Abstract
The pike Esox lucius is a large, long‐lived, iteroparous, top‐ predator fish species with a circumpolar distribution that occupies a broad range of aquatic environments. This study reports on a literature search and demonstrates that the publication rate of E. lucius research increases both in absolute terms and relative to total scientific output, and that the focus of investigation has changed over time from being dominated by studies on physiology and disease to being gradually replaced by studies on ecology and evolution. Esox lucius can be exploited as a model in future research for identifying causes and consequences of phenotypic and genetic variation at the levels of individuals, populations and species as well as for investigating community processes.
Keywords: adaptation, behaviour, Esocidae, evolution, fish, genetics
Research in ecology and evolution seeks to understand the causes and consequences of phenotypic and genetic biodiversity at the levels of individuals, populations or species. The usefulness of model species in providing insight into these issues is indisputable (Magurran, 2005; Merilä, 2013; Zuk et al., 2014), but little is known about the degree to which results and conclusions may be extrapolated from a given model to a wider spectrum of animals and environments. There are >25 000 species of teleosts, amounting to nearly half the extant vertebrate species (Nelson, 1994). This diversity can be exploited to gain further insights into how organisms cope with environmental challenges. To understand the complexity of ecology and evolution, it is crucial to combine several models, types of environments, investigations and experimental settings (Amundsen, 2003; Cossins & Crawford, 2005; Merilä, 2013; Schartl, 2014; Zuk et al., 2014). The Atlantic salmon Salmo salar L. 1758, the three‐spined stickleback Gasterosteus aculeatus L. 1758 and the guppy Poecilia reticulata Peters 1859 are examples of complementary fish model organisms that have gained their positions for partly different reasons. Do the features of pike Esox lucius L. 1758 make this non‐mainstream species a valuable addition to the set of already established fish models for studies of ecology and evolution?
Esox lucius is an iteroparous, large‐bodied (<130 cm) and long‐lived (>10 years), fish species that occupies eutrophic and oligotrophic lakes, rivers and brackish waters, it has been introduced to areas outside of its native range and it has a circumpolar distribution that spans c. 24° in latitude from northern Italy in the south to Murmansk in northern Russia (Craig, 1996, 2008; Larsson et al., 2015). It is a keystone predator that can exert top–down influence on fish communities (Craig, 1996). In part because of its size, wide distribution, occupancy of waters in urban areas and locally high abundance, E. lucius is important for recreational and commercial fishing (Pierce et al., 1995; Arlinghaus & Mehner, 2004; Lehtonen et al., 2009). This study reports on a literature search and publication trend analyses to assess the role of E. lucius as a model organism in ecology and evolutionary biology.
A topic search for ‘Esox lucius’ was conducted on 25 November 2014 using the ISI Web of Knowledge [Science Citation Index Expanded (1945 to present)]. The search generated 1684 studies. Each study was assigned to one of the six periods (published before 1970, 1970–1979, 1980–1989, 1990–1999, 2000–2009 and 2010–2014). Journals were assigned to one of the four categories: high ranked (with journal effect factor >5) and broad in scope; general ecology and evolution journals; organism (i.e. fish) or environment specific; miscellaneous (specializing in toxicology, physiology, development or chemistry).
Articles were classified with regard to focus of investigation based on title and assigned to one of the five sub‐disciplines: ecology and evolution (including life history, population dynamics and genetics); behaviour (including foraging and movement patterns); community ecology (including effects on species composition, energy flow, food webs and trophic relationships); physiology and disease; toxicology. To test whether scientific effect of published E. lucius articles depends on type of journal, total number of citations per article and number of citations per article per year were compared among journal categories.
Results uncovered that annual research output on E. lucius remained relatively low until the 1980s, but increased steadily thereafter, both in absolute terms (approaching 100 papers per year) and relative to the total research output summed across all scientific disciplines [Fig. 1(a), (b)].
Figure 1.
