Introduction
Multiple prospective randomized trials, now with long-term follow-up, have demonstrated that survival after breast conserving surgery (BCS) and whole breast radiotherapy is equivalent to mastectomy.1 Over time, rates of local recurrence after BCS have decreased and are now very similar to those seen after mastectomy.2, 3 In spite of this, a recent increase in rates of mastectomy in the United States has been observed after years of steady decline.4 In particular, women increasingly opt for bilateral instead of unilateral mastectomies even in the absence of a genetic predisposition or oncologic risk factor supporting the use of contralateral prophylactic mastectomy (CPM).5–7 This trend is particularly concerning as rates of contralateral breast cancer have also decreased due to the widespread use of adjuvant systemic therapy for early stage breast cancer, and there is no evidence that bilateral mastectomies with CPM prolong survival for women with sporadic breast cancer.8 Greater use of mastectomy, and particularly CPM, have been associated with younger age at diagnosis, greater educational attainment and socioeconomic status, race, higher histologic grade and in situ cancer (stage 0).4, 6, 9 While single-institution studies have shown an association between breast reconstruction and bilateral mastectomies with CPM, little is known about this relationship in larger and more representative patient samples.7
The Women’s Cancer and Health Rights Act (WCHRA) was enacted in 1998 to secure insurance coverage for breast reconstruction following mastectomy.10 Since the introduction of this legislation, rates of immediate breast reconstruction have increased gradually to approximately 38% of mastectomies.11 Greater access of immediate breast reconstruction may be an important unmeasured factor in women’s choice of surgical treatment for ESBC. For example, women who choose bilateral mastectomy have reconstruction rates approximately twice as high as women who choose unilateral mastectomy.11 The aim of the current study was to examine trends in the surgical management of ESBC while simultaneously assessing the role of breast reconstruction. We hypothesized that greater access to breast reconstruction is associated with the use of mastectomy for ESBC.
Methods
Data Source and Study Cohort
The primary data source was the National Cancer Data Base (NCDB), a joint initiative of the Commission on Cancer (CoC), the American College of Surgeons (ACoS) and the American Cancer Society. The NCDB is a nationwide oncology outcomes database for more than 1,500 Commission-accredited cancer programs. It includes information about patient and disease characteristics, treatment and outcomes for about 70% of all newly diagnosed cancers in the US and Puerto Rico.12 The study was approved by the CoC review board. The Commission on Cancer of the ACS does not require IRB approval for the current study since no patient identifiers are collected as part of the database.
The study cohort included women diagnosed with unilateral ESBC (stage 0, I or II of the American Joint Commission on Cancer (AJCC) staging criteria, 7th edition) from 1998 to 2011.13 Patients with synchronous bilateral cancers were excluded.
Outcomes and Predictors
The primary outcome was type of surgery, based on NCDB site-specific codes for breast conserving surgery (BCS), unilateral (UM), and bilateral procedures with contralateral prophylactic mastectomy (CPM). CPM was defined as bilateral mastectomy performed for unilateral breast cancer. Patients with unspecified or unknown type of surgery were excluded from analysis. The predictor of interest was the availability of breast reconstruction, based on annual rates of immediate, post-mastectomy breast reconstruction as recorded by the NCDB. All patients treated in a calendar year were assumed to have the same access to reconstruction.
Sociodemographic covariates and health characteristics included age at diagnosis, race, Charlson comorbidity score, median income and percent of non-high school graduates in the zip code of residence, type of health insurance, urban vs. rural residence, and facility geographic location. Disease characteristics included histology (lobular vs. ductal), tumor size, grade, invasion and the number of positive lymph nodes.
Statistical Analysis
Rates of each surgical procedure per 1,000 cases of ESBC were estimated for each year. Trends over time were analyzed using the Cochrane-Armitage test and Poisson regression. For the Poisson model, the dependent variable was the procedure rate, and the single independent variable was calendar year, with an observation for each year in the study period. The incidence rate ratio (IRR) estimated for year describes the trend in procedure rate over time, with values > 1.0 implying an increase and values < 1.0 suggesting a decrease. The influence of breast reconstruction rates on surgical treatment was estimated using a multinomial logistic regression model, controlling for sociodemographic and disease characteristics. In this model we estimated the impact of the predictor and covariates on the relative risk of CPM and the relative risk of UM, each compared with BCS. Variables were considered significant independent predictors of the outcome if p < 0.05.
In order to estimate the proportion of variability in CPM and UM use associated with each predictor, two separate multivariable logistic regression models for two outcomes were estimated: CPM (versus BCS) and UM (versus BCS). Changes in the pseudo-R2 for each model as each predictor was included and excluded were evaluated.14 All statistical analyses were performed using Stata 11.0 (Stata Corp., College Station, Texas).
