Abstract
Hepatic copper determination is an important test for the diagnosis of Wilson's disease (WD). However, the method has not been standardized, the diagnostic accuracy has not been evaluated prospectively, and the optimal cut‐off value remains controversial. Accordingly, we aimed to prospectively evaluate the diagnostic accuracy of hepatic copper content, as determined using the entire core of a liver biopsy sample. Patients for whom a liver biopsy was indicated were consecutively enrolled. Hepatic copper content was determined with atomic absorption spectroscopy. All assays were performed using careful quality control by a single technician. WD diagnosis was based on WD score or its combination with clinical follow‐up results. A total of 3,350 consecutive patients underwent liver biopsy. Six hundred ninety‐one patients, including 178 with WD, underwent two passes of liver biopsy with hepatic copper determination. Mean hepatic content in WD patients was 770.6 ± 393.2 μg/g dry weight (wt). Sensitivity, specificity, and positive and negative predictive values of hepatic copper content for WD diagnosis in the absence of primary biliary cirrhosis (PBC) or primary sclerosing cholangitis at the cut‐off value of 250 μg/g dry wt. were 94.4%, 96.8%, 91.8%, and 97.8%, respectively. The most useful cut‐off value was 209 μg/g dry wt, with a sensitivity and specificity of 99.4% and 96.1%, respectively. A total of 23.3% of patients without WD and PBC had hepatic copper content >75 μg/g dry wt. Conclusion: A liver biopsy sample of more than 1 mg dry wt may reliably reflect hepatic copper content and should be used for hepatic copper determination. Hepatic copper determination is a very valid procedure for the diagnosis of WD, and the most useful cut‐off value is 209 μg/g dry wt.(Hepatology 2015;62:1731–1741)
Abbreviations
- AIH
autoimmune hepatitis
- ALP
alkaline phosphatase
- CV
coefficient of variation
- GGT
gamma glutamyl transferase
- IPH
idiopathic portal hypertension
- K‐F rings
Kayser‐Fleischer rings
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- NPV
negative predictive value
- PBC
primary biliary cirrhosis
- PPV
positive predictive value
- PSC
primary sclerosing cholangitis
- ROC
receiver operating characteristic curves
- SD
standard deviation
- WD
Wilson's disease
- wt
weight
Wilson's disease (WD), first described by Kinnear Wilson in 1912, is an autosomal‐recessive inherited disorder of copper metabolism resulting in copper accumulation in the liver and other organs. As early as 1948, Cumings found elevated copper levels in both the liver and brain tissue of patients with WD and first discussed an etiological role for copper.1 Some other physicians and investigators, both before and after Cumings, have made similar observations. In the 1960s, Sternlieb et al.2 and Smallwood et al.3 found that hepatic copper concentrations are between 15 and 55 μg/g dry liver in normal individuals and are elevated in virtually all untreated patients with WD to values between 250 and 3,000 μg/g dry tissue. Then, Brewer4 stated that a liver copper value below diagnostic levels had not been found in their patients with WD. Therefore, a hepatic copper level of 250 μg/g dry tissue or more has been considered most valid for diagnosing WD.5, 6 However, the diagnostic accuracy of the test has never been prospectively investigated or validated in a sufficiently large group of patients with and without WD. Liver copper data in most studies have been collected over several decades from hospital records, and most information was from a small number of patients and the pathological conditions in these studies were limited. Furthermore, nondiagnostic hepatic copper levels in patients with confirmed WD have been reported by many researchers in the past two to three decades, including 3 of 12 patients with WD reported by Martins da Costa et al.,7 4 of 43 reported by Steindl et al.,8 1 of 19 reported by Gow et al.,9 4 of 55 reported by Merle et al.,10 and 2 of 30 patients reported by Niscastro et al.11 In a study reported by Ferenci et al.,12 liver copper concentration was below 250 μg/g dry weight (wt) in 19 of 114 patients with WD, and thus a threshold value of 75 μg/g dry wt has also been suggested. Unquestionably, the diagnostic accuracy and the optimal cut‐off value of hepatic copper content remains controversial, and further studies are necessary to resolve this matter.
Previous studies indicate that copper is unevenly distributed in the liver, and sample size is very important to correctly determine mean hepatic copper content.13, 14, 15 Brewer4 strongly emphasized that measuring hepatic copper levels from a 10‐ to 20‐mg needle biopsy sample requires a good laboratory and good quality control. However, few studies have focused on the method of hepatic copper determination, and thus the method has not yet been standardized. Hepatic copper content is usually determined using a part of a needle biopsy specimen,12 which may occasionally result in a false result because of sampling and measurement errors. Decreasing laboratory errors is a necessary prerequisite for study of the diagnostic accuracy of the test. Therefore, we strongly considered the test method and carefully designed the procedures. The aim of this study then was to prospectively investigate the diagnostic accuracy of liver copper content determined using the entire core of a liver biopsy sample and a good quality control for WD diagnosis in a large number of patients with WD and other liver diseases.
Patients and Methods
Design Overview, Setting, and Participants
The study was conducted between June 2007 and May 2014 at the Liver Disease Research Center, Second Xiangya Hospital, Central South University (Changsha, China). Patients referred to the Liver Disease Research Center were consecutively enrolled during the study period. The study inclusion criteria were as follows: (1) patients suspected of WD; (2) relatives of WD patients for whom liver biopsy was indicated; and (3) patients with various diseases for whom liver biopsy was indicated. Indications for liver biopsy were based on the guideline for liver biopsy.16 Patients with contraindications to percutaneous liver biopsy, such as severe coagulopathy and ascites, and patients who were uncooperative or refused to perform liver biopsy with copper determination were excluded from the study. Patients who had liver samples less than 1 mg/g dry wt, received chelator therapy more than 6 months preceding liver biopsy, or were lost to follow‐up before a diagnosis was made were also excluded.
