Abstract
Objectives
Clinical outcomes are worse for patients with heart failure (HF) and elevated depression symptoms. Depression related sympatho-immune dysregulation may be one mechanism leading to poorer HF prognosis. Sympathetically mediated adrenergic activity is known to regulate immune activity via β-adrenergic receptors (β-ARs). However, studies show conflicting relationships between leukocyte β-AR sensitivity and depression symptoms. The aim of this study was to determine in patients with HF the relationship of leukocyte β-AR sensitivity with two diverse measures of depression, self-report questionnaire versus clinical diagnostic interview.
Methods
Patients with HF (N=73, mean age = 56.3, S.D. = 13.0) completed the Beck Depression Inventory −1A (BDI) and a modified Structured Clinical Interview for the DSM-IV (SCID). Leukocyte β-AR sensitivity was determined from isoproterenol stimulated cyclic AMP levels; plasma norepinephrine and epinephrine were also assessed.
Results
Patients with major depression determined by SCID had significantly higher β-AR sensitivity than non-depressed (F(6, 72) = 9.27, p = .003, η2 = .12). Meanwhile, the BDI revealed a more complex relationship. Minimal, mild, and moderate-to-severe depression symptom groups had significant differences in β-AR sensitivity (F(7, 72) = 7.03, p = .002, η2 = .18), with mild symptoms appearing to correspond with reduced β-AR sensitivity and moderate-to-severe symptoms with higher β-AR sensitivity.
Conclusions
By deconstructing depression measurements a greater depth of information may be garnered to potentially reveal subtypes of depression symptoms and their relation to β-AR sensitivity in HF.
Keywords: Beta-adrenergic, heart failure, depression
INTRODUCTION
Between 20% and 65% of patients with symptomatic heart failure (HF) have clinically significant depression symptoms (1). In turn, elevated depression symptoms and major depression in patients with HF are associated with significantly increased risk of cardiovascular hospitalization and premature mortality, independent of HF severity (2-4). Neuroimmune factors have been proposed as mechanistic linkages between symptoms of depression and injury to the cardiovascular system (5, 6) that can worsen HF prognosis (7, 8). Both depression and HF are characterized by sympathetic nervous system (SNS) dysregulation (9-12). In HF, as a compensatory response to preserve perfusion during low-output states, autonomic hyperactivity and neurohormonal activation become pathologic over time, leading to deterioration of left ventricular function (13). Elevated depressive symptoms have also been associated with increased and prolonged sympathetic activation including, norepinephrine (NE), heart rate and blood pressure responses to stress and greater psychological stress-induced myocardial ischemia (9, 14). Although, the association between resting levels of NE and depression symptoms is less clear and have been reported in some studies to have a non-linear relationship (9). Sympathetic innervation is involved in regulating the immune system (15, 16), a relationship thought to be important in the development and progression of cardiovascular disease (CVD) (17). Sympathetic activity mediated through endogenous adrenergic activity is known to regulate cytokine production(18), immune cell proliferation (19) and mobilization (20) which can affect immune cell recruitment, infiltration and proliferation in myocardial and vascular tissue and prospectively influence cardiovascular remodeling (7, 8) and ventricular hypertrophy (21). Thus, multiple pathophysiological processes of SNS dysregulation associated with depression may exacerbate HF-related autonomic dysfunction.
However, definitive relationships between depressive symptoms and immune cell beta-adrenergic receptor (β-AR) function are unclear, and findings have been conflicting. Animal models of depression found an increment in leukocyte β-AR number and intracellular responses to a β-agonist (22) and increased leukocyte sensitivity to stress hormones, such as NE (23). Our research in patients with HF suggest that elevated depression symptoms are associated with augmented immune cell mobility to β-agonist following exercise (20), suggesting increased β-AR sensitivity. In contrast, other studies have found either no relationship(24), or reduced sensitivity of β-ARs in depressed patients (25). Inconsistent findings such as these may be attributable to the methods by which depression is measured and potentially the heterogeneity of depression itself, as well as the subject populations being studied (i.e. physically healthy versus patients with HF). Although many depression studies in humans present findings based on a continuum of depression symptom level scores, others present data based on a binomial, positive or negative diagnosis of major depression (e.g. meta-anlaysis: (26)). We investigated peripheral blood mononuclear cell (PBMC) β-AR sensitivity in relation to depression in patients with HF, which has not been previously examined to the best of our knowledge. Studies such as ours including both symptom severity and diagnostic measures of depression may offer improved insights into the depression- β -adrenergic activity relationship in patients with HF.
METHODS
Study participants
The study included 73 patients with HF assessed for β-AR sensitivity, depressive symptom level, major depression diagnostic criteria, sociodemographic variables and plasma NE and epinephrine (Epi) levels, from 2005 to 2010. Patients were recruited from the VA San Diego Healthcare System and the University of California-San Diego Medical Center as part of a larger study on the effects of depression on neuroimmunity in HF. Patients with HF fell within New York Heart Association (NYHA) functional classes II (n = 61) and III (n = 12), had symptoms of HF for at least 3 months, were optimally treated with diuretics, and angiotensin-converting enzyme inhibitors, and had systolic dysfunction, defined by an left ventricular ejection fraction (LVEF) < 45%, or HF with preserved EF (HFpEF). In order to reduce heterogeneity that may occur by including patients taking diverse β -blockers, 73 patients with HF were included in the present study that were taking carvedilol, a non-specific β - 1 and β -2 adrenergic receptor blocker frequently used to treat HF. LVEF was assessed by echocardiography. Exclusion criteria included recent myocardial infarction (1 month), significant cerebral neurological impairment, severe chronic obstructive pulmonary disease, benzodiazepines, steroidal medications, and psychiatric illnesses with the exception of major depression. The protocol was approved by the University of California-San Diego, Institutional Review Board, and participants gave written informed consent. The study was performed in accordance with the principles of the Declaration of Helsinki.
