Abstract
Background
The Stockholm III Trial randomized patients with primary operable rectal cancers to either short‐course radiotherapy (RT) with immediate surgery (SRT), short‐course RT with surgery delayed 4–8 weeks (SRT‐delay) or long‐course RT with surgery delayed 4–8 weeks. This preplanned interim analysis examined the pathological outcome of delaying surgery.
Methods
Patients randomized to the SRT and SRT‐delay arms in the Stockholm III Trial between October 1998 and November 2010 were included, and data were collected in a prospective register. Additional data regarding tumour regression grade, according to Dworak, and circumferential margin were obtained by reassessment of histopathological slides.
Results
A total of 462 of 545 randomized patients had specimens available for reassessment. Patients randomized to SRT‐delay had earlier ypT categories, and a higher rate of pathological complete responses (11·8 versus 1·7 per cent; P = 0·001) and Dworak grade 4 tumour regression (10·1 versus 1·7 per cent; P < 0·001) than patients randomized to SRT without delay. Positive circumferential resection margins were uncommon (6·3 per cent) and rates did not differ between the two treatment arms.
Conclusion
Short‐course RT induces tumour downstaging if surgery is performed after an interval of 4–8 weeks.
Short abstract
Short‐course therapy with delay causes downstaging
Introduction
Preoperative radiotherapy (RT) is recommended to many patients with rectal cancer as it leads to a reduced local recurrence rate1, 2, 3, 4, 5, 6 and, in some studies7, 8, improved overall survival. RT can induce downsizing and/or downstaging of the primary tumour and may increase sphincter preservation, although this is controversial9. A conventional, long‐course RT schedule (approximately 50 Gy over 6 weeks) combined with chemotherapy (CRT) induces tumour downstaging when surgery has to be delayed owing to the acute reaction caused by the treatment8, 10. In contrast, it had been thought that hypofractionated, short‐course RT (25 Gy in 1 week) did not induce downstaging11. However, two large trials4, 12 found tumour regression after a short delay (less than 1 week). In addition, in three retrospective13, 14, 15 and one prospective16 studies, short‐course RT induced downstaging if surgery was delayed for more than 4 weeks.
In 1998, the Stockholm Colorectal Cancer Study Group initiated the Stockholm III Trial to address the issues of fractionation and timing to surgery, with local recurrence rate as the primary endpoint. The multicentre randomized trial has recruited patients with primary resectable rectal cancers to one of three preoperative RT regimens: short‐course RT with surgery within a week (SRT), short‐course RT with surgery delayed for 4–8 weeks (SRT‐delay) and long‐course RT with surgery delayed for 4–8 weeks (LRT‐delay). The trial closed for further inclusion in February 2013. The aim of this second preplanned interim analysis was to compare the pathological outcomes in the two short‐course RT randomization arms after 500 included patients, with a special focus on T and N categories, involved resection margins and tumour regression.
Methods
The Stockholm III Trial
The Stockholm III Trial (ClinicalTrials.gov registration number NCT00904813) has been described previously17. The trial included patients with a primary rectal cancer, defined as an adenocarcinoma within 15 cm of the anal verge, and judged to be resectable. The patients were scheduled for an open abdominal procedure. Exclusion criteria were previous RT to the abdominal or pelvic regions, signs of severe ischaemic disease or symptoms of severe arteriosclerosis.
After giving informed consent, patients were randomized to SRT, SRT‐delay or LRT‐delay. A hospital could choose to participate in the three‐arm (SRT, SRT‐delay or LRT‐delay) or the two‐arm (SRT or SRT‐delay) comparison.
Preoperative radiotherapy
In patients randomized to short‐course RT (SRT and SRT‐delay), a total dose of 25 Gy was given over 5–7 consecutive days using a four‐field box technique, including the primary tumour and the primary and secondary lymph nodes in the pelvis. No individual tumour target was drawn in the few first years of the study, but this has become more common with time. After the first few years the anal canal was included in the target volume only if an abdominoperineal resection was planned. Otherwise, the lower limit of the beams was 3–4 cm above the anal verge or at least 5 cm below the lowest part of the visible tumour. The upper beam limit was initially typically at mid‐L5, or 1–1·5 cm above the promontory. In more recent years it was individualized. The dorsal limit of the lateral beams was behind the sacrum, and the anterior limit was sufficiently ventral to cover the obturator nodes, the entire mesorectum with tumour extension and the internal iliac nodes. The lateral limits of the anterior–posterior beams extended 1–1·5 cm outside the pelvic rim. In patients randomized to LRT‐delay the same target and technique was used, but with a daily fraction of 2 Gy in 25 fractions over 5 weeks, giving a total dose of 50 Gy. All treatments were given with high‐energy photons (8–20 Gy). Appropriate shielding of non‐target volumes was prescribed. Individual three‐dimensional dose planning of the tumour target volume and multileaf collimators were used at all hospitals during the latter part of the study, but not during the early years.
