Abstract
Background
Functional dyspepsia (FD) is one of the most common disorders of gastrointestinal (GI) diseases. However, no curable treatment is available for FD because the detailed mechanism of GI dysfunction in stressed conditions remains unclear. We aimed to clarify the association between endogenous acylated ghrelin signaling and gastric motor dysfunction and explore the possibility of a drug with ghrelin signal‐enhancing action for FD treatment.
Methods
Solid gastric emptying (GE) and plasma acylated ghrelin levels were evaluated in an urocortin1 (UCN1) ‐induced stress model. To clarify the role of acylated ghrelin on GI dysfunction in the model, exogenous acylated ghrelin, an endogenous ghrelin enhancer, rikkunshito, or an α 2‐adrenergic receptor (AR) antagonist was administered. Postprandial motor function was investigated using a strain gauge force transducer in a free‐moving condition.
Key Results
Exogenous acylated ghrelin supplementation restored UCN1‐induced delayed GE. Alpha2‐AR antagonist and rikkunshito inhibited the reduction in plasma acylated ghrelin and GE in the stress model. The action of rikkunshito on delayed GE was blocked by co‐administration of the ghrelin receptor antagonist. UCN1 decreased the amplitude of contraction in the antrum while increasing it in the duodenum. The motility index of the antrum but not the duodenum was significantly reduced by UCN1 treatment, which was improved by acylated ghrelin or rikkunshito.
Conclusions & Inferences
The UCN1‐induced gastric motility dysfunction was mediated by abnormal acylated ghrelin dynamics. Supplementation of exogenous acylated ghrelin or enhancement of endogenous acylated ghrelin secretion by rikkunshito may be effective in treating functional GI disorders.
Keywords: acylated ghrelin, gastric dysfunction, stress, rikkunshito, urocortin1, α2‐adrenergic receptor
Key Messages
This study aims to clarify the association between endogenous ghrelin signaling and gastric motor dysfunction in the stressed conditions and possibility of a drug with ghrelin signal enhancing action for functional dyspepsia treatment.
Stress hormone urocortin1‐induced gastric motility dysfunction was mediated by abnormal acylated ghrelin levels via α2‐adrenergic receptor activation in rats.
Supplementation of exogenous acylated ghrelin or enhancement of endogenous acylated ghrelin secretion by rikkunshito may be effective in treating functional gastrointestinal disorders.
Introduction
Excessive stress in modern society is supposed to be related to the development of functional gastrointestinal disorders.1 In particular, postprandial distress syndrome such as early satiety and postprandial fullness leads to a decreased quality of life in patients with functional dyspepsia (FD). Abnormalities of gastrointestinal (GI) motility have also been observed in experimental animal models, including restraint stress2 and water avoidance stress.3 However, the detailed mechanism underlying GI dysfunctions in a stressed condition remains unclear, and consequently, therapies which are available are not enough to treat FD.
Corticotropin‐releasing factor (CRF) plays a key role in coordinating the stress response by mediating hormonal and autonomic functions.4, 5 Enhanced CRF synthesis in stress‐related regions of the brain has been observed in various stress models.6, 7 Urocortin 1 (UCN1), a member of the mammalian CRF‐related peptides, is mainly localized in the Edinger–Westphal nucleus.8, 9 Evidence suggests that stress activates UCN1 neurons in the Edinger–Westphal nucleus.10 The actions of UCN1 are mediated by both CRF receptor 1 (CRF1R) and CRF receptor 2 (CRF2R). It has been well documented that UCN1 injected intracerebroventricularly (ICV) is more potent than CRF.11, 12, 13 Previous reports also indicate that CRF1R and CRF2R activation may result in abnormal GI function in some experimental stress models.14, 15, 16, 17
Stress causes excitement of the autonomic nervous system and modifies regulation of endocrine systems18, 19; therefore, it controls GI function and feeding behavior. On the other hand, ghrelin, an endogenous 28‐amino‐acid ligand of the growth hormone secretagogue receptor (GHS‐R1a), was discovered in the rat stomach.20, 21 Ghrelin regulates energy intake and consumption by positive regulation of growth hormone release, appetite, and GI motility, and it consequently plays an important role in physiological functions. Previous studies have shown that peripheral ghrelin dynamics are affected by acute/chronic stress.3, 22 In an UCN1‐induced stress model, plasma acylated ghrelin levels and food intake were reduced, and supplementation of exogenous acylated ghrelin abolished the decrease in food intake.23 The ghrelin deficiency effects may be mediated by peripheral adrenergic receptors (ARs), including α 2‐AR activation following central CRF2 receptor activation.24 However, whether α 2‐AR and peripheral acylated ghrelin levels are involved in delayed GE in an UCN1‐induced stress model remains unclear.
We therefore hypothesized that in a stress condition, postprandial distress syndrome involves in the suppression of acylated ghrelin via α 2‐AR activation. This study aimed to clarify the association between peripheral acylated ghrelin levels and gastric motor dysfunction via α 2‐AR activation in a UCN1‐induced stress model and to explore the possibility of the ghrelin enhancer rikkunshito for the treatment of FD disorders.