Depression
Depressive symptoms were assessed with the 21-item Beck Depression Inventory-1A (BDI), which is an instrument recommended for the measurement of depression in CVD (27). BDI scores were subcategorized into minimal (0-10), mild (11-19), and moderate-to-severe symptoms of depression (20+) (28). Various studies have used these categories in relation to CVD risk. For example, moderate to severe depression symptoms have been associated with subclinical alterations in left ventricular (LV) structure and function (29) and dose-response relationships have been found between depression symptoms and 5-year prognosis in post myocardial infarction patients (30). A modified Structured Clinical Interview for DSM-IV(31) was used to evaluate for major depressive disorder.
Blood draws
Blood was drawn into Vacutainer tubes (BD Biosciences, San Jose, California) coated with ethylenediaminetetraacetic acid after participants fasted for at least 12 hours, at approximately 0800 following a 10 minute rest period, while participants were in a sitting position.
β-adrenergic receptors
β-AR sensitivity was determined for peripheral blood mononuclear cells (PBMC's) as described earlier (32). Briefly, PBMCs were suspended in cold Dulbecco's Modified Eagle's Medium (DMEH). A 100 ul suspension of approximately 2×105 cells was brought up to 1 ml with 37 °C DMEH containing 100 mmol/l isobutylmethylxanthine (IBMX) to inhibit cyclic nucleotide phosphodiesterase activity. Half of the tubes were incubated with 10 μM isoproterenol. Upon reaction termination, the tubes were frozen at 4 pH at −20°C in sample kit Stop solution and later assayed for cyclic AMP (Perkin Elmer, Boston, MA) using a radio immune assay. Samples are stable for up to a year at −20°C. The assays were run within four weeks of receiving the kits. All kits were from the same lot number. Interassay and intra-assay coefficients of variation were < 10%, and < 11% respectively. The detection range is 500 – 50,000 fmol/mL Beta-adrenergic receptors can be in either a high-affinity, coupled (functionally capable of coupling to the G-protein-adenyl cyclase complex following agonist binding) or a low-affinity, uncoupled state. “Sensitivity” is used to signify the responsiveness of the β-receptor to isoproterenol stimulation and thus the ratio of isoproterenol-stimulated cyclic AMP to non-stimulated cyclic AMP was calculated as a stimulation index (SI).
NE and Epi measures
NE and Epi levels were determined as described earlier (33). Briefly, blood samples were centrifuged, and plasma was stored at −80°C until analysis. NE and Epi are stable in plasma stored below −70 degrees for one year. The plasma samples had catecholamines extracted by chelation with diphenylborate into a lipid layer and then extracted into dilute acetic acid to eliminate calcium and S-adenosylmethionine, which can interfere with the assay. The NE and Epi were converted to their radiolabelled metanephrine metabolites by catechol-O-methyltransferase, chromatographically separated, and the radioactivity counted. Sensitivity of the assay for NE and Epi is 10 and 6 pg/mL respectively in 1 ml of plasma. The intraassay coefficients of variation for NE and Epi are 4 and 13% and the interassay coefficients of variation for NE and Epi are 10 and 16% in human plasma. The major advantage of the assay is its sensitivity, which permits more accurate estimation of the low Epi levels in resting subjects.
Statistical analyses
Calculations were performed using SPSS version 21 (SPSS, Inc., Chicago, Illinois). Cases with missing data were excluded using list-wise deletion(34). Skewed data distribution was determined by the Kolmogorov- Smirnov test, and variables not normally distributed were log transformed. Analyses of categorical data were performed with Mann-Whitney U tests. Receiver operating characteristics (ROC) were computed to determine the relationship of BDI scores as related to the DSM-IV diagnosis of depression. A linear regression analysis was performed to examine the association between BDI scores and PBMC β-AR sensitivity. An additional regression analysis was performed with a quadratic function to determine whether there was a curvilinear relationship between BDI scores and PBMC β-AR sensitivity. Two analyses of covariance (ANCOVA) were performed to examine: 1) differences in major depression status for β-AR sensitivity, NE and Epi and 2) differences in BDI categories of depression severity (minimal, mild, and moderate-to-severe) for β-AR sensitivity, NE and Epi. Analyses were adjusted for cardiovascular risk factors including LVEF, NYHA class and HFpEF. Analyses were adjusted for antidepressant use due to their potential effects on depression scores, and race because of potential differences in β-AR sensitivity (35). The effect sizes are reported as Partial Eta Squared (η2). Cohen (1988), p. 283 suggests for η2 where 0.0099 constitutes a small effect, 0.0588 a medium effect and 0.1379 a large effect (36). Analyses were also rerun without LVEF as a covariate since contractility depends on SNS drive.
