Summary
Acoustic emission (AE) analysis allows nondestructive monitoring of embolism formation in plant xylem, but signal interpretation and agreement of acoustically measured hydraulic vulnerability with reference hydraulic techniques remain under debate.
We compared the hydraulic vulnerability of 16 species and three crop tree cultivars using hydraulic flow measurements and acoustic emission monitoring, proposing the use of time‐dependent AE rates as a novel parameter for AE analysis.
There was a linear correlation between the water potential (Ψ) at 50% loss of hydraulic conductivity (P50) and the Ψ at maximum AE activity (Pmaxrate), where species with lower P50 also had lower Pmaxrate (P < 0.001, R 2 = 0.76).
Using AE rates instead of cumulative counts for AE analysis allows more efficient estimation of P50, while excluding problematic AE at late stages of dehydration.
Keywords: acoustic activity, acoustic emission (AE), drought stress, hydraulic vulnerability, plant–water relations, xylem
Introduction
Acoustic emissions (AE) have long been used to detect cavitation (Milburn & Johnson, 1966) and estimate hydraulic vulnerability to drought or freezing stress (e.g. Tyree et al., 1984; Tyree & Sperry, 1989; Hacke et al., 2000; Mayr et al., 2007; Mayr & Sperry, 2010; Cochard et al., 2013; Charrier et al., 2014). Cavitating xylem conduits undergo rapid tension release when water vapour replaces water under negative pressure, which produces AE at both audible and ultrasonic frequencies (Milburn & Johnson, 1966; Tyree & Dixon, 1983; Ritman & Milburn, 1988). Modern measurement systems allow automated monitoring of AE in the high‐frequency range and analysis of multiple parameters, such as amplitude, energy and waveform features, but additional signals from nonconducting sources make AE analysis more complex (Tyree & Dixon, 1986; Ritman & Milburn, 1988; Cochard & Tyree, 1990; Hacke & Sauter, 1996; Kikuta, 2003; Mayr & Rosner, 2011; Wolkerstorfer et al., 2012).
In some species, especially conifers, quantitative analysis of cumulative acoustic emissions as an indirect measure of drought‐induced embolism was well correlated with hydraulically measured conductivity loss (e.g. Lo Gullo & Salleo, 1993; Hacke & Sauter, 1995; Hacke et al., 2000; Johnson et al., 2012), and the acoustic energy released during cavitation reflected conduit dimensions and the intensity of drought stress (Mayr & Rosner, 2011; Ponomarenko et al., 2014). However, other species, including many angiosperms, have revealed varying offsets between hydraulically and acoustically analysed hydraulic failure (e.g. Tyree & Dixon, 1986; Jackson & Grace, 1996; Nardini et al., 2001; Manoharan & Pammenter, 2005; Johnson et al., 2009). A common phenomenon among this second group of species is the fact that acoustic activity does not cease when samples are fully embolized (e.g. Tyree & Dixon, 1986; Hacke & Sauter, 1996; Kowalski & Smoczkiewicz, 2004; Wolkerstorfer et al., 2012; Rosner, 2015). Possible causes for these additional signals may be emissions from nonconducting cells or tissues such as sclerenchyma (Kikuta & Richter, 2003) or bark (Kikuta, 2003). Cavitating fibres (Tyree & Dixon, 1986; Jackson & Grace, 1996), ray cells (Tyree & Sperry, 1989) or shrinkage processes and related microfracturing of wood have also been reported to produce AE (Kikuta & Richter, 2003; Kowalski & Smoczkiewicz, 2004; Wolkerstorfer et al., 2012; Vergeynst et al., 2014b).
Recent improvements in AE analysis were made using acoustic parameters such as absolute signal energy (e.g. Rosner et al., 2006; Johnson et al., 2009; Mayr & Rosner, 2011; Wolkerstorfer et al., 2012; Vergeynst et al., 2014a). Other authors have manually set arbitrary end points for AE recording (Hacke & Sauter, 1996; Kikuta et al., 1997; Salleo et al., 2001). However, signal interpretation and analysis remain challenging, and thus the use of acoustic methods for hydraulic vulnerability analyses has remained limited. Meanwhile, the accuracy of hydraulic methods, including the benchtop dehydration (Sperry et al., 1988) and centrifuge techniques (Cochard, 2002), is also currently under debate (Jansen et al., 2015), as measurement artefacts were shown for both methods, especially with long‐vesselled species (e.g. Beikircher et al., 2010; Cochard et al., 2010, 2013; Wheeler et al., 2013; Torres‐Ruiz et al., 2015).
In this study, we focused on the use of acoustic activity as a function of time, rather than absolute cumulative emission counts. We hypothesized that the highest acoustic activity should occur near the steepest part of a typical vulnerability curve, that is, near its inflection point (P50), when most embolism is forming within a narrow range of water potential (Ψ). Therefore, the Ψ at maximum AE activity should be correlated to a species’ hydraulically measured P50.
