Abstract
Objective
Posaconazole prophylaxis during induction chemotherapy for acute myeloid leukaemia (AML) and myelodysplastic syndromes (MDS) has been shown to significantly decrease the incidence of invasive fungal disease (IFD) and increase overall survival in a trial setting, but only small real‐life studies have been published.
Methods
This was a retrospective cohort study including consecutive patients with AML/MDS treated with intensive induction chemotherapy; 176 patients received fluconazole prophylaxis 2008–2011 and 107 patients received posaconazole prophylaxis 2011–2013. Only proven and probable IFD according to the revised EORTC/MSG criteria were included in the analysis.
Results
The two cohorts were well matched without significant differences in patient characteristics. At day 100, patients receiving posaconazole had a significantly lower incidence of total IFD (0.9% vs. 10.8%, P < 0.01), invasive aspergillosis (0% vs. 5.7%, P = 0.02) and invasive candidiasis (0% vs. 4.0%, P < 0.05). There was no significant difference in overall survival, neither at day 100 (87% in the posaconazole group vs. 85% in the fluconazole group) nor at end of follow‐up (78% vs. 77%).
Conclusions
Posaconazole prophylaxis decreased the incidence of IFD but did not improve short‐term overall survival. Improved treatment efficacy of manifest IFD is likely to explain the lack of survival benefit.
Keywords: posaconazole, fluconazole, prophylaxis, neutropenia, acute myeloid leukaemia, invasive fungal disease, invasive aspergillosis
Invasive fungal disease (IFD) is a major complication of chemotherapy‐induced severe neutropenia during treatment for acute myeloid leukaemia (AML) 1, 2, 3. One potential way of reducing the incidence of IFD is the administration of primary antifungal prophylaxis (PAP) during neutropenia. In a randomised controlled trial conducted between 2002 and 2005, the efficacy of posaconazole, an oral triazole with activity against a broad spectrum of moulds, was compared to fluconazole/itraconazole as PAP in patients receiving intensive induction treatment for AML and myelodysplastic syndromes (MDS) 4. The results showed that posaconazole not only significantly reduced the incidence of IFD from 11% to 5%, but also increased overall survival. As a result, most guidelines for supportive care during AML treatment support the use of posaconazole as PAP 5, 6, 7.
Since then, three cohort studies investigating real‐life experiences with posaconazole vs. fluconazole prophylaxis in patients with AML/MDS have been published, all finding decreased incidence of IFD but no effect on overall survival 8, 9, 10. However, two of the studies were small and included only 130 and 125 patients, respectively 8, 9. The third study, a multicenter trial in China, included 234 patients, but young age (median 40 yr of age), lack of information regarding choice and dosing of chemotherapy, short duration of neutropenia (46% of patients had <14 d of neutropenia) and low overall mortality at day 100 (6.0% in fluconazole recipients compared to 21% in the randomised trial by Cornely et al.) make generalisation of the results difficult 10. The aim of this retrospective cohort study was therefore to investigate the effect of a change in PAP from fluconazole to posaconazole on IFD incidence and overall survival in a large contemporary cohort of patients with AML/MDS in a real‐life setting.
Patients and methods
This retrospective cohort study was performed at Karolinska University Hospital, Stockholm, Sweden. Patients were treated at two geographically different hospital sites, both full service hospitals with approximately 650 beds each. The study was approved by the Regional Ethical Review Board in Stockholm (DNR 2012/129‐31/1). All patients with AML or MDS who received intensive induction chemotherapy between January 2008 and March 2013 were eligible for inclusion. Exclusion criteria were previous IFD or not receiving the same antifungal prophylaxis during all cycles of chemotherapy (except when prophylaxis was changed to therapy of suspected or proven IFD). Clinical data were collected from the start of induction chemotherapy to 30 d after resolution of neutropenia after the last consolidation chemotherapy, to allogeneic stem cell transplantation (SCT), to conversion to palliative care or to death, whichever occurred first. As both suspected and undiagnosed IFD may influence the decision of conversion to palliative care, all proven and probable IFDs occurring within four weeks after the conversion were included in the analysis. The incidence of bacteraemia (excluding all isolates of coagulase‐negative staphylococci) was recorded as a non‐equivalent dependent variable; a variable independent of changes in fungal prophylaxis but otherwise having similar potential causal variables as IFD 11.