RESULTS
Sociodemographic and medical characteristics of the study groups
Table 1 presents sociodemographic and medical characteristics in relation to major depression status. The BDI scores, NE, E and β-AR sensitivity variables were not normally distributed (Kolmogorov-Smirnov tests p < .001). BDI scores, NE and E were normalized with log transformation. However, log transformation did not result in complete normalization of stimulated cAMP (β-AR sensitivity), with the Kologorov-Smirnov test yielding p = .042. Nonetheless, a normal probability P-P plot suggested that transformed β-AR sensitivity had a linear pattern with only minor deviations from the line fit to the points on the probability plot indicating that they approach a normal distribution. The ROC analysis (see Supplementary Figure 1) revealed that BDI scores significantly predicted diagnosis (area under the curve (AUC) = .94, SEM = .026, p < 0.001, r = .66). Additional analyses revealed that patients with HF with systolic dysfunction versus those with HFpEF did not differ in PBMC β-AR sensitivity (p = .87), BDI scores (p =0.85), NE (p = .52) and Epi (p = .48). There were no sex differences in PBMC β-AR sensitivity (p= .87), BDI scores (p = .64), NE (p = .38) and Epi (p = .93). There were no differences between NYHA II and III for β-AR sensitivity (p = .41), NE (p = .67) and Epi (p = .89), although there was a trend for higher BDI scores in NYHA III (p = .091). Patients with HF taking antidepressants compared with those not taking antidepressants did not differ in BDI scores (p =.38), or β-AR sensitivity (p = .21), or NE (p = .11), although there was a trend for patients taking antidepressants to have higher Epi levels (p = .06).
Table 1.
Patient characteristics
| Measure | Major Depression (N = 17) | No Major Depression (N = 56) | Total (N = 73) | p value |
|---|---|---|---|---|
| BNP, pg/mL (mean ± s.d) | 156.1 (178.4) | 237.5 (319.7) | 219.2 (294.7) | 0.21 |
| Age (mean ± s.d) | 52.9(12.6) | 57.4 (13.0) | 56.3 (13.0) | 0.15 |
| BMI (mean ± s.d) | 34.1 (10) | 31.5 (7.6) | 32.0 (8.2) | 0.45 |
| BDI (mean ± s.d) | 23.7 (8.6) | 9.3(5.0) | 12.6 (8.6) | < 0.001 |
| Women (N, %) | 7 (41%) | 11 (20%) | 18 (25%) | .065 |
| Antidepressants (N, %) | 4 (23%) | 5 (9%) | 9 (12%) | 0.10 |
| Current Smoker (N, %) | 2 (12%) | 9 (16%) | 11 (15%) | 0.68 |
| College grad (N, %) | 1 (6%) | 10 (18%) | 11 (15%) | 0.13 |
| White (N, %) | 10 (59%) | 39 (70%) | 49 (67%) | 0.82 |
| HF with preserved systolic function (N, %) | 2 (12%) | 3 (5%) | 5 (7%) | 0.35 |
| LVEF (%) | 32.2% | 29.5% | 30.1% | 0.35 |
| NYHA III (N, %) | 5 (29%) | 10 (18%) | 15 (20%) | 0.28 |
s.d. = standard deviation, BNP = brain- type natriuetic peptide, BMI = body mass index, BDI = Beck Depression Inventory, s.i. = stimulation index, Epi = epinephrine, NE = norepinephrine, HF = heart failure, EF = ejection fraction, NYHA = New York heart association.
Depressive symptoms and cAMP, NE and Epi
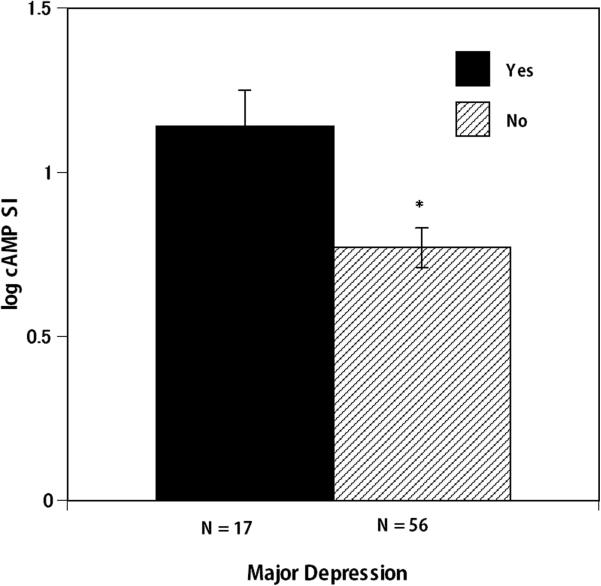
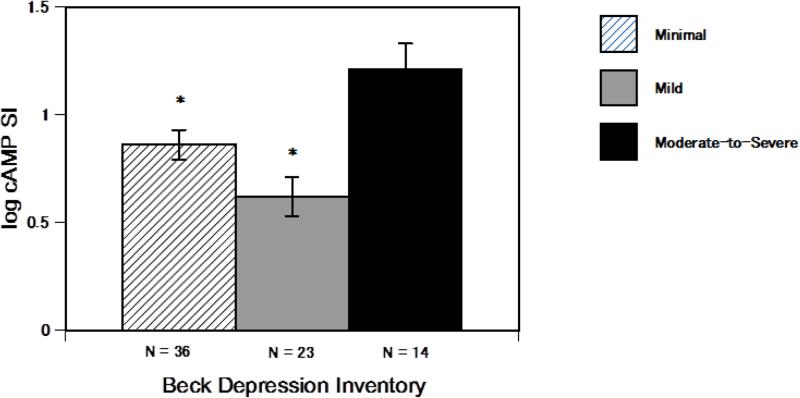
An ANCOVA revealed that in patients with HF, major depression (binomial categories: yes or no) was associated with differential PBMC β-AR sensitivity (F(6, 72) = 9.27, p = .003, η2 = .12) (see figure 1), whereby those with major depression (n = 17, 20% of the cohort) had increased β-AR sensitivity. Whereas, linear regression analyses revealed that BDI scores treated as a continuous independent variable of depression symptoms were not significantly related to β-AR sensitivity (p = .13, standardized β = .19). Adding a quadratic function to the regression equation revealed only a slight improvement in the fit of the model with the R2 increasing from .032 to .045 which was not significant (p = .37). This suggests that the relationship between BDI scores and β-AR sensitivity do not fit a simple curvilinear model (see Supplementary Figure 3). However, an ANCOVA comparing categories of depression symptom groups from BDI scores: minimal (n = 34), mild (n = 23), and moderate-to-severe (n = 14) revealed significant differences in β-AR sensitivity (F(7, 72) = 7.03, p = .002, η2 = .18) (see figure 2). Pair-wise comparisons revealed that those with moderate-to-severe depression symptoms had significantly