Abstract
Cadherin is a cell adhesion molecule widely expressed in the nervous system. Previously, we analyzed the expression of nine classic cadherins (Cdh4, Cdh6, Cdh7, Cdh8, Cdh9, Cdh10, Cdh11, Cdh12, and Cdh20) and T‐cadherin (Cdh13) in the developing postnatal common marmoset (Callithrix jacchus) brain, and found differential expressions between mice and marmosets. In this study, to explore primate‐specific cadherin expression at the embryonic stage, we extensively analyzed the expression of these cadherins in the developing embryonic marmoset brain. Each cadherin showed differential spatial and temporal expression and exhibited temporally complicated expression. Furthermore, the expression of some cadherins differed from that in rodent brains, even at the embryonic stage. These results suggest the possibility that the differential expressions of diverse cadherins are involved in primate specific cortical development, from the prenatal to postnatal period.
Keywords: cadherin, embryo, gene expression, marmoset, neocortex
Introduction
The neocortex (NCX) consists of many functional areas, and is indispensable for multiple complex cognitive functions. It has been recently shown that various genes are involved in cortical area formation (Rakic 2009; Krubitzer & Seelke 2012; Sun & Hevner 2014). Mounting evidence has been presented for molecular mechanisms of rodent NCX development. However, it has been recently demonstrated that the primate NCX has various distinct features compared with the rodent NCX (Lui et al. 2011; LaMonica et al. 2012; Zeng et al. 2012; Miller et al. 2014; Dehay et al. 2015). In addition, how each cortical area is established in the primate NCX and how cortical areas differ among mammalian species remains to be determined.
Cadherins are transmembrane glycoproteins originally isolated as cell adhesion molecules (Takeichi 2007; Hirano & Takeichi 2012). Currently, more than 100 proteins belong to the cadherin superfamily. Cadherins are widely expressed in the nervous system, and play multiple roles in neural development and function through homo‐ or heterotypic interactions (Hirano & Takeichi 2012; Redies et al. 2012). Among the family members, type II cadherins show area‐specific expression in relation to neural circuits (Suzuki et al. 1997; Suzuki & Takeichi 2008). Gain and loss‐of‐function analyses have revealed that type II cadherins are involved in neural patterning, cell migration, axon guidance, synapse formation, and synapse function (Nakagawa & Takeichi 1998; Manabe et al. 2000; Inoue et al. 2001; Price et al. 2002; Treubert‐Zimmermann et al. 2002; Suzuki et al. 2007; Barnes et al. 2010; Matsunaga et al. 2011a,b; Osterhout et al. 2011; Williams et al. 2011; Kuwako et al. 2014). Type II cadherin expression is spatially and temporally regulated during development and is species‐specific (Hatta & Takeichi 1986; Nakagawa & Takeichi 1998; Price et al. 2002; Ju et al. 2004; Matsunaga & Okanoya 2008, 2011, 2014; Neudert et al. 2008; Takahashi & Osumi 2008; Etzrodt et al. 2009; Krishna‐K et al. 2009; Barnes et al. 2010; Mashiko et al. 2012; Matsunaga et al. 2013). Thus, the expression of diverse cadherins might have been involved in brain evolution and diversity.
The common marmoset (Callithrix jacchus) is a small new world monkey found in the forests of Brazil. The marmoset uses various vocal communications and is able to learn complex cognitive tasks (Pistorio et al. 2006; Yamazaki et al. 2011). Because marmosets are easy to breed in the laboratory facility, it is easier to obtain embryos and juveniles at various developmental stages compared with other primates. Furthermore, genetic manipulation technologies in marmosets have been developed (Sasaki et al. 2009; Kishi et al. 2014). Thus, the marmoset is an emerging animal model for studying primate‐specific neural development and cognitive disorders.
Previously, we used the common marmoset as a model of primate NCX development, and performed in situ hybridization to analyze the expression of cadherins in the postnatal NCX and perinatal visual NCX (Matsunaga et al. 2013, 2014). We found that the expression of various cadherins differed between rodents and primates and was dynamically regulated, and in some cases, was related to the formation of primate‐specific visual circuits (Matsunaga et al. 2013, 2014). Here, to explore whether primate specific expression of cadherins is seen even in the embryonic stage, we performed a more extensive spatial and temporal analysis of cadherin expressions in the developing embryonic NCX.
Material and methods
Ethics
Research protocols were approved by the Animal Care and Use Committee of RIKEN and conform to the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Animals and sample preparation
Parental marmosets were purchased from the Research Resource Center (RRC) of RIKEN, and kept on a 12–12 h light–dark cycle, at 27°C with 50% humidity; they had ad libitum access to water and a standard marmoset diet (CMS‐1; Clea, Tokyo, Japan) with supplements. The dam was anesthetized by ketamine (15 mg/kg), followed by isoflurane, and embryonic marmosets were collected by cesarean section. After fixation in phosphate buffered saline (PBS, pH 7.4) with 4% paraformaldehyde, embryos were dissected and the brains were immersed in PBS with 20% sucrose. Brains were embedded in Tissue‐Tek optimal cutting temperature compound (Sakura Fine Technical, Tokyo, Japan) and frozen on dry ice. Serial sections (10–16 μm) were cut using a cryostat (Leica Microsystems, Wetzlar, Germany). Sections were used for either in situ hybridization or stained with thionine, for neuroanatomical reference. We used 2 gestational week (GW)10, 3 GW12, 2 gestational month (GM) 3.5 (around GW15), and 4 GM4 (around GW16 or 17) embryos of either sex. The stage of the embryos were estimated by the morphology of the forelimb or brain according to the reference (Hikishima et al. 2013).
