Summary
The gutless marine worm O lavius algarvensis lives in symbiosis with chemosynthetic bacteria that provide nutrition by fixing carbon dioxide (CO 2) into biomass using reduced sulfur compounds as energy sources. A recent metaproteomic analysis of the O . algarvensis symbiosis indicated that carbon monoxide (CO) and hydrogen (H 2) might also be used as energy sources.
We provide direct evidence that the O . algarvensis symbiosis consumes CO and H 2. Single cell imaging using nanoscale secondary ion mass spectrometry revealed that one of the symbionts, the γ3‐symbiont, uses the energy from CO oxidation to fix CO 2. Pore water analysis revealed considerable in‐situ concentrations of CO and H 2 in the O . algarvensis environment, Mediterranean seagrass sediments. Pore water H 2 concentrations (89–2147 nM) were up to two orders of magnitude higher than in seawater, and up to 36‐fold higher than previously known from shallow‐water marine sediments. Pore water CO concentrations (17–51 nM) were twice as high as in the overlying seawater (no literature data from other shallow‐water sediments are available for comparison). Ex‐situ incubation experiments showed that dead seagrass rhizomes produced large amounts of CO. CO production from decaying plant material could thus be a significant energy source for microbial primary production in seagrass sediments.
Introduction
Mutualistic symbioses between bacteria and animals are widespread, occur in almost all animal phyla and play major roles in the development, health and evolution of their hosts (McFall‐Ngai, 2002; Walker and Crossman, 2007; Moya et al., 2008; Fraune and Bosch, 2010; McFall‐Ngai et al., 2013). In many mutualistic symbioses, the function of the bacterial symbionts is to provide essential nutrients to their hosts (Moran, 2007; Moya et al., 2008). In chemosynthetic symbioses, the bacteria provide all or most of their host's nutrition using inorganic compounds such as sulfide or hydrogen (H2) as energy sources to fix carbon dioxide (CO2) into biomass (Stewart et al., 2005; DeChaine and Cavanaugh, 2006; Dubilier et al., 2008; Petersen et al., 2011; Kleiner et al., 2012a).
The marine oligochaete Olavius algarvensis does not have a digestive or excretory system and relies on its bacterial symbionts for nutrition and waste recycling (Dubilier et al., 2001; Giere and Erseus, 2002; Woyke et al., 2006; Ruehland et al., 2008; Kleiner et al., 2011; 2012b). It harbours two chemosynthetic sulfur‐oxidizing gammaproteobacterial symbionts (γ1 and γ3), two sulfate‐reducing deltaproteobacterial symbionts (δ1 and δ4) and a spirochaete between its cuticle and epidermal cells (Giere and Erseus, 2002; Ruehland et al., 2008). The energy sources that fuel the O. algarvensis symbiosis are still not well understood. The collection site for O. algarvensis in this study and previous studies from the same site, a shallow bay off the coast of the Island of Elba (Italy) in the Mediterranean Sea (Dubilier et al., 2001; Giere and Erseus, 2002; Woyke et al., 2006; Ruehland et al., 2008; Kleiner et al., 2012b), is characterized by Posidonia oceanica seagrass meadows and medium‐ to coarse‐grained sandy sediments that cover a thick, peat‐like structure consisting of dead seagrass rhizomes (Fig. 1). Concentrations of reduced sulfur compounds at this site are in the low nanomolar range, much lower than the micromolar concentrations that are usually present at sites with chemosynthetic symbioses (Dubilier et al., 2001; Kleiner et al., 2012b). In the O. algarvensis symbiosis, the reduced sulfur compounds required by the sulfur‐oxidizing γ‐symbionts are provided internally by the sulfate‐reducing δ‐symbionts (Dubilier et al., 2001). However, the external energy sources that power the symbiosis have remained enigmatic.
Figure 1.
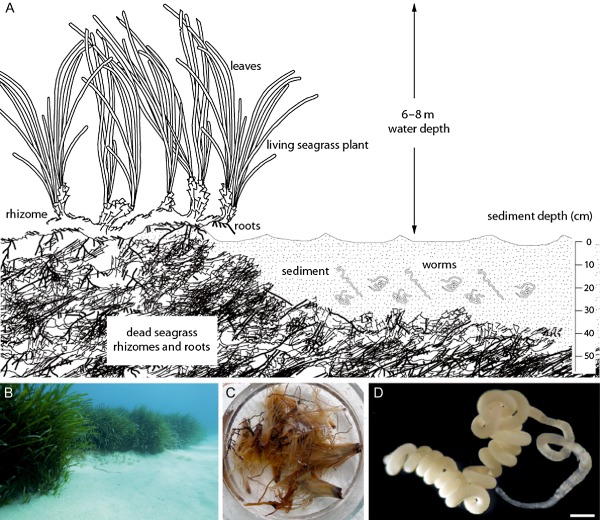
O lavius algarvensis and the Mediterranean seagrass sediments it inhabits.
A. The O . algarvensis environment is characterized by medium‐ to coarse‐grained silicate sediments and patches of the seagrass P osidonia oceanica. Subsurface roots and rhizomes (horizontal stems) stabilize the plants in the sediment. The roots and rhizomes form dense mats that are very stable, even after the seagrass has died, and can remain in the sediment for millennia (Mateo et al., 1997; Alcoverro et al., 2001; Duarte, 2002; Boudouresque et al., 2009; Gutiérrez et al., 2011). At the collection site for this study (Sant' Andrea in the north of the Island of Elba), reef‐like mats of dead rhizomes are buried underneath the sediment in the entire bay. The sediment overlying the rhizome mats is very poor in nutrients and energy sources (Kleiner et al., 2012b).
B. Image of the O . algarvensis collection site showing sandy sediments surrounded by seagrass beds in 5–6 m water depth.
C. Dead seagrass rhizomes from the O . algarvensis collection site.
D. Olavius algarvensis, scale bar = 0.4 mm.
Metaproteomic analyses of the O. algarvensis association showed that three of its symbionts may use carbon monoxide (CO) and H2 as energy sources (Kleiner et al., 2012b). Both sulfate‐reducing symbionts abundantly expressed anaerobic carbon monoxide dehydrogenases (CODHs), which enable the use of CO as an energy source, as well as hydrogenases for the use of H2 as an energy source (Kleiner et al., 2012b). The third symbiont, the sulfur‐oxidizing γ3‐symbiont, abundantly expressed an aerobic CODH (Kleiner et al., 2012b), an enzyme used by bacteria to oxidize CO with oxygen or nitrate (King and Weber, 2007). Because the symbionts are only separated from the environment by a thin cuticle, which is highly permeable for small molecules, they are unlikely to be limited in their access to dissolved gases in the worms' environment (Dubilier et al., 2006).
The goal of the current study was to test if the metaproteomic predictions by Kleiner and colleagues (2012b) that CO and H2 are used as energy sources by the O. algarvensis symbiosis are correct by examining the following questions: (1) Are CO and H2 consumed by the O. algarvensis symbiosis? (2) If so, is the energy gained from CO and H2 oxidation used for CO2 fixation? (3) Are CO and H2 present in the O. algarvensis habitat, and if so what is their source and distribution?
Results
The O . algarvensis symbiosis oxidizes CO to CO 2
In incubation experiments, CO consumption by live O. algarvensis worms began after 20–40 h and CO concentrations in the headspace of incubation bottles decreased from 3040 ± 30 ppm to 790 ± 680 ppm over 141 h (Fig. 2). No notable consumption of CO was observed in controls [dead worms, water that worms were washed in and pure artificial seawater (ASW) medium] (Fig. 2). The CO consumption rate of O. algarvensis was 2 ± 0.5 μmol g−1 (wet weight) h−1. In incubation experiments with 13C‐labelled CO, O. algarvensis worms almost completely oxidized 13CO to 13CO2 within 62 h, whereas no notable production of 13CO2 occurred in the controls (dead worms) (Fig. 3). The average blank‐corrected end‐point CO concentration in the incubations with 13CO was 6.5 ± 35.8 nM (equivalent to 9 ± 48 ppm headspace concentration).
Figure 2.