higher β-AR sensitivity than those with mild depression symptom levels (p = .001). Whereas, those with mild depression symptom levels had significantly lower β-AR sensitivity than those with minimal depression symptom levels (p = .049). This suggests that differential β-AR sensitivity may occur depending on the BDI severity category. Meanwhile, neither major depression status (p = .86 and p = .10 respectively) or BDI categories of depression symptom severity were related to Epi and NE (p = .66 and p = .49 respectively). Furthermore, β-AR sensitivity was not related to Epi and NE levels (p = .40, r = −.10 and p = .12, r = −.19 respectively). All analyses were performed adjusting for LVEF, NYHA class, antidepressant use, race and HFpEF. The analyses were repeated without statistically adjusting for LVEF, since ejection fraction depends on contractility that in turn depends on SNS drive. Results did not differ when LVEF was removed as covariate from the analyses.
Figure 1.
Heart failure patients with major depression had significantly higher beta 2- adrenergic receptor sensitivity (determined with cAMP stimulation index) compared with heart failure patients without major depression. Reported means are adjusted for LVEF, NYHA class and HF with preserved systolic function, antidepressant use and race. Error bars consist of standard error of the mean (SEM). log cAMP SI = log isoproteronol stimulated /log non-stimulated cAMP levels of peripheral blood mononuclear cells. * p < .05
Figure 2.
A comparison of heart failure patients that scored in the range of minimal, mild and moderate-to-severe depression symptoms using the Beck Depression Inventory for beta 2- adrenergic receptor sensitivity (determine with cAMP stimulation index). Patients with mild depression symptoms had significantly lower beta 2- adrenergic receptor sensitivity than those with minimal symptoms. Patients with moderate-to-severe depression symptoms had significantly higher beta 2- adrenergic receptor sensitivity than patients with mild depression symptoms. Reported means are adjusted for LVEF, NYHA class and HF with preserved systolic function, antidepressant use and race. Error bars consist of standard error of the mean (SEM). log cAMP SI = log isoproteronol stimulated /log non-stimulated cAMP levels of peripheral blood mononuclear cells. * p < .05
DISCUSSION
Patients with HF with major depression had increased PBMC β-AR sensitivity to an agonist. This relationship is consistent with our previous report that showed in response to exercise patients with HF with elevated depression levels had an increase in immune cell mobilization to β -agonist (20). Our results are consistent with research that psychological factors are associated with reduced β -blockade efficacy in CVD patients (37). However, our present findings are in contrast with investigations that found reduced β-AR sensitivity in physically healthy patients with major depression (25, 38). Conflicting findings in β-AR sensitivity may be due to HF-related sympathetic dysregulation in combination with major depression. As there are no other studies that we are aware of that have examined β-AR sensitivity in patients with HF and co-morbid major depression further study is needed to replicate our results. Heightened leukocyte β-AR sensitivity to agonists including catecholamines can potentially further affect cardiovascular physiology via leukocyte mobilization (20), especially to cardiac tissue which is related to greater risk of left ventricular hypertrophy (21), and cardiovascular remodeling (7, 8). Indeed, leukocyte mobilization to isoproterenol at rest described in our previous report (20) was significantly correlated with isoproterenol stimulated cAMP (p = .02, R2 change = .11) after adjusting for cardiovascular risk factors, and antidepressant use. Importantly, the present study suggests that some patients with HF with major depression may differentially respond to β -blockers since their PBMC β -adrenergic receptors are hyper-responsive to an agonist compared to those without current major depression. Thus, there may be clinical implications to this finding in this specific population although this still needs to be further investigated.