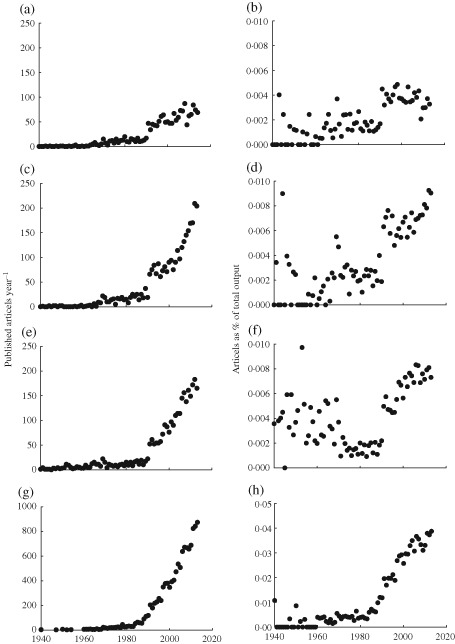
Trends in (a, c, e, g) absolute (number of papers published per year) and (b, d, f, h) relative research output (expressed as percentage of total research output summed across all scientific disciplines) for (a, b) Esox lucius, (c, d) Gasterosteus aculeatus, (e, f) Poecilia reticulata, formerly Lebistes reticulatus and (g, h) Salmo salar. The vertical axis for S. salar differs from that of the other three species. Data extracted from a topic search for each genus species conducted on 25 November 2014 from ISI Web of Science.
The focus of investigation in E. lucius studies has changed over time [Fig. 2(a)]. When pooled across all years, most studies concerned physiology and disease (34%), followed by ecology and evolution (31%), behaviour (16%), toxicology (11%) and community ecology (8%). While studies of physiology and disease dominated during the first half of the 20th century, these have declined since the 1970s. Conversely, studies on population ecology, evolution and behavioural ecology have increased and now dominate research on E. lucius. Studies on community ecology are less common but have increased since the 1990s. The proportion of toxicology studies has remained relatively stable [Fig. 2(a)].
Figure 2.
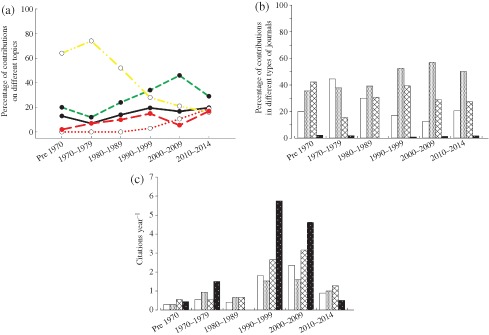
(a) Temporal shifts in focus of Esox lucius studies for five sub‐disciplines: ecology and evolution ( ), behaviour (
), behaviour ( ), community ecology (
), community ecology ( ), physiology and disease (
), physiology and disease ( ) and toxicology (
) and toxicology ( ). (b) The relative frequency distribution of papers that report on studies of E. lucius across four categories of scientific journals: miscellaneous (
). (b) The relative frequency distribution of papers that report on studies of E. lucius across four categories of scientific journals: miscellaneous ( ), organism or environment specific (
), organism or environment specific ( ), ecology and evolution oriented (
), ecology and evolution oriented ( ) and top ranked and general (
) and top ranked and general ( ), has changed over time (χ
2 = 84·9, d.f. = 15, P < 0·001). (c) Average number of citations year−1 for papers published in different categories of scientific journals [same as in panel (b)] in different decades (ANCOVA, effect of decade: F
1,1679 = 6·24, P < 0·05; effect of journal category: F
3,1679 = 10·43, P < 0·001).
), has changed over time (χ
2 = 84·9, d.f. = 15, P < 0·001). (c) Average number of citations year−1 for papers published in different categories of scientific journals [same as in panel (b)] in different decades (ANCOVA, effect of decade: F
1,1679 = 6·24, P < 0·05; effect of journal category: F
3,1679 = 10·43, P < 0·001).
Articles on E. lucius are typically published in specialized journals oriented to certain types of organisms (fish) or environments [Fig. 2(b)]. The frequency distribution of E. lucius articles among journal categories has changed somewhat, but the overall picture, that organism and environment‐specific journals dominate (864 of 1684, 51·3%) and that only a fraction (24 of 1684, 1·4%) of studies on E. lucius are published in top ranked journals, has remained unaffected [Fig. 2(b)].
Esox lucius articles published in specialized journals generally attract fewer citations, compared with articles in journals with a broader scope or in highly prestigious journals [Fig. 2(c)]. Number of citations per article depends on year of publication (ANCOVA, effect of year: F 1,1679 = 24·59, P < 0·001) and varies among journal categories (effect of category: F 3,1679 = 9·19, P < 0·001). Overall, E. lucius articles in top ranked journals attract c. 2·5 times as many citations (least‐squares means from ANCOVA = 46 citations) and articles in general ecology and evolution journals attract c. 1·5 times as many citations (32·1) compared with E. lucius articles in organism and environment oriented (19·3) or miscellaneous (18·9) journals. Number of citations per article per year varies among journal categories in a manner similar to total citations [Fig. 2(c)]. If more research on E. lucius is published in prestigious journals with a broader scope, this might increase scientific effect and contribute to the establishment of E. lucius as an influential model organism.
