Abstract
Objectives
To assess the diagnostic accuracy of placental growth factor (PlGF) and ultrasound parameters to predict delivery of a small‐for‐gestational‐age (SGA) infant in women presenting with reduced symphysis–fundus height (SFH).
Methods
This was a multicenter prospective observational study recruiting 601 women with a singleton pregnancy and reduced SFH between 24 and 37 weeks' gestation across 11 sites in the UK and Canada. Plasma PlGF concentration < 5th centile, estimated fetal weight (EFW) < 10th centile, umbilical artery Doppler pulsatility index > 95th centile and oligohydramnios (amniotic fluid index < 5 cm) were compared as predictors for a SGA infant < 3rd customized birth‐weight centile and adverse perinatal outcome. Test performance statistics were calculated for all parameters in isolation and in combination.
Results
Of the 601 women recruited, 592 were analyzed. For predicting delivery of SGA < 3rd centile (n = 78), EFW < 10th centile had 58% sensitivity (95% CI, 46–69%) and 93% negative predictive value (NPV) (95% CI, 90–95%), PlGF had 37% sensitivity (95% CI, 27–49%) and 90% NPV (95% CI, 87–93%); in combination, PlGF and EFW < 10th centile had 69% sensitivity (95% CI, 55–81%) and 93% NPV (95% CI, 89–96%). The equivalent receiver–operating characteristics (ROC) curve areas were 0.79 (95% CI, 0.74–0.84) for EFW < 10th centile, 0.70 (95% CI, 0.63–0.77) for low PlGF and 0.82 (95% CI, 0.77–0.86) in combination.
Conclusions
For women presenting with reduced SFH, ultrasound parameters had modest test performance for predicting delivery of SGA < 3rd centile. PlGF performed no better than EFW < 10th centile in determining delivery of a SGA infant. © 2015 The Authors. Ultrasound in Obstetrics & Gynecology published by John Wiley & Sons Ltd on behalf of the international society of ultrasound in obstetrics and gynecology.
Keywords: estimated fetal weight, fetal growth restriction, placental growth factor, small‐for‐gestational age
Short abstract
Linked Comment: Ultrasound Obstet Gynecol 2015; 46: 140–140
This article has been selected for Journal Club. Click here to view slides and discussion points.
INTRODUCTION
Fetal growth restriction (FGR) is a failure to fulfill growth potential, associated with an increased risk of stillbirth1, neonatal morbidity2, 3 and mortality4, 5, 6, 7. Complications can extend into adult life, with a greater risk of cardiovascular disease and Type 2 diabetes mellitus8. Small‐for‐gestational‐age (SGA) infants are defined typically as those with a birth weight < 3rd, < 5th or < 10th centile; these include constitutionally small infants and those with FGR and, as a group, these pregnancies are at increased risk of adverse neonatal outcome9.
Identifying SGA infants remains challenging in the low‐risk population, relying on imprecise techniques such as symphysis–fundus height (SFH) measurement10. If SGA is suspected, UK national guidance recommends ultrasound measurements of abdominal circumference (AC) or estimated fetal weight (EFW) < 10th centile to diagnose SGA11, 12. However, a large proportion of SGA infants are not detected antenatally (32% of 215 high‐risk women1 and 82% of 195 stillbirths with SGA13).
UK national guidance11 does not advocate routine ultrasound measurement in the third trimester as a screening tool for SGA owing to poor prediction (sensitivity, 38–51%)14, 15, 16, 17 and no evidence of improved neonatal outcome18. However, preliminary results from a recent large prospective cohort study reported increased sensitivity of screening (79%) vs selective (32%) sonography in the third trimester for prediction of severe SGA in an unselected nulliparous population19.
Whilst the pathophysiology of FGR is multifactorial, placental insufficiency is causative in many cases. Markers of placental function could provide adjuncts to current techniques to identify high‐risk pregnancies. Multiple biomarkers have been proposed to aid detection but none has sufficient accuracy for incorporation into clinical practice20. However, low levels of maternal serum placental growth factor (PlGF) can distinguish placental SGA from constitutionally small fetuses (sensitivity, 100%; specificity, 86%)21 and, in a high‐risk cohort with suspected preterm pre‐eclampsia (PE), can predict PE and delivery of a SGA infant (birth weight < 1st centile) with high sensitivity22.
We performed a large prospective multicenter cohort study in women with suspected SGA (reduced SFH measurement) with the aim of assessing the diagnostic accuracy of PlGF levels and ultrasound parameters to predict delivery of a SGA infant.
METHODS
Women were enrolled from 11 consultant‐led units across the UK and Canada, between December 2011 and July 2013 (approximate number of deliveries per year: St Thomas' Hospital London, 6650; St Mary's Hospital Manchester, 8200; Oxford, 6550; Leeds, 9550; Sheffield, 7000; St George's Hospital London, 4950; St Michael's Hospital Bristol, 5500; Lewisham, 4000; West Middlesex Hospital, 4700; Sunderland, 3200; Vancouver, 7000). Local audit data at St Thomas' Hospital London in the year prior to study commencement (2011) showed that approximately 1300 women were referred with reduced SFH. Of these women, 8% delivered an SGA infant with customized birth weight < 3rd centile for gestational age. Ethical approval was granted by East London Research Ethics Committee (ref. 10/H0701/117).
Women were eligible if they were ≥ 16 years of age, with a singleton pregnancy between 24 + 0 and 36 + 6 weeks' gestation and referred for suspected SGA because of either: (i) a SFH measuring > 2 cm less than the expected height for any given gestational age in completed weeks (e.g. measuring ≤ 33 cm at 36 weeks' gestation); or (ii) a SFH < 10th centile on a customized SFH chart. Women with SGA confirmed already (EFW < 10th customized centile), a major fetal anomaly (fetal malformations that affect viability and/or quality of life of the fetus and require intervention23) or confirmed rupture of amniotic membranes were excluded.