Treatment regimens for AML were similar throughout the whole study period and followed national guidelines 12. Standard induction therapy typically consisted of daunorubicin (60 mg/m2) on days 1–3 and high‐dose cytarabin (1000–2000 mg/m2 twice daily) on days 1–5 12.
Fungal prophylaxis was, in most cases, administered only during neutropenia (defined as absolute neutrophil count <0.5 × 109/L). Fluconazole, 100 or 200 mg once daily, was used as PAP between January 2008 and March 2011. During April to June 2011, PAP was gradually changed from fluconazole to posaconazole 200 mg three times daily, and after June 2011, only posaconazole was used. Therapeutic drug monitoring was not routinely conducted. The wards were not equipped with HEPA filters. Fungal surveillance with galactomannan or beta‐glucan assays was not performed. In case of fever more than 72 h or deterioration, despite broad spectrum antibiotics, the typical diagnostic workup consisted of blood cultures, a thoracic computed tomography (CT) and galactomannan testing in serum. In case of lesions on CT indicative of a fungal infection, investigation by broncho‐alveolar lavage was performed if feasible and, in most cases, treatment with voriconazole started. Empiric antifungal treatment was usually initiated after 4–5 d of neutropenic fever even if no infiltrates were seen on thoracic CT and consisted most often of caspofungin. Bacterial prophylaxis with ciprofloxacin, 500 mg twice daily, was used during neutropenia in approximately 80% of patients at the start of study period, increasing to above 90% at the end.
IFD was defined according to the revised 2008 EORTC/MSG definitions 13. An experienced thoracic radiologist (KC) re‐examined all thoracic CTs conducted during the study period. Lesions less than 1 cm were recorded as non‐significant. Primary outcome was incidence of proven and probable IFD. Secondary outcome was overall survival.
Statistical methods
All statistical data analysis was carried out in RStudio 0.97.551 (Boston, MA, USA) with R‐base version 3.0.2. Differences of proportions were tested using Fisher's exact test and the independent t‐test for differences in means. Kaplan–Meier curves were calculated for overall survival, and statistical comparisons were performed by log‐rank test. All statistical tests were two‐tailed, and P < 0.05 was defined as statistically significant.
Results
Patients
A total of 328 patients were eligible for inclusion. Twenty‐one patients that received neither of the two PAP regimens studied (17 received no prophylaxis, three received voriconazole, one unknown) and 24 patients that changed PAP during treatment cycles (in most cases due to changes in treatment recommendations during the study period) were excluded. Of the remaining 283 patients, 176 received prophylaxis with fluconazole and 107 posaconazole. The cohorts were well matched at baseline (Table 1). The incidence of bacteraemia during neutropenia was the same in both cohorts (0.8 episodes per patient), supporting the notion that besides changing fungal prophylaxis, no other significant changes in treatment were introduced during the study period.
Table 1.
Patient and disease characteristics
| Fluconazole (n = 176) | Posaconazole (n = 107) | P‐value | |
|---|---|---|---|
| Age (median; range) | 61 (21–85) | 58 (21–79) | 0.1 |
| Female | 81 (46%) | 46 (43%) | 0.7 |
| Primary leukaemia | 142 (81%) | 88 (82%) | 0.9 |
| AML | 154 (87%) | 92 (86%) | 0.9 |
| Neutropenia after induction therapy (mean days) | 19.9 | 21.8 | 0.06 |
| Total days of neutropenia (mean days) | 38.5 | 40.6 | 0.4 |
| Treatment cycles | 2.55 | 2.53 | 0.9 |
| Follow‐up (mean days)a | 117 | 119 | 0.7 |
| Remission, non‐allogeneic SCT | 78 (44%) | 46 (43%) | 0.9 |
| Allogeneic SCT | 51 (29%) | 31 (29%) | 0.9 |
| Palliative or dead during active treatment | 47 (27%) | 30 (28%) | 0.9 |
| Neutropenic feverb | 2.5 | 2.6 | 0.7 |
| Bacteraemiab | 0.8 | 0.8 | 0.8 |
| Empirical treatmentc | 50 (32%) | 22 (21%) | 0.05 |
| Possible IFD | 10 (5.7%) | 6 (5.6%) | 1.0 |
AML, acute myeloid leukaemia; SCT, stem cell transplantation; IFD, invasive fungal disease.