The above trends demonstrate that E. lucius is emerging as a model organism in ecology and evolution research. The increase in research output on E. lucius has not been as rapid, but parallels the development in S. salar, G. aculeatus and P. reticulata (Fig. 1). The last three mentioned species are well established as fish model organisms, but they differ in ecology and are generally used for addressing dissimilar questions and suitable for different types of approaches: For instance, S. salar has been widely used for field studies and questions concerning migration, life history and for its role in aquaculture, fishing industry and recreational fishing (Carvalho, 1993; Fleming, 1996); G. aculeatus occupies diverse habitats allowing for studies of population divergence and lends itself to studies of behaviour, population genetics, genomics and evolution (Schluter, 1993; Herczeg et al., 2009); P. reticulata is well suited for captive breeding and laboratory manipulation studies of developmental biology, physiology, behaviour ecology, reproductive life history and toxicity ( Carvalho, 1993; Rodd et al., 1997). Esox lucius shares certain characteristics with the aforementioned and other fish model species (Amundsen, 2003; Cossins & Crawford, 2005; Merilä, 2013; Schartl, 2014) but also differs in some important respects, and therefore provides a valuable addition to existing models. How the life history and other characteristics of E. lucius can be combined with various methodological approaches and genetic tools to answer interesting questions in ecology and evolutionary biology are briefly discussed.
Several methodological approaches have proven useful for studies of E. lucius, which contribute to its utility as a model organism. Because of their large size, E. lucius can be individually marked using external and internal tags that allow for monitoring of behaviour, movement patterns, depth and body temperature (Metcalfe, 2006). Esox lucius also have structures making them amenable for indirect study and reconstruction of behaviour and life history. For instance, analyses of trace elements in otoliths can inform about place of origin, migration movements and habitat use (Engstedt et al., 2014; Larsson et al., 2015). Analyses of annual growth rings in the otoliths, operculum or in the cleithrum (Casselman, 1987) enable age determination and reconstructions of past growth. This allows for quantification of growth trajectories and body size at the level of individuals or populations (Tibblin et al., 2015), which can be used to identify phenotypic, genetic and environmental correlates of growth rate.
The above mentioned approaches have uncovered different life‐history strategies for E. lucius making it suitable for research concerning consequences of habitat utilization strategies. In the Baltic Sea, the resident form of E. lucius spawns in brackish coastal waters (Lappalainen et al., 2008), whereas the anadromous form spawns in freshwater streams and wetlands, such that subpopulations are geographically separated during the early larval period but share a common coastal habitat during the majority of the life cycle (Müller, 1986; Larsson et al., 2015). Together with their homing behaviour (Miller et al., 2001; Larsson et al., 2015), this should enable investigations of causes and fitness consequences of resident and migratory strategies in E. lucius. Although such investigations appears to be lacking, important insights might be gained by comparing results of future E. lucius studies with findings in studies of resident and anadromous forms in salmonids (Fleming, 1996). The greater degree of iteroparity, in combination with the long lifespan and spawning site fidelity in E. lucius, also offer novel and unexplored opportunities for studies of inter and intra‐individual variation in timing of spawning migration behaviour, and for investigations of phenotypic correlates of survival.
That E. lucius has a wide distribution and occupies a broad range of habitats (Craig, 1996) opens up opportunities for future studies of population differentiation and for comparisons along environmental gradients to identify potential ecological drivers of adaptive population variation, for example, in age at maturity, body size and reproductive allocation strategies, akin to previous natural experiment studies on P. reticulata (Magurran, 2005). Surprisingly, little is known about the relative importance of genes and plasticity for population differentiation in vertebrates in general and in fishes in particular (Kuparinen & Merilä, 2007; Herczeg et al., 2009; Dmitriew, 2011). Esox lucius, however, can be raised in common‐garden experiments (using artificial fertilization) to estimate heritability and adaptive genetic divergence (Q ST) among populations (Tibblin et al., 2015). Furthermore, microsatellite markers allow for studies of population genetic structure and gene flow (Rousset, 1997; Bekkevold et al., 2014). These types of data can be combined for Q ST − F ST (or P ST − F ST) comparisons to evaluate the contribution of selection v. genetic drift to population differentiation (Holand et al., 2011; Leinonen et al., 2013; Tibblin et al., 2015). Such indirect approaches can be accompanied by reciprocal translocation experiments to more rigorously test for local adaptation and investigate whether genotypes perform better at ‘home’ than in ‘foreign’ environments (Kawecki & Ebert, 2004). Furthermore, the external fertilization of E. lucius offers hitherto unexplored possibilities to experimentally test for effects of parental genetic similarity and compatibility on offspring performance, and to examine the consequences of genetic admixture for population fitness; issues both of which are of fundamental scientific interest and key to successful aquaculture and management of biodiversity (Rius & Darling, 2014; Larsson et al., 2015).