Unexpectedly, we did not find relationships between depression symptoms and plasma catecholamines (NE and Epi). There may be various explanations for this lack of relationship in the present study. It is well documented that patients with HF have heightened cardiac sympathetic activation and increased central nervous system catecholamine turnover (12), which may mask associations with depression. The wide use of β -blockers to treat HF may further mask such a relationship. Additionally, other studies have similarly observed that depression symptoms were not related to resting levels of catecholamines, but instead found that elevated catecholamines were related to depression symptoms only in response to stress (14). Changes in β-AR sensitivity in response to stress were not examined in the present study. We also did not find a relationship between leukocyte β-AR sensitivity and catecholamines, which corresponds with studies that found a similar lack of association in depressed patients (25). Although plasma catecholamines reflect overall SNS activity, β-AR sensitivity of circulating mononuclear cells may be mediated locally in the spleen and lymphatic structures where sympathetic nerves have terminals in close apposition to sequestered cells (15). Other investigations have found local SNS activity to be associated with depression. Muscle SNS activity was elevated in major depression disorder (MDD) patients that had high depression symptoms compared with MDD patients with lower depression symptoms (10). Despite the lack of relationship between depression and catecholamines in the present study, observed differences in leukocyte β-AR sensitivity in relation to major depression may illustrate that functional measures of SNS may be important in depression studies for examining SNS activation.
In contrast to binomial assessments of major depression, when depression symptoms were examined as a continuous variable a relationship was not found between symptoms of depression and β-AR sensitivity. This confirms observations from other studies that also did not find a correspondence between depression as a continuous variable and β-AR sensitivity (24). However, some studies have found non-linear relationships between sympathetic activity and depression symptoms (10), 13). For example, the overall rate of spillover of NE to plasma did not differ between the healthy subjects and the patients with major depressive disorder (MDD); whereas the rate of NE spillover to plasma followed a bimodal distribution with a subset of MDD patients having very high sympathetic nervous activity, including sympathetic outflow to the heart (13). Upon further analysis, we found subcategories of depression symptom severity had differential relationships with β-AR sensitivity. Moderate-to-severe depression symptoms appeared to have greater β-AR sensitivity than patients with mild depression symptoms. Whereas, mild depression symptoms were associated with lower β-AR sensitivity compared with patients with minimal depression symptoms. However, our analyses did not determine a definitive curvilinear pattern of β-AR sensitivity in relation to continuous depression scores as might have been expected from the pattern exhibited from minimal, mild and moderate/severe depression symptom categories. This may be due to the small number of patients in the study with moderate/severe depressive symptoms and further investigation is needed to elucidate the precise parameters of the relationship. β-AR sensitivity differences did not appear to reflect antidepressant treatments, since β-AR sensitivity did not differ between those taking antidepressants and those who did not. Similar to our findings of reduced leukocyte β-AR sensitivity in patients with HF with mild depression symptoms, reduced β-AR sensitivity is found with chronic stress (39, 40) which has been linked with attenuated adrenergic immune regulation and corresponding augmented inflammation (41) that is related to chronic inflammatory diseases including CVD (40). However, more investigation is needed to determine whether mild depression symptoms associated with reductions in β-AR sensitivity indeed impact inflammation and whether this has an influence on HF prognosis. Heterogeneous patterns of SNS activity and depression severity should be further investigated, as differential depression symptomology may reflect a specific stage and/or etiology of depression, which may possess an array of differential neuroimmune characteristics from major depression. Although ROC analysis exhibited a high correlation between major depression diagnosis and BDI scores, this strong relationship may not extend to biological markers. Thus, the method of measuring depression may be an important factor when evaluating depression and β-AR sensitivity and may give disparate results depending on the depression measure used. We are not aware of other studies that have examined depression symptom score categories and β-AR sensitivity in healthy or clinical populations, and further investigation is needed.
Limitations of this study include the small number of patients with HF in the subcategories of depression symptom severity and major depression status. A larger scale investigation is needed to confirm and potentially strengthen our findings. In addition, the patients in our study were heterogeneous and included patients with HF with and without preserved systolic function. However, these groups did not differ in PBMC β-AR sensitivity or total BDI scores, and preserved systolic function was adjusted for in all analyses. The present investigation is likely limited in generalizability to patients with HF taking β -blockers. Since the current medical regimen for most patients with HF involves β -blockers it would be almost impossible to examine patients with HF that are not on β -blockers in order to separate out the effects of β -blockers versus the effects of HF itself on β-AR sensitivity in this cohort. The present study, however does suggest that some patients with major depression may be differentially responding to β -blockers since their β -adrenergic sensitivity appears to be increased compared with patients with HF without major depression. Another limitation is that there was a greater proportion of men in this study and thus, it is unknown if these findings are generalizable to women. However, there were no sex differences in PBMC β-AR sensitivity or BDI scores. Nonetheless, future studies should include larger populations of women to confirm that these findings do not differ by gender. It also should be noted that the measures of depression (BDI and SCID) together did not cleanly differentiate symptom severity (see Supplementary Figure 2). Some patients with major depression determined with the SCID also fell into the category of mild depression symptoms measured with the BDI (n = 5) and a couple of patients with moderate-to-severe depression symptoms were not found to have major depression (n = 2). Thus, major depression may not always result from of an accumulation of depression symptoms, but may have a qualitative component that is not measured with depression severity indexes such as the BDI (42). At the same time, self-report indexes of depression may provide added information and can provide a means of exploring categories, such as mild depression symptoms that may be missed with binomial determinations of major depression. Another potential limitation of this study was the lack of measurement of depression with a scale that eliminates the effects of somatic symptoms since this was a clinical population with underlying physical pathophysiology. However, our preliminary analyses did not find an association between somatic-affective symptoms of depression in the subscale of the BDI and β-AR sensitivity. Further investigation is needed in order to clarify the interactions among depression severity, subcategories of depressive symptoms, sympathetic activity, and HF status. Also, it is unknown whether these relationships are limited to patients with HF and may not generalize to other CVD populations. Additionally, research is needed to establish whether PBMC β-AR sensitivity is related to HF progression to reveal clinical implications of these results.