Surgery
Patients underwent anterior resection, abdominoperineal resection or Hartmann's procedure. The standard operation included total mesorectal excision, defined as removal of the rectum with the entire mesorectum by sharp dissection along the mesorectal fascia down to the pelvic floor. According to the protocol, patients randomized to the SRT group were to undergo surgery 1–7 days after the completion of RT. During the later phase of the study, it was stressed that it was preferable to carry out surgery within 1–4 days17, 18. In the two other groups (SRT‐delay and LRT‐delay) the surgery was undertaken 28–56 days after the completion of RT. Bowel preparation, and antiseptic and antithrombotic prophylaxis were administered according to local routines.
Follow‐up
Data on all patients with rectal cancer are reported continuously to the Swedish Rectal Cancer Register by the Regional Oncology Centres. The information in the register includes clinical patient characteristics, details of preoperative assessment, preoperative therapy, surgery and perioperative complications, the pathologists' and surgeons' assessments of clearance of primary tumours, postoperative mortality and morbidity, histopathology and follow‐up data on recurrences, metachronous metastases and cause of death. The Stockholm Regional Oncology Centre is responsible for the study database, which is validated continuously19.
Interim analyses of the Stockholm III Trial
Data from an interim analysis of the Stockholm III Trial after the first 303 randomized patients have been reported previously17. A second interim analysis was preplanned with the aim of comparing the downstaging and downsizing effects of the preoperative short‐course RT schedules after 500 included patients. The present study reports the findings regarding pathological tumour downstaging in the SRT and SRT‐delay arms. The LRT‐delay arm is not included in this interim analysis because it was expected that too few patients would have been included in the three‐arm randomization at this time. No formal power calculation for the analyses of the endpoints in the second interim analysis was done.
Present study
The present study identified patients randomized to the SRT and SRT‐delay arms from the trial start in October 1998 to November 2010. Demographic data, allocated treatment arm, RT received and surgical data were extracted from the Swedish Rectal Cancer Register. The specimens were originally dissected, prepared and assessed by eight different pathology departments according to local routines. For the present study, all available slides were retrieved for blinded reassessment by one pathologist. If the reassessment was impaired by technical difficulties, such as damaged slides or pale staining, the stage or circumferential resection margin (CRM) was recorded as not assessable. Patients for whom single whole‐mount sections of the tumour were missing were excluded from the analysis of CRM. The TNM staging system (6th edition)20, 21 was used for staging.
At pathological assessment, the CRM was defined as positive if the tumour involved the CRM or was 1 mm or less from the margin. It was judged to be negative if the distance from the tumour to the margin exceeded 1 mm.
The Dworak system22 was used for the assessment of tumour regression: grade 0, no regression; grade 1, dominant tumour mass with obvious fibrosis and/or vasculopathy; grade 2, dominantly fibrotic changes with few tumour cells or groups (easy to find); grade 3, very few (difficult to find microscopically) tumour cells in fibrotic tissue with or without mucous substance; grade 4, no tumour cells, only fibrotic mass (total regression or response).
Statistical analysis
Differences in distribution between the randomization arms regarding pathological outcomes of treatment were tested using Fisher's exact test or the Mann–Whitney U test. All analyses were carried out using Stata® version 11.2 (StataCorp LP, College Station, Texas, USA).
Results
From October 1998 to November 2010, 657 patients were randomized in the Stockholm III Trial; 112 were randomized to the LRT‐delay arm and were not analysed in the present study. Some 462 of 545 specimens were available for reassessment in the present study (Fig. 1). Clinical characteristics and surgical data are shown in Table 1.
Figure 1.