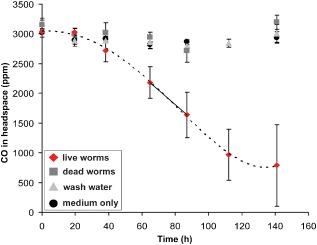
CO consumption by O . algarvensis. CO was consumed in incubations with live O . algarvensis worms, but not in controls. Consumption rates of live worms were calculated based on linear rates between 65 and 87 h (solid line). Mean values and standard deviations of three independent incubation bottles are plotted for each control and treatment.
Figure 3.
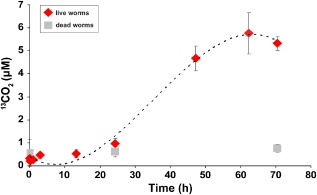
Oxidation of 13 CO to 13 CO 2 by O . algarvensis. 13 CO oxidation to 13 CO 2 in live and dead O . algarvensis worms was measured over 70 h after the addition of 13 CO (7 μM at start of incubations) and 13 CO 2 was produced. Mean values and standard deviations of four independent incubation bottles are plotted for each treatment and control. Standard deviations were very small in the first four time points and are therefore not visible.
The O . algarvensis symbiosis consumes H 2
H2 consumption by live O. algarvensis worms did not begin until after 40 h of incubation (Fig. 4A). After this lag phase, H2 consumption rates were high and H2 was nearly completely consumed after 86 h (from 2500 ± 320 ppm to 30 ± 20 ppm; Fig. 4A). A second injection of H2 into these incubations (t = 95.5 h) allowed us to better resolve H2 consumption over time. H2 decreased from 2630 ± 170 ppm to 270 ± 380 ppm within 17.5 h (Fig. 4B). The H2 consumption rate of the O. algarvensis symbiosis was 11 ± 1 μmol g−1 (wet weight) h−1. No notable consumption of H2 occurred in the controls (dead worms, water that worms were washed in and pure ASW medium).
Figure 4.
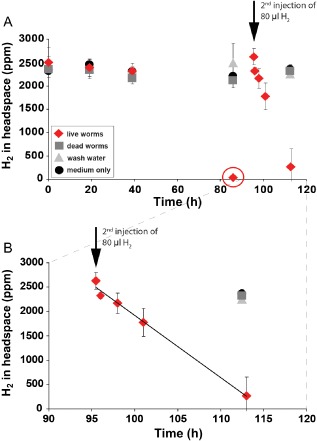
H2 consumption by the O . algarvensis symbiosis.
A. H 2 consumption by live worms began only after 40 h and was then completely consumed within 86 h (circled in red) in all three replicates (standard deviations at this time point were so small that they are not visible in this figure). A second injection of 80 μl of H 2 to the incubations with live worms was monitored in shorter intervals and revealed a linear consumption of H 2 by the O . algarvensis symbiosis.
B. Close‐up of A after second H2 injection (at 95.5 h after begin of incubations). Linear consumption is emphasized by solid line (R 2 = 0.99). Mean values and standard deviations of three independent incubation bottles are plotted for each control and treatment.
The γ3‐symbiont uses CO as an energy source to fix CO 2 into biomass
Our bulk analyses of 13CO2‐incorporation in whole O. algarvensis worms showed that live worms always incorporated significant amounts of 13CO2 compared with dead worms (Table 1). However, no significant differences in 13C‐content were detectable between live worms incubated with CO and H2 compared with control incubations with no experimentally added energy source (Table 1). Nanoscale secondary ion mass spectrometry (nanoSIMS) analyses of the symbionts at the single cell level revealed that in live worms all symbionts, except the δ4‐symbiont, had a higher 13C‐content compared with whole dead worms and the carbon signal from polycarbonate filter background (Table 1 and Table S1; Figs 5 and 6 and Fig. S1). This suggests that all symbionts except the δ4‐symbiont incorporated 13C under all three incubation conditions. However, because we could not measure 13C‐content of single symbiont cells from dead worm controls, the exact amount of 13C‐incorporation into individual symbionts could not be determined.
Table 1.
13 C‐content of whole worms based on bulk measurements in AT%
| Mean AT%c | SD AT% | Min. AT% | Max. AT% | n | P‐value t‐test versus deada | P‐value t‐test versus w/o e−‐donorb | |
|---|---|---|---|---|---|---|---|
| 13CO2 + CO | 1.284 | 0.013 | 1.274 | 1.299 | 3 | 5e‐10 | 0.56 |
| 13CO2 + H2 | 1.295 | 0.038 | 1.257 | 1.332 | 3 | 5.2e‐07 | 0.83 |
| 13CO2 w/o e−‐donor | 1.303 | 0.048 | 1.26 | 1.355 | 3 | 2.3e‐06 | – |
| Dead worms | 1.072 | 0.001 | 1.07 | 1.073 | 6 | – | – |
P‐values for comparisons of 13C‐content in worms from incubations versus dead worms (t‐test, one‐tailed, H0 = 13C‐content of live worms is not higher than that of dead worms). After Bonferroni correction for three comparisons the significance threshold P < 0.01 corresponds to P < 0.003.
P‐values for comparisons of 13C‐content in worms incubated without additional energy source versus incubations with CO or H2 added (t‐test, two‐tailed, H0 = means are equal).
The detailed data including the measurements on the standard caffeine can be found in Table S3.
AT%: atom percent [13C / (12C + 13C) × 100]. w/o e−‐donor: without an additional energy source added to the incubations. n: total number of bulk worm samples (each containing eight worms).
Figure 5.
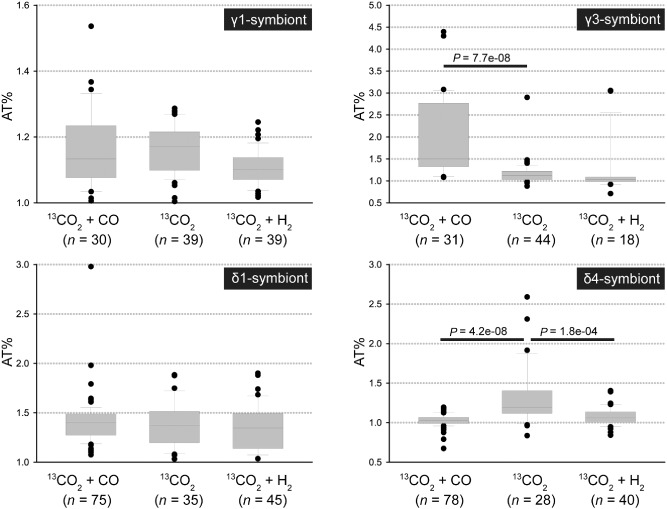
13C‐content of single symbiont cells based on nanoSIMS analysis. For all symbionts and treatments cells from three worms were analysed except for the γ3‐symbiont in the H 2 treatment, for which only cells from two worms were analysed. Horizontal bars with P‐values indicate significant differences based on a Kruskal–Wallis test. Due to the different 13 C‐contents of the four symbionts we used different scales for the y‐axis for optimal visualization of the data. AT%: atom percent [13 C / (12 C + 13 C) × 100]; n: total number of symbiont cells analysed. 13 C isotope content values for all individual cells can be found in Table S1.
Figure 6.
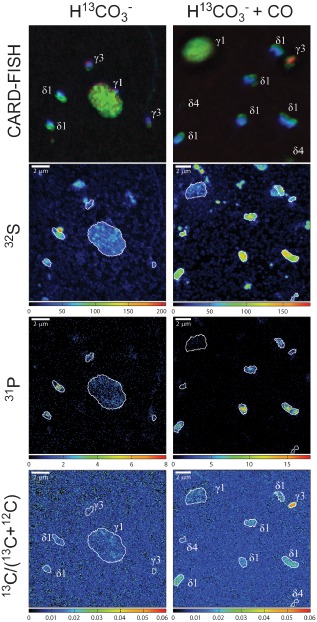
Comparison of 13 CO 2 fixation by single O . algarvensis symbiont cells in the presence and absence of CO. Increased carbon fixation in the presence of CO was only visible in the γ3‐symbionts (bottom right image). Images in the left and right columns show in the top row epifluorescence micrographs of O . algarvensis symbionts on a filter, followed by the corresponding nanoSIMS images for sulfur (32 S ‐, counts per pixel) in the second row, phosphorus (31 P ‐, counts per pixel) in the third row and 13 C‐content as 13 C / (13 C + 12 C) in the bottom row. In the epifluorescence images symbiont cells hybridized with the general eubacterial probe (EUB338I‐III) are green, the sulfur‐oxidizing symbionts targeted by the gammaproteobacterial probe (Gam42a) are red and the general DNA stain 4,6‐diamidino‐2‐phenylindole (DAPI) is shown in blue. The strong green fluorescence signal of the eubacterial probe (EUBI‐III) masks the red fluorescence signal of the Gam42a probe in the γ1‐symbiont (for images showing the single channels separately see Fig. S1).