In situ hybridization
The probes and procedure of in situ hybridization were as described previously (Matsunaga et al. 2013). Accordingly, we used the sense probe for Cdh10 as control, because longer probes induce stronger background signals in general and the probe length is the largest among the analyzed cadherins (we could not find any clear signals above the background staining with all cadherin sense probes.)
Results
We performed in situ hybridization analyses of nine classic cadherins (Cdh4, Cdh6, Cdh7, Cdh8, Cdh9, Cdh10, Cdh11, Cdh12, and Cdh20) and T‐cadherin (Cdh13) in the marmoset NCX from GW10 to GM4 (Figs 1, 2, 3, 4, 5, 6).
Figure 1.
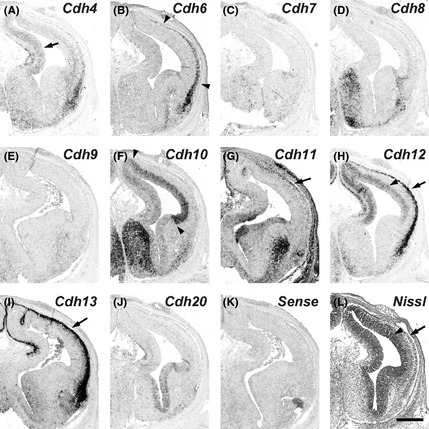
Expression patterns of cadherins in the gestational week 10 marmoset neocortex (NCX). In situ hybridization for cadherins on serial transverse sections of the GW10 marmoset brain. Cdh4 expression in the ventricular zone (VZ) (A, arrow). Cdh6 expression in the cortical plate (CP) (B, arrowheads). Cdh10 expression in the VZ (F, arrowheads). Cdh11 expression in the CP (G, arrow). Cdh12 expression in the VZ and CP (H, arrowhead and arrow, respectively). Cdh13 expression in the CP (I, arrow). The VZ and CP in a Nissl stained section (L, arrowhead and arrow, respectively). Scale bar is 500 μm (L).
Figure 2.
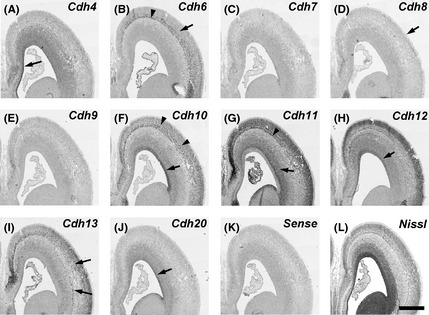
In situ hybridization for cadherins on serial transverse sections of the anterior part of gestational week 12 marmoset neocortex (NCX). Expression patterns of cadherins in the ventricular zone (VZ) (A, F, H, J, arrows) and subventricular zone (SVZ) (G, arrow). Expression of cadherins in the subplate (SP) (B, G, arrowhead). Expression of cadherinsin the cortical plate (CP) (B, arrow; F, arrowheads). No clear Cdh8 expression in the CP (D, arrow). Sparsely distributed cadherin‐expressing cells (I, arrows). Scale bar is 1 mm (L).
Figure 3.
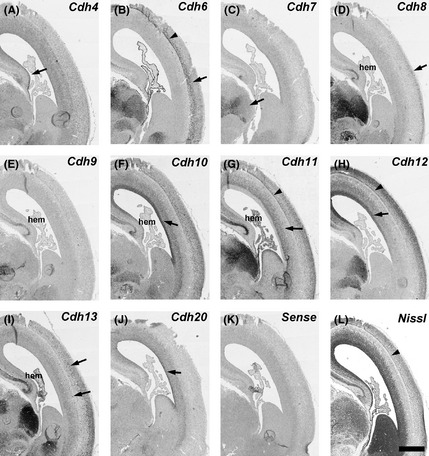
In situ hybridization for cadherins on serial transverse sections of the middle part of gestational week 12 marmoset neocortex (NCX). Expression patterns of cadherins in the ventricular zone (VZ) (A, F, H, J, arrows), subventricular zone (SVZ) (G, arrow), subplate (SP) (B, G, H, arrowheads), and cortical plate (CP) (B, D, arrows). Cdh7 expression in the thalamus (C). Cdh13‐expressing cells in the SVZ and CP (I, arrows). SP in a Nissl stained section (L, arrowhead). Scale bar is 1 mm (L).
Figure 4.
