Abstract
Many people with diabetes rely on insulin therapy to achieve optimal blood glucose control. A fundamental aim of such therapy is to mimic the pattern of ‘normal’ physiological insulin secretion, thereby controlling basal and meal‐time plasma glucose and fatty acid turnover. In people without diabetes, insulin release is modulated on a time base of 3–10 min, something that is impossible to replicate without intravascular glucose sensing and insulin delivery. Overnight physiological insulin delivery by islet β cells is unchanging, in contrast to requirements once any degree of hyperglycaemia occurs, when diurnal influences are evident. Subcutaneous pumped insulin or injected insulin analogues can approach the physiological profile, but there remains the challenge of responding to day‐to‐day changes in insulin sensitivity. Physiologically, meal‐time insulin release begins rapidly in response to reflex activity and incretins, continuing with the rise in glucose and amino acid concentrations. This rapid response reflects the need to fill the insulin space with maximum concentration as early as 30 min after starting the meal. Current meal‐time insulins, by contrast, are associated with a delay after injection before absorption begins, and a delay to peak because of tissue diffusion. While decay from peak for monomeric analogues is not dissimilar to average physiological needs, changes in meal type and, again, in day‐to‐day insulin sensitivity, are difficult to match. Recent and current developments in insulin depot technology are moving towards establishing flatter basal and closer‐to‐average physiological meal‐time plasma insulin profiles. The present article discusses the ideal physiological insulin profile, how this can be met by available and future insulin therapies and devices, and the challenges faced by healthcare professionals and people with diabetes in trying to achieve an optimum plasma insulin profile.
Keywords: analogue, basal, diabetes, insulin therapy, meal, physiology
Introduction
The benefit of good blood glucose control in diabetes is irrefutable 1, 2. For everyone with type 1 diabetes, and many with type 2 diabetes, insulin therapy is the means to achieve such control. One fundamental aim of insulin therapy is to mimic the pattern of physiological insulin secretion to control both basal and meal‐time plasma glucose levels. Attempts to mimic physiological profiles typically use long‐acting basal insulin or continuous subcutaneous insulin infusion (CSII) to modulate endogenous hepatic glucose production and adipose tissue fatty acid release 3. A meal‐time insulin analogue is used to suppress adipose tissue fatty acid release and hepatic glucose production, and enhance peripheral glucose uptake after eating 4. The major constraint to optimum control is hypoglycaemia, although high insulin doses and weight gain also contribute 5.
Since the 1920s, insulin therapy has evolved and our ability to mimic the average physiological profile, achieve good blood glucose control, minimize side effects and enhance user convenience has improved 6; however, average glucose control in clinical practice and even in randomized controlled trials (RCTs) remains poor, and hypoglycaemia is still a problem to nearly everyone with type 1 diabetes and many with type 2 diabetes 7, 8. In a US database analysis of people with type 2 diabetes starting basal insulin, the rate of hypoglycaemia requiring assistance was 11.2 per 100 person‐years 9. The incidence and fear of hypoglycaemia (especially nocturnal) both contribute to sub‐optimal adherence to insulin therapy. Other factors include the need for self‐monitoring and patient education, and the complexity of insulin regimens 10, 11.
The present review article discusses the ideal physiological insulin profile and the extent to which this can be met by available and future insulin therapies and devices.
Physiological Insulin Delivery: the Challenge
There is more to physiological insulin delivery than just achieving optimal average insulin profiles, and many of these issues are presently unapproachable. Most critically, individual insulin sensitivity varies from day to day with changes in lifestyle, both predictable and unpredictable (Table 1), while meal composition affects rates of gastric emptying and substrate absorption. Furthermore, there are underlying diurnal variations in insulin secretion and insulin action 12. Hypoglycaemia can increase insulin sensitivity for up to 24 h, partly via changes in lipolysis and partly through upregulation of glucose transporters 13, 14.
Table 1.
Factors determining acute insulin physiological need and effect.
| Factor Impact or effect |
|---|
| Nutritional |
| Rate of gut absorption of nutrients (fasting = zero) |
| Meal composition effects: |
| Gastric emptying |
| Nutrient absorption rate |
| Incretin secretion |
| Carbohydrate and fatty acid supply to liver |
| Hepatic autoregulation of glucose production |
| Glucose and amino‐acid effects at the islet β cell |
| Portion size |
| Alcohol inhibition of gluconeogenesis |
| Physiological state |
| Physical activity |
| Acute exercise |
| Previous activity affecting insulin sensitivity |
| Diurnal metabolic state |
| Meal glucose tolerance |
| Night‐time changes in hepatic glucose production |
| Hormonal cycles and state |
| Female monthly |
| Puberty‐related |
| Pregnancy |
| Emotional state affecting adrenergic nervous system |
| Pathophysiological and disruptive |
| Insulin insensitivity secondary to calorie excess, long‐term (‘obesity’) |
| Previous (within 24 h) hypoglycaemia |
| Metabolic stress (illness; trauma, including surgery) |
| Hormonal disturbance (adrenal axis; growth hormone) |
| Drug therapy |
| Glucose‐lowering |
| Non‐diabetes therapies (including hormonal, antipsychotics, retroviral) |
| Recreational |
| Travel and changes in time zones |
Physiologically, changes in these factors are modulated by the islet β‐cell response, increasing or reducing insulin secretion to match lower or higher plasma glucose concentrations, or in response to other hormonal changes or exercise 15, 16, 17. None of these responses can be replicated through the delivery of subcutaneous exogenous insulins with improved plasma profiles, although avoidance of hyperinsulinaemia (e.g. postprandially or in the middle of the night) can ameliorate the risk of low glucose levels. Future solutions will depend on feedback control of insulin delivery (‘closed‐loop’) 18, glucose‐sensitive insulins 19, or engineering of cells to mimic islet β cells 20.
Subcutaneously delivered insulin is absorbed into the peripheral rather than the portal circulation. Whether or not this is important will not be further discussed in the present review, but again it is not addressed by absorption profile. There are likely to be consequences, however, for nocturnal versus daytime hypoglycaemia and peripheral versus central fat deposition (and thus body weight) 21, 22.
Accordingly, average plasma glucose and insulin profiles are not typical of any individual, with further variance occurring in the same individual on different days; however, in trying to design an insulin delivery profile to give the best glucose control, it is appropriate to aim for average physiological profiles, while also aiming to minimize the variability of insulin absorption.
Average Physiological Basal Insulin Profile
In a lean person with a healthy pancreas, insulin is released continually at a near constant rate (ignoring pulsatility) during the basal state after food absorption ceases, often 3–5 h after a meal 23; thus, nocturnally, and before breakfast, plasma insulin concentrations remain constant in young adults, as do C‐peptide concentrations, suggesting unchanging insulin secretion (Figure 1) 23, 24. These individuals do not show any tendency towards reduced insulin secretion in the middle of the night, or increased insulin secretion or plasma glucose at the end of the night 23.
Figure 1.

(A) 24‐h serum insulin and C‐peptide profiles in healthy people and (B) overnight and peri‐breakfast serum insulin and blood glucose profiles in the same group of people (after Ref. 23, with permission). (C) 4‐h physiological plasma insulin profiles plotted together with pharmacokinetic profiles for insulin lispro and human insulin in type 1 diabetes; the insulin lispro profile is normalized for excursion to the physiological profile to allow direct comparison of shape (after Ref. 24, with permission).