Results
A total of 1,856,702 patients diagnosed with ESBC from 1998 to 2011were identified in the NCDB. The mean age at diagnosis was 60 years and 76% of patients were Caucasian (Table 1). Over 90% of patients had a Charlson comorbidity score of 0. More than half of the cohort (56%) had private health insurance, and only 2% were uninsured. Invasive cancer was present in 85% of cases, and of these, 60% of patients had a tumors less than 2 cm in size (T1). Only 14% of tumors were of lobular histology, and 79.5% did not have nodal involvement.
Table 1.
Characteristics of the cohort
| n | % | |
|---|---|---|
| Age (mean, SD) | 60.4 (13.3) | |
| Race | ||
| African-American | 161,972 | 8.7 |
| Caucasian | 1,406,389 | 75.8 |
| Asian | 44,192 | 2.4 |
| Hispanic | 225,528 | 12.6 |
| Other | 18,621 | 1.0 |
| Charlson comorbidity score | ||
| 0 | 1,693,848 | 91.2 |
| ≥1 | 162,854 | 8.8 |
| Zip code median income | ||
| <$30,000 | 201,285 | 11.4 |
| $31,000–34,999 | 287,626 | 16.3 |
| $35,000–45,999 | 481,254 | 27.3 |
| $46,000+ | 795,034 | 45.0 |
| Zip code population without high school diploma | ||
| 29%+ | 250,352 | 14.2 |
| 20.0–28.9% | 370,449 | 21.0 |
| 14.0–19.9 | 412,742 | 23.4 |
| <14.0% | 731,534 | 41.4 |
| Health insurance | ||
| Private | 1,040,948 | 56.1 |
| Medicaid | 74,774 | 4.0 |
| Medicare | 648,683 | 35.0 |
| Other public | 13,766 | 0.7 |
| Uninsured | 33,675 | 1.8 |
| Urban vs. rural residence | ||
| Urban | 1,726,267 | 98.3 |
| Rural | 30,456 | 1.7 |
| Facility location | ||
| Northeast | 409,269 | 22.0 |
| South | 651,354 | 35.0 |
| Midwest | 462,740 | 25.1 |
| West | 333,339 | 17.9 |
| Tumor size (T) | ||
| T0 (DCIS) | 286,481 | 15.4 |
| T1 (<2 cm) | 1,120,490 | 60.4 |
| T2 (2–4.9 cm) | 429,379 | 23.1 |
| T3 (>5 cm) | 20,352 | 1.1 |
| Positive lymph nodes | ||
| N0 (None) | 1,475,372 | 79.5 |
| N1 (1–3) | 381,330 | 20.5 |
| Tumor grade | ||
| Well differentiated | 380,124 | 20.5 |
| Moderately differentiated | 699,526 | 37.7 |
| Poorly differentiated | 535,519 | 28.8 |
| Undifferentiated | 24,194 | 1.3 |
| Carcinoma invasion | ||
| Invasive | 1,573,418 | 84.7 |
| DCIS | 238,284 | 15.3 |
| Lobular histology | ||
| No | 1,596,551 | 86.0 |
| Yes | 260,151 | 14.0 |
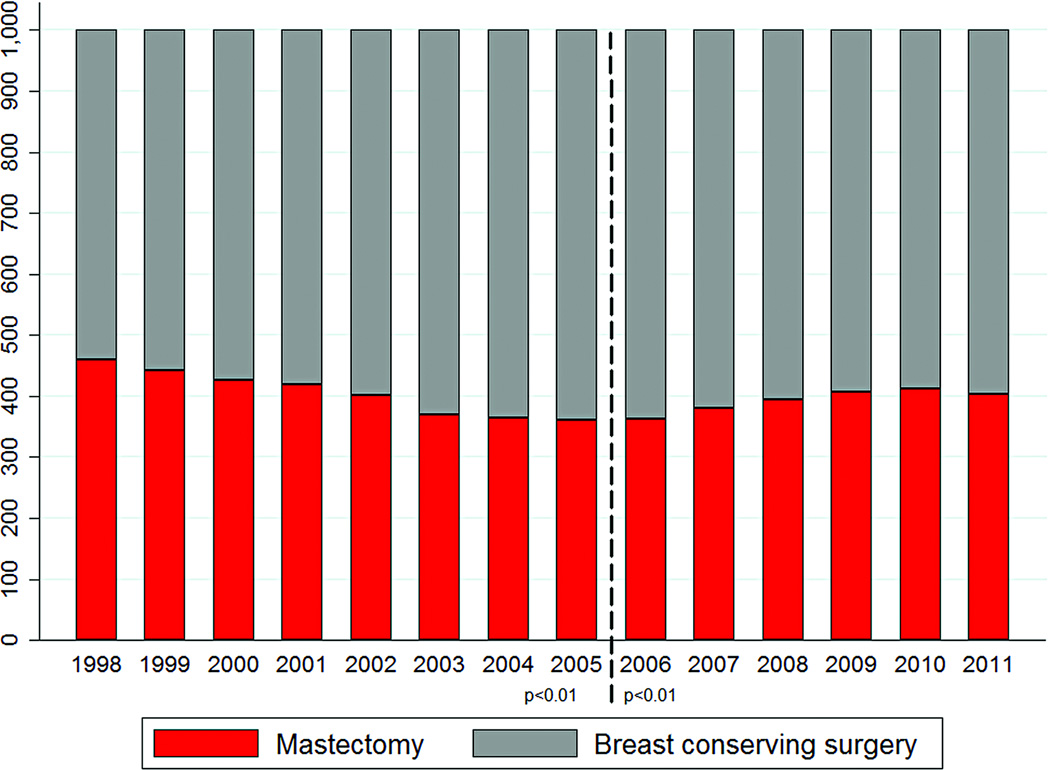
Figure 1 and Table 4 show rates of BCS and mastectomy per 1,000 cases of ESBC from 1998–2011. Mastectomy rates decreased from 459 per 1,000 in 1998 to a nadir of 361 per 1,000 in 2005 (p < 0.01 for trend). Thereafter, mastectomy rates steadily increased to 403 per 1,000 in 2011 (p < 0.01).