Patients suspected of WD underwent a slit‐lamp examination for Kayser‐Fleischer (K‐F) rings, neurological examination, measurement of serum ceruloplasmin level, and determination of 24‐hour urinary copper excretion before and after penicillamine challenge. Liver biopsy with copper determination was performed in most patients without contraindications, and owing to cost, gene analysis was performed in only some patients. Initial diagnosis of WD was based on the criteria described before4, 6 and the diagnostic scoring system published in 2003.17 Score was calculated according to a slightly modified scoring system (Table 1). Diagnosis of WD was accepted if the WD score was ≥4 in the absence of cholestasis. For patients in whom the diagnosis of WD could not be definitively confirmed or excluded at the outset, final diagnosis was based on a combination of WD score and clinical follow‐up results.
Table 1.
WD Scoring System Used in This Study
| Parameters | Score | ||||
|---|---|---|---|---|---|
| −1 | 0 | 1 | 2 | 4 | |
| Neuropsychiatric symptoms suggestive of WD | Absent | Present | |||
| K‐F rings | Absent | Present | |||
| Serum ceruloplasmin (mg/L) | >210 | 100‐210 | <100 | ||
| Urinary copper excretion (μg/24 hours) | <100 | 100‐200 | >200 | ||
| Urinary copper excretion after 2 × 0.5 g penicillamine (μg/24 hours) | <1,500 | 1,500‐2,500 | >2,500 | ||
| Hepatic copper level (μg/g dry wt) | <50 | 50‐99 | 100‐250 | >250 | |
| ATP7B mutation analysis (disease mutation number) | 0 | 1 | 2 | ||
This table was adapted from Ferenci et al.17 Only one item of urinary copper excretions with higher scores could be selected when calculating the WD scores. A score ≥4 indicates that WD is highly likely, a score of 2 or 3 indicates that disease is probable and further investigations are needed, and a score of 0 or 1 indicates that disease is unlikely.
WD patients were classified into the following phenotypes: “Pure hepatic” (H) was used when the patient had only liver dysfunction in the absence of any neurological symptoms upon a detailed neurological examination that was performed by a single experienced neurologist at the time of diagnosis; “hepatoneurological” (N1) was used when patients had hepatic and neurological and/or psychiatric dysfunctions simultaneously; “pure neurological” (N2) was used when patients presented with only neurological features in the absence of symptoms and signs related to liver disease and abnormal liver function tests; and “presymptomatic” (P) was used when patients had no symptoms or signs related to liver disease as well as normal liver functional tests. Diagnosis was established during screening of siblings from index cases or accidently found while performing routine laboratory tests or during the workup for another disease. Patients with viral hepatitis, autoimmune hepatitis (AIH), primary biliary cirrhosis (PBC), primary sclerosing cholangitis (PSC), alpha‐1‐antitrypsin deficiency, hemochromatosis, alcoholic liver disease, nonalcoholic steatohepatitis (NASH), Gilbert's syndrome, Dubin‐Johnson syndrome, polymyositis, extrahepatic causes of elevated serum aminotransferases, and so on, were diagnosed with conventional criteria and treated according to each respective etiology. Patients were considered to be affected by cryptogenic liver disease when the entity's origin was unknown.
An expert panel consisting of five hepatologists with extensive experience with WD and other liver diseases evaluated all enrolled patients. All patients were diagnosed and followed up by us. All data for each patient obtained at the diagnosis and follow‐up were recorded in a specially designed medical record. Written informed consent was obtained from all patients before the procedures. The study conformed to the 1975 Helsinki Declaration and was approved by the Second Xiangya Hospital Ethics Committee.
Laboratory Measurements
Routine laboratory data were obtained using standard methods. K‐F rings were examined under a slit lamp by a single ophthalmologist with extensive experience who was blind to the results of the other tests. Ceruloplasmin level in serum was measured using the nephelometric method (normal range: 210‐500 mg/L; Immage Immunochemistry System; Beckman Coulter, Brea, CA). A simple scoring system was used for histological grading and staging.16 Full sequencing of the ATP7B coding region and exon/intron boundaries was performed according to a previously published method.18
Patients underwent an ultrasound of the liver before biopsy. Liver biopsy procedures were performed by an experienced hepatologist. Two passes of biopsy were performed through the same skin puncture site with different needle inclinations using a disposable Tru‐Cut biopsy set. (Angiotech Medical Device Technologies Inc., Gainesville, FL). One sample of liver tissue obtained was sent for histology examination. Another sample of liver biopsy used for copper measurement was placed onto a 3 × 3‐cm clean white paper and dried in a small heating box (made with an electric bulb) at 68°C for 2 hours to constant weight. We accurately weighed the paper with dry liver tissue, scraped the liver tissue from the paper with a small knife, placed it into a plastic vial, and sealed the vial. We reweighed the paper to obtain the dry wt of the liver tissue. One biopsy sample more than 1 mg dry wt was used for copper measurement. Drying and weighing of liver samples were performed by a single physician.
Liver copper levels were determined in a special laboratory for copper determination. We carefully transferred liver tissue sample into a 5‐mL graduated glass test tube and added 0.5 mL of 4:1 mixed acid of concentrated nitric and perchloric acid (superpure grade) into the sample tube. The sample was left to digest at 150°C for 6‐8 hours until the white smoke of HClO4 evaporated and the liver tissue completely dissolved. The test tube was removed and left to cool, and then it was diluted accurately to 2 mL with 1% HNO3, mixed thoroughly by vortexing. The content of copper was determined with flame atomic absorption spectrophotometry at a wavelength of 324.7 nm (Model TAS‐986 atomic absorption spectrophotometer; Beijing Purkinje General Instruments Co., Ltd., Beijing, China). Two reference materials made of homogenized pig liver with a certified copper value of 70 μg/g dry wt and the liver of a patient who had died from fulminant WD, with a certified copper value of 980 μg/g dry wt, were analyzed simultaneously. All assays were performed using careful quality control by a single technician who was blind to the results of the other tests. The between‐batch coefficient of variation of the reference materials was less than 10%.
Statistical Analysis
All statistical analyses were performed using SPSS (version 16; SPSS, Inc., Chicago, IL). Patient characteristics are presented as mean ± standard deviation (SD). The t test and chi‐square test were used to compare continuous and categorical variables, respectively. Diagnostic accuracy of the test was assessed in terms of sensitivity, specificity, and positive and negative predictive value (PPV; NPV) and area under the receiver operating characteristic curve (ROC), which were calculated based on data from patients with final diagnosis of WD and patients with other various diseases in the absence of PBC and PSC. The optimal cut‐off value was determined with the ROC curve. Bar charts are used to show the mean liver copper level of patients with various liver diseases. Two‐tailed P values <0.05 were considered statistically significant.
