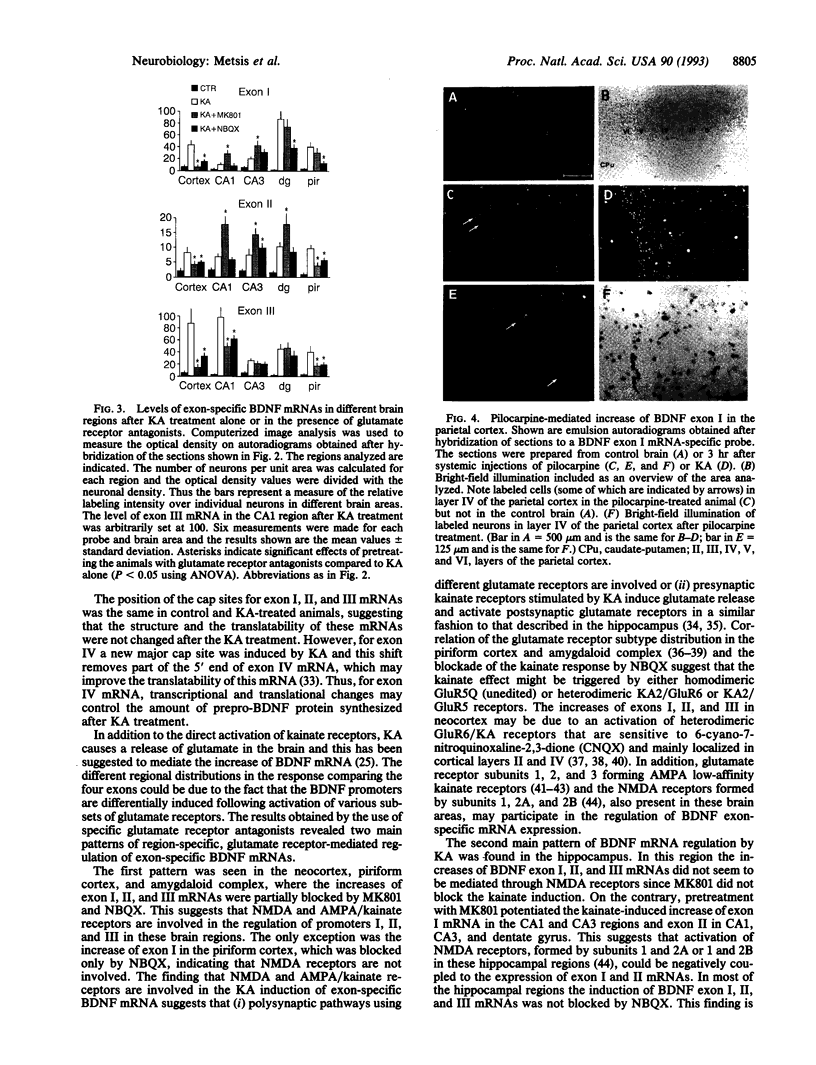
Abstract
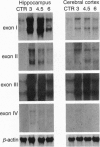
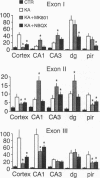
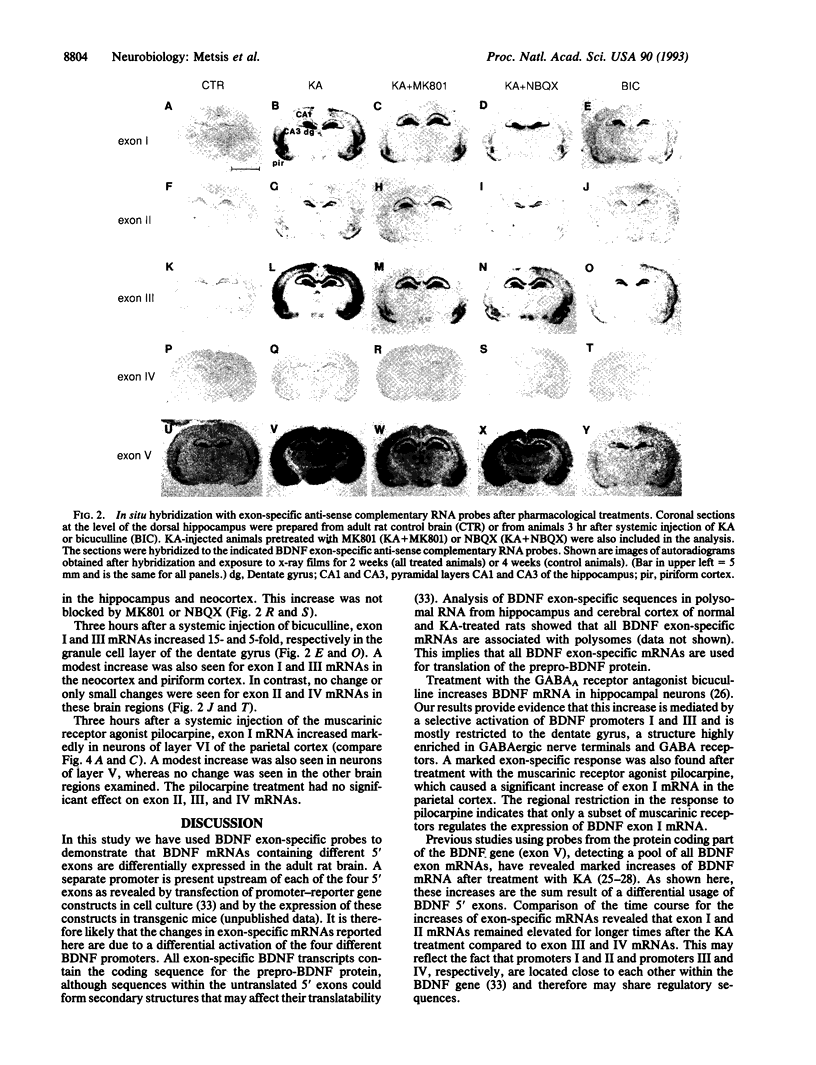
The rat brain-derived neurotropic factor (BDNF) gene consists of four 5' exons linked to separate promoters and one 3' exon encoding the prepro-BDNF protein. To gain insights into the regulation of BDNF mRNA expression, probes specific for the different 5' exons were used to study the expression of BDNF mRNA in the brain. Following a systemic injection of the glutamate analog kainic acid, exon I, II, and III mRNAs increased transiently in hippocampus and cerebral cortex. A modest increase was seen for exon IV, where a new transcription initiation site was induced by this treatment. Pretreatments with the N-methyl-D-aspartate (NMDA) receptor antagonist MK801 or the alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor antagonist 2,3-dihydroxy-6-nitrosulfanoylbenzo(f)quinoxaline revealed two region-specific patterns of glutamate receptor-mediated regulation. The first pattern found in neocortex, piriform cortex, and amygdala involves regulation of BDNF exon I, II, and III mRNAs through NMDA and AMPA/kainate receptors. The second pattern found in the hippocampus involves regulation of BDNF exon I, II, and III mRNAs by high-affinity kainate or metabotropic receptors. Treatment with the gamma-aminobutyric acid subtype A (GABAA) receptor antagonist bicuculline increased exon I and III mRNAs in the denate gyrus, and the muscarinic receptor agonist pilocarpine increased exon I mRNA mainly in the neocortex. These data show that the four BDNF promoters allow multiple points of BDNF mRNA regulation and suggest that the activation of different subtypes of glutamate receptors differentially regulates the expression of BDNF exon-specific mRNAs in the brain.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderson R. F., Alterman A. L., Barde Y. A., Lindsay R. M. Brain-derived neurotrophic factor increases survival and differentiated functions of rat septal cholinergic neurons in culture. Neuron. 1990 Sep;5(3):297–306. doi: 10.1016/0896-6273(90)90166-d. [DOI] [PubMed] [Google Scholar]
- Ballarín M., Ernfors P., Lindefors N., Persson H. Hippocampal damage and kainic acid injection induce a rapid increase in mRNA for BDNF and NGF in the rat brain. Exp Neurol. 1991 Oct;114(1):35–43. doi: 10.1016/0014-4886(91)90082-n. [DOI] [PubMed] [Google Scholar]