Figure 1.
Surgical treatment for early-stage breast cancer by year
*Dashed line represents the nadir of mastectomy rates
Table 4.
Annual rates of mastectomy* compared to breast conserving surgery (BCS) for the treatment of ESBC.
| Rates per 1,000 ESBC | |||
|---|---|---|---|
| Year | ESBC | BCS | Mastectomy* |
| 1998 | 122,178 | 540.8 | 459.2 |
| 1999 | 127,460 | 557.7 | 442.3 |
| 2000 | 129,240 | 574.1 | 425.9 |
| 2001 | 131,900 | 580.3 | 419.7 |
| 2002 | 132,758 | 598.6 | 401.4 |
| 2003 | 124,646 | 630.5 | 369.5 |
| 2004 | 122,815 | 635.3 | 364.7 |
| 2005 | 125,789 | 639.5 | 360.5 |
| 2006 | 131,545 | 637.4 | 362.6 |
| 2007 | 136,021 | 619.7 | 380.3 |
| 2008 | 141,313 | 604.8 | 395.2 |
| 2009 | 146,468 | 593.0 | 407.0 |
| 2010 | 140,553 | 587.3 | 412.7 |
| 2011 | 144,016 | 597.1 | 402.9 |
| Total | 1,856,702 | ||
Includes both UM and CPM
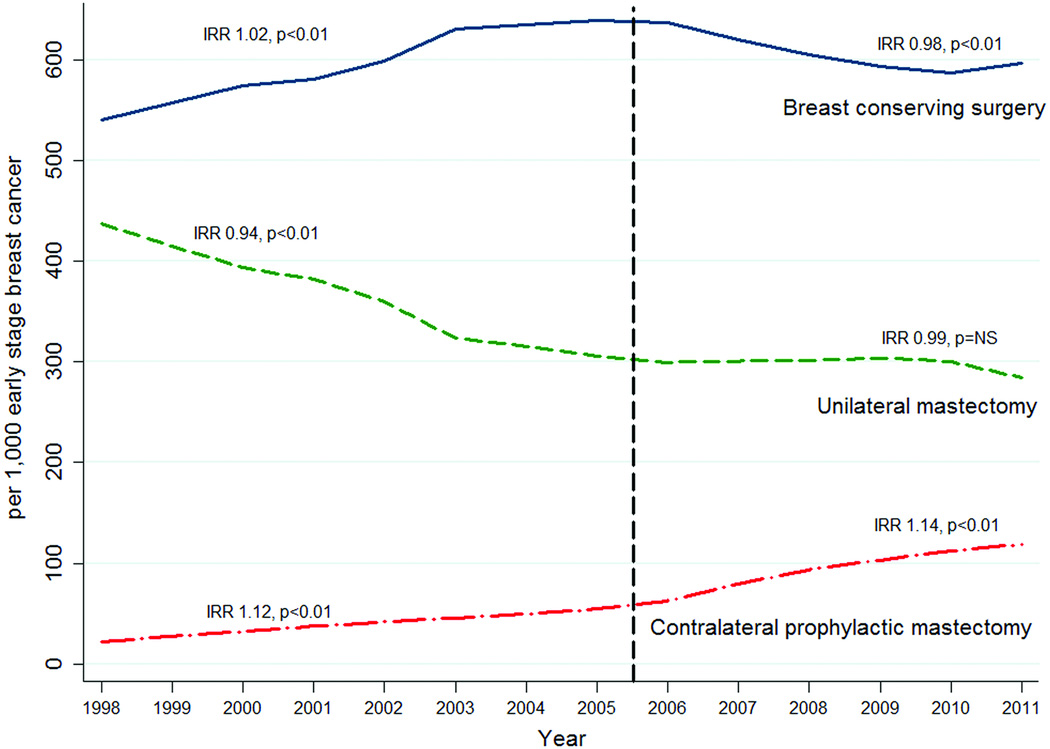
Figure 2 is a graphical representation of surgical trends for ESBC stratified by mastectomy type. From 1998 to 2005 BCS use increased from 540 to 639 per 1,000 ESBC (IRR 1.02, p < 0.01) while the rates of UM decreased from 437 to 306 per 1,000 ESBC (IRR 0.94, p < 0.01) (Figure 1). After 2005, the rates of BCS declined by 2% per year from 637 to 597 per 1,000 ESBC (IRR 0.98 p < 0.01), but without a significant corresponding increase in UM (IRR 0.99, p=NS). The rate of CPM increased significantly throughout the entire study period (IRR 1.13, p < 0.01). From 2005 to 2011, the rate of BCS decreased by 42 per 1,000 cases while there was a simultaneous increase in the rate of CPM of 64 per 1,000 ESBC (from 54 to 118 per 1,000 ESBC). This corresponds with a decrease in rates of UM by 22 per 1,000 cases during that time (from 306 to 284 per 1,000 ESBC).