Calculated from first day of induction chemotherapy.
Mean number of episodes per patient.
Patients receiving empirical mould‐active treatment without fulfilling criteria for proven or probable IFD.
Incidence of IFD
The incidence of all IFD was significantly decreased in patients receiving posaconazole both at day 100 and at end of follow‐up (0.9% vs. 10.7%, P < 0.01, and 2.8% vs. 12.5%, P < 0.01, respectively) (Fig. 1) (Table 2). The incidences of invasive aspergillosis and invasive candidiasis were both significantly decreased in posaconazole recipients at day 100 (0% vs. 5.7%, P = 0.01, and 0% vs. 4.0%, P < 0.05, respectively) (Table 2). There was no difference in invasive mould infections between patients with primary and relapsed leukaemia (fluconazole recipients 11/142 vs. 3/34, P = 0.74, posaconazole recipients 2/88 vs. 1/19, P = 0.45, all 13/240 vs. 4/53, P = 0.53).
Figure 1.
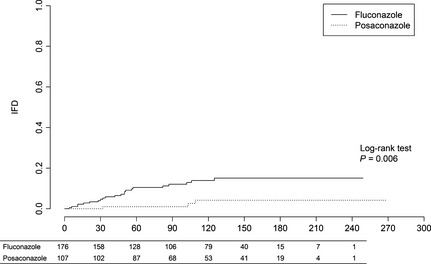
Cumulative incidence of invasive fungal disease. The table below graph shows the number of patients at risk.
Table 2.
Proven and probable invasive fungal disease at day 100 and at end of follow‐up
| Fluconazole (n = 176) | Posaconazole (n = 107) | P‐value | |
|---|---|---|---|
| Total IFD | |||
| Day 100 | 19 (10.8%) | 1 (0.9%) | <0.01 |
| End of follow‐up | 22 (12.5%) | 3 (2.8%) | <0.01 |
| Total mould | |||
| Day 100 | 11 (6.3%) | 1 (0.9%) | 0.03 |
| End of follow‐up | 14 (8.7%) | 3 (2.8%) | 0.1 |
| Aspergillus spp. | |||
| Day 100 | 10 (5.7%) | 0 (0%) | 0.01 |
| End of follow‐up | 11 (6.3%) | 2 (1.9%) | 0.14 |
| Candida spp | |||
| Day 100 | 7 (4.0%) | 0 (0%) | <0.05 |
| End of follow‐up | 7 (4.0%) | 0 (0%) | <0.05 |
| Species distribution | |||
| Mould | |||
| Aspergillus fumigatus | 2 | 1 | |
| Aspergillus niger | 1 | ||
| Aspergillus spp.a | 8 | 1 | |
| Rhizomucor pusillus | 1 | ||
| Rhizomucor miehei | 1 | ||
| Fusarium solani | 1 | ||
| Proven mould b | 1 | ||
| Yeast | |||
| Candida albicans | 2 | ||
| Candida krusei | 2 | ||
| Candida glabrata | 1 | ||
| Candida tropicalis | 1 | ||
| Candida parapsilosis | 1 | ||
| Blastoschizomyces capitatus | 1 | ||
IFD, invasive fungal disease.