Results
Patient Characteristics
During the study period, a total of 3,350 consecutive patients with various liver diseases underwent liver biopsy. Among them, 714 patients had two passes of liver biopsy with copper determination. Twenty‐three patients were excluded from the study: 8 had a liver sample less than 1 mg dry wt; 10 received chelator therapy more than 6 months preceding liver biopsy; and 5 were lost to follow‐up before the final diagnosis was made. We conducted the main analysis then on 691 patients and safety analysis on the entire group (Fig. 1). Demographics and hepatic copper content of study patients are summarized in Table 2.
Figure 1.
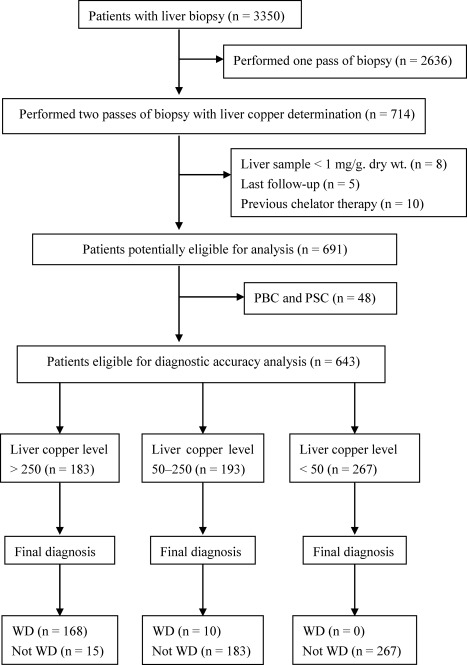
Subject selection flow chart. WD, Wilson disease; PBC, primary biliary cirrhosis; PSC, primary sclerosing cholangitis.
Table 2.
Characteristics and Hepatic Copper Content of Patients With Various Liver Diseases
| Etiology | No. of Patients | Gender (M/F) | Mean Age (Years) | Hepatic Copper Level of Patients (μg/g dry wt) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | 0– | 50– | 100– | 150– | 250+ | ||||
| WD | 178 | 104/74 | 19.7 ± 11.0 | 770.6 ± 393.2 | 0 | 0 | 1 | 9 | 168 |
| Heterozygote | 24 | 20/4 | 19.5 ± 8.0 | 110.7 ± 51.8 | 1 | 12 | 7 | 3 | 1 |
| PBC and PSC | 48 | 13/35 | 44.5 ± 13.1 | 318.2 ± 299.7 | 6 | 7 | 5 | 7 | 23 |
| Viral hepatitis | 198 | 181/17 | 33.4 ± 10.3 | 50.1 ± 35.3 | 126 | 53 | 14 | 5 | 0 |
| AIH | 50 | 22/28 | 38.0.2 ± 15.6 | 82.1 ± 66.0 | 19 | 20 | 6 | 3 | 2 |
| NASH | 40 | 35/5 | 33.5 ± 13.3 | 40.4 ± 28.1 | 31 | 8 | 1 | 0 | 0 |
| GS and DS | 28 | 24/4 | 22.5 ± 8.0 | 37.3 ± 19.7 | 23 | 5 | 0 | 0 | 0 |
| Hemochromatosis | 9 | 9/0 | 31.8 ± 10.1 | 55.4 ± 77.3 | 7 | 1 | 0 | 0 | 1 |
| IPH | 10 | 8/2 | 41.4 ± 14.0 | 59.8 ± 29.0 | 4 | 5 | 1 | 0 | 0 |
| Other diseases | 32 | 26/6 | 33.8 ± 18.0 | 50.2 ± 39.5 | 20 | 9 | 1 | 2 | 0 |
| Origin unknown | 74 | 50/24 | 31.0 ± 18.9 | 165.0 ± 320.6 | 36 | 17 | 6 | 5 | 10 |
| Total | 691 | 492/199 | 29.8 ± 14.2 | 272.8 ± 388.3 | 273 | 137 | 42 | 33 | 206 |
Viral hepatitis, hepatitis B (180), other viral hepatitis (18); GS (26) and DS (2); other diseases, including neurologic diseases other than WD (8), hemolytic jaundice (5), polymyositis (5), venous occlusive disease (4), Budd‐Chiari syndrome (3), alcoholic hepatitis (3), lymphoma (1), glycogen storage disease (1), congenital hepatic fibrosis (1), and schistosomiasis Japonica (1). Origin unknown refers to other diseases origin unknown.
Abbreviations: M, male; F, female; GS, Gilbert syndrome; DS, Dubin‐Johnson syndrome.
Hepatic Copper Levels in Patients With WD
The final diagnosis of WD was made in 178 patients, including 105 H patients (59.0%), 25 N1 patients (14.0%), 31 N2 patients (17.4%), and 17 P patients (9.6%). Among them, 174 (97.8%) had a WD score of at least 4, whereas only 4 had a WD score less than 4. Among these 4 patients, 3 were siblings of index cases, and they had mildly decreased ceruloplasmin levels and markedly increased liver copper levels. The remaining patient was evaluated because of elevated levels of alanine aminotransferase and serum aspartate aminotransferase for 3 years. This patient had a ceruloplasmin level of 272 mg/L and markedly elevated liver copper content of 1,125.0 μg/g dry wt. Four patients who refused to undergo genetic testing were treated with penicillamine or zinc, and their liver function tests gradually returned to normal. They were diagnosed with WD based on a combination of WD score, family history, and positive response to chelator therapy, even though their WD scores were less than 4.
Hepatic copper level in WD patients did not correlate with sex, the grade of hepatic inflammation, and stage of hepatic fibrosis, negatively correlated with age at diagnosis (r = −0.304; P = 0.001). Mean hepatic copper level was significantly higher in H patients than it was in other phenotypes (P = 0.001). It was also higher in patients 14 years old or younger than it was in older patients. All 105 H patients, including 60 patients 14 years old or younger, had hepatic copper levels over 250 μg/g dry wt. Among 31 N2 patients, 7 (22.6%) had hepatic copper levels below 250 μg/g dry wt (Table 3; Fig. 2).