Analyses of single‐cell 13CO2‐incorporation in each symbiont species for significant differences between the CO and H2 incubations compared with control incubations with no experimentally added energy source revealed: (1) The γ3‐symbiont incorporated significantly more 13C‐labelled CO2 in the presence of CO compared with incubations without an added energy source (Kruskal–Wallis, P = 7.7e‐08, Fig. 5). (2) The δ4‐symbiont incorporated less 13C‐labelled CO2 in the presence of CO or H2 compared with incubations without an added energy source (Kruskal–Wallis, P = 4.2e‐08 with CO and Kruskal–Wallis, P = 1.8e‐04 with H2). (3) In the γ1‐ and δ1‐symbionts, no significant differences in 13C‐content in the CO and H2 incubations compared with control incubations with no experimentally added energy source were observed.
It is important to note that the 13C content of the symbionts analysed with nanoSIMS was likely diluted because of the deposition of unlabelled carbon during the catalyzed reporter deposition fluorescence in situ hybridization (CARD‐FISH) treatment (Musat et al., 2014; Woebken et al., 2015) potentially obscuring additional significant differences. It is also noteworthy that 13C‐incorporation by the symbionts can only explain part of 13C‐incorporation into whole worms because 13C‐incorporation in individual symbionts was in a similar range as in whole worms and the symbionts only make up a small fraction of the total worm biomass (Fig. 5, Table 1). The additional 13C‐incorporation in whole worm bulk measurements is likely due to heterotrophic CO2 fixation by host tissues.
Elevated CO and H 2 concentrations in the O . algarvensis habitat
CO and H2 concentrations in sediment pore water where the worms were collected were much higher than in the seawater above the sediment (Fig. 7). Pore water CO concentrations (17–51 nM) were approximately twice as high as in seawater (8–16 nM), with the highest CO concentrations detected in pore water from within the dead rhizome mats (Figs 1 and 7). Pore water H2 concentrations (89–2147 nM) were up to two orders of magnitude higher than in the seawater (0–23 nM). In contrast to CO, the highest H2 concentrations were measured at 25 cm sediment depth and not in the deeper dead rhizome mats.
Figure 7.
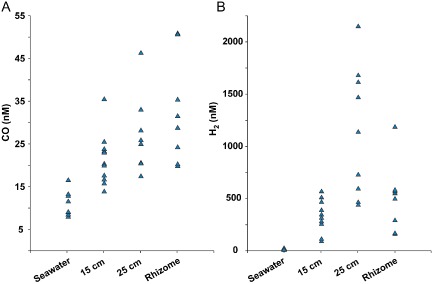
(A) CO and (B) H 2 concentrations at the O . algarvensis collection site. Concentrations were measured at 15 and 25 cm sediment depth, in the dead rhizome mats underlying the sediment, and in the seawater about 5 cm above the sediment. For each sediment depth and control at least eight independent samples were measured. Values are blank corrected.
Dead seagrass rhizomes release large amounts of CO
In incubations with dead seagrass rhizomes from the O. algarvensis habitat we observed high production rates of CO, whereas CO production rates in incubations with unfiltered seawater, and sediment with 0.2 μm‐filtered seawater were in the same range as in controls with double distilled water (Table 2). We measured the highest CO production rates in incubations with dead rhizomes to which ZnCl2 was added to stop biological activity (Table 2). Dead rhizomes produced up to two orders of magnitude more CO in incubations with ZnCl2 than in incubations without (3.64–55.60 versus 0.33–17.46 μmol CO kg−1 (dry weight rhizome material) day−1) (Table 2). We did not detect H2 production in any of the incubations with natural substrates from the O. algarvensis habitat.
Table 2.
Carbon monoxide release by components from the O . algarvensis habitat and double distilled water (ddH 2O) in μmol kg−1 (or l−1) day−1. ZnCl2 was added to incubations to stop biological activity. Mean values and standard deviations are given in parentheses
| Without ZnCl2 | With ZnCl2 | |
|---|---|---|
| Dead rhizomes (dry weight) | 0.33–17.46 (4.86 ± 6.95) | 3.64–55.60 (24.08 ± 19.12) |
| Dead rhizomes (wet weight) | 0.04–1.85 (0.55 ± 0.75) | 0.65–6.53 (3.42 ± 2.17) |
| Seawater (volume) | 0–0.04 | 0–0.01 |
| Sediment (dry weight) | 0 | 0 |
| ddH2O (volume) | 0.01–0.02 | 0.01–0.02 |
n ≥ 3 per incubation type.
Discussion
Is CO an energy source for autotrophic carbon fixation in the O . algarvensis symbiosis?
Our results indicate that part of the CO2 fixation in the O. algarvensis symbiosis is powered by the oxidation of CO by at least the γ3‐symbiont. This observation is in agreement with the abundant expression of an aerobic CODH and enzymes of the Calvin–Benson–Bassham (CBB) cycle under natural conditions in the γ3‐symbiont (Kleiner et al., 2012b). It suggests that the γ3‐symbiont uses its CODH (Fig. S2) to oxidize CO to CO2 and that it uses a part of the energy from CO oxidation to fix CO2 via the CBB cycle as described for other CO oxidizers (reviewed in King and Weber, 2007).
In contrast to the γ3‐symbiont, the sulfate‐reducing δ1‐ and δ4‐symbionts did not incorporate significantly more 13CO2 in the presence of CO compared with the control without CO (Fig. 5, Table S1), despite the fact that these symbionts express anaerobic CODHs under environmental conditions (Kleiner et al., 2012b). It is possible that the sulfate‐reducing symbionts use CO as an energy source for other metabolic functions, as known from ‘carboxydovores’, microorganisms that oxidize CO without coupling the energy gain to autotrophic growth (reviewed in King and Weber, 2007). The sulfate‐reducing symbionts could use the energy released by the oxidation of CO for lithoheterotrophic growth on organic substrates such as fatty acids that are abundantly produced by the host under anoxic conditions (Kleiner et al., 2012b).
Unexpectedly, the δ4‐symbiont incorporated significantly less labelled CO2 in the presence of CO or H2 compared with controls without an added energy source (Fig. 5). The δ4‐symbiont might have been inhibited by the oxygen concentrations used in this study, or by end products of the other co‐occurring symbionts. However, incubation conditions were similar in all three treatments, and 13CO2‐incorporation was clearly not inhibited in this symbiont in the control incubations without an external energy source (Fig. 5). We therefore do not have a satisfactory explanation for these results.
CO oxidation started after a lag phase of about 20 h (Fig. 2). This lag phase could be due to the CO‐free pre‐incubations of the worms (see Experimental Procedures). In aerobic CO‐oxidizing microorganisms the oxidation of CO is catalysed by an inducible CODH, which was shown to only be induced in the presence of CO (Meyer and Schlegel, 1983). It is thus likely that the symbionts did not have substantial amounts of CODH at the beginning of the incubation and had to produce it after exposure to CO.
CO oxidation rates have, to our knowledge, not been previously measured in an animal–bacterial symbiosis. In the O. algarvensis symbiosis, CO oxidation rates (2 ± 0.5 μmol g−1 (wet weight) h−1) were 10–100 times higher than those of bacterial CO oxidizers from coastal seawaters (0.02–0.23 μmol g−1 (wet weight) h−1) (Tolli et al., 2006), but comparable with rates of cultured carboxydotrophic microorganisms that can live on CO as their sole energy source (0.02 μmol to 18 mmol g−1 (wet weight) h−1) (Diekert and Thauer, 1978; Cypionka et al., 1980; Tolli et al., 2006). This comparison of CO oxidation rates has several limitations. These include the fact that CO oxidation rates for cultured CO oxidizers were determined at a range of different CO concentrations and that the mixing environment within the worm will greatly differ from a well‐shaken liquid culture.