This contrasts with pumped insulin delivery, where insulin requirements often change between the middle of the night and breakfast, with hepatic glucose production rising before breakfast 25; however, this only occurs when hepatic glucose production and plasma non‐esterified fatty acid levels are above normal, and if these are strictly normal, this ‘dawn phenomenon’ is not evident 25. In a study of night‐time open‐ and closed‐loop insulin delivery, there was no trend for change in insulin requirement overnight, although some individuals became hyperglycaemic on open‐loop insulin later in the night 18.
Clinically, most people are hypoinsulinized, as evidenced by hyperglycaemia 26, 27, so their ideal insulin profile is not the flat profile found physiologically. As changes in hormones, notably cortisol, could stress metabolism towards breakfast, any tendency for clinical insulin delivery to wane, as with neutral protamine Hagedorn (NPH) insulin, is likely to show exaggerated hyperglycaemia before breakfast 28, 29.
During meals, pumped insulin and injected depot insulin delivery differ. For a pumped insulin, the basal rate can be subsumed into the meal insulin delivery profile (in effect, switched off), while an injected basal insulin will continue to be absorbed. Physiologically, however, this should not matter, as a primary action of meal‐time insulin is to suppress hepatic glucose production, either directly or through suppression of peripheral fatty acid release; both basal and meal‐time injection can contribute to this 30. If this continuing basal insulin is included in any calculation, then it accounts for around half of total daily insulin secretion, consistent with modern insulin pump and injection studies 23.
Even a perfectly flat and unvarying basal insulin profile can cause hypoglycaemia, notably if hepatic insulin sensitivity is changed by previous exercise, or if previous hypoglycaemia blunts counter‐regulatory responses 31, 32. If a basal insulin profile is not flat, then either it will not control hepatic glucose production adequately – notably before breakfast – or it will oversuppress glucose production in the basal period. If, in any individual, insulin requirements are lower in the middle of the night and higher at the end of night, then a non‐flat insulin profile may be harnessed to good effect by timing the injection appropriately, e.g. by giving insulin glargine 100 U/ml during the evening 28, 33. Demonstrating this effect with insulin detemir, however, has been unsuccessful in type 2 diabetes 34. Pump regimens often adopt non‐flat profiles in type 1 diabetes to improve end‐of‐night control 35.
Average Meal‐Time Insulin Profile
As with basal insulin secretion, meal‐time insulin secretion is pulsatile, and regulated on a timescale of 3–10 min 36. Ingestion of an oral glucose load, or administration of intravenous (i.v.) glucose as a bolus or square wave, cannot imitate physiological insulin delivery at meal times 36. Glucose loads lack other nutrients, which may also affect stomach emptying (fat) or insulin secretion (amino acids) (Table 1). Food taken orally will result in ‘anticipatory’ insulin secretion through learnt reflexes, and notably by incretins secreted from the gut wall 37. It is estimated that as much as 70% of postprandial insulin secretion can be accounted for by the incretin effect 38; however, a primed i.v. dose of glucose will elicit a very rapid ‘first‐phase’ secretion of insulin, showing that the islet β cells contain a depot of presynthesized insulin ready for immediate secretion into the bloodstream 39.
Figure 1A shows a mean 24‐h insulin/C‐peptide profile in healthy individuals after high‐carbohydrate meals; Figure 1B focuses on the insulin/glucose profile during the overnight and breakfast period 23. These results will not necessarily apply to an individual's meal intake in the real‐world setting, where the composition and size of meals vary. Meal size might only affect the magnitude of the response, while meal composition, including the nature of the carbohydrate ingested 40, may affect the duration of the profile. Furthermore, insulin responses to meals may vary within individuals, with relative glucose intolerance in the afternoon 12, 41 and after other lifestyle changes, as well as between individuals (Table 1).
Pathophysiological Plasma Insulin Profile in People with Diabetes
Type 1 Diabetes
Destruction of pancreatic β cells in individuals with type 1 diabetes will generally progress to a state of near total insulin deficiency, although perhaps not at the rates previously believed 42, 43, 44. Consequently, exogenous insulin must attempt to fulfil all of the need for the absent endogenous insulin, while avoiding any significant risk of hypoglycaemia. This is currently an impossible challenge because of the multiple factors that influence insulin requirements (Table 1). The difficulty of achieving glucose control with exogenous insulin, even in the clinical laboratory, is highlighted by noting that both open‐ and closed‐loop insulin delivery can maintain average glucose control through the night, but with a much greater variance (high and low) for open‐loop control towards morning, and high hour‐to‐hour variation of closed‐loop insulin delivery 18.
Type 2 Diabetes
In type 2 diabetes, assessment of defects in insulin secretion is complicated by the marked degree of insulin insensitivity, both basally and after meals. Accordingly, plasma insulin concentrations are a poor guide to islet β‐cell dysfunction; indeed, plasma insulin concentrations may be high basally, when allowance for insulin insensitivity shows gross deficiency in insulin secretion 45 (Figure 2). Published insulin profiles after meals or glucose lead to the obvious conclusion that meal‐time insulin secretion is slow in rising, leading some to mistakenly conclude that this is the earliest observable defect in people progressing to type 2 diabetes 46. Studies using an i.v. glucose challenge show an absent initial response once fasting plasma glucose has increased to as little as 6.4 mmol/l 47, 48.
Figure 2.
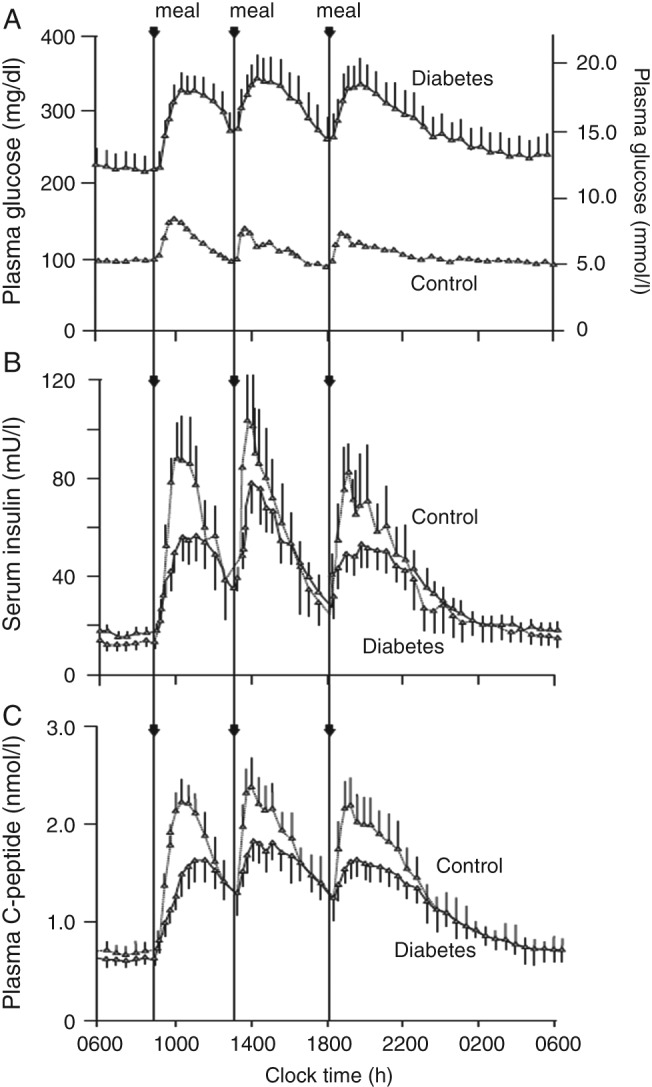
(A) 24‐h plasma glucose, (B) serum insulin, and (C) plasma C‐peptide profiles in people with type 2 diabetes and controls without diabetes. After Ref. 52, with permission.
