Abstract
Ubiquitin is a 76‐amino acid protein whose conjugation to protein targets is a form of post‐translational modification. Protein ubiquitylation is characterized by the covalent attachment of the COOH‐terminal carboxyl group of ubiquitin to an amino group of the substrate protein. Given that the NH2‐terminal amino group is usually masked, internal lysine residues are most often targeted for ubiquitylation. Polyubiquitylation refers to the formation of a polyubiquitin chain on the substrate as a result of the ubiquitylation of conjugated ubiquitin. The structures of such polyubiquitin chains depend on the specific lysine residues of ubiquitin targeted for ubiquitylation. Most of the polyubiquitin chains other than those linked via lysine‐63 and methionine‐1 of ubiquitin are recognized by the proteasome and serve as a trigger for substrate degradation. In contrast, polyubiquitin chains linked via lysine‐63 and methionine‐1 serve as a binding platform for proteins that function in immune signal transduction or DNA repair. With the exception of a few targets such as histones, the functions of protein monoubiquitylation have remained less clear. However, recent proteomics analysis has shown that monoubiquitylation occurs more frequently than polyubiquitylation, and studies are beginning to provide insight into its biologically important functions. Here, we summarize recent findings on protein monoubiquitylation to provide an overview of the targets and molecular functions of this modification.
Introduction
Ubiquitin is a highly conserved 76‐amino acid protein that plays key roles in many aspects of eukaryotic cell function as a protein modifier. Ubiquitin is synthesized as an inactive precursor protein from four distinct genes (UBA52, UBA80, UBB, UBC), and the precursor is then cleaved by a deubiquitinase (DUB) enzyme to yield ubiquitin with active diglycine residues exposed at its COOH‐terminus (Komander et al. 2009) (Fig. 1). In cultured mammalian cells, ~25% of ubiquitin molecules are estimated to exist in the free (unconjugated) form, with the remainder being covalently attached to other protein molecules, including ubiquitin itself (Kaiser et al. 2011). The formation of a peptide bond between ubiquitin and its target proteins requires the activation of ubiquitin by a ubiquitin‐activating enzyme (E1) and its subsequent conjugation to a ubiquitin‐conjugating enzyme (E2), with energy being stored in a thioester bond between a cysteine residue in E1 or E2 and the carboxyl group at the COOH‐terminus of ubiquitin. A ubiquitin ligase (E3) then catalyzes the transfer of ubiquitin to an amino group of the target protein and thereby determines target specificity (Nakayama & Nakayama 2006). Given that the amino group at the NH2‐terminus of most proteins is masked (Arnesen et al. 2009), internal lysine residues are targeted for ubiquitylation in most cases.
Figure 1.
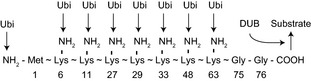
Schematic representation of amino (NH2) and carboxyl (COOH) groups of ubiquitin that play a role in ubiquitylation. Numbers indicate amino acid position. A deubiquitinase (DUB) cleaves the ubiquitin (Ubi)–substrate linkage.
E3 ligases have been divided into two major classes on the basis of a domain structure that reflects the catalytic mechanism (Metzger et al. 2012). One class consists of enzymes that possess a RING (really interesting new gene) domain or a related PHD (plant homeodomain), LAP (leukemia‐associated protein), or U‐box domain and which bind E2 through these domains and promote the direct transfer of ubiquitin from E2 to the substrate protein (Deshaies & Joazeiro 2009). The second class includes E3 ligases that contain a HECT (homologous to E6‐AP carboxyl terminus) domain and which receive ubiquitin from E2 at an active site cysteine residue in this domain before it is linked to the substrate (Rotin & Kumar 2009). The domain–mechanism relationship of E3 ligases was recently broadened by the RING–IBR (in‐between ring)–RING (RBR) domain which forms thioester bond with ubiquitin before transfer to the substrates although it has RING, but not HECT, domains (Smit & Sixma 2014).
E3 function requires simultaneous binding of both substrate and either E2‐ubiquitin (for RING‐type E3 ligases) or ubiquitin (for HECT‐type E3 ligases). Whereas some E3 ligases function as a monomer, most, especially those with a RING domain, exist as a complex that includes an E2‐ubiquitin binding module and a substrate‐binding module. Cullin‐RING E3 ligases (CRLs) are multisubunit E3 ligases that are capable of associating with various substrate‐binding proteins. For example, Cullin1‐based E3 ligases (CRL1) associate with proteins that contain an F‐box domain and which function as substrate‐binding subunits. Substrate‐binding proteins in CRLs are usually indicated by superscript notation, as in CRL1SKP2, in which SKP2 serves as the substrate‐binding subunit. Eight CRLs (CRL1, CRL2, CRL3, CRL4A, CRL4B, CRL5, CRL7, CRL9) have been identified in mammals (Marin 2009), some of which function in monoubiquitylation, as described below.
Polyubiquitylation is a form of ubiquitylation in which a polyubiquitin chain becomes attached to the substrate protein as a result of the ubiquitylation of conjugated ubiquitin. It is thought to be achieved either as a consequence of the processive nature of E3 ligase action—the attachment of multiple ubiquitin molecules to the substrate during a single binding event—or by the en bloc transfer of polyubiquitin to the substrate (Hochstrasser 2006). Given that eight amino groups are available as ubiquitylation sites in ubiquitin (Fig. 1), many different patterns of ubiquitin–ubiquitin linkage are possible. The best‐known function of polyubiquitylation is mediated by K6, K11, K27, K29, K33, and K48 linkage and is to provide a mark for substrate degradation by the proteasome. In contrast, polyubiquitylation mediated by M1 or K63 linkage serves to generate a binding platform for the organization of immune signal transduction or DNA repair machinery.
Compared with polyubiquitylation, the functions of monoubiquitylation of substrate proteins are in general less clear. However, recent proteomics analysis has shown that monoubiquitylation is more prevalent than polyubiquitylation even in cells in which proteasome activity is blocked, suggesting the importance of this modification (Kaiser et al. 2011). Furthermore, the accumulation of polyubiquitylated proteins that occurs in response to proteasome inhibition results in a rapid reduction in the extent of monoubiquitylation, suggesting that monoubiquitylation is reversible in a manner that is sensitive to changes in cellular state (Mimnaugh et al. 1997; Kim et al. 2011). In this review, we present recent findings related to monoubiquitylation, with a focus on the best‐studied mammalian systems, and we provide an overview of monoubiquitylated proteins and the effects of monoubiquitylation on substrates categorized according to their function.
Histone monoubiquitylation and transcriptional regulation
Eukaryotic DNA is packaged into chromatin, in which two copies each of the histones H2A, H2B, H3, and H4 form the nucleosome core particle around which DNA is wrapped. The histone tails are extruded from the nucleosome and are targeted for post‐translational modification including monoubiquitylation. Initially considered an abundant nonhistone chromatin protein because of its differential response to hepatectomy and different amino acid composition compared with histones, A24 was the first identified monoubiquitylated protein and was subsequently found to comprise histone H2A with a ubiquitin molecule attached to its K119 residue (Table 1). Such monoubiquitylation generated a Y‐shaped protein with one COOH‐terminus and two NH2‐termini (Goldknopf & Busch 1977). At least six ubiquitin ligases that catalyze the ubiquitylation of H2A at K119 have since been identified: RNF2 (also known as RING1B) in polycomb repressive complex (PRC) 1 (Wang et al. 2004), TRIM37 in PRC2 (Bhatnagar et al. 2014), BRCA1 in the BRCA1–BARD1 complex (Chen et al. 2002), 2A‐HUB (also known as hRUL138) in the 2A‐HUB complex (Zhou et al. 2008), CRL4A/BDDB2 (Guerrero‐Santoro et al. 2008) and CRL4BRBBP4/7 (Hu et al. 2012). Consistent with the function of these six ubiquitin ligase complexes as transcriptional repressors, monoubiquitylated H2A inhibits transcription by several mechanisms including down‐regulation of methylation of histone H3 at lysine 4 (H3K4) (Nakagawa et al. 2008), a mark of active promoters, and recruitment of PRC2 (Blackledge et al. 2014). Multiple DUBs that remove ubiquitin from H2A have also been identified and shown to be transcriptional activators, including ubiquitin‐specific peptidase (USP) 16 (Joo et al. 2007), USP21 (Nakagawa et al. 2008), BRCA1‐associated protein 1 (BAP1) (Scheuermann et al. 2010) and 2A‐DUB (Zhu et al. 2007). Although H2A is the most abundant ubiquitylated protein in multicellular organisms, ubiquitylated H2A has not been detected in yeast (Robzyk et al. 2000).
Table 1.
Monoubiquitylated substrates related to transcription
| Substrate | Ubi site | E3 ligase | DUB | Effect of monoubiquitylation |
|---|---|---|---|---|
| H2A | K119 | RNF2, TRIM37, BRCA1, 2A‐HUB, CRL4A/BDDB2, CRL4BRBBP4/7 | USP16, USP21, BAP1, 2A‐DUB | Transcriptional repression |
| H2B | K120 | RNF20, RNF40 | USP7, USP22 | Transcriptional activation |
| H1 | K46 | TAF1 | Not reported | Transcriptional activation |
| TET1 | K1537 | CRL4VprBP | Not reported | Recruitment to chromatin |
| TET2 | K1212 | CRL4VprBP | Not reported | Recruitment to chromatin |
| TET3 | K983 | CRL4VprBP | Not reported | Recruitment to chromatin |
| IкBα | Not reported | Not reported | Not reported | Prevention of association with IKK |
| p105 | Not reported | CRL1β‐TrCP | Not reported | Processing |
| NEMO | K277, K309 | cIAP1 | Not reported | Nuclear export |
| PKCε | K321 | RINCK1 | Not reported | Binding to NEMO |
| p53 | K351, K357, K370, K372, K373, K381, K382, K386 | MDM2, MSL2 | USP7, USP10 | Nuclear export |
| FOXO4 | K199, K211 | MDM2 | USP7 | Nuclear import |
| SMAD3 | K33, K53, K81 | Smurf2 | USP15 | Inhibition of DNA binding |
| SMAD4 | K507, K519 | Ectodermin | USP9X | Inhibition of R‐SMAD binding |
| TLE3 | Q domain | XIAP | Not reported | Inhibition of TCF binding |
| PAX3 | Not reported | TAF1 | Not reported | Degradation by proteasome |
In contrast to H2A, monoubiquitylation of H2B is conserved from yeast to mammals. It occurs at conserved lysine residues (K123 in yeast, K120 in mammals) and is catalyzed by the homologous E3 ligases Bre1 in budding yeast and RNF20 and RNF40 in mammals (Espinosa 2008). Genetic analysis in yeast has indicated that H2B monoubiquitylation at K123 is required for H3K4 methylation and consequent transcriptional activation. Most studies in mammals, however, support a function for H2B monoubiquitylation in transcriptional elongation. Monoubiquitylation of H2B is also dynamically regulated, with the ubiquitin moiety being removed by several DUBs including Ubp8 homologues in the SAGA complex (Ubp8 in yeast, USP22 in mammals) (Henry et al. 2003; Zhang et al. 2008), Ubp10 in budding yeast (Gardner et al. 2005) and USP7 in humans (Emre et al. 2005). Although histones H3 and H4 have been shown to undergo ubiquitylation, the function of their monoubiquitylated forms is unclear (Cao & Yan 2012), with the exception of H3 monoubiquitylated at K23 by UHRF1 as described below.
Histone H1 binds the linker region of DNA between nucleosome core particles and controls the dynamics of chromatin structure and gene activity as a result of its high mobility within the nucleus (Catez et al. 2006). In vitro studies have shown that the interaction of H1 with nucleosomes organizes the nucleosome arrays into a condensed 30‐nm chromatin fiber that inhibits DNA‐dependent activities such as transcription and replication (Li & Reinberg 2011). As with other histones, diverse post‐translational modifications including monoubiquitylation are thought to regulate H1 function. TAF1 [TATA box binding protein (TBP)‐associated factor 1] has been found to catalyze the monoubiquitylation of H1 (Pham & Sauer 2000), presumably at K46 (Wisniewski et al. 2007), and thereby to activate transcription. TAF1 monoubiquitylates H1 in the absence of other E1, E2 and E3 enzymes in vitro, suggesting that it may function as a combined E1/E2 enzyme and that H1 ubiquitylation does not require an E3 ligase. TAF1 also monoubiquitylates the transcription factor PAX3, as described below.