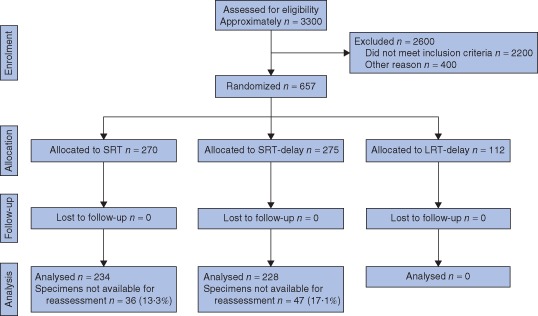
Flow diagram of randomization to the Stockholm III Trial, and patient selection to the present study of the trial arms with short‐course radiotherapy (RT) with immediate surgery (SRT) and short‐course RT with surgery delayed 4–8 weeks (SRT‐delay). The trial arm with long‐course RT and surgery delayed 4–8 weeks (LRT‐delay) was not analysed in the present study
Table 1.
Demographics and surgery
| SRT (n = 234) | SRT‐delay (n = 228) | |
|---|---|---|
| Age (years)* | 67 (35–89) | 67 (40–88) |
| Sex ratio (M : F) | 147 : 87 | 138 : 90 |
| Tumour height | ||
| Low (< 6 cm) | 88 (37·6) | 82 (36·0) |
| Medium (6–10 cm) | 90 (38·5) | 97 (42·5) |
| High (> 10 cm) | 56 (23·9) | 49 (21·5) |
| Type of surgery | ||
| Anterior resection | 143 (61·1) | 129 (56·6) |
| Abdominoperineal resection | 78 (33·3) | 87 (38·2) |
| Hartmann's procedure | 13 (5·6) | 12 (5·3) |
Values in parentheses are percentages unless indicated otherwise;
values are median (range). SRT, short‐course radiotherapy and immediate surgery; SRT‐delay, short‐course radiotherapy with surgery delayed 4–8 weeks.
RT was delivered according to the protocol in all 234 patients in the SRT group and in 226 (99·1 per cent) of 228 in the SRT‐delay group (P = 0·947). The overall treatment time (OTT; time from start of RT to surgery) was according to protocol in 221 (94·4 per cent) of 234 patients (range 6–98, mean 10, median 8, i.q.r. 3 days) and 198 (86·8 per cent) of 228 patients (range 7–428, mean 47, median 45, i.q.r. 13 days) respectively (P = 0·534). Reasons for protocol violations in the SRT‐delay group were a longer OTT in 16 patients and a shorter OTT than prescribed in the trial protocol in 14 patients.
Pathological outcomes are summarized in Table 2. There were statistically significant differences in distributions between the randomization arms regarding tumour stage and ypT category; both were lower in patients randomized to SRT‐delay. Node status did not differ significantly between the groups. There were differences in the rate of complete pathological response: 11·8 per cent in the SRT‐delay arm compared with 1·7 per cent for SRT. There was also a significant difference in tumour regression grade according to Dworak between the two groups (P < 0·001). Thirty‐four patients (14·9 per cent) in the SRT‐delay group had grade 3 or 4 tumour regression compared with six (2·6 per cent) in the SRT arm.
Table 2.