It has been suggested that postprandial hyperglycaemia is more marked than basal hyperglycaemia in early type 2 diabetes, although this has been disputed 49, 50; however, by the time insulin is started in clinical practice, the major defect is usually in basal blood glucose control 51. Figure 2 neatly illustrates the dilemma of interpretation here 52. The C‐peptide curves could be interpreted as showing a mostly meal‐time insulin secretory deficiency, of up to ∼50% of normal, with a slow rise to peak 52; however, it is clear from the plasma glucose curves that these individuals have a marked defect in basal blood glucose control, which accounts for most of the area under the curve above the normal glucose profile 52. Hyperglycaemic clamp data would suggest that raising plasma glucose in people without diabetes to these basal levels (16–17 mmol/l) would raise insulin secretion more than threefold in the steady state 53, in contrast to C‐peptide levels, which are essentially normal or barely raised. The same group reported elevated 24‐h insulin secretion rates compared with people without diabetes, but <60% of levels in an obese group with no diabetes 54.
Challenges in Mimicking the Physiological Insulin Profile
A goal of insulin therapy should then be to provide as close to possible optimal glycaemic control, in the absence of feedback control, by mimicking the physiological pattern of insulin secretion, in terms of both basal and prandial insulin profiles. It is not known to what extent perfect average glucose profiles would achieve near‐normal glucose control or reduce hypoglycaemia, and this would in any case vary by individual, but it is probably unrealistic to expect hypoglycaemia reductions of even 50% from current rates. Exogenous insulin delivery should perhaps reflect the continuous release of basal insulin by the pancreas, with allowance for diurnal requirements, and approximate both the rapid rise in secretion in anticipation of or during gut intake of food, and also the appropriate fall‐off in insulin secretion after meals (Figure 1, Table 2). The latter is important because insulin has a half‐time of action of ∼20 min, despite a plasma clearance of 4–5 min 24, so the meal‐time surge can result in hypoglycaemia after meals, even in people without diabetes (reactive hypoglycaemia) if gut absorption of foods is relatively brief 55.
Table 2.
Factors determining the effects of subcutaneously administered insulin.
| Variations in insulin requirement* |
| Minute‐to‐minute |
| Day‐to‐day |
| Longer‐term |
| Insulin dose |
| Insulin absorption profile |
| Basal |
| Activity through to 24–30 h |
| Peak to 24 h ratio |
| Inappropriate timing of peak |
| Meal‐time |
| Delay before absorption commences |
| Rate of rise to peak |
| Rate of fall back to basal levels (too long or short) |
| Overlap issues between basal and meal‐time insulins |
| Buffering ability of endogenous insulin supply |
| Islet β‐cell function |
| Progression of dysfunction |
| Variability of absorption (day‐to‐day or meal‐to‐meal) |
| Circulating pool (albumin‐bound insulins) |
| Injection site |
| Region |
| Injection‐site damage |
| Injection‐site blood flow |
| Insulin organ specificity |
See Table 1.
In addition to physiological changes in insulin sensitivity, diurnal variations, effect of meal composition, and effect of previous hypoglycaemia (discussed above), gastric emptying can also be disturbed in people with diabetes 56. In practice, however, all these issues can be difficult to address prospectively (Table 2). Hence, in type 1 diabetes, many clinicians advise an initial goal of a flat basal insulin profile, with rapid‐acting meal‐time insulin, and then develop the insulin regimen iteratively, according to glucose‐monitoring patterns.
The challenges of erratic insulin absorption or erratic control of unexplained cause both require a similar approach. Apart from feedback delivery by pancreas or islet transplantation, even a ‘perfect’ insulin profile will not resolve either issue, but clearly minimizing the deviation from such a profile will reduce the risk that other factors will raise or lower insulin levels to a degree that might result in hyper‐ or hypoglycaemia. Addressing conventional factors, such as avoiding scarred injection sites, injecting within one region of skin, reaching a consistent injection depth, avoiding leakage, and minimizing temperature fluctuations at injection sites remains appropriate (Table 2) 57, 58.
Achieving a Physiological Plasma Insulin Profile
Matching changing day‐to‐day requirements can then only be met by innovative approaches that restore feedback control and by providing the food reflex and incretin signals. Closed‐loop glucose control has a 40‐year history, but the delays involved with subcutaneous sensing and insulin delivery will continue to cause difficulties with meal‐time insulin delivery and acute exercise, i.v. sensing and insulin delivery still being impossible. Little published information yet exists in people with diabetes on the glucose‐sensitive insulins, or for engineered islet β cells 20.
Nevertheless, there have been notable improvements in available insulins for subcutaneous injection and infusion therapy, improvements that mostly address the issue of average plasma insulin profile, and to a lesser extent, variability of absorption (Table 3).
Table 3.
Approaches to achieving a more physiological profile from subcutaneous insulin delivery.
| Product | Mode of action |
|---|---|
| Basal insulins | |
| NPH insulin | Protamine crystal suspension |
| Lente insulin series | Zinc complexes, amorphous and crystalline |
| Pumped insulin | Continuously pumped insulin delivery |
| Insulin glargine 100 U/ml | Basic amino acid derivatization, microprecipitation on injection |
| Insulin glargine 300 U/ml | Basic amino acid derivatization, compact precipitation on injection |
| Insulin detemir | Fatty acid derivatization, tissue albumin binding |
| Insulin degludec | Fatty acid derivatization, tissue multihexamer formation |
| Pegylated lispro | PEG derivatization, tissue diffusion limited |
| Meal‐time insulins | |
| Insulin lispro | Amino acid substitutions, monomeric in tissues |
| Insulin aspart | Amino acid substitutions, monomeric in tissues |
| Insulin glulisine | Amino acid substitutions and reformulation, rapidly monomeric in tissues |
| EDTA/citrate human insulin | Chelation of metal ions, rapid dissociation of insulin hexamers |
| Insulin with hyaluronidase | Increased permeability of tissue injection site |
| Faster‐acting insulin aspart | Amino acid substitutions plus reformulation; rapid dissociation in tissues and possibly enhanced absorption into circulation |
| Controlled action insulin | |
| Smart insulins | Compete with glucose for lectin clearance from circulation, thus higher plasma concentration with hyperglycaemia |
| Closed‐loop pumped delivery | Glucose sensor‐controlled insulin pumps |
| Bioengineered islets | Restoration of feedback control of insulin secretion and synthesis |
Basal Insulin Therapy
Extensive attempts were made in the 1930s and 1940s to extend the action of unmodified insulin, but only protamine‐ (NPH insulin) and zinc‐based products stayed the course (Table 3). Zinc insulins have since largely been withdrawn because of incompatibility with fine‐bore injection needles; however, neither NPH nor the zinc (Lente) series achieve anything like a flat insulin delivery profile, typically with peak plasma insulin concentrations at ∼5 h after administration, and declining to ineffectual levels even by 10–12 h, although with high inter‐patient variation 59, 60. Such a profile is a bad mismatch with high insulin sensitivity during the night, or rising requirements at dawn, and the consequences are nocturnal hypoglycaemia and pre‐breakfast hyperglycaemia. Difficulties in resuspension of a complexed insulin may contribute to erratic insulin delivery. Nevertheless, the NPH approach has, until very recently, remained the standard for newer premixed insulins, albeit with the insulin in the complex being an insulin analogue.