Transcriptional regulation by monoubiquitylation of nonhistone proteins
DNA methylation at the C‐5 position of cytosine regulates gene expression and plays pivotal roles in numerous biological processes. The TET dioxygenases catalyze iterative oxidation of 5‐methylcytosine (5mC) that results in demethylation of 5mC, indicating that such DNA methylation is reversible (Wu & Zhang 2014). We recently found that CRL4A/BVprBP catalyzes the monoubiquitylation of all three mouse TET family proteins at a conserved lysine residue (K1537 in TET1, K1212 in TET2, K983 in TET3) (Table 1). Monoubiquitylation of TET proteins promotes their binding to DNA, which is essential for catalytic function of TET in cells (Nakagawa et al. 2015). How monoubiquitylation enhances TET binding to DNA is unclear. The level of TET monoubiquitylation is also regulated by at least one DUB, given that a DUB inhibitor increased TET monoubiquitylation in cells, although the identity of the responsible enzyme (or enzymes) remains unknown.
In the process of DNA replication, the pattern of DNA methylation is also replicated by a monoubiquitylation‐based mechanism. Immediately after DNA replication, the E3 ligase UHRF1 (ubiquitin‐like with PHD and RING finger domains 1) binds to the hemimethylated DNA and monoubiquitylates histone H3 at K23 to generate a binding site for DNA methyltransferase 1, which is responsible for DNA methylation on the daughter strand (Nishiyama et al. 2013).
The activity of transcription factors is also regulated by monoubiquitylation. The nuclear factor (NF)–κB family of transcription factors consists of five members—p50, p52, p65 (also known as RelA), c‐Rel, and RelB—that share an NH2‐terminal Rel homology domain (RHD) responsible for DNA binding as well as for homo‐ or heterodimerization (Hayden & Ghosh 2008). The active NF‐κB dimers bind to κB sites in DNA and thereby regulate transcription of target genes. In the absence of activating stimuli, NF‐κB is maintained inactive either by its association with a member of the IκB family of inhibitor proteins (IκBα, IκBβ, IκBɛ) or the precursor proteins p105 (precursor of p50) or p100 (precursor of p52). Cell stimulation results in the rapid phosphorylation of IκB by the IκB kinase (IKK) complex and its consequent polyubiquitylation by the CRL1β‐TrCP E3 ligase and degradation by the proteasome. The IKK complex, which comprises the kinase subunits IKKα (also known as IKK1) and IKKβ (IKK2) as well as the regulatory subunit IKKγ (NF‐κB essential modulator, or NEMO), is activated by TRAF proteins. Monoubiquitylation regulates NF‐κB activity at several steps. First, IκBα monoubiquitylation by an unidentified E3 ligase inhibits its association with and phosphorylation by IKK and thereby negatively regulates NF‐κB activity (Da Silva‐Ferrada et al. 2011). Second, p105 is monoubiquitylated at multiple lysine residues by CRL1β‐TrCP, which is necessary for its processing to active p50 by the proteasome (Kravtsova‐Ivantsiv et al. 2009). Third, monoubiquitylation of NEMO at K277 and K309 by the E3 ligase cIAP1 in response to genotoxic stress triggers its export from the nucleus and assembly into the IKK complex, resulting in NF‐κB activation (Jin et al. 2009). And fourth, monoubiquitylation of protein kinase Cε (PKCε) at K321 by the E3 ligase RINCK1 in response to growth factor stimulation promotes its association with NEMO, resulting in IKKβ phosphorylation and activation (Yang et al. 2012). Monoubiquitylation thus regulates the activity of NF‐κB in both positive and negative manners.
The tumor suppressor protein p53 is inactivated in more than half of all sporadic human cancers. It suppresses oncogenesis and cancer progression through transcriptional regulation of target genes related to the cell cycle, cellular senescence, apoptosis, metabolism, stem cell maintenance, metastasis and the interaction of tumor cells with their microenvironment (Bieging et al. 2014). Given that p53 hyperactivation as well as p53 loss is detrimental to cells, the amount and activity of this protein are tightly regulated by transcriptional and post‐transcriptional mechanisms including monoubiquitylation‐induced nuclear exclusion (Li et al. 2003; Kruse & Gu 2009). MDM2 and MSL2 each mediate monoubiquitylation of the COOH‐terminal region of p53 (at K370, K372, K373, K381, K382, or K386 by MDM2, and at K351 or K357 by MSL2), which results in a conformational change of the protein that exposes its nuclear export signal (Nie et al. 2007). Interestingly, over‐expression of MDM2 was shown to induce polyubiquitylation of p53, indicating that the amount of MDM2 determines whether p53 is monoubiquitylated or polyubiquitylated. Two DUBs influence the level of p53 monoubiquitylation: USP7 removes ubiquitin from p53 in the cytosol to allow p53 translocation to mitochondria (Marchenko et al. 2007), whereas USP10 does so in the nucleus to activate p53 (Yuan et al. 2010).
The FOX (Forkhead box) family of transcription factors is characterized by the presence of a DNA binding domain (known as the Forkhead domain) and contributes to several physiological processes including development, metabolism, and the immune response. The human genome encodes 50 FOX family proteins, which are divided into 19 subclasses (FOXA to FOXS) on the basis of sequence similarity of the Forkhead domain (Jackson et al. 2010). The FOXO family comprises four members (FOXO1, FOXO3, FOXO4, FOXO6), among which FOXO3 and FOXO4 are monoubiquitylated. The level of FOXO4 monoubiquitylation is determined by enzymes that also target p53 but with opposite consequences. MDM2 thus monoubiquitylates FOXO4 at K199 or K211 and thereby induces its nuclear translocation (rather than nuclear exclusion, as for p53) and activation in response to oxidative stress (Brenkman et al. 2008), and this monoubiquitylation is reversed by USP7‐catalyzed deubiquitylation (van der Horst et al. 2006). Regulation of FOXO3 by MDM2 and USP7 has not been described to date.
SMAD (homologs of the Caenorhabditis elegans protein SMA [small body size] and the Drosophila melanogaster protein MAD [mothers against decapentaplegic]) proteins, mediators of transforming growth factor–β (TGF‐β) signaling, are also monoubiquitylated (Dupont et al. 2012). On ligand activation, the TGF‐β receptor phosphorylates receptor‐regulated SMADs (R‐SMADs: SMAD1, SMAD2, SMAD3, SMAD5, and SMAD8), which then associate with SMAD4 to form an active transcriptional complex that regulates gene expression. Monoubiquitylation of lysine residues that are essential for DNA recognition by R‐SMADs (K33, K53, and K81 in SMAD3) results in the obstruction of DNA binding through steric hindrance. Smurf2 was shown to catalyze monoubiquitylation of SMAD3 (Tang et al. 2011), whereas USP15 deubiquitylates and thereby activates R‐SMADs (Inui et al. 2011). SMAD4 is also regulated by monoubiquitylation. Its E3 ligase, Ectodermin, was first identified in Xenopus embryos, in which this enzyme prevents ectodermal cells from differentiating into endodermal and mesodermal cells (Dupont et al. 2005). Subsequent studies showed that K507 or K519 of SMAD4 is monoubiquitylated by Ectodermin associated with chromatin and that monoubiquitylated SMAD4 dissociates from its active transcriptional complex with R‐SMAD, resulting in SMAD inactivation (Agricola et al. 2011). USP9X (also known as FAM) deubiquitylates SMAD4 and thereby sustains TGF‐β signaling (Dupont et al. 2009).
WNT (Wingless‐related integration site) signaling is also regulated by monoubiquitylation. Binding of WNT to its coreceptors Frizzled and LRP5/6 (LDL receptor‐related protein 5 or 6) results in stabilization of β‐catenin and its translocation to the nucleus, where it binds members of the TCF/LEF family of transcription factors to activate a WNT‐specific transcriptional program (Clevers & Nusse 2012). In the absence of WNT ligand, TCF/LEF factors are maintained inactive by binding to Groucho/TLE transcriptional corepressors. WNT stimulation induces the monoubiquitylation of TLE3 by the E3 ligase XIAP and its consequent dissociation from TCF/LEF (Hanson et al. 2012). Ubiquitylation sites as well as DUBs for TLE3 remain to be elucidated.
Although it is well established that the proteasome recognizes polyubiquitin chains composed of at least four ubiquitin molecules (Thrower et al. 2000), it is also reported that monoubiquitylation of model substrates can stimulate proteasomal degradation if the size of substrate is small (less than 150 amino acids) (Shabek et al. 2012). Consistently, in cells, evidence suggests that monoubiquitylated proteins can also be targeted by the proteasome, with one example being monoubiquitylated p105 as described above. Another example is monoubiquitylated PAX3 (Boutet et al. 2007). Nine PAX proteins (PAX1 to PAX9), which are characterized by the presence of a DNA binding domain known as the paired domain, play important roles in the development of various organs and tissues in mammals (Blake & Ziman 2014). PAX3 is transiently expressed in highly proliferative intermediate progenitor cells during postnatal myogenesis, with such expression declining at the differentiation stage (Conboy & Rando 2002) coincident with PAX3 monoubiquitylation by TAF1 and its delivery to the proteasome for degradation (Boutet et al. 2010). As with the case of histone H1 monoubiquitylation, PAX3 is monoubiquitylated by TAF1 alone in vitro, indicating that TAF1 can directly bind ubiquitin and transfer it to PAX3. DUBs and ubiquitylation sites for PAX3 remain to be identified.
Regulation of DNA damage repair and DNA replication by monoubiquitylation
The maintenance of genetic information is critical for proper execution of cellular function. Given that DNA is repeatedly exposed to genotoxic stressors such as ionizing radiation and ultraviolet light in sunlight, the detection and repair of DNA damage are central to such maintenance. Although K63‐linked polyubiquitylated forms of histones H2A and H2AX have been found to provide a signaling scaffold, RING1B and RNF20/RNF40 catalyze the monoubiquitylation of H2A/H2AX and H2B, respectively, at sites of DNA damage (Cao & Yan 2012). Monoubiquitylation of H2A and H2B is thought to interfere with chromatin compaction (as observed in transcriptional regulation) and thereby to facilitate assembly of the repair machinery at foci of DNA damage (Moyal et al. 2011), but the underlying mechanisms remain elusive.
Interstrand cross‐linking (ICL) occurs when chemotherapeutic drugs or certain by‐products of lipid peroxidation mediate the covalent joining of the two DNA strands of genomic DNA (Deans & West 2011). Central components of the ICL repair pathway include the products of 15 genes that are mutated in Fanconi anemia (FA), a rare recessive genetic disease (Kim & D'Andrea 2012). Eight of these 15 proteins (FANCA, FANCB, FANCC, FANCE, FANCF, FANCG, FANCL, FANCM) form the FA core complex which functions as an E3 ubiquitin ligase. Another two of these proteins, FANCD2 (at K561) and FANCI (at K523), collectively referred to as ID2, are monoubiquitylated in response to ICL (Table 2), which is detected on the basis of the association of FANCM with a stalled replication fork in S phase (Kim et al. 2008). FANCL contains a RING finger domain and is the catalytic subunit of the FA core complex. Monoubiquitylation induces ID2 translocation to chromatin foci containing ICL lesions (Moldovan & D'Andrea 2009). FAN1 (FANCD2/FANCI‐associated nuclease 1) is then recruited to monoubiquitylated ID2 via its UBZ4‐type ubiquitin binding domain (UBD) and cleaves the DNA, thereby initiating ICL repair. After the damage is fixed, monoubiquitylated ID2 is inactivated through deubiquitylation by USP1. Given that USP1 knockout mice show an FA‐like phenotype, the essential function of USP1 might be deubiquitylation of ID2 (Kim et al. 2009).
Table 2.
Monoubiquitylated substrates related to DNA repair, replication and segregation
| Substrate | Ubi site | E3 ligase | DUB | Effect of monoubiquitylation |
|---|---|---|---|---|
| FANCD2 | K561 | FANCL | USP1 | Recruitment to chromatin |
| FANCI | K523 | FANCL | USP1 | Recruitment to chromatin |
| PCNA | K164 | RAD18, CRL4A/BCDT2 | USP1 | Recruitment to TLS polymerase |
| Pol η | C‐terminus | Pirh2 | Not reported | Inhibition of PCNA binding |
| PAF15 | K15, K24 | CRL4A/BCDT2? | Not reported | Basis of polyubiquitylation |
| RFC2/4 | Not reported | RAD18 | Not reported | Not reported |
| ORC1 | Not reported | Not reported | Not reported | Nuclear export |
| CENP‐A | K124 | CRL4ACOPS8 | Not reported | Binding to HJURP |
Replication forks can bypass DNA damage by translesion synthesis (TLS), which allows DNA replication through a lesion with the use of specialized DNA polymerases. The recruitment of TLS polymerases to DNA is precisely regulated to prevent mutations caused by the low fidelity of these enzymes. Fifteen genes for DNA polymerases have been identified in mammals, among which three replicative polymerases (Pol α, Pol δ, Pol ε) are responsible for accurate replication. Seven polymerases (Pol η, Pol ι, Pol κ, REV1, Pol ζ, Pol θ, Pol ν) have been shown to possess TLS activity (Lange et al. 2011). Proliferating cell nuclear antigen (PCNA) functions as a homotrimeric ‘clamp’ that surrounds double‐stranded DNA and tethers replicative polymerases to the DNA template during replication. Stalling of the replication fork at a site of DNA damage results in the monoubiquitylation of PCNA at K164 by RAD18 or CRL4A/BCDT2 ubiquitin ligases and thereby leads to the recruitment of members of the Y‐family of TLS polymerases (Pol η, Pol ι, Pol κ, REV1), which harbor a PIP (PCNA‐interacting peptide) box and UBD (Bergink & Jentsch 2009; Terai et al. 2010). In the absence of DNA damage, monoubiquitylation of PCNA is counteracted by USP1, the same DUB involved in ICL repair, and the switch from replicative to TLS polymerases is inhibited (Huang et al. 2006).