Written informed consent was obtained from participants. A study‐specific database was designed and finalized before recruitment of the first participant. On the same day as the ultrasound scan, baseline demographic and pregnancy‐specific data were entered into the database and PlGF testing was performed. Blood was drawn into ethylenediamine tetra‐acetic acid and labeled with a study‐specific coded identifier. Samples were transported to the laboratory at the recruiting site and spun for 10 min at 1400 g. Plasma was extracted from each sample and stored at −80 °C until required for analysis. All samples were analyzed for PlGF at the recruiting site using the AlereTriage®PLGF (Alere, San Diego, CA, USA) test, according to the manufacturer's instructions. All laboratory staff received standardized training in sample processing, delivered by the study monitor. All meters were programmed to produce a blinded result, determining satisfactory test completion only, without revealing the value. All laboratory staff were blinded to the clinical diagnosis. The assay uses fluorescently labeled recombinant murine monoclonal antibodies and detects PlGF specifically and quantitatively, in the range of 12–3000 pg/mL, in approximately 15 min. The lower limit of detection of the assay is 12 pg/mL and PlGF results were classified as normal (PlGF ≥ 5th centile for gestational age), low (< 5th centile) and very low (< 12 pg/mL). To determine assay reproducibility, replicate samples were also tested at a central laboratory. The total precision (coefficient of variation) on plasma controls, at concentrations of 85 pg/mL and 1300 pg/mL, was 12.8% and 13.2%, respectively.
All case outcomes were adjudicated by two independent senior physicians, without knowledge of PlGF concentrations. SGA was defined as delivery of an infant with a birth weight < 3rd (or < 10th as a secondary analysis) customized birth‐weight centile, calculated using the Gestation Related Optimal Weight (GROW) method software24. A final maternal diagnosis was assigned using definitions from the American College of Obstetricians and Gynecologists' practice bulletin for maternal hypertensive disorders25 and the International and Australasian Societies for the Study of Hypertension in Pregnancy for atypical PE, as predefined in the study protocol26.
Any hospital attendances subsequent to enrolment were recorded in the study database, including repeat ultrasound assessments, details of delivery and adverse maternal and perinatal outcomes. Adverse maternal outcome was predefined as the presence of any of the following complications: maternal death; eclampsia; stroke; cortical blindness or retinal detachment; hypertensive encephalopathy; systolic blood pressure ≥ 160 mmHg; myocardial infarction; intubation (other than for Cesarean section); pulmonary edema; platelet count < 50 × 109/L (without transfusion); disseminated intravascular coagulation; thrombotic thrombocytopenic purpura/hemolytic uremic syndrome; hepatic dysfunction (alanine transaminase ≥ 70 IU/L); hepatic hematoma or rupture; acute fatty liver of pregnancy; creatinine > 150 µmol/L; renal dialysis; placental abruption; major postpartum hemorrhage; or major infection. Adverse perinatal outcome was defined as the presence of any of the following complications: antepartum/intrapartum fetal or neonatal death; neonatal unit admission for > 48 h following term delivery; intraventricular hemorrhage; periventricular leukomalacia; seizure; retinopathy of prematurity; respiratory distress syndrome; bronchopulmonary dysplasia; or necrotizing enterocolitis. An independent observer conducted regular data monitoring at all sites.
The study was powered on the basis of the number of cases needed to distinguish reliably good (80%) from moderate (60%) sensitivity. Fifty‐five cases were needed for 90% power and 5% significance. This number was met for all endpoints by recruiting 601 women, giving 78 cases of SGA < 3rd birth‐weight centile.
Statistical analysis
The predefined primary outcome (reference standard) was delivery of a SGA infant < 3rd customized birth‐weight centile, calculated using version 6.7 of the GROW calculator. SGA < 10th centile and adverse perinatal outcomes were considered as secondary outcomes.
PlGF centiles from a large low‐risk antenatal population, adjusted for gestational age, were used27. An abnormal result was defined as maternal PlGF concentration < 5th centile, as this has been shown previously to offer a combination of high sensitivity and acceptable specificity for detecting PE and SGA, with a high negative predictive value22. Levels of PlGF and three ultrasound parameters (EFW < 10th centile; oligohydramnios, defined as an amniotic fluid index < 5 cm; and umbilical artery Doppler pulsatility index > 95th centile) were compared, both in isolation and in combination, as predictors of delivery of a SGA infant < 3rd and < 10th customized centiles. Gestational‐age‐adjusted centiles were calculated for each observed value of umbilical artery Doppler pulsatility index (UA‐PI), based on a mean value of 0.405 – (0.0134 × gestational age (weeks)) and SD of 0.0794 for log10UA‐PI28. Sensitivity, specificity and positive and negative predictive values (PPV and NPV, respectively) were calculated with 95% CI. Receiver–operating characteristics (ROC) curve areas were also calculated for each individual parameter and their combinations, and in a predefined subgroup of patients who delivered within 6 weeks of PlGF sampling. Fisher's exact test was used to compare the event rate in women with normal and low PlGF measurements. Statistical analysis was carried out in the Stata statistical package (version 11.2; StataCorp, College Station, TX, USA). This study is reported in accordance with the STAndards for the Reporting of Diagnostic accuracy studies (STARD) guidelines (Table S1).
RESULTS
Six‐hundred and one women presenting with a suspected SGA fetus between 24 + 0 and 36 + 6 weeks' gestation were recruited across 11 sites between December 2011 and July 2013. We recruited all women who were approached, eligible and consented, but did not document women who declined to participate. No outcome data were available for two participants, and five women did not have PlGF results generated by the test meter. A further two women had no ultrasound data available at enrolment. After exclusion of these nine cases, 592 women were included in the subsequent analysis. Of these women, 192 delivered a SGA infant with birth weight < 10th customized centile and 78 had a birth weight < 3rd customized centile (Figure 1).
Figure 1.
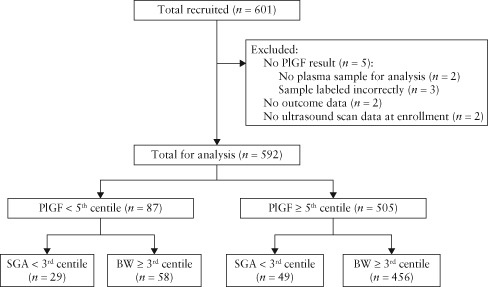
Flowchart of study population of women with singleton pregnancy presenting with reduced symphysis–fundus height. BW, birth weight; PlGF, placental growth factor; SGA, small‐for‐gestational age.