Materials and Methods
The study was conducted on a total of 16 woody species. The dataset includes both published (Beikircher et al., 2013) and unpublished hydraulic data, covering five temperate tree species, six shrub species from a dry temperate inner‐alpine forest, three high‐yield apple cultivars, three tropical rainforest species, and one species of woody vine (Table 1). Hydraulic reference measurements and AE testing were performed on plant material from the same populations or, when possible, from the same plants.
Table 1.
Study species and growth type, sampling location, hydraulic method and plant part used, and data source
| Species | Growth type | Sampling location (nearest town/city) | Hydraulic method | Branch/shoot | Study |
|---|---|---|---|---|---|
| Angiosperms | |||||
| Amelanchier ovalis | Shrub | Innsbruck, AT | Cavitron | Branch | This work |
| Berberis vulgaris | Shrub | Innsbruck, AT | Cavitron | Branch | This work |
| Dysoxylum papuanum | Tree | Cape Tribulation, AU | Sperry | Branch | M. Nolf et al. (unpublished) |
| Elaeocarpus grandis | Tree | Cape Tribulation, AU | Sperry | Branch | M. Nolf et al. (unpublished) |
| Hedera helix | Vine | Innsbruck, AT | Sperry | Shoot | This work |
| Lonicera xylosteum | Shrub | Innsbruck, AT | Cavitron | Branch | This work |
| Malus domestica var. Braeburn | Tree | Latsch, IT | Sperry | Branch | Beikircher et al. (2013) |
| Malus domestica var. Golden Delicious | Tree | Latsch, IT | Sperry | Branch | Beikircher et al. (2013) |
| Malus domestica var. Red Delicious | Tree | Latsch, IT | Sperry | Branch | Beikircher et al. (2013) |
| Populus alba | Tree | Vienna, AT | Sperry | Shoot | This work |
| Populus tremula | Tree | Vienna, AT | Sperry | Shoot | This work |
| Quercus petraea | Tree | Vienna, AT | Sperry | Shoot | This work |
| Sorbus aucuparia | Tree | Vienna, AT | Sperry | Branch | This work |
| Syzygium sayeri | Tree | Cape Tribulation, AU | Sperry | Branch | M. Nolf et al. (unpublished) |
| Viburnum lantana | Shrub | Innsbruck, AT | Cavitron | Branch | This work |
| Gymnosperms | |||||
| Juniperus communis | Shrub | Innsbruck, AT | Cavitron | Branch | This work |
| Picea abies | Tree | Innsbruck, AT | Sperry | Branch | This work |
| Pinus mugo | Shrub | Innsbruck, AT | Cavitron | Branch | This work |
Sample preparation and water potential determination
Long branches or shoots (typically 2–3 m long; Table 1) were collected from the field by cutting them under water (shrub species, apple cultivars), or cutting in air at full saturation early in the morning (tree species from Vienna, vine species) or before dawn (Australian rainforest species). All branches (sampled under tension and without tension) were immediately recut two to three times under water in the field, and one to two times in the laboratory, and rehydrated for at least 24 h while covered in plastic foil before benchtop drying or further sample preparation.
Water potential (Ψ) was measured on up to three unbagged leaves or small terminal branches on side twigs near the analysed section of the main stem with a pressure chamber (Model 1000 Pressure Chamber; PMS Instrument Co., Corvallis, OR, USA). These Ψ measurements were assumed to reflect the tension in the xylem of the main axis as a result of low transpiration under laboratory settings.
Hydraulic vulnerability analysis
Hydraulic vulnerability to drought‐induced embolism was analysed using either the benchtop drying technique with subsequent measurement of flow before and after flushing of embolism (Sperry et al., 1988) or saturation under partial vacuum (Hietz et al., 2008), or the Cavitron technique (Cochard, 2002), which allowed simultaneous application of water tension and measurement of flow (Table 1).
To avoid common artefacts in hydraulic techniques (e.g. Choat et al., 2010; Cochard et al., 2013), angiosperm species were only analysed with the centrifuge method when their maximum vessel length was smaller than the rotor diameter (Amelanchier ovalis, 19.7 ± 2.5 cm; Berberis vulgaris, 27.2 ± 2.5 cm; Lonicera xylosteum, 26.6 ± 4.5 cm; Viburnum lantana, 17.2 ± 4.3 cm; rotor diameter, 28 cm). Conifers were measured using a modified centrifuge method (Beikircher et al., 2010). Long‐vesselled angiosperm species were analysed via benchtop drying.
All sample segments were excised at least 50 cm from the branch's basal end and sequentially cut back, allowing for tension release before the final cutting and subsequent measurements. Samples from species that were collected under tension during the day (shrub species, apple cultivars) were re‐cut by at least twice their mean maximum vessel length to remove potentially introduced embolism in the analysed segments. Thus, we expect that cutting artefacts (Wheeler et al., 2013; Torres‐Ruiz et al., 2015) can be largely excluded from our analyses, although we note that despite these precautions, some embolism may have been introduced in some longer‐vesselled species measured with the benchtop drying technique.
We fitted the reparameterized Weibull function (Ogle et al., 2009) to vulnerability data using the fitplc package (Duursma, 2014) in R version 3.1.1 (R Core Team, 2014), except where hydraulic parameters were previously published. Hydraulic P50 was defined as the Ψ at 50% loss of conductivity, which coincides with the inflection point (i.e. the steepest part of the curve) of typical S‐shaped vulnerability curves.