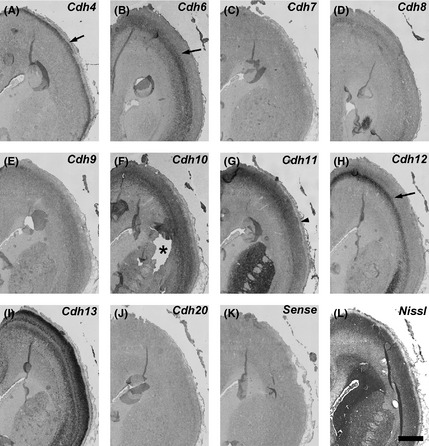
Expression patterns of cadherinsin the anterior part of gestational month 3.5 marmoset neocortex (NCX). Cadherin expressions in the upper layer of cortical plate (CP) (A, arrow) and deep layer of CP (B, H, arrows). The asterisk indicates the disintegrating part of the section (F). Strong Cdh11 expression in the dorsal NCX(G, arrowhead). Cdh12 shows a dorsal‐high and ventral‐low graded expression (H, arrow). Scale bar is 1 mm (L).
Figure 5.
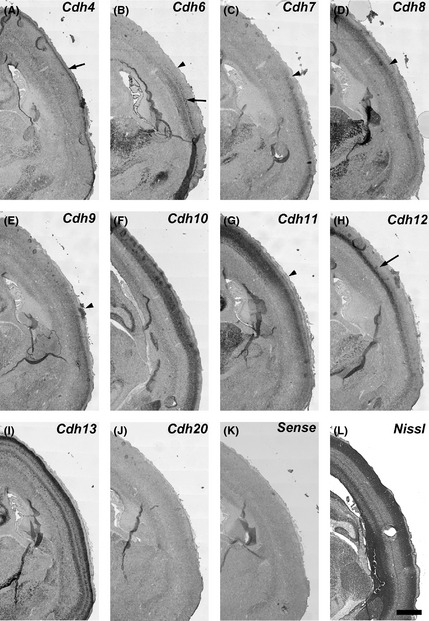
Expression patterns of cadherins in the middle part of gestational month 3.5 marmoset neocortex (NCX). Cdh4 expression in the upper layer of CP (A, arrow). Cdh6 expression in the deep layer of the CP (arrow); Cdh6 is strongly expressed in the ventral part of the CP (temporal cortex) (B). Cdh7 and Cdh9 expression in the ventral part of the CP (C, E). Cdh8 shows a complementary expression to Cdh6, Cdh7, and Cdh9 (B–E, arrowheads). Cdh11 expression in the middle and deep layer is strongly seen in the CP of the dorsal NCX (G, arrowhead). Cdh12 shows a dorsal‐high and ventral‐low graded expression in the deep layer (H, arrow). Scale bar is 1 mm (L).
Figure 6.
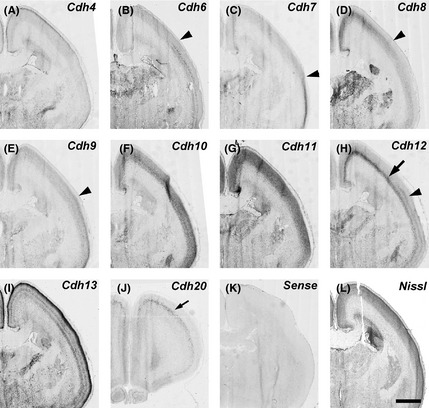
Expression patterns of cadherins in the middle part of gestational month 4 marmoset neocortex (NCX). Complementary expressions of Cdh6, Cdh7, Cdh9, and Cdh8 (B–E, arrowheads). Cdh12 shows a dorsal‐high and ventral‐low expression in the deep CP (arrow) and a ventral‐high expression in the middle CP (arrowhead) (H). Cdh20 expression in the anterior NCX (J, arrow). Scale bar is 2 mm (L).
Expression of each cadherin in the NCX
Cdh4
Cdh4 expression was weakly detected in the ventricular zone (VZ) of the NCX at GW10 (Fig. 1A). Its expression was strongly observed in the medial NCX, but faintly in the lateral NCX at GW10–12 (Figs 1A, 2A, 3A, arrows). By GM3.5 (around GW15), strong Cdh4 expression was broadly seen in the upper layer of the NCX (Figs 4A, 5A, arrows). The strong expression in the upper layer continued into the neonatal stage (Fig. 6A; Matsunaga et al. 2013).
Cdh6
Cdh6 expression was not seen in the VZ, but seen in the cortical plate (CP) at GW10 (Fig. 1B, arrowheads). At GW12 (GM3), Cdh6 expression was seen in the subplate (SP) and the deep layer of CP of the NCX (Figs 2B, 3B, arrowhead and arrow, respectively). At GM3.5, Cdh6 expression was seen in the deep layer of NCX (Figs 4B, 5B, arrow). Clear Cdh6 expression was seen in the deep layer of the temporal NCX (Fig. 5B, arrow). Around GM4, Cdh6 expression started to be seen in the middle layer of some brain areas such as the middle temporal visual area (MT) (data not shown; Matsunaga et al. 2014).