Materials and Methods
Experimental animals
Eight‐week‐old male Sprague–Dawley rats (weight, 240–280 g) were purchased from Japan SLC, Inc. (Shizuoka, Japan). All animals were housed in polycarbonate cages in a room with controlled conditions of ambient temperature (23 ± 3 °C), humidity (50 ± 20%), and lighting (12‐h light : dark cycle). Animals were maintained with water and standard laboratory food ad libitum. Access to standard laboratory food was removed 16 h before experiments. All experimental procedures were performed according to the Guidelines for the Care and Use of Laboratory Animals and approved by the Laboratory Animal Committee (Permit nos. 10‐113, 10‐139, 11‐031, 11‐046, 13‐080, and 14‐008) of Tsumura & Co. (Tokyo, Japan).
Surgery
Intracerebroventricular cannula
Rats under sodium pentobarbital (50 mg/kg, intraperitoneal, IP) anesthesia were placed on a stereotaxic apparatus (SLC, Inc., Shizuoka, Japan). A stainless steel guide cannula (AG‐8; Eicom, Kyoto, Japan) was implanted into the right lateral ventricle using the following coordinates derived from the rat brain atlas25: 0.8 mm posterior and 1.4 mm lateral from the bregma and 3.4 mm ventral from the skull surface. Coordinate validity was confirmed by extracting and partitioning the brain after injecting the dye and checking the distribution of the dye. Rats were singly housed after the surgery and had a recovery period of at least 5 days before treatment initiation.
Animal preparation for strain gauge force transducer test
The surgery was performed at least 10 days after ICV cannulation as described previously26 in pentobarbital‐anesthetized (50 mg/kg, IP; Abbott Laboratories, North Chicago, IL, USA) rats that had been fasted overnight. After laparotomy, a strain gauge force transducer (F‐08IS; Star Medical, Inc., Tokyo, Japan) was placed on the serosal surface of the antrum and duodenum for measuring circular muscle contractions. The wire of the transducer was led out from the back of the neck via the subcutaneous part of the back. In order to inject acylated ghrelin intravenously, a vessel catheter was inserted into the right jugular vein and led out from the back of the neck. The catheter was filled with heparinized saline (100 U/mL) to avoid blood coagulation. The wire and catheter were led through a protective coil. Measurements were performed under free‐moving conditions in individual cages after a 5‐day postoperative recovery period.
Drugs and treatments
Rat UCN1 and rat acylated ghrelin were purchased from Peptide Institute, Inc. (Osaka, Japan). Yohimbine hydrochloride, a selective α 2‐AR antagonist, was purchased from Sigma‐Aldrich Chemical Co. (St. Louis, MO, USA). The GHS‐R1a antagonist, [D‐Lys3]‐GHRP‐6, was purchased from Bachem, Inc. (Torrance, CA, USA). These compounds were dissolved in saline when injected by IP and intravenous (IV) routes. Rikkunshito, a Japanese traditional Kampo medicine, was supplied from Tsumura & Co. in the form of a powdered extract obtained by spray‐drying a hot water extract mixture of the following eight crude drugs: Atractylodis lanceae rhizoma (4.0 g), Ginseng radix (4.0 g), Pinelliae tuber (4.0 g), Poria (4.0 g), Zizyphi fructus (2.0 g), Citri unshiu pericarpium (2.0 g), Glycyrrhizae radix (1.0 g), and Zingiberis rhizoma (0.5 g). Rikkunshito was dissolved in distilled water when injected by orogastric administration (PO). Other analytical reagents included commercially available highest‐purity products.
Treatments were performed on unanesthetized and lightly hand‐restrained rats using the following volumes: 10 μL/rat for ICV injection, 1 mL/kg for IP or IV injection through the tail vein, and 10 mL/kg for PO.
Acylated ghrelin determination
Rats were euthanized by decapitation, and trunk blood (approximately 4 mL) was collected in cold polypropylene tubes containing 8.0 mg ethylenediaminetetraacetic acid and 0.8 mg aprotinin. Samples were centrifuged at 10,000 × g at 4 °C for 3 min. The supernatant was acidified with 1 mol/L HCl (1/10 volume) and stored at −80 °C until the acylated ghrelin assays were performed. Plasma acylated ghrelin levels were determined using acylated ghrelin enzyme‐linked immunoassay kits (Mitsubishi Chemical Corp., Tokyo, Japan).
Experimental protocols
All experiments were performed using 16‐h fasted rats with no access to food before ICV injection, except otherwise mentioned.
Gastric emptying test (1): Effects of exogenous acylated ghrelin or yohimbine on gastric emptying
Rats fasted for longer than 16 h received phosphate‐buffered saline (PBS) or UCN1 (300 pmol/rat, ICV) as previously reported.23 One hour later, all the groups were gavaged with the test meal (1 mL/rat of the meal composed of standard powdered chow [24 g, MF; Oriental Yeast, Tokyo, Japan] and 30 g of glass bead [0.2‐mm diameter, BZ‐02; AS One, Osaka, Japan] in 60 mL of distilled water), followed by IV injection of vehicle or rat acylated ghrelin (3 nmol/rat). In another part of the experiment, rats fasted for longer than 16 h received yohimbine (5 mg/kg, IP) 15 min before ICV injection of PBS or UCN1. Following this treatment, 1 h later, all groups were gavaged with the test meal (1 mL/rat), and 2 h thereafter, the rats were euthanized to determine the percentage of GE.
