Abstract
Objectives
Neurological worsening in acute ischaemic stroke patients is common with significant morbidity and mortality.
Aims
To determine the factors associated with early neurological worsening within the first 9 h after onset of acute ischaemic stroke.
Materials & methods
The National Institute of Health Stroke Scale (NIHSS) was used to assess stroke severity. Early neurological worsening was defined as NIHSS score increase ≥4 NIHSS points within 9 h of symptom onset compared to NIHSS score within 3 h of symptom onset. Patients with early neurological worsening were compared to patients with unchanged or improved NIHSS scores.
Results
Of the 2484 patients admitted with ischaemic stroke, 552 patients had NIHSS score within 3 h of symptom onset, and 44 (8.0%) experienced early neurological worsening. The median NIHSS on admission was 8.4 in both groups. Early neurological worsening was associated with low body temperature on admission (P = 0.01), proximal compared to distal MCA occlusion (P = 0.007) and with ipsilateral internal carotid artery stenosis >50% or occlusion (P = 0.04). Early neurological worsening was associated with higher NIHSS day 7 (P < 0.001) and higher mortality within 7 days of stroke onset (P = 0.005).
Conclusions
Early neurological worsening has serious consequences for the short‐term outcome for patients with acute ischaemic stroke and is associated with low body temperature on admission, and with extracranially and intracranially large‐vessel stenosis or occlusion.
Keywords: acute ischaemic stroke, body temperature, Early neurological worsening, NIHSS
Introduction
Neurological worsening in acute ischaemic stroke patients is common with significant morbidity and mortality. The prevalence varies between 13% and 38% 1. Previous studies have focused on neurological worsening within 48 h and up to the first week after stroke onset and showed that large‐vessel occlusion, insufficient collateralization, clot progression, haemorrhagic transformation, raised intracranial pressure, seizures, infection or pre‐existing comorbid conditions are potential factors associated with neurological worsening 2, 3, 4, 5, 6. Nocturnal cardiac modulation after acute ischaemic stroke may be responsible for sudden neurological worsening 7, and baroreflex sensitivity is a prospective marker for acute neurological worsening 5. Heart–brain interactions in sleep may have important implications for preventing stroke worsening and sudden death 7.
However, many issues are unresolved regarding neurological worsening, and this pertains especially to early neurological worsening. There have been no studies performed on neurological worsening in the first few hours after stroke onset. Getting worse in the hospital despite early admission after symptom onset and receiving acute treatment is a frustration for doctors and patients 8.
We focused our study on neurological worsening during the first 9 h in patients with acute ischaemic stroke admitted to our stroke unit ≤3 h after symptoms onset, because these first hours are critical as to recanalization and possible progression of infarction. Increasing ischaemia due to late recanalization may be important in the early stages, while other factors are important more than 6–9 h after ischaemic stroke onset.
The aim of this study was to identify the possible mechanisms and causes leading to early neurological worsening in acute phase of ischaemic stroke. We hypothesize that different mechanisms may influence neurological worsening in the acute early phase vs late phase of ischaemic stroke.
Materials and methods
In total, 2484 consecutive patients with acute cerebral infarction (the index stroke) admitted to the Stroke Unit, Department of Neurology, Haukeland University Hospital between February 2006 and February 2013, were prospectively registered in a database (The Bergen NORSTROKE Registry). Of them, 552 (22%) patients had first NIHSS score within 3 h of symptom onset. Cerebral infarction was defined as neurological deficits lasting more than 24 h because of ischaemic lesions or transient ischaemic attacks where computer tomography (CT) or magnetic resonance imaging (MRI) showed infarctions related to the clinical findings 9. The National Institute of Health Stroke Scale (NIHSS) was used to assess stroke severity on admission. All patients were transferred to the stroke unit where repeated NIHSS scores were obtained during the first 24 h by experienced stroke nurses and stroke doctors. Exact time of each scoring was registered.
Early neurological worsening was defined as NIHSS score increase of ≥4 points within 9 h of symptom onset compared to NIHSS score within 3 h of symptom onset. Patients with early neurological worsening were compared to patients with first NIHSS score within 3 h of symptom onset who experienced neurological improvement or no neurological change during the hospital stay. Patients with NIHSS score increase ≤3 points (35%) within 9 h of symptom onset were not analysed in this study. Body temperature was measured with an infrared tympanic temperature device (LighTouch‐LTX; Exergen Corp, Watertown, MA) on admission to the hospital. No temperature intervention (such as paracetamol or intravenous fluids administration) was provided prior to temperature measurement.
