Abstract
Satellite cells are maintained in an undifferentiated quiescent state, but during muscle regeneration they acquire an activated stage, and initiate to proliferate and differentiate as myoblasts. The transmembrane protein teneurin‐4 (Ten‐4) is specifically expressed in the quiescent satellite cells; however, its cellular and molecular functions remain unknown. We therefore aimed to elucidate the function of Ten‐4 in muscle satellite cells. In the tibialis anterior (TA) muscle of Ten‐4‐deficient mice, the number and the size of myofibers, as well as the population of satellite cells, were reduced with/without induction of muscle regeneration. Furthermore, we found an accelerated activation of satellite cells in the regenerated Ten‐4‐deficient TA muscle. The cell culture analysis using primary satellite cells showed that Ten‐4 suppressed the progression of myogenic differentiation. Together, our findings revealed that Ten‐4 functions as a crucial player in maintaining the quiescence of muscle satellite cells. Stem Cells 2015;33:3017–3027
Keywords: Satellite cell, Muscle stem cell, Regeneration, Muscle, Quiescence, Teneurin‐4/Odz4
Significance Statement .
The transmembrane protein teneurin‐4 (Ten‐4) is specifically expressed in the quiescent satellite cells; however, its cellular and molecular function remains unknown. Our results provided evidences that Ten‐4 possesses a suppressive function in the satellite cell quiescence and myogenic differentiation. This is the first report to our knowledge that demonstrates the biological function of Ten‐4 in muscle satellite cells. Our findings will facilitate a better understanding of the molecular mechanism of muscle satellite cell biology.
Introduction
Skeletal muscle is essential for the physical support of the animal body and for the proper body movement. In human, the mass of the skeletal muscle occupies ∼38% and ∼30% of the total body mass of men and women, respectively 1. Defects in the formation and/or maintenance of skeletal muscle tissues, in some cases, cause severe disorders, such as muscular dystrophy and muscle atrophy 2. During embryonic development, somites differentiate to the sclerotome and dermomyotome, and the dermomyotome tissue gives rise to myogenic progenitor cells, which are specified with the expression of paired box transcription factors Pax3 and Pax7 3, 4, 5. Subsequently, myogenic progenitor cells differentiate into myoblasts, positive for the transcription factor myogenic differentiation 1, Myod1/MyoD. The fusion of numerous myoblasts results in the formation of cylindrical and multinucleated myofibers abundant with filaments of actin and myosin heavy chains (MHCs). The myofibers are eventually surrounded by basement membranes and matured as functional units 6.
Muscle satellite cells, myogenic stem cells, are found between a myofiber sarcolemma and the basement membrane, accompanying with the expression of its marker Pax7 6, 7. Satellite cells exert their function at two main occasions, the postnatal development and the regeneration process. During postnatal stages, satellite cells contribute to the growth and maturation of myofibers, by supplying additional myonuclei 8. Satellite cell function is critical at the stages. In this sense, in Pax7 knockout mice, satellite cell number is dramatically reduced a few weeks after birth, and as a result, myofibers have small diameter and the muscle weakens 9, 10, 11. In the adult skeletal muscle, satellite cells remain in the quiescent phase. However, once the muscle tissue is damaged, satellite cells become activated and begin to proliferate, followed by differentiation into myoblasts. Then, multinucleated myofibers are formed to complete the tissue repair 12. Some of satellite cells do not commit to the differentiation process, but self‐renew to maintain their own population 12. Ablation of CSL (CBF1/suppressor of hairless/LAG‐1) (RBP‐J) or Hesr1 and Hesr3, which are key molecules in Notch signaling for the quiescence of satellite cells, forces to exit from the quiescent phase, and promotes proliferation and differentiation. This causes impairment in the tissue repair 13, 14, 15, 16. In addition, microRNA‐489 also maintains the quiescent phase of satellite cells 17. However, the detailed molecular mechanism for the satellite cell quiescence is poorly understood.
Teneurin‐4 (Ten‐4) is a member of the teneurin (Ten‐m/Odz) family that encodes type II transmembrane proteins 18. Ten‐4 is highly expressed in the central nervous system, but its expression is observed in various tissues, including mesenchymal tissues 19. During cartilage development, Ten‐4 is expressed in the mesenchymal condensation area, where chondrogenic stem cells reside, and suppresses chondrogenic differentiation 20. In skeletal muscle, the specific expression of Ten‐4 is found in quiescent satellite cells, and it disappears after the activation of satellite cells during the tissue regeneration process 21, 22. However, the cellular and molecular functions of Ten‐4 in satellite cells remain unknown.
In this study, we elucidated the biological function of Ten‐4 in muscle satellite cells. We analyzed the musculature in the skeletal muscle tissue of Ten‐4‐deficient (−/−) mice with/without cardiotoxin (CTX) injection and the character of cultured satellite cells isolated from the mutant mice. We found defects in the myofiber formation in Ten‐4−/− mice and increased activation of Ten‐4‐deficient satellite cells. Our findings present Ten‐4 as a novel player in the satellite cell biology.
Materials and Methods
Mice
The Ten‐4‐deficient furue mouse line was kindly provided by Dr. Yoshihiko Yamada from NIDCR, NIH 23. Littermates or age‐matched mice between different genotypes were used for experiments 8–12 weeks after birth. All procedures for experimental animals were approved by the Institutional Animal Care and Use Committees of Tokyo Medical and Dental University and Keio University.
Cryosections
Tibialis anterior (TA) muscles were dissected out and frozen in liquid nitrogen‐cooled isopentane (Wako, Osaka, Japan, www.wako-chem.co.jp). Using a cryostat (Leica, Wetzlar, Germany, www.leica-microsystems.com), the frozen TA muscles were sectioned transversely at a 10 µm thickness, and sections from the widest part in the TA muscles were attached on MAS‐coated slide glasses (MATSUNAMI, Kishiwada, Japan, www.matsunami-glass.co.jp). The cryosections were kept at −80°C until they were used for immunostaining.