Vulnerability curves obtained with the benchtop drying technique were fitted to pooled data across up to 127 measurements per species per cultivar, owing to the destructive nature of the technique. By contrast, the Cavitron technique allowed for curve‐fitting of three to five individual samples per species and subsequent averaging of computed hydraulic parameters.
AE analysis
Acoustic emission was monitored with either a PCI‐based system (PAC‐125 PCI‐2 AE System, 18‐bit A/D, 40 MHz) with 150 kHz resonant sensors (R15) and 20/40/60 dB external preamplifiers set to 40 dB, or a USB‐based system (1283 USB AE node, 18‐bit A/D, 20 MHz) with 150 kHz resonant sensors (PK15I, 26 dB integrated preamplifiers; all components from Physical Acoustics Corp., Wolfegg, Germany). Peak definition time, hit definition time, and detection threshold were set to 100–200 μs, 300–400 μs and 35–45 dB, respectively.
Sensors were attached directly to exposed xylem of the main (branch) stem using spring‐loaded clamps (Wolfcraft, Kempenich, Germany), where a small section (1–2 cm2) of bark had been removed and covered with silicone grease (Wacker, Burghausen, Germany; or RS Components Ltd, Corby, UK) to improve acoustic coupling and prevent local water loss. AE was recorded from the xylem of dehydrating branches using AEWin for PCI2 version E3.50 (PCI system) or AEWin for USB version E3.35 (USB system; both systems: Physical Acoustics Corp.), respectively, and Ψ was determined periodically. AE with an amplitude of 45 dB or higher was used for analysis in R version 3.1.1 (R Core Team, 2014).
Acoustic activity was calculated as AE min−1 (averaged across 15 min at 1 min increments). Maximum peaks of activity were determined by fitting the Savitzky–Golay smoothing function (function sgolayfilt in R; Savitzky & Golay, 1964) to emission rates with a filter order of five, thus reducing variation but preserving the original shape of our data. Finally, the Ψ at maximum AE activity (Pmaxrate) was estimated by linear interpolation between the nearest previous and following Ψ measurements (Figs 1, 2), whereby interpolated Ψ was within 0.3 MPa of the nearest measured Ψ.
Figure 1.
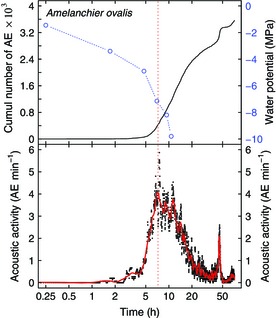
Example graph illustrating the relationship of cumulative acoustic emission (AE) counts and acoustic activity. Upper panel, curve of total cumulative AE (solid curve), water potential (blue open circles) and linear interpolation (dashed blue line) vs time. Lower panel, raw (black squares) and filtered acoustic activity (solid red curve). The red dotted vertical line indicates the timing of maximum rate and Ψ at maximum AE activity (Pmaxrate). Mean SE between parallel water potential measurements was ≤ 0.2 MPa. Note that time is presented on a logarithmic scale.
Figure 2.
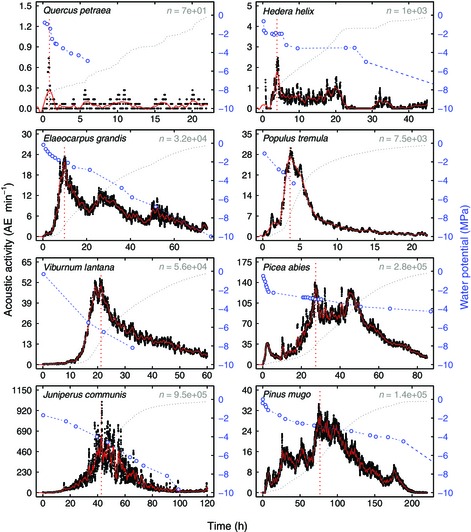
Representative example graphs illustrating the progression of acoustic activity during dehydration in eight species: raw (black circles) and filtered acoustic activity (red solid curves), timing of maximum rate (red dotted vertical lines), water potential data (blue open circles) and linear interpolation (blue dashed lines). Grey dotted lines illustrate corresponding cumulative acoustic emission (AE) curves (linear scale) vs time, whereas the total number of recorded AE (n) is given in the top right corner. Mean SE between parallel water potential measurements was ≤ 0.3 MPa in all species.
Statistics
Hydraulic P50 and Pmaxrate were tested for least‐squares linear correlation using standard statistical methods (function lm) and model diagnostics (analysis of residuals, leverage, and Cook's distance) in R version 3.1.1 (R Core Team, 2014). All tests were performed at a probability level of P < 0.05.
Confidence intervals (95% CI) for P50 obtained from pooled data (benchtop drying technique) were calculated by fitting vulnerability curves to resampled data (999 bootstrap replicates). For all other data, CIs were calculated from the sample mean and SE (Whitley & Ball, 2002):
| (Eqn 1) |
where CIs are the upper and lower confidence intervals, respectively. SE values, and therefore CIs, were not computed when n < 3. Values are given as mean, lower CI, and upper CI.
