Abstract
Objectives
This study examined the differential impact of two telehealth programs for women caring for an older adult with a neurocognitive disorder. Outcomes examined were depressive symptoms, upset following disruptive behaviors, anxious and angry mood states, and caregiving self‐efficacy.
Methods
Women cohabitating with a family member diagnosed with a neurocognitive disorder were assigned via random allocation to either of the following: (1) a 14‐week behavioral intervention using video instructional materials, workbook and telephone coaching in behavioral management, pleasant events scheduling, and relaxation or (2) a basic education guide and telephone support comparison condition. Telephone assessments were conducted by interviewers blind to treatment condition at pre‐intervention, post‐intervention, and 6 months following intervention.
Results
For those providing in‐home care at post‐treatment, depressive symptoms, upset following disruptive behaviors, and negative mood states were statistically lower in the behavioral coaching condition than in the basic education and support condition. Reliable change index analyses for Beck Depression Inventory II scores favored the behavioral coaching condition. Caregiving self‐efficacy scores for obtaining respite and for managing patient behavioral disturbances were significantly higher in the coaching condition. Effect sizes were moderate but not maintained at the 6‐month follow‐up.
Conclusions
This study provides some initial evidence for the efficacy of a telehealth behavioral coaching intervention compared with basic education and telephone support. Carers' abilities to maintain strategy use during progressive disorders such as Alzheimer's disease likely require longer intervention contact than provided in the current study. Dementia carers, including those living in rural areas, can benefit from accessible and empirically supported interventions that can be easily disseminated across distances at modest cost. © 2015 The Authors. International Journal of Geriatric Psychiatry Published by John Wiley & Sons, Ltd.
Keywords: family caregiving, neurocognitive disorders, behavioral treatments, depression
Introduction
Family carers of individuals with Alzheimer's disease or another progressive neurocognitive disorder (NCD) experience increased risk for distress, depression, and negative health outcomes (Schulz and Monin, 2012; Mausbach et al., 2013; Adelman et al., 2014; Joling et al., 2014). NCD carers can reduce distress and improve functioning using psychologically derived treatments that meet evidence‐based criteria (Mittelman et al., 2004; Van Mierlo et al., 2012), including skills training interventions utilizing cognitive‐behavioral principles and strategies (Coon et al., 2003).
Community‐based programs are often inaccessible or burdensome because of the care recipient's need for ongoing supervision. Promising interventions often involve psychoeducational groups requiring travel to the program (Losada et al., 2011; Savundranayagam et al., 2011; Kurz et al., 2013). Rural areas have additional challenges of a sparse population, few agencies with trained professionals, and uncoordinated services (Buckwalter et al., 2002). In‐home programs are important and can affect a number of care outcomes (Huang et al., 2003; Gitlin et al., 2003a, 2003b; Kuo et al., 2013) but also include challenges such as cost of individualized services and staff travel.
The current study sought to reduce these barriers through evaluating telehealth interventions for emotionally distressed female NCD family carers. We hypothesized that, compared with basic education and telephone support, a video instruction and telephone coaching condition would be more efficacious in reducing distress following problematic patient behaviors, depression, and negative mood, as well as increasing caregiving self‐efficacy.
Methods
Procedure and sample
A single, blind, randomized controlled design was used, and all participants provided informed consent; the protocol was approved and followed ethical standards of the institutional review board of the principal investigator's university (IRB# 991123S).
Prospective participants were screened by telephone, with the following criteria: (1) female aged 30+ years; (2) responsible for a cohabitating family member diagnosed with an NCD; (3) ≥2 upsetting care‐recipient memory/behavior problems (Teri et al., 1992) in previous week; (4) three positive depressive symptoms on Center for Epidemiologic Studies‐Depression Boston short form (Kohout et al., 1992) in previous week; (5) no plans for placement in nursing care/hospice in the next 6 months; (6) no history of suicide attempts or current ideation; (7) alcohol use ≤2 drinks/day; and (8) receiving primary care. Women were exclusively targeted because of literature documenting gender differences in caregiving responses and preferences (Lauderdale and Gallagher‐Thompson, 2002). Because of carers' experience of unique stressors when living with an NCD patient, we focused on behavioral and cognitive strategies to increase respite utilization and reduce upset following disruptive behaviors. Including only those living with a diagnosed individual allowed us to evaluate intervention impact on respite self‐efficacy.
Inclusion criteria for the care recipient were as follows: (1) dementia/neurocognitive diagnosis confirmed by primary care clinician; (2) receiving primary care; and no lifetime history of (3) schizophrenia, (4) bipolar disorder, (5) suicide attempts, (6) Huntington's disease, (7) Korsakoff's disease, (8) multiple sclerosis, (9) HIV, or (10) alcohol dependence. Recruitment was via Alzheimer's Association Chapters and Area Agencies on Aging throughout the Midwestern United States. Project staff activity occurred in a university lab setting, with telephone and mail/email contact with participants.