In unstressed cells, Pol η is monoubiquitylated at its COOH‐terminus by Pirh2, resulting in inhibition of the interaction of Pol η with PCNA (Bienko et al. 2010; Jung et al. 2011). Monoubiquitylation thus has both positive and negative effects on DNA repair. The level of Pol η ubiquitylation declines during the repair of DNA damage, suggesting Pol η is deubiquitylated, but the DUB responsible for this process has not been identified.
PCNA‐associated factor 15 (PAF15) also prevents inopportune binding of Pol η to PCNA and is monoubiquitylated at K15 and K24 in a PCNA‐dependent manner (Povlsen et al. 2012), possibly by CRL4A/BCDT2 (Havens & Walter 2011). Monoubiquitylation of PAF15 is not directly related to its function, however, instead serving as the basis for polyubiquitylation and consequent degradation at the site of DNA damage. A DUB for PAF15 also remains to be identified.
The doughnut‐like structure of PCNA must be opened by the clamp loader complex RFC (comprised of RFC1 to RFC5) in order for PCNA to be able to encircle DNA and initiate DNA replication (Indiani & O'Donnell 2006). At sites of DNA damage, the PCNA‐like complex 9‐1‐1 (RAD9‐HUS1‐RAD1) is loaded by another clamp loader complex (comprising RAD17 and RFC2 to RFC5) to initiate the DNA damage response (Cimprich & Cortez 2008). The shared clamp loader proteins RFC2 and RFC4 are monoubiquitylated on chromatin by RAD18 in response to DNA damage (Tomida et al. 2008). The monoubiquitylation of these proteins has been suggested to be related to the switching of clamp loader complexes, but the corresponding ubiquitylation sites and DUBs have not been identified.
Potential replication initiation sites are marked by the formation of a prereplication complex that begins with loading of the proteins ORC1 to ORC6. ORC1 was shown to be exported from the nucleus in response to its monoubiquitylation to prevent the re‐formation of the prereplication complex during S phase, although the corresponding ubiquitylation sites, E3 ligases, and DUBs remain unknown (Saha et al. 2006).
From the various findings presented so far, it is clear that monoubiquitylation serves to fine‐tune DNA‐based processes including replication, transcription and repair. Emerging evidence also suggests that epigenetic regulators such as TET proteins are among the ranks of monoubiquitylated proteins. The histone H3 variant CENP‐A, which is specifically loaded onto the centromere to build the kinetochore, was thus recently shown to be monoubiquitylated at K124 by CRL4ACOPS8 (Niikura et al. 2015). Monoubiquitylation of CENP‐A promotes its association with the CENP‐A‐specific chromatin assembly factor HJURP and is required for CENP‐A loading. This finding thus also implicates monoubiquitylation in regulation of mitotic events.
Regulation of membrane‐associated processes by monoubiquitylation
Protein monoubiquitylation also contributes to the regulation of membrane‐associated processes, among which its role in endocytosis is the most well characterized (Table 3). The abundance of cell surface receptors and channels is strictly controlled to ensure the appropriate timing and duration of ligand‐induced signaling—with activated receptors having been shown to induce their own internalization and subsequent lysosomal degradation, for example. The activation of receptor tyrosine kinases (RTKs) such as the epidermal growth factor receptor (EGFR), hepatocyte growth factor receptor (MET), and stem cell factor receptor (c‐KIT) by cognate growth factors results in receptor autophosphorylation as well as the receptor‐mediated phosphorylation of tyrosine residues in other cytoplasmic proteins. The phosphorylated tyrosine residues in RTKs are recognized by the E3 ligase Cbl, which then mediates receptor monoubiquitylation on multiple lysine residues. The multiple monoubiquitin moieties on RTKs recruit endocytic adaptor proteins that possess a UBD and are necessary for endocytosis (Haglund & Dikic 2012). Of note, many of these adaptor proteins [EPS15, EPS15R, epsin 1, epsin 2, HRS, STAM, RabGEF1 (also known as Rabex5), STS1, STS2, ZFYVE28] are themselves negatively regulated by monoubiquitylation, which induces an intramolecular UBD‐monoubiquitin interaction (Hoeller et al. 2006). Two E3 ligases, NEDD4 and Parkin, have been implicated in the monoubiquitylation of EPS15 (Fallon et al. 2006; Woelk et al. 2006). The E3 ligases responsible for the monoubiquitylation of other adaptor proteins remain to be identified, however.
Table 3.
Monoubiquitylated substrates related to membrane‐associated processes
| Substrate | Ubi site | E3 ligase | DUB | Effect of monoubiquitylation |
|---|---|---|---|---|
| RTKs | Not reported | Cbl | Not reported | Endocytosis |
| Endocytic adaptors | Not reported | NEDD4, Parkin | Not reported | Self‐inactivation |
| TRPC4 | Not reported | ITCH | Not reported | Endocytosis |
| TRPV4 | Not reported | ITCH | Not reported | Endocytosis |
| ENaC | Not reported | NEDD4‐2 | Not reported | Endocytosis |
| Kir1.1 | Not reported | SH3RF1 | Not reported | Endocytosis |
| Cx43 | Not reported | NEDD4 | Not reported | Endocytosis |
| CXCR4 | Not reported | ITCH | Not reported | Endocytosis |
| TfR | Not reported | Not reported | Not reported | Endocytosis |
| NOTCH1 | K1749 | Not reported | Not reported | Endocytosis |
| SEC31 | Not reported | CRL3KLHL12 | Not reported | Enlargement of COPII |
| H‐Ras | K117, K147, K170, K185 | Rabex5 | Not reported | Endosomal sorting |
| N‐Ras | Not reported | Rabex5 | Not reported | Endosomal sorting |
| K‐Ras | K104, K147 | Not reported | Not reported | Activation |
| PLD1 | Not reported | Not reported | Not reported | Localization to near Golgi |
| Spartin | Not reported | WWP1 | Not reported | Dissociation from lipid droplets |
| PEX5 | C11 | PEX12 | USP9X | Extraction from peroxisomes |
Ion channels also undergo endocytosis in response to the monoubiquitylation of multiple lysine residues, although this process is less well characterized. TRPC4 and TRPV4, two members of the transient receptor potential (TRP) family of Ca2+ channels, as well as the epithelial Na+ channel (ENaC) and the K+ channel Kir1.1 (also known as ROMK), are thus internalized as a result of monoubiquitylation by the E3 ligases ITCH (also known as AIP4) (Wegierski et al. 2006), NEDD4‐2 (Eaton et al. 2010) and SH3RF1 (also known as POSH) (Lin et al. 2005), respectively.
Regulation of endocytosis by monoubiquitylation also extends beyond RTKs and channels, with other examples including that of the gap‐junction protein connexin 43 (Cx43) by NEDD4 (Girao et al. 2009), the chemokine receptor CXCR4 by ITCH (Marchese et al. 2003), the transferrin receptor (TfR) by an unknown E3 ligase (Raiborg et al. 2002) and NOTCH1 at K1749 also by an unidentified E3 ligase (Gupta‐Rossi et al. 2004).
Monoubiquitylation also participates in the regulation of protein secretion. Most secreted proteins are not directly released from the cytosol but instead enter the endoplasmic reticulum cotranslationally and then move to the Golgi complex before their delivery to the cell surface. Transport of cargo proteins from the endoplasmic reticulum to the Golgi is mediated by COPII vesicles, which consist of five cytosolic proteins of the SAR1 GTPase, SEC23‐SEC24 adaptors, and SEC13‐SEC31 outer‐layer heterotetramers (Jensen & Schekman 2011), among which SEC31 is monoubiquitylated by the E3 ligase CRL3KLHL12 (Jin et al. 2012). SEC31 monoubiquitylation is necessary for the enlargement of COPII vesicles by an unknown mechanism that is required to accommodate large secretory cargos such as procollagen fibers and chylomicrons.
Monoubiquitylation plays an important role in the function of membrane‐associated proteins. The Ras superfamily of proteins comprises >150 members in humans and has been divided into five major branches: Ras, Rho, Rab, Ran and Arf. The activity of these proteins is regulated by membrane association dependent on a COOH‐terminal lipid modification and by the binding of GTP or GDP (Wennerberg et al. 2005). The founding member of this superfamily, Ras itself, is frequently mutated in human cancers and transmits proliferation, differentiation and survival signals in response to diverse extracellular stimuli. Mammalian cells express three closely related Ras isoforms designated H‐, K‐ and N‐Ras. At the steady state, H‐Ras and N‐Ras are localized at the plasma membrane, endosomes and Golgi membrane, and their activity is regulated differentially depending on their location, whereas K‐Ras is mostly present at the plasma membrane. Monoubiquitylation of Ras has been shown to influence its subcellular localization and activity. The endosomal E3 ligase RabGEF1 (also known as Rabex5) catalyzes monoubiquitylation of H‐Ras (at K117, K147, K170, or K185) and N‐Ras, resulting in their anchoring to the endosomal membrane (Jura et al. 2006; Xu et al. 2010), whereas monoubiquitylation of K‐Ras at K104 or K147 by an unknown E3 ligase does not affect its intracellular localization but rather promotes the binding and activation of the downstream effector proteins RAF and phosphoinositide 3‐kinase by an unknown mechanism (Sasaki et al. 2011).
Phospholipase D (PLD) catalyzes the hydrolysis of phosphatidylcholine to yield phosphatidic acid and free choline, with the former product and its derivative diacylglycerol both serving as signaling molecules. Mammalian cells contain two PLD isoforms, PLD1 and PLD2. PLD1 localizes to a perinuclear structure, the Golgi complex, endosomes and the plasma membrane, whereas PLD2 is present at the plasma membrane (Jenkins & Frohman 2005). Intriguingly, PLD1, but not PLD2, has been found to be monoubiquitylated in a manner dependent on its own lipase activity. Forced monoubiquitylation of PLD1 at its NH2‐terminus resulted in the formation of enlarged vesicles near the Golgi complex and its localization there (Yin et al. 2010). Such forced monoubiquitylation of a catalytically inactive mutant of PLD1 also resulted in its localization near the Golgi, but the formation of enlarged vesicles was less pronounced. These observations suggested that the production of phosphatidic acid by PLD near vesicles might induce vesicle enlargement, whereas the localization of PLD1 is dependent on its monoubiquitylation. Of note, PLD1, phosphatidic acid and the Golgi complex are all thought to play an important role in the formation of lipid droplets (Hesse et al., 2013), suggesting that PLD1 monoubiquitylation might be involved in this process.
Lipid droplets are dynamic structures that store fat in the form of neutral lipids such as triacylglycerol and cholesteryl esters. They are surrounded by a monolayer of amphipathic lipids (phospholipids and cholesterol) that is coated with PAT proteins. Dissociation of PAT proteins from lipid droplets is induced by competition with Spartin (also known as SPG20) for binding to the droplets and results in droplet turnover. The E3 ligase WWP1 monoubiquitylates Spartin and thereby reduces its abundance both at the droplet surface and overall (Eastman et al. 2009), although the mechanism of this effect remains unknown. Given that knockdown of Spartin induces the accumulation of lipid droplets, WWP1 might be expected to regulate droplet size through Spartin monoubiquitylation, although such regulation has not yet been shown. Interestingly, a recent study showed that Spartin directly binds to mono‐ and diubiquitin as well as localizes to another subcellular compartment, DALIS (dendritic aggresome‐like induced structures), in lipopolysaccharide‐stimulated macrophages (Karlsson et al. 2014). It was not examined whether monoubiquitylation inhibits the ubiquitin binding function of Spartin or its ability to localize to DALIS in a manner similar to that operative for endocytic adaptor proteins.