Pathological outcomes
| SRT (n = 234) | SRT‐delay (n = 228) | P ¶ | |
|---|---|---|---|
| Tumour stage | 0·001 | ||
| yp0 | 4 (1·7) | 27 (11·8) | |
| ypI | 69 (29·5) | 76 (33·3) | |
| ypII | 71 (30·3) | 53 (23·2) | |
| ypIII | 74 (31·6) | 55 (24·1) | |
| ypIV | 5 (2·1) | 6 (2·6) | |
| ypx† | 11 (4·7) | 11 (4·8) | |
| Tumour category | < 0·001 | ||
| ypT0 | 5 (2·1) | 27 (11·8) | |
| ypT1 | 12 (5·1) | 27 (11·8) | |
| ypT2 | 74 (31·6) | 60 (26·3) | |
| ypT3‡ | |||
| ypT3ab | 88 (37·6) | 67 (29·4) | |
| ypT3cd | 41 (17·5) | 26 (11·4) | |
| ypT3x | 3 (1·3) | 1 (0·4) | |
| ypT4‡ | |||
| ypT4a | 1 (0·4) | 5 (2·2) | |
| ypT4b | 3 (1·3) | 3 (1·3) | |
| ypTx† | 7 (3·0) | 12 (5·3) | |
| Node category | 0·059 | ||
| ypN0 | 149 (63·7) | 163 (71·5) | |
| yp N1 | 52 (22·2) | 41 (18·0) | |
| ypN2 | 28 (12·0) | 19 (8·3) | |
| ypNx† | 5 (2·1) | 5 (2·2) | |
| Tumour regression* | < 0·001 | ||
| Grade 0 | 17 (7·3) | 15 (6·6) | |
| Grade 1 | 165 (70·5) | 104 (45·6) | |
| Grade 2 | 41 (17·5) | 64 (28·1) | |
| Grade 3 | 2 (0·9) | 11 (4·8) | |
| Grade 4 | 4 (1·7) | 23 (10·1) | |
| Grade x† | 5 (2·1) | 11 (4·8) | |
| Circumferential resection margin§ | n = 170 | n = 150 | 1·000# |
| Positive (≤ 1 mm) | 11 | 9 | |
| Negative (> 1 mm) | 159 | 141 |
Values in parentheses are percentages.
Dworak regression grading system. SRT, short‐course radiotherapy and immediate surgery; SRT‐delay, short‐course radiotherapy with surgery delayed 4–8 weeks.
Not included in statistical analysis;
subcategorization not used in statistical analysis;
142 patients with missing data excluded from analysis.
Mann–Whitney U test, except
Fisher's exact test.
Patients without single whole‐mount sections of the tumour were excluded from the analysis of CRM; 170 (72·6 per cent) of 234 patients were analysed in the SRT arm, and 150 (65·8 per cent) of 228 in the SRT‐delay arm (P = 0·499). CRM positivity and node status again did not differ significantly between the treatment arms. The median (range) total number of examined lymph nodes in the SRT and SRT‐delay groups were 11 (0–56) and 12 (0–39) respectively, and did not differ between the groups (P = 0·733).
Discussion
The present interim analysis comparing pathological outcomes between the two arms of short‐course RT in the randomized Stockholm III Trial showed that patients in the SRT‐delay group had a lower tumour stage (ypTNM stage), a lower ypT category, a higher rate of complete pathological response and a greater degree of tumour regression than patients in the SRT group. This effect is not seen with traditional short‐course RT with surgery within a week unless surgery is postponed23. Short‐course RT with a delay to surgery has been used outside trials for patients who were not suitable for CRT. Retrospective outcome analyses13, 14, 15 of these patients have indicated a downstaging and downsizing effect similar to that seen here.
In primary resectable rectal cancer, without an involved or threatened mesorectal fascia indicating the risk of a positive CRM, tumour regression per se is not an important endpoint after RT. However, more advanced cT3 lesions with a threatened margin, or cT4 tumours demonstrated on preoperative MRI24, may require tumour regression (downstaging) to allow radical surgery. Several studies have indicated a better prognosis in patients who have shown significant tumour regression after CRT, especially in those with a pathological complete response25, 26.
The proportion of pathological complete responses in the SRT‐delay arm in the present study is at the same level as those reported in studies on CRT9, 27, 28. The Stockholm III Trial will not answer whether the tumoricidal effect differs between short‐course RT and CRT, as chemotherapy is not included in the trial. However, two other medium‐sized randomized studies29, 30 did not find any difference in local recurrence rates after SRT or CRT, indicating that the effects on local control are similar. Final results from the Stockholm III Trial will probably give an answer to the relative cell kill effect of short‐course RT and LRT‐delay (50 Gy without concurrent chemotherapy). However, in this report comparison between short‐course RT and LRT‐delay was not feasible as some hospitals participating in the trial randomized only between SRT and SRT‐delay, but not LRT‐delay. Hence, too few patients had been included in the LRT‐delay arm to allow an interim analysis.
The retrospective reassessment of pathology is one limitation of this study. The standards and routines of specimen preparation, assessment and reporting have gradually improved over time31. During the early years of the trial some pathology laboratories had routines that might be considered substandard today. Owing to the lack of single whole‐mount sections or few regular slides from the tumour, the pathology could not always be reassessed adequately, and so data were missing for some patients, especially regarding the CRM. However, there was no difference in the proportion of missing CRM data between the randomization arms; the missing data contribute mainly to loss of power, but do not introduce selection bias. The reassessment of specimens by a single pathologist, blinded to the original pathology report, is the strength of this study. Thus, there was no introduction of information bias between the treatment arms in the partly subjective evaluation of tumour stage and regression grading.