Of the 253 carers screened, 104 (41%) were eligible; 30 (29%) declined participation, resulting in a randomized sample of 74 participants. The primary reason for ineligibility was insufficient emotional distress following behavior problems; the primary reason for declining participation was unwillingness to commit the time required. A stratified random assignment procedure used computerized random number generator with blocks of 50 participants to maintain equal proportions of metropolitan versus nonmetropolitan residents across conditions. Allocation concealment involved separate individuals setting up randomization and enrollment. Randomized participants included 33 carers in the behavioral coaching intervention and 41 in the basic education condition; differences in group size were due to stratified allocation procedures and limited project time to recruit the full target of 100 participants (50 per group). The sample was split between spousal carers (52%) and adult children (48%). Participants reported mild to moderate levels of depressive symptoms, as measured by the Beck Depression Inventory II (BDI‐II; Beck et al., 1996; M = 15.7; SD = 8.3). Sample characteristics are shown in Tables 1 and 2.
Table 1.
Participant characteristics (N = 74)
| Mean (SD) | Range | |
|---|---|---|
| Length of caregiving (months) | 33.4 (23.4) | 3–95 |
| Age (years) | 60.3 (10.8) | 35–87 |
| Income (median) | $40 000 | $5–70K |
| Education (years) | 14 (1.8) | (9–17) |
| N | Percent | |
| Female | 74 | 100 |
| Began living with care recipient to provide care | 32 | 43 |
| Race | ||
| African‐American | 15 | 20 |
| White | 59 | 80 |
| Census designation | ||
| Metropolitan | 51 | 68.9 |
| Nonmetropolitan | 23 | 31.1 |
| Marital status | ||
| Never married | 6 | 8.0 |
| Currently married/cohabitating | 50 | 67.6 |
| Divorced/separated | 13 | 17.6 |
| Widowed | 5 | 6.8 |
| Employment status | ||
| Homemaker | 18 | 24.3 |
| Working full time outside home | 18 | 24.3 |
| Working part time outside home | 9 | 12.2 |
| Retired | 27 | 36.5 |
| Unemployed—looking for work | 2 | 2.7 |
| Affordability of basics | ||
| Not difficult at all | 28 | 37.8 |
| Not very difficult | 16 | 21.6 |
| Somewhat difficult | 22 | 29.7 |
| Very difficult | 8 | 10.9 |
Table 2.
Care‐recipient characteristics (N = 74)
| Mean (SD) | Range | |
|---|---|---|
| Age (years) | 77.4 (9.4) | (56–95) |
| Activities of daily living impairment | 2.0 (1.8) | (0–6) |
| No. of memory and behavior problems | 11.5 (3.3) | (4–20) |
| Relationship to caregiver | N | Percent |
| Husband/partner | 35 | 52.2 |
| Mother | 28 | 37.3 |
| Father | 4 | 6.0 |
| Other | 7 | 9.5 |
| Diagnosis | ||
| Alzheimer's | 46 | 62.2 |
| Vascular | 6 | 8.0 |
| Mixed Alzheimer's and vascular | 3 | 4 |
| Dementia with Lewy bodies | 2 | 2.7 |
| Parkinson's dementia | 2 | 2.7 |
| Unspecified dementia | 15 | 20.3 |
| Health status (per caregiver report) | ||
| Excellent | 4 | 5.4 |
| Very good | 11 | 14.9 |
| Good | 28 | 37.7 |
| Fair | 24 | 32.4 |
| Poor | 7 | 9.5 |
Interventions
Behavioral coaching condition (n = 33) participants received 10 video segments, each lasting 30 min, a workbook, 10 weekly telephone calls, and 2 maintenance calls from a trained coach. The multi‐component video series (DVD or VHS) and workbook included instruction and examples of female carers utilizing strategies for increasing the following: (a) behavioral activation for both the carer and care recipient (Teri et al., 1997; Moore et al., 2013); (b) management of disruptive behaviors (Teri et al., 1998; Burgio et al., 2003); (c) relaxation during caregiving situations (Coon et al., 2003); and (d) caregiving self‐efficacy (Bandura, 2004). The workbook provided didactic and experiential materials reinforcing each video and weekly monitoring forms. Participant received weekly 30‐ to 50‐min phone calls by their designated coach. The first 10 weekly calls reviewed material in each video session, workbook chapter, and completed worksheets and troubleshooted challenging patient behaviors. The last two calls were biweekly and emphasized maintenance of skills developed during the intervention. Staff used a coach manual providing scripts for reviewing didactic materials/assignments, assessing homework compliance, and applying concepts to specific problems. Calls averaged 40 min in length.
The basic education and support condition (n = 41) was designed to function as a “treatment as usual” comparison, using information and support interventions common to nonprofit agencies (Corbett et al., 2012). Because services typically rely on standardized content and less intensive staff contact than in psychoactive interventions, this condition is intentionally weaker in content and staff contact than the coaching intervention. Participants in this condition received by mail a 37‐page Basic Care Guide (Alzheimer's Association Education Institute, 2005), which included information on dementia and suggestions for responding to specific care challenges. Suggestions for using this educational booklet were provided, with specified pages matching the concerns reported during intake. Carers received seven telephone calls scheduled every other week; staff checked on the safety of the carer and family member, discussed suggestions from the guide, and responded to questions. Calls averaged 20 min in length.