It is likely that CO oxidation rates of the carboxydotrophic O. algarvensis symbionts are even higher than 2 ± 0.5 μmol g−1 (wet weight) h−1, because we estimated rates based on the biomass of the entire worm. If we assume that only the γ3‐symbiont oxidized CO, based on the observation that it was the only symbiont that incorporated significantly more 13CO2 in the presence of CO (Fig. 5), and estimate its abundance at 5% of the total biomass of whole worms (Giere and Erseus, 2002; Ruehland et al., 2008), CO oxidation rates would be as high as 40 μmol g−1 (wet weight) h−1. However, in‐situ CO oxidation rates of the O. algarvensis symbiosis are likely to be lower because of the lower in‐situ concentrations of CO compared with the concentrations used in our incubations.
Is H 2 also an energy source for the O . algarvensis symbiosis?
H2 consumption rates of the O. algarvensis symbiosis (11 ± 1 μmol g−1 (wet weight) h−1) were higher than those measured in the deep‐sea hydrothermal vent mussel Bathymodiolus symbiosis (∼3 μmol g−1 (wet weight of gill tissue) h−1) despite similar incubation H2 concentrations of 1800 ppm (Petersen et al., 2011). H2 consumption rates of free‐living and cultivated microorganisms are higher than the O. algarvensis symbiosis and range from ∼ 100 μmol g−1 (wet weight) h−1 to several mmol g−1 (wet weight) h−1 (Vmax) at H2 concentrations between 1 ppm and 20 000 ppm (Häring and Conrad, 1991; Klüber and Conrad, 1993; Perner et al., 2010). To adequately compare these rates to the H2 consumption rate of the O. algarvensis symbiosis the reaction kinetics of H2 oxidation would need to be characterized for the O. algarvensis symbiosis in future experiments. As discussed for CO, it is likely that the H2 oxidation rates of the O. algarvensis symbionts are considerably higher, because we calculated these rates based on whole worm wet weight, instead of the biomass of the two sulfate‐reducing symbionts predicted to be able to oxidize H2 (Kleiner et al., 2012b). If we assume that only both deltaproteobacterial symbionts oxidized H2 based on the observation that they possess hydrogenases (Kleiner et al., 2012b), and estimate their abundance at 10% of the total biomass of whole worms (Giere and Erseus, 2002; Ruehland et al., 2008), H2 oxidation rates would be 110 μmol g−1 (wet weight) h−1, which is in the range of those of free‐living and cultivated bacteria (Häring and Conrad, 1991; Klüber and Conrad, 1993; Perner et al., 2010).
The lag phase in H2 consumption during the first 40 h of incubation (Fig. 4) could be due to the oxic pre‐incubations of the worms and the relatively high oxygen concentrations at the beginning of the experiments (see Experimental Procedures). The expression of hydrogenases in anaerobic H2‐oxidizing microorganisms is generally only induced in the presence of H2 and repressed by oxygen (reviewed in Vignais and Billoud, 2007). It is thus likely that the symbionts did not have substantial amounts of hydrogenases at the beginning of the incubation and had to produce these after exposure to H2 and suboxic conditions in the incubation vessels. Once H2 consumption began, H2 was consumed down to 9 ppm in one incubation bottle, which corresponds to 5.4 nM H2 in solution (Fig. 4A), indicating that the O. algarvensis symbionts have hydrogenases that can take up H2 down to very low concentrations. This is in agreement with the metaproteome study of Kleiner and colleagues (2012b), which showed that the expressed uptake hydrogenases of the deltaproteobacterial symbionts are closely related to hydrogenases characterized as having a high‐affinity for H2 (Kleiner et al., 2012b).
Despite high H2 consumption rates of live O. algarvensis worms, none of the symbionts showed increased CO2 incorporation in the presence of H2 in the nanoSIMS analyses (Fig. 5). This suggests that H2 was not used as an energy source for autotrophic CO2 fixation by the hydrogenase‐possessing δ‐symbionts under the applied incubation conditions. The δ‐symbionts may have instead used the energy released by the oxidation of H2 for lithoheterotrophic growth as discussed for CO above.
What are the sources of CO and H 2 in the habitat of O . algarvensis?
In the photic zone of the ocean, CO is produced through non‐biological processes (abiotically) during the photochemical lysis of organic material. CO concentrations in the seawater above our study site were in the same low nanomolar range as those measured in other studies, and was most likely produced through photolytic processes (summarized and discussed in Tolli et al., 2006 and Moran and Miller, 2007). Surprisingly, there is currently no data on CO concentrations in marine sediments. In this study, we found CO concentrations in sediment pore waters that were twice as high as those in the overlying seawater, with the highest concentrations measured in the dead rhizome mats at sediment depths of 25 cm and more where photolysis of organic material is not possible (Figs 1 and 7). Accordingly, our results from incubations with different components from the O. algarvensis habitat showed that dead seagrass rhizomes incubated in the dark released considerable amounts of CO (Table 2). We therefore hypothesize that the large mats of dead seagrass rhizomes in the O. algarvensis habitat are a source of aphotically produced CO. Based on our observation that up to two orders of magnitude more CO was produced in the rhizome incubations in which biological activity was stopped with ZnCl2 than in the rhizome incubations without ZnCl2, we hypothesize that (1) CO production occurred abiotically, i.e. in the absence of live organisms, and (2) in the rhizome incubations without ZnCl2, CO was not only produced, but also consumed by microorganisms associated with the dead rhizomes. This hypothesis is supported by earlier studies that found abiotic production of CO from humic acids, phenolic compounds and decaying plant material in soils in the absence of light and the presence of an oxidant (Conrad and Seiler, 1980; 1982; 1985). These authors observed that CO production increased with increasing temperature, moisture content and alkaline conditions (higher pH), indicating that a thermochemical process is involved in the production of CO from decaying plant material in soils. The exact reaction mechanism behind this process remains unknown.
CO production from organic material could also explain the widespread presence of microorganisms with the genetic potential to oxidize CO in the aphotic zones of the ocean. Several metagenomic and one metatranscriptomic study found high frequencies of CODH genes used for CO oxidation in the Mediterranean, Pacific and Atlantic Oceans at water depths between 200 and 6000 m, but the CO source remained elusive (Martin‐Cuadrado et al., 2009; Quaiser et al., 2011; Smedile et al., 2013). We hypothesize that CO production from decaying organic matter is not limited to soils as described by Conrad and Seiler (1980; 1982; 1985) but could also explain CO production in the bathypelagic. This hypothesis is supported by studies showing ‘dark production’ of CO (i.e. not from photolytic processes) in coastal surface waters that can make up as much as 25% of the total CO production budget (Zhang et al., 2008; Day and Faloona, 2009).
In contrast to CO, none of the components from the O. algarvensis environment produced H2 under the aerobic conditions used in this study. Potential sources for the high H2 concentrations in the sediment pore waters at our collection site are the anaerobic oxidation of CO by carboxydotrophs that use protons as electron acceptors and thus release H2 (Kerby et al., 1995; Maness et al., 2005; Oelgeschlager and Rother, 2008), and microbial fermentation that would produce H2 as a by‐product (Schwartz and Friedrich, 2006). Difficult to explain are the unusually high H2 concentrations in the O. algarvensis sediments of 89–2147 nM because H2 concentrations in aquatic sediments are usually very low (<60 nM) because of rapid oxidation by free‐living H2 oxidizers (Goodwin et al., 1988; Novelli et al., 1988).
Could CO production by dead seagrass rhizomes in the Mediterranean sediments support the O . algarvensis symbiosis?
CO concentrations in the pore waters of the O. algarvensis collection site (17–51 nM) correspond to atmospheric mixing ratios of 23–68 ppm. Such low concentrations (10–100 ppm) have been successfully used to incubate and isolate CO oxidizers from soils and seawater (Hendrickson and Kubiseski, 1991; Hardy and King, 2001; King, 2007; Weber and King, 2010).
In our incubations, O. algarvensis was also able to oxidize CO down to concentrations as low as 16 ppm (concentration at the end of one of the CO incubations) and down to 6.5 ± 35.8 nM in the incubations with 13CO (average and standard deviation for four parallel 13CO incubations), indicating that the symbionts would be able to take up CO at the concentrations measured in the worm's environment. Additionally, we consider it likely that the symbionts experience fluctuating conditions of CO and H2 supply and that CO concentrations may often be higher than those that we measured in the pore waters for two reasons. First, based on our data we hypothesize that CO flux in the sediments close to the dead rhizomes is very high despite the low concentrations measured in pore waters. We base this assumption on the fact that on average five times more CO was produced in dead rhizome incubations with ZnCl2 compared with without ZnCl2 (Table 2), suggesting that an active CO‐oxidizing microbial community rapidly oxidized the CO produced by the dead rhizomes. Second, the high variability in the measured pore water CO (and H2) concentrations indicate a high spatial variation and suggest that small pockets with high concentrations may exist in the sediment. However, our measuring method, which required large sample volumes, would not have allowed the detection of such fine spatial differences.