Many attempts were made from 1970 to 2000 to develop new basal insulins with longer – and thus flatter – profiles, but these largely floundered because of erratic absorption and poor bioavailability 61. Some success was gained around 1995 by the approaches used for insulin glargine (soluble in vitro at acidic pH, microprecipitation at neutral pH in tissues) and insulin detemir (derivatization with a fatty acid moiety to promote albumin binding, thus delaying absorption), both having flatter profiles than NPH insulin (Table 3) 60, 62. While neither is a true 24‐h insulin in people with type 1 diabetes 63, both will ensure night‐time coverage if given in the evening, and the shorter absorption profile of insulin detemir may be compensated for by the intravascular albumin binding that will buffer erratic changes in insulin absorption 60, 64.
A problem with long‐acting analogues is assessment of duration of action. Logically, it might seem that glucose‐clamp glucose requirement at 24 h or beyond is the appropriate measure; however, by that time, the study participant's metabolism is abnormal because of prolonged fasting (apart from clamp glucose infusions). As a result, while comparative studies may show differences between insulins, absolute duration (ability to control glucose levels to normal at 24 h in normal life) is not measurable, although could be achieved by injecting in the morning, feeding as normal during the day and clamping only overnight. Researchers often report time to inability to maintain plasma glucose using a level well above target levels, thus biasing the study in favour of the insulin 60, 65. An additional problem is that, while the ideal platform might seem to be totally insulin‐deficient type 1 diabetes, reported clamp glucose requirements in this population are often rather erratic 66. Indeed, European regulators suggest that clinically unacceptable 95% confidence intervals of 80–125% are pragmatically acceptable as showing similarity between insulin products 67. Accordingly, better judgement might be based on pre‐breakfast plasma glucose control 24 h from the last injection.
When used with rapid‐acting insulin analogues, both insulin glargine and insulin detemir provide improved glycated haemoglobin (HbA1c) and less nocturnal hypoglycaemia in people with type 1 diabetes when compared with human insulin regimens 33, 62, 68. A recent manufacturer‐supported systematic review showed similar or better glycaemic control, reduced within‐person variability, similar or reduced frequency of hypoglycaemia and less weight gain with insulin detemir compared with NPH insulin 69. In type 2 diabetes, the situation is complicated by the buffering effect of endogenous insulin secretion, and some reimbursement authorities believe that basal analogues have no advantage over NPH insulin, despite treat‐to‐target studies showing less nocturnal hypoglycaemia. In a Cochrane analysis, no significant difference was found in glycaemic control between insulins glargine and detemir in people with type 2 diabetes, as measured by HbA1c, between‐day variability of fasting plasma glucose, or consistency of glucose concentrations over 24 h 70. Detemir was associated with lower weight gain, whereas glargine was associated with a lower basal insulin dose 70.
Because neither insulin detemir nor insulin glargine 100 U/ml are true 24‐h insulins in people with type 1 diabetes, a single injection may not provide full coverage in some individuals. Furthermore, they may not be suitable for morning injection or allow the injection‐time interval to be beyond 24 h, as sometimes occurs in normal life; however, these insulins are better matched to diurnal changes in insulin sensitivity if given in the evening, perhaps even more so than would be the case with a perfectly flat insulin profile. Furthermore, it is still evident that within‐individual variability is a problem from the average pre‐breakfast glucose levels reported in clinical trials – still around two‐thirds higher than the normal level 27, 71, 72. Accordingly, attempts have been made to extend action further, for example, with the development of insulin degludec 27, 71, 73, insulin glargine 300 U/ml 65, 72, and pegylated lispro 21, 22.
The absorption profile of insulin degludec, an acylated analogue of human insulin, is convincingly longer than 24 h, with a half‐life of ∼25 h (vs 13 h for insulin glargine 100 U/ml) 74. This is confirmed by studies of extreme flexibility of injection times, which show no major detriment 75. Interestingly, the mechanism of delayed absorption is very different from that of insulin detemir, the cartridge/vial dihexamers self‐associating subcutaneously into long‐chain multihexamers, which slowly disassociate from their ends 76. This provides essentially zero order kinetics, not changing with depletion of the injection depot until towards the end of the profile. The acylation should mean that, as with detemir, there is some albumin buffering of any erratic absorption. This may also be reflected in the results found in controlled trials versus insulin glargine 100 U/ml, where HbA1c is unchanged (because of a treat‐to‐target approach) but nocturnal hypoglycaemia is reduced 27, 71, 73. Insulin doses do not rise with insulin degludec despite the longer subcutaneous residence time 26, 27. Speculatively, this may be because the multihexamer structures pack tightly and consistently with fatty acid chains externally 76, perhaps protecting against tissue peptidases.
Insulin glargine 300 U/ml shows a much lower peak‐to‐trough ratio than glargine 100 U/ml in clamp studies and, thus, better 24‐h efficacy in glucose control 65. Published studies in very obese, high‐dose‐requiring people with type 2 diabetes show reduced nocturnal hypoglycaemia in the context of unchanged overall glucose control (HbA1c) 72, 77. Further studies appear underpowered for any putative advantage for hypoglycaemia 78, 79, although comparison of continuous glucose monitoring profiles in type 1 diabetes versus glargine 100 U/ml does suggest much more consistent 24‐h action after one injection 80.
Pegylated insulin lispro is an ultra‐long acting basal insulin, but is also designed to be hepato‐selective (not discussed here, but not necessarily an advantage). Again, clamp data appear to confirm an advantage over insulin glargine 100 U/ml 81, 82, with an apparently very long duration of action. Phase II and III trials show a reduction in nocturnal hypoglycaemia, but daytime events may be increased 21, 83, presumably because of suppression of counter‐regulatory hepatic glucose production.
Meal‐Time Insulins
The kinetic properties of the rapid‐acting insulin analogues have been reviewed elsewhere 24. These insulins incorporate amino acid substitutions that result in a primarily monomeric form in subcutaneous tissue (Table 3). The pharmacokinetic profiles are much more similar in duration to the average meal‐time endogenous insulin profile than for unmodified human insulin, although still with a lag after injection and delayed time to peak concentrations (Figure 1C) 24. These properties have been used to promote administration closer to meal‐times, provide better postprandial plasma glucose control and a lower risk of late‐postprandial hypoglycaemia compared with unmodified human insulin 24. Glulisine, because of formulation changes, shows faster onset of action in pharmacokinetic and pharmacodynamic studies than insulin aspart or insulin lispro, especially in obese people, but it has not been possible to show that these differences are clinically meaningful, such that the overall plasma glucose profile appears similar 24. Currently, when used with the long‐acting basal insulins and compared with human insulin (meal‐time and NPH), aspart and lispro have been shown to be superior for both HbA1c and nocturnal hypoglycaemia (in the same study) in RCTs in people with type 1 diabetes 33, 62, 68.