Cdh7
No clear Cdh7 expression in the NCX was seen at GW10–12 (Figs 1C, 2C, 3C), although its expression was seen in the thalamus (Fig. 3C, arrow). By GM3.5, Cdh7 expression started to be seen in the temporal NCX (Fig. 5C, arrowhead), although its expression was area‐specific (Figs 4C, 5C). By GM4, Cdh7 expression was visible in the motor cortex in addition to the temporal cortex (data not shown).
Cdh8
Clear Cdh8 expression in the NCX was first seen in the CP at GW12 (Fig. 3D). Its expression was area‐specific (Figs 2D, 3D, arrow). At GM3.5, Cdh8 expression was seen in the middle layer of the dorsal NCX (Fig. 5D, arrowhead) with a complementary expression to Cdh6 and Cdh7 (Fig. 5B, C, arrowheads). However, only faint expression was seen in the anterior NCX (Fig. 4D). By GM4, strong Cdh8 expression was also seen in the ventral region of the anterior NCX (data not shown).
Cdh9
Similar to Cdh7, no clear Cdh9 expression was seen at GW10 (Fig. 1E). At GW12, Cdh9 expression was still lacking in the NCX (Figs 2E, 3E). At GM3.5, Cdh9 expression was detected in the temporal area (Fig. 5E, arrow) but not in other cortical areas (Fig. 4E), similar to Cdh7. However, in contrast to Cdh7, by the neonatal stage, Cdh9 expression was broadly seen in the NCX (Matsunaga et al. 2013).
Cdh10
Cdh10 was strongly expressed in the VZ of the lateral NCX and weakly expressed in the medial NCX at GW10 (Fig. 1F, arrowheads). Its expression in the VZ was maintained at GW12 (Figs 2F, 3F, arrows). Cdh10 was also sparsely expressed in the deep layer of the CP (Figs 2F, 3F). By GM3.5, Cdh10 expression was seen from the upper to the deep layer of NCX (Figs 4F, 5F). Its expression continued into the neonatal stage (Fig. 6F; Matsunaga et al. 2013).
Cdh11
Sparse Cdh11 expression was seen in the CP at GW10 (Fig. 1G, arrow). At GW12, Cdh11 expression was widely seen in the subventricular zone (SVZ) and CP (Fig. 2G). Clear Cdh11 expression was seen in the SVZ (arrow), SP (arrowhead), and CP (Figs 2G, 3G). At GM3.5, Cdh11 was broadly expressed both in the upper and deep layer of the dorsal NCX, but only in the upper layer of the ventral NCX (Figs 4G, 5G, arrowhead). By GM4, Cdh11 expression was broadly seen in both the upper and deep layer of the NCX (Fig. 6G).
Cdh12
Expression of Cdh12 was detected in the VZ of NCX at GW10 (Fig. 1H, arrowhead). Clear Cdh12 expression was also seen in the CP (Fig. 1H, arrow). At GW12, Cdh12 expression was seen in the VZ (arrow) and CP including the SP (arrowhead) (Figs 2H, 3H). At GM3.5, Cdh12 showed a graded expression in the deep layer of NCX in a dorsal‐high and ventral‐low manner (Figs 4H, 5H, arrows). At GM4, Cdh12 expression in the deep layer was maintained but its expression in the middle layer started to appear from the ventral part of the NCX (Fig. 6H). The Cdh12 expression in the middle layer (presumptive layer IV) was seen in the temporal or occipital NCX, as previously described (Matsunaga et al. 2014), but not in the parietal NCX. However, by the neonatal stage, Cdh12 expression in layer IV became clear in the parietal NCX (Matsunaga et al. 2013).
Cdh13
Cdh13 was strongly expressed in the CP at GW10 (Fig. 1I, arrow). At GW12, Cdh13 expressing cells were sparsely distributed in the SVZ and CP (Figs 2I, 3I, arrows). By GM3.5, Cdh13 was expressed from the upper to the deep layer in the neonatal brain (Figs 4I, 5I; Matsunaga et al. 2013).
Cdh20
No clear Cdh20 expression was detected in the NCX at GW10 (Fig. 1J). By GW12, Cdh20 expression was seen in the VZ and SVZ (Fig. 3J, arrow). At GM3.5, Cdh20 expression in the CP was seen only in a small part of the anterior NCX (data not shown). By GM4, Cdh20 expression was evident in the deep layer (the presumptive layer V) of the anterior NCX (Fig. 6J). Its expression in other areas was evident by the neonatal stage (Matsunaga et al. 2013).
Cadherin expression in the hippocampal area
By GW12, clear Cdh4, Cdh8, Cdh9, Cdh10, Cdh11, and Cdh13 expression was detected in the cortical hem, the presumptive area of the hippocampus (Fig. 3A,D,E,F,G,I). Weak Cdh12 and Cdh20 expression was also visible by this stage (Fig. 3H,J).
