Abstract
Objectives
Age dependency of acute ischaemic stroke aetiology and vascular risk factors have not been adequately evaluated in stroke patients in Norway. Aims of this study were to evaluate how stroke subtypes and vascular risk factors vary with age in a western Norway stroke population.
Materials and methods
Patients aged 15–100 years consecutively admitted to our neurovascular centre with acute ischaemic stroke between 2006 and 2012 were included. The study population was categorized as young (15–49 years), middle‐aged (50–74 years) or elderly (≥75 years). Stroke aetiology was defined by TOAST criteria. Risk factors and history of cardiovascular disease were recorded.
Results
In total, 2484 patients with acute cerebral infarction were included: 1418 were males (57.3%). Mean age was 70.8 years (SD ± 14.9), 228 patients were young, 1126 middle‐aged, and 1130 were elderly. The proportion of large‐artery atherosclerosis and of small‐vessel occlusion was highest among middle‐aged patients. The proportion of cardioembolism was high at all ages, especially among the elderly. The proportion of stroke of other determined cause was highest among young patients. Some risk factors (diabetes mellitus, active smoking, angina pectoris, prior stroke and peripheral artery disease) decreased among the elderly. The proportions of several potential causes increased with age.
Conclusion
The proportion of stroke subtypes and vascular risk factors are age dependent. Age 50–74 years constitutes the period in life where cardiovascular risk factors become manifest and stroke subtypes change.
Keywords: age, cardiovascular disease, ischaemic stroke, stroke subtypes, stroke aetiology, trial of org 10172 in acute stroke treatment, vascular risk factors
Introduction
The aetiology of stroke is influenced by sex, race and age, as well as by cultural and geographic factors 1, 2, 3, 4, 5, 6. Stroke subtypes are commonly categorized as large‐artery atherosclerosis (LAA), cardioembolism (CE), small‐vessel occlusion (SVO), stroke of other determined cause (SOC) and stroke of undetermined cause (SUC) (TOAST criteria) 7. Pathogenesis, prognosis and treatment vary among these subtypes, and evaluating vascular risk factors within each subtype may improve acute stroke treatment and secondary prevention 8.
Vascular risk factors may be classified as non‐modifiable (e.g. age, sex, race, genetics), modifiable and well documented (e.g. cardiovascular disease, hypertension, diabetes mellitus, dyslipidaemia, atrial fibrillation, smoking), or potentially modifiable and less well documented (e.g. metabolic syndrome, hyperhomocysteinemia, migraine, malignancy) 9, 10. Risk factor profiles change with increasing age, as does the risk factor burden 11, 12. In Norway, recent studies have revealed a rather high‐risk factor burden in the general population 13.
Adequate data on age dependency of acute ischaemic stroke aetiology and the prevalence of vascular risk factors are lacking in Norway. The aim of this study was to evaluate how ischaemic stroke subtypes and vascular risk factors vary with age in a well‐defined western Norway ischaemic stroke population.
Materials and methods
Ischaemic stroke population and aetiology
The presented study is based on data from 2484 consecutive ischaemic stroke patients aged 15–100 years and admitted to Bergen Centre for neurovascular diseases between 2006 and 2012. All patients were prospectively registered in the Bergen NORSTROKE Registry. The study population was categorized as young (15–49 years), middle‐aged (50–74 years) or elderly (≥75 years). The index stroke was documented by magnetic resonance imaging (MRI) or computed tomography (CT) confirming a lesion related to the clinical symptoms. Routine blood samples were collected on admission. Stroke aetiology was classified by an experienced stroke neurologist (HN) according to TOAST criteria 7. The proportion of different stroke aetiologies stratified by 10 years of age intervals is shown in Figs 1 and 2.
Figure 1.