Rapid‐acting insulin analogues appear to be advantageous compared with human insulin in CSII. A meta‐analysis of studies comparing these approaches concluded that analogues provide modest but significantly better reductions in HbA1c and are preferred by users 84; however, the half‐time of absorption of insulin analogues is not changed between pumps and injections (∼45 min), meaning that it is still many times that of clearance of i.v. insulin (<5 min), and thus imposing limitations of the speed of response of closed‐loop systems 85.
Unlike CSII, which when properly managed can give near‐normal average pre‐breakfast glucose levels, current injection therapy with basal analogues results in fasting hyperglycaemia in type 1 diabetes, indicating hypoinsulinaemia 27, 71, 72. Accordingly, breakfast meal‐time insulin has a dual task in dealing with the breakfast calorie load and correcting basal hypoinsulinaemia. In this situation, the failure of current rapid‐acting insulin analogues to provide a physiologically rapid rise in plasma insulin concentrations is a double problem, even if the duration of action of ∼4 h is appropriate (Figure 1C). Hyperglycaemia after breakfast is therefore usual, and can cause problems later in the day, as the breakfast insulin injection dose needs to be higher than otherwise necessary.
Current developmental approaches to the problem of faster onset of absorption include modified excipients and enabling of tissue diffusion (Table 3). Afrezza® human insulin inhalation powder is now marketed in the USA 86. Absorption across the respiratory epithelium is ultra‐rapid, but also of short duration, providing effective control of postprandial plasma glucose in some people with type 2 diabetes 87. Issues remain over dose flexibility, administration of larger doses, use in type 1 diabetes, and safety concerns regarding the lung 88. Another approach under investigation is the addition of hyaluronidase to insulin preparations. A clamp study examining the addition of recombinant human hyaluronidase to a rapid‐acting analogue demonstrated a 13–25‐min faster onset and 40–49‐min shorter mean duration of insulin action 89. Biodel insulins (such as BIOD‐123 and BIOD‐531) use EDTA/citrate to promote tissue dispersion of insulin monomers, and have been reported to have rapid absorption and decline from peak concentrations, or better post‐meal glucose control, compared with insulin lispro 90, 91.
Faster‐acting insulin aspart is a new formulation that uses arginine as a pharmaceutical stabilizer and nicotinamide to enhance initial absorption after injection 92. As well as earlier plasma insulin exposure after injection in people with type 1 diabetes, this insulin produces a significantly greater early glucose‐lowering effect after a test meal than conventional insulin aspart 92; clamp glucose infusion rates suggest that insulin action continues for ≥4 h. Very preliminary results from clinical trials appear encouraging 93.
Conclusions
The ideal insulin profile for any individual with diabetes will change from day to day and also within the day, due to lifestyle changes and metabolic influences, such as previous hypoglycaemia, and will only ever be attained using technologies sensitive to immediate changes in glucose concentration. In the meantime, it is logical to aim for average physiological profiles, with an awareness that hyperglycaemia can itself alter the diurnal pattern of insulin requirement. Current approaches include the accomplishment of basal insulins with completely flat insulin delivery over an extended day, and targeting of post‐meal glucose control by faster‐onset meal‐time insulin analogues; however, other targets for insulin therapy, including minimizing day‐to‐day variation in absorption, potential to cause weight gain, and perhaps organ selectivity, also deserve consideration. New types and formulations of ultra‐long‐acting analogues and, for prandial control, fast‐acting analogues based on more active dispersion of insulin monomers, enhanced absorption, or alternative delivery routes, exemplify such approaches. While these improvements are very welcome, glucose control in most people with type 1 diabetes still results in levels that are far from normal, emphasizing the need for continued investment in supporting activities to improve patient education and provide more informative glucose monitoring. The scope for continued technological innovation remains broad.
Conflict of Interest
P. H., or institutions with which he is associated, receive funding from potential and actual insulin manufacturers Antriabio, Biocon/Mylan, Eli Lilly, Hanmi, Merck (MSD), Novo Nordisk and Sanofi, and competitor companies with non‐insulin products, for his research, advisory and lecturing activities.
P. H. wrote most of the text of this manuscript, contributed the ideas and opinions that are guaranteed as his own or endorsed by him when referenced to others, has published some of the original research underpinning the topic, and directed choice of source materials and referencing.
Acknowledgements
The author is grateful to AXON Communications for editorial assistance in the development of this manuscript. That assistance was funded by Novo Nordisk. Novo Nordisk was also invited to comment to the author on medical and scientific accuracy.
References
- 1. The Diabetes Control and Complications Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med 1993; 329: 977–986. [DOI] [PubMed] [Google Scholar]
- 2. UK Prospective Diabetes Study (UKPDS) Group . Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998; 352: 837–853. [PubMed] [Google Scholar]
- 3. Moore MC, Smith MS, Turney MK, Boysen S, Williams PE. Comparison of insulins detemir and glargine: effects on glucose disposal, hepatic glucose release and the central nervous system. Diabetes Obes Metab 2011; 13: 832–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Homko C, Deluzio A, Jimenez C, Kolaczynski JW, Boden G. Comparison of insulin aspart and lispro: pharmacokinetic and metabolic effects. Diabetes Care 2003; 26: 2027–2031. [DOI] [PubMed] [Google Scholar]
- 5. Meneghini LF. Insulin therapy for type 2 diabetes. Endocrine 2013; 43: 529–534. [DOI] [PubMed] [Google Scholar]
- 6. Grunberger G. The need for better insulin therapy. Diabetes Obes Metab 2013; 15(Suppl. 1): 1–5. [DOI] [PubMed] [Google Scholar]
- 7. Riddle MC, Rosenstock J, Gerich J, Insulin Glargine Study Investigators . The treat‐to‐target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care 2003; 26: 3080–3086. [DOI] [PubMed] [Google Scholar]
- 8. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Research Group , Nathan DM, Zinman B et al. Modern‐day clinical course of type 1 diabetes mellitus after 30 years' duration: the diabetes control and complications trial/epidemiology of diabetes interventions and complications and Pittsburgh epidemiology of diabetes complications experience (1983–2005). Arch Intern Med 2009; 169: 1307–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ganz ML, Wintfeld NS, Li Q, Lee YC, Gatt E, Huang JC. Severe hypoglycemia rates and associated costs among type 2 diabetics starting basal insulin therapy in the United States. Curr Med Res Opin 2014; 30: 1991–2000. [DOI] [PubMed] [Google Scholar]
- 10. Hartman I. Insulin analogs: impact on treatment success, satisfaction, quality of life, and adherence. Clin Med Res 2008; 6: 54–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. National Institute for Health and Care Excellence (NICE) . Diagnosis and management of type 1 diabetes in children, young people and adults. NICE clinical guideline 15. 2004 (last modified July 2014). 2004. Available from URL: https://www.nice.org.uk/guidance/cg15/resources/guidance‐type‐1‐diabetes‐pdf. Accessed 8 April 2015.