Table 3.
Hepatic Copper Levels in Various Phenotypes and Age Groups of WD Patients
| Groups | No. | Hepatic Copper Level (μg/g dry wt) Mean ± SD 95% CI Range | P Value |
|---|---|---|---|
| Phenotype | |||
| Pure liver disease, H | 105 | 915.4 ± 365.3 844.7‐986.1 337.0‐1,968.0 | 0.006 |
| Liver and neurological disease, N1 | 25 | 702. 3 ± 389.3 541.6‐863.0 118.0‐1,540.0 | |
| Pure neurological, N2 | 31 | 411.3 ± 262.9 313.3‐509.5 210.0‐1,363.0 | 0.001 |
| Presymptomatic, P | 17 | 737.8 ± 245.0 607.2‐868.4 357.0‐1,149.0 | |
| Age group, years old | |||
| 0‐7 | 16 | 1,113.3 ± 289.8 958.9‐1,267.7 639.0‐1,650.0 | 0.001 |
| 8‐14 | 55 | 927.0 ± 403.8 817.8‐1,036.1 118.0‐1,968.0 | 0.001 |
| 15‐21 | 47 | 626.3 ± 338.1 527.0‐725.6 212.0‐1,732.0 | |
| 22‐27 | 26 | 661.0 ± 354.0 518.0‐804.0 213.0‐1,540.0 | |
| 28‐35 | 13 | 745.8 ± 281.5 475.7‐915.9 445.0‐1,356.7 | |
| ≥36 | 21 | 639.4 ± 366.6 472.5‐806.2 233.0‐1,504.0 |
Abbreviation: CI, confidence interval.
Figure 2.
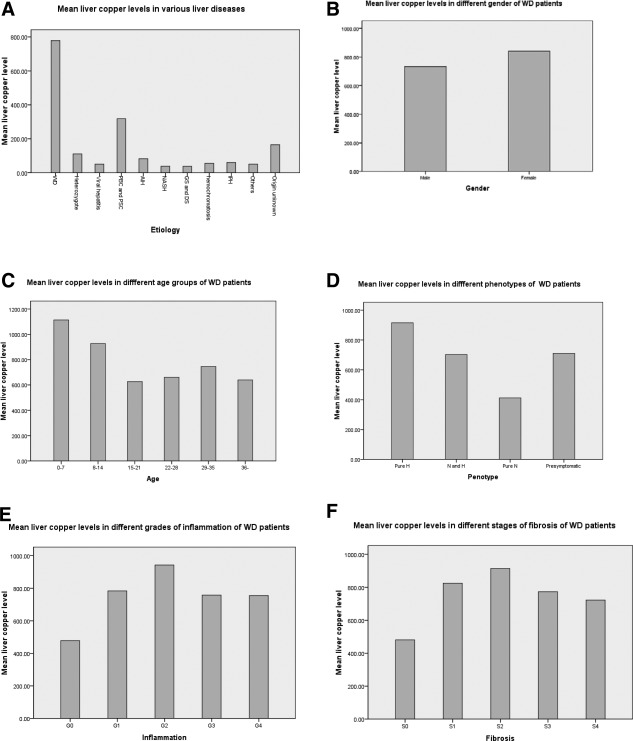
Mean liver copper level in various liver diseases and by gender, age, phenotype, grade of imflammation, and stage of fibrosis of WD patients. WD, Wilson disease; PBC, primary biliary cirrhosis; PSC, primary sclerosing cholangitis; AIH, autoimmune hepatitis; NASH, nonalcoholic steatohepatitis.
Correlation Between the Precision of Copper Measurement and the Sample Size, Hepatic Copper Content and Stage of Fibrosis
To assess the magnitude of hepatic copper heterogeneity in a given sample according to dry weight of biopsy specimen, we assessed the coefficient of variation (CV) of the hepatic copper measurement in liver biopsy samples with different dry weights. The CV of the copper measurement in the liver biopsy sample with 1.0‐1.5 mg of dry weight was 76.0%; this decreased to 39.2% in the liver biopsy sample with a dry weight of more than 3 mg. A significant trend was observed in which the CV decreased with the increase in liver sample weight. There was no difference in the CV of hepatic copper measurements between patients with and without cirrhosis (Table 4).
Table 4.
Variability of the Copper Determination in Different Samples
| Group | No. of Patients | Mean Hepatic Copper Level (μg/dry wt) | SD (μg/dry wt) | CV (%) |
|---|---|---|---|---|
| Dry weight, mg | ||||
| 1.0‐1.5 | 22 | 618.4 | 469.8 | 76.0 |
| 1.6‐2.0 | 46 | 856.3 | 436.9 | 51.0 |
| 2.1‐2.5 | 40 | 710.9 | 368.4 | 51.8 |
| 2.6‐3.0 | 37 | 829.9 | 340.0 | 41.0 |
| ≥3.1 | 33 | 800.4 | 313.9 | 39.2 |
| Fibrosis | ||||
| S0 | 15 | 481.2 | 261.6 | 54.4 |
| S1 | 29 | 824.9 | 408.3 | 49.5 |
| S2 | 45 | 913.8 | 358.6 | 39.2 |
| S3 | 39 | 773.2 | 408.9 | 52.9 |
| S4 | 50 | 722.5 | 376.9 | 52.2 |
| Inflammation | ||||
| G0 | 15 | 478.3 | 264.4 | 55.3 |
| G1 | 27 | 783.9 | 366.9 | 46.8 |
| G2 | 29 | 942.5 | 366.4 | 41.0 |
| G3 | 67 | 757.9 | 387.8 | 51.1 |
| G4 | 30 | 755.5 | 392.1 | 51.9 |
| Total | 178 | 778.3 | 390.3 | 50.1 |
Abbreviations: S, stage of fibrosis; G, grade of inflammation.