Immunostaining
Cryosections described above were used for immunohistochemistry. For immunocytochemistry, primary satellite cells were cultured on eight‐well chamber slides (MATSUNAMI) coated with Matrigel (BD Biosciences, San Jose, California, www.bd.com). Tissue sections or cells were fixed in 4% paraformaldehyde in PBS for 10 minutes at room temperature, and then permeabilized with 0.2% Triton X‐100 (Sigma‐Aldrich, St. Louis, Missouri, www.sigmaaldrich.com) in phosphate buffered saline (PBS) for 15 minutes at room temperature. After blocking with Power Block Universal Blocking Reagent (BioGenex, Fremont, California, http://biogenex.comLaboratories) or M.O.M. kit (Vector Laboratories, Burlingame, California, www.vectorlabs.com), the fixed cells were incubated with primary antibodies overnight at 4°C. After washing, bound primary antibodies were labeled with fluorescence‐conjugated secondary antibodies for 1 hour at room temperature. The immunostained samples were mounted with Mounting medium for fluorescence with DAPI (Vector Laboratories). Primary and secondary antibodies were as follows: anti‐laminin α2 (Sigma‐Aldrich), anti‐Pax7 (Developmental Studies Hybridoma Bank, Iowa City, Iowa, http://dshb.biology.uiowa.edu), anti‐Ki67 (Leica or BD Biosciences), anti‐MHC (Leica), and mouse/rabbit/rat IgG‐Alexa488, ‐Alexa594, or Alexa647 (Life Technologies, St. Aubin, France, www.lifetech.com).
Muscle Injury
To induce regeneration of skeletal muscle, mice were anesthetized with isoflurane, and hairs in their hind limbs were shaved. One hundred microliters of CTX (10 µM in 0.9% NaCl; Sigma‐Aldrich) was injected into the TA muscle using a 29‐gauge needle. Seven or fourteen days after injections, mice were euthanized and the frozen tissue sections were prepared for the analysis as described above.
Quantification of Myofibers and Satellite Cells on Immunostained Tissue Sections
Immunofluorescent images of laminin α2 chain were taken, and myofibers surrounded by the laminin α2 signal in TA cross‐sections were analyzed using the MetaMorph 7.5 software (Molecular Devices, Wokingham‐Berkshire, United Kingdom, www.moleculardevices.com). The signal was thresholded, and the number of myofibers in whole areas of the cross‐sections was counted using the Integrated Morphometry Analysis program of the software. The thresholded images were also used for measurement of areas of individual myofibers by the program. Five hundreds to one thousand fibers per mouse were analyzed for the measurement of individual fiber areas. For counting satellite cells, cells positive for Pax7 and DAPI staining and located between a myofiber and the laminin α2 signal were counted as satellite cells. The number of satellite cells per 100 myofibers was measured in each genotype.
Flow Cytometric Analysis of Muscle Satellite Cells
Skeletal muscles from both fore‐limbs and hind limbs were dissected out and digested with 0.2% collagenase type II (Worthington Biochemical CorporaAon, Lakewood, Washington, www.worthington-biochem.com) for 1 hour at 37°C. Then, the digested tissue was filtered through 100 µm‐ and 40 µm‐cell strainers (BD Biosciences). The filtered mononuclear cells were stained with phycoerythrin (PE)‐conjugated anti‐CD31 (BD Biosciences), PE‐conjugated anti‐CD45 (BD Biosciences), FITC‐conjugated anti‐Sca‐1 (BD Biosciences), and biotinylated SM/C‐2.6 antibodies 24 on ice for 30 minutes. After washing, streptavidin‐allophycocyanin (BD Biosciences) was added to the cells labeled with biotinylated SM/C‐2.6 antibody and incubated on ice for 30 minutes. All the cells were resuspended in HBSS (−) and propidium iodide. Cell sorting was performed using MoFlo flow cytometer (BeckMan, Brea, California, www.beckmancoulter.com), and CD31−, CD45−, Sca‐1−, and SM/C‐2.6+ cells were collected as satellite cells 24. Percentage of satellite cells in the total mononuclear cells, except for CD31‐positive endothelial cells and CD45‐positive lymphocytes/leukocytes, was calculated for analyzing satellite cell population.
Primary Culture
Isolated satellite cells were plated on plastic dishes or glass chamber slides coated with Matrigel. For proliferative condition, satellite cells were cultured in Dulbecco's modified Eagle's medium (DMEM) with GlutaMAX (Life Technologies) containing 20% fetal bovine serum (Sigma‐Aldrich), 1% Chick Embryo Extract (U.S. Biological, SwampscoW, MassachuseWs, www.usbio.net), 100 units/ml penicillin and 100 µg/ml streptomycin (Life Technologies), and 5 ng/ml basic‐FGF (ReproCell, Yokohama, Japan, www.reprocell.com) under 5% CO2 at 37°C. For differentiation assay, satellite cells were cultured in differentiation medium consisting of DMEM with GlutaMAX supplemented with 5% horse serum (Life Technologies), 100 units/ml penicillin, and 100 µg/ml streptomycin.
Results
Defects in the Formation or Maintenance of Myofibers and Satellite Cells in Ten‐4‐Deficient Mice
For analyses of the Ten‐4 function in satellite cells, we used the furue (fur) mutant mouse line whose Ten‐4 expression is absent because of a transgene insertion in the Ten‐4 gene (Odz4/Tenm4) 23. The mutant fur/fur mice are hereafter referred to as Ten‐4−/− mice. Since one of main functions of satellite cells is promoting muscle growth after birth, we first analyzed the body size and weight of Ten‐4−/− mice during postnatal stages. At postnatal day (P)0–1, no difference in body weight between wild‐type (WT), Ten‐4 heterozygous (+/−), and Ten‐4−/− mice was observed. However, a delay of the body growth was found at P2 and became obvious in Ten‐4−/− mice during postnatal stages (Fig. 1A). The body size of Ten‐4−/− mice was smaller than that of WT and Ten‐4+/− mice at P5 (Fig. 1B). These results suggest that the postnatal body growth of Ten‐4−/− mice was interfered.
Figure 1.