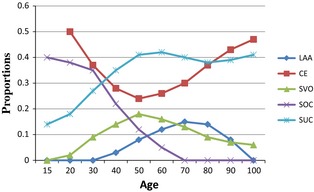
Proportions of TOAST subtypes stratified by age group. TOAST, Trial of Org 10172 in Acute Stroke Treatment; LAA, large‐artery atherosclerosis; CE, cardioembolism; SVO, small‐vessel occlusion; SOC, stroke of other determined cause; SUC, stroke of undetermined cause.
Figure 2.
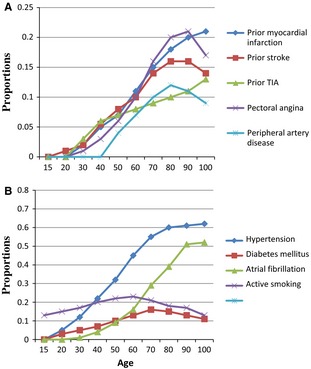
Proportions of prior cardiovascular disease (A) and vascular risk factors (B) stratified by age group. TIA, transitory ischaemic attack.
Vascular risk factors
Hypertension, diabetes mellitus, paroxysmal or chronic atrial fibrillation were considered present if diagnosed and/or treated prior to the index stroke or during the hospital stay (fasting plasma glucose >7.7 mmol/l; at least one ECG documenting atrial fibrillation). Current smoking was defined as smoking one or more cigarettes per day. A history of prior stroke, transitory ischaemic attack (TIA), myocardial infarction, pectoral angina or peripheral artery disease was considered present if diagnosed by a physician any time prior to index stroke onset.
The study was approved by the local ethics committee (REK Vest).
Statistics
STATA 13.1 (Statacorp 4905 Lakeway Drive, College Station, TX, USA) was used for analysis. Chi‐square test, Student's t‐test, Spearman's correlation and Mann–Whitney U‐test were used when appropriate. The proportion of TOAST subtypes was displayed by means of the Lowess function. Figures showing the proportion of prior cardiovascular disease (Fig. 2A) and vascular risk factors (Fig. 2B) stratified by age decades were made based on Lowess analyses.
Results
In total, 2484 patients with acute ischaemic stroke were included. Of these, 1418 were males (57.3%). The mean age was 70.8 years (SD ± 14.9), 228 patients were young, 1126 were middle‐aged, and 1130 were elderly. Socio‐demographic characteristics, CT and MRI imaging stratified by age group are given in Table 1. TOAST classification stratified by age group is shown in Fig. 1. In the total study population, SUC was the most frequent stroke subtype (40.2%), and CE (32.5%) dominated the determined causes (Table 2). Including only patients that underwent full assessment with CT, MRI, ECG, echocardiography and ultrasound of the neck vessels (in total 32% of the patients) showed similar curves as in Fig. 1, except that of the frequency in LAA in patients >80 years old was not so steep and the frequency of SUC decreased in the elderly.
Table 1.
Socio‐demographic characteristics and imaging stratified by age group
| 15–49 years n = 228 | 50–74 years n = 1126 | ≥75 years n = 1130 | P | |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | P a | |
| Socio‐demographics | ||||
| Females | 76 (33.3) | 362 (32.1) | 618 (54.7) | <0.001 |
| Married | 147 (64.5) | 808 (71.8) | 452 (40) | <0.001 |
| Employed | 183 (80.3) | 431 (38.3) | 20 (1.7) | <0.001 |
| Imaging | ||||
| Computed tomography | 178 (78.07) | 932 (82.77) | 1056 (93.45) | |
| Magnetic resonance imaging | 214 (93.86) | 1013 (90) | 815 (72.12) | |
Chi‐square test.
Table 2.