Results
We found a linear correlation (R 2 = 0.76) between hydraulically measured P50 and Pmaxrate across all studied species, with a correlation coefficient of 1.19 (P < 0.001) and an intercept of −0.11 (P ≥ 0.05; Fig. 3). Correlation parameters and significance levels changed only minimally after removal of the most influential observations (high leverage and Cook's distance, respectively) or of one species where n = 2, so all data points were included in the analysis.
Figure 3.
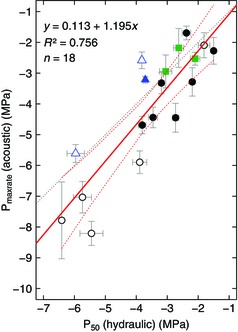
Linear correlation of 50% loss of hydraulic conductivity (P50) and the water potential (Ψ) at maximum acoustic emission (AE) activity (Pmaxrate) across 16 species and three crop cultivars of trees (closed symbols) and shrubs or vines (open symbols) of temperate angiosperms (black circles), tropical angiosperms (green squares) and gymnosperms (blue triangles), respectively. Lines indicate the least‐squares linear regression (red solid) and 95% confidence intervals (red dashed), and a 1 : 1 relationship (dotted). Whiskers show ± 1 SE of sample means.
Hydraulically measured P50 ranged between −1.5 and −6.4 MPa across all species, with a mean P50 of −3.5 ± 0.3 MPa (Fig. 3; Table 2). As expected, as a result of their habitat, shrub species from the dry forest showed the highest resistance to drought‐induced embolism among the species studied.
Table 2.
Hydraulic vulnerability (P50) and water potential at maximum acoustic activity (Pmaxrate)
| Species | P50 | CI | n | Pmaxrate | CI | n |
|---|---|---|---|---|---|---|
| Angiosperms | ||||||
| Amelanchier ovalis | −5.45 | −6.18, −4.72 | 5 | −8.21 | −8.60, −7.82 | 6 |
| Berberis vulgaris | −5.74 | −6.31, −5.17 | 4 | −7.03 | −7.55, −6.52 | 5 |
| Dysoxylum papuanum | −2.63 | −2.97, −2.34 | (21) | −2.18 | −2.81, −1.55 | 3 |
| Elaeocarpus grandis | −3.05 | −3.41, −2.63 | (21) | −2.94 | −3.48, −2.41 | 3 |
| Hedera helix | −1.81 | −2.19, −1.51 | (18) | −2.09 | −2.50, −1.69 | 3 |
| Lonicera xylosteum | −3.89 | −4.34, −3.44 | 5 | −5.90 | −6.26, −5.53 | 5 |
| Malus domestica var. Braeburn | −3.46 | −3.75, −3.17 | (49) | −4.44 | −4.77, −4.11 | 4 |
| Malus domestica var. Golden Delicious | −3.81 | −4.05, −3.57 | (46) | −4.69 | −4.97, −4.40 | 5 |
| Malus domestica var. Red Delicious | −2.73 | −2.93, −2.53 | (40) | −4.45 | −4.92, −3.98 | 4 |
| Populus alba | −1.50 | −1.60, −1.39 | (92) | −2.27 | −2.71, −1.84 | 5 |
| Populus tremula | −2.19 | −2.33, −2.04 | (56) | −3.29 | −3.75, −2.82 | 8 |
| Quercus petraea | −2.38 | −2.64, −2.10 | (35) | −1.69 | −1.92, −1.47 | 3 |
| Sorbus aucuparia | −3.19 | −3.36, −3.01 | (33) | −3.32 | −3.67, −2.97 | 3 |
| Syzygium sayeri | −2.10 | −2.47, −1.87 | (19) | −2.53 | − | 2 |
| Viburnum lantana | −6.41 | −6.55, −6.27 | 4 | −7.79 | −9.03, −6.54 | 4 |
| Gymnosperms | ||||||
| Juniperus communis | −5.96 | −6.51, −5.41 | 5 | −5.61 | −5.90, −5.32 | 13 |
| Picea abies | −3.71 | −3.81, −3.59 | (127) | −3.21 | −3.32, −3.11 | 3 |
| Pinus mugo | −3.82 | −3.94, −3.70 | 3 | −2.58 | −2.85, −2.31 | 5 |
Mean, 95% confidence interval (CI, lower and upper), and number of samples. Values in parentheses are the number of individual measurements used for pooled curve‐fitting. Confidence intervals were not calculated when n < 3.
Pmaxrate was between −1.7 and −8.2 MPa across all species (average, −4.1 ± 0.5 MPa), and was more negative than P50 in all but three angiosperms, but less negative than P50 in the three conifer species (Figs 3, 4). A comparison of the analysis of total cumulative AE vs AE activity is presented in Fig. 1.
Figure 4.