Gastric emptying test (2): Effects of rikkunshito
Rats fasted for longer than 16 h received an orogastric gavage (10 mL/rat) of vehicle or rikkunshito (1000 mg/kg). Following this, 1 h later, rats received an IV injection of either saline or [D‐Lys3]‐GHRP‐6 (4 μmol/kg), followed by ICV PBS or UCN1 (300 pmol/rat) 1 min later. After 1 h, all groups were gavaged with the test meal (1 mL/rat), and 2 h thereafter, the rats were euthanized to determine the percentage of GE.
Effects of yohimbine and rikkunshito on plasma acylated ghrelin levels
In the same sets of experiment described in above gastric emptying tests, after euthanasia, trunk blood was collected for analysis of acylated ghrelin plasma levels.
Measurement of gastroduodenal motility
The connector of the strain gauge force transducer placed in the fasted rats was connected to a preamplifier, FS‐04M (Star Medical, Inc.), via a bridge box FB‐01 (Star Medical, Inc.) to allow measurement of antrum and duodenum movements. Data were recorded using an MP150 (BIOPAC Systems, Aero Camino Goleta, CA, USA). The experiment was initiated when the fasted gastric contraction had stabilized, 3 h after the initial measurement. The system was calibrated before each experiment using a calibrator (Star Medical Equipment, Inc.), and contractions were expressed in grams. The motility index (MI) was determined as the area under the curve (AUC) in the antrum and duodenum for a 30‐min period and is shown as a percentage (% MI = 100 × (AUC posttreatment/AUC pretreatment).
Statistical analysis
All values are presented as the mean ± SEM. Statistical analyses of the mean values of different test groups were performed using Student's t‐test or the Aspin–Welch t‐test. The mean values of multiple groups were determined by one‐way anova, followed by the Dunnett post hoc test. For all tests, probability (p) values <0.05 were considered statistically significant.
Results
Effects of IV injection of exogenous acylated ghrelin or IP injection of α 2‐AR antagonist on ICV UCN1‐induced delayed gastric emptying
Gastric emptying after ICV UCN1 was significantly delayed compared with that after ICV PBS (Control: IV saline, ICV PBS: 60.0 ± 6.7%/2 h; IV saline, ICV UCN1: 11.3 ± 2.1%/2 h, p < 0.001; Fig. 1A; N = 7/group). The administration of acylated ghrelin 1 h after ICV significantly improved delayed GE (IV acylated ghrelin, ICV UCN1: 38.6 ± 9.7%/2 h, p < 0.05; Fig. 1A; N = 8/group).
Figure 1.
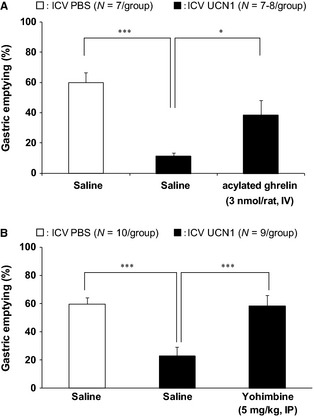
Effects of (A) intravenous (IV) administration of acylated ghrelin (3 nmol/rat, N = 7–8/group) and (B) intraperitoneal (IP) administration of selective α 2‐AR antagonists (5 m/kg, N = 9/group) on delayed gastric emptying in ICV UCN1‐treated rats. All groups were gavaged with the test meal (1 mL/rat) 1 h after ICV UCN1 (300 pmol/rat) or vehicle. Two hours thereafter, animals were euthanized to determine the percentage of gastric emptying. IV injections were performed immediately after ICV UCN1 or vehicle. IP injections were performed 15 min before ICV UCN1 or vehicle. All values are presented as the mean ± SEM (N = 7–8/group or N = 9–10 group). Significance was identified using the Dunnett's post hoc test following one‐way anova. *p < 0.05, ***p < 0.001.
In addition, IP pretreatment with the selective α 2‐AR antagonist (yohimbine) 15 min before ICV UCN1, prevents delayed GE by UCN1 (IP saline, ICV UCN1: 22.9 ± 6.5%/2 h, IP yohimbine, ICV UCN1: 58.5 ± 7.6%/2 h, p < 0.001; Fig. 1B; N = 9/group).
Effects of rikkunshito on gastric emptying and acylated ghrelin in ICV UCN1‐treated rats
Intracerebroventricular UCN1 induced a significant reduction in the 2‐h GE response (control: PO distilled water (DW), ICV PBS: 55.3 ± 3.8%/2 h; PO DW, ICV UCN1: 17.5 ± 2.5%/2 h, p < 0.001; Fig. 2A; N = 12/group). Oral administration of rikkunshito partially inhibited the delayed GE by UCN1 (PO rikkunshito, ICV UCN1: 28.2 ± 4.0%/2 h, p < 0.05; Fig. 2A; N = 12/group). [D‐Lys3]‐GHRP‐6 injected IV did not influence the 2‐h GE in ICV PBS‐treated rats (data not shown), and when [D‐Lys3]‐GHRP‐6 was also added, the effect of rikkunshito was completely abolished by [D‐Lys3]‐GHRP‐6 (PO rikkunshito, IV [D‐Lys3]‐GHRP‐6, ICV UCN1: 11.8 ± 1.5%/2 h; Fig. 2A, N = 12/group).