CONCLUSIONS
Our results suggest that depending on the category of depression symptoms, PBMC β-AR sensitivity may be differentially affected. Thus, patients with HF with major depression may have disparate sympathetic regulatory functions than those with mild depression symptoms. Patients with HF with major depression appear to have increased PBMC β-AR sensitivity, which may indicate that psychological disorders may undermine medications prescribed to reduce sympathetic activity. By deconstructing measurements of depression a greater depth of information may be garnered to potentially reveal subtypes of depression symptoms and their relation to β -adrenergic sensitivity in HF. This potentially reveals the necessity for diverse treatments of depression in patients with HF since they may be related to different mechanistic pathways to worsening HF. More research is needed to determine the clinical significance of these differences in PBMC β-AR sensitivity in HF.
Supplementary Material
Acknowledgments
Funding source: NIH: National Heart, Lung and Blood: R01 HL057265-08, R01 HL096784
Acronyms
- β-AR
beta-adrenergic receptor
- SCID
Structured Clinical Interview for the DSM-IV
- HF
heart failure
- MDD
major depressive disorder
- NE
norepinephrine
- Epi
epinephrine
- CVD
cardiovascular disease
Footnotes
Conflicts of Interest: there are no conflicts of interest to declare in the manuscript, including financial, consultant, institutional and other relationships that might lead to bias or a conflict of interest.
References
- 1.Rutledge T, Reis VA, Linke SE, Greenberg BH, Mills PJ. Depression in heart failure a meta-analytic review of prevalence, intervention effects, and associations with clinical outcomes. J Am Coll Cardiol. 2006;48:1527–37. doi: 10.1016/j.jacc.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 2.Jiang W, Kuchibhatla M, Clary GL, Cuffe MS, Christopher EJ, Alexander JD, Califf RM, Krishnan RR, O'Connor CM. Relationship between depressive symptoms and long-term mortality in patients with heart failure. Am Heart J. 2007;154:102–8. doi: 10.1016/j.ahj.2007.03.043. [DOI] [PubMed] [Google Scholar]
- 3.Friedmann E, Thomas SA, Liu F, Morton PG, Chapa D, Gottlieb SS. Relationship of depression, anxiety, and social isolation to chronic heart failure outpatient mortality. Am Heart J. 2006;152:940, e1–8. doi: 10.1016/j.ahj.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 4.Sherwood A, Blumenthal JA, Trivedi R, Johnson KS, O'Connor CM, Adams KF, Jr., Dupree CS, Waugh RA, Bensimhon DR, Gaulden L, Christenson RH, Koch GG, Hinderliter AL. Relationship of depression to death or hospitalization in patients with heart failure. Arch Intern Med. 2007;167:367–73. doi: 10.1001/archinte.167.4.367. [DOI] [PubMed] [Google Scholar]
- 5.Irwin MR. Human psychoneuroimmunology: 20 years of discovery. Brain Behav Immun. 2008;22:129–39. doi: 10.1016/j.bbi.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 6.Pace TW, Mletzko TC, Alagbe O, Musselman DL, Nemeroff CB, Miller AH, Heim CM. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am J Psychiatry. 2006;163:1630–3. doi: 10.1176/ajp.2006.163.9.1630. [DOI] [PubMed] [Google Scholar]
- 7.Pyo RT, Sui J, Dhume A, Palomeque J, Blaxall BC, Diaz G, Tunstead J, Logothetis DE, Hajjar RJ, Schecter AD. CXCR4 modulates contractility in adult cardiac myocytes. J Mol Cell Cardiol. 2006;41:834–44. doi: 10.1016/j.yjmcc.2006.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frangogiannis NG, Dewald O, Xia Y, Ren G, Haudek S, Leucker T, Kraemer D, Taffet G, Rollins BJ, Entman ML. Critical role of monocyte chemoattractant protein-1/CC chemokine ligand 2 in the pathogenesis of ischemic cardiomyopathy. Circulation. 2007;115:584–92. doi: 10.1161/CIRCULATIONAHA.106.646091. [DOI] [PubMed] [Google Scholar]
- 9.Barton DA, Dawood T, Lambert EA, Esler MD, Haikerwal D, Brenchley C, Socratous F, Kaye DM, Schlaich MP, Hickie I, Lambert GW. Sympathetic activity in major depressive disorder: identifying those at increased cardiac risk? J Hypertens. 2007;25:2117–24. doi: 10.1097/HJH.0b013e32829baae7. [DOI] [PubMed] [Google Scholar]
- 10.Scalco AZ, Rondon MU, Trombetta IC, Laterza MC, Azul JB, Pullenayegum EM, Scalco MZ, Kuniyoshi FH, Wajngarten M, Negrão CE, Lotufo-Neto F. Muscle sympathetic nervous activity in depressed patients before and after treatment with sertraline. J Hypertens. 2009;27:2429–36. doi: 10.1097/HJH.0b013e3283310ece. [DOI] [PubMed] [Google Scholar]