Peroxisomes are eukaryotic intracellular organelles with essential functions in lipid metabolism and reduction in reactive oxygen species (Ma et al. 2011). Given that these organelles are bounded by a single membrane, peroxisomal matrix proteins must be actively imported from the cytosol. The peroxisomal shuttling receptor PEX5 recognizes newly synthesized proteins with a peroxisomal targeting sequence and delivers them to the translocation machinery on the peroxisomal membrane. The PEX5–cargo complex is then imported into peroxisomes, the cargo is released, and PEX5 is recycled back to the cytosol. This process is dependent on monoubiquitylation of PEX5, which occurs at a conserved cysteine residue (C11), rather than at a lysine, and is mediated by the ubiquitin‐conjugating enzyme PEX4 and the ligase PEX12 in peroxisomes. The monoubiquitylated PEX5 is extracted by the receptor export module comprising the ATPases PEX1 and PEX6 and the peroxisomal membrane protein PEX26. Monoubiquitylated PEX5 is then deubiquitylated in the cytosol by USP9X, regenerating unmodified PEX5, which initiates another round of peroxisomal matrix protein import (Grou et al. 2012). The monoubiquitylation of PEX5 in peroxisomes suggests that other intra‐organellar ubiquitylation systems might exist, although no evidence to support this possibility has been obtained to date. Monoubiquitylation of PEX5 in peroxisomes is also unusual in that the target residue is a cysteine.
Regulation of neuronal proteins by monoubiquitylation
Several proteins mutated in neurological disorders are subject to regulation by monoubiquitylation (Table 4). Parkinson's disease is the second most prevalent neurodegenerative disorder, after Alzheimer's disease. It is usually diagnosed after the sixth decade of life and is characterized by motor dysfunction and cognitive decline due to progressive degeneration of dopaminergic neurons in the substantia nigra and other monoaminergic neurons in the brainstem. Genetic analysis has identified >10 genes or loci that are mutated in familial as well as sporadic forms of Parkinson's disease (Corti et al. 2011).
Table 4.
Monoubiquitylated substrates related to neuronal, metabolic and apoptotic processes
| Substrate | Ubi site | E3 ligase | DUB | Effect of monoubiquitylation |
|---|---|---|---|---|
| Hsp70 | Not reported | Parkin | Not reported | Not reported |
| Hsc70 | Not reported | Parkin | Not reported | Not reported |
| Bcl‐2 | Not reported | Parkin | Not reported | Stabilization |
| PICK1 | Not reported | Parkin | Not reported | Inactivation |
| α‐Synuclein | K10, K12, K21, K23, K34, K43, K96 | SIAH | USP9X | Aggregation |
| UCH‐L1 | K4, K65, K71, K157 | Not reported | UCH‐L1 | Self‐inactivation |
| α4 | Not reported | MID1 | Not reported | Cleavage |
| DLG3 | SH3 domain | NEDD4, MEDD4‐2 | Not reported | Localization to apical membrane |
| CPEB3 | Not reported | Neuralized1 | Not reported | Activation |
| PGM‐B | Not reported | Not reported | Not reported | Not reported |
| LDH‐A | Not reported | Not reported | Not reported | Lysosomal degradation |
| Ferritin | Not reported | Not reported | Not reported | Disassembly |
| Caspase‐3/7 | Not reported | cIAP2 | Not reported | Not reported |
| DEDD | Not reported | cIAP1, cIAP2 | Not reported | Nuclear export |
| PTEN | K289 | NEDD4, XIAP | USP7 | Nuclear import |
Among the genes associated with Parkinson's disease, PARK2 encodes the E3 ligase Parkin, which mediates polyubiquitylation or monoubiquitylation depending on the substrate. At least five proteins have been shown to be monoubiquitylated by Parkin. Two of these substrates are protein chaperones of the heat‐shock protein (Hsp) 70 family—Hsp70 and Hsc70—that facilitate the folding of newly synthesized proteins as well as the refolding of misfolded and aggregated proteins (Mayer & Bukau 2005; Moore et al. 2008). Although the effects of monoubiquitylation of these proteins are unclear, the observation that the solubility of Hsp70 is reduced in Parkin‐deficient brain tissue suggests that monoubiquitylation may be a determinant of protein solubility. Parkin has also been found to have anti‐apoptotic effects, which are mediated in part by Parkin‐dependent monoubiquitylation and consequent stabilization of the anti‐apoptotic protein Bcl‐2 (Chen et al. 2010). Parkin also attenuates autophagy through Bcl‐2 stabilization, consistent with the role of Bcl‐2 as an inhibitor not only of apoptosis but also of autophagy (Pattingre et al. 2005). Another mechanism by which Parkin inhibits apoptosis was shown by the finding that Parkin monoubiquitylates and thereby inactivates the endocytic adaptor protein EPS15 (Fallon et al. 2006). EPS15 is an important regulator of growth factor receptor internalization, as described above, and its inactivation by Parkin therefore enhances survival signaling emanating from cell surface receptors—enhancement that is compromised by Parkin mutations. Excessive stimulation of neurons can lead to cell death as a result of Na+ and Ca2+ overload. The ion channel‐associated protein PICK1 (protein interacting with C‐kinase 1) is another substrate for Parkin‐mediated monoubiquitylation (Joch et al. 2007). The channel ASIC2a (acid‐sensing ion channel 2a) is activated by PICK1, whereas this function of PICK1 is lost as a result of Parkin‐catalyzed monoubiquitylation. Given that PICK1 also interacts with several ion channels implicated in neurological diseases (Focant & Hermans 2013), whether Parkin‐mediated monoubiquitylation of PICK1 also affects the activity of these channels warrants further investigation.
α‐Synuclein, which is encoded by the Parkinson's disease‐associated loci PARK1 and PARK4, is a component of the Lewy bodies characteristic of brain tissue in affected individuals. The processes of α‐synuclein oligomerization and fibril growth play central roles in the pathogenesis of Parkinson's disease (Lashuel et al. 2013) and are influenced by monoubiquitylation. Monoubiquitylation by the E3 ligase SIAH (at K10, K12, K21, K23, K34, K43 or K96) promotes α‐synuclein aggregation (Rott et al. 2008), whereas deubiquitylation by USP9X attenuates it (Rott et al. 2011). Although the mechanism underlying this effect of α‐synuclein monoubiquitylation remains unclear, these observations suggest that manipulation of such monoubiquitylation is a potential therapeutic approach to Parkinson's disease.
Another Parkinson's disease‐associated protein, UCH‐L1, which is encoded by the PARK5 locus, is regulated by monoubiquitylation. UCH‐L1 is one of four members of the UCH (ubiquitin COOH‐terminal hydrolase) family of DUB proteins that hydrolyze small ubiquitin chains or possibly short COOH‐terminal extensions of polymeric ubiquitin precursors, with this specificity being due to the confined structure of the active site (Komander et al. 2009). The DUB activity of UCH‐L1 is negatively regulated by monoubiquitylation at K4, K65, K71 or K157, all of which residues are located near the active site (Meray & Lansbury 2007). Monoubiquitin attached to these lysine residues binds to the ubiquitin binding region of UCH‐L1 itself and thereby prevents its association with ubiquitylated substrates. Although not confirmed, the E3 ligase for UCH‐L1 monoubiquitylation is likely to be UCH‐L1 itself, given that dimerized UCH‐L1 shows E3 ligase activity (Liu et al. 2002). The DUB for UCH‐L1 is also UCH‐L1. These observations indicate that UCH‐L1 regulates its own activity by cycles of intramolecular monoubiquitylation and deubiquitylation, but the role of such autoregulation remains unclear. Importantly, the physiological substrates of UCH‐L1 are still unidentified, with the result that the mechanism of UCH‐L1‐related Parkinson's disease pathogenesis is also unknown. Possible insight into the pathological function of UCH‐L1 has been provided by Gad (gracile axonal degeneration) mice, which do not express UCH‐L1 as a result of a corresponding gene mutation (Saigoh et al. 1999). The axonal degeneration apparent in these mice suggests that UCH‐L1 is essential for functional maintenance of axons.
Intellectual disability is a developmental brain disorder characterized by limitations in both intellectual functioning and adaptive behavior. The skewed male‐to‐female ratio of affected individuals and the identification of families showing X‐linked disease segregation are suggestive of the presence of causative genes on the X chromosome. Indeed, >100 such genes have been identified to date (Piton et al. 2013). The products of at least two validated X‐linked intellectual disability genes are involved in monoubiquitylation. Loss‐of‐function mutation of the E3 ligase MID1 thus causes Opitz syndrome, one form of X‐linked intellectual disability. MID1 forms a ternary complex with the protein α4 and the catalytic subunit of protein phosphatase 2 (PP2c) on microtubules, with α4 being necessary for PP2c stabilization. However, MID1‐catalyzed monoubiquitylation of α4 at unidentified lysine residues induces its cleavage at Phe255–Gly256 by calpain, negating its effect on PP2c stabilization (Watkins et al. 2012). Interestingly, MID1 was also shown to polyubiquitylate PP2c and thereby to mark it for degradation (Trockenbacher et al. 2001). However, the cleavage of α4 induced by MID1 releases MID1 from the ternary complex, suggesting that other E3 ligases are responsible for PP2c polyubiquitylation in the absence of associated MID1.
Mammalian cells contain four DLG (disks large) family proteins that play an important role in the establishment of epithelial cell polarity at the basolateral membrane below the apical junction (Roberts et al. 2012). The DLG3 gene is located on the X chromosome and is mutated in some individuals with X‐linked intellectual disability. Monoubiquitylation of DLG3 by the E3 ligases NEDD4 and NEDD4‐2 at unidentified lysine residues in its Src homology 3 (SH3) domain was shown to be required for the binding to motor proteins that recruit DLG3 to the apical membrane at the site of tight‐junction formation (Van Campenhout et al. 2011). Interestingly, other DLG proteins (DLG1, DLG2, DLG4) do not bind to NEDD4 or NEDD4‐2, indicating that regulation of localization by monoubiquitylation is specific to DLG3. Whether monoubiquitylation of DLG3 is relevant to the pathogenesis of intellectual disability remains unknown.
Efficient neurotransmission is dependent on specialized neuronal structures including the cell body (soma), dendrites and axon. In general, neurons receive signals from other neurons at dendrites and then transmit this information through the soma to the axon, the terminal of which makes contact with dendrites of other neurons. Formation of an appropriate structure is thus of utmost importance for neuronal function. Although no mutations in the corresponding gene have been associated with neurological diseases, the E3 ligase Neuralized1 has been shown to regulate the development of dendrites by monoubiquitylation. Neuralized1 monoubiquitylates CPEB3, which binds mRNA and therefore regulates translation. This monoubiquitylation of CPEB3 results in its stabilization and activation and the consequent induction of CPEB target proteins such as the GluA1 and GluA2 subunits of AMPA‐type glutamate receptors as well as the formation of new dendritic spines (Pavlopoulos et al. 2011). Overexpression of Neuralized1 in mouse brain enhances memory formation and synaptic plasticity in parallel with up‐regulation of GluA1 and GluA2 expression and formation of functional synapses. Neuralized1 is induced by neuronal activity and thereby promotes the function of active neurons by monoubiquitylation of CPEB3. The ubiquitylation sites and DUBs for CPEB3 remain to be identified.
Regulation of metabolism by monoubiquitylation
In a screen for cancer‐specific ubiquitylated proteins, monoubiquitylated phosphoglycerate mutase‐B (PGM‐B) was detected in colorectal cancer specimens but not in normal colon tissue, suggesting that PGM‐B monoubiquitylation may play a role in colorectal carcinogenesis (Usuba et al. 2001). PGM is an enzyme of the glycolytic pathway and catalyzes the reversible conversion of 3‐phosphoglycerate to 2‐phosphoglycerate. It is a dimeric protein with two different subunits, PGM‐B and PGM‐M, and the combination of subunits is tissue dependent—with PGM‐BB being present in colorectal tissue and PGM‐MM in skeletal muscle, for example. Although PGM enzymatic activity is higher in colorectal cancer than in adjacent normal tissue (Durany et al. 1997), the possible role of monoubiquitylation in this difference has not been reported (Table 4). Another glycolytic enzyme, lactate dehydrogenase (LDH), supplies NADH by reducing NAD+ in parallel with oxidation of pyruvate, the final product of glycolysis, under conditions of high energy demand associated with anaerobic metabolism. LDH forms a tetramer from combinations of three different subunits—LDH‐A, LDH‐B, and LDH‐C—giving rise to six different isoforms: LDH‐B4 (LDH1), LDH‐A1B3 (LDH2), LDH‐A2B2 (LDH3), LDH‐A3B1 (LDH4), LDH‐A4 (LDH5) and LDH‐C4. As with PGM, the expression of these three subunits is tissue dependent, with LDH‐A being expressed in skeletal muscle and LDH‐B in cardiac muscle, for example. A screen for proteins ubiquitylated in response to oxidative stress in skeletal muscle identified monoubiquitylated LDH‐A (Onishi et al. 2005). Oxidative stress induced a transient increase in LDH‐A monoubiquitylation, and this effect was prolonged by treatment with a lysosomal protease inhibitor, suggesting that LDH‐A monoubiquitylation induces lysosomal sorting and consequent degradation of LDH‐A. Whether other isoforms of LDH are also regulated by monoubiquitylation remains unknown.