Figure 2.
Trends in surgery for early-stage breast cancer, 1998–2011
Reconstruction use varied by year and by type of surgery (Table 5). Women who had CPM were more than twice as likely to have reconstruction as their peers who had UM. Immediate reconstruction rates after UM increased from 10% in 1998 to 27% in 2011, while reconstruction after CPM increased from 37% to 57%.
Table 5.
Immediate reconstruction rates by type of mastectomy, 1998–2011
| Year | Unilateral mastectomy (n) |
UM reconstructive rate (%) |
Contralateral prophylactic mastectomy (n) |
CPM reconstructive rate (%) |
|---|---|---|---|---|
| 1998 | 53,411 | 10.3 | 2,690 | 37.1 |
| 1999 | 52,870 | 13.1 | 3,510 | 44.0 |
| 2000 | 50,874 | 14.2 | 4,164 | 45.2 |
| 2001 | 50,385 | 14.8 | 4,969 | 44.6 |
| 2002 | 47,734 | 14.5 | 5,556 | 43.8 |
| 2003 | 40,357 | 14.9 | 5,704 | 42.8 |
| 2004 | 38,724 | 15.6 | 6,069 | 45.4 |
| 2005 | 38,483 | 16.6 | 6,862 | 46.8 |
| 2006 | 39,419 | 17.5 | 8,274 | 48.8 |
| 2007 | 40,910 | 19.8 | 10,822 | 50.5 |
| 2008 | 42,601 | 21.7 | 13,241 | 51.8 |
| 2009 | 44,521 | 25.0 | 15,088 | 53.1 |
| 2010 | 42,215 | 26.1 | 15,787 | 55.5 |
| 2011 | 40,928 | 27.4 | 17,090 | 56.7 |
UM: Unilateral mastectomy, CPM: contralateral prophylactic mastectomy
Independent predictors of the use of CPM compared to BCS were identified using a multinomial logistic regression model (Table 2). After adjustment for other factors, multivariable analysis demonstrated a significant association between the decision to pursue a CPM and breast reconstruction rates (RRR 1.07, 95% CI 1.05-1.07, p < 0.01), Young age, race other than African American, lower education level, rural area of residency, facility location, presence of comorbidities, large tumor size (> 5 cm), positive lymph nodes, DCIS, higher grade, and lobular histology were also significantly associated with a women’s decision to undergo CPM. The relative contribution of each factor to the likelihood of CPM is shown in Table 3. The three factors most associated with CPM were young age (32.2%), breast reconstruction (28.6%), and stage 0 (DCIS) (4.6%).
Table 2.
Impact of breast reconstruction availability on odds of UM and CPM (compared to BCS), adjusted by patient, disease and geography characteristics
| Adjusted RRR CPM |
95% CI | p | Adjusted RRR UM |
95% CI | p | |
|---|---|---|---|---|---|---|
| Breast reconstruction rates | 1.07 | 1.05–1.07 | <0.01 | 0.98 | 0.97–0.98 | <0.01 |
| Age | 0.94 | 0.94–0.95 | <0.01 | 1.01 | 1.00–1.01 | <0.01 |
| Race | <0.01 | <0.01 | ||||
| African-American | 1.00 | 1.00 | ||||
| Caucasian | 2.04 | 1.99–2.10 | 1.09 | 1.08–1.11 | ||
| Asian | 1.00 | 0.95–1.06 | 1.66 | 1.62–1.70 | ||
| Hispanic | 1.80 | 1.75–1.86 | 1.14 | 1.12–1.16 | ||
| Other | 1.55 | 1.45–1.66 | 1.21 | 1.17–1.25 | ||
| Charlson comorbidity score | <0.01 | <0.01 | ||||
| 0 | 1.00 | 1.00 | ||||
| ≥1 | 1.36 | 1.33–1.40 | 1.34 | 1.32–1.35 | ||
| Zip code median income | <0.01 | <0.01 | ||||
| <$30,000 | 1.00 | 1.00 | ||||
| $31,000–34,999 | 1.06 | 1.03–1.09 | 0.95 | 0.93–0.96 | ||
| $35,000–45,999 | 0.99 | 0.96–1.02 | 0.91 | 0.89–0.92 | ||
| $46,000+ | 0.97 | 0.94–1.00 | 0.85 | 0.84–0.87 | ||
| Zip code population without high school diploma | ||||||
| <0.01 | <0.01 | |||||
| 29%+ | 1.00 | 1.00 | ||||
| 20.0–28.9% | 1.14 | 1.11–1.17 | 0.94 | 0.93–0.95 | ||