Diagnostic Accuracy of Hepatic Copper Content for WD Diagnosis
Mean hepatic content in 178 WD patients was 770.6 ± 393.2 μg/g dry wt (range, 118‐1,860), which was significantly greater than that in other groups, including PBC and PSC (P = 0.001; Fig. 1A). Among them, hepatic copper level was ≥210 μg/g dry wt in 177 (99.4%), ≥250 μg/g dry wt in 168 (94.4%), ≥500 μg/g dry wt in 127 (71.3%), and ≥1,000 μg/g dry wt in 51 (28.7%). Sensitivity, specificity, PPV, and NPV of hepatic copper level for the diagnosis of WD in the absence of PBC and at the conventional cut‐off value of 250 μg/g dry wt were 94.4%, 96.8%, 91.8%, and 97.8%, respectively. Subgroup analysis revealed that sensitivities of hepatic copper level for the diagnosis of WD was 100% in patients presenting with liver disease and those 14 years old or younger. An ROC curve of hepatic copper content for diagnosis of WD was constructed using the data of 178 patients with WD and 465 patients without PBC and PSC. The area under the curve was 0.987 (0.979‐0.995). The ROC curve suggests that the most useful cut‐off value was 209 μg/g dry wt, where the sensitivity was 99.4% and specificity was 96.1%. This provides the highest diagnostic accuracy for the diagnosis of WD (Table 5; Fig. 3).
Table 5.
Diagnostic Accuracy of Hepatic Copper Content at the Conventional Cut‐off Value in the Absence of PBC
| A. Diagnostic Accuracy of Hepatic Copper Content in the Whole Group | |||||||
|---|---|---|---|---|---|---|---|
| No. of Patients | |||||||
| Liver Copper Content | Diagnosed | Sensitivity | Specificity | PPV | NPV | ||
| (μg/g dry wt) | WD | Non‐WD | Total | % (95% CI) | % (95% CI) | % (95% CI) | % (95% CI) |
| ≥250 | 168 | 15 | 183 | 94.4 | 96.8 | 91.8 | 97.8 |
| (97.9‐91.3) | (98.4‐95.2) | (95.8‐87.8) | (99.1‐96.5) | ||||
| <250 | 10 | 450 | 460 | ||||
| Total | 178 | 465 | 643 | ||||
| B. Diagnostic Accuracy of the Hepatic Copper Content in Patients Presenting With Pure Liver Disease | |||||||
|---|---|---|---|---|---|---|---|
| No. of Patients | |||||||
| Liver Copper Content | Diagnosed | Sensitivity | Specificity | PPV | NPV | ||
| (μg/g dry wt) | WD | Non‐WD | Total | % (95% CI) | % (95% CI) | % (95% CI) | % (95% CI) |
| ≥250 | 105 | 15 | 120 | 100.0 | 96.8 | 87.5 | 100.0 |
| (100.0) | (98.4‐95.2) | (93.4‐81.6) | (100.0) | ||||
| <250 | 0 | 450 | 450 | ||||
| Total | 105 | 465 | 570 | ||||
| C. Diagnostic Accuracy of Hepatic Copper Content in WD Patients 14 Years or Younger Presenting With Liver Disease | |||||||
|---|---|---|---|---|---|---|---|
| No. of Patients | |||||||
| Liver Copper Content | Diagnosed | Sensitivity | Specificity | PPV | NPV | ||
| (μg/g dry wt) | WD | Non‐WD | Total | % (95% CI) | % (95% CI) | % (95% CI) | % (95% CI) |
| ≥250 | 60 | 15 | 75 | 100.0 | 96.8 | 80.0 | 100.0 |
| (100.0) | (98.4‐95.2) | (89.1‐70.9) | (100.0) | ||||
| <250 | 0 | 450 | 450 | ||||
| Total | 60 | 465 | 525 | ||||
Abbreviation: CI, confidence interval.
Figure 3.
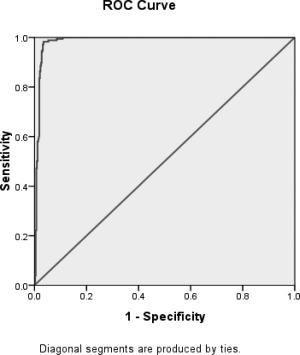
ROC curve of liver copper content for the diagnosis of WD. The curve was constructed with data from 178 patients with final diagnosis of WD and 465 patients without WD in the absence of PBC. SPSS automatically generated the curve and calculated the area under the curve (0.987) as well as serial data of test sensitivity and specificity at different cut‐off values (from the lowest liver copper level to the highest liver copper level). The maximum sum of sensitivity and specificity was obtained at the cut‐off value of 209 μg/g dry wt.; therefore, the optimal test cutoff value was 209 μg/g dry wt. WD, Wilson disease; ROC, receiver operating characteristic; PBC, primary biliary cirrhosis.
Hepatic Copper Levels in Other Patients
Mean hepatic copper level was lower than normal in NASH, Gilbert syndrome, Dubin‐Johnson syndrome, neurological diseases other than WD, hemolytic jaundice, polymyositis, and so on, and it was normal or mildly elevated in heterozygotes of WD, viral hepatitis, idiopathic portal hypertension (IPH), hemochromatosis, venous occlusive disease, Budd‐Chiari syndrome, and so on. Among 48 patients with PBC or PSC, 23 (47.9%) had liver copper values ≥250 μg/g dry wt and 2 had WD scores of 4. None of them were diagnosed with WD because of the presence of typical clinical and biochemical features related to PBC or PSC. Among 465 patients without WD and PBC, 108 (23.2%) had hepatic copper content over 75 μg/g dry wt, 32 (6.9%) had over 150 μg/g dry wt, and 15 (3.2%) had over 250 μg/g dry wt. Among 24 heterozygotes of WD, 19 individuals (79.2%) had hepatic copper levels over 75 μg/g dry wt and 4 (16.7%) had over 150 μg/g dry wt. One individual, the father of an index case, had normal liver function tests, normal liver histology, and hepatic copper content of 253 μg/g dry wt, which was confirmed with a repeat biopsy 2 years later. He did not develop any WD‐related symptoms during the 5‐year follow‐up period and was finally diagnosed as WD heterozygous. Among 50 patients with AIH, 19 (38.0%) had hepatic copper levels over 75 μg/g dry wt and 2 had over 250 μg/g dry wt. The possibility of WD had been considered in patients with above‐normal hepatic copper levels; however, none had WD scores more than 3 or developed symptoms related to WD during the study period. Accordingly, WD diagnosis could be dismissed with certainty in all cases.