- Barde Y. A., Edgar D., Thoenen H. Purification of a new neurotrophic factor from mammalian brain. EMBO J. 1982;1(5):549–553. doi: 10.1002/j.1460-2075.1982.tb01207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkemeier L. R., Winslow J. W., Kaplan D. R., Nikolics K., Goeddel D. V., Rosenthal A. Neurotrophin-5: a novel neurotrophic factor that activates trk and trkB. Neuron. 1991 Nov;7(5):857–866. doi: 10.1016/0896-6273(91)90287-a. [DOI] [PubMed] [Google Scholar]
- Bettler B., Boulter J., Hermans-Borgmeyer I., O'Shea-Greenfield A., Deneris E. S., Moll C., Borgmeyer U., Hollmann M., Heinemann S. Cloning of a novel glutamate receptor subunit, GluR5: expression in the nervous system during development. Neuron. 1990 Nov;5(5):583–595. doi: 10.1016/0896-6273(90)90213-y. [DOI] [PubMed] [Google Scholar]
- Boulter J., Hollmann M., O'Shea-Greenfield A., Hartley M., Deneris E., Maron C., Heinemann S. Molecular cloning and functional expression of glutamate receptor subunit genes. Science. 1990 Aug 31;249(4972):1033–1037. doi: 10.1126/science.2168579. [DOI] [PubMed] [Google Scholar]
- Boulter J., Hollmann M., O'Shea-Greenfield A., Hartley M., Deneris E., Maron C., Heinemann S. Molecular cloning and functional expression of glutamate receptor subunit genes. Science. 1990 Aug 31;249(4972):1033–1037. doi: 10.1126/science.2168579. [DOI] [PubMed] [Google Scholar]
- Dugich-Djordjevic M. M., Tocco G., Lapchak P. A., Pasinetti G. M., Najm I., Baudry M., Hefti F. Regionally specific and rapid increases in brain-derived neurotrophic factor messenger RNA in the adult rat brain following seizures induced by systemic administration of kainic acid. Neuroscience. 1992;47(2):303–315. doi: 10.1016/0306-4522(92)90246-x. [DOI] [PubMed] [Google Scholar]
- Egebjerg J., Bettler B., Hermans-Borgmeyer I., Heinemann S. Cloning of a cDNA for a glutamate receptor subunit activated by kainate but not AMPA. Nature. 1991 Jun 27;351(6329):745–748. doi: 10.1038/351745a0. [DOI] [PubMed] [Google Scholar]
- Ernfors P., Bengzon J., Kokaia Z., Persson H., Lindvall O. Increased levels of messenger RNAs for neurotrophic factors in the brain during kindling epileptogenesis. Neuron. 1991 Jul;7(1):165–176. doi: 10.1016/0896-6273(91)90084-d. [DOI] [PubMed] [Google Scholar]
- Ernfors P., Ibáez C. F., Ebendal T., Olson L., Persson H. Molecular cloning and neurotrophic activities of a protein with structural similarities to nerve growth factor: developmental and topographical expression in the brain. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5454–5458. doi: 10.1073/pnas.87.14.5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernfors P., Wetmore C., Olson L., Persson H. Identification of cells in rat brain and peripheral tissues expressing mRNA for members of the nerve growth factor family. Neuron. 1990 Oct;5(4):511–526. doi: 10.1016/0896-6273(90)90090-3. [DOI] [PubMed] [Google Scholar]