Hypertension was defined as treatment with antihypertensive drugs because of hypertension before the index stroke. Atrial fibrillation was considered present if there was history of chronic or paroxysmal atrial fibrillation confirmed by at least 1 ECG any time prior to stroke onset, or presence of atrial fibrillation during hospitalization. Other comorbid conditions were considered present if they were diagnosed by a physician any time before stroke onset or during hospital stay. Intravenous tissue plasminogen activator (tPA) was administered to eligible patients according to the SITS protocol 10. Aetiology was determined by the TOAST classification as large‐artery atherosclerosis, cardio‐embolism, small‐vessel disease, other and unknown 11. CT and CT angiography (CTA) were performed on admission and MRI, and MRA was performed within 24 h. Intracranial middle cerebral artery (MCA) occlusions were divided into proximal (MCA1) distal (MCA2) or MCA3 occlusion based on MRI findings. Duplex ultrasound examinations of carotid and vertebral arteries were carried out within 24 h after admission. Carotid stenosis was defined based on flow velocities and area reduction either as none, 20–50% stenosis, 51–70% stenosis, 71–99% stenosis or occlusion. Short‐term outcomes were defined by the NIHSS score on day 7 or at discharge (if discharged earlier). The study was approved by the REK Vest ethics committee.
Statistics: Chi‐square, Student's t‐test, Mann–Whitney's tests were used when appropriate.
Stepwise backward logistic regression with early neurological worsening (≥4 NIHSS points) vs no neurological worsening as dependent variable was performed to analyse factors associated with neurological worsening based on univariate analyses from Table 1 and keeping age, sex and initial NIHSS score in the final analysis. Variables on vessel occlusions were not included in the logistic regression analyses because this excluded too many patients. Temperature, blood analyses and blood pressure were treated as continuous variables. STATA 11.0 (Statacorp 4905 Lakeway Drive, College Station, Texas 77845, USA) was used for analyses.
Table 1.
Characteristics of patients with early neurological worsening (≥4 NIHSS points) and patients without early neurological worsening
| Early neurological worsening N = 44 | No neurological worsening N = 315 | P‐value | |
|---|---|---|---|
| Ageb | 70.8 (14.7) | 70.0 (14.8) | 0.72 |
| Malesa | 23 (52) | 198 (63) | 0.18 |
| NIHSS on admissionb | 8.4 (6.9) | 8.4 (7.6) | 0.99 |
| NIHSS 3–6 hb | 11.8 (7.0) | 6.0 (6.3) | <0.001 |
| NIHSS 6–9 hb | 13.4 (6.5) | 5.3 (6.4) | <0.001 |
| NIHSS 9–12 hb | 14.2 (8.2) | 5.0 (6.0) | <0.001 |
| NIHSS 24 hb | 11.1 (7.1) | 4.2 (5.7) | <0.001 |
| NIHSS 7 daysb | 12.7 (9.9) | 3.7 (5.8) | <0.001 |
| Dead by 7 daysa | 6 (13.6) | 12 (3.8) | 0.005 |
| Systolic blood pressure, mm Hgb | 164 (28) | 160 (29) | 0.33 |
| Body temperature, Celsius, °Cb | 36.3 (.53) | 36.6 (.58) | 0.01 |
| Glucose, mmol/lb | 7.0 (2.0) | 6.8 (2.3) | 0.74 |
| D‐dimer, mg/Lb | 1.4 (1.1) | 1.8 (3.2) | 0.51 |
| Fibrinogen, mmol/Lb | 3.8 (1.3) | 3.6 (.9) | 0.33 |
| CRP, mg/Lb | 9.2 (24) | 6.6 (10) | 0.48 |
| Prior cerebral infarctiona | 5 (11) | 38 (12) | 0.87 |
| Prior myocardial infarctiona | 6 (14) | 50 (16) | 0.70 |
| Hypertensiona | 16 (36) | 158 (50) | 0.08 |
| Diabetes mellitusa | 5 (12) | 41 (13) | 0.81 |
| Atrial fibrillationa | 17 (37) | 98 (31) | 0.32 |
| Active smokinga | 7 (18) | 74 (25) | 0.33 |
| Symptomatic internal carotid stenosis of at least 50%a | 10 (29.4) | 41 (15.3) | 0.04 |
| Atherosclerosisa | 4 (9) | 44 (14) | 0.37 |
| Cardiac embolisma | 24 (55) | 133 (42) | 0.12 |
| Small‐vessel diseasea | 2 (5) | 17 (5) | 0.81 |
| Othera | 3 (7) | 6 (2) | 0.05 |
| Unknowna | 11 (25) | 113 (36) | 0.16 |
| Thrombolysisa | 28 (64) | 187 (60) | 0.61 |
| Initial MCA3 vs MCA2 or MCA1 occlusiona | 1 (3.6) | 48 (27.0) | 0.007 |
| Lacunar infarction on MRIa | 4 (11.4) | 34 (13.3) | 0.76 |
| Hospital stay, daysa | 12.3 (7.6) | 6.5 (5.1) | <0.001 |
| Posterior vs anterior circulation (OCSP)a | 2 (4.6) | 44 (14) | 0.08 |
NIHSS, National Institute of Health Stroke Scale; MRI, magnetic resonance imaging; OCSP, Oxford Community Stroke Project classification; MCA, middle cerebral artery; CRP, C‐reactive protein.