Characteristics of participants at booking are given in Table 1; higher rates of smoking were observed in women who delivered a SGA infant. Table 2 displays baseline characteristics at study enrolment. Details of maternal and neonatal outcomes and final adjudicated maternal diagnoses are shown in Table 3. The majority of women (n = 555) experienced no maternal complications during their pregnancy. Whilst the number of cases complicated by PE was small (n = 16), most of these women delivered a SGA infant (n = 12). Of the 13 cases with adverse perinatal outcome, there was one stillbirth, four cases of respiratory distress syndrome and nine infants admitted to the neonatal unit at term for > 48 h (one of whom had respiratory distress syndrome).
Table 1.
Maternal characteristics of 592 women with singleton pregnancy and reduced symphysis–fundus height at booking, according to subsequent birth‐weight (BW) centile of infant
| Characteristic | SGA < 3rd centile (n = 78) | SGA < 10th centile (n = 192) | BW ≥ 10th centile (n = 400) | All women (n = 592) |
|---|---|---|---|---|
| Maternal age (years) | 29.1 (24.1–32.9) | 29.6 (24.8–33.5) | 30.0 (25.3–33.7) | 29.9 (25.2–33.6) |
| BMI (kg/m2) | 22.9 (20.3–25.2) | 21.7 (20.1–24.1) | 21.5 (20.0–23.4) | 21.5 (20.0–23.6) |
| White ethnicity | 52 (66.7) | 122 (63.5) | 266 (66.5) | 388 (65.5) |
| Nulliparous | 65 (83.3) | 163 (84.9) | 344 (86.0) | 507 (85.6) |
| Highest first‐trimester systolic BP (mmHg) | 105 (100–114) | 105 (100–114) | 104 (100–112) | 105 (100–112) |
| Highest first‐trimester diastolic BP (mmHg) | 63 (60–70) | 62 (60–70) | 60 (60–69) | 61 (60–70) |
| Smoking status | ||||
| Never smoked | 46 (59.0) | 128 (66.7) | 306 (76.5) | 434 (73.3) |
| Quit smoking before pregnancy | 9 (11.5) | 22 (11.5) | 31 (7.8) | 53 (8.9) |
| Quit smoking during pregnancy | 10 (12.8) | 16 (8.3) | 24 (6.0) | 40 (6.7) |
| Current smoker | 13 (16.7) | 26 (13.5) | 39 (9.8) | 65 (11.0) |
| Drug use | ||||
| History of drug use* | 5 (6.4) | 6 (3.1) | 3 (0.8) | 9 (1.5) |
| Current drug user† | 1 (1.3) | 2 (1.0) | 0 (0) | 2 (0.3) |
| Medical history | ||||
| PE requiring delivery at < 34 weeks | 0 (0) | 0 (0) | 1 (0.3) | 1 (0.2) |
| Chronic hypertension | 0 (0) | 1 (0.5) | 1 (0.3) | 2 (0.3) |
| SLE/APS | 1 (1.3) | 1 (0.5) | 0 (0) | 1 (0.2) |
| Pre‐existing diabetes mellitus | 0 (0) | 0 (0) | 1 (0.3) | 1 (0.2) |
| Renal disease | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Self‐report of previous small baby | 9 (11.5) | 22 (11.5) | 27 (6.8) | 49 (8.3) |
Data are given as median (interquartile range) or n (%).
Including cannabis, cocaine, ecstasy, amphetamines (speed and/or crystal meth) and heroin.
Cannabis only (rare or occasional use). APS, antiphospholipid syndrome; BMI, body mass index; BP, blood pressure; PE, pre‐eclampsia; SGA, small‐for‐gestational age; SLE, systemic lupus erythematosus.
Table 2.
Baseline characteristics of 592 women with singleton pregnancy presenting with reduced symphysis–fundus height at study enrolment, according to birth‐weight (BW) centile of infant
| Characteristic | SGA < 3rd centile (n = 78) | SGA < 10th centile (n = 192) | BW ≥ 10th centile (n = 400) | All women (n = 592) |
|---|---|---|---|---|
| Gestational age (days) | 238 (221–250) | 235 (213–250) | 236 (214–250) | 236 (213–250) |
| Maternal BP | ||||
| Highest systolic BP (mmHg) | 118 (109–129) | 115 (102–121) | 110 (101–118) | 110 (101–120) |
| Highest diastolic BP (mmHg) | 70 (60–81) | 70 (60–80) | 67 (60–73) | 68 (60–74) |
| Dipstick proteinuria | ||||
| Not done | 11 (14.1) | 29 (15.1) | 61 (15.3) | 90 (15.2) |
| Negative | 58 (74.4) | 148 (77.1) | 322 (80.5) | 470 (79.4) |
| Positive* | 9 (11.5) | 15 (7.8) | 17 (4.3) | 32 (5.4) |
| Complications in current pregnancy | ||||
| Gestational hypertension | 4 (5.1) | 4 (2.1) | 0 (0) | 4 (0.7) |
| Pre‐eclampsia | 0 (0) | 1 (0.5) | 1 (0.3) | 2 (0.3) |
| Gestational diabetes | 1 (1.3) | 3 (1.5) | 4 (1.0) | 7 (1.2) |
| Intrahepatic cholestasis of pregnancy | 0 (0.0) | 1 (0.5) | 2 (0.5) | 3 (0.5) |
| Fetal characteristics | ||||
| EFW < 10th centile | 44 (57.9) | 88 (47.1) | 64 (16.3) | 152 (25.9) |
| Oligohydramnios (AFI < 5 cm) | 2 (3.6) (n = 54) | 4 (3.3) (n = 118) | 1 (0.4) (n = 228) | 5 (1.4) (n = 346) |
| Absent/reversed UA flow | 1 (1.3) (n = 76) | 1 (0.6) (n = 176) | 1 (0.3) (n = 358) | 2 (0.4) (n = 534) |
| UA‐PI > 95th centile | 10 (16.1) (n = 61) | 12 (8.2) (n = 147) | 14 (4.5) (n = 312) | 26 (5.7) (n = 458) |
Data are given as median (interquartile range) or n (%).
+1 or greater. AFI, amniotic fluid index; BP, blood pressure; EFW, estimated fetal weight; PI, pulsatility index; SGA, small‐for‐gestational age; UA, umbilical artery.
Table 3.