With a difference in tumour stage, T category and tumour regression grade between the two groups, a difference in the positive CRM rate might also have been expected, although this was not observed. The proportion of involved CRMs in the reassessed specimen was low in both arms. The likely reason for this is that patients with locally advanced T3 tumours involving the mesorectal fascia or T4 tumours, judged with present terminology as unresectable, were not eligible and therefore not included. Downstaging per se is not important in primary resectable tumours. However, the downstaging effect of hypofractionated RT may be important in locally advanced unresectable tumours (often cT4). The use of SRT‐delay in these locally advanced tumours when the patient is not fit for CRT has been reported previously13, 14, 15.
The inclusion of some patients with early tumours in the present study is also illustrated by the fact that about 30 per cent of patients in the SRT arm had stage I disease. Early tumours, besides cancer in a polyp, were not an exclusion criterion in the trial protocol. However, there has been a gradual shift during the study towards exclusion of these tumours, especially in the upper and mid rectum, owing to the low risk of local recurrence after surgery alone32. In many hospitals there was a lack of appropriate pretreatment local tumour staging with MRI in the early years of the study, and the use of high‐standard MRI protocols in all hospitals has been achieved only recently. Pretreatment clinical staging was not recorded in the Swedish Rectal Cancer Register before 2008 and so there is insufficient information on pretreatment cT category and threatened or involved mesorectal fascia to assess whether there were any differences between the groups before RT. An ongoing analysis within the Stockholm III Trial of MRI images before and after RT (correlated with pathological outcome) will provide information on this matter.
Editor's comments

Acknowledgements
The authors thank H. Johansson (Department of Oncology and Pathology, Karolinska University Hospital, Stockholm) for help with statistical calculations, T. Singnomklao (Regional Cancer Centre, Stockholm) for help with the collection and validation of register data, and all members of the Stockholm Colorectal Cancer Study Group and hospitals outside the Stockholm/Gotland region for recruiting patients to the Stockholm III Trial and for providing support during the reassessment of pathological specimens: L. Blomqvist (Karolinska University Hospital, Stockholm); J. Dalén (St Göran's Hospital, Stockholm); L. Franzén (Medilab, Stockholm); M. Goldinger (St Göran's Hospital, Stockholm); M. Bragmark (Danderyds Hospital, Stockholm); G. Lindgren (Södertälje Hospital, Södertälje); N. Lundqvist (Norrtälje Hospital, Norrtälje); M. Machado (Ersta Hospital, Stockholm); Y. Raab (South Hospital, Stockholm); P. Nygren, Å. Berglund, L. Påhlman (University Hospital, Uppsala); A. Nihlberg (Falu Hospital, Falun); R. Heuman (Mora Hospital, Mora); G. Lindmark (Helsingborg Hospital, Helsingborg); I. Syk (Skåne University Hospital, Malmö); G. Ljung (Mälarsjukhuset Hospital, Eskilstuna); O. Hallböök, (Linköping University Hospital, Linköping); P. Loftås (Vrinnevi Hospital, Norrköping); P. Gustavsson (Visby Hospital, Visby).
The study was supported financially by the Swedish Research Council, the Swedish Cancer Society and the Stockholm Cancer Society. Financial support was also provided through the regional agreement on medical training and clinical research (ALF) between the Stockholm County Council and Karolinska Institute.
Disclosure: The authors declare no conflict of interest.