Values are anumbers (N) with percentages (%) in parentheses and bmedian (IQR).
Results
In total, 552 patients with acute ischaemic stroke patients obtained the first NIHSS score within 3 h of symptom onset (217 (39%) females and 335 (61%) males). Among these, 44 (8.0%) experienced early neurological worsening (NIHSS score worsening ≥4 points), whereas 315 (57%) experienced either improvement or no change as to NIHSS scores during the first 9 h of stroke onset. Table 1 shows the demographic data, past medical history, laboratory parameters, and clinical characteristics of patients with early neurological worsening (≥4 NIHSS points) and no neurological worsening. The median age was 70 years in both groups. The median NIHSS on admission was 8.4 in both groups. Patients in the worsening group continued to get worse during the first 9 h after symptoms onset with NIHSS score 11.8 between 3 and 6 h, 13.4 between 6 and 9 h and 14.2 between 9 and 12 h after stroke onset (all P < 0.001). Fig. 1 shows that the most marked worsening occurred the first 6 h and slowed down 9–12 h after ischaemic stroke onset. There was no association between the level of consciousness on admission and neurological worsening (P = 0.66).
Figure 1.
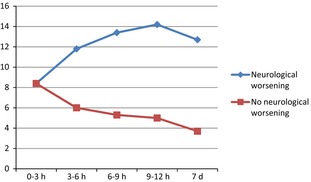
Development of NIHSS scores over time in patients with and without early neurological worsening.
Early neurological worsening was associated with low body temperature on admission (P = 0.01). There was a trend towards hypertension being associated with neurological worsening (P = 0.08). Proximal middle cerebral artery (MCA)1 or MCA2 occlusion compared to MCA3 occlusion was associated with neurological worsening (P = 0.007). Neurological worsening was associated with ipsilateral internal carotid artery stenosis >50% or occlusion (P = 0.04). Lacunar infarction (compared to embolic infarction) was not associated with neurological worsening (P = 0.76). There was no difference in the frequency thrombolytic treatment between patients with and without early neurological worsening (P = 0.61).
Early neurological worsening was associated with poor short‐term outcome as measured by NIHSS score day 7 (12.7 vs 3.7, P < 0.001), with longer duration of hospital stay (P < 0.001), and with increased mortality within 7 days of stroke onset (P = 0.005).
Logistic regression with early neurological worsening vs no neurological worsening as dependent variable showed that early neurological worsening was associated with low body temperature on admission (P = 0.006) (Table 2).
Table 2.
Logistic regression with early neurological worsening (≥4 NIHSS points) vs no neurological worsening as dependent variable
| Odds ratio | 95% Confidence interval | P‐value | |
|---|---|---|---|
| Age | 1.0 | 0.97–1.02 | 0.84 |
| Female | 1.8 | 0.9–3.5 | 0.11 |
| NIHSS scorea | 1.0 | 0.95–1.04 | 0.91 |
| Body temperaturea | 0.41 | 0.22–0.77 | 0.006 |
NIHSS, National Institutes of Health Stroke Scale.
On admission.