Iron plays essential roles in various biochemical activities such as oxygen transport, energy metabolism and demethylation of DNA and histones. Given that iron is also a hazardous biometal, its metabolism is tightly regulated. After its delivery to cells by transferrin, iron is transported into mitochondria for the synthesis of heme or iron–sulfur clusters as well as to the nucleus for DNA and histone demethylation. Excess iron is stored and detoxified in cytosolic nanocages consisting of 24 heavy and light ferritin subunits (Wang & Pantopoulos 2011). In response to iron deficiency, ferritin nanocages are disassembled and degraded by the proteasome and lysosomes. This disassembly process was reported to be promoted by ferritin monoubiquitylation (De Domenico et al. 2006).
Collectively, these various observations implicate monoubiquitylation in the regulation of metabolic processes. However, it seems premature to conclude that monoubiquitylation plays a prominent role in the control of metabolism on the basis of these few examples uncovered to date.
Regulation of apoptosis by monoubiquitylation
Apoptosis is a form of programmed cell death by which multicellular organisms eliminate excess or damaged cells. The caspase family of proteases plays an essential role in the execution of apoptosis. Apoptotic caspases have been categorized as initiators (caspase‐2, caspase‐8, caspase‐9, and caspase‐10) and effectors (caspase‐3, caspase‐6, and caspase‐7). Two effector caspases, caspase‐3 and caspase‐7, were shown to be monoubiquitylated by the E3 ligase cIAP2 in vitro (Huang et al. 2000) (Table 4). Although XIAP, a paralog of cIAP2, directly binds to and inhibits the activation of caspase‐3/7, neither cIAP1 nor cIAP2 appear to share this function (Eckelman et al. 2006). However, cIAP2‐deficient macrophages were found to be more susceptible to apoptosis than wild‐type cells (Conte et al. 2006), suggesting that cIAP2 might inhibit caspase‐3/7 activation by monoubiquitylation in these cells. Curiously, cIAP1 was shown to polyubiquitylate and thereby to promote the proteasomal degradation of caspase‐3/7, whereas the polyubiquitylation activity of cIAP2 toward caspase‐3/7 in cells was much weaker (Choi et al. 2009). Both cIAP1 and cIAP2 monoubiquitylate DEDD (death effector domain‐containing DNA binding protein) in the nucleolus and thereby regulate apoptosis. Monoubiquitylation of DEDD induces its translocation to the cytosol, where it facilitates caspase‐3/7 activation (Lee et al. 2005). These findings indicate that cIAP1/2 exert both anti‐apoptotic and proapoptotic functions through monoubiquitylation. The ubiquitylation sites and DUBs for caspases and DEDD have not been identified. As for its role in metabolic control, there is currently too little evidence to judge the importance of monoubiquitylation in the regulation of apoptosis. Both cIAP1 and cIAP2 were recently shown to inhibit another form of programmed cell death, necroptosis, by an unknown mechanism (McComb et al. 2012), suggesting that monoubiquitylation might also contribute to the regulation of this death pathway.
Regulation of PTEN by monoubiquitylation
Phosphatase and tensin homologue (PTEN) is a tumor suppressor that is frequently lost or mutated in a variety of human tumors. The primary substrate of PTEN phosphatase activity is phosphatidylinositol 3,4,5‐trisphosphate localized in the plasma membrane, which is required for membrane recruitment and activation of the protein kinase AKT. PTEN thus antagonizes AKT‐dependent cellular activities such as survival, growth and proliferation (Hopkins et al. 2014). However, PTEN also has nuclear functions that are thought to be regulated by monoubiquitylation.
There are at least four E3 ligases for PTEN [NEDD4, XIAP, WWP2, TRIM27 (also known as RFP)], among which NEDD4 and XIAP were shown to monoubiquitylate PTEN at K289 and to induce its nuclear translocation (Trotman et al. 2007; Van Themsche et al. 2009) (Table 4). Ubiquitin fusion rescued the nuclear import defect of the K289E mutant of PTEN, indicative of a key role for monoubiquitylation in nuclear import. NEDD4 knockout did not substantially affect the nuclear localization of PTEN (Fouladkou et al. 2008), whereas XIAP‐deficient cells show reduced amounts of PTEN in the nucleus, suggesting that XIAP is the major E3 ligase for regulation of the nuclear localization of PTEN by monoubiquitylation. Importantly, these E3 ligases also polyubiquitylate PTEN and thereby mark it for degradation. Although the mechanisms underlying the regulation of monoubiquitylation vs polyubiquitylation of PTEN remain to be determined, the expression level of E3 ligases might play an important role, analogous to regulation of p53 by MDM2. Two DUBs have been found to regulate the ubiquitylation of PTEN: USP7 targets monoubiquitylated PTEN to counteract its nuclear translocation (Song et al. 2008), whereas USP13 removes polyubiquitin from and thereby stabilizes PTEN (Zhang et al. 2013). The different subcellular localizations of these DUBs—USP7 in the nucleus and USP13 in the cytosol—may allow the targeting of different populations of ubiquitylated PTEN, a scenario that is again reminiscent of p53 regulation by DUBs.
Concluding remarks
We have here provided an overview of studies addressing the substrates and functions of monoubiquitylation. These studies have uncovered several characteristic functions of monoubiquitylation such as regulation of the subcellular localization, activity and stability of protein targets (Fig. 2). Future work should allow many of the ‘Not reported’ entries in Tables 1, 2, 3, 4 to be replaced with the relevant information.
Figure 2.
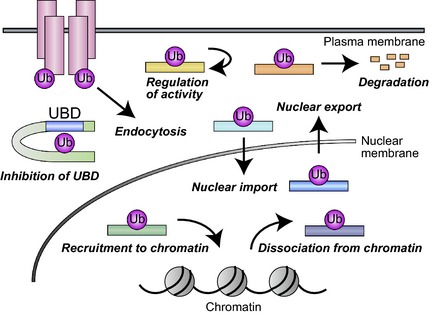
Representative functions of monoubiquitylation. Ub, ubiquitin.
As a post‐translational protein modification, monoubiquitylation is similar to acetylation, which is also reversible, targets lysine residues as well as the NH2‐terminal methionine, and regulates the subcellular localization, activity and stability of substrate proteins (Xiong & Guan 2012). Studies that address the relation between monoubiquitylation and acetylation might be expected to shed light on the biological and functional differences between these modifications.
One of the biggest unsolved problems in the ubiquitin field is how the ubiquitin conjugation machinery distinctively conjugates monoubiquitin or ubiquitin chains onto the substrate proteins. We consider several potential strategies, based on the characters of substrates, E3 ligases, DUBs or trans‐acting factors such as UBD proteins. First, only monoubiquitylation might be allowed due to the structural restriction of substrate. Second, E3 ligases might only conjugate single ubiquitin molecule due to its low processivity. Third, monoubiquitylation could be the most preferred form in the dynamic equilibrium between ubiquitylation and deubiquitylation. Fourth, several DUBs might only deubiquitylate ubiquitin–ubiquitin linkage but not be able to remove ubiquitin directly conjugated to the substrate. Fifth, monoubiquitin on the substrate might be immediately recognized by UBD protein which prevents further ubiquitin from being attached to the monoubiquitin moiety. In some cases, the choice of E2s might also contribute to mono‐, but not poly‐, ubiquitylation (Ye & Rape 2009; Ramanathan & Ye 2012).
Another important issue to be resolved is how monoubiquitin conjugated to a protein target is interpreted for subsequent functional changes. For some UBD‐containing proteins, such monoubiquitin moieties engage in an intramolecular interaction with the UBD and thereby prevent it from binding to other monoubiquitylated proteins. Monoubiquitin recognition by UBD proteins clearly contributes to regulation of protein function (Husnjak & Dikic 2012). Characterization of the mechanisms by which different UBD proteins recognize their cognate monoubiquitylated proteins will be key to gaining further insight into such regulation. The diverse outcomes of monoubiquitylation indicate that the surrounding structure of the monoubiquitin moiety is also recognized. Another biochemical consequence of monoubiquitylation is structural interference, as exemplified by inhibition of the binding of SMAD3 to DNA. In this instance, no UBD protein is required, and this mechanism of action might be more prevalent than is currently appreciated.
Compared with the study of polyubiquitylation, whose function in most cases is to mark a protein for degradation, research on monoubiquitylation has progressed more slowly, which is due in part to the more diverse functions of this modification as well as to methodological challenges. Knowledge of the functions of monoubiquitylation uncovered to date, as surveyed in this review, may serve as the basis for hypothesis generation regarding the role of novel instances of protein monoubiquitylation. Forced ubiquitin fusion has provided key insights into the function of monoubiquitylation for some proteins but not others, the latter probably as a result of structural differences between artificially fused and native monoubiquitylated conjugates. New methodological approaches that allow specific modification of target lysine residues with monoubiquitin may circumvent such problems. Manipulation of E3 ligases or DUBs as a means to uncover the functions of monoubiquitylation might lead to changes in the ubiquitylation level of unrelated proteins, whereas lysine mutation might affect not only ubiquitylation but also other modifications such as acetylation, stressing the necessity of caution in practicing these methods.
Given the large number of monoubiquitylated proteins estimated by proteomics data, many such proteins remain to be identified and characterized. The identification of novel targets of monoubiquitylation should be facilitated by large‐scale proteomics studies of ubiquitylated sites and proteins based on mass spectrometry. One such study, based on the fact that the abundance of monoubiquitylated histones is decreased in cells treated with a proteasome inhibitor, identified >20 monoubiquitylated nonhistone proteins that were also down‐regulated in response to such treatment (Kim et al. 2011). With such technical advances in hand, more monoubiquitylated proteins and their functions are certain to be identified, and such knowledge will lead to an increased understanding of the monoubiquitin world.