Interventionists were one licensed doctoral‐level clinical psychologist and five masters‐trained clinicians. All assessment and intervention phone contacts were audiotaped. To confirm treatment adherence and competence, we randomly identified one session each for a random selection of 10 behavioral coaching participants and 10 basic education participants. These 20 tapes were evaluated by a clinical geropsychologist unaffiliated with the project, using adherence and competence scales developed for this study. All sessions met protocol requirements.
Measures
The three assessment points were intake, post‐intervention, and 6 months post‐intervention. Outcomes reported in this paper were obtained during audiotaped telephone interviews by staff blind to intervention condition; additional measures not included in this report were collected via paper‐and‐pencil questionnaires sent to participants and returned. To assist telephone interviews, participants received response cards prior to the interview.
Demographic and care‐related variables
Assessed characteristics included carer and care‐recipient ethnicity, marital status, education, age, and relationship characteristics. The Index of Independence in Activities of Daily Living Scale (Katz et al., 1963) measured reports of care recipients' functional impairments in basic activities (e.g., bathing and dressing). The living and residential status of the patient was tracked at each assessment as follows: (1) living with the same family carer, (2) moved to live with another family member, (3) moved to long‐term care, or (4) deceased. When a patient entered long‐term care or died during the intervention, carers were discontinued from the intervention but followed at all assessment points and offered referrals for support resources in their local community.
Patient diagnostic status
Participants signed an authorization for the patient's primary care clinician or specialist (e.g., psychiatrist and neurologist) to inform project staff of the dementia diagnosis. This authorization was mailed to the provider to report diagnosis and then sign and return. Rate of return was 96% (71/74), all signed by an MD physician. For the three cases not returned, authorizations were faxed to the office nurse who provided verbal diagnostic information to our staff. Participants started intervention prior to confirmation, and no cases were later deemed ineligible.
Primary outcome measures
Upset following memory and behavior problems
The Revised Memory and Behavior Problem Checklist (RMBPC; Teri et al., 1992) assessed bother or upset following specific patient behaviors over the previous week (Likert scale of 0, not at all, to 4, extremely). A total score was created by summing (rather than averaging) these upset ratings, as recommended by Roth et al. (2003).
Depressive symptomatology
The BDI‐II (Beck et al., 1996) measured depressive symptomatology over the past 2 weeks.
Secondary outcome measures
Negative mood
Level of global negative mood was assessed by the Negative Affect Scale (Watson et al., 1988); 10 adjectives representing negative emotions were rated for the past 2 weeks and totaled. Self‐reported levels of state anxiety and state hostility were assessed with the short version of the Multiple Affect Adjective Check List‐Revised (MAACL‐R) Anxiety and Hostility subscales (Zuckerman and Lubin, 1985) assessed over the previous 2 weeks.
Caregiving self‐efficacy
The Obtaining Respite and Responding to Disruptive Behaviors subscales of the Revised Scale for Caregiving Self‐efficacy (Steffen et al., 2002) assessed carers' confidence in managing specific care challenges on a scale of 0 to 100.
Analysis
A series of independent t‐tests and chi‐square analyses evaluated group differences in demographic and baseline variables. When cell sizes were <5 for categorical variables, Fisher's exact tests were used. Any baseline scores that varied between groups at p ≤ .10 were included as potential covariates and retained if significant in the model. Intervention impact was tested using two‐group (behavioral coaching/basic education) repeated‐measures multivariate analysis of variance (MANOVA) or multivariate analysis of covariance, with p ≤ .05 signifying statistical significance. The sample size was lower at 6‐month follow‐up because of changes in patient living/residential status; analyses were run separately for pre‐intervention to post‐intervention and for pre‐intervention to 6‐month follow‐up. Cohen's d effect size was estimated for outcome variables, using Cohen's (1988) method for converting partial eta squared values to Cohen's f and d values [f = sqr(η 2/(1 − η 2)); d = 2 × f], where effect sizes of 0.2, 0.5, and 0.8 are considered small, medium, and large, respectively. We used the reliable change index (RCI) to assess the clinical significance of pre‐intervention to post‐intervention changes for each participant (Jacobson and Truax, 1991). An RCI critical value was formulated for the BDI‐II (11.35) using descriptive data from the pre‐intervention assessment (mean and standard deviation) as clinical norms, along with community norms (mean and standard deviation) for older women reported by Segal et al. (2008). For RCI analyses, participants were coded by whether scores at post‐intervention and 6‐month follow‐up did the following: (a) moved from above to below 11.35, (b) moved below to above 11.35, (c) stayed above 11.35, or (d) stayed below 11.35.