To estimate how many O. algarvensis worms could be sustained by using CO as sole energy source for growth, we calculated the amount of carbon that could be fixed with the CO produced in the O. algarvensis habitat assuming that (1) between 0.02 and 0.164 mol CO2 can be fixed autotrophically per mole CO oxidized using oxygen as terminal electron acceptor (Moersdorf et al., 1992); (2) an average O. algarvensis individual has a carbon content of 5 μmol; (3) 20 kg (dry weight) dead seagrass rhizome are buried in 1 m2 of sediment (rough estimate based on samples taken for incubations) which produce 36.5 mmol CO per year [using a conservative mean CO production value of 5 μmol kg−1 (dry weight) per day by dead rhizomes in their native state, Table 2]; and (4) CO production by dead rhizomes is constant. We calculated that up to 6 mmol carbon could be fixed per year and square meter using CO as an energy source, which is equal to the carbon content of 1200 worms. These estimates indicate that CO production from decaying plant material could be a significant energy source for microbial primary production in marine seagrass sediments in the Mediterranean Sea and possibly in other coastal regions with large amounts of decaying plant material.
Experimental procedures
Specimen collection and preparation for incubations
Worms were collected by scuba diving in October 2009 and October 2011 off Capo di Sant' Andrea, Elba in Italy (geographic position: 42°48′29.38′N, 10°8′31.57′E; 6–8 m of water depth). Only intact specimens were used in incubation experiments. Sexually mature worms that were identified as O. ilvae, a co‐occurring less abundant gutless oligochaete species, were sorted out and not used in the experiments (Giere and Erseus, 2002).
Internally stored sulfur in the γ1‐symbionts (Giere and Erseus, 2002) was removed by pre‐incubating all worms in large glass bowls containing 0.2 μM‐filtered oxic seawater and a thin (3 mm) layer of glass beads for a week. This pre‐treatment was necessary because the γ1‐symbionts use their stored sulfur for CO2 fixation, and this would have masked differences in CO2 fixation between treatments. After this pre‐incubation, the symbionts had lost most of their stored sulfur as determined by the change of worm colour from bright white to transparent and decreased CO2 fixation rates in test incubations without an external energy source (Fig. S3).
Incubation experiments with 13 C‐labelled bicarbonate and CO, H 2 or no external energy source
We compared uptake rates of 13C‐labelled bicarbonate in O. algarvensis worms under three conditions: (1) in the presence of CO, (2) in the presence of H2 and (3) in the absence of an externally added energy source. ASW with a salinity of 39‰ was prepared as previously described (Widdel and Bak, 1992) (Supporting Information). The pH of the ASW was adjusted to 7.5 corresponding to the conditions in the O. algarvensis habitat. 13C‐labelled NaHCO3 − was added to detect CO2 fixation in the symbionts.
Incubation bottles (serum bottles) were flushed with N2 gas prior to filling with 20 ml of ASW to create microaerobic conditions. Oxygen concentrations were measured at the end of the incubations with an amperometric microelectrode (Revsbech, 1989) and were 0.18 mM in the control bottles and 0.11 mM in the bottles containing live worms.
All incubations were run in triplicates with 35 live worms added to each serum bottle, whereas control incubations contained 35 dead worms (see Supporting Information), 5 μl of wash water (see Supporting Information) or only ASW. To start the incubation, either 80 μl of CO (purity level 3.7; Air Liquide, Düsseldorf, Germany) or 80 μl of H2 (purity level 5.0; Air Liquide) were injected into the headspace of the serum bottles; in the incubations without an external energy source nothing was added to the headspace. Serum bottles were stored at 22°C and gently tilted back and forth (18× per minute) to allow mixing and to reduce diffusion limitation. At given time points, subsamples from the headspace were taken with gas‐tight syringes and CO and H2 concentrations were measured with a Shimadzu GC‐8A gas chromatograph equipped with a Molecular Sieve 5A column and an RGD2 Reduction Gas Detector (Trace Analytical, Menlo Park, CA, USA) as described previously (Pohorelic et al., 2002). To stay within the linear range of the detector samples were diluted with pure nitrogen gas if needed. H2 and CO standards were produced from pure H2 (purity level 5.0; Air Liquide) and CO gas (purity level 3.7; Air Liquide) in pure nitrogen gas (purity level 5.0; Air Liquide). To control for potential instrument drift, standards were measured in each measurement run and used to calculate sample concentrations within each run (Table S2). After H2 was completely consumed, we added an additional 80 μl of H2 to the serum bottles containing live worms for a better time resolution of H2 consumption by the O. algarvensis symbiosis. At the end of the incubations, worms were processed for bulk tissue analyses and nanoSIMS (see below). CO and H2 consumption rates were calculated based on the linear CO consumption between 65 and 87 h (Fig. 2) and the linear H2 consumption during the last 17.5 h of the incubation using the average wet weight of one worm (0.5 mg) and the molar volume of an ideal gas at 22 °C (24.54 l mol−1).
Bulk analysis of 13 C‐incorporation in whole worms
To determine incorporation of 13C‐labelled bicarbonate in whole worms, eight worms from each replicate were killed in 3 ml of ASW and 100 μl of aqueous zinc chloride (ZnCl2) solution (50% v/w). Worms were washed in ASW, rinsed briefly in 0.1% HCl to remove unfixed labelled bicarbonate, washed again in ASW, placed in tin cups and their wet weight measured. Dead worms from control incubations were treated the same way. Tin cups with worms were dried over night at 70°C and stored at room temperature until further processing. Carbon isotope composition of the worms was analysed using an automated elemental analyzer (Thermo Flash EA 1112) coupled to an isotopic ratio mass spectrometer (Thermo Delta Plus XP, Thermo Fisher Scientific) 13C isotope content in the worms was calculated as atom percent (AT% = 13C / (12C + 13C) × 100). Caffeine (Sigma‐Aldrich) was used as a standard for isotope calibration and quantification (Table S3).
nanoSIMS analysis of 13 C‐incorporation into single symbiont cells
To determine the amount of 13C assimilated by each symbiont we analysed the carbon isotope composition of single symbiont cells using CARD‐FISH combined with nanoSIMS imaging (see Supporting Information for more details) (Musat et al., 2012; Polerecky et al., 2012).
Incubation experiments with 13 CO
To investigate whether CO consumption was caused by oxidation to CO2 or by CO assimilation, labelled 13CO (99 AT% 13C, < 5 AT% 18O, Sigma‐Aldrich Cat. No. 388505) was added to incubations that were prepared as described above but with unlabelled 12C‐bicarbonate (Sigma‐Aldrich) added to the ASW. Incubations were done in 12 ml glass vials (Exetainers, Labco, High Wycombe, UK) without headspace, with four parallel incubations for each time point. Four worms were placed in an incubation vial and the incubation started by adding CO to a final concentration of 7 μM. At given time points, samples were killed by addition of ZnCl2 (50% w/v). Six milliliters of medium were transferred to 6 ml glass vials (Exetainers). Two milliliters of medium were taken with pure helium (He) gas (purity level 5.0; Air Liquide) and transferred to new He‐flushed 6 ml glass vials (Exetainers). For outgassing of CO2 into the headspace 0.2 ml of 85% phosphoric acid were injected. Two hundred fifty microlitres of the headspace were analysed with a gas chromatograph – isotope ratio mass spectrometer (VG Optima, Manchester, UK). Pure CO2 (purity 4.5; Air Liquide) was used as a standard for isotope calibration and quantification.
CO end‐point concentrations were measured after outgassing of CO into the 2 ml He headspace created in the 12 ml glass vials after the medium for the CO2 analyses was removed. The same measurement setup as for the CO and H2 worm incubations was used. The average CO concentration in the incubations with worms was blank corrected using the average CO concentration in control vessels without added CO to account for CO production by the rubber septa after killing of the worms.