Discussion
This is the first study, to our knowledge, that looked at neurological worsening during the first 9 h after acute ischaemic stroke symptoms onset. In this study, early neurological worsening occurred in 8.0% of the patients. This frequency is similar to another study 4, where worsening of ≥4 points occurred in 7.5% of patients. However, that study included patients admitted more than 3 h after symptom onset and comparison should be made with caution.
We found that patients with early neurological worsening had lower body temperature on admission and that this worsening translates into poor short‐term outcome. Previously, we have reported that higher body temperature on admission is associated with major neurological improvement in severe ischaemic stroke patients treated with tPA 12. A possible cause is that clot lysis occurs faster in patients with high body temperature. The inverse relationship between temperature and outcome was found among patients not receiving tPA. However, the study included patients admitted within 6 h of stroke onset and is therefore not fully comparable to the present study. Recanalization is important in early phase of ischaemic stroke. High body temperature may have a beneficial effect on clot lysis in the early phase of ischaemic stroke; after that, high body temperature effect on clot lysis is maybe over. It is possible that high body temperature in late phase of ischaemic stroke have a detrimental effect on neurons in ischaemic lesions. The present study supports the association between poor prognosis and low body temperature among patients admitted within 3 h of stroke onset. The mechanism may include early neurological worsening due to late recanalization. A recent study supports that higher temperature on admission in stroke patients is associated with less severe neurological deficits and reduced final infarct volumes 13.
In our study, there was a trend on univariate analysis towards known hypertension on admission to be associated with no early neurological worsening. A possible explanation mechanism is that mild hypertension in the acute phase of ischaemic stroke can increase collateral cerebral blood flow and oxygenation and improves cerebral metabolic rate of oxygen in the core and penumbra. This has been shown in an animal study with induced hypertension 14.
We found that initial MCA1 or MCA2 occlusion was associated with early neurological worsening. This is in agreement with another study showing that large‐vessel occlusion may be a major independent risk factor for early neurological worsening occurring in 38% of patients with large‐vessel occlusion, but in only 3% of those with no occlusion 3.
Early neurological worsening in patients with MCA infarction was associated with ipsilateral internal carotid artery stenosis >50% or occlusion in our study. Possible mechanisms include hemodynamic effects on the ischaemic region. It is possible that poor collateral function may explain this finding. Another possibility is that low blood pressure makes patients with carotid artery stenosis or occlusion especially prone to neurological worsening. However, we did not find that blood pressure on admission was associated with neurological worsening.
Our study showed that early neurological worsening is an independent predictor for poor short‐term outcome in patients with ischaemic stroke. This is in agreement with other studies 15, 16. Because of that, these patients require additional expenses and longer hospital stay. Early neurological worsening is associated with increased mortality within 7 days of stroke onset. Longer duration of hospitalization, higher rates of death during hospitalization and lower rate of functional independence in neurological worsening group were observed in another study 16.
Early neurological worsening associated with pre‐existing comorbid conditions (such as prior stroke or myocardial infarction, diabetes mellitus, atrial fibrillation, atherosclerosis, small‐vessel disease) did not reach statistical significance in our study.
The strength of this study is its focus on the neurological worsening in the first 9 h after symptom onset in patients with first NIHSS score within 3 h of stroke onset, the repeated NIHSS scoring as a part of the routine in our stroke unit and the relatively large number of patients with early admission. The study demonstrates the value of carefully monitoring patients with acute ischaemic stroke. Repeated NIHSS score identifies patients with early neurological worsening and may define a therapeutic time window for ameliorating disastrous events early in their course 11. Another strength of this study is that we included and studied patients admitted early compared with other studies. Most other studies have focused on neurological worsening during 48–72 h and up to the first week after stroke onset 3, 4, 12, 14, 15. Such time windows, however, miss the crucial early hours, when the evolving ischaemic damage is most profound, as shown in our study. Other mechanisms and causes such as infection, raised intracranial pressure, haemorrhagic transformation, seizures may lead to neurological worsening after 9 h of ischaemic stroke onset. Limitations of our study include lack of repeated measurement of body temperature and blood pressure.
Conclusions
Early neurological worsening is associated with low body temperature on admission, extracranially and intracranially large‐vessel stenosis or occlusions and has serious consequences for the short‐term outcome for patients with acute ischaemic stroke.