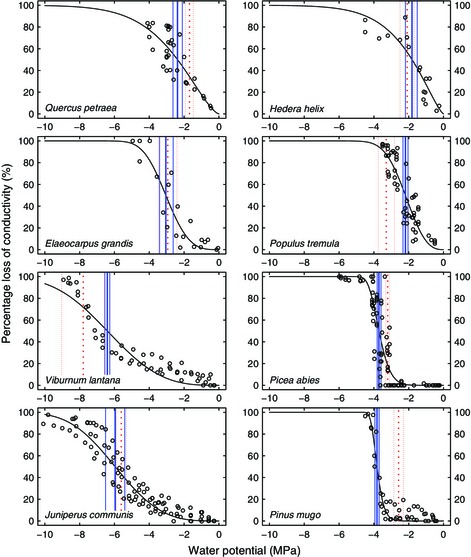
Representative example graphs illustrating the relationship of 50% loss of hydraulic conductivity (P50) and the water potential (Ψ) at maximum acoustic emission (AE) activity (Pmaxrate) in hydraulic vulnerability curves in eight species according to Fig. 2: hydraulic measurements (open circles) and fitted Weibull curves (black solid lines). Blue solid lines indicate P50 (thick lines) and 95% confidence intervals (CIs, thin lines), and red dotted lines indicate Pmaxrate (thick lines) and 95% CI (thin lines).
Example graphs illustrating the progression of AE activity and analysis of maximum peaks for ring‐porous angiosperms (Quercus petraea, temperate tree; Hedera helix, temperate vine, semi‐ring‐porous), diffuse‐porous angiosperms (Elaeocarpus grandis, tropical tree; Populus tremula, temperate tree; V. lantana, temperate shrub) and conifers (Picea abies, temperate tree; Juniperus communis, temperate tree or shrub; Pinus mugo, temperate shrub) are shown in Fig. 2.
Discussion
We showed that a species’ hydraulic vulnerability can be estimated by determining the temporal density of acoustic emissions during increasing amounts of drought stress. As hypothesized, the maximum acoustic activity (Pmaxrate) was reached at a Ψ corresponding to the Ψ at or near the steepest point of hydraulic vulnerability curves in most species, and therefore was correlated to hydraulically measured P50.
Our analysis covers a broad range of P50 spanning 4.9 MPa. All hydraulic vulnerability curves were S‐shaped, and an exemplary selection is presented in Fig. 4. The most vulnerable species were P. tremula and the vine H. helix (P50 of −1.5 and −1.8 MPa, respectively), and V. lantana and J. communis were the least vulnerable species (P50 of −6.4 and −6.0 MPa).
Acoustic emission testing offers a less laborious and noninvasive way to answer plant hydraulic questions in the laboratory and in situ. The method enables monitoring of the occurrence and timing of cavitation events in plants noninvasively, and has been successfully used to estimate plant hydraulic vulnerability in some species (Lo Gullo & Salleo, 1993; Hacke & Sauter, 1995; Hacke et al., 2000; Rosner et al., 2006; Vergeynst et al., 2013, 2014a).
In many species, plots of cumulative AE vs time first resemble an exponential‐sigmoid curve similar to S‐shaped hydraulic vulnerability curves, but later deviate from that shape and continue rising despite reaching complete hydraulic failure (Rosner, 2015; Figs 1, 2). This additional AE occurring at late stages of dehydration often cannot be distinguished from AE that originates in conducting elements, and thus exclusion from quantitative vulnerability analysis remains difficult (Rosner, 2012). Unless this emission can be accounted for, the higher total number of recorded signals should result in lower estimated hydraulic parameters, such as P12, P50 and P88. Authors have thus been looking for ways to minimize the effects of noise on vulnerability analysis, for example by manually setting end points of recording (Hacke & Sauter, 1996; Kikuta et al., 1997; Salleo et al., 2001) or by looking at amplitudes or energy properties rather than signal amplitudes (Rosner et al., 2006, 2009; Mayr & Rosner, 2011; Johnson et al., 2012), but a universal, automatable procedure is still lacking.
Our method offers a convenient way to avoid the effect of these late‐stage signals by looking at acoustic activity over time, rather than cumulative emissions per Ψ step. This approach allowed us to determine the Ψ at which most cavitation occurred within a short time‐frame (Pmaxrate) during dehydration, while minimizing the influence of late‐stage signals below the physiologically relevant range in Ψ (Figs 1, 2). P50 values, the points at which most embolism forms per Ψ decrement, were thus correctly estimated using Pmaxrate.
Interestingly, Pmaxrate values were more negative than P50 in all but three angiosperm species, indicating that the highest density of cavitation events occurs at Ψ values slightly lower than hydraulic P50. By contrast, all conifer species revealed Pmaxrate to be higher than P50 (Figs 3, 4; Table 2). We believe the differences between P50 and Pmaxrate, and the differences between angiosperms and gymnosperms, result from several contributing factors.