Improved sequencing technologies enable genomic resources to be generated with increasing efficiency and speed, such that non‐mainstream fish species can now be exploited as models. The National Center for Biotechnology Information database (NCBI, 2014) currently includes genome sequence assemblies for 45 teleost species. Esox lucius, one of the few long‐lived iteroparous fish species sequenced so far (Rondeau et al., 2014), can thus be included in comparative genomics studies among populations in different environments and across species with different characteristics.
In conclusion, E. lucius is suitable for many lines of investigation, is on its way to becoming an important model organism and has potential to contribute new knowledge and a better understanding of ecology and evolutionary biology.
This work was supported by grants from Linnaeus University, the Swedish Research Council (grant to A.F.), the Swedish Research Council Formas (via Ecochange grant to P.L.) and from Stiftelsen Olle Engqvist, Byggmästare (grant to A.F. and P.L.). We thank A. Svensson for discussion and two anonymous reviewers for comments on the manuscript.
References
References
- Amundsen, T. (2003). Fishes as models in studies of sexual selection and parental care. Journal of Fish Biology 63, 17–52. [Google Scholar]
- Arlinghaus, R. & Mehner, T. (2004). A management‐orientated comparative analysis of urban and rural anglers living in a metropolis (Berlin, Germany). Environmental Management 33, 331–344. [DOI] [PubMed] [Google Scholar]
- Bekkevold, D. , Jacobsen, L. , Hansen, J. H. , Berg, S. & Skov, C. (2014). From regionally predictable to locally complex population structure in a freshwater top predator: river systems are not always the unit of connectivity in Northern Pike Esox lucius . Ecology of Freshwater Fish 24, 305–316. doi: 10.1111/eff.12149 [Google Scholar]
- Carvalho, G. R. (1993). Evolutionary aspects of fish distribution: genetic variability and adaptation. Journal of Fish Biology 43, 53–73. [Google Scholar]
- Casselman, J. M. (1987). Determination of age and growth In The Biology of Fish Growth (Weatherly A. H. & Gill H. S., eds), pp. 209–242. London: Academic Press. [Google Scholar]
- Cossins, A. R. & Crawford, D. L. (2005). Fish as models for environmental genomics. Nature Reviews Genetics 6, 324–333. [DOI] [PubMed] [Google Scholar]
- Craig, J. F. (1996). Pike – Biology and Exploitation. London: Chapman & Hall. [Google Scholar]
- Craig, J. F. (2008). A short review of pike ecology. Hydrobiologia 601, 5–16. [Google Scholar]
- Dmitriew, C. L. (2011). The evolution of growth trajectories: what limits growth rate? Biological Reviews 86, 97–116. [DOI] [PubMed] [Google Scholar]
- Engstedt, O. , Engkvist, R. & Larsson, P. (2014). Elemental fingerprinting in otoliths reveals natal homing of anadromous Baltic Sea pike (Esox lucius L.). Ecology of Freshwater Fish 23, 313–321. [Google Scholar]
- Fleming, I. A. (1996). Reproductive strategies of Atlantic salmon: ecology and evolution. Reviews in Fish Biology and Fisheries 6, 379–416. [Google Scholar]
- Herczeg, G. , Gonda, A. & Merilä, J. (2009). Evolution of gigantism in nine‐spined sticklebacks. Evolution 63, 3190–3200. [DOI] [PubMed] [Google Scholar]
- Holand, A. M. , Jensen, H. , Tufto, J. & Moe, R. (2011). Does selection or genetic drift explain geographic differentiation of morphological characters in house sparrows Passer domesticus? Genetics Research 93, 367–379. [DOI] [PubMed] [Google Scholar]
- Kawecki, T. J. & Ebert, D. (2004). Conceptual issues in local adaptation. Ecology Letters 7, 1225–1241. [Google Scholar]
- Kuparinen, A. & Merilä, J. (2007). Detecting and managing fisheries induced evolution. Trends in Ecology and Evolution 22, 652–659. [DOI] [PubMed] [Google Scholar]
- Lappalainen, A. , Harma, M. , Kuningas, S. & Urho, L. (2008). Reproduction of pike (Esox lucius) in reed belt shores of the SW coast of Finland, Baltic Sea: a new survey approach. Boreal Environment Research 13, 370–380. [Google Scholar]