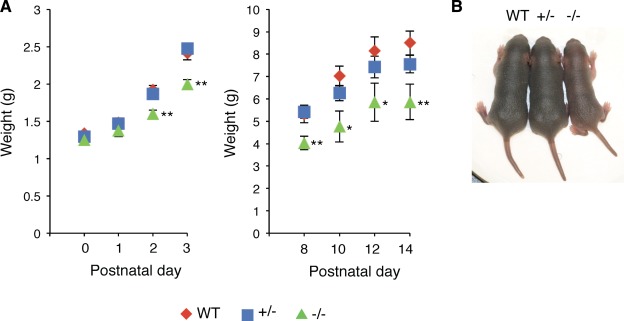
Reduced body weight and size of Ten‐4‐deficient mice. (A): Reduced weight of WT, Ten‐4 heterozygous (+/−), and Ten‐4 homozygous (−/−) mice. The body weight of postnatal Ten‐4−/− mice was measured (left: P0–3; right: P8–14). Error bars, SEM; *, p < .05; **, p < .01. (B): Smaller body size of Ten‐4−/− mice at P5. The body size of Ten‐4−/− mice was smaller than that of WT and Ten‐4+/− mice. The onset of the reduced body size was early postnatal stage, around P2. Abbreviation: WT, wild type.
We next examined weight and musculature of the TA muscle of adult Ten‐4−/− mice (8–12‐weeks old). The TA weight of Ten‐4−/− mice was lighter than that of WT mice (Fig. 2A). To analyze the myofiber formation in the TA muscle tissue, immunostaining of laminin α2 chain, a basement membrane protein, on transverse sections of TA was performed. The whole area of the TA sections of Ten‐4+/− and Ten‐4−/− mice was smaller, compared with that of WT (Fig. 2B: left images, Fig. 2C: left graph). Less number of the myofiber number was observed in the whole area of the Ten‐4−/− TA section, relative to that in the WT section (Fig. 2B: left images, Fig. 2C: center graph). In addition, the area of individual myofibers was reduced in Ten‐4−/− mice (Fig. 2B: right images, Fig. 2C: right graph). These observations indicate that Ten‐4 was required for production of the normal number and growth of myofibers.
Figure 2.
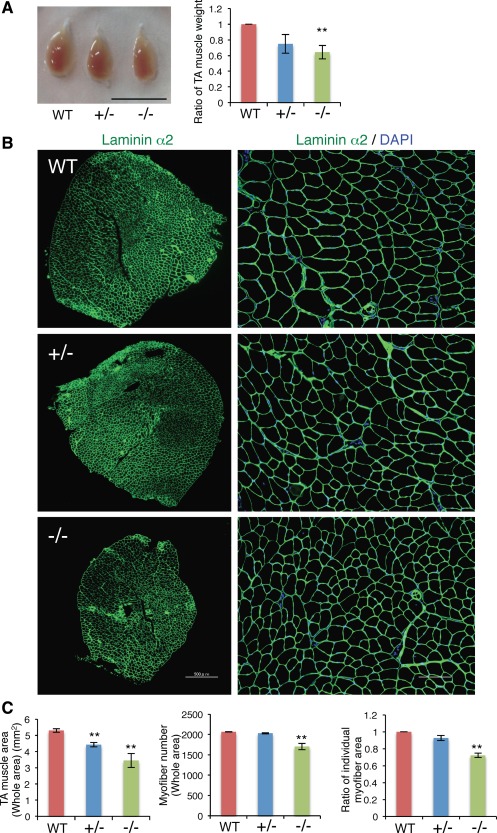
Defects in the muscle tissue of Ten‐4‐deficient mice. (A): Size and weight of the TA muscle from WT, Ten‐4+/−, and Ten‐4−/− mice. The TA muscle was dissected out and weighed. The weight of the WT TA muscle was set as 1.0. Error bars, SEM; **, p < .01. Scale bar = 10 mm. (B): Immunostaining of laminin α2 (green) in the TA muscle tissue from WT, Ten‐4+/−, and Ten‐4−/− mice. DAPI staining (blue) was used to visualize nuclei. Scale bar = 500 µm in lower magnification images (left); 100 µm in higher magnification images (right). (C): The whole area (left), the total myofiber number (center), and the area of individual myofibers (right) in transverse TA sections. The individual myofiber area of WT mice was set as 1.0. Error bars, SEM; **, p < .01. Abbreviations: TA, tibialis anterior; WT, wild type.
We further analyzed the population of satellite cells by immunostaining of Pax7, a marker for satellite cells, on TA transverse sections. In Ten‐4+/− and Ten‐4−/− tissues, the number of Pax7‐positive satellite cells per 100 myofibers was attenuated (Fig. 3A). Moreover, the population of TA satellite cells was assessed by flow cytometry using the antibody SM/C‐2.6, which recognizes satellite cells specifically 24. After mononuclear cells negative for CD31 and CD45, markers for endothelial cells and lymphocytes/leukocytes, respectively, were gated in the total cells from TA tissues, the population of satellite cells, positive and negative for SM/C‐2.6 antibody and Sca‐1, respectively, was analyzed. We found that the percentage of Ten‐4−/− satellite cell population per the total mononuclear cells, except for endothelial cells and lymphocytes/leukocytes, was significantly decreased, compared to that of WT (Fig. 3B). These data indicate that Ten‐4 was required for myofiber formation and production or maintenance of satellite cells during normal development.
Figure 3.
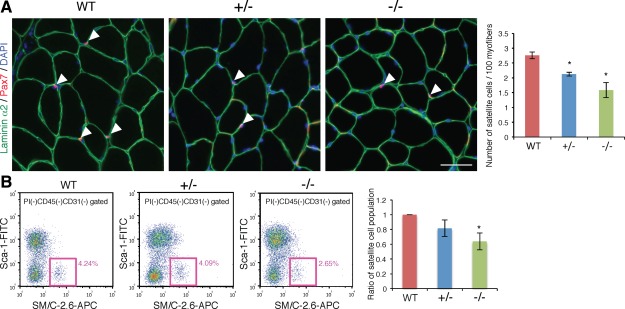
Decrease of satellite cells in Ten‐4‐deficient mice. (A): Immunohistochemistry of Pax7 (red) in WT, Ten‐4+/−, and Ten‐4−/− TA muscle sections. Arrowheads indicate Pax7‐positive cells located between a myofiber and basement membrane labeled with laminin α2 staining (green). DAPI staining (blue) was used to visualize nuclei. Scale bar = 50 µm. The number of satellite cells per cross‐sectional 100 myofibers was counted. Error bars, SEM; *, p < .05. (B): Population of satellite cells from WT, Ten‐4+/−, and Ten‐4−/− TA muscles by flow cytometry. The CD31−CD45−Sca‐1−SM/C‐2.6+ cells were analyzed as muscle satellite cells using the MoFlo flow cytometer. Pink boxes represent the satellite cell population, and values in pink denote percentages of satellite cells in total mononuclear cells, except for CD45+ lymphocytes/leukocytes and CD31+ endothelial cells. The percentage of the WT satellite cell population was set as 1.0. Error bars, SEM; *, p < .05. Abbreviations: APC, allophycocyanin; TA, tibialis anterior; WT, wild type.