Results
Randomization check
Independent t‐tests and chi‐square analyses revealed no significant group differences (i.e., p < .05) at intake for participant age, education, ethnicity, difficulty paying for household expenses, relation to care recipient, months as a carer, number of memory and behavior problems in the care recipient, or pre‐treatment values for outcome variables. Because two outcomes approached significance for group differences at pre‐treatment (i.e., BDI‐II and RMBPC Upset), these intake values were entered as covariates in relevant analyses.
Completion rates
Figure 1 depicts flow of participants through the study. Of 74 randomized carers, 11 (15%) did not complete the intervention phase; withdrawals were due to three patient deaths, four nursing home placements, and four dropouts for other reasons (e.g., poor timing and disinterest in continuing). There were no differences between the behavioral coaching intervention (n = 6; 18%) and the basic education condition (n = 5; 12%) in distribution of non‐completers (X 2(1, N = 74) = 0.52, p = .47). Non‐completers continued to be assessed; one withdrawn participant was lost to follow‐up.
Figure 1.
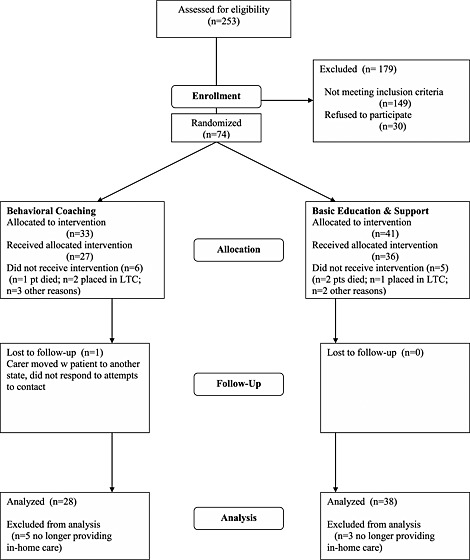
CONSORT study flowchart. LTC, long‐term care.
Outcome analyses utilized all participants known to be providing in‐home care at the time of post‐intervention assessment (n = 66); this included three of the five participants who withdrew for any reason other than placement or death of patient. One patient was placed with another family member, and one carer was lost to follow‐up. An intent‐to‐treat approach (pre‐intervention scores carried forward) was used to handle the missing data from the one lost case; linear mixed modeling was not deemed necessary given the small extent of missing data.
Primary post‐intervention outcomes
Depressive symptoms
A repeated‐measures analysis of covariance (ANCOVA) tested change in BDI‐II scores, using a 2 (condition: behavioral coaching/basic education) × 2 (time: baseline/post‐intervention) model with intake BDI‐II scores as a significant covariate. Post‐intervention Cohen's d, adjusted cell means, and standard deviations are shown in Table 3. The Condition × Time statistic for BDI‐II was significant (Wilk's F(1, 63) = 4.11, p ≤ .05, ηp 2 = .06), indicating greater efficacy of the behavioral coaching condition in reducing depressive symptoms across the active intervention phase of the study; Cohen's d = 0.50 represents a medium effect.
Table 3.
Post‐intervention Cohen's d, means, and standard deviations by intervention condition
| Behavioral coaching (n = 28) | Basic education and support (n = 38) | |||||
|---|---|---|---|---|---|---|
| Intake | Post | 6 months | Intake | Post | 6 months | |
| (n = 28) | (n = 28) | (n = 22) | (n = 38) | (n = 38) | (n = 30) | |
| Primary outcomesa | ||||||
| BDI‐II (Cohen's d = 0.50) | 15.4 | 9.8 | 10.3 | 15.4 | 13.2 | 9.4 |
| (0.0) | (1.3) | (1.3) | (0.0) | (1.1) | (1.1) | |
| RMBPC upset (Cohen's d = 0.50) | 19.3 | 10.7 | 10.1 | 19.3 | 14.5 | 13.3 |
| (0.0) | (1.4) | (1.8) | (0.0) | (1.2) | (1.5) | |
| Secondary outcomesb | ||||||
| Negative Affect Scale (Cohen's d = 0.66) | 24.5 | 17.6 | 20.1 | 24.1 | 22.0 | 21.7 |
| (6.9) | (4.4) | (7.3) | (7.3) | (7.0) | (7.6) | |
| MAACL Anxiety (Cohen's d = 0.63) | 4.8 | 3.5 | 4.4 | 4.6 | 5.0 | 4.2 |
| (2.3) | (2.2) | (2.6) | (2.3) | (2.7) | (2.6) | |
| MAACL Hostility (Cohen's d = 0.39) | 5.1 | 3.9 | 4.3 | 6.3 | 6.2 | 5.5 |
| (2.6) | (2.6) | (2.9) | (3.2) | (3.2) | (3.5) | |
| Self‐efficacy: Respite (Cohen's d = 0.55) | 47.3 | 57.4 | 49.0 | 53.5 | 50.1 | 58.6 |
| (26.0) | (30.8) | (31.9) | (30.5) | (28.6) | (31.1) | |
| Self‐efficacy: Behavioral Management (Cohen's d = 0.46) | 68.1 | 75.6 | 76.4 | 65.9 | 66.6 | 68.5 |
| (20.1) | (16.9) | (17.7) | (20.8) | (19.7) | (19.8) | |
BDI‐II, Beck Depression Inventory II; RMBPC, Revised Memory and Behavior Problem Checklist; MAACL, Multiple Affect Adjective Checklist.