Figure 2.
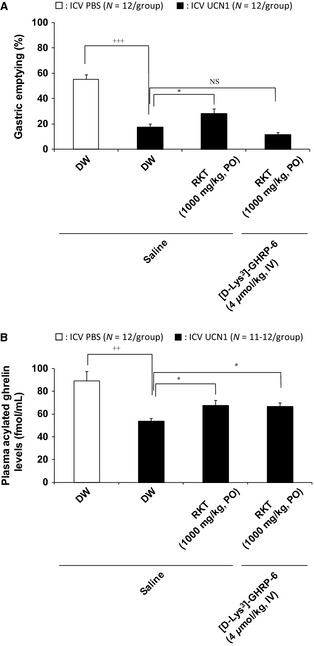
Effects of administration of rikkunshito (N = 12/group) alone and in combination with a ghrelin receptor antagonist on delayed gastric emptying (A) and plasma acylated ghrelin levels (B) in ICV UCN1‐treated rats. Distilled water (DW) or rikkunshito was orally administered ICV 1 h before. In addition, saline or ghrelin receptor antagonist [D‐Lys3]‐GHRP‐6 (4 μmol/mL/kg) was intravenously administered into the tail vein 1 min after ICV administration of PBS or UCN1 (300 pmol/rat). One hour after ICV, all groups were gavaged with the test meal (1 mL/rat), and 2 h thereafter, animals were euthanized to determine the percentage of gastric emptying and plasma acylated ghrelin levels. Each bar represents the mean ± SEM (N = 12/group). Significance was identified using the Student's t‐test or Aspin–Welch t‐test. ++ p < 0.01, +++ p < 0.001 compared with the PBS‐treated group. Significance of multiple groups were determined by one‐way anova, followed by Dunnett's post hoc test. *p < 0.05 compared with the UCN1‐treated group. RKT: rikkunshito.
In addition, UCN1 induced a significant reduction in the plasma acylated ghrelin levels (control: PO DW, ICV PBS: 89.0 ± 8.4 fmol/mL/2 h; PO DW, ICV UCN1: 53.7 ± 2.6 fmol/mL/2 h, p < 0.01; Fig. 2B; N = 12/group). Oral administration of rikkunshito partially inhibited the decreased acylated ghrelin by ICV UCN1 (PO rikkunshito, ICV UCN1: 67.8 ± 4.5 fmol/mL/2 h, p < 0.05; Fig. 2B; N = 12/group). [D‐Lys3]‐GHRP‐6 injected IV did not influence plasma acylated ghrelin levels in vehicle‐treated control rats (data not shown) or the effect of rikkunshito on plasma acylated ghrelin (PO rikkunshito, IV [D‐Lys3]‐GHRP‐6, ICV UCN1: 66.6 ± 3.5 fmol/mL/2 h; Fig. 2B; N = 11/group).
GI motility
Effect of ICV UCN1 on gastroduodenal motility
Strain gauge force transducer measurements revealed a typical type of contractions, called phase III‐like contractions, in the antrum and duodenum in fasted rats. After feeding of 3 g standard chow, phase III‐like contractions disappeared and increased the amplitude of contraction in the antrum. The fasted patterns changed into the fed‐like motor patterns immediately after feeding in both the antrum and duodenum (Fig. 3A–C). In ICV PBS control rats, postprandial contractions in the antrum and duodenum continued even after treatment (Fig. 3A). However, ICV UCN1 decreased the amplitude of contraction in the antrum while increasing the amplitude of contraction in the duodenum (Fig. 3B). The percentage changes in MI of the antrum over 30 min were significantly lower in the ICV UCN1‐treated group than in the ICV PBS‐treated control group (Fig. 3D). Percentage changes in MI of the duodenum over 30 min tended to increase, albeit not significantly (Fig. 3E).
Figure 3.
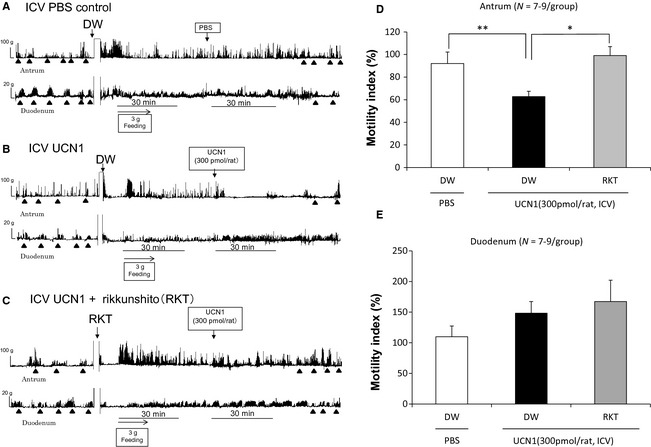
Effects of ICV PBS, and ICV UCN1 alone or in combination with rikkunshito on gastroduodenal motility (A–C) and the percentual changes in MI of the antrum (D) or the duodenum (E). ▲: phase III‐like contractions. Each bar represents the mean ± SEM (N = 7–9/group). Significance was determined using one‐way anova, followed by Dunnett's post hoc test. *p < 0.05, **p < 0.01 compared with the UCN1/DW group.