Vascular risk factors, history of cardiovascular disease and stroke subtypes stratified by age group
| 15–49 years n = 228 | 50–74 years n = 1126 | ≥75 years n = 1130 | P a | |
|---|---|---|---|---|
| Vascular risk factors | ||||
| Hypertension | 53 (23.2) | 550 (48.9) | 696 (61.6) | <0.001 |
| Diabetes mellitus | 16 (7.0) | 161 (14.3) | 173 (15.3) | 0.004 |
| Atrial fibrillation | 9 (3.9) | 203 (18.0) | 487 (43.1) | <0.001 |
| Active smoking | 81 (35.5) | 406 (36.1) | 117 (10.3) | <0.001 |
| History of CVD | ||||
| Prior stroke | 10 (4.4) | 148 (13.1) | 187 (16.5) | <0.001 |
| Prior TIA | 9 (3.9) | 75 (6.7) | 105 (9.3) | 0.005 |
| Myocardial infarction | 8 (3.5) | 126 (11.2) | 215 (19.0) | <0.001 |
| Pectoral angina | 6 (2.6) | 102 (9.1) | 204 (18.0) | <0.001 |
| Peripheral artery disease | 3 (1.3) | 81 (7.2) | 83 (7.4) | 0.002 |
| Stroke subtype (TOAST) | ||||
| Large‐artery atherosclerosis | 7 (3.1) | 159 (14.1) | 135 (11.9) | <0.001 |
| Cardioembolism | 65 (28.5) | 280 (24.9) | 460 (40.7) | <0.001 |
| Small‐vessel occlusion | 32 (14.0) | 181 (16.1) | 93 (8.2) | <0.001 |
| Stroke of other determined cause | 44 (19.3) | 21 (1.9) | 3 (0.2) | <0.001 |
| Stroke of undetermined cause | 80 (35.1) | 485 (43.1) | 429 (37.9) | 0.02 |
TIA, transient ischaemic attack; CVD, cardiovascular disease; TOAST, Trial of Org 10172 in Acute Stroke Treatment 1.
Chi‐square test.
Figure 2 and Table 3 show the proportion of TOAST subtypes in accordance with age. LAA increased among middle‐aged patients and declined among the elderly, with a peak proportion between 70 and 80 years. CE was a frequent cause at all ages and showed u‐shaped correlation with decrease among the young, an increase among the middle‐aged and among elderly patients (Table 3). The lowest proportion of CE was between 50 and 60 years. The SVO proportion peaked around 50 years and decreased thereafter both among mid‐aged and elderly patients (Table 3). The SOC proportion was highest among the youngest patients with a peak proportion between 20 and 30 years and declined in the middle‐aged. The SUC proportion increased among the young, peaked between age 50 and 60, and kept a steady proportion among the elderly (Table 3). The proportion of several potential causes in SUC increased with age, from 1% in the young, 9% in the middle‐aged to 20% in the elderly (correlation factor 0.17, P < 0.001).
Table 3.
Spearman's correlation between proportions of risk factors and increasing age within three different age groups
| 15–49 years | P | 50–74 years | P | ≥75 years | P | |
|---|---|---|---|---|---|---|
| Risk factors | ||||||
| Hypertension | 0.19 | 0.005 | 0.16 | <0.001 | −0.01 | 0.60 |
| Diabetes mellitus | 0.11 | 0.09 | 0.07 | 0.01 | −0.06 | 0.03 |
| Atrial fibrillation | 0.02 | 0.71 | 0.12 | <0.001 | 0.02 | 0.37 |
| Active smoking | 0.14 | 0.03 | −0.12 | <0.001 | −0.21 | <0.001 |
| History of CVD | ||||||
| Prior stroke | −0.05 | 0.41 | 0.07 | 0.01 | 0.007 | 0.79 |
| Prior TIA | 0.06 | 0.32 | 0.06 | 0.04 | 0.02 | 0.40 |
| Myocardial infarction | 0.05 | 0.38 | 0.07 | 0.01 | 0.03 | 0.31 |
| Pectoral angina | 0.06 | 0.31 | 0.11 | <0.001 | 0.05 | 0.05 |
| Peripheral artery disease | 0.03 | 0.64 | 0.12 | <0.001 | −0.03 | 0.29 |
| Stroke subtype (TOAST) | ||||||
| Large‐artery atherosclerosis | 0.08 | 0.20 | 0.12 | <0.001 | −0.13 | <0.001 |
| Cardioembolism | −0.26 | <0.001 | 0.08 | 0.004 | 0.09 | <0.001 |
| Small‐vessel occlusion | 0.12 | 0.06 | −0.11 | <0.001 | −0.06 | 0.02 |
| Stroke of other determined cause | −0.10 | 0.12 | −0.14 | <0.001 | 0.0007 | 0.10 |
| Stroke of undetermined cause | 0.21 | <0.001 | −0.03 | 0.21 | 0.03 | 0.26 |
TIA, transient ischaemic attack; CVD, cardiovascular disease; TOAST, Trial of Org 10172 in Acute Stroke Treatment 1.