Defective Tissue Regeneration in the Ten‐4‐Deficient Muscle
The regeneration of injured tissues in skeletal muscle is another important function of satellite cells. Therefore, we carried out a muscle regeneration experiment by injection of CTX. After CTX was injected into TA muscle, myofiber formation and satellite cell population were analyzed. Seven days after the CTX injection, the weight of the TA muscle was diminished in Ten‐4+/− and Ten‐4−/− mice, in comparison with WT (Fig. 4A). The whole area of transverse TA sections and the number of regenerated myofibers, whose nuclei were centralized in the fibers, were reduced in Ten‐4−/− TA muscles (Fig. 4B: left images, Fig. 4C: left and center graphs). The area of individual regenerated myofibers was smaller in Ten‐4+/− and Ten‐4−/− TA muscles (Fig. 4B: right images, Fig. 4C: right graph). From these results, we concluded that the deficiency of Ten‐4 perturbed the tissue repair.
Figure 4.
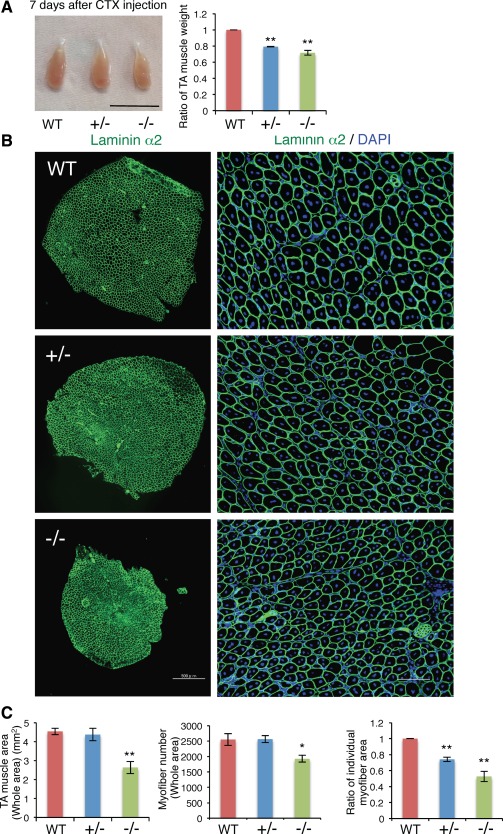
Abnormalities in the regenerated muscle tissue of Ten‐4‐deficient mice. (A): Weight of TA muscles from WT, Ten‐4+/−, and Ten‐4−/− mice. TA muscles were dissected out 7 days after the injection of CTX and were analyzed. The weight of the WT TA muscle was set as 1.0. Error bars, SEM; **, p < .01. Scale bar = 10 mm. (B): Immunostaining of laminin α2 (green) in the TA muscle tissue from WT, Ten‐4+/−, and Ten‐4−/− mice, 7 days after the injection of CTX. DAPI staining (blue) was used to visualize nuclei. Scale bar = 500 µm in lower magnification images (left); 100 µm in higher magnification images (right). (C): The whole area (left), the total myofiber number (center), and the area of individual myofibers (right) in transverse TA sections consisting of regenerated muscle fibers, whose nuclei were in the center. The individual myofiber area of WT tissue was set as 1.0. Error bars, SEM; *, p < .05; **, p < .01. Abbreviations: CTX, cardiotoxin; TA, tibialis anterior; WT, wild type.
To address the self‐renewal capacity of satellite cells, we analyzed the population of satellite cells in regenerated TA tissues after the CTX injection. Immunostaining of Pax7 showed the decreased number of satellite cells in the Ten‐4−/− regenerated tissue, relative to the WT tissue (Fig. 5A). Similarly, by flow cytometric analysis using SM/C‐2.6 antibody, a decrease of the satellite cell population was observed in the regenerated TA tissue of Ten‐4−/− mice (Fig. 5B). In the both cases of with and without CTX injection, however, the percentages of Ten‐4−/− satellite cells to WT satellite cells set as 100% were similar, and no statistical difference was observed between the TA tissues with and without CTX injection (immunostaining: with CTX: 60.7% ± 4.1%, without CTX: 57.4% ± 9.1%; flow cytometry: with CTX: 89.5% ± 3.1%, without CTX: 63.9% ± 11.5%, in Ten‐4−/− mice, relative to 100% for in WT mice) (Figs. 3, 5). This evidence indicates that the satellite cell population was maintained in the Ten‐4−/− muscle after the regeneration. From these observations, we found that in the Ten‐4−/− TA muscle, the tissue repair was defective, albeit the self‐renewal of satellite cells occurred normally.
Figure 5.
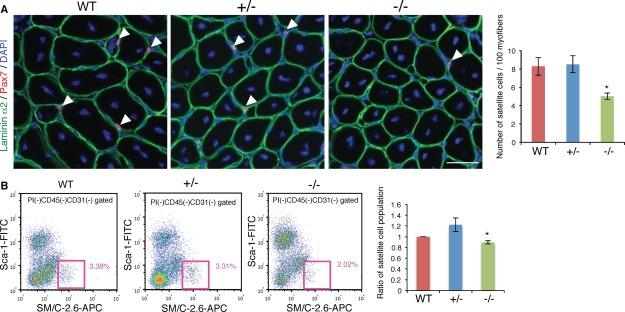
Reduced number of satellite cells in regenerated muscle tissues of Ten‐4‐deficient mice. (A): Immunohistochemistry of Pax7 (red) in regenerated TA muscle sections, 7 days after CTX injection. Arrowheads show Pax7‐positive satellite cells between the laminin α2‐containing basement membrane (green) and a regenerated myofiber, whose nuclei are centralized. DAPI staining (blue) was used to visualize nuclei. Scale bar = 50 µm. The number of satellite cells per cross‐sectional 100 myofibers was counted and quantified. Error bars, SEM; *, p < .05. (B): Flow cytometer profiles of satellite cells from regenerated TA muscles of WT, Ten‐4+/−, and Ten‐4−/− mice. The CD31−CD45−Sca‐1−SM/C‐2.6+ cells were analyzed as muscle satellite cells using the MoFlo flow cytometer, 14 days after injection of CTX. Pink boxes exhibit the population of satellite cells, and percentages of satellite cells out of total mononuclear cells, except for CD45+ lymphocytes/leukocytes and CD31+ endothelial cells are indicated in pink. The percentage of the WT satellite cell population was set as 1.0. Error bars, SEM; *, p < .05. Abbreviations: APC, allophycocyanin; CTX, cardiotoxin; TA, tibialis anterior; WT, wild type.