Discussion
In the present study, we analyzed the expression of 10 cadherins in the developing embryonic NCX and found complex and diverse cadherin expression patterns. The expression patterns are summarized in Figure 7.
Figure 7.
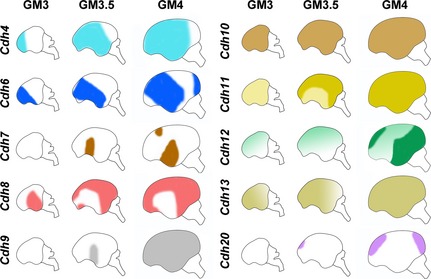
Schematic representation of expression patterns of cadherins in the developing cortical plate of embryonic marmosets at gestational month (GM)3, GM3.5, and GM4. Cdh11 expression in the upper layer was broadly seen in the whole neocortex (NCX) at GM3 (light yellow), whereas Cdh11 expression in the deep CP is seen in the dorsal NCX at GM3.5 (dark yellow). Cdh12 expression in the deep CP shows a dorsal‐high and ventral low graded expression (light green) whereas Cdh12 expression in the middle CP shows a strong expression in the ventroposterior NCX (dark green).
Complex regulation of various cadherins in embryonic marmosets
Cadherin expression patterns are divided into at least four types (Fig. 7): (i) expression commencing from anterior to posterior (Cdh4, Cdh13, Cdh20), (ii) starting from dorsal to ventral (Cdh11), (iii) initiating from middle ventral region (temporal cortex) (Cdh7, Cdh9), and (iv) complicated regulation of expression (others). For example, Cdh12 expression in the deep layer starts from the dorsal NCX by GW10, whereas Cdh12 expression in the middle layer starts from the ventral region at around GM4. It appears that there are at least two mechanisms controlling Cdh12 expression. First, expression in the deep layer may expand from dorsal to ventral by the signal from the cortical hem. Second, expression in the middle layer may expand from ventral to dorsal by the signal form the anti‐hem. It has been suggested that some neurons migrate tangentially, such as the GABAergic neurons and some populations of subplate neurons from the ventral region outside the NCX (Hoerder‐Suabedissen & Molnár 2013; Kessaris et al. 2014). Further analysis involving identification of individual cadherin‐expressing cells with various cell‐type specific markers is needed to examine whether Cdh12‐expressing cells in the deep layer and middle layer are derived from the same origin.
It has been suggested that cortical areas are formed by the combination of various signals from the anterior neural ridge (ANR), cortical hem, and anti‐hem (Alfano & Studer 2013). Interestingly, expression of Cdh7 and Cdh9 started to be seen in the middle part of the NCX, an area surrounding the lateral sulcus (LS), noticeable in the primate brain. It is possible that a signal center exists in the presumptive area of LS, which induces temporal area‐specific expression of cadherins.
Species differences in cadherin expression
Previously, we examined expression of various cadherins in the postnatal marmoset cerebral cortex and found differences in their expression between mouse and marmoset NCX. In this study, we found distinct expression of cadherins already in the embryonic stage. For example, expression of cadherins in the VZ differed between the mouse and marmoset NCX. In contrast to previously reported expression of cadherins in the mouse NCX (Inoue et al. 1998; Lefkovics et al. 2012), no clear Cdh6, Cdh7, and Cdh9 expression was seen in the marmoset VZ. In the rodent brain, both Cdh7 and Cdh9 expression was already broadly seen in the NCX before the start of corticogenesis (Takahashi & Osumi 2008; Lefkovics et al. 2012). However, in the marmoset brain, Cdh7 and Cdh9 expression started only after the initiation of NCX differentiation and that too in a limited area. Such differential expression of cadherins may result in formation of distinct brain structures and function in rodents and primates.
Some type II cadherins share homologous sequences (Fig. S1), and it has been demonstrated that type II cadherins are capable of heterotypic binding and show functional redundancy among some family members (Shimoyama et al. 2000; Lefkovics et al. 2012). Therefore, a lack of specific expression of cadherins in the embryonic stage may be rescued by other cadherin expressions, and therefore, differential cadherin expressions in the embryonic stage may only have subtle effects on brain development. However, since expression of cadherins in the marmoset NCX tend to segregate during postnatal development (Matsunaga et al. 2013), expression of diverse cadherins may gradually cause differences in neural connections and electrophysiological properties during the later developmental stages, for example, the formation of intracontico‐cortical connections, that is highly evolved in the primate NCX.
Supporting information
Fig. S1. Phylogenetic tree of mouse (m) or marmoset (cj) coding sequence of each cadherin gene.
Acknowledgments
We thank Dr Kimie Niimi (RIKEN BSI RRC) for marmoset cesarean section. This research was supported by RIKEN Incentive Research Projects, JSPS KAKENHI Grant number 25750403 (E.M.), and the Brain Mapping by Integrated Neurotechnologies for Disease Studies (Brain/MINDS) by the Ministry of Education, Culture, Sports, Science and Technology of Japan (to A.I.).