The proportions of prior cardiovascular disease according to age are shown in Fig. 2A and Table 3. The proportions of previous stroke, TIA, myocardial infarction, pectoral angina and peripheral artery disease consistently increased with age, predominantly in the middle‐aged group (Table 3).
The proportion of vascular risk factors according to age is shown in Fig. 2B and Table 3. The proportion of hypertension increased consistently with age among young and middle‐aged patients. Diabetes mellitus increased in the mid‐age group and decreased in the elderly group, peaking between 70 and 80 years. The proportion of atrial fibrillation increased consistently with age. The proportion of active smoking increased with age among the young, and decreased among the middle‐aged and elderly, with a peak proportion between 50 and 60 years.
Discussion
Our data demonstrate the correlation between age and different aetiologies and risk factors of ischaemic stroke for a well‐defined western Norway population.
Cardioembolism was the most frequent determined cause of stroke in all age groups. This constellation may be explained by high rates of cardiac disorders with low or uncertain risk, such as patent foramen ovale, among young patients 5, 19, 20, whereas high‐risk disorders, such as atrial fibrillation, increased dramatically with age 19, 20. The present data support this, as we identified AF as an important risk factor from the 4th decade, thereafter consistently increasing throughout the 10th decade. However, our data may be biased by diagnostics focusing mostly on cardiac arrhythmias among older patients, whereas investigation for right–left shunt with transesophageal echocardiography and neurosonographic bubble test was mostly performed among younger patients.
Stroke of other determined cause, predominantly cervical artery dissections 19, 21, has been described as the second most frequent cause of stroke in the young. In our study, the proportion of this subtype declined steeply during mid‐age and was non‐existent among the elderly. Other young stroke studies also support a decline of stroke of other determined cause towards mid‐age, although their rates declined more gently than in our study 5, 17.
Small‐vessel occlusion followed an inversed u‐shaped curve with a peak in the 6th decade. This is in line with recent studies on young and middle‐aged patients 5, 17, while others have observed a further increase in small‐vessel occlusion at older ages 22. Large‐artery atherosclerosis followed a similar time course as small‐vessel occlusions. This corresponds to that of other stroke populations 18, 22. However, while some studies have observed higher proportions of large‐artery atherosclerosis earlier in life 23, 24, our population shows that large‐artery atherosclerosis appears in the 3rd decade, but was not a common finding before the 5th decade and thereafter increased until the 8th decade before decreasing again.
Vascular risk factors influence both small‐vessel occlusion and large‐artery atherosclerosis 25, 26. In our study population, all risk factors (except smoking and diabetes mellitus) increased consistently throughout the lifetime, but most distinctly among middle‐aged patients. Risk factors increasing with age have been reported previously 10, 11, 27. We found hypertension as the most frequent risk factor at all ages. Hypertension plays a particular role in small‐vessel occlusion 25 and is also frequently present in large‐artery atherosclerosis 18, 22. There are some indications that large‐artery atherosclerosis and small‐vessel occlusion are coexisting subtypes of stroke 26, supporting the partly atherosclerotic aetiology of small‐vessel occlusion. However, small‐vessel occlusion is an imprecise subtype, which besides occlusions due to small artery microatheroma, lipohyalinosis and fibrinoid necrosis, most likely also includes misinterpreted occlusions due to embolic sources 28, 29, 30, 31, 32.