Promoted Activation of Ten‐4‐Deficient Satellite Cells
To address the mechanism of Ten‐4 function in satellite cells, we analyzed activation of satellite cells in Ten‐4‐deficient mice by immunostaining of Pax7 and Ki67, a marker for proliferative cells. We counted Pax7‐ and Ki67‐dual positive cells as activated satellite cells (Fig. 6, arrows) in TA tissues, 7 days after CTX injection. The ratio of Pax7‐ and Ki67‐dual positive cells to total Pax7‐positive cells in Ten‐4+/− and Ten‐4−/− tissues was significantly higher, in comparison with that in WT (Fig. 6). This result suggests that the deficiency of Ten‐4 accelerated activation of satellite cells. In normal TA tissues without injection of CTX, the ratio of Pax7‐ and Ki67‐dual positive cells to total Pax7‐positive cells was lower than that in regenerated tissues (Fig 6 and Supporting Information Fig. S1), and there was no difference in the ratios between WT, Ten‐4+/−, and Ten‐4−/− mice (Supporting Information Fig. S1). As activation of satellite cells in normal adult tissue occurs less frequently, it was probably hard to detect the promoting effect of Ten‐4 deficiency by immunostaining. However, we found that mRNA expression level of MyoD was elevated in Ten‐4−/− satellite cells from TA tissue without CTX injection, relative to in WT satellite cells (Supporting Information Fig. S2), indicating that activation was promoted in Ten‐4−/− satellite cells. From these observations, Ten‐4 is required for maintaining of quiescence of satellite cells.
Figure 6.
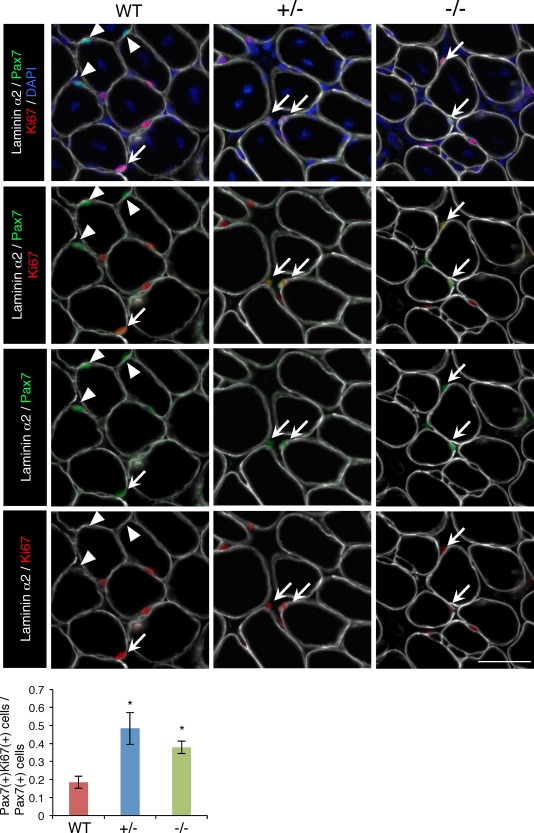
Increased activated satellite cells in regenerated TA muscle from WT, Ten‐4+/−, and Ten‐4−/− mice. Immunostaining of laminin α2 (white), Pax7 (green), and Ki67 (red) in the TA muscle tissue of WT, Ten‐4+/−, and Ten‐4−/− mice, 7 days after CTX injection. Arrows indicate Pax7‐ and Ki67‐double positive cells, and arrowheads represent Pax7‐single positive cells. DAPI staining (blue) was used to visualize nuclei. The ratio of the numbers of Pax7‐ and Ki67‐dual positive cells/Pax7‐single positive cells was quantified. Error bars, SEM; *, p < .05. Scale bar = 50 µm. Abbreviation: CTX, cardiotoxin; TA, tibialis anterior; WT, wild type.
Promotion of Proliferation and Differentiation in the Ten‐4‐Deficient Cell Culture
We further analyzed Ten‐4 function in proliferation and differentiation of primary satellite cell culture. Satellite cells were purified from fore‐limb and hind limb skeletal muscles by flow cytometry using the SM/C‐2.6 antibody (Fig. 7A). The population of Ten‐4+/− and Ten‐4−/− satellite cells in the skeletal muscles from fore‐limb and hind limbs was less than that of WT cells (Fig. 7A), suggesting that satellite cells were reduced in not only the TA muscle but also other skeletal muscles of mutant mice. We then cultured the purified satellite cells and analyzed cell proliferation and differentiation into myotubes. When cells were cultured under the proliferative condition for 3 days, ∼70% of WT cells were positive for Ki67, indicating that these Ki67‐positive cells were in the activated phase, as reported previously 21. In the Ten‐4−/− culture, the number of proliferating cells, labeled with anti‐Ki67 antibody, was increased, compared with in the WT culture (Fig. 7B). This data show an accelerated activation of Ten‐4−/− satellite cells, and agrees with the in vivo immunohistochemistry result (Fig. 6). At this stage, most proliferating cells were positive for MyoD and represented spread and typical myoblast‐like morphology (data not shown), meaning that activated satellite cells were differentiating into myoblasts. We further performed the differentiation assay into myotubes. Two days after induction of differentiation, the MHC‐positive myotubes with multiple nuclei from Ten‐4+/− and Ten‐4−/− cells were increased compared with those from WT cells (Fig. 7C). In contrast, a decrease of MHC‐positive cells with single nucleus was observed in the Ten‐4+/− and −/− cultures (Fig. 7C). This abnormally early differentiation of Ten‐4‐deficient cells may be due to the accelerated activation in satellite cells, since Ten‐4 is specifically expressed in satellite cells 22. However, it is also possible that Ten‐4 deficiency might have promoted differentiation of myoblasts into myotubes. Taken all together, Ten‐4 is a novel suppressor of satellite cell activation and possibly of myogenic differentiation as well, and is required for the formation of the normal and regenerated muscle tissues.