References
- Alfano, C. & Studer, M. 2013. Neocortical arealization: evolution, mechanisms, and open questions. Dev. Neurobiol. 73, 411–447. [DOI] [PubMed] [Google Scholar]
- Barnes, S. H. , Price, S. R. , Wentzel, C. & Guthrie, S. C. 2010. Cadherin‐7 and cadherin‐6B differentially regulate the growth, branching and guidance of cranial motor axons. Development 137, 805–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehay, C. , Kennedy, H. , Kosik, K. S. , Lyon, I. & Lyon, D. 2015. Review the outer subventricular zone and primate‐specific cortical complexification. Neuron 85, 683–694. [DOI] [PubMed] [Google Scholar]
- Etzrodt, J. , Krishna‐K, K. & Redies, C. 2009. Expression of classic cadherins and delta‐protocadherins in the developing ferret retina. BMC Neurosci. 10, 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatta, K. & Takeichi, M. 1986. Expression of N‐cadherin adhesion molecules associated with early morphogenetic events in chick development. Nature 320, 447–449. [DOI] [PubMed] [Google Scholar]
- Hikishima, K. , Sawada, K. , Murayama, a. Y. , Komaki, Y. , Kawai, K. , Sato, N. , Inoue, T. , Itoh, T. , Momoshima, S. , Iriki, A. , Okano, H. J. , Sasaki, E. & Okano, H. 2013. Atlas of the developing brain of the marmoset monkey constructed using magnetic resonance histology. Neuroscience 230, 102–113. [DOI] [PubMed] [Google Scholar]
- Hirano, S. & Takeichi, M. 2012. Cadherins in brain morphogenesis and wiring. Physiol. Rev. 92, 597–634. [DOI] [PubMed] [Google Scholar]
- Hoerder‐Suabedissen, A. & Molnár, Z. 2013. Molecular diversity of early‐born subplate neurons. Cereb. Cortex 23, 1473–1483. [DOI] [PubMed] [Google Scholar]
- Inoue, T. , Tanaka, T. , Suzuki, S. C. & Takeichi, M. 1998. Cadherin‐6 in the developing mouse brain: expression along restricted connection systems and synaptic localization suggest a potential role in neuronal circuitry. Dev. Dyn. 211, 338–351. [DOI] [PubMed] [Google Scholar]
- Inoue, T. , Tanaka, T. , Takeichi, M. , Chisaka, O. , Nakamura, S. & Osumi, N. 2001. Role of cadherins in maintaining the compartment boundary between the cortex and striatum during development. Development 128, 561–569. [DOI] [PubMed] [Google Scholar]
- Ju, M. J. , Aroca, P. , Luo, J. , Puelles, L. & Redies, C. 2004. Molecular profiling indicates avian branchiomotor nuclei invade the hindbrain alar plate. Neuroscience 128, 785–796. [DOI] [PubMed] [Google Scholar]
- Kessaris, N. , Magno, L. , Rubin, A. N. & Oliveira, M. G. 2014. Genetic programs controlling cortical interneuron fate. Curr. Opin. Neurobiol. 26, 79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi, N. , Sato, K. , Sasaki, E. & Okano, H. 2014. Common marmoset as a new model animal for neuroscience research and genome editing technology. Dev. Growth Differ. 56, 53–62. [DOI] [PubMed] [Google Scholar]
- Krishna‐K, K. , Nuernberger, M. , Weth, F. & Redies, C. 2009. Layer‐specific expression of multiple cadherins in the developing visual cortex (V1) of the ferret. Cereb. Cortex 19, 388–401. [DOI] [PubMed] [Google Scholar]
- Krubitzer, L. A. & Seelke, A. M. H. 2012. Cortical evolution in mammals: the bane and beauty of phenotypic variability. Proc. Natl Acad. Sci. USA 109(Suppl), 10647–10654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwako, K. , Nishimoto, Y. , Kawase, S. , Okano, H. J. & Okano, H. 2014. Cadherin‐7 regulates mossy fiber connectivity in the cerebellum. Cell Rep. 9, 311–323. [DOI] [PubMed] [Google Scholar]
- LaMonica, B. E. , Lui, J. H. , Wang, X. & Kriegstein, A. R. 2012. OSVZ progenitors in the human cortex: an updated perspective on neurodevelopmental disease. Curr. Opin. Neurobiol. 22, 747–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefkovics, K. , Mayer, M. , Bercsényi, K. , Szabó, G. & Lele, Z. 2012. Comparative analysis of type II classic cadherin mRNA distribution patterns in the developing and adult mouse somatosensory cortex and hippocampus suggests significant functional redundancy. J. Comp. Neurol. 520, 1387–1405. [DOI] [PubMed] [Google Scholar]