The diagnosis was challenging in 10 patients grouped as origin unknown. They were referred for cryptogenic liver disease to our center from hospitals all over China. The common causes of liver disease, such as viral hepatitis, drug‐induced liver disease, toxic hepatitis, alcoholic hepatitis, nonalcoholic fatty liver disease (NAFLD), alpha‐1‐antitrypsin deficiency, and so on, had all been excluded. They had markedly elevated gamma‐glutamyl transferase (GGT) and alkaline phosphatase (ALP), but did not fulfill the conventional diagnostic criteria for PBC or PSC. They had normal serum ceruloplasmin levels and markedly elevated liver copper content. Three patients had identified K‐F rings, 2 had WD scores of 6, and 5 had WD scores of 4. All but 1 patient had no identified disease mutations. They were initially suspected as WD and treated with D‐penicillamine for 6‐20 months with no adverse effects, but did not improve symptomatically, and 2 patients died. Most were eventually diagnosed as atypical PBC, PSC, or other cholestatic disorders.
Complications of Liver Biopsy
Minor complications after liver biopsy, including transient, localized discomfort at the biopsy site with dull, mild, and brief pain in the right upper quadrant or right shoulder, mild transient hypotension, and mild subcapsular hematoma requiring no hospital admission or special treatment, were the same in both groups. One patient who had one pass of liver biopsy developed pneumothorax, which was managed conservatively (0.06%). There was no biopsy‐related mortality in either group.
Discussion
It is well accepted that the distribution of copper in the liver is inhomogeneous and that the accuracy of the liver copper measurement is improved with an adequately sized specimen.6 However, the method of hepatic copper determination has not yet been standardized, though liver copper content is usually determined in part of a needle biopsy specimen.12 The threshold length recommended for avoiding sampling errors is arbitrary; to our knowledge, no studies have sought to define this threshold on a rational basis. Whether the entire core of a liver biopsy sample is large enough for a correct copper determination is another unresolved question. Our main measures in hepatic copper determination are as follows: (1) Two passes of liver biopsy should be performed, and an entire core of liver biopsy sample should be used for copper determination. The entire core of a biopsy liver sample may contain regions with low and high copper concentrations, which will decrease sampling variability; (2) the liver biopsy sample should be placed on a small piece of paper for drying, which can more accurately measure the dry wt of liver sample because the weight of paper is very light (because of very small tare weight); and (3) a single technician with extensive experience in liver copper determination should perform using good quality control.
Our study demonstrated that all 178 WD patients but 1 had liver copper content ≥210 μg/g dry wt. There was no significant difference in mean liver copper content, SD of liver copper content, or the CV of copper determination between WD patients with or without cirrhosis. These results do not support the idea that patients with cirrhosis have a more irregular distribution of copper in the liver, which may have an influence on copper content and the precise measurement of the liver copper content. This discrepancy may be related to the histochemical staining method that was used for the copper distribution study; the staining method will only be positive when copper is sequestered into organelles. If the copper is diffusely cytoplasmic, the stain will be negative even though the patient has a very high level of hepatic copper.4 Therefore, we speculate that the irregular distribution of liver copper observed by histochemical stains does not equal the actual variation of copper content. However, our study showed that the size of the liver biopsy specimen has a major influence on the precise determination of copper content. The CV of the copper measurement in the liver biopsy sample, with a dry weight of more than 3 mg, was 39.2%; this increased to 76.0% with 1.0‐1.5 mg of dry weight. There is a clear trend that the larger the biopsy specimen, the lower the risk for an erroneous measurement of copper (Table 3). These results indicate that our measures of the liver copper content are both effective and reliable. An entire core of liver biopsy sample may reliably reflect hepatic copper content, which is both essential and sufficient for a correct determination of liver copper content; this is true regardless of the fact that copper is unevenly distributed in the liver. If liver copper content is determined in a part of biopsy specimen (as is usually done), the dry weight of the liver sample, in some patients, will be less than 1 mg; this may lead to either an under‐ or overestimation of copper stores. Because the complications after liver biopsy were the same in both groups of patients who had undergone one or two passes of liver biopsy, we recommend that an entire core liver biopsy sample be used for hepatic copper determination.
A hepatic copper level ≥250 μg/g dry wt in the absence of cholestasis has been considered as the most valid evidence for the diagnosis of WD since the late 1960s.2, 3, 4 However, this threshold value has been criticized recently by Ferenci et al.12 as being too high and based on relatively few cases. They stated that the sensitivity of hepatic copper level for the diagnosis of WD in the absence of cholestasis at the cut‐off value of 250 μg/g dry wt was 83.3%, and the most useful threshold value was 75 μg/g dry wt. This cut‐off value has been cited by the American Association for the Study of Liver Diseases and European Association for the Study of the Liver guidelines on WD, as well as some articles.5, 6 However, whether this cut‐off value is appropriate has not yet been evidenced by other studies.
Our study shows that the sensitivity, specificity, PPV, and NPV of hepatic copper level for the diagnosis of WD in the absence of PBC and PSC at the conventional cut‐off value of 250 μg/g dry wt were 94.4%, 96.8%, 91.8%, and 97.8%, respectively (Table 4A). The ROC curve suggests that the most useful cut‐off value was 209 μg/g dry wt, with a sensitivity of 99.4% and specificity of 96.1% (Fig. 2). Furthermore, our study showed that hepatic copper content ≥75 μg/g dry wt was found in 23.3% of patients without WD and PBC, in 42.2% of patients with AIH, and in 79.6% of heterozygotes. Thus, lowering the cut‐off value to 75 μg/g dry wt will further increase the overlap between WD patients and other patients. In order to improve the diagnostic accuracy of the test, it is important to increase the precision of the test with an adequate liver sample, rather than significantly lowering the cut‐off value. Two factors could account for the difference between the two studies. First, they used 3‐ to 5‐mm parts of the core of fresh liver biopsy specimens for hepatic copper determination, which sometimes might result in a false result because of greater likelihood of sampling error, as mentioned above. Second, it seems that their study did not include enough patients and conditions for adequate differentiation from WD. Our study is the largest, most comprehensive clinical liver copper study conducted thus far, given that hepatic copper content was determined consecutively in 178 patients with WD and 513 patients with various liver diseases, who closely resembled actual patients for whom the test is intended. This suggests that our estimates of diagnostic accuracy may have better clinical generalizability. Our study confirms that hepatic copper content determined with the entire core of a liver biopsy sample is a very valid procedure for the diagnosis of WD, and the most useful cut‐off value is 209 μg/g dry wt.