Characteristics of delivery and maternal and neonatal outcome in 592 women with singleton pregnancy presenting with reduced symphysis–fundus height, according to birth‐weight (BW) centile of infant
| Characteristic | SGA < 3rd centile (n = 78) | SGA < 10th centile (n = 192) | BW ≥ 10th centile (n = 400) | All women (n = 592) |
|---|---|---|---|---|
| GA at delivery (weeks) | 38.7 (37.1–40.1) | 39.4 (38.0–40.4) | 40.0 (39.0–40.9) | 39.9 (38.9–40.7) |
| Maternal diagnosis | ||||
| No new maternal disease in pregnancy | 68 (86.3) | 173 (89.2) | 382 (95.5) | 555 (93.4) |
| Pre‐eclampsia | 8 (10.0) | 12 (6.2) | 4 (0.99) | 16 (2.7) |
| Gestational hypertension | 0 (0) | 0 (0) | 8 (1.9) | 8 (1.3) |
| Chronic hypertension | 0 (0) | 2 (1.0) | 0 (0) | 2 (0.3) |
| Other diagnosis | 2 (2.5) | 5 (2.6) | 6 (1.5) | 11 (1.8) |
| Maternal medication | ||||
| Dexamethasone | 5 (6.4) | 7 (3.6) | 4 (1.0) | 11 (1.8) |
| Betamethasone | 2 (2.6) | 4 (2.1) | 0 (0) | 4 (0.7) |
| Methyldopa | 2 (2.6) | 2 (1.0) | 0 (0) | 2 (0.3) |
| Labetalol | 6 (7.7) | 9 (4.7) | 2 (0.5) | 11 (1.8) |
| Heparin | 1 (1.3) | 2 (1.0) | 3 (0.8) | 5 (0.8) |
| Nifedipine | 1 (1.3) | 2 (1.0) | 1 (0.3) | 3 (0.5) |
| Aspirin | 3 (3.8) | 4 (2.1) | 8 (2.0) | 12 (2.0) |
| Oral corticosteroids | 0 (0) | 3 (1.6) | 2 (0.5) | 5 (0.8) |
| Onset of labor | ||||
| Spontaneous | 24 (30.8) | 99 (51.6) | 300 (75.0) | 399 (67.4) |
| Induced | 41 (52.6) | 67 (34.9) | 66 (16.5) | 133 (22.5) |
| Prelabor Cesarean section | 13 (16.7) | 26 (13.5) | 34 (8.5) | 60 (10.1) |
| Mode of delivery | ||||
| Spontaneous | 48 (61.5) | 125 (65.1) | 279 (69.8) | 404 (68.2) |
| Assisted vaginal delivery | 8 (10.3) | 23 (12.0) | 66 (16.5) | 89 (15.0) |
| Cesarean section | 22 (28.2) | 44 (22.9) | 55 (13.8) | 99 (16.7) |
| Adverse maternal outcome* | 5 (6.4) | 9 (4.7) | 10 (2.5) | 19 (3.2) |
| Postpartum hemorrhage | 2 (2.6) | 5 (2.6) | 7 (1.8) | 12 (2.0) |
| Placental abruption | 1 (1.3) | 1 (0.5) | 1 (0.3) | 2 (0.3) |
| HELLP | 0 (0) | 0 (0) | 1 (0.3) | 1 (0.2) |
| Fetal outcome | ||||
| Fetal death | 0 (0) | 0 (0) | 1 (0.3) | 1 (0.2) |
| Neonatal death | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Birth weight (g) | 2375 (2100–2610) | 2660 (2360–2854) | 3214 (3000–3470) | 3050 (2740–3329) |
| Adverse perinatal outcome† | 4 (5.1) | 6 (3.1) | 7 (1.8) | 13 (2.2) |
Data are given as median (interquartile range) or n (%).
Defined as presence of any of the following complications: maternal death, eclampsia, stroke, cortical blindness or retinal detachment, hypertensive encephalopathy, systolic blood pressure ≥ 160 mmHg, myocardial infarction, intubation (other than for Cesarean section), pulmonary edema, platelet count < 50 × 109/L (without transfusion), disseminated intravascular coagulation, thrombotic thrombocytopenic purpura/hemolytic uremic syndrome, hepatic dysfunction (alanine transaminase ≥ 70 IU/L), hepatic hematoma or rupture, acute fatty liver of pregnancy, creatinine > 150 µmol/L, renal dialysis, placental abruption, major postpartum hemorrhage, major infection.
Defined as presence of any of the following complications: antepartum/intrapartum fetal or neonatal death, neonatal unit admission for > 48 h at term, intraventricular hemorrhage, periventricular leukomalacia, seizure, retinopathy of prematurity, respiratory distress syndrome, bronchopulmonary dysplasia or necrotizing enterocolitis. GA, gestational age; HELLP, hemolysis, elevated liver enzymes, low platelets; SGA, small‐for‐gestational age.
Induction of labor and Cesarean section occurred more frequently in SGA pregnancies compared with those with birth weights appropriate‐for‐gestational age. Maternal and perinatal adverse outcomes were reported in 3.2% of women and in 2.2% of infants, respectively. Both complications were higher in pregnancies with delivery of a SGA infant (4.7% and 3.1%, respectively).
The median concentration of PlGF according to birth weight was 94.5 (interquartile range (IQR), 36.3–324) pg/mL for SGA < 3rd centile, 253 (IQR, 125–631) pg/mL for SGA < 10th centile and 311 (IQR, 131–742) pg/mL for birth weight ≥ 10th centile. The diagnostic accuracy of PlGF and ultrasound parameters to determine SGA < 3rd and < 10th centile are shown in Table 4, with EFW having the highest sensitivity and NPV of all parameters assessed alone. Addition of PlGF to current ultrasound parameters utilized altered the test sensitivity from 58% to 69% (NPV was unchanged at 93%) in determining SGA < 3rd centile and from 47% to 57% (NPV increased from 77% to 78%) in determining SGA < 10th centile. For women presenting with reduced SFH before 37 weeks' gestation and in whom EFW was measured as ≥ 10th centile, low PlGF concentrations at the time of scanning (< 5th centile) would have detected an additional nine women with subsequent SGA < 3rd centile. This difference in SGA < 3rd centile between those with normal PlGF (5.9%; 23/390) compared with those with low PlGF (20.5%; 9/44) was significant (P = 0.002; Fisher's exact test).
Table 4.
