 1993;14:424–442. [DOI] [PubMed] [Google Scholar]
- 53. Rawadi G, Vayssière B, Dunn F, Baron R, Roman‐Roman S. BMP‐2 controls alkaline phosphatase expression and osteoblast mineralization by a wnt autocrine loop. J Bone Miner Res 2003;18:1842–1853. [DOI] [PubMed] [Google Scholar]
- 54. Aubin JE. Bone stem cells. J Cell Biochem Suppl 1998;30‐31:73–82. [PubMed] [Google Scholar]
- 55. Ogata Y, Yamauchi M, Kim RH, Li JJ, Freedman LP, Sodek J. Glucocorticoid regulation of bone sialoprotein (BSP) gene expression. Identification of a glucocorticoid response element in the bone sialoprotein gene promoter. Eur J Biochem 1995;230:183–192. [DOI] [PubMed] [Google Scholar]
- 56. Hutmacher DW. Scaffolds in tissue engineering bone and cartilage. Biomaterials 2000;21:2529–2543. [DOI] [PubMed] [Google Scholar]