Ferenci et al.'s and Nicastro et al.'s studies suggest that, unlike urinary copper excretion, which is age related, liver copper concentration does not seem to be influenced by patient age.11, 12, 19 One unexpected finding of this study was that hepatic copper content was actually negatively correlated with age of patients with WD at time of diagnosis. Specifically, mean hepatic copper content in 14‐year‐old or younger patients was significantly higher than that in older patients (Fig. 3); all patients 10 years old or younger had hepatic copper levels much greater than 250 μg/g dry wt. This finding contradicts the conventional belief that copper continuously accumulates over time in untreated patients with WD. We have three potential explanations for this finding: (1) Liver copper accumulation always precedes the increase of urinary copper excretion for a considerable time. Meanwhile, liver copper levels are calculated per gram of liver tissue, but urinary copper excretion is calculated according to the total urinary excretion per 24 hours, which is directly correlated with patient age; (2) early‐onset patients may have more‐serious mutations of the WD gene, which may lead to more‐rapid accumulation of copper in the liver20, 21, 22; and (3) patients with WD may have similar age‐dependent changes in hepatic copper concentrations as animal models of WD. To specify, copper concentration increased dramatically in the liver of a rodent model of WD with a plateau in the first few months of life, did not change significantly for the next few months, and, subsequently, copper levels decreased. The observed decline in copper concentration is likely owing to age‐dependent up‐regulation of copper‐handling mechanisms independent of ATP7B.23, 24, 25 However, whether results obtained from a rodent model of WD are necessarily representative of the human mechanism of copper accumulation is unclear.
Another unexpected finding of this study was that mean liver copper content in patients presenting with liver disease was significantly higher than that in other phenotypes. All 105 WD patients who presented with liver disease had a liver copper content ≥250 μg/g dry wt. At present, we have no explanation for this finding, which requires further investigation. Establishing a diagnosis of WD is often challenging in patients presenting with liver disease, given that ceruloplasmin levels may be normal, K‐F rings may be absent, and urinary copper excretions may be unreliable.8, 9 Establishing a diagnosis of WD is especially challenging in young, presymptomatic patients because the conventional criteria established for adults are not always appropriate for children.11, 26, 27 Genetic analysis has become an important diagnostic method, especially when routine testing is equivocal for WD. However, the test is not always diagnostic, and in many previous studies, only approximately 65% of all affected alleles were identified in patients.28 Unlike other diagnostic tests of WD, sensitivity of hepatic copper content is almost 100% at the conventional cut‐off value in children and patients presenting with liver disease, and therefore it is particularly useful for the diagnosis of WD in these patients.
This study demonstrated that hepatic copper concentrations in patients with NAFLD were lower than normal. This result is consistent with studies by Aigner et al.32 Ten patients with cryptogenic liver diseases were initially suspected as WD because of elevated hepatic copper levels and were eventually diagnosed as atypical PBC, PSC, or other cholestatic disorders. These cases suggest that how to differentiate cryptogenic liver disease from WD remains a challenging task facing clinical physicians. The possibility of cryptogenic cholestasis should be considered when making a diagnosis of WD in patients with cryptogenic liver diseases.
The strengths of this study include the prospective study design, accurate method of liver copper determination, the largest cohort of patients with WD and other various liver diseases necessary to differentiate from WD, and the collection of detailed data, including long‐term follow‐up data. However, the study had one limitation, as do all other studies on the diagnostic accuracy of WD: A true gold standard was absent. However, at present, there is no better reference standard than a combination of WD score and clinical follow‐up results.
In conclusion, hepatic copper content determined with the entire core of a liver biopsy sample is a very valid test for the diagnosis of WD, especially in children and patients presenting with liver disease. Hepatic copper levels in WD patients do not correlate with stage of hepatic fibrosis. Size of the liver biopsy specimen has a major influence on the precise determination of copper content.
Potential conflict of interest: Nothing to report.
This work was supported by the National Natural Science Foundation of China (grant no.: 30170852) and the Research Fund for the Doctoral Program of Higher Education of China (grant no.: 20050533025).