| 14.0–19.9 | 1.17 | 1.14–1.20 | 0.91 | 0.89–0.92 | ||
| <14.0% | 1.33 | 1.29–1.36 | 0.89 | 0.88–0.90 | ||
| Health insurance | <0.01 | <0.01 | ||||
| Private | 1.00 | 1.00 | ||||
| Medicaid | 0.69 | 0.66–0.71 | 1.26 | 1.24–1.29 | ||
| Medicare | 0.98 | 0.96–1.00 | 1.25 | 1.23–1.26 | ||
| Other public | 0.98 | 0.92–1.04 | 1.09 | 1.05–1.13 | ||
| Uninsured | 0.73 | 0.68–0.76 | 1.26 | 1.18–1.24 | ||
| Urban vs. rural residence | <0.01 | <0.01 | ||||
| Urban | 1.00 | 1.00 | ||||
| Rural | 1.24 | 1.18–1.31 | 1.18 | 1.15–1.21 | ||
| Facility location | <0.01 | <0.01 | ||||
| Northeast | 1.00 | 1.00 | ||||
| South | 1.90 | 1.87–1.94 | 1.49 | 1.48–1.51 | ||
| Midwest | 1.55 | 1.52–1.58 | 1.35 | 1.34–1.37 | ||
| West | 1.60 | 1.57–1.64 | 1.14 | 1.12–1.15 | ||
| Tumor size (T) | <0.01 | <0.01 | ||||
| T0 (DCIS) | 1.00 | 1.00 | ||||
| T1 (<2 cm) | 0.47 | 0.44–0.51 | 0.48 | 0.46–0.50 | ||
| T2 (2–4.9 cm) | 0.72 | 0.66–0.78 | 1.01 | 0.96–1.04 | ||
| T3 (>5 cm) | 2.48 | 2.25–2.72 | 4.39 | 4.15–4.65 | ||
| Positive lymph nodes | <0.01 | <0.01 | ||||
| N0 (None) | 1.00 | 1.00 | ||||
| N1 (1–3) | 1.42 | 1.40–1.44 | 1.67 | 1.66–1.69 | ||
| Tumor grade | <0.01 | <0.01 | ||||
| Well differentiated | 1.00 | 1.00 | ||||
| Moderately differentiated | 1.18 | 1.16–1.20 | 1.24 | 1.23–1.26 | ||
| Poorly differentiated | 1.33 | 1.31–1.36 | 1.40 | 1.38–1.41 | ||
| Undifferentiated | 1.48 | 1.40–1.57 | 1.56 | 1.52–1.61 | ||
| Carcinoma invasion | <0.01 | <0.01 | ||||
| DCIS | 1.00 | 1.00 | ||||
| Invasive | 1.38 | 1.26–1.47 | 1.46 | 1.39–1.52 | ||
| Lobular histology | <0.01 | <0.01 | ||||
| No | 1.00 | 1.00 | ||||
| Yes | 1.83 | 1.80–1.86 | 1.30 | 1.29–1.31 | ||
Notes
RRR: Relative risk ratio, 95% CI: 95% Confidence interval
RRRs estimated in multinomial regression, with all characteristics included as predictors.
Table 3.
Variability in contralateral prophylactic mastectomy use (compared to BCS) explained by patient, disease and area characteristics
| Characteristic | % of variation explained |
|---|---|
| Young age | 32 |
| Breast reconstruction | 29 |
| Stage 0 (DCIS) | 5 |
| Lobular histology | 4 |
| Race | 4 |
| Tumor size | 4 |
| Facility location | 3 |
Percent of variation explained by each characteristic based on change in logistic regression pseudo-R2, with and without each characteristic. Only variables that changed the pseudo R2 by ≥2% are shown here. The full model included all characteristics shown in Table 3, and had a pseudo R2 of 0.1274, c-statistic 0.75, n=1,149,395.
Independent predictors for the use of UM compared to BCS were also examined in the multinomial logistic regression model (Table 2). Factors significantly associated with UM were older age, all races compared to African Americans, comorbidities, lower income, lower education status, rural area of residency, facility location, Medicaid, Medicare and other governmental insurances compared to private insurance, large tumors (> 5cm), positive lymph nodes, higher tumor grade, stage 0 (DCIS), and lobular histology. Breast reconstruction was negatively associated with UM (RRR 0.98, p < 0.01). The relative contribution of each factor to the likelihood to undergo UM is shown in Table 6. The most relevant factors were tumor size (43.4%), presence of DCIS (24.2%) and positive lymph nodes (13.5%).
Table 6.