Effect of rikkunshito on ICV UCN1‐induced change in gastroduodenal motility
The effects of rikkunshito on the ICV UCN1‐induced contractions in the antrum and duodenum are shown in Fig. 3C. Rikkushito improved the decreasing amplitude of contraction in the antrum upon UCN1 treatment. The percentage changes in MI of the antrum in the rikkunshito‐treated group were significantly increased compared with those in the group treated with ICV UCN1 alone (Fig. 3D). The percentage changes in MI of the duodenum were not influenced by rikkunshito treatment (Fig. 3E).
Discussion
We found that the supplementation of exogenous acylated ghrelin or enhancement of endogenous acylated ghrelin secretion by rikkunshito improved the reduction of GE and postprandial antrum motility in the UCN1‐induced stress model. These findings suggest that the ghrelin signal‐enhancing action is effective in treating functional GI disorders.
Because early satiety and abdominal fullness after eating are characteristics of the syndrome in FD patients and symptoms are possibly due to a disturbance in GE and postprandial antrum motility, we investigated GE after the administration of a test meal composed of nutritive food in an UCN1‐induced stress model. As a result, ICV UCN1 significantly reduced GE of the test meal, which was possibly associated with decreased plasma acylated ghrelin levels. Acylated ghrelin promotes eating behavior by transmitting signals to the brain via GHS‐R expressed on the termini of the afferent vagus nerves in the stomach.27, 28 In addition, it has been reported that acylated ghrelin has a strong enhancing activity on GI motility and it stimulates the actions depending on the plasma levels.26 Our previous research indicated that ICV UCN1 significantly induced non‐nutritive liquid GE delay in fasted rats and that emptying returned to normal levels upon administration of a CRF2R antagonist; therefore, we demonstrated that UCN1 suppressed GE via CRF2R.23 In addition, peripheral acylated ghrelin secretion is regulated via central CRF2R.23 These findings suggest that postprandial GI dysfunction in the UCN1‐induced stress model is involved in the suppression of the secretion of acylated ghrelin via central CRF2R activation.
The administration of rikkunshito, a traditional Japanese Kampo medicine, resulted in significant inhibition of the delayed GE through an increase in endogenous acylated ghrelin in the UCN1‐induced stress model. It has been reported that rikkunshito elicits a continuously increased acylated ghrelin signal in GHS‐R‐expressing cells and a decreased afferent activity of the gastric vagus nerve.26, 29 Furthermore, the study in the restraint stress model demonstrated that supplementation of exogenous acylated ghrelin or enhancement of endogenous ghrelin signaling by rikkunshito improved GI dysfunction.30 These findings indicate that the improvement in delayed GE by rikkunshito is mediated by the inhibition of a decrease in endogenous acylated ghrelin and an enhanced acylated ghrelin signal. In addition, the administration of exogenous acylated ghrelin could improve ICV UCN1‐induced GE delays. Plasma acylated ghrelin increased 2 h after the treatment (IV saline, ICV UCN1: 56.0 ± 7.6 fmol/mL; IV acylated ghrelin, ICV UCN1: 85.4 ± 7.5 fmol/mL, p < 0.05; N = 7–8/group, data not shown in figures or tables), when feeding and GE recovered. In our investigation, this was overall within the physiological range (during fasting: 68.0 ± 5.1 fmol/mL to 102.8 ± 9.6 fmol/mL). These findings evidence that the abnormality of acylated ghrelin dynamics related to delayed GE in an UCN1‐induced stress model.
Alpha2‐AR mRNA has a more abundant expression of the message in gastric mucosa.31 Shujaa et al. confirmed that α 2‐AR stimulation and, in particular, stimulation of α 2A‐AR, hindered gastric motility in mice.32 In addition, we demonstrated that α 2‐AR blocking accelerated GE in normal rats. UCN1 acts on central CRF2R,23 transmits signals to the periphery via the activation of the sympathetic nervous system efferent pathway, and negatively controls acylated ghrelin secretion via peripheral α 2‐ARs.24 The administration of the α 2‐AR antagonist yohimbine, but not β‐AR antagonist, significantly inhibits the reduction of plasma acylated ghrelin levels induced by UCN1 in fasted rats.24 Our experiments in this study indicated that UCN1‐induced delayed GE was restored by the administration of yohimbine through antagonism to α 2‐AR. Simultaneously, plasma acylated ghrelin levels were significantly increased by yohimbine, supporting previous results (IP saline, ICV UCN1: 48.3 ± 3.6 fmol/mL/2 h; IP yohimbine, ICV UCN1: 82.1 ± 7.5 fmol/mL/2 h, p < 0.01; N = 9/group, data not shown in figures and tables). These findings indicate that delayed GE may be mediated by a decrease in endogenous acylated ghrelin via peripheral α 2‐AR activation.