- Larsson, P. , Tibblin, P. , Koch‐Schmidt, P. , Engstedt, O. , Nilsson, J. , Nordahl, O. & Forsman, A. (2015). Ecology, evolution and management strategies of northern pike populations in the Baltic Sea. Ambio 44(Suppl. 3), S451–S461. doi: 10.1007/s13280-015-0664-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtonen, H. , Leskinen, E. , Selen, R. & Reinikainen, M. (2009). Potential reasons for the changes in the abundance of pike, Esox lucius, in the western Gulf of Finland, 1939–2007. Fisheries Management and Ecology 16, 484–491. [Google Scholar]
- Leinonen, T. , McCairns, R. J. S. , O'Hara, R. B. & Merila, J. (2013). QST‐FST comparisons: evolutionary and ecological insights from genomic heterogeneity. Nature Reviews Genetics 14, 179–190. [DOI] [PubMed] [Google Scholar]
- Magurran, A. E. (2005). Evolutionary Ecology: The Trinidadian Guppy. Oxford: Oxford University Press. [Google Scholar]
- Merilä, J. (2013). Nine‐spined stickleback (Pungitius pungitius): an emerging model for evolutionary biology research. Annals of the New York Academy of Sciences 1289, 18–35. [DOI] [PubMed] [Google Scholar]
- Metcalfe, J. D. (2006). Fish population structuring in the North Sea: understanding processes and mechanisms from studies of the movements of adults. Journal of Fish Biology 69, 48–65. [Google Scholar]
- Miller, L. M. , Kallemeyn, L. & Senanan, W. (2001). Spawning‐site and natal‐site fidelity by northern pike in a large lake: mark–recapture and genetic evidence. Transactions of the American Fisheries Society 130, 307–316. [Google Scholar]
- Müller, K. (1986). Seasonal anadromous migration of the pike (Esox lucius L.) in coastal areas of the northern Bothnian sea. Archiv für Hydrobiologie 107, 315–330. [Google Scholar]
- Nelson, J. (1994). Fishes of the World, 3rd edn. New York, NY: Wiley. [Google Scholar]
- Pierce, R. B. , Tomcko, C. M. & Schupp, D. H. (1995). Exploitation of northern pike in seven small north‐central Minnesota lakes. North American Journal of Fisheries Management 15, 601–609. [Google Scholar]
- Rius, M. & Darling, J. A. (2014). How important is intraspecific genetic admixture to the success of colonising populations? Trends in Ecology and Evolution 29, 233–242. [DOI] [PubMed] [Google Scholar]
- Rodd, F. H. , Reznick, D. N. & Sokolowski, M. B. (1997). Phenotypic plasticity in the life history traits of guppies: responses to social environment. Ecology 78, 419–433. [Google Scholar]
- Rondeau, E. B. , Minkley, D. R. , Leong, J. S. , Messmer, A. M. , Jantzen, J. R. , von Schalburg, K. R. , Lemon, C. , Bird, N. H. & Koop, B. F. (2014). The genome and linkage map of the Northern pike (Esox lucius): conserved synteny revealed between the salmonid sister group and the Neoteleostei. PLoS One 9, e102089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousset, F. (1997). Genetic differentiation and estimation of gene flow from F‐statistics under isolation by distance. Genetics 145, 1219–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schartl, M. (2014). Beyond the zebrafish: diverse fish species for modeling human disease. Disease Models and Mechanisms 7, 181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluter, D. (1993). Adaptive radiation in sticklebacks–size, shape, and habitat use efficiency. Ecology 74, 699–709. [Google Scholar]
- Tibblin, P. , Forsman, A. , Koch‐Schmidt, P. , Nordahl, O. , Johannessen, P. , Nilsson, J. & Larsson, P. (2015). Evolutionary divergence of adult body size and juvenile growth in sympatric subpopulations of a top predator in aquatic ecosystems. American Naturalist 186(on‐line). doi: 10.1086/681597 [DOI] [PubMed] [Google Scholar]
- Zuk, M. , Garcia‐Gonzalez, F. , Herberstein, M. E. & Simmons, L. W. (2014). Model systems, taxonomic bias, and sexual selection: beyond Drosophila . Annual Review of Entomology 59, 321–338. [DOI] [PubMed] [Google Scholar]
Electronic Reference
- NCBI (2014). The National Center for Biotechnology Information. Genome Information by Organism Available at http://www.ncbi.nlm.nih.gov/genome/browse/ (last accessed 10 December 2014).