First, AE provides information about cavitation events and not their effects on hydraulic conductivity. This distinction is especially important in angiosperms, where conduits in a xylem cross‐section are heterogeneous in size and differ in their hydraulic conductivity, as cavitation in both a small conduit and a much larger vessel is expected to result in one AE causing embolism (Jackson & Grace, 1996). Emissions might also be caused by nanobubble formation, which may or may not expand to form embolism (Schenk et al., 2015). Wide conduits also cavitate at more moderate Ψ than smaller conduits (Tyree & Sperry, 1989; Tyree & Zimmermann, 2002), so we expect that more relative conductivity is lost in angiosperms during early cavitation, while later stages should produce more signals causing considerably lower hydraulic decline. In conifers, the high number of small, vulnerable and hydraulically less important tracheids in latewood and compression wood (Mayr & Cochard, 2003) may have caused high acoustic activity before P50 was reached.
Second, AE can originate in both conducting and nonconducting tissues, such as fibre tracheids, ray cells and sclerenchyma (Tyree & Dixon, 1986; Jackson & Grace, 1996; Kikuta, 2003; Kikuta & Richter, 2003), which means that not every individual signal necessarily represents an increase in xylem embolism.
Third, xylem is a heterogeneous medium for sound, and sound propagation velocities vary for air (embolized conduits), water (xylem sap) and solid materials (cell walls). Sound waves are influenced by these factors, resulting in complex acoustic sound conduction and attenuation effects before signals arrive at the sensor, which further complicates analysis (Tyree & Sperry, 1989; Jackson & Grace, 1996; Kikuta et al., 1997; Bucur, 2006; Mayr & Rosner, 2011). Such acoustic effects, in combination with the variability of sample anatomy, sample size or geometry, and acoustic coupling of the AE sensor, also influence the total number of recorded AEs (Fig. 2), which adds importance to the use of relative AE counts or rates.
Finally, despite the precautions taken, some artificial embolism may have been introduced in species with longer vessels during sampling or Ψ determination. This would mean that some angiosperm species might be less vulnerable to embolism formation than hydraulically measured, and could explain part of the negative shift in Pmaxrate (Figs 3, 4). Furthermore, our rate‐based analysis requires careful evaluation when the Ψ decrease is not constant, as AE may simply occur on a shorter timescale for part of the measurement, rather than revealing a higher number of signals per Ψ decrement (e.g. for Q. petraea in Fig. 2).
When viewed across a sample set of 18 data points (16 species and three cultivars), the expected linear correlation between P50 and Pmaxrate is clearly present (Fig. 3), but as a result of the varying shift between acoustic and hydraulic parameters (Fig. 4), hydraulic measurements may still be required for precise comparison between samples of similar hydraulic vulnerability. While our study does not allow evaluation of whether variation in Pmaxrate is a result of real variation in P50 or of measurement uncertainty, such covariation between P50 and Pmaxrate deserves testing in further experiments.
We believe that the use of AE rates instead of counts may be a valuable option for noninvasive monitoring of cavitation in situ: peaking rates may indicate the timing of considerable drought stress under controlled conditions, whereby possible temperature effects need to be considered. The presented analysis of AE activity allows an estimate of the hydraulic P50 of a study species or population and can provide new insights into the timing of drought stress and embolism formation.
Acknowledgements
We thank Chiara De Cesare, Peter Schmid and Verena Zublasing for participation in the measurements, and Birgit Dämon for excellent technical assistance. This study was supported by the Austrian Science Fund (FWF), projects L556‐B16, I826‐B25, T‐667, and V146‐B16, and by the COST Action FP1106 STReESS. M.N. is a recipient of a DOC‐fellowship of the Austrian Academy of Sciences.
References
- Beikircher B, Ameglio T, Cochard H, Mayr S. 2010. Limitation of the Cavitron technique by conifer pit aspiration. Journal of Experimental Botany 61: 3385–3393. [DOI] [PubMed] [Google Scholar]
- Beikircher B, De Cesare C, Mayr S. 2013. Hydraulics of high‐yield orchard trees: a case study of three Malus domestica cultivars. Tree Physiology 33: 1296–1307. [DOI] [PubMed] [Google Scholar]
- Bucur V. 2006. Acoustics of wood. Vol. 1431, No. 8563 Berlin, Heidelberg, Germnay: Springer. [Google Scholar]
- Charrier G, Charra‐Vaskou K, Kasuga J, Cochard H, Mayr S, Ameglio T. 2014. Freeze–thaw stress: effects of temperature on hydraulic conductivity and ultrasonic activity in ten woody angiosperms. Plant Physiology 164: 992–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choat B, Drayton WM, Brodersen C, Matthews MA, Shackel KA, Wada H, McElrone AJ. 2010. Measurement of vulnerability to water stress‐induced cavitation in grapevine: a comparison of four techniques applied to a long‐vesseled species. Plant, Cell & Environment 33: 1502–1512. [DOI] [PubMed] [Google Scholar]
- Cochard H. 2002. A technique for measuring xylem hydraulic conductance under high negative pressures. Plant, Cell & Environment 25: 815–819. [Google Scholar]
- Cochard H, Badel E, Herbette S, Delzon S, Choat B, Jansen S. 2013. Methods for measuring plant vulnerability to cavitation: a critical review. Journal of Experimental Botany 64: 4779–4791. [DOI] [PubMed] [Google Scholar]
- Cochard H, Herbette S, Barigah T, Badel E, Ennajeh M, Vilagrosa A. 2010. Does sample length influence the shape of xylem embolism vulnerability curves? A test with the Cavitron spinning technique. Plant, Cell & Environment 33: 1543–1552. [DOI] [PubMed] [Google Scholar]
- Cochard H, Tyree MT. 1990. Xylem dysfunction in Quercus: vessel sizes, tyloses, cavitation and seasonal changes in embolism. Tree Physiology 6: 393–407. [DOI] [PubMed] [Google Scholar]
- Duursma R. 2014. Fit vulnerability curves in R (R package). [WWW document] URL https://bitbucket.org/remkoduursma/fitplc/ [accessed 10 January 2015].