Presented in part to the European Society of Coloproctology, Vienna, Austria, September 2012
References
- 1. Peeters KC, Marijnen CA, Nagtegaal ID, Kranenbarg EK, Putter H, Wiggers T et al The TME trial after a median follow‐up of 6 years: increased local control but no survival benefit in irradiated patients with resectable rectal carcinoma. Ann Surg 2007; 246: 693–701. [DOI] [PubMed] [Google Scholar]
- 2. Kapiteijn E, Marijnen CA, Nagtegaal ID, Putter H, Steup WH, Wiggers T et al Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med 2001; 345: 638–646. [DOI] [PubMed] [Google Scholar]
- 3. Pahlman L, Glimelius B. Pre‐ or postoperative radiotherapy in rectal and rectosigmoid carcinoma. Report from a randomized multicenter trial. Ann Surg 1990; 211: 187–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Improved survival with preoperative radiotherapy in resectable rectal cancer. Swedish Rectal Cancer Trial [see comments]. N Engl J Med 1997; 336: 980–987 [DOI] [PubMed] [Google Scholar]; [published erratum appears in N Engl J Med 1997; 336: 1539]. [Google Scholar]
- 5. Martling A, Holm T, Johansson H, Rutqvist L, Cedermark B. The Stockholm II trial on preoperative radiotherapy in rectal carcinoma. Cancer 2001; 92: 896–902. [DOI] [PubMed] [Google Scholar]
- 6. Cedermark B, Johansson H, Rutqvist LE, Wilking N. The Stockholm I trial of preoperative short term radiotherapy in operable rectal carcinoma. A prospective randomized trial. Stockholm Colorectal Cancer Study Group. Cancer 1995; 75: 2269–2275. [DOI] [PubMed] [Google Scholar]
- 7. Colorectal Cancer Collaborative Group . Adjuvant radiotherapy for rectal cancer: a systematic overview of 8507 patients from 22 randomised trials. Lancet 2001; 358: 1291–1304. [DOI] [PubMed] [Google Scholar]
- 8. Glimelius B, Grönberg H, Järhult J, Wallgren A, Cavallin‐Ståhl E. A systematic overview of radiation therapy effects in rectal cancer. Acta Oncol 2003; 42: 476–492. [DOI] [PubMed] [Google Scholar]
- 9. Bujko K, Nowacki MP, Nasierowska‐Guttmejer A, Michalski W, Bebenek M, Pudelko M et al Sphincter preservation following preoperative radiotherapy for rectal cancer: report of a randomised trial comparing short‐term radiotherapy vs. conventionally fractionated radiochemotherapy. Radiother Oncol 2004; 72: 15–24. [DOI] [PubMed] [Google Scholar]
- 10. Glimelius B, Holm T, Blomqvist L. Chemotherapy in addition to preoperative radiotherapy in locally advanced rectal cancer – a systematic overview. Rev Recent Clin Trials 2008; 3: 204–211. [DOI] [PubMed] [Google Scholar]
- 11. Marijnen CA, Nagtegaal ID, Klein Kranenbarg E, Hermans J, van de Velde CJ, Leer JW et al No downstaging after short‐term preoperative radiotherapy in rectal cancer patients. J Clin Oncol 2001; 19: 1976–1984. [DOI] [PubMed] [Google Scholar]
- 12. Sebag‐Montefiore D, Stephens RJ, Steele R, Monson J, Grieve R, Khanna S et al Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC‐CTG C016): a multicentre, randomised trial. Lancet 2009; 373: 811–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hatfield P, Hingorani M, Radhakrishna G, Cooper R, Melcher A, Crellin A et al Short‐course radiotherapy, with elective delay prior to surgery, in patients with unresectable rectal cancer who have poor performance status or significant co‐morbidity. Radiother Oncol 2009; 92: 210–214. [DOI] [PubMed] [Google Scholar]
- 14. Radu C, Berglund A, Pâhlman L, Glimelius B. Short‐course preoperative radiotherapy with delayed surgery in rectal cancer – a retrospective study. Radiother Oncol 2008; 87: 343–349. [DOI] [PubMed] [Google Scholar]
- 15. Pettersson D, Holm T, Iversen H, Blomqvist L, Glimelius B, Martling A. Preoperative short‐course radiotherapy with delayed surgery in primary rectal cancer. Br J Surg 2012; 99: 577–583. [DOI] [PubMed] [Google Scholar]