- Flood J. F., Baker M. L., Davis J. L. Modulation of memory processing by glutamic acid receptor agonists and antagonists. Brain Res. 1990 Jun 25;521(1-2):197–202. doi: 10.1016/0006-8993(90)91543-p. [DOI] [PubMed] [Google Scholar]
- Gannon R. L., Terrian D. M. Presynaptic modulation of glutamate and dynorphin release by excitatory amino acids in the guinea-pig hippocampus. Neuroscience. 1991;41(2-3):401–410. doi: 10.1016/0306-4522(91)90336-m. [DOI] [PubMed] [Google Scholar]
- Hallbök F., Ibáez C. F., Persson H. Evolutionary studies of the nerve growth factor family reveal a novel member abundantly expressed in Xenopus ovary. Neuron. 1991 May;6(5):845–858. doi: 10.1016/0896-6273(91)90180-8. [DOI] [PubMed] [Google Scholar]
- Herb A., Burnashev N., Werner P., Sakmann B., Wisden W., Seeburg P. H. The KA-2 subunit of excitatory amino acid receptors shows widespread expression in brain and forms ion channels with distantly related subunits. Neuron. 1992 Apr;8(4):775–785. doi: 10.1016/0896-6273(92)90098-x. [DOI] [PubMed] [Google Scholar]
- Hofer M. M., Barde Y. A. Brain-derived neurotrophic factor prevents neuronal death in vivo. Nature. 1988 Jan 21;331(6153):261–262. doi: 10.1038/331261a0. [DOI] [PubMed] [Google Scholar]
- Hofer M., Pagliusi S. R., Hohn A., Leibrock J., Barde Y. A. Regional distribution of brain-derived neurotrophic factor mRNA in the adult mouse brain. EMBO J. 1990 Aug;9(8):2459–2464. doi: 10.1002/j.1460-2075.1990.tb07423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohn A., Leibrock J., Bailey K., Barde Y. A. Identification and characterization of a novel member of the nerve growth factor/brain-derived neurotrophic factor family. Nature. 1990 Mar 22;344(6264):339–341. doi: 10.1038/344339a0. [DOI] [PubMed] [Google Scholar]
- Hyman C., Hofer M., Barde Y. A., Juhasz M., Yancopoulos G. D., Squinto S. P., Lindsay R. M. BDNF is a neurotrophic factor for dopaminergic neurons of the substantia nigra. Nature. 1991 Mar 21;350(6315):230–232. doi: 10.1038/350230a0. [DOI] [PubMed] [Google Scholar]