Variability in unilateral mastectomy use (compared to BCS) explained by patient, disease and area characteristics
| Variable | % |
|---|---|
| Tumor size | 43 |
| Stage 0 (DCIS) | 24 |
| Positive lymph nodes | 14 |
| Breast reconstruction rates | 5 |
| Health insurance | 3 |
| Comorbidities | 2 |
Percent of variation explained by each characteristic based on change in logistic regression pseudo-R2, with and without each characteristic. Only variables that changed the pseudo R2 by ≥2% are shown here. The full model included all characteristics shown in Table 3, and had a pseudo R2 of 0.0495, c-statistic 0.64, n= 1,651,924
Discussion
Most women with ESBC can be treated safely with BCS with the added benefit of preserving the native breast. However, surgical treatment for ESBC is a “preference-sensitive” decision that should be made together by the patient and her breast surgeon considering individual clinical factors in conjunction with the patient’s values and preferences. The current study confirms, in a large, diverse patient sample, that after several years of decreasing use, rates of mastectomy for ESBC have risen since 2005. The trend of increased mastectomy rates identified in the current study is in agreement with a recent report from the SEER database.4 In that study, the choice for mastectomy was associated with a variety of sociodemographic and oncologic variables; however, there was no evaluation of mastectomy type (CPM versus UM) or breast reconstruction. The current report is novel in that it demonstrates a decrease of BCS, but without a corresponding increase in UM. Instead, patients may be deciding between BCS and removal of both breasts (CPM) when diagnosed with ESBC (Figure 2).
Why more women are choosing an aggressive surgical treatment for ESBC when less invasive alternatives are available is unclear.25 Most bilateral mastectomies with CPM are performed in patients who are at low-risk of developing contralateral cancer.7 Although the 10-year risk of contralateral cancer is approximately 5%, newly diagnosed patients tend to overestimate their level of risk.15, 16 Other reasons patients cite for choosing CPM include achieving “peace of mind”, avoidance of ongoing surveillance and diagnostic procedures, and desire for breast symmetry following reconstruction.17, 18 While in the past access to breast reconstruction was limited, breast reconstruction is now more available with coverage mandated through federal and state legislation. Furthermore, improvements in both mastectomy (e.g. skin sparing and nipple sparing) and reconstructive (e.g. silicone implant safety and shape) techniques may make CPM an increasingly attractive option for women. Other reasons that could partially explain the decision of undergo CPM over BCS are avoidance of radiotherapy and chance of recurrence.
While a variety of sociodemographics and oncologic factors impact decision-making for the surgical treatment of ESBC, breast reconstruction needs to be considered. In this study, breast reconstruction rates were the second most important factor associated with undergoing CPM compared to BCS (explaining 28% of the variability). Only patient age was more strongly associated with the use of bilateral mastectomies with CPM. Interestingly, breast reconstruction was negatively associated with the decision for UM compared to BCS. The choice for UM for ESBC is better explained by oncologic factors (tumor size, DCIS, and positive lymph nodes). The strength of the relationship between mastectomy type and breast reconstruction is evidenced by the reconstructive rates for CPM, which are more than double those for UM. Breast reconstruction appears to substantially influence patient choice of bilateral mastectomy for ESBC. In a study of 206 patients who underwent CPM, 59% of them indicated that the availability of breast reconstruction was an influencing factor in the decision.19 A population-based survey of 1,178 women from two major metropolitan areas, showed that patients who discussed breast reconstruction with their general surgeon were 2 times more likely to consider mastectomy and 4 times more likely to receive a mastectomy.20 Greenberg et al, using a patient sample from the National Comprehensive Cancer Network found that greater numbers of plastic surgeons and a shorter waiting time to mastectomy with reconstruction were significantly associated with the use of mastectomy rather than BCS, although they did not analyze UM and CPM separately.14 Along with cancer fear, “Desire to have both breasts look the same after surgery” (57%) and “Desire to make breasts look better” (27%) are considerations for CPM cited by women when asked about important reasons for undergoing this procedure.17, 21
In breast cancer surgery, the quality of the decisions can be estimated by the extent to which patients are informed, involved in decision-making, and undergo treatments that reflect their values.22 Greater patient involvement has been associated with increased likelihood of mastectomy; however, greater involvement is separate from health literacy.23 Approximately 35–40% of ESBC patients have adequate knowledge about survival or recurrence rates following BCS and mastectomy.22 Furthermore, the risk of developing contralateral cancer is overestimated by women.16 Patients may also have unrealistic expectations about the reconstructive benefits of CPM. A multicenter study showed that 21–33% of patients who underwent CPM felt that the number of surgical procedures, cosmetic results, complications and recovery from reconstructive surgery were worse than expected.21 Patients should be aware of the increased complication rates following bilateral mastectomies, 7.6% compared to 4.2% in unilateral procedures.24 Improved preoperative education is needed to ensure high quality decisions are made while simultaneously setting realistic expectations.