Post‐intervention and 6‐month follow‐up scores adjusted for intake value.
Original means are shown.
Although participants were not required to have clinically elevated depressive symptoms to be eligible for the study, we examined whether conditions differed in proportions of carers with post‐intervention BDI‐II scores above or below the RCI cutoff of 11.35. At the post‐intervention assessment, a higher proportion of behavioral coaching participants (71.4%) than basic education participants (42.1%) had reliably low BDI‐II scores (X 2(1, N = 66) = 5.59, p ≤ .05). This represents a greater proportion of carers in the basic education than in the behavioral coaching condition whose BDI‐II scores either remained clinically significant (52.6% vs. 28.6%) or changed from low to high (5.3% vs. 0%) (X 2(3, N = 66) = 8.76, p ≤ .05).
Upset following behavior problems
A repeated‐measures ANCOVA was run with a 2 (condition: behavioral coaching/basic education) × 2 (time: baseline/post‐intervention) model retaining intake RMBPC upset scores as a significant covariate. Cohen's d, adjusted cell means, and standard deviations are shown in Table 3. The multivariate Condition × Time statistic was significant (Wilk's F(1, 63) = 4.29, p ≤ .05, ηp 2 = .06) with greater post‐intervention reductions in upset for participants in the behavioral coaching condition; Cohen's d = 0.50 represents a medium effect.
Secondary post‐intervention outcomes
Negative mood
A repeated‐measures MANOVA with a 2 (condition: behavioral coaching/basic education) × 2 (time: baseline/post‐intervention) design evaluated intervention effects for the Negative Affect Scale, MAACL Anxiety, and MAACL Hostility scores. No covariates were included; Table 3 displays post‐intervention Cohen's d, unadjusted cell means, and standard deviations. The multivariate Condition × Time statistic was significant (Wilk's F(3, 62) = 2.82, p ≤ .05, ηp 2 = .12). Univariate tests revealed a significant time‐by‐condition interaction for Positive and Negative Affect Schedule (PANAS) Negative Affect scores (F(1, 64) = 6.92, p ≤ .05, ηp 2 = .10) and MAACL anxiety scores (F(1, 64) = 6.34, p ≤ .05, ηp 2 = .09), with lower levels of negative mood and anxiety at post‐intervention for participants in the behavioral coaching condition. Cohen's d scores were moderate for Negative Affect (d = 0.66) and MAACL Anxiety (d = 0.63) and smaller for MAACL Hostility (d = 0.39).
Caregiving self‐efficacy
A repeated‐measures MANOVA with a 2 (condition: behavioral coaching/basic education) × 2 (time: baseline/post‐intervention) design evaluated intervention effects for the Respite and Behavior Management subscales of the Revised Scale for Caregiving Self‐efficacy (SE). No covariates were included; Table 3 displays post‐intervention Cohen's d, unadjusted cell means, and standard deviations. The multivariate Condition × Time statistic was significant (Wilk's F(2, 63) = 3.74, p ≤ .05, ηp 2 = .11). Univariate tests revealed a significant time‐by‐condition interaction for SE‐Respite scores (F(1, 64) = 4.43, p ≤ .05, ηp 2 = .07) and that approached significance for SE‐Behavior Management scores (F(1, 64) = 3.61, p = .06, ηp 2 = .05), with relatively greater increases in caregiving self‐efficacy at post‐intervention for participants in the behavioral coaching condition. Cohen's d scores indicated a moderate effect for SE‐Respite (d = 0.55) and for SE‐Behavior Management (d = 0.46).
Caregiving status
The two intervention conditions did not differ in caregiving status changes, either immediately post‐intervention (X 2(2, N = 74) = 1.70, p = .43) or 6 months post‐intervention (X 2(2, N = 73) = 0.22, p = .90).
Six‐month follow‐up
At the 6‐month follow‐up, 52 (70%) participants continued as in‐home carers. The behavioral coaching (M = 4.8, SD = 2.9) and basic education (M = 5.8, SD = 2.6) conditions were similar in months from intake to placement for patients entering long‐term care (t(1, 14) = 0.65, p = .53). Using RCI analysis, behavioral coaching and basic education participants were equally likely at 6 months post‐intervention to have reliable decreases, increases, or stability in BDI‐II scores (X 2(3, N = 52) = 4.84, p = .18).
Repeated‐measures MANOVA and multivariate analysis of covariance examined changes in primary and secondary outcome variables from pre‐intervention to 6 months post‐intervention. Multivariate Condition × Time statistics were non‐significant, with groups similar to each other in depressive symptoms (Wilk's F(1, 49) = 0.26, p = .61, ηp 2 = .01), level of upset following patient behavior problems (Wilk's F(1, 49) = 1.87, p = 18, ηp 2 = .04), negative mood (Wilk's F(3, 48) = 0.21, p = .89, ηp 2 = .01), and caregiving self‐efficacy (Wilk's F(2, 49) = 0.43, p = .66, ηp 2 = .02). Examination of cell means suggests that, in general, behavioral coaching participants maintained post‐intervention gains while basic education participants continued to improve.