Large‐artery atherosclerosis is classified according to rather rigid TOAST criteria, which requires occlusion or ≥50% stenosis in a related artery 7, not paying attention to atherosclerotic plaque instability which may lead to arterio‐arterial embolism even from stenosis of low or moderate degree 33, 34. Hence, true large‐artery atherosclerosis is in general likely to be underestimated due to an arbitrary TOAST definition.
In our total study population, stroke of undetermined cause was the most frequent subtype. This illustrates a well‐known limitation of the TOAST classification, as the stroke of undetermined cause category not only includes cases of truly unknown cause, but also cases with several potential causes 14, 15. Stroke of undetermined cause comprised the largest subtype among young and middle‐aged patients, which is in accordance with other studies 16, 17, whereas stroke of undetermined cause was outnumbered by cardioembolism among the elderly 18. The lowest proportion of stroke of undetermined cause was found among those younger than 30 years.
The clarification of stroke aetiology has an impact on optimal acute treatment, as well as optimal secondary prevention 8, 35, and thus comprises a crucial part of stroke workup. Our data show that ischaemic stroke entities change depending on age in western Norway, and stroke investigation needs accordingly to be focused in different directions during different periods of life.
The proportions of some risk factors (diabetes mellitus, active smoking, angina pectoris, prior stroke and peripheral artery disease) decreased among the elderly. One possible explanation is that many patients with these risk factors died before reaching old age.
The total number of patients in the mid‐age and elderly group was approximately the same, but the proportions of cardiovascular disease and vascular risk factors tended to decrease with increasing age among the elderly as opposed to the middle‐aged. This indicates that there may be important differences as to the underlying pathophysiological mechanisms between these age groups.
The strength of this study is a relatively large study population, investigated in a single stroke centre according to a predefined protocol. True stroke subtype classification is negatively affected by the TOAST classification's own limitations, such as oversizing the stroke of undetermined cause subgroup and undersizing the large‐artery atherosclerosis subgroup. Another limitation is that TOE and neurosonographic bubble test were mainly performed among patients younger than 60 years, which may have introduced some bias by overlooking right–left shunts in the elderly. Also, elderly patients were examined with MRA in only 72% of the patients and some dissections, albeit rare, may have been missed. In stroke of other determined cause, we may have failed to look for some rare causes. This bias is likely small because of the rare occurrence of diseases such as Fabry's disease.
Conclusions
This study demonstrates the close correlation between age and ischaemic stroke subtypes as well as vascular risk factors. Mid‐age constitutes the period of life where risk factors become manifest and the proportions of risk factors and stroke subtypes profoundly change among ischaemic stroke patients in western Norway.
Authors' contributions
AN and AF contributed equally for this manuscript and both are first authors. HN and AN are responsible for the idea and design of the study. The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors and were fully responsible for all content and editorial decisions. All authors critically reviewed the manuscript and approved the submitted version.
Conflict of interest and sources of funding
The authors declare no conflict of interests or sources of funding.
Acknowledgments
The authors thank research nurse Maren Inselseth for her excellent work and assistance with data registration.
Nacu A, Fromm A, Sand KM, Waje‐Andreassen U, Thomassen L, Naess H. Age dependency of ischaemic stroke subtypes and vascular risk factors in western Norway: the Bergen Norwegian Stroke Cooperation Study. Acta Neurol Scand 2016: 133: 202–207. © 2015 The Authors. Acta Neurologica Scandinavica Published by John Wiley & Sons Ltd.
The copyright line for this article was changed on 1 December 2015 after original online publication.