Conflict of interest and sources of funding
The authors declare no conflict of interests or sources of funding.
Authors' contributions
The authors met the criteria for authorship as recommended by the International Committee of Medical Journal Editors and were fully responsible for all content and editorial decisions. All authors critically reviewed the manuscript and approved the submitted version.
Acknowledgments
The authors thank research nurse Maren Inselseth for her excellent work and assistance with data registration.
Nacu A, Bringeland GH, Khanevski A, Thomassen L, Waje‐Andreassen U, Naess H. Early neurological worsening in acute ischaemic stroke patients. Acta Neurol Scand 2016: 133: 25–29. © 2015 The Authors. Acta Neurologica Scandinavica Published by John Wiley & Sons Ltd.
References
- 1. Tsivgoulis G, Apostolidou N, Giannopoulos S et al. Hemodynamic causes of deterioration in acute ischemic stroke. Perspect Med 2012;1:177–84. [Google Scholar]
- 2. Thanvi B, Treadwell S, Robinson T. Early neurological deterioration in acute ischaemic stroke: predictors, mechanisms and management. Postgrad Med J 2008;84:412–7. [DOI] [PubMed] [Google Scholar]
- 3. Rajajee V, Kidwell C, Starkman S et al. Early MRI and outcomes of untreated patients with mild or improving ischemic stroke. Neurology 2006;67:980–4. [DOI] [PubMed] [Google Scholar]
- 4. Weimar C, Mieck T, Buchthal J et al. Neurologic worsening during the acute phase of ischemic stroke. Arch Neurol 2005;62:393–7. [DOI] [PubMed] [Google Scholar]
- 5. Davalos A, Toni D, Iweins F et al. Neurological deterioration in acute ischemic stroke: potential predictors and associated factors in the European cooperative acute stroke study (ECASS) I. Stroke 1999;30:2631–6. [DOI] [PubMed] [Google Scholar]
- 6. Balami JS, Chen RL, Grunwald IQ, Buchan AM. Neurological complications of acute ischaemic stroke. Lancet Neurol 2011;10:357–71. [DOI] [PubMed] [Google Scholar]
- 7. Palamarchuk I, Kimpinski K, Lippert C, Hachinski V. Nocturnal deterioration after ischemic stroke and autonomic dysfunction: hypothesis and implications. Cerebrovasc Dis 2013;36:454–61. [DOI] [PubMed] [Google Scholar]
- 8. Caplan LR. Worsening in ischemic stroke patients: is it time for a new strategy? Stroke 2002;33:1443–5. [DOI] [PubMed] [Google Scholar]
- 9. Rohr J, Kittner S, Feeser B et al. Traditional risk factors and ischemic stroke in young adults: the Baltimore‐Washington cooperative young stroke study. Arch Neurol 1996;53:603–7. [DOI] [PubMed] [Google Scholar]
- 10. Wahlgren N, Ahmed N, Davalos A et al. Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke‐Monitoring Study (SITS‐MOST): an observational study. Lancet 2007;369:275–82. [DOI] [PubMed] [Google Scholar]
- 11. Adams HP, Jr , Bendixen BH, Kappelle LJ et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. Toast. Trial of org 10172 in acute stroke treatment. Stroke 1993;24:35–41. [DOI] [PubMed] [Google Scholar]
- 12. Kvistad CE, Thomassen L, Waje‐Andreassen U et al. Body temperature and major neurological improvement in tpa‐treated stroke patients. Acta Neurol Scand 2014;129:325–9. [DOI] [PubMed] [Google Scholar]
- 13. Kim SH, Saver JL. Initial body temperature in ischemic stroke: nonpotentiation of tissue‐type plasminogen activator benefit and inverse association with severity. Stroke 2015;46:132–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shin HK, Nishimura M, Jones PB et al. Mild induced hypertension improves blood flow and oxygen metabolism in transient focal cerebral ischemia. Stroke 2008;39:1548–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Saposnik G, Hill MD, O'Donnell M et al. Variables associated with 7‐day, 30‐day, and 1‐year fatality after ischemic stroke. Stroke 2008;39:2318–24. [DOI] [PubMed] [Google Scholar]
- 16. Kwan J, Hand P. Early neurological deterioration in acute stroke: clinical characteristics and impact on outcome. QJM 2006;99:625–33. [DOI] [PubMed] [Google Scholar]