It seems counterintuitive that in an era of minimally invasive surgery, many women with ESBC are choosing more extensive treatment. The current patient-centered healthcare model has empowered patients to become active participants in their care decisions undergoing services based on individual needs/preferences.25 A possible explanation for the evolution in women’s surgical choice from BCS to bilateral mastectomy is that both treatments share the property of theoretical symmetry. Although not all patients who have BCS or bilateral mastectomy end up with symmetry, patients who are deciding about surgery likely consider these options as maximizing symmetry (and UM as not preserving symmetry). Another possible explanation for re-framing of the surgical choice may be a form of decision momentum.26 Once the patient knows she will not have BCS, either by choice or medical necessity, she may begin to put less value on the importance of preserving her contralateral breast, consciously or unconsciously. People are known to respond to adverse circumstances or loss of choice by reducing their cognitive dissonance through adaptation or even preference reversal.27–30
The current study has limitations. The NCDB is not a population-based registry, although the large numbers of ESBC patients included in the current analysis may confer generalizability. The trends reported herein are also concordant with findings using the SEER database, further supporting their validity.4 Other limitations include a lack of information on previous attempts at BCS, incidence of multicentricity, BRCA mutation status, high familiar/genetic risk or preoperative MRI use, all factors which influence the decision for mastectomy. The information presented here demonstrates an association between breast reconstruction rates and surgical treatment for ESBC, but does not imply causality. Further insight about the role of breast reconstruction on the decision-making process for CPM needs to be obtained through qualitative interviews with patients. Another limitation is that NCDB has no information on delayed reconstruction. The association between reconstruction and CPM may be stronger if delayed reconstructions were included.
In conclusion, since 2005 an increasing proportion of patients with ESBC are choosing mastectomy for their surgical treatment. The observed increase in mastectomy rates is attributable to a shift towards bilateral mastectomy with CPM, not UM. While a variety of oncologic factors influence decision-making, wider breast reconstruction access and acceptance may facilitate the option for more radical surgery. Evolution of the surgical treatment for ESBC has important implications for patient care, the design of decision support tools, and health care policy.
Supplementary Material
Acknowledgments
“The data used in the study are derived from a de-identified NCDB file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigator”
Footnotes
Disclosures: The authors have no disclosures
References
- 1.Early Breast Cancer Trialists' Collaborative, G. Darby S, McGale P, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378:1707–1716. doi: 10.1016/S0140-6736(11)61629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson SJ, Wapnir I, Dignam JJ, et al. Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in patients treated by breast-conserving therapy in five National Surgical Adjuvant Breast and Bowel Project protocols of node-negative breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:2466–2473. doi: 10.1200/JCO.2008.19.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zumsteg ZS, Morrow M, Arnold B, et al. Breast-conserving therapy achieves locoregional outcomes comparable to mastectomy in women with T1-2N0 triple-negative breast cancer. Annals of surgical oncology. 2013;20:3469–3476. doi: 10.1245/s10434-013-3011-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mahmood U, Hanlon AL, Koshy M, et al. Increasing national mastectomy rates for the treatment of early stage breast cancer. Annals of surgical oncology. 2013;20:1436–1443. doi: 10.1245/s10434-012-2732-5. [DOI] [PubMed] [Google Scholar]
- 5.Tuttle TM, Habermann EB, Grund EH, et al. Increasing use of contralateral prophylactic mastectomy for breast cancer patients: a trend toward more aggressive surgical treatment. J Clin Oncol. 2007;25:5203–5209. doi: 10.1200/JCO.2007.12.3141. [DOI] [PubMed] [Google Scholar]
- 6.Tuttle TM, Jarosek S, Habermann EB, et al. Increasing rates of contralateral prophylactic mastectomy among patients with ductal carcinoma in situ. J Clin Oncol. 2009;27:1362–1367. doi: 10.1200/JCO.2008.20.1681. [DOI] [PubMed] [Google Scholar]
- 7.King TA, Sakr R, Patil S, et al. Clinical management factors contribute to the decision for contralateral prophylactic mastectomy. J Clin Oncol. 2011;29:2158–2164. doi: 10.1200/JCO.2010.29.4041. [DOI] [PubMed] [Google Scholar]
- 8.Nichols HB, Berrington de Gonzalez A, Lacey JV, Jr, et al. Declining incidence of contralateral breast cancer in the United States from 1975 to 2006. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:1564–1569. doi: 10.1200/JCO.2010.32.7395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arrington AK, Jarosek SL, Virnig BA, et al. Patient and surgeon characteristics associated with increased use of contralateral prophylactic mastectomy in patients with breast cancer. Ann Surg Oncol. 2009;16:2697–2704. doi: 10.1245/s10434-009-0641-z. [DOI] [PubMed] [Google Scholar]
- 10.Congress of the United Stated of America. Women's Health and Cancer Rights Act of 1998. 1998 http://www.cms.gov/Regulations-and-Guidance/Health-Insurance-Reform/HealthInsReformforConsume/downloads/WHCRA_Statute.pdf Consulted January 6, 2013.