References
- 1. Cuming JN. The copper and iron content of brain and liver in the normal and in hepatolenticular degeneration. Brain 1948;71:410‐415. [DOI] [PubMed] [Google Scholar]
- 2. Sternlieb I, Scheinberg IH. Prevention of Wilson's disease in asymptomatic patients. N Engl J Med 1968;278:352‐359. [DOI] [PubMed] [Google Scholar]
- 3. Smallwood RA. Williams HA, Rosenoer VM, Sherlock S. Liver‐copper levels in liver disease: studies using neutron activation analysis. Lancet 1968;2:1310‐1313. [DOI] [PubMed] [Google Scholar]
- 4. Brewer GJ. Wilson's Disease: A Clinician's Guide to Recognition, Diagnosis, and Management. Norwell, MA: Kluwer Academic; 2001. [Google Scholar]
- 5. European Association for the Study of the Liver . EASL Clinical Practice Guidelines: Wilson's disease. J Hepatol 2012;56:671‐685. [DOI] [PubMed] [Google Scholar]
- 6. Roberts EA, Schilsky ML. Diagnosis and treatment of Wilson disease: an update. Hepatology 2008;47:2089‐2111. [DOI] [PubMed] [Google Scholar]
- 7. Martins da Costa C, Baldwin D, Portmann B, Lolin Y, Mowat AP, Mieli‐Vergani G. Value of urinary copper excretion after penicillamine challenge in the diagnosis of Wilson's disease. Hepatology 1992;15:609‐615. [DOI] [PubMed] [Google Scholar]
- 8. Steindl P, Ferenci P, Dienes HP, Grimm G, Pabinger I, Madl C, et al. Wilson's disease in patients presenting with liver disease: a diagnostic challenge. Gastroenterology 1997;113:212‐218. [DOI] [PubMed] [Google Scholar]
- 9. Gow PJ, Smallwood RA, Angus PW, Smith AL, Wall AJ, Sewell RB. Diagnosis of Wilson's disease: an experience over three decades. Gut 2000;46:415‐419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Merle U, Schaefer M, Ferenci P, Stremmel W. Clinical presentation, diagnosis and long‐term outcome of Wilson's disease: a cohort study. Gut 2007;56:115‐120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nicastro E, Ranucci G, Vajro P, Vegnente A, Iorio R. Re‐evaluation of the diagnostic criteria for Wilson disease in children with mild liver disease. Hepatology 2010;52:1948‐1956. [DOI] [PubMed] [Google Scholar]
- 12. Ferenci P, Steindl‐Munda P, Vogel W, Jessner W, Gschwantler M, Stauber R. et al. Diagnostic value of quantitative hepatic copper determination in patients with Wilson's disease. Clin Gastroenterol Hepatol 2005;3:811‐818. [DOI] [PubMed] [Google Scholar]
- 13. Faa G, Diaz G, Farci G, Lai ML, Pilleri G, Liguori C, et al. Variability of copper levels in biopsy tissue from a cirrhotic liver. J Trace Elem Electrolytes Health Dis 1990;4:49‐50. [PubMed] [Google Scholar]
- 14. Faa G, Nurchi V, Demelia L, Ambu R, Parodo G, Congiu T, et al. Uneven hepatic copper distribution in Wilson's disease. J Hepatol 1995;22:303‐308. [DOI] [PubMed] [Google Scholar]
- 15. Sternilieb I. Perspectives on Wilson's disease. Hepatology 1990;12:1234‐1239. [DOI] [PubMed] [Google Scholar]
- 16. Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD. Liver biopsy. Hepatology 2009;49:1017‐1044. [DOI] [PubMed] [Google Scholar]
- 17. Ferenci P, Caca K, Loudianos G, Mieli‐Vergani G, Tanner S, Sternlieb I, et al. Diagnosis and phenotypic classification of Wilson disease. Liver Int 2003;23:139‐142. [DOI] [PubMed] [Google Scholar]
- 18. He G, Yang X, Luo KZ. A study of the liver pathology and direct sequencing of all exons of WD gene in a patient with fulminate Wilson disease. Chin J Hepatol 2007;15:712‐713. [PubMed] [Google Scholar]
- 19. Nicastro E, Loudianos G, Zancan L, D'Antiga L, Maggiore G, Marcellini M, et al. Genotype‐phenotype correlation in Italian children with Wilson's disease. J Hepatol 2009;50:555‐561. [DOI] [PubMed] [Google Scholar]
- 20. Wilson DC, Phillips MJ, Cox DW, Roberts EA. Severe hepatic Wilson's disease in preschool‐aged children. J Pediatrics 2000;137:719‐722. [DOI] [PubMed] [Google Scholar]
- 21. Giacchino R, Marazzi MG, Barabino A, Fasce L, Ciravegna B, Famularo L, et al. Syndromic variability of Wilson's disease in children. Clinical study of 44 cases. Ital J Gastroenterol Hepatol 1997;29:155‐161. [PubMed] [Google Scholar]
- 22. Iorio R, D'Ambrosi M, Mazzarella G, Varrella F, Vecchione R, Vegnente A. Early occurrence of hypertransaminasemia in a 13‐month‐old child with Wilson disease. J Pediatr Gastroenterol Nutr 2003;36:637‐638. [DOI] [PubMed] [Google Scholar]
- 23. Huster D, Finegold MJ, Morgan CT, Burkhead JL, Nixon R, Vanderwerf SM, et al. Consequences of copper accumulation in the livers of the Atp7b‐/‐ (Wilson disease gene) knockout mice. Am J Pathol 2006;168:423‐434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Haywood S, Loughran M, Batt RM. Copper toxicosis and tolerance in the rat. III. Intracellular localization of copper in the liver and kidney. Exp Mol Pathol 1985;43:209‐219. [DOI] [PubMed] [Google Scholar]
- 25. Biempica L, Rauch H, Quintana N, Sternlieb I. Morphologic and chemical studies on a murine mutation (toxic milk mice) resulting in hepatic copper toxicosis. Lab Invest 1988;59:500‐508. [PubMed] [Google Scholar]
- 26. Rosencrantz R, Schilsky ML. Mining for a diagnosis of Wilson's disease in children: genetics, score, and ore. Hepatology 2010;52:1872‐1874. [DOI] [PubMed] [Google Scholar]
- 27. Iorio R, Porzio S, Mazzarella G, Fusco G, Vegnente A. Wilson disease: diagnostic dilemmas? J Pediatr Gastroenterol Nutr 2000;31:93. [DOI] [PubMed] [Google Scholar]
- 28. Schilsky ML. Wilson disease: current status and the future. Biochimie 2009;91:1278‐1281. [DOI] [PubMed] [Google Scholar]
- 29. Ramraj R, Finegold MJ, Karpen SJ. Progressive familial intrahepatic cholestasis type 3–overlapping presentation with Wilson disease. Clin Pediatr (Phila) 2012;51:689‐691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Heathcote ET. Diagnosis and management of cholestatic liver disease. Clin Gastroenterol Hepatol 2007;5:776‐782. [DOI] [PubMed] [Google Scholar]
- 31. European Association for the Study of the Liver . EASL Clinical Practice Guidelines: management of cholestatic liver diseases. J Hepatol 2009;51:237‐267. [DOI] [PubMed] [Google Scholar]
- 32. Aigner E, Strasser M, Haufe H, Sonnweber Y, Hohla F, Stadlmaryr A, et al. A role for low hepatic copper concentrations in nonalcoholic fatty liver disease. Am J Gastroenterol 2010;105:1978‐1985. [DOI] [PubMed] [Google Scholar]