Figure 7.
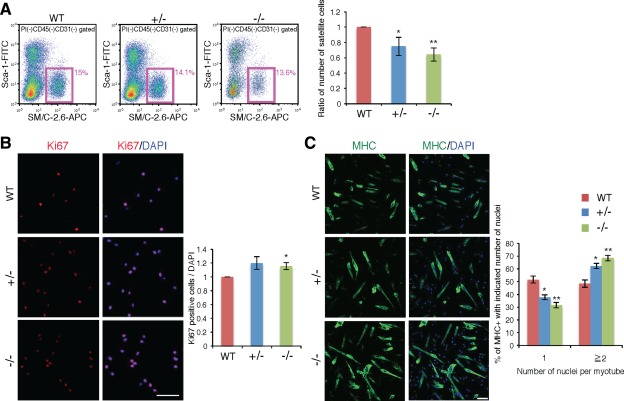
Promoted cell proliferation and differentiation in the culture of purified satellite cells from Ten‐4‐deficient mice. (A): Flow cytometry profiles of satellite cells in WT, Ten‐4+/−, and Ten‐4−/− mouse skeletal muscles from fore‐limb and hind limbs. The CD31−CD45−Sca‐1−SM/C‐2.6+ cells were analyzed and sorted out as muscle satellite cells using the MoFlo flow cytometer. Pink boxes represent the satellite cell population, and values in pink denote percentages of satellite cells in total mononuclear cells, except for CD45+ lymphocytes/leukocytes and CD31+ endothelial cells. The percentage of WT satellite cells was set as 1.0. Error bars, SEM; *, p < .05; **, p < .01. (B): Immunostaining of Ki67 in WT, Ten‐4+/−, and Ten‐4−/− cells cultured for 3 days with the proliferation medium. Cells were stained with anti‐Ki67 antibody (red) and DAPI (blue) to label proliferative cells and nuclei, respectively. The relative numbers of proliferative cells were calculated. The cell number of the WT culture was set as 1.0. Error bars, SEM; *, p < .05; Scale bar = 100 µm. (C): Immunostaining of MHC in WT, Ten‐4+/−, and Ten‐4−/− cell cultures 2 days after induction of differentiation. Cells were stained with anti‐MHC antibody (green) and DAPI (blue) to visualize differentiating myoblasts/myotubes and nuclei, respectively. The number of nuclei in MHC‐positive myotubes or cells was measured and quantified. Error bars, SEM; *, p < .05; **, p < .01. Scale bar = 100 µm. Abbreviations: APC, allophycocyanin; MHC, myosin heavy chain; WT, wild type.
Discussion
In this study, we showed a critical role of Ten‐4 in skeletal muscle satellite cells. In Ten‐4−/− mice with/without the injection of CTX, the number and size of muscle fibers were smaller, and the satellite cell population was decreased. In addition, activation of satellite cells was promoted in Ten‐4−/− satellite cells. From these observations, Ten‐4 functioned as a suppressor of quiescence in satellite cells.
A genome‐wide gene expression analysis revealed that a specific expression of Ten‐4 is detected in quiescent satellite cells, and that the expression is diminished in activated satellite cells, similar to that of calcitonin receptor, a marker for quiescent satellite cells 21. Analysis of Ten‐4 expression pattern by immunostaining showed that all the quiescent satellite cells expressed Ten‐4 at the neonatal stage, while the calcitonin receptor was expressed only in part of the cells 22. These evidences indicate that Ten‐4 is a new marker of quiescent satellite cells, and its expression pattern is broader than that of calcitonin receptor. Here, we found that the population of satellite cells was lower in Ten‐4−/− mice than in WT mice (Figs. 3, 5). Therefore, Ten‐4 was required for production and/or maintenance of the normal satellite cell population. The loss of satellite cells perturbs the postnatal muscle growth, because of defects in the myonucleus fusion, in Pax7 null mice 9, 10, 11. Furthermore, a reduced number and size of myofibers are observed in Pax7 knockout mice after the regeneration process 9, 25. In this study, the caliber size of myofibers and the weight of the TA muscle were decreased in both normal and regenerated cases, due to the deficiency of Ten‐4 (Figs. 2, 4). Also, we found an attenuation of the postnatal body growth of Ten‐4−/− mice (Fig. 1). These defects were presumably caused by the declined number of Ten‐4−/− satellite cells, similar to the phenotype in Pax7 knockout mice.
There are several studies regarding the mechanism of maintaining quiescent satellite cells. In the conditional knockout mice of CSL, a key molecule of Notch signaling, and the double knockout mice of Hesr1 and Hesr3, target genes of Notch, a decrease of satellite cells is observed, which results in the impairment of both normal and regenerated muscle formation 13, 14, 15. Moreover, in the satellite cells of the both cases, a spontaneous exit from the quiescent phase occurs, indicating that these pathways are necessary for maintaining the quiescence. These mutant satellite cells undergo abnormally early differentiation and fail to self‐renew properly 13, 14, 15. Our data in this study showed the increased number of Pax7‐ and Ki67‐dual positive cells in Ten‐4−/− tissue (Fig. 6) and the higher mRNA expression level of MyoD in Ten‐4−/− satellite cells (Supporting Information Fig. S2), which indicates that an abnormally accelerated differentiation occurred in satellite cells due to the Ten‐4 deficiency. In the Ten‐4−/− culture of primary satellite cells, furthermore, there was an increase of activated Ki67‐positive proliferating cells in a few days after plated (Fig. 7). However, the percentage of Ten‐4−/− satellite cell population between with and without regeneration was not changed, although it was lower than that of WT in both cases (Figs. 3, 5). These observations suggest that Ten‐4 maintained the quiescence of satellite cells and was dispensable for the self‐renewal during regeneration. These evidences lead us to hypothesize that a premature differentiation did not allow to reach a proper cell number, and disturbed the proper process of myofiber formation, which resulted in a reduced number and size of myofibers in Ten‐4−/− muscles. In addition, MHC‐positive multinucleated myotubes were increased in the cultures of Ten‐4−/− primary cells (Fig. 7). This may be caused by the accelerated activation in the Ten‐4−/− satellite cells, as the specific expression is found in satellite cells 22. However, it is also possible that Ten‐4 may be weakly expressed during myoblast and/or myotube stages and regulates their differentiation and maturation as a suppressor.