- Lui, J. H. , Hansen, D. V. & Kriegstein, A. R. 2011. Development and evolution of the human neocortex. Cell 146, 18–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manabe, T. , Togashi, H. , Uchida, N. , Suzuki, S. C. , Hayakawa, Y. , Yamamoto, M. , Yoda, H. , Miyakawa, T. , Takeichi, M. & Chisaka, O. 2000. Loss of cadherin‐11 adhesion receptor enhances plastic changes in hippocampal synapses and modifies behavioral responses. Mol. Cell Neurosci. 15, 534–546. [DOI] [PubMed] [Google Scholar]
- Mashiko, H. , Yoshida, A. C. , Kikuchi, S. S. , Niimi, K. , Takahashi, E. , Aruga, J. , Okano, H. & Shimogori, T. 2012. Comparative anatomy of marmoset and mouse cortex from genomic expression. J. Neurosci. 32, 5039–5053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunaga, E. & Okanoya, K. 2008. Expression analysis of cadherins in the songbird brain: relationship to vocal system development. J. Comp. Neurol. 508, 329–342. [DOI] [PubMed] [Google Scholar]
- Matsunaga, E. & Okanoya, K. 2011. Comparative gene expression analysis among vocal learners (bengalese finch and budgerigar) and non‐learners (quail and ring dove) reveals variable cadherin expressions in the vocal system. Front. Neuroanat. 5, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunaga, E. & Okanoya, K. 2014. Cadherins: potential regulators in the faculty of language. Curr. Opin. Neurobiol. 28, 28–33. [DOI] [PubMed] [Google Scholar]
- Matsunaga, E. , Kurotani, T. , Suzuki, K. & Okanoya, K. 2011a. Type‐II cadherins modulate neural activity in cultured rat hippocampal neurons. NeuroReport 22, 629–632. [DOI] [PubMed] [Google Scholar]
- Matsunaga, E. , Suzuki, K. , Kato, S. , Kurotani, T. , Kobayashi, K. & Okanoya, K. 2011b. Dynamic expression of cadherins regulates vocal development in a songbird. PLoS ONE 6, e25272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunaga, E. , Nambu, S. , Oka, M. & Iriki, A. 2013. Differential cadherin expression in the developing postnatal telencephalon of a new world monkey. J. Comp. Neurol. 521, 4027–4060. [DOI] [PubMed] [Google Scholar]
- Matsunaga, E. , Nambu, S. , Oka, M. & Iriki, A. 2014. Complementary and dynamic type II cadherin expression associated with development of the primate visual system. Dev. Growth Differ. 56, 535–543. [DOI] [PubMed] [Google Scholar]
- Miller, J. A. , Ding, S.‐L. , Sunkin, S. M. , Smith, K. A. , Ng, L. , Szafer, A. , Ebbert, A. , Riley, Z. L. , Royall, J. J. , Aiona, K. , Arnold, J. M. , Bennet, C. , Bertagnolli, D. , Brouner, K. , Butler, S. , Caldejon, S. , Carey, A. , Cuhaciyan, C. , Dalley, R. A. , Dee, N. , Dolbeare, T. A. , Facer, B. A. C. , Feng, D. , Fliss, T. P. , Gee, G. , Goldy, J. , Gourley, L. , Gregor, B. W. , Gu, G. , Howard, R. E. , Jochim, J. M. , Kuan, C. L. , Lau, C. , Lee, C.‐K. , Lee, F. , Lemon, T. A. , Lesnar, P. , McMurray, B. , Mastan, N. , Mosqueda, N. , Naluai‐Cecchini, T. , Ngo, N.‐K. , Nyhus, J. , Oldre, A. , Olson, E. , Parente, J. , Parker, P. D. , Parry, S. E. , Stevens, A. , Pletikos, M. , Reding, M. , Roll, K. , Sandman, D. , Sarreal, M. , Shapouri, S. , Shapovalova, N. V. , Shen, E. H. , Sjoquist, N. , Slaughterbeck, C. R. , Smith, M. , Sodt, A. J. , Williams, D. , Zöllei, L. , Fischl, B. , Gerstein, M. B. , Geschwind, D. H. , Glass, I. A. , Hawrylycz, M. J. , Hevner, R. F. , Huang, H. , Jones, A. R. , Knowles, J. A. , Levitt, P. , Phillips, J. W. , Sestan, N. , Wohnoutka, P. , Dang, C. , Bernard, A. , Hohmann, J. G. & Lein, E. S. 2014. Transcriptional landscape of the prenatal human brain. Nature 508, 199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa, S. & Takeichi, M. 1998. Neural crest emigration from the neural tube depends on regulated cadherin expression. Development 125, 2963–2971. [DOI] [PubMed] [Google Scholar]
- Neudert, F. , Krishna, K. , Neurnberger, M. & Redies, C. 2008. Comparative analysis of cadherin expression and connectivity patterns in the cerebellar system of ferret and mouse. J. Comp. Neurol. 511, 736–752. [DOI] [PubMed] [Google Scholar]
- Osterhout, J. A. , Josten, N. , Yamada, J. , Pan, F. , Wu, S. , Nguyen, P. L. , Panagiotakos, G. , Inoue, Y. U. , Egusa, S. F. , Volgyi, B. , Inoue, T. , Bloomfield, S. A. , Barres, B. A. , Berson, D. M. , Feldheim, D. A. & Huberman, A. D. 2011. Cadherin‐6 mediates axon‐target matching in a non‐image‐forming visual circuit. Neuron 71, 632–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pistorio, A. L. , Vintch, B. & Wang, X. 2006. Acoustic analysis of vocal development in a New World primate, the common marmoset (Callithrix jacchus). J. Acoust. Soc. Am. 120, 1655–1670. [DOI] [PubMed] [Google Scholar]