- 11.Cohn JN. Unloading the heart in congestive heart failure. Am J Med. 1984;77:67–70. doi: 10.1016/s0002-9343(84)80060-1. [DOI] [PubMed] [Google Scholar]
- 12.Kaye DM, Lambert GW, Lefkovits J, Morris M, Jennings G, Esler MD. Neurochemical evidence of cardiac sympathetic activation and increased central nervous system norepinephrine turnover in severe congestive heart failure. J Am Coll Cardiol. 1994;23:570–8. doi: 10.1016/0735-1097(94)90738-2. [DOI] [PubMed] [Google Scholar]
- 13.Nair N, Farmer C, Gongora E, Dehmer GJ. Commonality between depression and heart failure. Am J Cardiol. 2012;109:768–72. doi: 10.1016/j.amjcard.2011.10.039. [DOI] [PubMed] [Google Scholar]
- 14.Weinstein A, Deuster P, Francis J, Bonsall R, Tracy R, Kop W. Neurohormonal and inflammatory hyper-responsiveness to acute mental stress in depression. Biol Psychol. 2010 doi: 10.1016/j.biopsycho.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Straub RH, Mayer M, Kreutz M, Leeb S, Scholmerich J, Falk W. Neurotransmitters of the sympathetic nerve terminal are powerful chemoattractants for monocytes. J Leukoc Biol. 2000;67:553–8. doi: 10.1002/jlb.67.4.553. [DOI] [PubMed] [Google Scholar]
- 16.Nance DM, Sanders VM. Autonomic innervation and regulation of the immune system (1987-2007). Brain Behav Immun. 2007;21:736–45. doi: 10.1016/j.bbi.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruzicka M, Floras JS, McReynolds AJ, Coletta E, Haddad H, Davies R, Leenen FH. Do high doses of AT1-receptor blockers attenuate central sympathetic outflow in humans with chronic heart failure? Clin Sci (Lond) 2012 doi: 10.1042/CS20120437. [DOI] [PubMed] [Google Scholar]
- 18.Elenkov IJ, Kvetnansky R, Hashiramoto A, Bakalov VK, Link AA, Zachman K, Crane M, Jezova D, Rovensky J, Dimitrov MA, Gold PW, Bonini S, Fleisher T, Chrousos GP, Wilder RL. Low-versus high-baseline epinephrine output shapes opposite innate cytokine profiles: presence of Lewis- and Fischer-like neurohormonal immune phenotypes in humans? J Immunol. 2008;181:1737–45. doi: 10.4049/jimmunol.181.3.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Redwine L, Jenkins F, Baum A. Relation between beta-adrenergic receptor density and lymphocyte proliferation associates with acute stress. Int J Behav Med. 1996;3:337–53. doi: 10.1207/s15327558ijbm0304_4. [DOI] [PubMed] [Google Scholar]
- 20.Redwine LS, Wirtz PH, Hong S, Bosch J, Ziegler MG, Greenberg B, Mills PJ. Depression as a potential modulator of Beta-adrenergic-associated leukocyte mobilization in heart failure patients. J Am Coll Cardiol. 2010;56:1720–7. doi: 10.1016/j.jacc.2010.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park S, Choi D, Kim C. Hypertensive left ventricular hypertrophy: relation to beta-adrenergic receptor kinase-1 (betaARK1) in peripheral lymphocytes. J Hypertens. 2004;22:1025–32. doi: 10.1097/00004872-200405000-00026. [DOI] [PubMed] [Google Scholar]
- 22.Edgar VA, Cremaschi GA, Sterin-Borda L, Genaro AM. Altered expression of autonomic neurotransmitter receptors and proliferative responses in lymphocytes from a chronic mild stress model of depression: effects of fluoxetine. Brain Behav Immun. 2002;16:333–50. doi: 10.1006/brbi.2001.0632. [DOI] [PubMed] [Google Scholar]
- 23.Silberman D, Ayelli-Edgar V, Zorrilla-Zubilete M, Zieher L, Genaro A. Impaired T-cell dependent humoral response and its relationship with T lymphocyte sensitivity to stress hormones in a chronic mild stress model of depression. Brain Behav Immun. 2004;18:81–90. doi: 10.1016/s0889-1591(03)00109-0. [DOI] [PubMed] [Google Scholar]
- 24.Yu BH, Kang EH, Ziegler MG, Mills PJ, Dimsdale JE. Mood states, sympathetic activity, and in vivo beta-adrenergic receptor function in a normal population. Depress Anxiety. 2008;25:559–64. doi: 10.1002/da.20338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mann JJ, Halper JP, Wilner PJ, Sweeney JA, Mieczkowski TA, Chen JS, Stokes PE, Brown RP. Subsensitivity of adenylyl cyclase-coupled receptors on mononuclear leukocytes from drug-free inpatients with a major depressive episode. Biol Psychiatry. 1997;42:859–70. doi: 10.1016/S0006-3223(97)00154-6. [DOI] [PubMed] [Google Scholar]
- 26.Rutledge T, Redwine LS, Linke SE, Mills PJ. A Meta-Analysis of Mental Health Treatments and Cardiac Rehabilitation for Improving Clinical Outcomes and Depression Among Patients With Coronary Heart Disease. Psychosom Med. 2013 doi: 10.1097/PSY.0b013e318291d798. [DOI] [PubMed] [Google Scholar]