References
- 1. Gutierrez J, Koch S, Dong C et al. Racial and ethnic disparities in stroke subtypes: a multiethnic sample of patients with stroke. Neurol Sci 2014;35:577–82. [DOI] [PubMed] [Google Scholar]
- 2. Carod‐Artal FJ, Casanova Lanchipa JO, Cruz Ramirez LM et al. Stroke subtypes and comorbidity among ischemic stroke patients in Brasilia and Cuenca: a Brazilian‐Spanish cross‐cultural study. J Stroke Cerebrovasc Dis 2014;23:140–7. [DOI] [PubMed] [Google Scholar]
- 3. Palm F, Urbanek C, Wolf J et al. Etiology, risk factors and sex differences in ischemic stroke in the Ludwigshafen Stroke Study, a population‐based stroke registry. Cerebrovasc Dis 2012;33:69–75. [DOI] [PubMed] [Google Scholar]
- 4. Rosamond WD, Folsom AR, Chambless LE et al. Stroke incidence and survival among middle‐aged adults: 9‐year follow‐up of the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke 1999;30:736–43. [DOI] [PubMed] [Google Scholar]
- 5. Yesilot Barlas N, Putaala J et al. Etiology of first‐ever ischaemic stroke in European young adults: the 15 cities young stroke study. Eur J Neurol 2013;20:1431–9. [DOI] [PubMed] [Google Scholar]
- 6. European Registers of Stroke (EROS) Investigators , Heuschmann PU, Di Carlo A et al. Incidence of stroke in Europe at the beginning of the 21st century. Stroke 2009;40:1557–63. [DOI] [PubMed] [Google Scholar]
- 7. Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE 3rd. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993;24:35–41. [DOI] [PubMed] [Google Scholar]
- 8. Ohira T, Shahar E, Chambless LE, Rosamond WD, Mosley TH Jr, Folsom AR. Risk factors for ischemic stroke subtypes: the Atherosclerosis Risk in Communities study. Stroke 2006;37:2493–8. [DOI] [PubMed] [Google Scholar]
- 9. Goldstein LB, Bushnell CD, Adams RJ et al. Guidelines for the primary prevention of stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011;42:517–84. [DOI] [PubMed] [Google Scholar]
- 10. Von Sarnowski B, Putaala J, Grittner U et al. Lifestyle risk factors for ischemic stroke and transient ischemic attack in young adults in the Stroke in Young Fabry Patients study. Stroke 2013;44:119–25. [DOI] [PubMed] [Google Scholar]
- 11. Berry JD, Dyer A, Cai X et al. Lifetime risks of cardiovascular disease. N Engl J Med 2012;366:321–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Anderden KK, Andersen ZJ, Olsen TS. Age‐ and gender‐specific prevalence of cardiovascular risk factors in 40,102 patients with first‐ever ischemic stroke: a Nationwide Danish Study. Stroke 2010;41:2768–74. [DOI] [PubMed] [Google Scholar]
- 13. Jenum AK, Graff‐Iversen S, Selmer R, Sogaard AJ. Risk factors for cardiovascular disease and diabetes through three decades. Tidsskr Nor Laegeforen 2007;127:2532–6. [PubMed] [Google Scholar]
- 14. Ay H. Advances in the diagnosis of etiologic subtypes of ischemic stroke. Curr Neurol Neurosci Rep 2010;10:14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Amarenco P, Bogousslavsky J, Caplan LR, Donnan GA, Hennerici MG. Classification of stroke subtypes. Cerebrovasc Dis 2009;27:493–501. [DOI] [PubMed] [Google Scholar]
- 16. Putaala J, Metso AJ, Metso TM et al. Analysis of 1008 consecutive patients aged 15 to 49 with first‐ever ischemic stroke: the Helsinki young stroke registry. Stroke 2009;40:1195–203. [DOI] [PubMed] [Google Scholar]
- 17. Rolfs A, Fazekas F, Grittner U et al. Acute cerebrovascular disease in the young: the Stroke in Young Fabry Patients study. Stroke 2013;44:340–9. [DOI] [PubMed] [Google Scholar]