Communicated by: Mitsuhiro Yanagida
References
- Agricola, E. , Randall, R.A. , Gaarenstroom, T. , Dupont, S. & Hill, C.S. (2011) Recruitment of TIF1gamma to chromatin via its PHD finger‐bromodomain activates its ubiquitin ligase and transcriptional repressor activities. Mol. Cell 43, 85–96. [DOI] [PubMed] [Google Scholar]
- Arnesen, T. , Van Damme, P. , Polevoda, B. , Helsens, K. , Evjenth, R. , Colaert, N. , Varhaug, J.E. , Vandekerckhove, J. , Lillehaug, J.R. , Sherman, F. & Gevaert, K. (2009) Proteomics analyses reveal the evolutionary conservation and divergence of N‐terminal acetyltransferases from yeast and humans. Proc. Natl Acad. Sci. USA 106, 8157–8162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergink, S. & Jentsch, S. (2009) Principles of ubiquitin and SUMO modifications in DNA repair. Nature 458, 461–467. [DOI] [PubMed] [Google Scholar]
- Bhatnagar, S. , Gazin, C. , Chamberlain, L. , Ou, J. , Zhu, X. , Tushir, J.S. , Virbasius, C.M. , Lin, L. , Zhu, L.J. , Wajapeyee, N. & Green, M.R. (2014) TRIM37 is a new histone H2A ubiquitin ligase and breast cancer oncoprotein. Nature 516, 116–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieging, K.T. , Mello, S.S. & Attardi, L.D. (2014) Unravelling mechanisms of p53‐mediated tumour suppression. Nat. Rev. Cancer 14, 359–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienko, M. , Green, C.M. , Sabbioneda, S. , Crosetto, N. , Matic, I. , Hibbert, R.G. , Begovic, T. , Niimi, A. , Mann, M. , Lehmann, A.R. & Dikic, I. (2010) Regulation of translesion synthesis DNA polymerase eta by monoubiquitination. Mol. Cell 37, 396–407. [DOI] [PubMed] [Google Scholar]
- Blackledge, N.P. , Farcas, A.M. , Kondo, T. et al (2014) Variant PRC1 complex‐dependent H2A ubiquitylation drives PRC2 recruitment and polycomb domain formation. Cell 157, 1445–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake, J.A. & Ziman, M.R. (2014) Pax genes: regulators of lineage specification and progenitor cell maintenance. Development 141, 737–751. [DOI] [PubMed] [Google Scholar]
- Boutet, S.C. , Biressi, S. , Iori, K. , Natu, V. & Rando, T.A. (2010) Taf1 regulates Pax3 protein by monoubiquitination in skeletal muscle progenitors. Mol. Cell 40, 749–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutet, S.C. , Disatnik, M.H. , Chan, L.S. , Iori, K. & Rando, T.A. (2007) Regulation of Pax3 by proteasomal degradation of monoubiquitinated protein in skeletal muscle progenitors. Cell 130, 349–362. [DOI] [PubMed] [Google Scholar]
- Brenkman, A.B. , de Keizer, P.L. , van den Broek, N.J. , Jochemsen, A.G. & Burgering, B.M. (2008) Mdm2 induces mono‐ubiquitination of FOXO4. PLoS ONE 3, e2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, J. & Yan, Q. (2012) Histone ubiquitination and deubiquitination in transcription, DNA damage response, and cancer. Front. Oncol. 2, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catez, F. , Ueda, T. & Bustin, M. (2006) Determinants of histone H1 mobility and chromatin binding in living cells. Nat. Struct. Mol. Biol. 13, 305–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, A. , Kleiman, F.E. , Manley, J.L. , Ouchi, T. & Pan, Z.Q. (2002) Autoubiquitination of the BRCA1*BARD1 RING ubiquitin ligase. J. Biol. Chem. 277, 22085–22092. [DOI] [PubMed] [Google Scholar]
- Chen, D. , Gao, F. , Li, B. , Wang, H. , Xu, Y. , Zhu, C. & Wang, G. (2010) Parkin mono‐ubiquitinates Bcl‐2 and regulates autophagy. J. Biol. Chem. 285, 38214–38223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, Y.E. , Butterworth, M. , Malladi, S. , Duckett, C.S. , Cohen, G.M. & Bratton, S.B. (2009) The E3 ubiquitin ligase cIAP1 binds and ubiquitinates caspase‐3 and ‐7 via unique mechanisms at distinct steps in their processing. J. Biol. Chem. 284, 12772–12782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimprich, K.A. & Cortez, D. (2008) ATR: an essential regulator of genome integrity. Nat. Rev. Mol. Cell Biol. 9, 616–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers, H. & Nusse, R. (2012) Wnt/beta‐catenin signaling and disease. Cell 149, 1192–1205. [DOI] [PubMed] [Google Scholar]
- Conboy, I.M. & Rando, T.A. (2002) The regulation of Notch signaling controls satellite cell activation and cell fate determination in postnatal myogenesis. Dev. Cell 3, 397–409. [DOI] [PubMed] [Google Scholar]
- Conte, D. , Holcik, M. , Lefebvre, C.A. , Lacasse, E. , Picketts, D.J. , Wright, K.E. & Korneluk, R.G. (2006) Inhibitor of apoptosis protein cIAP2 is essential for lipopolysaccharide‐induced macrophage survival. Mol. Cell. Biol. 26, 699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corti, O. , Lesage, S. & Brice, A. (2011) What genetics tells us about the causes and mechanisms of Parkinson's disease. Physiol. Rev. 91, 1161–1218. [DOI] [PubMed] [Google Scholar]
- Da Silva‐Ferrada, E. , Torres‐Ramos, M. , Aillet, F. , Campagna, M. , Matute, C. , Rivas, C. , Rodriguez, M.S. & Lang, V. (2011) Role of monoubiquitylation on the control of IkappaBalpha degradation and NF‐kappaB activity. PLoS ONE 6, e25397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Domenico, I. , Vaughn, M.B. , Li, L. , Bagley, D. , Musci, G. , Ward, D.M. & Kaplan, J. (2006) Ferroportin‐mediated mobilization of ferritin iron precedes ferritin degradation by the proteasome. EMBO J. 25, 5396–5404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deans, A.J. & West, S.C. (2011) DNA interstrand crosslink repair and cancer. Nat. Rev. Cancer 11, 467–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshaies, R.J. & Joazeiro, C.A. (2009) RING domain E3 ubiquitin ligases. Annu. Rev. Biochem. 78, 399–434. [DOI] [PubMed] [Google Scholar]
- Dupont, S. , Inui, M. & Newfeld, S.J. (2012) Regulation of TGF‐beta signal transduction by mono‐ and deubiquitylation of Smads. FEBS Lett. 586, 1913–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont, S. , Mamidi, A. , Cordenonsi, M. et al (2009) FAM/USP9x, a deubiquitinating enzyme essential for TGFbeta signaling, controls Smad4 monoubiquitination. Cell 136, 123–135. [DOI] [PubMed] [Google Scholar]
- Dupont, S. , Zacchigna, L. , Cordenonsi, M. , Soligo, S. , Adorno, M. , Rugge, M. & Piccolo, S. (2005) Germ‐layer specification and control of cell growth by Ectodermin, a Smad4 ubiquitin ligase. Cell 121, 87–99. [DOI] [PubMed] [Google Scholar]
- Durany, N. , Joseph, J. , Campo, E. , Molina, R. & Carreras, J. (1997) Phosphoglycerate mutase, 2,3‐bisphosphoglycerate phosphatase and enolase activity and isoenzymes in lung, colon and liver carcinomas. Br. J. Cancer 75, 969–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastman, S.W. , Yassaee, M. & Bieniasz, P.D. (2009) A role for ubiquitin ligases and Spartin/SPG20 in lipid droplet turnover. J. Cell Biol. 184, 881–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton, D.C. , Malik, B. , Bao, H.F. , Yu, L. & Jain, L. (2010) Regulation of epithelial sodium channel trafficking by ubiquitination. Proc. Am. Thorac. Soc. 7, 54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckelman, B.P. , Salvesen, G.S. & Scott, F.L. (2006) Human inhibitor of apoptosis proteins: why XIAP is the black sheep of the family. EMBO Rep. 7, 988–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emre, N.C. , Ingvarsdottir, K. , Wyce, A. , Wood, A. , Krogan, N.J. , Henry, K.W. , Li, K. , Marmorstein, R. , Greenblatt, J.F. , Shilatifard, A. & Berger, S.L. (2005) Maintenance of low histone ubiquitylation by Ubp10 correlates with telomere‐proximal Sir2 association and gene silencing. Mol. Cell 17, 585–594. [DOI] [PubMed] [Google Scholar]
- Espinosa, J.M. (2008) Histone H2B ubiquitination: the cancer connection. Genes Dev. 22, 2743–2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon, L. , Belanger, C.M. , Corera, A.T. , Kontogiannea, M. , Regan‐Klapisz, E. , Moreau, F. , Voortman, J. , Haber, M. , Rouleau, G. , Thorarinsdottir, T. , Brice, A. , van Bergen En Henegouwen, P.M. & Fon, E.A. (2006) A regulated interaction with the UIM protein Eps15 implicates parkin in EGF receptor trafficking and PI(3)K‐Akt signalling. Nat. Cell Biol. 8, 834–842. [DOI] [PubMed] [Google Scholar]
- Focant, M.C. & Hermans, E. (2013) Protein interacting with C kinase and neurological disorders. Synapse 67, 532–540. [DOI] [PubMed] [Google Scholar]
- Fouladkou, F. , Landry, T. , Kawabe, H. , Neeb, A. , Lu, C. , Brose, N. , Stambolic, V. & Rotin, D. (2008) The ubiquitin ligase Nedd4‐1 is dispensable for the regulation of PTEN stability and localization. Proc. Natl Acad. Sci. USA 105, 8585–8590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner, R.G. , Nelson, Z.W. & Gottschling, D.E. (2005) Ubp10/Dot4p regulates the persistence of ubiquitinated histone H2B: distinct roles in telomeric silencing and general chromatin. Mol. Cell. Biol. 25, 6123–6139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girao, H. , Catarino, S. & Pereira, P. (2009) Eps15 interacts with ubiquitinated Cx43 and mediates its internalization. Exp. Cell Res. 315, 3587–3597. [DOI] [PubMed] [Google Scholar]
- Goldknopf, I.L. & Busch, H. (1977) Isopeptide linkage between nonhistone and histone 2A polypeptides of chromosomal conjugate‐protein A24. Proc. Natl Acad. Sci. USA 74, 864–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grou, C.P. , Francisco, T. , Rodrigues, T.A. , Freitas, M.O. , Pinto, M.P. , Carvalho, A.F. , Domingues, P. , Wood, S.A. , Rodriguez‐Borges, J.E. , Sa‐Miranda, C. , Fransen, M. & Azevedo, J.E. (2012) Identification of ubiquitin‐specific protease 9X (USP9X) as a deubiquitinase acting on ubiquitin‐peroxin 5 (PEX5) thioester conjugate. J. Biol. Chem. 287, 12815–12827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero‐Santoro, J. , Kapetanaki, M.G. , Hsieh, C.L. , Gorbachinsky, I. , Levine, A.S. & Rapic‐Otrin, V. (2008) The cullin 4B‐based UV‐damaged DNA‐binding protein ligase binds to UV‐damaged chromatin and ubiquitinates histone H2A. Cancer Res. 68, 5014–5022. [DOI] [PubMed] [Google Scholar]
- Gupta‐Rossi, N. , Six, E. , LeBail, O. , Logeat, F. , Chastagner, P. , Olry, A. , Israel, A. & Brou, C. (2004) Monoubiquitination and endocytosis direct gamma‐secretase cleavage of activated Notch receptor. J. Cell Biol. 166, 73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haglund, K. & Dikic, I. (2012) The role of ubiquitylation in receptor endocytosis and endosomal sorting. J. Cell Sci. 125, 265–275. [DOI] [PubMed] [Google Scholar]
- Hanson, A.J. , Wallace, H.A. , Freeman, T.J. , Beauchamp, R.D. , Lee, L.A. & Lee, E. (2012) XIAP monoubiquitylates Groucho/TLE to promote canonical Wnt signaling. Mol. Cell 45, 619–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havens, C.G. & Walter, J.C. (2011) Mechanism of CRL4(Cdt2), a PCNA‐dependent E3 ubiquitin ligase. Genes Dev. 25, 1568–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden, M.S. & Ghosh, S. (2008) Shared principles in NF‐kappaB signaling. Cell 132, 344–362. [DOI] [PubMed] [Google Scholar]
- Henry, K.W. , Wyce, A. , Lo, W.S. , Duggan, L.J. , Emre, N.C. , Kao, C.F. , Pillus, L. , Shilatifard, A. , Osley, M.A. & Berger, S.L. (2003) Transcriptional activation via sequential histone H2B ubiquitylation and deubiquitylation, mediated by SAGA‐associated Ubp8. Genes Dev. 17, 2648–2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse, D. , Jaschke, A. , Chung, B. & Schürmann, A. (2013) Trans‐Golgi proteins participate in the control of lipid droplet and chylomicron formation. Biosci. Rep. 33, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochstrasser, M. (2006) Lingering mysteries of ubiquitin‐chain assembly. Cell 124, 27–34. [DOI] [PubMed] [Google Scholar]
- Hoeller, D. , Crosetto, N. , Blagoev, B. , Raiborg, C. , Tikkanen, R. , Wagner, S. , Kowanetz, K. , Breitling, R. , Mann, M. , Stenmark, H. & Dikic, I. (2006) Regulation of ubiquitin‐binding proteins by monoubiquitination. Nat. Cell Biol. 8, 163–169. [DOI] [PubMed] [Google Scholar]