- 12. Saad A, Dalla Man C, Nandy DK et al. Diurnal pattern to insulin secretion and insulin action in healthy individuals. Diabetes 2012; 61: 2691–2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lucidi P, Rossetti P, Porcellati F et al. Mechanisms of insulin resistance after insulin‐induced hypoglycemia in humans: the role of lipolysis. Diabetes 2010; 59: 1349–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bourey RE, Koranyi L, James DE, Mueckler M, Permutt MA. Effects of altered glucose homeostasis on glucose transporter expression in skeletal muscle of the rat. J Clin Invest 1990; 86: 542–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pørksen N, Juhl C, Hollingdal M et al. Concordant induction of rapid in vivo pulsatile insulin secretion by recurrent punctuated glucose infusions. Am J Physiol Endocrinol Metab 2000; 278: E162–170. [DOI] [PubMed] [Google Scholar]
- 16. Pørksen N. The in vivo regulation of pulsatile insulin secretion. Diabetologia 2002; 45: 3–20. [DOI] [PubMed] [Google Scholar]
- 17. Wahren J, Felig P, Ahlborg G, Jorfeldt L. Glucose metabolism during leg exercise in man. J Clin Invest 1971; 50: 2715–2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hovorka R, Kumareswaran K, Harris J et al. Overnight closed loop insulin delivery (artificial pancreas) in adults with type 1 diabetes: crossover randomised controlled studies. BMJ 2011; 342: d1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sun L, Zhang X, Zheng C, Wu Z, Li C. A pH gated, glucose‐sensitive nanoparticle based on worm‐like mesoporous silica for controlled insulin release. J Phys Chem B 2013; 117: 3852–3860. [DOI] [PubMed] [Google Scholar]
- 20. Pagliuca FW, Millman JR, Gurtler M et al. Generation of functional human pancreatic beta cells in vitro. Cell 2014; 159: 428–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rosenstock J, Bergenstal RM, Blevins TC et al. Better glycemic control and weight loss with the novel long‐acting basal insulin LY2605541 compared with insulin glargine in type 1 diabetes: a randomized, crossover study. Diabetes Care 2013; 36: 522–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jacober SJ, Rosenstock J, Bergenstal RM, Prince MJ, Qu Y, Beals JM. Contrasting weight changes with LY2605541, a novel long‐acting insulin, and insulin glargine despite similar improved glycaemic control in T1DM and T2DM. Diabetes Obes Metab 2014; 16: 351–356. [DOI] [PubMed] [Google Scholar]
- 23. Kruszynska YT, Home PD, Hanning I, Alberti KG. Basal and 24‐h C‐peptide and insulin secretion rate in normal man. Diabetologia 1987; 30: 16–21. [DOI] [PubMed] [Google Scholar]
- 24. Home PD. The pharmacokinetics and pharmacodynamics of rapid‐acting insulin analogues and their clinical consequences. Diabetes Obes Metab 2012; 14: 780–788. [DOI] [PubMed] [Google Scholar]
- 25. Koivisto VA, Yki‐Jarvinen H, Helve E, Karonen SL, Pelkonen R. Pathogenesis and prevention of the dawn phenomenon in diabetic patients treated with CSII. Diabetes 1986; 35: 78–82. [DOI] [PubMed] [Google Scholar]
- 26. Birkeland KI, Home PD, Wendisch U et al. Insulin degludec in type 1 diabetes: a randomized controlled trial of a new‐generation ultra‐long‐acting insulin compared with insulin glargine. Diabetes Care 2011; 34: 661–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Heller S, Buse J, Fisher M et al. Insulin degludec, an ultra‐longacting basal insulin, versus insulin glargine in basal‐bolus treatment with mealtime insulin aspart in type 1 diabetes (BEGIN Basal‐Bolus Type 1): a phase 3, randomised, open‐label, treat‐to‐target non‐inferiority trial. Lancet 2012; 379: 1489–1497. [DOI] [PubMed] [Google Scholar]
- 28. Francis AJ, Home PD, Hanning I, Alberti KG, Tunbridge WM. Intermediate acting insulin given at bedtime: effect on blood glucose concentrations before and after breakfast. Br Med J (Clin Res Ed) 1983; 286: 1173–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gale EA, Kurtz AB, Tattersall RB. In search of the Somogyi effect. Lancet 1980; 2: 279–282. [DOI] [PubMed] [Google Scholar]
- 30. Mitrakou A, Kelley D, Veneman T et al. Contribution of abnormal muscle and liver glucose metabolism to postprandial hyperglycemia in NIDDM. Diabetes 1990; 39: 1381–1390. [DOI] [PubMed] [Google Scholar]
- 31. Kemmer F. Chapter 35. Exercise In: Alberti K, DeFronzo R, Keen H, Zimmet P, eds. International Textbook of Diabetes Mellitus. 2nd edn. Chicester: John Wiley; 1997, 799–815. [Google Scholar]
- 32. Dagogo‐Jack SE, Craft S, Cryer PE. Hypoglycemia‐associated autonomic failure in insulin‐dependent diabetes mellitus. Recent antecedent hypoglycemia reduces autonomic responses to, symptoms of, and defense against subsequent hypoglycemia. J Clin Invest 1993; 91: 819–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ashwell SG, Amiel SA, Bilous RW et al. Improved glycaemic control with insulin glargine plus insulin lispro: a multicentre, randomized, cross‐over trial in people with Type 1 diabetes. Diabet Med 2006; 23: 285–292. [DOI] [PubMed] [Google Scholar]
- 34. Rosenstock J, Davies M, Home PD, Larsen J, Koenen C, Schernthaner G. A randomised, 52‐week, treat‐to‐target trial comparing insulin detemir with insulin glargine when administered as add‐on to glucose‐lowering drugs in insulin‐naive people with type 2 diabetes. Diabetologia 2008; 51: 408–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pickup J, Keen H. Continuous subcutaneous insulin infusion at 25 years: evidence base for the expanding use of insulin pump therapy in type 1 diabetes. Diabetes Care 2002; 25: 593–598. [DOI] [PubMed] [Google Scholar]
- 36. Caumo A, Luzi L. First‐phase insulin secretion: does it exist in real life? Considerations on shape and function. Am J Physiol Endocrinol Metab 2004; 287: E371–385. [DOI] [PubMed] [Google Scholar]
- 37. Nauck MA, Homberger E, Siegel EG et al. Incretin effects of increasing glucose loads in man calculated from venous insulin and C‐peptide responses. J Clin Endocrinol Metab 1986; 63: 492–498. [DOI] [PubMed] [Google Scholar]
- 38. Diakogiannaki E, Gribble FM, Reimann F. Nutrient detection by incretin hormone secreting cells. Physiol Behav 2012; 106: 387–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rhodes CJ, Halban PA. Newly synthesized proinsulin/insulin and stored insulin are released from pancreatic B cells predominantly via a regulated, rather than a constitutive, pathway. J Cell Biol 1987; 105: 145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Riccardi G, Rivellese AA. Effects of dietary fiber and carbohydrate on glucose and lipoprotein metabolism in diabetic patients. Diabetes Care 1991; 14: 1115–1125. [DOI] [PubMed] [Google Scholar]