- Hacke UG, Sauter JJ. 1995. Vulnerability of xylem to embolism in relation to leaf water potential and stomatal conductance in Fagus sylvatica f. purpurea and Populus balsamifera . Journal of Experimental Botany 46: 1177–1183. [Google Scholar]
- Hacke UG, Sauter JJ. 1996. Drought‐induced xylem dysfunction in petioles, branches, and roots of Populus balsamifera L. and Alnus glutinosa (L.) Gaertn. Plant Physiology 111: 413–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacke UG, Sperry JS, Pittermann J. 2000. Drought experience and cavitation resistance in six shrubs from the Great Basin, Utah. Basic and Applied Ecology 1: 31–41. [Google Scholar]
- Hietz P, Rosner S, Sorz J, Mayr S. 2008. Comparison of methods to quantify loss of hydraulic conductivity in Norway spruce. Annals of Forest Science 65: 502–508. [Google Scholar]
- Jackson GE, Grace J. 1996. Field measurements of xylem cavitation: are acoustic emissions useful? Journal of Experimental Botany 47: 1643–1650. [Google Scholar]
- Jansen S, Schuldt B, Choat B. 2015. Current controversies and challenges in applying plant hydraulic techniques. New Phytologist 205: 961–964. [DOI] [PubMed] [Google Scholar]
- Johnson DM, McCulloh KA, Woodruff DR, Meinzer FC. 2012. Evidence for xylem embolism as a primary factor in dehydration‐induced declines in leaf hydraulic conductance. Plant, Cell & Environment 35: 760–769. [DOI] [PubMed] [Google Scholar]
- Johnson DM, Meinzer FC, Woodruff DR, McCulloh KA. 2009. Leaf xylem embolism, detected acoustically and by cryo‐SEM, corresponds to decreases in leaf hydraulic conductance in four evergreen species. Plant, Cell & Environment 32: 828–836. [DOI] [PubMed] [Google Scholar]
- Kikuta SB. 2003. Ultrasound acoustic emissions from bark samples differing in anatomical characteristics. Phyton 43: 161–178. [Google Scholar]
- Kikuta SB, Lo Gullo MA, Nardini A, Richter H, Salleo S. 1997. Ultrasound acoustic emissions from dehydrating leaves of deciduous and evergreen trees. Plant, Cell & Environment 20: 1381–1390. [Google Scholar]
- Kikuta SB, Richter H. 2003. Ultrasound acoustic emissions from freezing xylem. Plant, Cell & Environment 26: 383–388. [Google Scholar]
- Kowalski SJ, Smoczkiewicz A. 2004. Acoustic emission in wood under drying. Seria B, Zeszyt 35: 59–71. [Google Scholar]
- Lo Gullo MA, Salleo S. 1993. Different vulnerabilities of Quercus ilex L. to freeze‐ and summer drought‐induced xylem embolism: an ecological interpretation. Plant, Cell & Environment 16: 511–519. [Google Scholar]
- Manoharan P, Pammenter NW. 2005. Comparison between ultrasonic acoustic emission (UAE) and hydraulically measured loss of hydraulic conductance in Eucalyptus spp. clone GU210. Journal of Science of the University of Kelaniya Sri Lanka 2: 85–104. [Google Scholar]
- Mayr S, Cochard H. 2003. A new method for vulnerability analysis of small xylem areas reveals that compression wood of Norway spruce has lower hydraulic safety than opposite wood. Plant, Cell & Environment 26: 1365–1371. [Google Scholar]
- Mayr S, Cochard H, Ameglio T, Kikuta SB. 2007. Embolism formation during freezing in the wood of Picea abies . Plant Physiology 143: 60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr S, Rosner S. 2011. Cavitation in dehydrating xylem of Picea abies: energy properties of ultrasonic emissions reflect tracheid dimensions. Tree Physiology 31: 59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr S, Sperry JS. 2010. Freeze‐thaw‐induced embolism in Pinus contorta: centrifuge experiments validate the ‘thaw‐expansion hypothesis’ but conflict with ultrasonic emission data. New Phytologist 185: 1016–1024. [DOI] [PubMed] [Google Scholar]
- Milburn JA, Johnson RPC. 1966. The conduction of Sap. II. Detection of vibration produced by sap cavitation in Ricinus xylem. Planta 69: 43–52. [DOI] [PubMed] [Google Scholar]
- Nardini A, Tyree MT, Salleo S. 2001. Xylem cavitation in the leaf of Prunus laurocerasus and its impact on leaf hydraulics. Plant Physiology 125: 1700–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogle K, Barber JJ, Willson C, Thompson B. 2009. Hierarchical statistical modeling of xylem vulnerability to cavitation. New Phytologist 182: 541–554. [DOI] [PubMed] [Google Scholar]