Measurement of CO and H 2 concentrations in the worms' habitat
Sediment pore water and seawater above the sediment were collected at the worm collection site by scuba diving and measured as previously described (Kleiner et al., 2012b). Nine profiles were sampled within an area of approx. one hundred square metres at sediment depths of 15 cm, 25 cm and in the dead seagrass rhizomes (Fig. 1). Seawater samples were collected ∼5 cm above the sediment surface. H2 and CO concentrations were measured with an RGA3 reduction gas analyser (Trace Analytical) using ultra pure nitrogen (purity level 5.0; SOL s.p.a., Monza, Italy) as carrier gas. To control for potential instrument drift, standards were measured in each measurement run and used to calculate sample concentrations within each run (Table S2).
Eight blanks with ddH2O were created, processed and measured in the same way as the pore water samples. Average CO concentrations (3.1 nM) and H2 concentrations (21.5 nM) in blanks were used for blank correction of pore water and seawater concentrations. All concentrations in this study are given as blank‐corrected values.
CO and H 2 production of different components from the O . algarvensis habitat
To identify the sources of CO and H2 in the O. algarvensis environment, we incubated dead seagrass rhizome, sediment and seawater from six different locations at the worm collection site (Fig. 1). From each location, 70–100 g (wet weight) rhizome material (collected from the layer of dead rhizome mats at ≥ 25 cm sediment depth), 250 ml of sediment or unfiltered seawater were added to 0.5 l Schott bottles. We filled bottles completely with sterile filtered seawater, closed them with rubber septa and then withdrew 20 ml of seawater using a syringe to create a headspace. Bottles with ddH2O were used as controls. Bottles were incubated in a water bath set at 23°C for ∼ 3.5 h in the dark to avoid photochemical CO production (King and Weber, 2007). Ten minutes before measuring H2 and CO concentrations, bottles were shaken thoroughly to allow produced H2 and CO gas to equilibrate with the headspace. H2 and CO were measured using the same setup as for the pore water samples (see above). Rhizome samples and sediment samples were rinsed in freshwater and dried after the incubation to determine their dry weight.
To distinguish biotic from abiotic CO and H2 production, samples were either incubated in their native state or amended with ZnCl2 (50% w/v) to a final concentration of 61 mM to stop biological activity. The pH was adjusted to the pH of the native seawater (pH 7.7–7.8) in ZnCl2‐amended samples. The minimum inhibitory concentration of ZnCl2 for microbial activity is about 1 mM (Winslow and Haywood, 1931; He et al., 2002; Choi et al., 2010). We are therefore confident that the 61‐fold higher concentration in our experiments efficiently stopped most biological activity.
Supporting information
Fig. S1. Epifluorescence images of O. algarvensis symbionts on a filter. Symbiont cells are the same as in Fig. 6. Images in the left and right columns show in the top row the composite CARD‐FISH signals of all fluorescence channels, followed in the second row by the epifluorescence images of symbiont cells with the sulfur‐oxidizing symbionts targeted by the gammaproteobacterial probe (Gam42a) in red. The third row shows epifluorescence images of all symbiont cells targeted by the general eubacterial probe (EUB338I‐III) in green and the fourth row epifluorescence images of the general DNA stain DAPI in blue.
Fig. S2. Comparison of the aerobic form I CODH operons in known CO oxidizers and the γ3‐symbiont. While the metagenomic sequences of the γ3‐symbiont CODH genes used in the metaproteomic study of the O. algarvensis symbionts were fragmented and distributed among two genome contigs (Woyke et al., 2006; Kleiner et al., 2012), we recovered the complete and uninterrupted CODH operon of the γ3‐symbiont as part of our 2012 Community Sequencing Project (CSP) with the US Department of Energy Joint Genome Institute (see Acknowledgments). The bottom three microorganisms are known to oxidize CO at very low concentrations (<1000 ppm). Bold letters highlight the functional subunits of the CODH (coxMSL), while the other genes are accessory proteins (e.g. coxDEF). Genes shown in white do not belong to the CODH operon and genes labeled with # indicate a gene found in many CODH operons, but not in the O. carboxidovorans genome. This gene (#) is annotated as ‘CTP:molybdopterin cytidylytransferase’ according to RAST. Genes are not drawn to scale. The CSP 2012 contig is available upon request.
Fig. S3. Comparison of 13C isotope content in white and pale worms after incubation with 13C‐labeled bicarbonate, nitrate and oxygen, but no additional external energy source for 36 h. The γ1‐symbionts in white worms contain large amounts of stored elemental sulfur, which they use for CO2 fixation under oxic conditions (Giere, 2006). In pale worms, the sulfur stores of the γ1‐symbionts are reduced or depleted leading to less CO2 fixation. Pale worms were obtained by oxic pre‐incubations (see Experimental procedures). Mean values and standard deviations of five (for the white worms) and three (for the pale worms) independent incubations are shown. 13C isotope content values are given in atomic percentage (AT%=(13C/(12C+13C)x100).
Table S1. 13C isotope fraction data for individual regions of interest (ROIs).
Table S2. Values for individual measurements of CO and H2 standards.
Table S3. Results from bulk analyses of whole worms.
Acknowledgements
We thank the team of the HYDRA Institute on Elba for their extensive support with sample collection and onsite experiments; Lubos Polerecky for help with the Look@NanoSIMS software; all members of the Symbiosis Group for helping sorting out worms of the sediment; Silke Wetzel, Agnes Zimmer and Nadine Lehnen for excellent technical assistance; Hannah Marchant and Tim Kalvelage for help with GC‐measurements; Richard Hahnke for his help with everyday challenges; and Anne‐Christine Kreutzmann for valuable comments on the experiments. Alex Copeland is acknowledged for assembling the CODH operon of the γ3‐symbiont as part of a CSP2012 project. We also thank the two anonymous reviewers for detailed and thoughtful feedback that helped improve this manuscript significantly. Sequencing was conducted by the U.S. Department of Energy Joint Genome Institute and is supported by the Office of Science of the U.S. Department of Energy under Contract No. DE‐AC02‐05CH11231. CW and MK were supported by scholarships of the Studienstiftung des deutschen Volkes. Funding for this study was provided by the Gordon and Betty Moore Foundation through Grant No. GBMF3811 to ND and the Max Planck Society.