Ten‐4+/− mice exhibited defects in several analyses shown in this study, while significant abnormalities in Ten‐4−/− mice were found in all the analyses (Figs. 1, 2, 3, 4, 5, 6, 7). In regenerating Ten‐4+/− TA, for instance, the weight was lighter than WT, whereas the size and the entire cross‐sectioned area of TA were normal (Fig. 4). This may be due to a delay of muscle growth and maturation in the regenerating Ten‐4+/− tissue. There is presumably a difference in an increase/decrease of downstream signaling and/or compensational feedback discussed below between hetero‐ and homo‐zygosity of Ten‐4.
The Notch signaling participates in the satellite cell quiescence 26. Inhibition of the Notch signaling in satellite cells, as well as the deficiency of Ten‐4 as shown in this study, forces to exit the quiescent state 13, 14, 15. Brohl et al. reported that the Ten‐4 expression level was significantly reduced in the double knockout mice of RBP‐J and MyoD, where Notch signaling was deactivated. Furthermore, Ten‐4 was upregulated in satellite cells by stimulating the Notch signaling with its ligand Dll1, suggesting that Ten‐4 is a downstream molecule of the Notch signaling 27. Our preliminary data showed that the expression levels of Notch pathway molecules, including Hesr1 and Hesr3, were elevated in Ten‐4−/− satellite cells. This increased expression may have been due to a feedback. In addition, genes encoding cell adhesion molecules and basement membrane components, such as integrin alpha‐7, dystroglycan, alpha‐1 chain of type XVIII collagen, and alpha‐2 chain of type IV collagen, were also upregulated and downregulated in satellite cells by the stimulus with Dll1 and by the deficiency of RBP‐J and MyoD, respectively 27. Adhesion to the basement membrane and the myofiber plasma membrane is crucial for the quiescence of satellite cells in their niche 28. We have previously reported that Ten‐4 regulates the activation of focal adhesion kinase, a critical downstream molecule of integrins and dystroglycan, in neural and glial cells 23, 28, 29. Therefore, Ten‐4 may play a key role in satellite cells at the quiescent state together with these proteins.
There are four members in the vertebrate teneurin family (Ten‐1 to Ten‐4). Some cell types express specific teneurin members, but the others express all the members 20, 29, 30, 31. In muscle development, no detailed expression analysis of teneurin family members, except for Ten‐4, has been reported. However, in a genome‐wide expression analysis by microarray, Ten‐3 is expressed in quiescent satellite cells, and an attenuation of its expression level is observed in activated satellite cells 21. This expression pattern of Ten‐3 is similar to that of Ten‐4. In addition, both, Ten‐4 and Ten‐3, are expressed in somites during somitogenesis, where and when Pax3‐ and Pax7‐positive myogenic progenitor cells appear 6, 30. Since teneurins share a high homology in their sequence and bind homophilically or heterophilically between their family members 32, it is possible that Ten‐4 and Ten‐3 redundantly regulate critical signaling for the quiescence of satellite cells. The absence of Ten‐4 significantly diminished the function of satellite cells, but did not eliminate it completely (Figs. 2, 3, 4, 5, 6). Ten‐3 and/or the other teneurins may have partially compensated for the lack of Ten‐4.
Summary
In summary, our results provided evidences that Ten‐4 possesses a suppressive function in the satellite cell quiescence and myogenic differentiation. This is the first report to our knowledge that demonstrates the biological function of Ten‐4 in muscle satellite cells. Our findings will facilitate a better understanding of the molecular mechanism of muscle satellite cell biology.
Author Contributions
K.I.: conception and design, collection and/or assembly of data, data analysis and interpretation, and manuscript writing; N.S.: conception and design, financial support, collection and/or assembly of data, data analysis and interpretation, and manuscript writing; Y.M.: collection and/or assembly of data, financial support, and data analysis and interpretation; N.I. and S.T.: conception and design and data analysis and interpretation; N.K.: collection and/or assembly of data; S.F.: provision of study material or patients and data analysis and interpretation; H.O.: provision of study material or patients; C.A.: conception and design, financial support, data analysis and interpretation, manuscript writing, and final approval of manuscript. K.I. and N.S. contributed equally to this work.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
Supporting information
Supplementary Figure 1
Supplementary Figure 2
Acknowledgments
We thank Dr. Yoshihiko Yamada (NIDCR, NIH, Bethesda, MD) and Dr. Shinsuke Shibata (Keio University, Tokyo, Japan) for providing the furue mouse line and maintaining the line, respectively. We also thank Dr. Hidetoshi Sakurai (CiRA, Kyoto University, Kyoto, Japan) and Dr. Susana de Vega (Juntendo University, Tokyo, Japan) for his advice regarding the experiment and for critical reading the manuscript, respectively. This work was supported by the Grant‐in‐Aid for Scientific Research (C) of the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) in Japan (25505001) (C.A., N.S., and Y.M.), the Grant‐in‐Aid for Young Scientists of the MEXT in Japan (25860701) (N.S.), and the Grant‐in‐Aid from the Ministry of Health, Labor and Welfare of Japan (2260201) (C.A.).