- 41. Zimmet PZ, Wall JR, Rome R, Stimmler L, Jarrett RJ. Diurnal variation in glucose tolerance: associated changes in plasma insulin, growth hormone, and non‐esterified fatty acids. Br Med J 1974; 1: 485–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Oram RA, Jones AG, Besser RE et al. The majority of patients with long‐duration type 1 diabetes are insulin microsecretors and have functioning beta cells. Diabetologia 2014; 57: 187–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. American Diabetes Association . Diagnosis and classification of diabetes mellitus. Diabetes Care 2014; 37(Suppl. 1): S81–90. [DOI] [PubMed] [Google Scholar]
- 44. Davis AK, DuBose SN, Haller MJ et al. Prevalence of detectable C‐Peptide according to age at diagnosis and duration of type 1 diabetes. Diabetes Care 2015; 38: 476–481. [DOI] [PubMed] [Google Scholar]
- 45. Kahn SE. The relative contributions of insulin resistance and beta‐cell dysfunction to the pathophysiology of Type 2 diabetes. Diabetologia 2003; 46: 3–19. [DOI] [PubMed] [Google Scholar]
- 46. Gerich JE. Is reduced first‐phase insulin release the earliest detectable abnormality in individuals destined to develop type 2 diabetes? Diabetes 2002; 51(Suppl. 1): S117–121. [DOI] [PubMed] [Google Scholar]
- 47. Kahn SE, Carr DB, Faulenbach MV, Utzschneider KM. An examination of beta‐cell function measures and their potential use for estimating beta‐cell mass. Diabetes Obes Metab 2008; 10(Suppl. 4): 63–76. [DOI] [PubMed] [Google Scholar]
- 48. Brunzell JD, Robertson RP, Lerner RL et al. Relationships between fasting plasma glucose levels and insulin secretion during intravenous glucose tolerance tests. J Clin Endocrinol Metab 1976; 42: 222–229. [DOI] [PubMed] [Google Scholar]
- 49. Monnier L, Lapinski H, Colette C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: variations with increasing levels of HbA1c . Diabetes Care 2003; 26: 881–885. [DOI] [PubMed] [Google Scholar]
- 50. Riddle M, Umpierrez G, DiGenio A, Zhou R, Rosenstock J. Contributions of basal and postprandial hyperglycemia over a wide range of A1C levels before and after treatment intensification in type 2 diabetes. Diabetes Care 2011; 34: 2508–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Holman RR, Turner RC. Optimizing blood glucose control in type 2 diabetes: an approach based on fasting blood glucose measurements. Diabet Med 1988; 5: 582–588. [DOI] [PubMed] [Google Scholar]
- 52. Polonsky KS, Given BD, Hirsch LJ et al. Abnormal patterns of insulin secretion in non‐insulin‐dependent diabetes mellitus. N Engl J Med 1988; 318: 1231–1239. [DOI] [PubMed] [Google Scholar]
- 53. Elahi D. In praise of the hyperglycemic clamp. A method for assessment of beta‐cell sensitivity and insulin resistance. Diabetes Care 1996; 19: 278–286. [DOI] [PubMed] [Google Scholar]
- 54. Van Cauter E, Mestrez F, Sturis J, Polonsky KS. Estimation of insulin secretion rates from C‐peptide levels. Comparison of individual and standard kinetic parameters for C‐peptide clearance. Diabetes 1992; 41: 368–377. [DOI] [PubMed] [Google Scholar]
- 55. Lefebvre PJ, Luyckx AS, Lecomte MJ. Studies on the pathogenesis of reactive hypoglycemia: role of insulin and glucagon. Horm Metab Res 1976; Suppl 6: 91–98. [PubMed] [Google Scholar]
- 56. Rayner CK, Samsom M, Jones KL, Horowitz M. Relationships of upper gastrointestinal motor and sensory function with glycemic control. Diabetes Care 2001; 24: 371–381. [DOI] [PubMed] [Google Scholar]
- 57. Binder C, Lauritzen T, Faber O, Pramming S. Insulin pharmacokinetics. Diabetes Care 1984; 7: 188–199. [DOI] [PubMed] [Google Scholar]
- 58. Sindelka G, Heinemann L, Berger M, Frenck W, Chantelau E. Effect of insulin concentration, subcutaneous fat thickness and skin temperature on subcutaneous insulin absorption in healthy subjects. Diabetologia 1994; 37: 377–380. [DOI] [PubMed] [Google Scholar]
- 59. Heinemann L, Linkeschova R, Rave K, Hompesch B, Sedlak M, Heise T. Time‐action profile of the long‐acting insulin analog insulin glargine (HOE901) in comparison with those of NPH insulin and placebo. Diabetes Care 2000; 23: 644–649. [DOI] [PubMed] [Google Scholar]
- 60. Lepore M, Pampanelli S, Fanelli C et al. Pharmacokinetics and pharmacodynamics of subcutaneous injection of long‐acting human insulin analog glargine, NPH insulin, and ultralente human insulin and continuous subcutaneous infusion of insulin lispro. Diabetes 2000; 49: 2142–2148. [DOI] [PubMed] [Google Scholar]
- 61. Home PD, Hanning I, Capaldo B, Alberti KG. Bioavailability of highly purified bovine ultralente insulin. Diabetes Care 1983; 6: 210. [DOI] [PubMed] [Google Scholar]
- 62. Hermansen K, Fontaine P, Kukolja KK, Peterkova V, Leth G, Gall MA. Insulin analogues (insulin detemir and insulin aspart) versus traditional human insulins (NPH insulin and regular human insulin) in basal‐bolus therapy for patients with type 1 diabetes. Diabetologia 2004; 47: 622–629. [DOI] [PubMed] [Google Scholar]
- 63. Ashwell SG, Gebbie J, Home PD. Twice‐daily compared with once‐daily insulin glargine in people with Type 1 diabetes using meal‐time insulin aspart. Diabet Med 2006; 23: 879–886. [DOI] [PubMed] [Google Scholar]
- 64. Kurtzhals P. Engineering predictability and protraction in a basal insulin analogue: the pharmacology of insulin detemir. Int J Obes Relat Metab Disord 2004; 28(Suppl. 2): S23–28. [DOI] [PubMed] [Google Scholar]
- 65. Becker RH, Dahmen R, Bergmann K, Lehmann A, Jax T, Heise T. New insulin glargine 300 units.mL‐1 provides a more even activity profile and prolonged glycemic control at steady state compared with insulin glargine 100 units.mL‐1 . Diabetes Care 2014; 38: 637–643. [DOI] [PubMed] [Google Scholar]
- 66. Heise T, Zhang X, Quin Lam E et al. Duration of action of 2 insulin glargine products, LY2963016 and Lantus®, in subjects with type 1 diabetes mellitus (T1DM) (Abstract 891‐P). Diabetes 2014; 63(Suppl. 1): A228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. European Medicines Agency . Guideline on non‐clinical and clinical development of 4 similar biological medicinal products containing 5 recombinant human insulin and insulin analogues. 2014. Available from URL: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2014/04/WC500165988.pdf. Accessed 8 April 2015.