References
- Alcoverro, T. , Manzanera, M. , and Romero, J. (2001) Annual metabolic carbon balance of the seagrass Posidonia oceanica: the importance of carbohydrate reserves. Mar Ecol Prog Ser 211: 105–116. [Google Scholar]
- Boudouresque, C.F. , Bernard, G. , Pergent, G. , Shili, A. , and Verlaque, M. (2009) Regression of Mediterranean seagrasses caused by natural processes and anthropogenic disturbances and stress: a critical review. Botanica Marina 52: 395–418. [Google Scholar]
- Choi, E.K. , Lee, H.H. , Kang, M.S. , Kim, B.G. , Lim, H.S. , Kim, S.M. , and Kang, I.C. (2010) Potentiation of bacterial killing activity of zinc chloride by pyrrolidine dithiocarbamate. J Microbiol 48: 40–43. [DOI] [PubMed] [Google Scholar]
- Conrad, R. , and Seiler, W. (1980) Role of microorganisms in the consumption and production of atmospheric carbon monoxide by soil. Appl Environ Microbiol 40: 437–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad, R. , and Seiler, W. (1982) Arid soils as a source of atmospheric carbon‐monoxide. Geophys Res Lett 9: 1353–1356. [Google Scholar]
- Conrad, R. , and Seiler, W. (1985) Characteristics of abiological carbon monoxide formation from soil organic matter, humic acids, and phenolic compounds. Environ Sci Technol 19: 1165–1169. [DOI] [PubMed] [Google Scholar]
- Cypionka, H. , Meyer, O. , and Schlegel, H.G. (1980) Physiological‐characteristics of various species of strains of carboxydobacteria. Arch Microbiol 127: 301–307. [Google Scholar]
- Day, D.A. , and Faloona, I. (2009) Carbon monoxide and chromophoric dissolved organic matter cycles in the shelf waters of the northern California upwelling system. J Geophys Res Oceans 114: doi: 10.1029/2007JC004590. [Google Scholar]
- DeChaine, E.G. , and Cavanaugh, C.M. (2006) Symbioses of methanotrophs and deep‐sea mussels (Mytilidae: Bathymodiolinae). Prog Mol Subcell Biol 41: 227–249. [DOI] [PubMed] [Google Scholar]
- Diekert, G.B. , and Thauer, R.K. (1978) Carbon monoxide oxidation by Clostridium thermoaceticum and Clostridium formicoaceticum . J Bacteriol 136: 597–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte, C.M. (2002) The future of seagrass meadows. Environ Conserv 29: 192–206. [Google Scholar]
- Dubilier, N. , Mulders, C. , Ferdelman, T. , de Beer, D. , Pernthaler, A. , Klein, M. , et al (2001) Endosymbiotic sulphate‐reducing and sulphide‐oxidizing bacteria in an oligochaete worm. Nature 411: 298–302. [DOI] [PubMed] [Google Scholar]
- Dubilier, N. , Blazejak, A. , and Rühland, C. (2006) Symbioses between bacteria and gutless marine oligochaetes In Molecular Basis of Symbiosis. Overmann J. (ed.). Berlin, Heidelberg: Springer‐Verlag, pp. 251–275. [DOI] [PubMed] [Google Scholar]
- Dubilier, N. , Bergin, C. , and Lott, C. (2008) Symbiotic diversity in marine animals: the art of harnessing chemosynthesis. Nat Rev Microbiol 6: 725–740. [DOI] [PubMed] [Google Scholar]
- Fraune, S. , and Bosch, T.C.G. (2010) Why bacteria matter in animal development and evolution. Bioessays 32: 571–580. [DOI] [PubMed] [Google Scholar]
- Giere, O. , and Erseus, C. (2002) Taxonomy and new bacterial symbioses of gutless marine Tubificidae (Annelida, Oligochaeta) from the Island of Elba (Italy). Org Divers Evol 2: 289–297. [Google Scholar]
- Goodwin, S. , Conrad, R. , and Zeikus, J.G. (1988) Influence of pH on microbial hydrogen metabolism in diverse sedimentary ecosystems. Appl Environ Microbiol 54: 590–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez, J.L. , Jones, C.G. , Byers, J.E. , Arkema, K.K. , Berkenbusch, K. , Commito, J.A. , et al (2011) Functioning of estuaries and coastal ecosystems In Treatise on Estuarine and Coastal Science. Heip C.H.R., Philippart C.J.M., and Middelburg J.J. (eds). Elsevier, doi: 10.1016/B978‐0‐12‐374711‐2.00705‐1. [Google Scholar]
- Hardy, K.R. , and King, G.M. (2001) Enrichment of high‐affinity CO oxidizers in Maine forest soil. Appl Environ Microbiol 67: 3671–3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häring, V. , and Conrad, R. (1991) Kinetics of H2‐oxidation in respiring and denitrifying Paracoccus denitrificans . FEMS Microbiol Lett 78: 259–264. [Google Scholar]
- He, G. , Pearce, E.I.F. , and Sissons, C.H. (2002) Inhibitory effect of ZnCl2 on glycolysis in human oral microbes. Arch Oral Biol 47: 117–129. [DOI] [PubMed] [Google Scholar]
- Hendrickson, O.Q. , and Kubiseski, T. (1991) Soil microbial activity at high‐levels of carbon‐monoxide. J Environ Qual 20: 675–678. [Google Scholar]
- Kerby, R.L. , Ludden, P.W. , and Roberts, G.P. (1995) Carbon monoxide‐dependent growth of Rhodospirillum rubrum . J Bacteriol 177: 2241–2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King, G.M. (2007) Microbial carbon monoxide consumption in salt marsh sediments. FEMS Microbiol Ecol 59: 2–9. [DOI] [PubMed] [Google Scholar]
- King, G.M. , and Weber, C.F. (2007) Distribution, diversity and ecology of aerobic CO‐oxidizing bacteria. Nat Rev Microbiol 5: 107–118. [DOI] [PubMed] [Google Scholar]
- Kleiner, M. , Woyke, T. , Ruehland, C. , and Dubilier, N. (2011) The Olavius algarvensis metagenome revisited: lessons learned from the analysis of the low‐diversity microbial consortium of a gutless marine worm In Handbook of Molecular Microbial Ecology II: Metagenomics in Different Habitats. de Brujin F.J. (ed.). New York: Wiley‐Blackwell, pp. 321–333. [Google Scholar]
- Kleiner, M. , Petersen, J.M. , and Dubilier, N. (2012a) Convergent and divergent evolution of metabolism in sulfur‐oxidizing symbionts and the role of horizontal gene transfer. Curr Opin Microbiol 15: 621–631. [DOI] [PubMed] [Google Scholar]
- Kleiner, M. , Wentrup, C. , Lott, C. , Teeling, H. , Wetzel, S. , Young, J. , et al (2012b) Metaproteomics of a gutless marine worm and its symbiotic microbial community reveal unusual pathways for carbon and energy use. Proc Natl Acad Sci USA 109: E1173–E1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klüber, H.D. , and Conrad, R. (1993) Ferric iron‐reducing Shewanella putrefaciens and N2‐fixing Bradyrhizobium japonicum with uptake hydrogenase are unable to oxidize atmospheric H2 . FEMS Microbiol Lett 111: 337–341. [Google Scholar]
- McFall‐Ngai, M. , Hadfield, M.G. , Bosch, T.C.G. , Carey, H.V. , Domazet‐Loso, T. , Douglas, A.E. , et al (2013) Animals in a bacterial world, a new imperative for the life sciences. Proc Natl Acad Sci USA 110: 3229–3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFall‐Ngai, M.J. (2002) Unseen forces: the influence of bacteria on animal development. Dev Biol 242: 1–14. [DOI] [PubMed] [Google Scholar]
- Maness, P.C. , Huang, J. , Smolinski, S. , Tek, V. , and Vanzin, G. (2005) Energy generation from the CO oxidation–hydrogen production pathway in Rubrivivax gelatinosus . Appl Environ Microbiol 71: 2870–2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin‐Cuadrado, A.B. , Ghai, R. , Gonzaga, A. , and Rodriguez‐Valera, F. (2009) CO dehydrogenase genes found in metagenomic fosmid clones from the deep Mediterranean Sea. Appl Environ Microbiol 75: 7436–7444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateo, M.A. , Romero, J. , Perez, M. , Littler, M.M. , and Littler, D.S. (1997) Dynamics of millenary organic deposits resulting from the growth of the Mediterranean seagrass Posidonia oceanica . Estuar Coast Shelf Sci 44: 103–110. [Google Scholar]
- Meyer, O. , and Schlegel, H.G. (1983) Biology of aerobic carbon monoxide oxidizing bacteria. Annu Rev Microbiol 37: 277–310. [DOI] [PubMed] [Google Scholar]
- Moersdorf, G. , Frunzke, K. , Gadkari, D. , and Meyer, O. (1992) Microbial growth on carbon monoxide In Biodegradation. Netherlands: Kluwer Academic Publishers, pp. 61–82. [Google Scholar]
- Moran, M.A. , and Miller, W.L. (2007) Resourceful heterotrophs make the most of light in the coastal ocean. Nat Rev Microbiol 5: 792–800. [DOI] [PubMed] [Google Scholar]
- Moran, N.A. (2007) Symbiosis as an adaptive process and source of phenotypic complexity. Proc Natl Acad Sci USA 104: 8627–8633. doi: 10.1073/pnas.0611659104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moya, A. , Pereto, J. , Gil, R. , and Latorre, A. (2008) Learning how to live together: genomic insights into prokaryote‐animal symbioses. Nat Rev Genet 9: 218–229. [DOI] [PubMed] [Google Scholar]