Diagnosed with positive galactomannan test. None of the patients had received pipercillin + tazobactam within four days of the positive test. Five patients had a positive galactomannan test in BAL, two patients had positive tests both in BAL and serum, one patient had two positive tests in serum, and one patient had a single positive test in serum.
Hyphae in lung biopsy, culture negative.
Five of 17 invasive mould infections occurred during the first induction therapy, four during re‐induction therapy, four during the first consolidation therapy, three during the second consolidation therapy and one during the third consolidation therapy. There was a trend towards longer mean time to first fungal episode in patients receiving posaconazole (81 vs. 48 d, P = 0.3). Antifungal therapy was used more often in the fluconazole group than in the posaconazole group, measured both as mean days with antifungal therapy (4 vs. 25, P < 0.01) and proportion of patients receiving antifungal therapy (23% vs. 40%, P < 0.01).
Overall survival
There was no significant difference in overall survival at day 100 (87% in the posaconazole group vs. 86% in the fluconazole group [relative risk (RR) 0.99, 95% CI 0.90–1.09]) and at end of follow‐up [78% in the fluconazole group vs. 77% in the posaconazole group (RR 1.02, 95% CI 0.90–1.17)] (Fig. 2). Four patients in the fluconazole group with proven or probable IFD died within 100 d: one patient died because of mucormycosis; one patient in a combination of invasive aspergillosis and refractory leukaemia; one patient in a combination of candidemia and refractory leukaemia; and one patient with candidemia died because of brain oedema of unknown origin. In the posaconazole group, one patient died from a combination of mucormycosis and refractory leukaemia.
Figure 2.
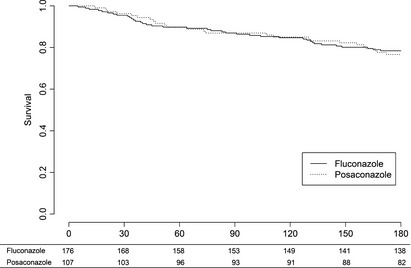
Kaplan–Meier estimates of overall survival. There was no difference in overall survival between the groups using the log‐rank test. The table below graph shows the number of patients at risk.
Discussion
Primary prophylaxis with posaconazole has, in contrast to fluconazole, been shown to reduce IFD in neutropenic AML/MDS patients, both in a large randomised trial and in small real‐life cohorts 4, 8, 9, 10, 14, 15. In the Nordic countries, including Sweden, the incidence of mould infections has been thought to be low and many centres have continued using fluconazole as primary prophylaxis. Prophylaxis guidelines were changed at our institution during 2011, based on a perceived increase in the number of patients with IFD, possibly due to building activities at the hospital sites. This change enabled us to perform this large real‐life study investigating posaconazole vs. fluconazole as primary fungal prophylaxis in consecutive patients with AML/MDS receiving induction therapy. We found a significantly lower incidence of proven and probable IFD in patients receiving posaconazole compared to fluconazole prophylaxis, both at day 100 and at end of follow‐up. The decrease in incidence was even larger than that observed in the randomised trial by Cornely et al. (present study 11 to 1%, Cornely study 11 to 3% when excluding itraconazole recipients) 4. In accordance with earlier reports, prophylaxis with posaconazole significantly reduced the requirement for other antifungal treatment 8. Interestingly, 47% of the mould infections were diagnosed during consolidation therapy. Earlier studies and guidelines have often focused on the risk for mould infections and need for mould‐active prophylaxis during induction therapy 4, 7. The results in this study indicate that it might be appropriate to use mould‐active prophylaxis during all periods of neutropenia.