- 18. Grau AJ, Weimar C, Buggle F et al. Risk factors, outcome, and treatment in subtypes of ischemic stroke: the German stroke data bank. Stroke 2001;32:2559–66. [DOI] [PubMed] [Google Scholar]
- 19. Fromm A, Waje‐Andreassen U, Thomassen L, Naess H. Comparison between ischemic stroke patients < 50 years and ≥50 years admitted to a single centre: the Bergen Stroke study. Stroke Res Treat 2011;2011:183256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cotter PE, Belham M, Martin PJ. Stroke in younger patients: the heart of the matter. J Neurol 2010;257:1777–87. [DOI] [PubMed] [Google Scholar]
- 21. Debette S, Grond‐Ginsbach C, Bodenant M et al. Differential features of carotid and vertebral artery dissections: the CADISP study. Neurology 2011;77:1174–81. [DOI] [PubMed] [Google Scholar]
- 22. Kolominsky‐Rabas PL, Weber M, Gefeller O, Neundoerfer B, Heuschmann PU. Epidemiology of ischemic stroke subtypes according to TOAST criteria: incidence, recurrence, and long‐term survival in ischemic stroke subtypes: a population‐based study. Stroke 2001;32:2735–40. [DOI] [PubMed] [Google Scholar]
- 23. Varona JF, Guerra JM, Bermejo F, Molina JA, Gomez de LA, Camara A. Causes of ischemic stroke in young adults, and evolution of the etiological diagnosis over the long term. Eur Neurol 2007;57:212–8. [DOI] [PubMed] [Google Scholar]
- 24. Cerrato P, Grasso M, Imperiale D et al. Stroke in young patients: etiopathogenesis and risk factors in different age classes. Cerebrovasc Dis 2004;18:154–9. [DOI] [PubMed] [Google Scholar]
- 25. Arboix A, Marti‐Vilalta JL. Lacunar stroke. Expert Rev Neurother 2009;9:179–96. [DOI] [PubMed] [Google Scholar]
- 26. Chatzikonstantinou A, Krissak R, Schaefer A, Schoenberg SO, Fink C, Hennerici MG. Coexisting large and small vessel disease in patients with ischemic stroke of undetermined cause. Eur Neurol 2012;68:162–5. [DOI] [PubMed] [Google Scholar]
- 27. Fromm A, Haaland OA, Naess H, Thomassen L, Waje‐andreassen U. Risk factors and their impact on carotid intima‐media thickness in young and middle‐aged ischemic stroke patients and controls: the Norwegian Stroke in the Young Study. BMC Res Notes 2014;7:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Barnett HJM, Stein BM, Mohr JP, Yatsu FM, eds. Stroke: pathophysiology, diagnosis and management. New York, NY: Churchill Livingstone, 1986: 377–450. [Google Scholar]
- 29. Futrell N. Lacunar infarction: embolism is the key. Stroke 2004;35:1778–9. [DOI] [PubMed] [Google Scholar]
- 30. Norrving B. Lacunar infarction: embolism is the key: against. Stroke 2004;35:1779–80. [DOI] [PubMed] [Google Scholar]
- 31. Norrving B. Lacunar infarcts: no black holes in the brain are benign. Pract Neurol 2008;8:222–8. [DOI] [PubMed] [Google Scholar]
- 32. Ay H, Oliveira‐Filho J, Buonanno FS et al. Diffusion‐weighted imaging identifies a subset of lacunar infarction associated with embolic source. Stroke 1999;30:2644–50. [DOI] [PubMed] [Google Scholar]
- 33. Rothwell PM, Eliasziw M, Gutnikov SA et al. Analysis of pooled data from the randomised controlled trials of endarterectomy for symptomatic carotid stenosis. Lancet 2003;361:107–16. [DOI] [PubMed] [Google Scholar]
- 34. Mathiesen EB, Bonaa KH, Joakimsen O. Echolucent plaques are associated with high risk of ischemic cerebrovascular events in carotid stenosis: the tromso study. Circulation 2001;103:2171–5. [DOI] [PubMed] [Google Scholar]
- 35. Kirshner HS. Differentiating ischemic stroke subtypes: risk factors and secondary prevention. J Neurol Sci 2009;279:1–8. [DOI] [PubMed] [Google Scholar]