- Price, S. R. , De Marco Garcia, N. V. , Ranscht, B. & Jessell, T. M. 2002. Regulation of motor neuron pool sorting by differential expression of type II cadherins. Cell 109, 205–216. [DOI] [PubMed] [Google Scholar]
- Rakic, P. 2009. Evolution of the neocortex: a perspective from developmental biology. Nat. Rev. Neurosci. 10, 724–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redies, C. , Hertel, N. & Hübner, C. A. 2012. Cadherins and neuropsychiatric disorders. Brain Res. 1470, 130–144. [DOI] [PubMed] [Google Scholar]
- Sasaki, E. , Suemizu, H. , Shimada, A. , Hanazawa, K. , Oiwa, R. , Kamioka, M. , Tomioka, I. , Sotomaru, Y. , Hirakawa, R. , Eto, T. , Shiozawa, S. , Maeda, T. , Ito, M. , Ito, R. , Kito, C. , Yagihashi, C. , Kawai, K. , Miyoshi, H. , Tanioka, Y. , Tamaoki, N. , Habu, S. , Okano, H. & Nomura, T. 2009. Generation of transgenic non‐human primates with germline transmission. Nature 459, 523–527. [DOI] [PubMed] [Google Scholar]
- Shimoyama, Y. , Tsujimoto, G. , Kitajima, M. & Natri, M. 2000. Identification of three human type‐II classic cadherins and frequent heterophilic interactions between different subclasses of type‐II classic cadherins. Biochem. J. 167, 159–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, T. & Hevner, R. F. 2014. Growth and folding of the mammalian cerebral cortex: from molecules to malformations. Nat. Rev. Neurosci. 15, 217–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki, S. C. & Takeichi, M. 2008. Cadherins in neuronal morphogenesis and function. Dev. Growth Differ. 50, S119–S130. [DOI] [PubMed] [Google Scholar]
- Suzuki, S. C. , Inoue, T. , Kimura, Y. , Tanaka, T. & Takeichi, M. 1997. Neuronal circuits are subdivided by differential expression of type‐II classic cadherins in postnatal mouse brains. Mol. Cell Neurosci. 447, 433–447. [DOI] [PubMed] [Google Scholar]
- Suzuki, S. C. , Furue, H. , Koga, K. , Jiang, N. , Nohmi, M. , Shimazaki, Y. , Katoh‐Fukui, Y. , Yokoyama, M. , Yoshimura, M. & Takeichi, M. 2007. Cadherin‐8 is required for the first relay synapses to receive functional inputs from primary sensory afferents for cold sensation. J. Neurosci. 27, 3466–3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, M. & Osumi, N. 2008. Expression study of cadherin7 and cadherin20 in the embryonic and adult rat central nervous system. BMC Dev. Biol. 8, 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeichi, M. 2007. The cadherin superfamily in neuronal connections and interactions. Nat. Rev. Neurosci. 8, 11–20. [DOI] [PubMed] [Google Scholar]
- Treubert‐Zimmermann, U. , Heyers, D. & Redies, C. 2002. Targeting axons to specific fiber tracts in vivo by altering cadherin expression. J. Neurosci. 22, 7617–7626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, M. E. , Wilke, S. A. , Daggett, A. , Davis, E. , Otto, S. , Ravi, D. , Ripley, B. , Bushong, E. A. , Ellisman, M. H. , Klein, G. & Ghosh, A. 2011. Cadherin‐9 regulates synapse‐specific differentiation in the developing hippocampus. Neuron 71, 640–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki, Y. , Echigo, C. , Saiki, M. , Inada, M. , Watanabe, S. & Iriki, A. 2011. Tool‐use learning by common marmosets (Callithrix jacchus). Exp. Brain Res. 213, 63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng, H. , Shen, E. H. , Hohmann, J. G. , Oh, S. W. , Bernard, A. , Royall, J. J. , Glattfelder, K. J. , Sunkin, S. M. , Morris, J. A. , Guillozet‐Bongaarts, A. L. , Smith, K. A. , Ebbert, A. J. , Swanson, B. , Kuan, L. , Page, D. T. , Overly, C. C. , Lein, E. S. , Hawrylycz, M. J. , Hof, P. R. , Hyde, T. M. , Kleinman, J. E. & Jones, A. R. 2012. Large‐scale cellular‐resolution gene profiling in human neocortex reveals species‐specific molecular signatures. Cell 149, 483–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Phylogenetic tree of mouse (m) or marmoset (cj) coding sequence of each cadherin gene.