We did not find any trend towards an improvement of overall survival with posaconazole prophylaxis. This was somewhat unexpected because the decrease in mould infections in posaconazole recipients at day 100 was similar to that in the Cornely study: 6.3 to 0.9% in the present study and 7.1 to 1.0% in the Cornely study. A likely explanation could be the lower attributably mortality of IFD in the current study compared to the Cornely study (1.7% vs. 5%) 4. Other real‐life studies in patients with AML/MDS have also reported a significant decrease in IFD incidence in patients receiving posaconazole prophylaxis with no impact on overall survival 8, 9, 10. One possible explanation for the lower attributable mortality is that treatment with voriconazole is associated with improved prognosis of invasive aspergillosis 16, 17. This was not known when the trial by Cornely was initiated and patients with proven or probable breakthrough infections were treated at the discretion of the local physicians. Another contributing factor might be the continuing improvement of supportive care, whereas earlier diagnosis of invasive aspergillosis appears to be a less probably explanation because no fungal surveillance with galactomannan (or beta‐glucan) tests was performed during the study period. However, it is important to acknowledge that one effect of reducing the frequency of IFD is that patients eligible for allogeneic SCT could be transplanted without delay due to IFD. Since the patients were censored at the time of allogeneic SCT, the potential positive effect of this factor could not be analysed. In addition, postponing of consolidation therapy because of IFD has been shown to negatively impact long‐term survival 18. Considering that the number of patients needed to treat to avoid one probable or proven mould infection was relatively low, 19 at day 100, the severity of invasive‐mould infections and the low frequency of severe side effect of the drug, we believe that prophylaxis with posaconazole during chemotherapy‐induced neutropenia in patients with AML/MDS is indicated despite no clear beneficial effect on short‐term survival.
There are limitations to the study, the most important being its retrospective nature. We surmised that the short time span (5.5 yr) together with inclusion of all patients receiving induction chemotherapy would render the two cohorts comparable. This is supported by the fact that the cohorts were well matched at baseline. In addition, the incidence of bacteraemia during the two study periods was found to be unchanged. A lower dose of fluconazole, 100 or 200 mg, was used compared to the 400 mg in the Cornely study. This might explain the significant difference in the incidence of candidemia seen in the present study, especially as several of the strains isolated were Candida albicans sensitive to fluconazole. The sensitivity of the galactomannan test has been shown to be decreased by mould‐active prophylaxis 19. This might decrease the number of probable cases with a corresponding increase of possible cases, as, in most studies, a positive galactomannan test is the most common mycological criterion to be fulfilled. Such an effect may have been true also in the present study with the majority of the probable aspergillosis infections in fluconazole recipients being diagnosed with a positive galactomannan test, even if there were no difference in possible invasive‐mould infections between the groups.
To conclude, we found that primary posaconazole prophylaxis significantly decreased the incidence of IFD, but had no impact on short‐term overall survival, seemingly due to a low attributable mortality of IFD in patients receiving fluconazole.
Conflict of interest and Sources of funding
This work was supported by unrestricted grants from Merck & Co and Adolf H. Lundin Charitable Foundation. M Kalin has previously received lecture fees and M Björkholm consultation fees from Merck & Co. Per Ljungman has been an advisor for Merck & Co regarding planning of a clinical trial. O Blennow has previously received an unrestricted grant from Merck & Co. All other authors: none to declare.
Acknowledgements
Part of the results presented in the article has been presented as an oral presentation at the 24th ECCMID, 10–13 May 2014, in Barcelona, Spain.
The copyright line for this article was changed on 5 June 2015 after original online publication.
References
- 1. Michallet M, Bénet T, Sobh M, et al Invasive aspergillosis: an important risk factor on the short‐ and long‐term survival of acute myeloid leukemia (AML) patients. Eur J Clin Microbiol Infect Dis 2012;31:991–7. [DOI] [PubMed] [Google Scholar]
- 2. Neofytos D, Lu K, Hatfield‐Seung A, Marr K, Treadway S, Ostrander D, Nussenblatt V, Karp J. Epidemiology, outcomes, and risk factors of invasive fungal infections in adult patients with acute myelogenous leukemia after induction chemotherapy. Diagn Microbiol Infect Dis 2013;75:144–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nicolle MC, Benet T, Thiebaut A, et al Invasive aspergillosis in patients with hematologic malignancies: incidence and description of 127 cases enrolled in a single institution prospective survey from 2004 to 2009. Haematologica 2011;96:1685–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cornely O, Maertens J, Winston DJ, et al Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med 2007;356:348–59. [DOI] [PubMed] [Google Scholar]
- 5. National Comprehensive Cancer Network . NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Acute Myeloid Leukemia. 2013.