The improvement in delayed GE by rikkunshito or exogenous acylated ghrelin was only a blunted response unlike the α 2‐AR antagonist yohimbine. The plasma acylated ghrelin concentration also did not recover sufficiently. Because the effects of rikkunshito on GE are cancelled by concurrent administration of ghrelin receptor antagonists, decreased acylated ghrelin may be partially involved in delayed GE induced by ICV UCN1. Sufficient recovery may not have been exerted by rikkunshito because its α 2‐AR antagonizing effects are weaker than those of yohimbine. However, GI hormones other than ghrelin such as cholecystokinin (CCK), Peptide YY (PYY), and somatostatin are also known to be involved in delayed GE.33, 34, 35 Furthermore, because it has also been reported that CCK and PYY plasma levels are increased in FD patients,36 the influence of these also needs to be investigated in the future.
As shown in Figure 3, the typical phase III‐like contractions disappeared in the antrum and duodenum after feeding, transitioning to a postprandial pattern. Intracerebroventricularly UCN1 resulted in the disappearance of antrum contractions and an increase in continuous duodenal contractions in the postprandial phase. This result is consistent with a previous report by Fujimiya et al.37 Acylated ghrelin caused a transition of postprandial GI motility to a phase III‐like contraction28; however, ghrelin receptor antagonist administration resulted in the disappearance of phase III‐like contractions under normal conditions.38 In addition, in the UCN1‐induced stress model, IV administration of exogenous acylated ghrelin reversed abnormal antrum and duodenum contractions to normal postprandial motility (Fig. S1). These results strongly indicate that abnormality of acylated ghrelin is linked to abnormal GI motility upon UCN1 treatment. The administration of rikkunshito also recovered decreased gastric motility in ICV UCN1‐treated rats, suggesting the enhancement of endogenous acylated ghrelin. On the other hand, duodenal motility showed a tendency to increase by UCN1 treatment; however, this was not significant. A previous study37 has shown that UCN1 administration induced decreased gastric motility in both fed and fasting states while displaying increased duodenal motility. This difference between ours and previous study may depend on differences in the UCN1 dosage and/or evaluation time.
Our previous report demonstrated that several components of the ghrelin enhancer rikkunshito had α 2‐AR antagonist activity,24 suggesting that the increased plasma ghrelin level and improved gastric motility by rikkunshito involve α 2‐AR blockade in the sympathetic nerves of the stomach. Alpha2‐AR‐mediated inhibition of ghrelin secretion plays an important role in regulating gastric motor function. The effect of rikkunshito on GE was blocked by the co‐administration of ghrelin receptor antagonist, and supplementation of exogenous acylated ghrelin augmented GE, which is mediated by vagal nerve activity in the brain‐gut axis. Several studies have suggested that the oral administration of rikkunshito acts on the central nervous system.39, 40, 41, 42 However, decreased peripheral ghrelin levels in the UCN1‐induced stress model have been reported that involve the stimulation of peripheral, rather than central α 2‐AR.24 Accordingly, the effects of rikkunshito in this study may be peripheral effects. However, further investigation is required.
In conclusion, delayed GE during the postprandial phase was observed in an UCN1‐induced stress model and was related to acylated ghrelin dynamics. Thus, the supplementation of exogenous acylated ghrelin or the endogenous acylated ghrelin enhancer rikkunshito may be effective in treating postprandial distress syndrome in stress‐induced FD.
Funding
This work was supported in part by Saitama Medical University (Japan), a grant from Tsumura & Co. (Japan).
Disclosure
K. Yakabi received grant support from Tsumura & Co. Y. Harada, N. Fujitsuka, and T. Hattori are employed by Tsumura & Co. S. Ro, M. Ochiai, K. Hayashi, and E. Hosomi have nothing to declare.
Author Contribution
YH performed the research, analyzed the data, and drafted the manuscript; NF performed the research and analyzed the data; SR, MO, KH, and EH analyzed and reviewed the data; TH analyzed and reviewed the data, and supervised; KY designed the research study and drafted the manuscript.
Supporting information
Figure S1 Effects of UCN1 (1000 pmol/rat, ICV) alone (A) or in combination with acylated ghrelin (B) on change in gastroduodenal motility.