- 16. Pach R, Kulig J, Richter P, Gach T, Szura M, Kowalska T. Randomized clinical trial on preoperative radiotherapy 25 Gy in rectal cancer – treatment results at 5‐year follow‐up. Langenbecks Arch Surg 2012; 397: 801–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pettersson D, Cedermark B, Holm T, Radu C, Pâhlman L, Glimelius B et al Interim analysis of the Stockholm III trial of preoperative radiotherapy regimens for rectal cancer. Br J Surg 2010; 97: 580–587. [DOI] [PubMed] [Google Scholar]
- 18. Fokstuen T, Holm T, Glimelius B. Postoperative morbidity and mortality in relation to leukocyte counts and time to surgery after short‐course preoperative radiotherapy for rectal cancer. Radiother Oncol 2009; 93: 293–297. [DOI] [PubMed] [Google Scholar]
- 19. Jörgren F, Johansson R, Damber L, Lindmark G. Validity of the Swedish Rectal Cancer Registry for patients treated with major abdominal surgery between 1995 and 1997. Acta Oncol 2013; 52: 1707–1714. [DOI] [PubMed] [Google Scholar]
- 20. Sobin LH, Wittekind C. International Union Against Cancer TNM Classification of Malignant Tumours (6th edn). John Wiley & Sons: New York, 2002. [Google Scholar]
- 21. Greene FL, Flemming ID, Fritz A, Balch CM, Haller DG, Morrow M. (eds). AJCC Cancer Staging Manual (6th edn). Springer: New York, 2002. [Google Scholar]
- 22. Dworak O, Keilholz L, Hoffmann A. Pathological features of rectal cancer after preoperative radiochemotherapy. Int J Colorectal Dis 1997; 12: 19–23. [DOI] [PubMed] [Google Scholar]
- 23. Graf W, Dahlberg M, Osman MM, Holmberg L, Pahlman L, Glimelius B. Short‐term preoperative radiotherapy results in down‐staging of rectal cancer: a study of 1316 patients. Radiother Oncol 1997; 43: 133–137. [DOI] [PubMed] [Google Scholar]
- 24. Glimelius B, Beets‐Tan R, Blomqvist L, Brown G, Nagtegaal I, Pahlman L et al Mesorectal fascia instead of circumferential resection margin in preoperative staging of rectal cancer. J Clin Oncol 2011; 29: 2142–2143. [DOI] [PubMed] [Google Scholar]
- 25. Maas M, Nelemans PJ, Valentini V, Das P, Rödel C, Kuo L‐J et al Long‐term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol 2010; 11: 835–844. [DOI] [PubMed] [Google Scholar]
- 26. Rödel C, Martus P, Papadoupolos T, Füzesi L, Klimpfinger M, Fietkau R et al Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J Clin Oncol 2005; 23: 8688–8696. [DOI] [PubMed] [Google Scholar]
- 27. Gerard JP, Conroy T, Bonnetain F, Bouche O, Chapet O, Closon‐Dejardin MT et al Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3–4 rectal cancers: results of FFCD 9203. J Clin Oncol 2006; 24: 4620–4625. [DOI] [PubMed] [Google Scholar]
- 28. Bosset J‐F, Calais G, Mineur L, Maingon P, Stojanovic‐Rundic S, Bensadoun R‐J et al Fluorouracil‐based adjuvant chemotherapy after preoperative chemoradiotherapy in rectal cancer: long‐term results of the EORTC 22921 randomised study. Lancet Oncol 2014;15: 184–190. [DOI] [PubMed] [Google Scholar]
- 29. Bujko K, Nowacki MP, Nasierowska‐Guttmejer A, Michalski W, Bebenek M, Kryj M. Long‐term results of a randomized trial comparing preoperative short‐course radiotherapy with preoperative conventionally fractionated chemoradiation for rectal cancer. Br J Surg 2006; 93: 1215–1223. [DOI] [PubMed] [Google Scholar]
- 30. Bujko K, Bujko M. Point: short‐course radiation therapy is preferable in the neoadjuvant treatment of rectal cancer. Semin Radiat Oncol 2011; 21: 220–227. [DOI] [PubMed] [Google Scholar]
- 31. Valentini V, Aristei C, Glimelius B, Minsky BD, Beets‐Tan R, Borras JM et al Multidisciplinary Rectal Cancer Management: 2nd European Rectal Cancer Consensus Conference (EURECA‐CC2). Radiother Oncol 2009; 92: 148–163. [DOI] [PubMed] [Google Scholar]
- 32. Glimelius B, Tiret E, Cervantes A, Arnold D; ESMO Guidelines Working Group . Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2013; 24(Suppl 6): vi81–vi88. [DOI] [PubMed] [Google Scholar]