- 6. Döhner H, Estey EH, Amadori S, et al Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood 2010;115:453–74. [DOI] [PubMed] [Google Scholar]
- 7. Maertens J, Marchetti O, Herbrecht R, et al European guidelines for antifungal management in leukemia and hematopoietic stem cell transplant recipients: summary of the ECIL 3 – 2009 update. Bone Marrow Transplant 2011;46:709–18. [DOI] [PubMed] [Google Scholar]
- 8. Ananda‐Rajah MR, Grigg A, Downey MT, Bajel A, Spelman T, Cheng A, Thursky KT, Vincent J, Slavin M. Comparative clinical effectiveness of prophylactic voriconazole/posaconazole to fluconazole/itraconazole in patients with acute myeloid leukemia/myelodysplastic syndrome undergoing cytotoxic chemotherapy over a 12‐year period. Haematologica 2012;97:459–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kung HC, Johnson MD, Drew RH, Saha‐Chaudhuri P, Perfect JR. Clinical effectiveness of posaconazole versus fluconazole as antifungal prophylaxis in hematology‐oncology patients: a retrospective cohort study. Cancer Med 2014;3:667–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shen Y, Huang X‐J, Wang J‐X, et al Posaconazole vs. fluconazole as invasive fungal infection prophylaxis in China: a multicenter, randomized, open‐label study. Int J Clin Pharmacol Ther 2013;51:738–45. [DOI] [PubMed] [Google Scholar]
- 11. Harris AD, Lautenbach E, Perencevich E. A systematic review of quasi‐experimental study designs in the fields of infection control and antibiotic resistance. Clin Infect Dis 2005;41:77–82. [DOI] [PubMed] [Google Scholar]
- 12. Derolf AR, Kristinsson SY, Andersson TM, Landgren O, Dickman PW, Bjorkholm M. Improved patient survival for acute myeloid leukemia: a population‐based study of 9729 patients diagnosed in Sweden between 1973 and 2005. Blood 2009;113:3666–72. [DOI] [PubMed] [Google Scholar]
- 13. De Pauw B, Walsh TJ, Donnelly JP, et al Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis 2008;46:1813–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Girmenia C, Frustaci AM, Gentile G, et al Posaconazole prophylaxis during front‐line chemotherapy of acute myeloid leukemia: a single‐center, real‐life experience. Haematologica 2012;97:560–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vehreschild JJ, Rüping MJGT, Wisplinghoff H, et al Clinical effectiveness of posaconazole prophylaxis in patients with acute myelogenous leukaemia (AML): a 6 year experience of the Cologne AML cohort. J Antimicrob Chemother 2010;65:1466–71. [DOI] [PubMed] [Google Scholar]
- 16. Herbrecht R, Denning DW, Patterson TF, et al Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med 2002;347:408–15. [DOI] [PubMed] [Google Scholar]
- 17. Nivoix Y, Velten M, Letscher‐Bru V, et al Factors associated with overall and attributable mortality in invasive aspergillosis. Clin Infect Dis 2008;47:1176–84. [DOI] [PubMed] [Google Scholar]
- 18. Even C, Bastuji‐Garin S, Hicheri Y, Pautas C, Botterel F, Maury S, Cabanne L, Bretagne S, Cordonnier C. Impact of invasive fungal disease on the chemotherapy schedule and event‐free survival in acute leukemia patients who survived fungal disease: a case‐control study. Haematologica 2011;96:337–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Marr KA, Laverdiere M, Gugel A, Leisenring W. Antifungal therapy decreases sensitivity of the Aspergillus galactomannan enzyme immunoassay. Clin Infect Dis 2005;40:1762–9. [DOI] [PubMed] [Google Scholar]