Diagnostic performance of placental growth factor (PlGF) and ultrasound parameters to predict small‐for‐gestational age (SGA) < 3rd and < 10th centiles in women presenting with reduced symphysis–fundus height (n = 592)
| Biomarker/clinical indicator | Sensitivity (% (95% CI)) n/N | Specificity (% (95% CI)) n/N | PPV (% (95% CI)) n/N | NPV (% (95% CI)) n/N |
|---|---|---|---|---|
| SGA < 3rd centile | ||||
| EFW < 10th centile | 57.9 (46.0–69.1) 44/76 | 78.8 (75.0–82.3) 402/510 | 28.9 (21.9–36.8) 44/152 | 92.6 (89.8–94.9) 402/434 |
| Oligohydramnios* | 3.7 (0.5–12.7) 2/54 | 99.0 (97.0–99.8) 289/292 | 40.0 (5.3–85.3) 2/5 | 84.8 (80.5–88.4) 289/341 |
| UA‐PI > 95th centile | 16.4 (8.2–28.1) 10/61 | 96.0 (93.5–97.7) 381/397 | 38.5 (20.2–59.4) 10/26 | 88.2 (84.8–91.1) 381/432 |
| PlGF < 5th centile | 37.2 (26.5–48.9) 29/78 | 88.7 (85.7–91.3) 456/514 | 33.3 (23.6–44.3) 29/87 | 90.3 (87.4–92.7) 456/505 |
| Abnormal AFI or EFW | 57.7 (43.2–71.3) 30/52 | 79.0 (73.9–83.6) 230/291 | 33.0 (23.5–43.6) 30/91 | 91.3 (87.1–94.4) 230/252 |
| Abnormal PlGF or AFI or EFW | 69.2 (54.9–81.3) 36/52 | 72.2 (66.6–77.2) 210/291 | 30.8 (22.6–40.0) 36/117 | 92.9 (88.8–95.9) 210/226 |
| SGA < 10th centile | ||||
| EFW < 10th centile | 47.1 (39.7–54.5) 88/187 | 84.0 (80.0–87.4) 335/399 | 57.9 (49.6–65.8) 88/152 | 77.2 (72.9–81.1) 335/434 |
| Oligohydramnios* | 3.4 (0.9–8.5) 4/118 | 99.6 (97.6–100) 227/228 | 80.0 (28.4–99.5) 4/5 | 66.6 (61.3–71.6) 227/341 |
| UA‐PI > 95th centile | 8.2 (4.3–13.8) 12/147 | 95.5 (92.6–97.5) 297/311 | 46.2 (26.6–66.6) 12/26 | 68.8 (64.1–73.1) 297/432 |
| PlGF < 5th centile | 24.5 (18.6–31.2) 47/192 | 90.0 (86.6–92.8) 360/400 | 54.0 (43.0–64.8) 47/87 | 71.3 (67.1–75.2) 360/505 |
| Abnormal AFI or EFW | 48.7 (39.3–58.2) 56/115 | 84.6 (79.3–89.1) 193/228 | 61.5 (50.8– 71.6) 56/91 | 76.6 (70.9–81.7) 193/252 |
| Abnormal PlGF or AFI or EFW | 57.4 (47.8–66.6) 66/115 | 77.6 (71.7–82.9) 177/228 | 56.4 (46.9–65.6) 66/117 | 78.3 (72.4–83.5) 177/226 |
Amniotic fluid index (AFI), estimated fetal weight (EFW) and umbilical artery (UA) Doppler were not recorded in all subjects.
AFI < 5 cm. NPV, negative predictive value; PI, pulsatility index; PPV, positive predictive value.
In the whole cohort, the ROC area was greater for EFW < 10th centile (0.79 (95% CI, 0.74–0.84)) than for low PlGF levels (0.70 (95% CI, 0.63–0.77)) for the prediction of SGA < 3rd centile; when used in combination, this increased to 0.82 (95% CI, 0.77–0.86) (Figure 2a). In a planned subgroup analysis of 267 women in whom delivery occurred within 6 weeks of PlGF sampling (Table S2), ROC areas were 0.76 (95% CI, 0.69–0.84), 0.74 (95% CI, 0.66–0.83) and 0.81 (95% CI, 0.72–0.88) for EFW < 10th centile, low PlGF and a combination of both parameters, respectively (Figure 2b).
Figure 2.
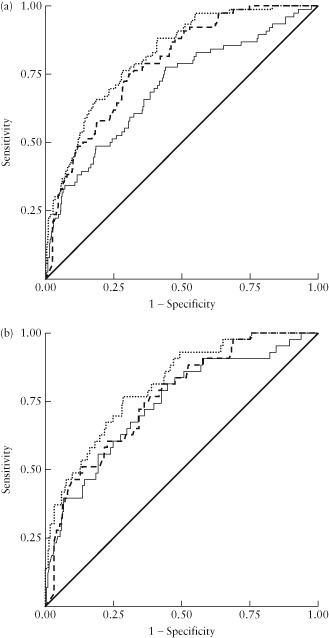
Receiver–operating characteristics curves for low placental growth factor (PlGF) ( ), low estimated fetal weight (EFW) < 10th centile (
), low estimated fetal weight (EFW) < 10th centile ( ) and a combination of these parameters (
) and a combination of these parameters ( ) to predict delivery of a small‐for‐gestational‐age infant with birth weight < 3rd centile in: (a) all women (n = 592); and (b) women who delivered within 6 weeks of PlGF sampling (n = 267). (a) Area under the curve (AUC) for: low PlGF = 0.70 (95% CI, 0.63–0.77), EFW < 10th centile = 0.79 (95% CI, 0.74–0.84) and their combination = 0.82 (95% CI, 0.77–0.86). (b) AUC for low PIGF = 0.74 (95% CI, 0.66–0.83), low EFW < 10th centile = 0.76 (95% CI, 0.69–0.84) and their combination = 0.81 (95% CI, 0.72–0.88).
) to predict delivery of a small‐for‐gestational‐age infant with birth weight < 3rd centile in: (a) all women (n = 592); and (b) women who delivered within 6 weeks of PlGF sampling (n = 267). (a) Area under the curve (AUC) for: low PlGF = 0.70 (95% CI, 0.63–0.77), EFW < 10th centile = 0.79 (95% CI, 0.74–0.84) and their combination = 0.82 (95% CI, 0.77–0.86). (b) AUC for low PIGF = 0.74 (95% CI, 0.66–0.83), low EFW < 10th centile = 0.76 (95% CI, 0.69–0.84) and their combination = 0.81 (95% CI, 0.72–0.88).