- 68. Pedersen‐Bjergaard U, Kristensen PL, Beck‐Nielsen H et al. Effect of insulin analogues on risk of severe hypoglycaemia in patients with type 1 diabetes prone to recurrent severe hypoglycaemia (HypoAna trial): a prospective, randomised, open‐label, blinded‐endpoint crossover trial. Lancet Diabetes Endocrinol 2014; 2: 553–561. [DOI] [PubMed] [Google Scholar]
- 69. Frier BM, Russell‐Jones D, Heise T. A comparison of insulin detemir and neutral protamine Hagedorn (isophane) insulin in the treatment of diabetes: a systematic review. Diabetes Obes Metab 2013; 15: 978–986. [DOI] [PubMed] [Google Scholar]
- 70. Swinnen SG, Simon AC, Holleman F, Hoekstra JB, Devries JH. Insulin detemir versus insulin glargine for type 2 diabetes mellitus. Cochrane Database Syst Rev 2011: CD006383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Bode BW, Buse JB, Fisher M et al. Insulin degludec improves glycaemic control with lower nocturnal hypoglycaemia risk than insulin glargine in basal‐bolus treatment with mealtime insulin aspart in Type 1 diabetes (BEGIN® Basal‐Bolus Type 1): 2‐year results of a randomized clinical trial. Diabet Med 2013; 30: 1293–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Riddle MC, Bolli GB, Ziemen M, Muehlen‐Bartmer I, Bizet F, Home PD. New insulin glargine 300 units/mL versus glargine 100 units/mL in people with type 2 diabetes using basal and mealtime insulin: glucose control and hypoglycemia in a 6‐month randomized controlled trial (EDITION 1). Diabetes Care 2014; 37: 2755–2762. [DOI] [PubMed] [Google Scholar]
- 73. Zinman B, Philis‐Tsimikas A, Cariou B et al. Insulin degludec versus insulin glargine in insulin‐naive patients with type 2 diabetes: a 1‐year, randomized, treat‐to‐target trial (BEGIN Once Long). Diabetes Care 2012; 35: 2464–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Heise T, Nosek L, Bottcher SG, Hastrup H, Haahr H. Ultra‐long‐acting insulin degludec has a flat and stable glucose‐lowering effect in type 2 diabetes. Diabetes Obes Metab 2012; 14: 944–950. [DOI] [PubMed] [Google Scholar]
- 75. Mathieu C, Hollander P, Miranda‐Palma B et al. Efficacy and safety of insulin degludec in a flexible dosing regimen vs insulin glargine in patients with type 1 diabetes (BEGIN: Flex T1): a 26‐week randomized, treat‐to‐target trial with a 26‐week extension. J Clin Endocrinol Metab 2013; 98: 1154–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Jonassen I, Havelund S, Hoeg‐Jensen T, Steensgaard DB, Wahlund PO, Ribel U. Design of the novel protraction mechanism of insulin degludec, an ultra‐long‐acting basal insulin. Pharm Res 2012; 29: 2104–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Yki‐Jarvinen H, Bergenstal R, Ziemen M et al. New insulin glargine 300 units/mL versus glargine 100 units/mL in people with type 2 diabetes using oral agents and basal insulin: glucose control and hypoglycemia in a 6‐month randomized controlled trial (EDITION 2). Diabetes Care 2014; 37: 3235–3243. [DOI] [PubMed] [Google Scholar]
- 78. Bolli GB, Riddle MC, Bergenstal RM et al. New insulin glargine 300 U/ml compared with glargine 100 U/ml in insulin‐naive people with type 2 diabetes on oral glucose‐lowering drugs: a randomized controlled trial (EDITION 3). Diabetes Obes Metab 2015; 17: 386–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Home PD, Bergenstal RM, Bolli GB et al. New insulin glargine 300 units/mL versus glargine 100 units/mL in people with type 1 diabetes: a randomized, phase 3a, open‐label clinical trial (EDITION 4). Diabetes Care 2015; 38; DOI: 10.2337/dc2315-0249 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 80. Bergenstal RM, Bailey TS, Rodbard D et al. Insulin glargine 300 U/ml vs 100 U/ml: glucose profiles of morning vs evening injections in adults with type 1 diabetes mellitus measured with continuous glucose monitoring (CGM) (Abstract 949). Diabetologia 2014; 57(Suppl. 1): S388. [Google Scholar]
- 81. Sinha VP, Choi SL, Soon DK et al. Single‐dose pharmacokinetics and glucodynamics of the novel, long‐acting basal insulin LY2605541 in healthy subjects. J Clin Pharmacol 2014; 54: 792–799. [DOI] [PubMed] [Google Scholar]
- 82. Sinha VP, Howey DC, Choi SL, Mace KF, Heise T. Steady‐state pharmacokinetics and glucodynamics of the novel, long‐acting basal insulin LY2605541 dosed once‐daily in patients with type 2 diabetes mellitus. Diabetes Obes Metab 2014; 16: 344–350. [DOI] [PubMed] [Google Scholar]
- 83. Bergenstal RM, Rosenstock J, Arakaki RF et al. A randomized, controlled study of once‐daily LY2605541, a novel long‐acting basal insulin, versus insulin glargine in basal insulin‐treated patients with type 2 diabetes. Diabetes Care 2012; 35: 2140–2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Colquitt J, Royle P, Waugh N. Are analogue insulins better than soluble in continuous subcutaneous insulin infusion? Results of a meta‐analysis. Diabet Med 2003; 20: 863–866. [DOI] [PubMed] [Google Scholar]
- 85. Rebrin K, Sheppard NF Jr, Steil GM. Use of subcutaneous interstitial fluid glucose to estimate blood glucose: revisiting delay and sensor offset. J Diabetes Sci Technol 2010; 4: 1087–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. US Food and Drug Administration . FDA news release: FDA approves Afrezza to treat diabetes. 2014. Available from URL: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm403122.htm. Accessed 8 April 2015.
- 87. Zisser H, Jovanovic L, Markova K et al. Technosphere insulin effectively controls postprandial glycemia in patients with type 2 diabetes mellitus. Diabetes Technol Ther 2012; 14: 997–1001. [DOI] [PubMed] [Google Scholar]
- 88. US Food and Drug Administration . Summary minutes of the Endocrinologic and Metabolic Drugs Advisory Committee Meeting. April 1, 2014. 2014. Available from URL: http://www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/ucm386727.htm. Accessed 8 April 2015.
- 89. Morrow L, Muchmore DB, Hompesch M, Ludington EA, Vaughn DE. Comparative pharmacokinetics and insulin action for three rapid‐acting insulin analogs injected subcutaneously with and without hyaluronidase. Diabetes Care 2013; 36: 273–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Krasner A, Brazg RL, Blevins TC et al. Safety and efficacy of ultra‐rapid‐acting human insulin formulation BIOD‐123 in patients with type 1 diabetes (Abstract 130‐OR). Diabetes 2014; 63(Suppl. 1): A34. [Google Scholar]
- 91. Morrow L, Krasner A, Canney L, Hompesch M, Pichotta P, Souza ED. Biphasic pharmacokinetic and pharmacodynamic profiles associated with concentrated insulin BIOD‐531 show rapid onset and basal duration of action (Abstract 937). Diabetologia 2014; 57(Suppl. 1): S383. [Google Scholar]
- 92. Heise T, Hövelmann U, Brøndsted L, Adrian CL, Nosek L, Haahr H. Faster‐acting insulin aspart: earlier onset of appearance and greater early pharmacokinetic and pharmacodynamic effects than insulin aspart. Diabetes Obes Metab 2015; 17: 682–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Novo Nordisk . Novo Nordisk completes phase 3a trials comparing faster‐acting insulin aspart with NovoRapid® in people with type 1 and type 2 diabetes. 2015. Available from URL: http://www.novonordisk.com/bin/getPDF.1906174.pdf. Accessed 8 April 2015.