References
- 1. Tack J, Lee KJ. Pathophysiology and treatment of functional dyspepsia. J Clin Gastroenterol 2005; 39: S211–S216. [DOI] [PubMed] [Google Scholar]
- 2. Zheng J, Dobner A, Babygirija R, Ludwig K, Takahashi T. Effects of repeated restraint stress on gastric motility in rats. Am J Physiol Regul Integr Comp Physiol 2009; 296: R1358–R1365. [DOI] [PubMed] [Google Scholar]
- 3. Ochi M, Tominaga K, Tanaka F, Tanigawa T, Shiba M, Watanabe T, Fujiwara Y, Oshitani N et al Effect of chronic stress on gastric emptying and plasma ghrelin levels in rats. Life Sci 2008; 82: 862–868. [DOI] [PubMed] [Google Scholar]
- 4. Stengel A, Tache Y. Corticotropin‐releasing factor signaling and visceral response to stress. Exp Biol Med (Maywood) 2010; 235: 1168–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stengel A, Goebel M, Luckey A, Yuan PQ, Wang L, Tache Y. Cold ambient temperature reverses abdominal surgery‐induced delayed gastric emptying and decreased plasma ghrelin levels in rats. Peptides 2010; 31: 2229–2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lu A, Steiner MA, Whittle N, Vogl AM, Walser SM, Ableitner M, Refojo D, Ekker M et al Conditional mouse mutants highlight mechanisms of corticotropin‐releasing hormone effects on stress‐coping behavior. Mol Psychiatry 2008; 13: 1028–1042. [DOI] [PubMed] [Google Scholar]
- 7. Stengel A, Tache Y. CRF and urocortin peptides as modulators of energy balance and feeding behavior during stress. Front Neurosci 2014; 8: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shah NS, Pugh PC, Nam H, Rosenthal DT, van Wijk D, Gaszner B, Kozicz T, Kerman IA. A subset of presympathetic‐premotor neurons within the centrally projecting Edinger‐Westphal nucleus expresses urocortin‐1. J Chem Neuroanat 2013; 52: 25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kozicz T, Yanaihara H, Arimura A. Distribution of urocortin‐like immunoreactivity in the central nervous system of the rat. J Comp Neurol 1998; 391: 1–10. [DOI] [PubMed] [Google Scholar]
- 10. Gaszner B, Csernus V, Kozicz T. Urocortinergic neurons respond in a differentiated manner to various acute stressors in the Edinger‐Westphal nucleus in the rat. J Comp Neurol 2004; 480: 170–179. [DOI] [PubMed] [Google Scholar]
- 11. Benoit SC, Thiele TE, Heinrichs SC, Rushing PA, Blake KA, Steeley RJ. Comparison of central administration of corticotropin‐releasing hormone and urocortin on food intake, conditioned taste aversion, and c‐Fos expression. Peptides 2000; 21: 345–351. [DOI] [PubMed] [Google Scholar]
- 12. Smagin GN, Howell LA, Ryan DH, De Souza EB, Harris RB. The role of CRF2 receptors in corticotropin‐releasing factor‐ and urocortin‐induced anorexia. Neuroreport 1998; 9: 1601–1606. [DOI] [PubMed] [Google Scholar]
- 13. Spina M, Merlo‐Pich E, Chan RK, Basso AM, Rivier J, Vale W, Koob GF. Appetite‐suppressing effects of urocortin, a CRF‐related neuropeptide. Science 1996; 273: 1561–1564. [DOI] [PubMed] [Google Scholar]
- 14. Maillot C, Million M, Wei JY, Gauthier A, Tache Y. Peripheral corticotropin‐releasing factor and stress‐stimulated colonic motor activity involve type 1 receptor in rats. Gastroenterology 2000; 119: 1569–1579. [DOI] [PubMed] [Google Scholar]
- 15. Nakade Y, Tsuchida D, Fukuda H, Iwa M, Pappas TN, Takahashi T. Restraint stress delays solid gastric emptying via a central CRF and peripheral sympathetic neuron in rats. Am J Physiol Regul Integr Comp Physiol 2005; 288: R427–R32. [DOI] [PubMed] [Google Scholar]
- 16. Tache Y, Bonaz B. Corticotropin‐releasing factor receptors and stress‐related alterations of gut motor function. J Clin Invest 2007; 117: 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Asakawa A, Inui A, Ueno N, Makino S, Fujino MA, Kasuga M. Urocortin reduces food intake and gastric emptying in lean and ob/ob obese mice. Gastroenterology 1999; 116: 1287–1292. [DOI] [PubMed] [Google Scholar]
- 18. Hosoda H, Kangawa K. The autonomic nervous system regulates gastric ghrelin secretion in rats. Regul Pept 2008; 146: 12–18. [DOI] [PubMed] [Google Scholar]
- 19. Yang LZ, Tovote P, Rayner M, Kockskamper J, Pieske B, Spiess J. Corticotropin‐releasing factor receptors and urocortins, links between the brain and the heart. Eur J Pharmacol 2010; 632: 1–6. [DOI] [PubMed] [Google Scholar]
- 20. Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth‐hormone‐releasing acylated peptide from stomach. Nature 1999; 402: 656–660. [DOI] [PubMed] [Google Scholar]
- 21. Kojima M, Kangawa K. Ghrelin: structure and function. Physiol Rev 2005; 85: 495–522. [DOI] [PubMed] [Google Scholar]