The outcomes of 16 participants with a very low PlGF concentration (<12 pg/mL; below the level of assay detection) at enrolment are shown in Table S3. Seven women had hypertensive complications of pregnancy (7/16 (44%) vs 17/576 (3%) in the rest of the cohort) and 11 women delivered a SGA infant with birth weight < 10th customized centile.
There were no adverse events associated with blood sampling for PlGF measurement.
DISCUSSION
In this multicenter prospective cohort study of women presenting with reduced SFH, ultrasound parameters utilized currently, including EFW < 10th centile, had modest test performance for predicting delivery of a SGA infant. Maternal PlGF measurement performed no better than these ultrasound parameters and provided only minimal increments in overall test performance when used in combination. This contrasts with the findings of our previous study, assessing the diagnostic accuracy of PlGF levels in women with suspected PE, which reported excellent performance (sensitivity, 93%; NPV, 96%) in predicting SGA in women presenting at < 35 weeks' gestation22.
There are several possible explanations for the differences observed in these studies. The majority of women recruited into this study had no maternal complications in pregnancy (555/592; 93%) and only 24 (4%) had a new hypertensive disorder. This contrasts with our previous high‐risk cohort, in which 61% of women enrolled at < 35 weeks' gestation developed PE22. Differing pathological processes may occur in the placentae of pregnancies complicated by hypertensive disease, particularly if early onset, and in those who remain normotensive but deliver a SGA infant29. The gestational age at delivery of SGA infants < 3rd centile in this study was 38.7 weeks (with 5% adverse perinatal outcome), compared with 33.8 weeks (with 39% adverse perinatal outcome) in the previous study, emphasizing the probably different placental pathophysiology. The median gestational age at PlGF sampling and at delivery was 34 weeks and 40 weeks, respectively. PlGF appears to have limited clinical utility in women presenting with reduced SFH late in pregnancy and delivering near term. This may reflect convergence of PlGF measurements between normal and pathological pregnancies with advancing gestation27 and the heterogeneous etiology of SGA, even when categorized as birth weight < 3rd customized centile. PlGF is an angiogenic factor produced principally by trophoblasts. Low maternal plasma PlGF concentrations reflect placental dysfunction and have been described in early‐onset PE and SGA, associated with abnormal placental pathology21.
It is particularly notable that adverse perinatal outcome occurred infrequently (2.2%) in this study; this makes conclusions regarding the ability of PlGF to determine adverse outcomes impossible. The single case of stillbirth had a normal PlGF concentration and was not SGA; therefore, placental insufficiency is an unlikely etiology. The neonatal characteristics in this study (Table 3) are markedly different from those described in the previous PlGF study, in which nine (2.1%) cases of stillbirth/neonatal death were reported, with adverse perinatal outcome in 19%22.
This is the largest reported prospective study evaluating the ability of third‐trimester PlGF concentration to predict delivery of a SGA infant in women presenting with reduced SFH. Recruitment from 11 centers across the UK and Canada provided a diverse ethnic and geographical population. PlGF was measured at the recruiting site, as would occur if adopted into clinical practice. The PlGF results were concealed until assignment of a final maternal diagnosis at study completion. The study entry criterion, reduced SFH, was selected for clinical relevance, reflecting current referral practice in the UK. A primary endpoint of delivering an infant < 3rd customized birth‐weight centile was selected as it includes fewer constitutionally small infants and has a stronger association with perinatal mortality7.
This study included only PlGF measurement at study enrolment. Serial measurements to assess whether longitudinal changes in PlGF correlate with evolving placental dysfunction could be informative. When routine antenatal third‐trimester ultrasound in low‐risk women is performed, the findings of this study may be less applicable.
A systematic review evaluating biomarkers for predicting FGR identified 13 studies that reported test performance for PlGF in predicting delivery of a SGA infant20. In a subgroup of studies recruiting women after 20 weeks' gestation, the pooled PlGF sensitivity (at various thresholds) for prediction of intrauterine growth restriction (using differing definitions) was 49% (95% CI, 44–53%). Comparisons were difficult because of heterogeneity between studies. The majority were case–control studies, with only two cohort studies recruiting women over 20 weeks' gestation. Of these, one was in an abnormal population (abnormal uterine artery Doppler waveforms at 20 weeks' gestation), whilst, in the other, delivery of a SGA infant was a secondary endpoint. No cohort studies recruiting in the third trimester were evaluated. A recent study evaluated maternal PlGF concentration at a fixed time point (30–34 weeks' gestation) and reported increased adjusted odds ratio for PlGF combined with other angiogenic factors in the prediction of delivering a SGA infant, but did not provide test performance statistics to enable comparison30.
The capabilities of current standard ultrasound parameters to determine delivery of a SGA infant must also be considered. A large study published a sensitivity of 27% for SFH measurement to predict delivery of a SGA infant10. Reported test performance of EFW < 10th centile to predict pregnancies delivering a SGA infant (sensitivity, 21–46%; NPV, 90–94%)14, 17 are similar to those published in this cohort (sensitivity, 47%; NPV, 77%). Three Cochrane systematic reviews evaluating SFH31, routine ultrasound measurement (including EFW)18 and fetal and umbilical artery Doppler assessment in low‐risk pregnancy32 concluded that none of these techniques reduced adverse perinatal outcome. Use of customized SFH charts and EFW centiles, which adjust for maternal characteristics, may improve SGA detection33, prediction of delivering a SGA infant13, 34 and adverse outcome, including stillbirth35 and neonatal death36. Implementation of customized charts in conjunction with accredited training is associated with a reduction in stillbirth rates in areas of high uptake37 but has not been validated in a randomized control trial.
A systematic review and meta‐analysis assessing amniotic fluid index reported a strong correlation between oligohydramnios and delivery of a SGA infant (birth weight < 10th centile) and mortality, but the predictive accuracy for perinatal outcome was poor38. This agrees with our findings of high specificity for delivery of a SGA infant (99.6% (95% CI, 97.6–100%)) but low sensitivity (3.4% (95% CI, 0.9–8.5%)), limiting clinical application without incorporating other clinical factors. Novel ultrasound parameters, such as the cerebroplacental ratio, have been reported as potentially useful in predicting neonatal status, and validation is awaited39.