- Ponomarenko A, Vincent O, Pietriga A, Cochard H, Badel E, Marmottant P. 2014. Ultrasonic emissions reveal individual cavitation bubbles in water‐stressed wood. Journal of the Royal Society Interface 11: 20140480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team . 2014. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; URL http://www.R-project.org/. [Google Scholar]
- Ritman KT, Milburn JA. 1988. Acoustic emissions from plants: ultrasonic and audible compared. Journal of Experimental Botany 39: 1237–1248. [Google Scholar]
- Rosner S. 2012. Waveform features of acoustic emission provide information about reversible and irreversible processes during spruce sapwood drying. BioResources 7: 1253–1263. [Google Scholar]
- Rosner S. 2015. A new type of vulnerability curve: is there truth in vine? Tree Physiology 35: 410–414. [DOI] [PubMed] [Google Scholar]
- Rosner S, Karlsson B, Konnerth J, Hansmann C. 2009. Shrinkage processes in standard‐size Norway spruce wood specimens with different vulnerability to cavitation. Tree Physiology 29: 1419–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner S, Klein A, Wimmer R, Karlsson B. 2006. Extraction of features from ultrasound acoustic emissions: a tool to assess the hydraulic vulnerability of Norway spruce trunkwood? New Phytologist 171: 105–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salleo S, Lo Gullo MA, Raimondo F, Nardini A. 2001. Vulnerability to cavitation of leaf minor veins: any impact on leaf gas exchange? Plant, Cell & Environment 24: 851–859. [Google Scholar]
- Savitzky A, Golay MJE. 1964. Smoothing and differentiation of data by simplified least squares procedures. Analytical Chemistry 36: 1627–1639. [DOI] [PubMed] [Google Scholar]
- Schenk HJ, Steppe K, Jansen S. 2015. Nanobubbles: a new paradigm for air‐seeding in xylem. Trends in Plant Science 20: 199–205. [DOI] [PubMed] [Google Scholar]
- Sperry JS, Donnely JR, Tyree MT. 1988. A method for measuring hydraulic conductivity and embolism in xylem. Plant, Cell & Environment 11: 35–40. [Google Scholar]
- Torres‐Ruiz JM, Jansen S, Choat B, McElrone AJ, Cochard H, Brodribb TJ, Badel E, Burlett R, Bouche PS, Brodersen CR et al 2015. Direct X‐ray microtomography observation confirms the induction of embolism upon xylem cutting under tension. Plant Physiology 167: 40–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyree MT, Dixon MA. 1983. Cavitation events in Thuja occidentalis L.? Plant Physiology 72: 1094–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyree MT, Dixon MA. 1986. Water stress induced cavitation and embolism in some woody plants. Physiologia Plantarum 66: 397–405. [Google Scholar]
- Tyree MT, Dixon MA, Thompson RG. 1984. Ultrasonic acoustic emissions from the sapwood of Thuja occidentalis measured inside a pressure bomb. Plant Physiology 74: 1046–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyree MT, Sperry JS. 1989. Vulnerability of xylem to cavitation and embolism. Annual Review of Plant Physiology and Plant Molecular Biology 40: 19–38. [Google Scholar]
- Tyree MT, Zimmermann MH. 2002. Xylem structure and the ascent of sap. Berlin, Germany: Springer. [Google Scholar]
- Vergeynst L, Bogaerts J, Baert A, Kips L, Steppe K. 2013. New type of vulnerability curve gives insight in the hydraulic capacitance and conductivity of the xylem. Acta Horticulturae 991: 341–347. [Google Scholar]
- Vergeynst LL, Dierick M, Bogaerts JA, Cnudde V, Steppe K. 2014a. Cavitation: a blessing in disguise? New method to establish vulnerability curves and assess hydraulic capacitance of woody tissues. Tree Physiology 35: 400–409. [DOI] [PubMed] [Google Scholar]
- Vergeynst LL, Sause MG, Steppe K. 2014b. Acoustic emission signal detection in drought‐stressed trees: beyond counting hits. 31st conference of the European Working Group on Acoustic Emission (EWGAE). Dresden, Germany: German Society for Nondestructive Testing. [Google Scholar]
- Wheeler JK, Huggett BA, Tofte AN, Rockwell FE, Holbrook NM. 2013. Cutting xylem under tension or supersaturated with gas can generate PLC and the appearance of rapid recovery from embolism. Plant, Cell & Environment 36: 1938–1949. [DOI] [PubMed] [Google Scholar]
- Whitley E, Ball J. 2002. Statistics review 2: samples and populations. Critical Care 6: 143–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolkerstorfer SV, Rosner S, Hietz P. 2012. An improved method and data analysis for ultrasound acoustic emissions and xylem vulnerability in conifer wood. Physiologia Plantarum 146: 184–191. [DOI] [PubMed] [Google Scholar]