- 22. Lutter M, Sakata I, Osborne‐Lawrence S, Rovinsky SA, Anderson JG, Jung S, Birnbaum S, Yanagisawa M et al The orexigenic hormone ghrelin defends against depressive symptoms of chronic stress. Nat Neurosci 2008; 11: 752–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yakabi K, Noguchi M, Ohno S, Ro S, Onouchi T, Ochiai M, Takabayashi H, Takayama K et al Urocortin 1 reduces food intake and ghrelin secretion via CRF(2) receptors. Am J Physiol Endocrinol Metab 2011; 301: E72–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yakabi K, Harada Y, Takayama K, Ro S, Ochiai M, Iizuka S, Hattori T, Wang L et al Peripheral alpha‐beta adrenergic interactions mediate the ghrelin response to brain urocortin 1 in rats. Psychoneuroendocrinology 2014; 50C: 300–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Paxinos G, Watson C. The rat brain in stereotaxic coordinates, 4th ed San Diego: Academic Press, 1998. [Google Scholar]
- 26. Fujitsuka N, Asakawa A, Hayashi M, Sameshima M, Amitani H, Kojima S, Fujimiya M, Inui A. Selective serotonin reuptake inhibitors modify physiological gastrointestinal motor activities via 5‐HT2c receptor and acyl ghrelin. Biol Psychiatry 2009; 65: 748–759. [DOI] [PubMed] [Google Scholar]
- 27. Date Y, Murakami N, Toshinai K, Matsukura S, Niijima A, Matsuo H, Kangawa K, Nakazato M. The role of the gastric afferent vagal nerve in ghrelin‐induced feeding and growth hormone secretion in rats. Gastroenterology 2002; 123: 1120–1128. [DOI] [PubMed] [Google Scholar]
- 28. Fujino K, Inui A, Asakawa A, Kihara N, Fujimura M, Fujimiya M. Ghrelin induces fasted motor activity of the gastrointestinal tract in conscious fed rats. J Physiol 2003; 550: 227–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Takeda H, Muto S, Nakagawa K, Ohnishi S, Sadakane C, Saegusa Y, Nahata M, Hattori T et al Rikkunshito as a ghrelin enhancer. Methods Enzymol 2012; 514: 333–351. [DOI] [PubMed] [Google Scholar]
- 30. Nahata M, Saegusa Y, Sadakane C, Yamada C, Nakagawa K, Okubo N, Ohnishi S, Hattori T et al Administration of exogenous acylated ghrelin or rikkunshito, an endogenous ghrelin enhancer, improves the decrease in postprandial gastric motility in an acute restraint stress mouse model. Neurogastroenterol Motil 2014; 26: 821–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gyires K, Zadori ZS, Shujaa N, Minorics R, Falkay G, Matyus P. Analysis of the role of central and peripheral alpha2‐adrenoceptor subtypes in gastric mucosal defense in the rat. Neurochem Int 2007; 51: 289–296. [DOI] [PubMed] [Google Scholar]
- 32. Shujaa N, Al‐Khrasani M, Zadori ZS, Rossi M, Matyus P, Nemeth J, Hein L, Gyires K. alpha(2)‐adrenoceptor agonist‐induced inhibition of gastric motor activity is mediated by alpha(2A)‐adrenoceptor subtype in the mouse. Neurochem Int 2011; 58: 708–713. [DOI] [PubMed] [Google Scholar]
- 33. Takahashi T, Owyang C. Mechanism of cholecystokinin‐induced relaxation of the rat stomach. J Auton Nerv Syst 1999; 75: 123–130. [DOI] [PubMed] [Google Scholar]
- 34. De Ponti F, Azpiroz F, Malagelada JR. Reflex gastric relaxation in response to distention of the duodenum. Am J Physiol 1987; 252: G595–G601. [DOI] [PubMed] [Google Scholar]
- 35. Geraghty JG, Angerson WJ, Carter DC. A study of regional gastric mucosal blood flow in a rat model of hepatic cirrhosis. Am J Physiol 1992; 262: G727–G731. [DOI] [PubMed] [Google Scholar]
- 36. Khoo J, Rayner CK, Feinle‐Bisset C, Jones KL, Horowitz M. Gastrointestinal hormonal dysfunction in gastroparesis and functional dyspepsia. Neurogastroenterol Motil 2010; 22: 1270–1278. [DOI] [PubMed] [Google Scholar]
- 37. Kihara N, Fujimura M, Yamamoto I, Itoh E, Inui A, Fujimiya M. Effects of central and peripheral urocortin on fed and fasted gastroduodenal motor activity in conscious rats. Am J Physiol Gastrointest Liver Physiol 2001; 280: G406–G419. [DOI] [PubMed] [Google Scholar]
- 38. Ariga H, Tsukamoto K, Chen C, Mantyh C, Pappas TN, Takahashi T. Endogenous acyl ghrelin is involved in mediating spontaneous phase III‐like contractions of the rat stomach. Neurogastroenterol Motil 2007; 19: 675–680. [DOI] [PubMed] [Google Scholar]
- 39. Takeda H, Sadakane C, Hattori T, Katsurada T, Ohkawara T, Nagai K, Asaka M. Rikkunshito, an herbal medicine, suppresses cisplatin‐induced anorexia in rats via 5‐HT2 receptor antagonism. Gastroenterology 2008; 134: 2004–2013. [DOI] [PubMed] [Google Scholar]
- 40. Yakabi K, Sadakane C, Noguchi M, Ohno S, Ro S, Chinen K, Aoyama T, Sakurada T et al Reduced ghrelin secretion in the hypothalamus of rats due to cisplatin‐induced anorexia. Endocrinology 2010; 151: 3773–3782. [DOI] [PubMed] [Google Scholar]
- 41. Yada T, Kohno D, Maejima Y, Sedbazar U, Arai T, Toriya M, Maekawa F, Kurita H et al Neurohormones, rikkunshito and hypothalamic neurons interactively control appetite and anorexia. Curr Pharm Des 2012; 18: 4854–4864. [DOI] [PubMed] [Google Scholar]
- 42. Nahata M, Muto S, Nakagawa K, Ohnishi S, Sadakane C, Saegusa Y, Iizuka S, Hattori T et al Serotonin 2C receptor antagonism ameliorates novelty‐induced hypophagia in aged mice. Psychoneuroendocrinology 2013; 38: 2051–2064. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Effects of UCN1 (1000 pmol/rat, ICV) alone (A) or in combination with acylated ghrelin (B) on change in gastroduodenal motility.