We previously suggested PlGF measurement as a useful adjunct to current clinical practice in women with suspected preterm PE, but the findings from this study cannot support its use in women with reduced SFH. Whilst EFW < 10th centile has only modest test performance for prediction of SGA, addition of PlGF measurement does not improve test performance significantly. This study highlights the need for caution when generalizing findings from one population to another and alerts against the overenthusiastic adoption of novel biomarkers without appropriate evaluation.
Supporting information
Table S1 STARD checklist for reporting of studies of diagnostic accuracy
Table S2 Diagnostic performance for placental growth factor (PlGF) and ultrasound parameters to predict small‐for‐gestational age (SGA) < 3rd centile when PlGF was sampled within 6 weeks of delivery (n = 267) in women with reduced symphysis–fundus height
Table S3 Maternal outcome in 16 women with very low placental growth factor levels (<12 pg/mL) at sampling
ACKNOWLEDGMENTS
We thank the doctors, midwives and sonographers at the study centers for their work and the women who participated.
Disclosures
C.R., A.H.S., N.S. and J.M. have been paid as consultants for Alere; A.H.S. has also been paid as a consultant for Roche and PerkinElmer. We acknowledge funding support from Tommy's Charity (registered charity no. 1060508 and SCO39280) and from Alere (San Diego, California, USA) for providing the PlGF assays. This was an investigator‐led study and neither funder had any role in study design, patient recruitment, data collection, analysis or interpretation, in the writing of the manuscript or in the decision to submit it for publication. No author was paid to write this article by these or any other funders. J.M. is supported by Action Medical Research Endowment Fund, the Manchester Biomedical Research Centre and the Greater Manchester Comprehensive Local Research Network. P.T.S.'s salary is supported by Tommy's Charity.
REFERENCES
- 1. Gardosi J, Madurasinghe V, Williams M, Malik A, Francis A. Maternal and fetal risk factors for stillbirth: population based study. BMJ 2013. ; 346 : f108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bernstein IM, Horbar JD, Badger GJ, Ohlsson A, Golan A. Morbidity and mortality among very‐low‐birth‐weight neonates with intrauterine growth restriction. The Vermont Oxford Network. Am J Obstet Gynecol 2013. ; 182(1 Pt 1): 198–206. [DOI] [PubMed] [Google Scholar]
- 3. McIntire DD, Bloom SL, Casey BM, Leveno KJ. Birth weight in relation to morbidity and mortality among newborn infants. N Engl J Med 1999. ; 340 : 1234–1238. [DOI] [PubMed] [Google Scholar]
- 4. Frøen JF, Gardosi JO, Thurmann A, Francis A, Stray‐Pedersen B. Restricted fetal growth in sudden intrauterine unexplained death. Acta Obstet Gynecol Scand 2004. ; 83 : 801–807. [DOI] [PubMed] [Google Scholar]
- 5. Lawn JE, Cousens S, Zupan J. 4 million neonatal deaths: when? Where? Why? Lancet 2005. ; 365 : 891–900. [DOI] [PubMed] [Google Scholar]
- 6. Lackman F, Capewell V, Richardson B, daSilva O, Gagnon R. The risks of spontaneous preterm delivery and perinatal mortality in relation to size at birth according to fetal versus neonatal growth standards. Am J Obstet Gynecol 2001. ; 184 : 946–953. [DOI] [PubMed] [Google Scholar]
- 7. Moraitis AA, Wood AM, Fleming M, Smith GC. Birth weight percentile and the risk of term perinatal death. Obstet Gynecol 2014. ; 124(2 Pt 1): 274–283. [DOI] [PubMed] [Google Scholar]
- 8. Godfrey KM, Barker DJ. Fetal nutrition and adult disease. Am J Clin Nutr 2000. ; 71(5 Suppl): 1344S–1352S. [DOI] [PubMed] [Google Scholar]
- 9. Malin GL, Morris RK, Riley R, Teune MJ, Khan KS. When is birthweight at term abnormally low? A systematic review and meta‐analysis of the association and predictive ability of current birthweight standards for neonatal outcomes. BJOG 2014. ; 121 : 515–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Persson B, Stangenberg M, Lunell NO, Brodin U, Holmberg NG, Vaclavinkova V. Prediction of size of infants at birth by measurement of symphysis fundus height. Br J Obstet Gynaecol 1986. ; 93 : 206–211. [DOI] [PubMed] [Google Scholar]
- 11. Robson SC, Martin WL, Morris RK. The Investigation and Management of the Small‐for‐Gestational‐Age Fetus (2nd edn). RCOG Green‐top guidelines: London, 2013; 1–34. [Google Scholar]
- 12. Chang TC, Robson SC, Boys RJ, Spencer JA. Prediction of the small for gestational age infant: which ultrasonic measurement is best? Obstet Gynecol 1992. ; 80 : 1030–1038. [PubMed] [Google Scholar]
- 13. De Jong CL, Francis A, Van Geijn HP, Gardosi J. Customized fetal weight limits for antenatal detection of fetal growth restriction. Ultrasound Obstet Gynecol 2000. ; 15 : 36–40. [DOI] [PubMed] [Google Scholar]
- 14. Ben‐Haroush A, Yogev Y, Hod M, Bar J. Predictive value of a single early fetal weight estimate in normal pregnancies. Eur J Obstet Gynecol and Reprod Biol 2007. ; 130 : 187–192. [DOI] [PubMed] [Google Scholar]
- 15. Secher NJ, Hansen PK, Lenstrup C, Eriksen PS. Controlled trial of ultrasound screening for light for gestational age (LGA) infants in late pregnancy. Eur J Obst Gynaecol 1986. ; 23 : 307–313. [DOI] [PubMed] [Google Scholar]
- 16. Souka AP, Papastefanou I, Pilalis A, Michalitsi V, Kassanos D. Performance of third‐trimester ultrasound for prediction of small‐for‐gestational‐age neonates and evaluation of contingency screening policies. Ultrasound Obstet Gynecol 2012. ; 39 : 535–542. [DOI] [PubMed] [Google Scholar]
- 17. David C, Tagliavini G, Pilu G, Rudenholz A, Bovicelli L. Receiver‐operator characteristic curves for the ultrasonographic prediction of small‐for‐gestational‐age fetuses in low‐risk pregnancies. Am J Obstet Gynecol 1996. ; 174 : 1037–1042. [DOI] [PubMed] [Google Scholar]