- Hopkins, B.D. , Hodakoski, C. , Barrows, D. , Mense, S.M. & Parsons, R.E. (2014) PTEN function: the long and the short of it. Trends Biochem. Sci. 39, 183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Horst, A. , de Vries‐Smits, A.M. , Brenkman, A.B. , van Triest, M.H. , van den Broek, N. , Colland, F. , Maurice, M.M. & Burgering, B.M. (2006) FOXO4 transcriptional activity is regulated by monoubiquitination and USP7/HAUSP. Nat. Cell Biol. 8, 1064–1073. [DOI] [PubMed] [Google Scholar]
- Hu, H. , Yang, Y. , Ji, Q. , Zhao, W. , Jiang, B. , Liu, R. , Yuan, J. , Liu, Q. , Li, X. , Zou, Y. , Shao, C. , Shang, Y. , Wang, Y. & Gong, Y. (2012) CRL4B catalyzes H2AK119 monoubiquitination and coordinates with PRC2 to promote tumorigenesis. Cancer Cell 22, 781–795. [DOI] [PubMed] [Google Scholar]
- Huang, H. , Joazeiro, C.A. , Bonfoco, E. , Kamada, S. , Leverson, J.D. & Hunter, T. (2000) The inhibitor of apoptosis, cIAP2, functions as a ubiquitin‐protein ligase and promotes in vitro monoubiquitination of caspases 3 and 7. J. Biol. Chem. 275, 26661–26664. [DOI] [PubMed] [Google Scholar]
- Huang, T.T. , Nijman, S.M. , Mirchandani, K.D. , Galardy, P.J. , Cohn, M.A. , Haas, W. , Gygi, S.P. , Ploegh, H.L. , Bernards, R. & D'Andrea, A.D. (2006) Regulation of monoubiquitinated PCNA by DUB autocleavage. Nat. Cell Biol. 8, 339–347. [DOI] [PubMed] [Google Scholar]
- Husnjak, K. & Dikic, I. (2012) Ubiquitin‐binding proteins: decoders of ubiquitin‐mediated cellular functions. Annu. Rev. Biochem. 81, 291–322. [DOI] [PubMed] [Google Scholar]
- Indiani, C. & O'Donnell, M. (2006) The replication clamp‐loading machine at work in the three domains of life. Nat. Rev. Mol. Cell Biol. 7, 751–761. [DOI] [PubMed] [Google Scholar]
- Inui, M. , Manfrin, A. , Mamidi, A. , Martello, G. , Morsut, L. , Soligo, S. , Enzo, E. , Moro, S. , Polo, S. , Dupont, S. , Cordenonsi, M. & Piccolo, S. (2011) USP15 is a deubiquitylating enzyme for receptor‐activated SMADs. Nat. Cell Biol. 13, 1368–1375. [DOI] [PubMed] [Google Scholar]
- Jackson, B.C. , Carpenter, C. , Nebert, D.W. & Vasiliou, V. (2010) Update of human and mouse forkhead box (FOX) gene families. Hum. Genomics 4, 345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins, G.M. & Frohman, M.A. (2005) Phospholipase D: a lipid centric review. Cell. Mol. Life Sci. 62, 2305–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen, D. & Schekman, R. (2011) COPII‐mediated vesicle formation at a glance. J. Cell Sci. 124, 1–4. [DOI] [PubMed] [Google Scholar]
- Jin, H.S. , Lee, D.H. , Kim, D.H. , Chung, J.H. , Lee, S.J. & Lee, T.H. (2009) cIAP1, cIAP2, and XIAP act cooperatively via nonredundant pathways to regulate genotoxic stress‐induced nuclear factor‐kappaB activation. Cancer Res. 69, 1782–1791. [DOI] [PubMed] [Google Scholar]
- Jin, L. , Pahuja, K.B. , Wickliffe, K.E. , Gorur, A. , Baumgartel, C. , Schekman, R. & Rape, M. (2012) Ubiquitin‐dependent regulation of COPII coat size and function. Nature 482, 495–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joch, M. , Ase, A.R. , Chen, C.X. , MacDonald, P.A. , Kontogiannea, M. , Corera, A.T. , Brice, A. , Seguela, P. & Fon, E.A. (2007) Parkin‐mediated monoubiquitination of the PDZ protein PICK1 regulates the activity of acid‐sensing ion channels. Mol. Biol. Cell 18, 3105–3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo, H.Y. , Zhai, L. , Yang, C. , Nie, S. , Erdjument‐Bromage, H. , Tempst, P. , Chang, C. & Wang, H. (2007) Regulation of cell cycle progression and gene expression by H2A deubiquitination. Nature 449, 1068–1072. [DOI] [PubMed] [Google Scholar]
- Jung, Y.S. , Hakem, A. , Hakem, R. & Chen, X. (2011) Pirh2 E3 ubiquitin ligase monoubiquitinates DNA polymerase eta to suppress translesion DNA synthesis. Mol. Cell. Biol. 31, 3997–4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jura, N. , Scotto‐Lavino, E. , Sobczyk, A. & Bar‐Sagi, D. (2006) Differential modification of Ras proteins by ubiquitination. Mol. Cell 21, 679–687. [DOI] [PubMed] [Google Scholar]
- Kaiser, S.E. , Riley, B.E. , Shaler, T.A. , Trevino, R.S. , Becker, C.H. , Schulman, H. & Kopito, R.R. (2011) Protein standard absolute quantification (PSAQ) method for the measurement of cellular ubiquitin pools. Nat. Methods 8, 691–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson, A.B. , Washington, J. , Dimitrova, V. , Hooper, C. , Shekhtman, A. & Bakowska, J.C. (2014) The role of spartin and its novel ubiquitin binding region in DALIS occurrence. Mol. Biol. Cell 25, 1355–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, H. & D'Andrea, A.D. (2012) Regulation of DNA cross‐link repair by the Fanconi anemia/BRCA pathway. Genes Dev. 26, 1393–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J.M. , Kee, Y. , Gurtan, A. & D'Andrea, A.D. (2008) Cell cycle‐dependent chromatin loading of the Fanconi anemia core complex by FANCM/FAAP24. Blood 111, 5215–5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J.M. , Parmar, K. , Huang, M. , Weinstock, D.M. , Ruit, C.A. , Kutok, J.L. & D'Andrea, A.D. (2009) Inactivation of murine Usp1 results in genomic instability and a Fanconi anemia phenotype. Dev. Cell 16, 314–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, W. , Bennett, E.J. , Huttlin, E.L. , Guo, A. , Li, J. , Possemato, A. , Sowa, M.E. , Rad, R. , Rush, J. , Comb, M.J. , Harper, J.W. & Gygi, S.P. (2011) Systematic and quantitative assessment of the ubiquitin‐modified proteome. Mol. Cell 44, 325–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komander, D. , Clague, M.J. & Urbe, S. (2009) Breaking the chains: structure and function of the deubiquitinases. Nat. Rev. Mol. Cell Biol. 10, 550–563. [DOI] [PubMed] [Google Scholar]
- Kravtsova‐Ivantsiv, Y. , Cohen, S. & Ciechanover, A. (2009) Modification by single ubiquitin moieties rather than polyubiquitination is sufficient for proteasomal processing of the p105 NF‐kappaB precursor. Mol. Cell 33, 496–504. [DOI] [PubMed] [Google Scholar]
- Kruse, J.P. & Gu, W. (2009) MSL2 promotes Mdm2‐independent cytoplasmic localization of p53. J. Biol. Chem. 284, 3250–3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange, S.S. , Takata, K. & Wood, R.D. (2011) DNA polymerases and cancer. Nat. Rev. Cancer 11, 96–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lashuel, H.A. , Overk, C.R. , Oueslati, A. & Masliah, E. (2013) The many faces of alpha‐synuclein: from structure and toxicity to therapeutic target. Nat. Rev. Neurosci. 14, 38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J.C. , Wang, G.X. , Schickling, O. & Peter, M.E. (2005) Fusing DEDD with ubiquitin changes its intracellular localization and apoptotic potential. Apoptosis 10, 1483–1495. [DOI] [PubMed] [Google Scholar]
- Li, G. & Reinberg, D. (2011) Chromatin higher‐order structures and gene regulation. Curr. Opin. Genet. Dev. 21, 175–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, M. , Brooks, C.L. , Wu‐Baer, F. , Chen, D. , Baer, R. & Gu, W. (2003) Mono‐ versus polyubiquitination: differential control of p53 fate by Mdm2. Science 302, 1972–1975. [DOI] [PubMed] [Google Scholar]
- Lin, D.H. , Sterling, H. , Wang, Z. , Babilonia, E. , Yang, B. , Dong, K. , Hebert, S.C. , Giebisch, G. & Wang, W.H. (2005) ROMK1 channel activity is regulated by monoubiquitination. Proc. Natl Acad. Sci. USA 102, 4306–4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y. , Fallon, L. , Lashuel, H.A. , Liu, Z. & Lansbury, P.T. Jr (2002) The UCH‐L1 gene encodes two opposing enzymatic activities that affect alpha‐synuclein degradation and Parkinson's disease susceptibility. Cell 111, 209–218. [DOI] [PubMed] [Google Scholar]
- Ma, C. , Agrawal, G. & Subramani, S. (2011) Peroxisome assembly: matrix and membrane protein biogenesis. J. Cell Biol. 193, 7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchenko, N.D. , Wolff, S. , Erster, S. , Becker, K. & Moll, U.M. (2007) Monoubiquitylation promotes mitochondrial p53 translocation. EMBO J. 26, 923–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchese, A. , Raiborg, C. , Santini, F. , Keen, J.H. , Stenmark, H. & Benovic, J.L. (2003) The E3 ubiquitin ligase AIP4 mediates ubiquitination and sorting of the G protein‐coupled receptor CXCR4. Dev. Cell 5, 709–722. [DOI] [PubMed] [Google Scholar]
- Marin, I. (2009) Diversification of the cullin family. BMC Evol. Biol. 9, 267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer, M.P. & Bukau, B. (2005) Hsp70 chaperones: cellular functions and molecular mechanism. Cell. Mol. Life Sci. 62, 670–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McComb, S. , Cheung, H.H. , Korneluk, R.G. , Wang, S. , Krishnan, L. & Sad, S. (2012) cIAP1 and cIAP2 limit macrophage necroptosis by inhibiting Rip1 and Rip3 activation. Cell Death Differ. 19, 1791–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meray, R.K. & Lansbury, P.T. Jr (2007) Reversible monoubiquitination regulates the Parkinson disease‐associated ubiquitin hydrolase UCH‐L1. J. Biol. Chem. 282, 10567–10575. [DOI] [PubMed] [Google Scholar]
- Metzger, M.B. , Hristova, V.A. & Weissman, A.M. (2012) HECT and RING finger families of E3 ubiquitin ligases at a glance. J. Cell Sci. 125, 531–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimnaugh, E.G. , Chen, H.Y. , Davie, J.R. , Celis, J.E. & Neckers, L. (1997) Rapid deubiquitination of nucleosomal histones in human tumor cells caused by proteasome inhibitors and stress response inducers: effects on replication, transcription, translation, and the cellular stress response. Biochemistry 36, 14418–14429. [DOI] [PubMed] [Google Scholar]
- Moldovan, G.L. & D'Andrea, A.D. (2009) How the fanconi anemia pathway guards the genome. Annu. Rev. Genet. 43, 223–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore, D.J. , West, A.B. , Dikeman, D.A. , Dawson, V.L. & Dawson, T.M. (2008) Parkin mediates the degradation‐independent ubiquitination of Hsp70. J. Neurochem. 105, 1806–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyal, L. , Lerenthal, Y. , Gana‐Weisz, M. et al (2011) Requirement of ATM‐dependent monoubiquitylation of histone H2B for timely repair of DNA double‐strand breaks. Mol. Cell 41, 529–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa, T. , Kajitani, T. , Togo, S. , Masuko, N. , Ohdan, H. , Hishikawa, Y. , Koji, T. , Matsuyama, T. , Ikura, T. , Muramatsu, M. & Ito, T. (2008) Deubiquitylation of histone H2A activates transcriptional initiation via trans‐histone cross‐talk with H3K4 di‐ and trimethylation. Genes Dev. 22, 37–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa, T. , Lv, L. , Nakagawa, M. , Yu, Y. , Yu, C. , D'Alessio, A.C. , Nakayama, K. , Fan, H.Y. , Chen, X. & Xiong, Y. (2015) CRL4(VprBP) E3 ligase promotes monoubiquitylation and chromatin binding of TET dioxygenases. Mol. Cell 57, 247–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama, K.I. & Nakayama, K. (2006) Ubiquitin ligases: cell‐cycle control and cancer. Nat. Rev. Cancer 6, 369–381. [DOI] [PubMed] [Google Scholar]
- Nie, L. , Sasaki, M. & Maki, C.G. (2007) Regulation of p53 nuclear export through sequential changes in conformation and ubiquitination. J. Biol. Chem. 282, 14616–14625. [DOI] [PubMed] [Google Scholar]
- Niikura, Y. , Kitagawa, R. , Ogi, H. , Abdulle, R. , Pagala, V. & Kitagawa, K. (2015) CENP‐A K124 ubiquitylation is required for CENP‐A deposition at the centromere. Dev. Cell 32, 589–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama, A. , Yamaguchi, L. , Sharif, J. , Johmura, Y. , Kawamura, T. , Nakanishi, K. , Shimamura, S. , Arita, K. , Kodama, T. , Ishikawa, F. , Koseki, H. & Nakanishi, M. (2013) Uhrf1‐dependent H3K23 ubiquitylation couples maintenance DNA methylation and replication. Nature 502, 249–253. [DOI] [PubMed] [Google Scholar]