Discussion
A telehealth behavioral coaching intervention was evaluated (in a randomized trial format) for female NCD family members actively involved with in‐home care and experiencing emotional distress. This behavioral approach identified specific concerns and tailored strategies to individual needs, consistent with the empirical literature (Gallagher‐Thompson and Coon, 2007) and meeting practice guidelines for dementia care (Vasse et al., 2012). Carers report such types of support as important and preferred (Alwin et al., 2010). Participants reported viewing segments and complying with between‐session assignments.
Compared with the basic education and support condition, the video/workbook/telephone coaching intervention led to statistically significant reductions in depressive symptoms, negative mood, and upset following disruptive patient behaviors. RCI analyses of BDI‐II scores indicate that the behavioral coaching intervention was reliably superior to the basic education/support condition in pre‐intervention to post‐intervention change of depressive symptoms. The behavioral coaching intervention also showed greater improvements in caregiving self‐efficacy to respond to disruptive behaviors and seek respite assistance. Effect sizes were moderate for the primary outcome measures of BDI and RMBPC upset scores, as well as for most of the secondary outcomes (i.e., negative affect, anxiety, and caregiving self‐efficacy). Thus, the study provides support for a telehealth mechanism to deliver psychoeducational interventions for NCD carers. These initial improvements were generally maintained at the 6‐month follow‐up for behavioral coaching participants, at which time carers in the comparison condition also reported lower distress. “Booster” sessions were not included during follow‐up; future studies of similar interventions should consider strategies for building upon initial post‐intervention benefits.
Among the limitations to this research is the small sample that reduced power and precluded additional analyses such as examining moderator effects (e.g., spousal versus adult children and ethnically diverse subgroups). To strengthen recruitment and generalizability, eligibility criteria for dementia diagnoses included “unspecified dementia,” which comprised 20% of our sample. Dementia detection without a documented NCD diagnosis is alarmingly frequent (Connolly et al., 2011); excluding such cases would have rendered our sample different from families struggling with current diagnostic practices in primary care. Timely diagnosis and documentation of NCDs are important for the health of individuals and families (Connolly et al., 2011; George and Steffen, 2014) and economically important for healthcare systems (Brooker et al., 2014). Interventions that facilitate appropriate diagnostic and documentation practices need immediate attention by investigators and policy makers.
Focusing on women living with the identified dementia patient increased our recruitment challenges during this relatively brief pilot trial and limited generalizability of findings. It is unclear whether cohabitation is needed to effectively target respite and behavioral management domains associated with dementia caregiving or if other contact requirements would improve recruitment and yield comparable results. Families of cognitively impaired elders in a variety of settings have needs; future interventions might focus on communication between families and health providers and effective management of chronic health conditions in older adults with NCDs, among other outcomes.
The behavioral coaching intervention was designed for accessibility and dissemination to community agencies. For such programs to be sustainable, agencies require business models for staffing telephone coaches. We found masters‐level students across a range of disciplines (e.g., clinical and counseling psychology, social work, nursing, and occupational therapy) to be effective behavioral coaches when provided training and supervision. Utilizing volunteer paraprofessionals is worth future exploration.
Distance‐based interventions (e.g., telephone, video, Internet, and bibliotherapy) hold promise for family carers, especially those living in rural or metropolitan areas with limited transportation. Similar to the findings of Davis et al. (2011) in their study of telephone interventions, carers reported valuing the time they spent discussing their concerns with intervention staff. The development and evaluation of telehealth interventions easily accessible to NCD family carers continue to be important and worth the sustained effort.
Conflict of interest
None declared.
Key points.
This study examined the differential impact of two telehealth programs for women caring for an older adult with a neurocognitive disorder.
Outcomes including depressive symptoms, upset following disruptive behaviors, and negative mood states were statistically lower in the behavioral coaching condition than in the basic education and support condition. Caregiving self‐efficacy for obtaining respite and managing patient behavioral disturbances was significantly higher in the coaching condition.
Dementia carers, including those living in rural areas, can benefit from accessible and empirically supported interventions that can be easily disseminated across distances at modest cost.
Acknowledgements
This project was funded by grant #R21 MH061956 from the US National Institute of Mental Health and also supported by grant #IIRG 5987 from the US National Alzheimer's Association. We wish to thank Dolores Gallagher‐Thompson, Ph.D., Larry Thompson, Ph.D., Louis Burgio, Ph.D., Suzann Ogland‐Hand, ph.D., and Antonette Zeiss, Ph.D. for their support and expert advice. We are also grateful for the efforts of Area Agencies on Aging and Alzheimer's Association staff members who assisted with this project and the family carers who participated in this study.
Steffen, A. M. , and Gant, J. R. (2016) A telehealth behavioral coaching intervention for neurocognitive disorder family carers. Int J Geriatr Psychiatry, 31: 195–203. doi: 10.1002/gps.4312.