- 18. Bricker L, Neilson JP, Dowswell T. Routine ultrasound in late pregnancy (after 24 weeks' gestation). Cochrane Database Syst Rev 2008. (4): CD001451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sovio U, Smith G, Dacey A. Level 1 evidence for the diagnostic effectiveness of routine sonography as a screening test for small for gestational age (SGA) infants. Am J Obstet Gynecol 2014. ; 210 ( S ): S408. [Google Scholar]
- 20. Conde‐Agudelo A, Papageorghiou AT, Kennedy SH, Villar J. Novel biomarkers for predicting intrauterine growth restriction: a systematic review and meta‐analysis. BJOG 2013. ; 120 : 681–694. [DOI] [PubMed] [Google Scholar]
- 21. Benton SJ, Hu Y, Xie F, Kupfer K, Lee SW, Magee LA, von Dadelszen P. Can placental growth factor in maternal circulation identify fetuses with placental intrauterine growth restriction? Am J Obstet Gynecol 2012. ; 206: 163.e1–e7. [DOI] [PubMed] [Google Scholar]
- 22. Chappell LC, Duckworth S, Seed PT, Griffin M, Myers J, Mackillop L, Simpson N, Waugh J, Anumba D, Kenny LC, Redman CW, Shennan AH. Diagnostic accuracy of placental growth factor in women with suspected preeclampsia: a prospective multicenter study. Circulation 2013. ; 128 : 2121–2131. [DOI] [PubMed] [Google Scholar]
- 23. Addor MC, Pescia G, Schorderet DF. Registration of congenital anomalies in Switzerland by EUROCAT. Schweiz Med Wochenschr 2000. ; 130 : 1319–1325. [PubMed] [Google Scholar]
- 24. Gardosi J, Francis A. Customised weight centile calculator. GROW Version 6.7, 2013. [Google Scholar]
- 25. ACOG Committee on Practice Bulletins – Obstetrics . ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. Obstet Gynecol 2002. ; 99 : 159–167. [DOI] [PubMed] [Google Scholar]
- 26. Brown MA, Lindheimer MD, de Swiet M, Van Assche A, Moutquin JM. The classification and diagnosis of the hypertensive disorders of pregnancy: statement from the International Society for the Study of Hypertension in Pregnancy (ISSHP). Hypertens Pregnancy 2001. ; 20 : IX–XIV. [DOI] [PubMed] [Google Scholar]
- 27. Knudsen UB, Kronborg CS, von Dadelszen P, Kupfer K, Lee SW, Vittinghus E, Allen JG, Redman CW. A single rapid point‐of‐care placental growth factor determination as an aid in the diagnosis of preeclampsia. Pregnancy Hypertens 2012. ; 2 : 8–15. [DOI] [PubMed] [Google Scholar]
- 28. Parra‐Cordero M, Lees C, Missfelder‐Lobos H, Seed P, Harris C. Fetal arterial and venous Doppler pulsatility index and time averaged velocity ranges. Prenat Diagn 2007. ; 27 : 1251–1257. [DOI] [PubMed] [Google Scholar]
- 29. Redline RW. Placental pathology: a systematic approach with clinical correlations. Placenta 2008. ; 29 : 86–91. [DOI] [PubMed] [Google Scholar]
- 30. Chaiworapongsa T, Romero R, Korzeniewski SJ, Kusanovic JP, Soto E, Lam J, Dong Z, Than NG, Yeo L, Hernandez‐Andrade E, Conde‐Agudelo A, Hassan SS. Maternal plasma concentrations of angiogenic/antiangiogenic factors in the third trimester of pregnancy to identify the patient at risk for stillbirth at or near term and severe late preeclampsia. Am J Obstet Gynecol 2013. ; 208 : 287.e1–e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Neilson JP. Symphysis‐fundal height measurement in pregnancy. Cochrane Database Syst Rev 2000. (2): CD000944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Alfirevic Z, Stampalija T, Gyte GM. Fetal and umbilical Doppler ultrasound in normal pregnancy. Cochrane Database Syst Rev 2010. (8): CD001450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gardosi J, Francis A. Controlled trial of fundal height measurement plotted on customised antenatal growth charts. Br J Obstet Gynaecol 1999. ; 106 : 309–317. [DOI] [PubMed] [Google Scholar]
- 34. Gardosi J, Chang A, Kalyan B, Sahota D, Symonds EM. Customised antenatal growth charts. Lancet 1992. ; 339 : 283–287. [DOI] [PubMed] [Google Scholar]
- 35. Odibo AO, Cahill AG, Odibo L, Roehl K, Macones GA. Prediction of intrauterine fetal death in small‐for gestational‐age fetuses: impact of including ultrasound biometry in customized models. Ultrasound Obstet Gynecol 2011. ; 39 : 288–292. [DOI] [PubMed] [Google Scholar]
- 36. Clausson B, Gardosi J, Francis A, Cnattingius S. Perinatal outcome in SGA births defined by customised versus population‐based birthweight standards. BJOG 2001. ; 108 : 830–834. [DOI] [PubMed] [Google Scholar]
- 37. Gardosi J, Giddings S, Clifford S, Wood L, Francis A. Association between reduced stillbirth rates in England and regional uptake of accreditation training in customised fetal growth assessment. BMJ Open 2013. ; 3 : e003942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Morris RK, Meller CH, Tamblyn J, Malin GM, Riley RD, Kilby MD, Robson SC, Khan KS. Association and prediction of amniotic fluid measurements for adverse pregnancy outcome: systematic review and meta‐analysis. BJOG 2014. ; 121 : 686–699. [DOI] [PubMed] [Google Scholar]
- 39. Morales‐Roselló J, Khalil A, Morlando M, Bhide A, Papageorghiou A, Thilaganathan B. Poor neonatal acid–base status in term fetuses with low cerebroplacental ratio. Ultrasound Obstet Gynecol 2015. ; 45: 156–161. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 STARD checklist for reporting of studies of diagnostic accuracy
Table S2 Diagnostic performance for placental growth factor (PlGF) and ultrasound parameters to predict small‐for‐gestational age (SGA) < 3rd centile when PlGF was sampled within 6 weeks of delivery (n = 267) in women with reduced symphysis–fundus height
Table S3 Maternal outcome in 16 women with very low placental growth factor levels (<12 pg/mL) at sampling