- Musat, N. , Foster, R. , Vagner, T. , Adam, B. , and Kuypers, M.M. (2012) Detecting metabolic activities in single cells, with emphasis on nanoSIMS. FEMS Microbiol Rev 36: 486–511. [DOI] [PubMed] [Google Scholar]
- Musat, N. , Stryhanyuk, H. , Bombach, P. , Lorenz, A. , Audinot, J.N. , and Richnow, H.H. (2014) The effect of FISH and CARD‐FISH on the isotopic composition of 13C‐ and 15N‐labeled Pseudomonas putida cells measured by nanoSIMS. Syst Appl Microbiol 37: 267–276. [DOI] [PubMed] [Google Scholar]
- Novelli, P.C. , Michelson, A.R. , Scranton, M.I. , Banta, G.T. , Hobbie, J.E. , and Howarth, R.W. (1988) Hydrogen and acetate cycling in 2 sulfate‐reducing sediments – Buzzards Bay and Town Cove, Mass. Geochim Cosmochim Acta 52: 2477–2486. [Google Scholar]
- Oelgeschlager, E. , and Rother, M. (2008) Carbon monoxide‐dependent energy metabolism in anaerobic bacteria and archaea. Arch Microbiol 190: 257–269. [DOI] [PubMed] [Google Scholar]
- Perner, M. , Petersen, J.M. , Zielinski, F. , Gennerich, H.H. , and Seifert, R. (2010) Geochemical constraints on the diversity and activity of H2‐oxidizing microorganisms in diffuse hydrothermal fluids from a basalt‐ and an ultramafic‐hosted vent. FEMS Microbiol Ecol 74: 55–71. [DOI] [PubMed] [Google Scholar]
- Petersen, J.M. , Zielinski, F.U. , Pape, T. , Seifert, R. , Moraru, C. , Amann, R. , et al (2011) Hydrogen is an energy source for hydrothermal vent symbioses. Nature 476: 176–180. [DOI] [PubMed] [Google Scholar]
- Pohorelic, B.K.J. , Voordouw, J.K. , Lojou, E. , Dolla, A. , Harder, J. , and Voordouw, G. (2002) Effects of deletion of genes encoding Fe‐only hydrogenase of Desulfovibrio vulgaris Hildenborough on hydrogen and lactate metabolism. J Bacteriol 184: 679–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polerecky, L. , Adam, B. , Milucka, J. , Musat, N. , Vagner, T. , and Kuypers, M.M. (2012) Look@NanoSIMS – a tool for the analysis of nanoSIMS data in environmental microbiology. Environ Microbiol 14: 1009–1023. [DOI] [PubMed] [Google Scholar]
- Quaiser, A. , Zivanovic, Y. , Moreira, D. , and Lopez‐Garcia, P. (2011) Comparative metagenomics of bathypelagic plankton and bottom sediment from the Sea of Marmara. ISME J 5: 285–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revsbech, N.P. (1989) An oxygen microsensor with a guard cathode. Limnol Oceanogr 34: 474–478. [Google Scholar]
- Ruehland, C. , Blazejak, A. , Lott, C. , Loy, A. , Erseus, C. , and Dubilier, N. (2008) Multiple bacterial symbionts in two species of co‐occurring gutless oligochaete worms from Mediterranean seagrass sediments. Environ Microbiol 10: 3404–3416. [DOI] [PubMed] [Google Scholar]
- Schwartz, E. , and Friedrich, B. (2006) The H2‐metabolizing Prokaryotes In The Prokaryotes. Dworkin M., Falkow S.I., Rosenberg E., Schleifer K.‐H., and Stackebrandt E. (eds). New York: Springer, pp. 496–563. [Google Scholar]
- Smedile, F. , Messina, E. , La Cono, V. , Tsoy, O. , Monticelli, L.S. , Borghini, M. , et al (2013) Metagenomic analysis of hadopelagic microbial assemblages thriving at the deepest part of Mediterranean Sea, Matapan–Vavilov Deep. Environ Microbiol 15: 167–182. [DOI] [PubMed] [Google Scholar]
- Stewart, F.J. , Newton, I.L. , and Cavanaugh, C.M. (2005) Chemosynthetic endosymbioses: adaptations to oxic–anoxic interfaces. Trends Microbiol 13: 439–448. [DOI] [PubMed] [Google Scholar]
- Tolli, J.D. , Sievert, S.M. , and Taylor, C.D. (2006) Unexpected diversity of bacteria capable of carbon monoxide oxidation in a coastal marine environment, and contribution of the Roseobacter‐associated clade to total CO oxidation. Appl Environ Microbiol 72: 1966–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignais, P.M. , and Billoud, B. (2007) Occurrence, classification, and biological function of hydrogenases: an overview. Chem Rev 107: 4206–4272. [DOI] [PubMed] [Google Scholar]
- Walker, A. , and Crossman, L.C. (2007) This place is big enough for both of us. Nat Rev Microbiol 5: 90–92. [DOI] [PubMed] [Google Scholar]
- Weber, C.F. , and King, G.M. (2010) Distribution and diversity of carbon monoxide‐oxidizing bacteria and bulk bacterial communities across a succession gradient on a Hawaiian volcanic deposit. Environ Microbiol 12: 1855–1867. [DOI] [PubMed] [Google Scholar]
- Widdel, F. , and Bak, F. (1992) Gram‐negative mesophilic sulfate‐reducing bacteria In The Prokaryotes. Balows A., Trüper H.G., Dworkin M., Harder W., and Schleifer K.‐H. (eds). New York: Springer, pp. 3352–3378. [Google Scholar]
- Winslow, C.‐E.A. , and Haywood, E.T. (1931) The specific potency of certain cations with reference to their effect on bacterial viability. J Bacteriol 22: 49–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woebken, D. , Burow, L.C. , Behnam, F. , Mayali, X. , Schintlmeister, A. , Fleming, E.D. , et al (2015) Revisiting N2 fixation in Guerrero Negro intertidal microbial mats with a functional single‐cell approach. ISME J 9: 485–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woyke, T. , Teeling, H. , Ivanova, N.N. , Huntemann, M. , Richter, M. , Gloeckner, F.O. , et al (2006) Symbiosis insights through metagenomic analysis of a microbial consortium. Nature 443: 950–955. [DOI] [PubMed] [Google Scholar]
- Zhang, Y. , Xie, H.X. , Fichot, C.G. , and Chen, G.H. (2008) Dark production of carbon monoxide (CO) from dissolved organic matter in the St. Lawrence estuarine system: implication for the global coastal and blue water CO budgets. J Geophys Res Oceans 113: DOI: 10.1029/2008JC004811. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Epifluorescence images of O. algarvensis symbionts on a filter. Symbiont cells are the same as in Fig. 6. Images in the left and right columns show in the top row the composite CARD‐FISH signals of all fluorescence channels, followed in the second row by the epifluorescence images of symbiont cells with the sulfur‐oxidizing symbionts targeted by the gammaproteobacterial probe (Gam42a) in red. The third row shows epifluorescence images of all symbiont cells targeted by the general eubacterial probe (EUB338I‐III) in green and the fourth row epifluorescence images of the general DNA stain DAPI in blue.
Fig. S2. Comparison of the aerobic form I CODH operons in known CO oxidizers and the γ3‐symbiont. While the metagenomic sequences of the γ3‐symbiont CODH genes used in the metaproteomic study of the O. algarvensis symbionts were fragmented and distributed among two genome contigs (Woyke et al., 2006; Kleiner et al., 2012), we recovered the complete and uninterrupted CODH operon of the γ3‐symbiont as part of our 2012 Community Sequencing Project (CSP) with the US Department of Energy Joint Genome Institute (see Acknowledgments). The bottom three microorganisms are known to oxidize CO at very low concentrations (<1000 ppm). Bold letters highlight the functional subunits of the CODH (coxMSL), while the other genes are accessory proteins (e.g. coxDEF). Genes shown in white do not belong to the CODH operon and genes labeled with # indicate a gene found in many CODH operons, but not in the O. carboxidovorans genome. This gene (#) is annotated as ‘CTP:molybdopterin cytidylytransferase’ according to RAST. Genes are not drawn to scale. The CSP 2012 contig is available upon request.
Fig. S3. Comparison of 13C isotope content in white and pale worms after incubation with 13C‐labeled bicarbonate, nitrate and oxygen, but no additional external energy source for 36 h. The γ1‐symbionts in white worms contain large amounts of stored elemental sulfur, which they use for CO2 fixation under oxic conditions (Giere, 2006). In pale worms, the sulfur stores of the γ1‐symbionts are reduced or depleted leading to less CO2 fixation. Pale worms were obtained by oxic pre‐incubations (see Experimental procedures). Mean values and standard deviations of five (for the white worms) and three (for the pale worms) independent incubations are shown. 13C isotope content values are given in atomic percentage (AT%=(13C/(12C+13C)x100).
Table S1. 13C isotope fraction data for individual regions of interest (ROIs).
Table S2. Values for individual measurements of CO and H2 standards.
Table S3. Results from bulk analyses of whole worms.