References
- Adelman RD, Tmanova LL, Delgado D, Dion S, Lachs MS. 2014. Caregiver burden: a clinical review. JAMA 311(10): 1052–1060. [DOI] [PubMed] [Google Scholar]
- Alwin J, Öberg B, Krevers B. 2010. Support/services among family caregivers of persons with dementia—perceived importance and services received. Int J Geriatr Psychiatry 25: 240–248. [DOI] [PubMed] [Google Scholar]
- Bandura A. 2004. Health promotion by social cognitive means. Health Educ Behav 31: 143–164. [DOI] [PubMed] [Google Scholar]
- Beck A, Speer R, Brown G. 1996. Beck Depression Inventory: Second Edition Manual. The Psychological Corporation: San Antonio. [Google Scholar]
- Brooker D, La Fontaine J, Evans S, Bray J, Saad K. 2014. Public health guidance to facilitate timely diagnosis of dementia: Alzheimer's Cooperative Valuation in Europe recommendations. Int J Geriatr Psychiatry 29: 682–693. [DOI] [PubMed] [Google Scholar]
- Buckwalter KC, Davis LL, Wakefield BJ, Kienzle MG, Murray MA. 2002. Telehealth for elders and their caregivers in rural communities. Fam Comm Health 25: 31–40. [DOI] [PubMed] [Google Scholar]
- Burgio L, Stevens A, Guy D, Roth DL, Haley WE. 2003. Impact of two psychosocial interventions on White and African American family caregivers of individuals with dementia. Gerontologist 43: 568–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. 1988. Statistical Power Analysis for the Behavioral Sciences. 2nd edn. Lawrence Erlbaum Associates, Inc.: New Jersey. [Google Scholar]
- Connolly A, Gaehl E, Martin H, Morris J, Purandare N. 2011. Underdiagnosis of dementia in primary care: variations in the observed prevalence and comparisons to the expected prevalence. Aging Ment Health 15: 978–984. [DOI] [PubMed] [Google Scholar]
- Coon DW, Thompson L, Steffen A, Sorocco K, Gallagher‐Thompson D. 2003. Anger and depression management: psychoeducational skill training interventions for women caregivers of a relative with dementia. Gerontologist 26: 678–689. [DOI] [PubMed] [Google Scholar]
- Corbett A, Stevens J, Aarsland D, et al. 2012. Systematic review of services providing information and/or advice to people with dementia and/or their caregivers. Int J Geriatr Psychiatry 27: 628–636. [DOI] [PubMed] [Google Scholar]
- Davis JD, Tremont G, Bishop DS, Fortinsky R. 2011. A telephone‐delivered psychosocial intervention improves dementia caregiver adjustment following nursing home placement. Int J Geriatr Psychiatry 26: 380–387. [DOI] [PubMed] [Google Scholar]
- Education Institute . 2005. Basic Dementia Care Guide. Alzheimer's Association: St. Louis, Mo. [Google Scholar]
- Gallagher‐Thompson D, Coon D. 2007. Evidence‐based psychological treatments for distress in family caregivers of older adults. Psychol Aging 22: 37–51. [DOI] [PubMed] [Google Scholar]
- George NR, Steffen AM. 2014. Promoting medication adherence in older adults through early diagnosis of neurocognitive disorders. Prim Care Companion CNS Disord 16, doi: 10.4088/PCC.14m01686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitlin LN, Belle SH, Burgio LD, et al. 2003a. Effect of multicomponent interventions on caregiver burden and depression: the REACH multisite initiative at 6‐month follow‐up. Psychol Aging 18: 361–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitlin LN, Winter L, Corcoran M, et al. 2003b. Effects of the home environment skill‐building program on the caregiver–care recipient dyad: 6‐month outcomes from the Philadelphia REACH initiative. Gerontologist 43(4): 532–546. [DOI] [PubMed] [Google Scholar]
- Huang HL, Shyu YI, Chen MC, Chen ST, Lin LC. 2003. A pilot study on a home‐based caregiver training program for improving caregiver self‐efficacy and decreasing the behavioral problems of elders with dementia in Taiwan. Int J Geriatr Psychiatry 18(4): 337–345. [DOI] [PubMed] [Google Scholar]
- Jacobson N, Truax P. 1991. Clinical significance: a statistical approach to defining meaningful change in psychotherapy research. J Consult Clin Psych 59: 12–19. [DOI] [PubMed] [Google Scholar]
- Joling KJ, van Marwijk HW, Veldhuijzen AE, et al. 2014. The two‐year incidence of depression and anxiety disorders in spousal caregivers of persons with dementia: who is at the greatest risk? Am J Geriatr Psychiatry S1064(14): 146–148. [DOI] [PubMed] [Google Scholar]
- Katz S, Ford AB, Moskowitz RW, Jackson BW, Jaffe M. 1963. Studies of illness in the aged: the index of ADL, a standardized measure of biological and psychosocial function. JAMA 185: 914–919. [DOI] [PubMed] [Google Scholar]