- 27.Davidson KW, Kupfer DJ, Bigger JT, Califf RM, Carney RM, Coyne JC, Czajkowski SM, Frank E, Frasure-Smith N, Freedland KE, Froelicher ES, Glassman AH, Katon WJ, Kaufmann PG, Kessler RC, Kraemer HC, Krishnan KR, Lespérance F, Rieckmann N, Sheps DS, Suls JM. National Heart Ln, and Blood Institute Working Group. Assessment and treatment of depression in patients with cardiovascular disease: National Heart, Lung, and Blood Institute Working Group Report. Psychosom Med. 2006;68:645–50. doi: 10.1097/01.psy.0000233233.48738.22. [DOI] [PubMed] [Google Scholar]
- 28.Linke S, Rutledge T, Johnson B, Vaccarino V, Bittner V, Cornell C, Eteiba W, Sheps D, Krantz D, Parashar S, Bairey Merz C. Depressive symptom dimensions and cardiovascular prognosis among women with suspected myocardial ischemia: A report from the National Heart, Lung, and Blood Institute-sponsored Women's Ischemia Syndrome Evaluation. Arch Gen Psychiatry. 2009;66:499–507. doi: 10.1001/archgenpsychiatry.2009.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim YH, Kim SH, Lim SY, Cho GY, Baik IK, Lim HE, Na JO, Han SW, Ko YH, Shin C. Relationship between depression and subclinical left ventricular changes in the general population. Heart. 2012;98:1378–83. doi: 10.1136/heartjnl-2012-302180. [DOI] [PubMed] [Google Scholar]
- 30.Lespérance F, Frasure-Smith N, Talajic M, Bourassa MG. Five-year risk of cardiac mortality in relation to initial severity and one-year changes in depression symptoms after myocardial infarction. Circulation. 2002;105:1049–53. doi: 10.1161/hc0902.104707. [DOI] [PubMed] [Google Scholar]
- 31.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th ed. American Psychiatric Association; Washington DC: 1994. [Google Scholar]
- 32.Mills PJ, Dimsdale JE. Anger suppression: its relationship to beta-adrenergic receptor sensitivity and stress-induced changes in blood pressure. Psychol Med. 1993;23:673–8. doi: 10.1017/s0033291700025459. [DOI] [PubMed] [Google Scholar]
- 33.Kennedy B, Ziegler MG. A more sensitive and specific radioenzymatic assay for catecholamines. Life Sci. 1990;47:2143–53. doi: 10.1016/0024-3205(90)90314-h. [DOI] [PubMed] [Google Scholar]
- 34.Allison P. Missing data techniques for structural equation modeling. J Abnorm Psychol. 2003;112:545–57. doi: 10.1037/0021-843X.112.4.545. [DOI] [PubMed] [Google Scholar]
- 35.Euteneuer F, Ziegler MG, Mills PJ, Rief W, Dimsdale JE. In Vivo β-Adrenergic Receptor Responsiveness: Ethnic Differences in the Relationship with Symptoms of Depression and Fatigue. Int J Behav Med. 2013 doi: 10.1007/s12529-013-9359-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.J. C. Statistical power analysis for the behavior sciences. 2nd ed. Lawrence Erlbaum Association, Inc.; Hillsdale, New Jersey: 1988. [Google Scholar]
- 37.Rutledge T, Linden W, Davies RF. Psychological risk factors may moderate pharmacological treatment effects among ischemic heart disease patients. Canadian Amlodipine/Atenolol in Silent Ischemia Study (CASIS) Investigators. Psychosom Med. 1999;61:834–41. doi: 10.1097/00006842-199911000-00018. [DOI] [PubMed] [Google Scholar]
- 38.Carstens ME, Engelbrecht AH, Russell VA, Aalbers C, Gagiano CA, Chalton DO, Taljaard JJ. Beta-adrenoceptors on lymphocytes of patients with major depressive disorder. Psychiatry Res. 1987;20:239–48. doi: 10.1016/0165-1781(87)90084-9. [DOI] [PubMed] [Google Scholar]
- 39.Dimsdale JE, Mills P, Patterson T, Ziegler M, Dillon E. Effects of chronic stress on beta-adrenergic receptors in the homeless. Psychosom Med. 1994;56:290–5. doi: 10.1097/00006842-199407000-00003. [DOI] [PubMed] [Google Scholar]
- 40.Mausbach BT, Aschbacher K, Mills PJ, Roepke SK, von Känel R, Patterson TL, Dimsdale JE, Ziegler MG, Ancoli-Israel S, Grant I. A 5-year longitudinal study of the relationships between stress, coping, and immune cell beta(2)-adrenergic receptor sensitivity. Psychiatry Res. 2008;160:247–55. doi: 10.1016/j.psychres.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kohm AP, Sanders VM. Norepinephrine and beta 2-adrenergic receptor stimulation regulate CD4+ T and B lymphocyte function in vitro and in vivo. Pharmacol Rev. 2001;53:487–525. [PubMed] [Google Scholar]
- 42.Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009;71:171–86. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.