References
- 1. Janssen I, Heymsfield SB, Wang ZM et al. Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. J Appl Physiol (1985) 2000;89:81–88. [DOI] [PubMed] [Google Scholar]
- 2. Shin J, Tajrishi MM, Ogura Y et al. Wasting mechanisms in muscular dystrophy. Int J Biochem Cell Biol 2013;45:2266–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gros J, Manceau M, Thome V et al. A common somitic origin for embryonic muscle progenitors and satellite cells. Nature 2005;435:954–958. [DOI] [PubMed] [Google Scholar]
- 4. Kassar‐Duchossoy L, Giacone E, Gayraud‐Morel B et al. Pax3/Pax7 mark a novel population of primitive myogenic cells during development. Genes Dev 2005;19:1426–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Relaix F, Rocancourt D, Mansouri A et al. A Pax3/Pax7‐dependent population of skeletal muscle progenitor cells. Nature 2005;435:948–953. [DOI] [PubMed] [Google Scholar]
- 6. Relaix F, Zammit PS. Satellite cells are essential for skeletal muscle regeneration: The cell on the edge returns centre stage. Development 2012;139:2845–2856. [DOI] [PubMed] [Google Scholar]
- 7. Zammit PS, Relaix F, Nagata Y et al. Pax7 and myogenic progression in skeletal muscle satellite cells. J Cell Sci 2006;119:1824–1832. [DOI] [PubMed] [Google Scholar]
- 8. White RB, Bierinx AS, Gnocchi VF et al. Dynamics of muscle fibre growth during postnatal mouse development. BMC Dev Biol 2010;10:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kuang S, Charge SB, Seale P et al. Distinct roles for Pax7 and Pax3 in adult regenerative myogenesis. J Cell Biol 2006;172:103–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Relaix F, Montarras D, Zaffran S et al. Pax3 and Pax7 have distinct and overlapping functions in adult muscle progenitor cells. J Cell Biol 2006;172:91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Seale P, Sabourin LA, Girgis‐Gabardo A et al. Pax7 is required for the specification of myogenic satellite cells. Cell 2000;102:777–786. [DOI] [PubMed] [Google Scholar]
- 12. Kuang S, Gillespie MA, Rudnicki MA. Niche regulation of muscle satellite cell self‐renewal and differentiation. Cell Stem Cell 2008;2:22–31. [DOI] [PubMed] [Google Scholar]
- 13. Mourikis P, Sambasivan R, Castel D et al. A critical requirement for notch signaling in maintenance of the quiescent skeletal muscle stem cell state. Stem Cells 2012;30:243–252. [DOI] [PubMed] [Google Scholar]
- 14. Bjornson CR, Cheung TH, Liu L et al. Notch signaling is necessary to maintain quiescence in adult muscle stem cells. Stem Cells 2012;30:232–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fukada S, Yamaguchi M, Kokubo H et al. Hesr1 and Hesr3 are essential to generate undifferentiated quiescent satellite cells and to maintain satellite cell numbers. Development 2011;138:4609–4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wen Y, Bi P, Liu W et al. Constitutive Notch activation upregulates Pax7 and promotes the self‐renewal of skeletal muscle satellite cells. Mol Cell Biol 2012;32:2300–2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cheung TH, Quach NL, Charville GW et al. Maintenance of muscle stem‐cell quiescence by microRNA‐489. Nature 2012;482:524–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tucker RP, Chiquet‐Ehrismann R. Teneurins: A conserved family of transmembrane proteins involved in intercellular signaling during development. Dev Biol 2006;290:237–245. [DOI] [PubMed] [Google Scholar]
- 19. Kenzelmann‐Broz D, Tucker RP, Leachman NT et al. The expression of teneurin‐4 in the avian embryo: Potential roles in patterning of the limb and nervous system. Int J Dev Biol 2010;54:1509–1516. [DOI] [PubMed] [Google Scholar]
- 20. Suzuki N, Mizuniwa C, Ishii K et al. Teneurin‐4, a transmembrane protein, is a novel regulator that suppresses chondrogenic differentiation. J Orthop Res 2014;32:915–922. [DOI] [PubMed] [Google Scholar]
- 21. Fukada S, Uezumi A, Ikemoto M et al. Molecular signature of quiescent satellite cells in adult skeletal muscle. Stem Cells 2007;25:2448–2459. [DOI] [PubMed] [Google Scholar]
- 22. Yamaguchi M, Ogawa R, Watanabe Y et al. Calcitonin receptor and Odz4 are differently expressed in Pax7‐positive cells during skeletal muscle regeneration. J Mol Histol 2012;43:581–587. [DOI] [PubMed] [Google Scholar]
- 23. Suzuki N, Fukushi M, Kosaki K et al. Teneurin‐4 is a novel regulator of oligodendrocyte differentiation and myelination of small‐diameter axons in the CNS. J Neurosci 2012;32:11586–11599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fukada S, Higuchi S, Segawa M et al. Purification and cell‐surface marker characterization of quiescent satellite cells from murine skeletal muscle by a novel monoclonal antibody. Exp Cell Res 2004;296:245–255. [DOI] [PubMed] [Google Scholar]
- 25. Oustanina S, Hause G, Braun T. Pax7 directs postnatal renewal and propagation of myogenic satellite cells but not their specification. EMBO J 2004;23:3430–3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fujimaki S, Machida M, Hidaka R et al. Intrinsic ability of adult stem cell in skeletal muscle: An effective and replenishable resource to the establishment of pluripotent stem cells. Stem Cells Int 2013;2013:420164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brohl D, Vasyutina E, Czajkowski MT et al. Colonization of the satellite cell niche by skeletal muscle progenitor cells depends on Notch signals. Dev Cell 2012;23:469–481. [DOI] [PubMed] [Google Scholar]
- 28. Chacon MR, Fazzari P. FAK: Dynamic integration of guidance signals at the growth cone. Cell Adh Migr 2011;5:52–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Suzuki N, Numakawa T, Chou J et al. Teneurin‐4 promotes cellular protrusion formation and neurite outgrowth through focal adhesion kinase signaling. FASEB J 2014;28:1386–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhou XH, Brandau O, Feng K et al. The murine Ten‐m/Odz genes show distinct but overlapping expression patterns during development and in adult brain. Gene Expr Patterns 2003;3:397–405. [DOI] [PubMed] [Google Scholar]
- 31. Li H, Bishop KM, O'Leary DD. Potential target genes of EMX2 include Odz/Ten‐M and other gene families with implications for cortical patterning. Mol Cell Neurosci 2006;33:136–149. [DOI] [PubMed] [Google Scholar]
- 32. Feng K, Zhou XH, Oohashi T et al. All four members of the Ten‐m/Odz family of transmembrane proteins form dimers. J Biol Chem 2002;277:26128–26135. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1
Supplementary Figure 2