- Kohout F, Berkman L, Evans D, et al. 1992. Two shorter forms of the CES‐D depression symptoms index. J Aging Health 5: 179–193. [DOI] [PubMed] [Google Scholar]
- Kuo LM, Huang HL, Huang HL, et al. 2013. A home‐based training program improves Taiwanese family caregivers' quality of life and decreases their risk for depression; a randomized controlled trial. Int J Geriatr Psychiatry 28: 504–513. [DOI] [PubMed] [Google Scholar]
- Kurz A, Wagenpfeil S, Hallauer J, Schneider‐Schelte H, Jansen S. 2013. Evaluation of a brief educational program for dementia carers: the AENEAS study. Int J Geriatr Psychiatry 25: 861–865. [DOI] [PubMed] [Google Scholar]
- Lauderdale S, Gallagher‐Thompson D. 2002. Men providing care: what do they need and how can we do it? Clin Gerontologist 26: 53–70. [Google Scholar]
- Losada A, Márquez‐González M, Romero‐Moreno R. 2011. Mechanisms of action of a psychological intervention for dementia caregivers: effects of behavioral activation and modification of dysfunctional thoughts. Int J Geriatr Psychiatry 26: 1119–1127. [DOI] [PubMed] [Google Scholar]
- Mausbach B, Chattillion E, Roepke S, Patterson T, Grant I. 2013. A comparison of psychosocial outcomes in elderly Alzheimer's caregivers and noncaregivers. Am J Geriatr Psychiatry 31: 5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittelman MS, Roth DL, Coon DW, Haley WE. 2004. Sustained benefit of supportive intervention for depressive symptoms in caregivers of patients with Alzheimer's disease. Am J Psychiatry 161(5): 850–856. [DOI] [PubMed] [Google Scholar]
- Moore RC, Chattillion EA, Ceglowski J, et al. 2013. A randomized clinical trial of behavioral activation (BA) therapy for improving psychological and physical health in dementia caregivers: results of the Pleasant Events Program (PEP). Behav Res Therapy 51: 623–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth D, Burgio L, Gitlin L, et al. 2003. Psychometric analysis of the Revised Memory and Behavior Problems Checklist: factor structure of occurrence and reaction ratings. Psychol Aging 18: 906–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savundranayagam MY, Montgomery RJV, Kosloski K, Little TD. 2011. Impact of a psychoeducational program on three types of caregiver burden among spouses. Int J Geriatr Psychiatry 26: 388–396. [DOI] [PubMed] [Google Scholar]
- Schulz R, Monin LM. 2012. Family caregiving of persons with dementia: prevalence, health effects, and support strategies In Moving beyond Self‐interest: Perspectives from Evolutionary Biology, Neuroscience, and the Social Sciences, Brown S, Brown R, Penner LA. (eds.). Oxford University Press: New York. [Google Scholar]
- Segal D, Coolidge FL, Cahill BS, O'Riley AA. 2008. Psychometric properties of the Beck Depression Inventory‐II (BDI‐II) among community‐dwelling older adults. Behav Modif 32: 3–20. [DOI] [PubMed] [Google Scholar]
- Steffen AM, McKibbin C, Zeiss A, Gallagher‐Thompson D, Bandura A. 2002. The Revised Scale for Caregiving Self‐efficacy: two reliability and validity studies. J Gerontol B Psychol Sci Soc Sci 57: 74–86. [DOI] [PubMed] [Google Scholar]
- Teri L, Logsdon R, Uomoto J, et al. 1997. Behavioral treatment of depression in dementia patients: a controlled clinical trial. J Gerontol B Psychol Sci Soc Sci 52: 159–166. [DOI] [PubMed] [Google Scholar]
- Teri L, Logsdon R, Weiner M, et al. 1998. Treatment for agitation in dementia patients: a behavior management approach. Psychother 35: 436–443. [Google Scholar]
- Teri L, Truaz P, Logsdon R, et al. 1992. Assessment of behavioral problems in dementia: the Revised Memory and Behavior Checklist. Psychol Aging 7: 622–631. [DOI] [PubMed] [Google Scholar]
- Van Mierlo LD, Meiland FJM, Van der Roest HG, Dröes RM. 2012. Personalised caregiver support: effectiveness of psychosocial interventions in subgroups of caregivers of people with dementia. Int J Geriatr Psychiatry 27: 1–14. [DOI] [PubMed] [Google Scholar]
- Vasse E, Vernooiu‐Dassen M, Cantegreil I, et al. 2012. Guidelines for psychosocial interventions in dementia care: a European survey and comparison. Int J Geriatr Psychiatry 27: 40–48. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark L, Tellegen A. 1988. Development and validation of brief measures of positive and negative affect: the PANAS Scales. J Pers Social Psychol 54: 1063–1070. [DOI] [PubMed] [Google Scholar]
- Zuckerman M, Lubin B. 1985. Manual for the MAACL‐R: The Multiple Affect Adjective Checklist‐Revised. EdITS: San Diego, CA. [Google Scholar]