- 11.Albornoz CR, Bach PB, Mehrara BJ, et al. A paradigm shift in u.s. Breast reconstruction: increasing implant rates. Plast Reconstr Surg. 2013;131:15–23. doi: 10.1097/PRS.0b013e3182729cde. [DOI] [PubMed] [Google Scholar]
- 12.American College of Surgeons. National Cancer Database. 2013 http://www.facs.org/cancer/ncdb/index.html# Consulted September 1, 2013.
- 13.American Joint Committee on Cancer (AJJCC) Breast Cancer Staging, 7th edition. 2010 http://cancerstaging.org/references-tools/quickreferences/Documents/BreastMedium.pdf Consulted December 1, 2013.
- 14.Greenberg CC, Lipsitz SR, Hughes ME, et al. Institutional variation in the surgical treatment of breast cancer: a study of the NCCN. Annals of surgery. 2011;254:339–345. doi: 10.1097/SLA.0b013e3182263bb0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao X, Fisher SG, Emami B. Risk of second primary cancer in the contralateral breast in women treated for early-stage breast cancer: a population-based study. International journal of radiation oncology, biology, physics. 2003;56:1038–1045. doi: 10.1016/s0360-3016(03)00203-7. [DOI] [PubMed] [Google Scholar]
- 16.Abbott A, Rueth N, Pappas-Varco S, et al. Perceptions of contralateral breast cancer: an overestimation of risk. Annals of surgical oncology. 2011;18:3129–3136. doi: 10.1245/s10434-011-1914-x. [DOI] [PubMed] [Google Scholar]
- 17.Han E, Johnson N, Glissmeyer M, et al. Increasing incidence of bilateral mastectomies: the patient perspective. American journal of surgery. 2011;201:615–618. doi: 10.1016/j.amjsurg.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 18.Katz SJ, Morrow M. Contralateral prophylactic mastectomy for breast cancer: addressing peace of mind. JAMA : the journal of the American Medical Association. 2013;310:793–794. doi: 10.1001/jama.2013.101055. [DOI] [PubMed] [Google Scholar]
- 19.Soran A, Ibrahim A, Kanbour M, et al. Decision Making and Factors Influencing Long-term Satisfaction With Prophylactic Mastectomy in Women With Breast Cancer. American journal of clinical oncology. 2013 doi: 10.1097/COC.0b013e318292f8a7. [DOI] [PubMed] [Google Scholar]
- 20.Alderman AK, Hawley ST, Waljee J, et al. Understanding the impact of breast reconstruction on the surgical decision-making process for breast cancer. Cancer. 2008;112:489–494. doi: 10.1002/cncr.23214. [DOI] [PubMed] [Google Scholar]
- 21.Rosenberg SM, Tracy MS, Meyer ME, et al. Perceptions, knowledge, and satisfaction with contralateral prophylactic mastectomy among young women with breast cancer: a cross-sectional survey. Annals of internal medicine. 2013;159:373–381. doi: 10.7326/0003-4819-159-6-201309170-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee CN, Chang Y, Adimorah N, et al. Decision making about surgery for early-stage breast cancer. Journal of the American College of Surgeons. 2012;214:1–10. doi: 10.1016/j.jamcollsurg.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katz SJ, Lantz PM, Janz NK, et al. Patient involvement in surgery treatment decisions for breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23:5526–5533. doi: 10.1200/JCO.2005.06.217. [DOI] [PubMed] [Google Scholar]
- 24.Osman F, Saleh F, Jackson TD, et al. Increased postoperative complications in bilateral mastectomy patients compared to unilateral mastectomy: an analysis of the NSQIP database. Annals of surgical oncology. 2013;20:3212–3217. doi: 10.1245/s10434-013-3116-1. [DOI] [PubMed] [Google Scholar]
- 25.Agency for Healthcare Research and Quality, R., MD. Expanding Patient-Centered Care To Empower Patients and Assist Providers: Research in Action. 2002 http://www.ahrq.gov/research/findings/factsheets/patient-centered/ria-issue5/index.html Consulted January 31, 2014.
- 26.Dhar R, Huber J, Khan U. The Shopping Momentum Effect. Journal of Marketing Research. 2007;44:370–378. [Google Scholar]
- 27.Gilbert DT, Pinel EC, Wilson TD, et al. Immune neglect: a source of durability bias in affective forecasting. Journal of personality and social psychology. 1998;75:617–638. doi: 10.1037//0022-3514.75.3.617. [DOI] [PubMed] [Google Scholar]
- 28.Sharot T, Velasquez CM, Dolan RJ. Do decisions shape preference? Evidence from blind choice. Psychological science. 2010;21:1231–1235. doi: 10.1177/0956797610379235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brehm JW. Postdecision changes in the desirability of alternatives. Journal of abnormal psychology. 1956;52:384–389. doi: 10.1037/h0041006. [DOI] [PubMed] [Google Scholar]
- 30.Festinger L. Cognitive dissonance. Scientific American. 1962;207:93–102. doi: 10.1038/scientificamerican1062-93. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.