- Onishi, Y. , Hirasaka, K. , Ishihara, I. , Oarada, M. , Goto, J. , Ogawa, T. , Suzue, N. , Nakano, S. , Furochi, H. , Ishidoh, K. , Kishi, K. & Nikawa, T. (2005) Identification of mono‐ubiquitinated LDH‐A in skeletal muscle cells exposed to oxidative stress. Biochem. Biophys. Res. Commun. 336, 799–806. [DOI] [PubMed] [Google Scholar]
- Pattingre, S. , Tassa, A. , Qu, X. , Garuti, R. , Liang, X.H. , Mizushima, N. , Packer, M. , Schneider, M.D. & Levine, B. (2005) Bcl‐2 antiapoptotic proteins inhibit Beclin 1‐dependent autophagy. Cell 122, 927–939. [DOI] [PubMed] [Google Scholar]
- Pavlopoulos, E. , Trifilieff, P. , Chevaleyre, V. , Fioriti, L. , Zairis, S. , Pagano, A. , Malleret, G. & Kandel, E.R. (2011) Neuralized1 activates CPEB3: a function for nonproteolytic ubiquitin in synaptic plasticity and memory storage. Cell 147, 1369–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham, A.D. & Sauer, F. (2000) Ubiquitin‐activating/conjugating activity of TAFII250, a mediator of activation of gene expression in Drosophila. Science 289, 2357–2360. [DOI] [PubMed] [Google Scholar]
- Piton, A. , Redin, C. & Mandel, J.L. (2013) XLID‐causing mutations and associated genes challenged in light of data from large‐scale human exome sequencing. Am. J. Hum. Genet. 93, 368–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povlsen, L.K. , Beli, P. , Wagner, S.A. , Poulsen, S.L. , Sylvestersen, K.B. , Poulsen, J.W. , Nielsen, M.L. , Bekker‐Jensen, S. , Mailand, N. & Choudhary, C. (2012) Systems‐wide analysis of ubiquitylation dynamics reveals a key role for PAF15 ubiquitylation in DNA‐damage bypass. Nat. Cell Biol. 14, 1089–1098. [DOI] [PubMed] [Google Scholar]
- Raiborg, C. , Bache, K.G. , Gillooly, D.J. , Madshus, I.H. , Stang, E. & Stenmark, H. (2002) Hrs sorts ubiquitinated proteins into clathrin‐coated microdomains of early endosomes. Nat. Cell Biol. 4, 394–398. [DOI] [PubMed] [Google Scholar]
- Ramanathan, H.N. & Ye, Y. (2012) Cellular strategies for making monoubiquitin signals. Crit. Rev. Biochem. Mol. Biol. 47, 17–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts, S. , Delury, C. & Marsh, E. (2012) The PDZ protein discs‐large (DLG): the ‘Jekyll and Hyde’ of the epithelial polarity proteins. FEBS J. 279, 3549–3558. [DOI] [PubMed] [Google Scholar]
- Robzyk, K. , Recht, J. & Osley, M.A. (2000) Rad6‐dependent ubiquitination of histone H2B in yeast. Science 287, 501–504. [DOI] [PubMed] [Google Scholar]
- Rotin, D. & Kumar, S. (2009) Physiological functions of the HECT family of ubiquitin ligases. Nat. Rev. Mol. Cell Biol. 10, 398–409. [DOI] [PubMed] [Google Scholar]
- Rott, R. , Szargel, R. , Haskin, J. , Bandopadhyay, R. , Lees, A.J. , Shani, V. & Engelender, S. (2011) alpha‐Synuclein fate is determined by USP9X‐regulated monoubiquitination. Proc. Natl Acad. Sci. USA 108, 18666–18671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rott, R. , Szargel, R. , Haskin, J. , Shani, V. , Shainskaya, A. , Manov, I. , Liani, E. , Avraham, E. & Engelender, S. (2008) Monoubiquitylation of alpha‐synuclein by seven in absentia homolog (SIAH) promotes its aggregation in dopaminergic cells. J. Biol. Chem. 283, 3316–3328. [DOI] [PubMed] [Google Scholar]
- Saha, T. , Ghosh, S. , Vassilev, A. & DePamphilis, M.L. (2006) Ubiquitylation, phosphorylation and Orc2 modulate the subcellular location of Orc1 and prevent it from inducing apoptosis. J. Cell Sci. 119, 1371–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saigoh, K. , Wang, Y.L. , Suh, J.G. , Yamanishi, T. , Sakai, Y. , Kiyosawa, H. , Harada, T. , Ichihara, N. , Wakana, S. , Kikuchi, T. & Wada, K. (1999) Intragenic deletion in the gene encoding ubiquitin carboxy‐terminal hydrolase in gad mice. Nat. Genet. 23, 47–51. [DOI] [PubMed] [Google Scholar]
- Sasaki, A.T. , Carracedo, A. , Locasale, J.W. , Anastasiou, D. , Takeuchi, K. , Kahoud, E.R. , Haviv, S. , Asara, J.M. , Pandolfi, P.P. & Cantley, L.C. (2011) Ubiquitination of K‐Ras enhances activation and facilitates binding to select downstream effectors. Sci. Signal. 4, ra13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuermann, J.C. , de Ayala Alonso, A.G. , Oktaba, K. , Ly‐Hartig, N. , McGinty, R.K. , Fraterman, S. , Wilm, M. , Muir, T.W. & Muller, J. (2010) Histone H2A deubiquitinase activity of the Polycomb repressive complex PR‐DUB. Nature 465, 243–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabek, N. , Herman‐Bachinsky, Y. , Buchsbaum, S. , Lewinson, O. , Haj‐Yahya, M. , Hejjaoui, M. , Lashuel, H.A. , Sommer, T. , Brik, A. & Ciechanover, A. (2012) The size of the proteasomal substrate determines whether its degradation will be mediated by mono‐ or polyubiquitylation. Mol. Cell 48, 87–97. [DOI] [PubMed] [Google Scholar]
- Smit, J.J. & Sixma, T.K. (2014) RBR E3‐ligases at work. EMBO Rep. 15, 142–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, M.S. , Salmena, L. , Carracedo, A. , Egia, A. , Lo‐Coco, F. , Teruya‐Feldstein, J. & Pandolfi, P.P. (2008) The deubiquitinylation and localization of PTEN are regulated by a HAUSP‐PML network. Nature 455, 813–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, L.Y. , Yamashita, M. , Coussens, N.P. , Tang, Y. , Wang, X. , Li, C. , Deng, C.X. , Cheng, S.Y. & Zhang, Y.E. (2011) Ablation of Smurf2 reveals an inhibition in TGF‐beta signalling through multiple mono‐ubiquitination of Smad3. EMBO J. 30, 4777–4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terai, K. , Abbas, T. , Jazaeri, A.A. & Dutta, A. (2010) CRL4(Cdt2) E3 ubiquitin ligase monoubiquitinates PCNA to promote translesion DNA synthesis. Mol. Cell 37, 143–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrower, J.S. , Hoffman, L. , Rechsteiner, M. & Pickart, C.M. (2000) Recognition of the polyubiquitin proteolytic signal. EMBO J. 19, 94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomida, J. , Masuda, Y. , Hiroaki, H. , Ishikawa, T. , Song, I. , Tsurimoto, T. , Tateishi, S. , Shiomi, T. , Kamei, Y. , Kim, J. , Kamiya, K. , Vaziri, C. , Ohmori, H. & Todo, T. (2008) DNA damage‐induced ubiquitylation of RFC2 subunit of replication factor C complex. J. Biol. Chem. 283, 9071–9079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trockenbacher, A. , Suckow, V. , Foerster, J. , Winter, J. , Krauss, S. , Ropers, H.H. , Schneider, R. & Schweiger, S. (2001) MID1, mutated in Opitz syndrome, encodes an ubiquitin ligase that targets phosphatase 2A for degradation. Nat. Genet. 29, 287–294. [DOI] [PubMed] [Google Scholar]
- Trotman, L.C. , Wang, X. , Alimonti, A. et al (2007) Ubiquitination regulates PTEN nuclear import and tumor suppression. Cell 128, 141–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usuba, T. , Ishibashi, Y. , Okawa, Y. , Hirakawa, T. , Takada, K. & Ohkawa, K. (2001) Purification and identification of monoubiquitin‐phosphoglycerate mutase B complex from human colorectal cancer tissues. Int. J. Cancer 94, 662–668. [DOI] [PubMed] [Google Scholar]
- Van Campenhout, C.A. , Eitelhuber, A. , Gloeckner, C.J. , Giallonardo, P. , Gegg, M. , Oller, H. , Grant, S.G. , Krappmann, D. , Ueffing, M. & Lickert, H. (2011) Dlg3 trafficking and apical tight junction formation is regulated by nedd4 and nedd4‐2 e3 ubiquitin ligases. Dev. Cell 21, 479–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Themsche, C. , Leblanc, V. , Parent, S. & Asselin, E. (2009) X‐linked inhibitor of apoptosis protein (XIAP) regulates PTEN ubiquitination, content, and compartmentalization. J. Biol. Chem. 284, 20462–20466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, H. , Wang, L. , Erdjument‐Bromage, H. , Vidal, M. , Tempst, P. , Jones, R.S. & Zhang, Y. (2004) Role of histone H2A ubiquitination in Polycomb silencing. Nature 431, 873–878. [DOI] [PubMed] [Google Scholar]
- Wang, J. & Pantopoulos, K. (2011) Regulation of cellular iron metabolism. Biochem. J. 434, 365–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins, G.R. , Wang, N. , Mazalouskas, M.D. , Gomez, R.J. , Guthrie, C.R. , Kraemer, B.C. , Schweiger, S. , Spiller, B.W. & Wadzinski, B.E. (2012) Monoubiquitination promotes calpain cleavage of the protein phosphatase 2A (PP2A) regulatory subunit alpha4, altering PP2A stability and microtubule‐associated protein phosphorylation. J. Biol. Chem. 287, 24207–24215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegierski, T. , Hill, K. , Schaefer, M. & Walz, G. (2006) The HECT ubiquitin ligase AIP4 regulates the cell surface expression of select TRP channels. EMBO J. 25, 5659–5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wennerberg, K. , Rossman, K.L. & Der, C.J. (2005) The Ras superfamily at a glance. J. Cell Sci. 118, 843–846. [DOI] [PubMed] [Google Scholar]
- Wisniewski, J.R. , Zougman, A. , Kruger, S. & Mann, M. (2007) Mass spectrometric mapping of linker histone H1 variants reveals multiple acetylations, methylations, and phosphorylation as well as differences between cell culture and tissue. Mol. Cell Proteomics 6, 72–87. [DOI] [PubMed] [Google Scholar]
- Woelk, T. , Oldrini, B. , Maspero, E. , Confalonieri, S. , Cavallaro, E. , Di Fiore, P.P. & Polo, S. (2006) Molecular mechanisms of coupled monoubiquitination. Nat. Cell Biol. 8, 1246–1254. [DOI] [PubMed] [Google Scholar]
- Wu, H. & Zhang, Y. (2014) Reversing DNA methylation: mechanisms, genomics, and biological functions. Cell 156, 45–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong, Y. & Guan, K.L. (2012) Mechanistic insights into the regulation of metabolic enzymes by acetylation. J. Cell Biol. 198, 155–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, L. , Lubkov, V. , Taylor, L.J. & Bar‐Sagi, D. (2010) Feedback regulation of Ras signaling by Rabex‐5‐mediated ubiquitination. Curr. Biol. 20, 1372–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, W. , Xia, Y. , Cao, Y. , Zheng, Y. , Bu, W. , Zhang, L. , You, M.J. , Koh, M.Y. , Cote, G. , Aldape, K. , Li, Y. , Verma, I.M. , Chiao, P.J. & Lu, Z. (2012) EGFR‐induced and PKCepsilon monoubiquitylation‐dependent NF‐kappaB activation upregulates PKM2 expression and promotes tumorigenesis. Mol. Cell 48, 771–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye, Y. & Rape, M. (2009) Building ubiquitin chains: E2 enzymes at work. Nat. Rev. Mol. Cell Biol. 10, 755–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin, H. , Gui, Y. , Du, G. , Frohman, M.A. & Zheng, X.L. (2010) Dependence of phospholipase D1 multi‐monoubiquitination on its enzymatic activity and palmitoylation. J. Biol. Chem. 285, 13580–13588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan, J. , Luo, K. , Zhang, L. , Cheville, J.C. & Lou, Z. (2010) USP10 regulates p53 localization and stability by deubiquitinating p53. Cell 140, 384–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J. , Zhang, P. , Wei, Y. , Piao, H.L. , Wang, W. , Maddika, S. , Wang, M. , Chen, D. , Sun, Y. , Hung, M.C. , Chen, J. & Ma, L. (2013) Deubiquitylation and stabilization of PTEN by USP13. Nat. Cell Biol. 15, 1486–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X.Y. , Varthi, M. , Sykes, S.M. , Phillips, C. , Warzecha, C. , Zhu, W. , Wyce, A. , Thorne, A.W. , Berger, S.L. & McMahon, S.B. (2008) The putative cancer stem cell marker USP22 is a subunit of the human SAGA complex required for activated transcription and cell‐cycle progression. Mol. Cell 29, 102–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, W. , Zhu, P. , Wang, J. , Pascual, G. , Ohgi, K.A. , Lozach, J. , Glass, C.K. & Rosenfeld, M.G. (2008) Histone H2A monoubiquitination represses transcription by inhibiting RNA polymerase II transcriptional elongation. Mol. Cell 29, 69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, P. , Zhou, W. , Wang, J. , Puc, J. , Ohgi, K.A. , Erdjument‐Bromage, H. , Tempst, P. , Glass, C.K. & Rosenfeld, M.G. (2007) A histone H2A deubiquitinase complex coordinating histone acetylation and H1 dissociation in transcriptional regulation. Mol. Cell 27, 609–621. [DOI] [PMC free article] [PubMed] [Google Scholar]