- Ip N. Y., Ibáez C. F., Nye S. H., McClain J., Jones P. F., Gies D. R., Belluscio L., Le Beau M. M., Espinosa R., 3rd, Squinto S. P. Mammalian neurotrophin-4: structure, chromosomal localization, tissue distribution, and receptor specificity. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):3060–3064. doi: 10.1073/pnas.89.7.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isackson P. J., Huntsman M. M., Murray K. D., Gall C. M. BDNF mRNA expression is increased in adult rat forebrain after limbic seizures: temporal patterns of induction distinct from NGF. Neuron. 1991 Jun;6(6):937–948. doi: 10.1016/0896-6273(91)90234-q. [DOI] [PubMed] [Google Scholar]
- Izquierdo I., Medina J. H. GABAA receptor modulation of memory: the role of endogenous benzodiazepines. Trends Pharmacol Sci. 1991 Jul;12(7):260–265. doi: 10.1016/0165-6147(91)90567-c. [DOI] [PubMed] [Google Scholar]
- Johnson J. E., Barde Y. A., Schwab M., Thoenen H. Brain-derived neurotrophic factor supports the survival of cultured rat retinal ganglion cells. J Neurosci. 1986 Oct;6(10):3031–3038. doi: 10.1523/JNEUROSCI.06-10-03031.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K. R., Reichardt L. F. Molecular cloning of a human gene that is a member of the nerve growth factor family. Proc Natl Acad Sci U S A. 1990 Oct;87(20):8060–8064. doi: 10.1073/pnas.87.20.8060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaisho Y., Yoshimura K., Nakahama K. Cloning and expression of a cDNA encoding a novel human neurotrophic factor. FEBS Lett. 1990 Jun 18;266(1-2):187–191. doi: 10.1016/0014-5793(90)81536-w. [DOI] [PubMed] [Google Scholar]
- Keinänen K., Wisden W., Sommer B., Werner P., Herb A., Verdoorn T. A., Sakmann B., Seeburg P. H. A family of AMPA-selective glutamate receptors. Science. 1990 Aug 3;249(4968):556–560. doi: 10.1126/science.2166337. [DOI] [PubMed] [Google Scholar]
- Knüsel B., Winslow J. W., Rosenthal A., Burton L. E., Seid D. P., Nikolics K., Hefti F. Promotion of central cholinergic and dopaminergic neuron differentiation by brain-derived neurotrophic factor but not neurotrophin 3. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):961–965. doi: 10.1073/pnas.88.3.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibrock J., Lottspeich F., Hohn A., Hofer M., Hengerer B., Masiakowski P., Thoenen H., Barde Y. A. Molecular cloning and expression of brain-derived neurotrophic factor. Nature. 1989 Sep 14;341(6238):149–152. doi: 10.1038/341149a0. [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R. The nerve growth factor 35 years later. Science. 1987 Sep 4;237(4819):1154–1162. doi: 10.1126/science.3306916. [DOI] [PubMed] [Google Scholar]
- Maisonpierre P. C., Belluscio L., Squinto S., Ip N. Y., Furth M. E., Lindsay R. M., Yancopoulos G. D. Neurotrophin-3: a neurotrophic factor related to NGF and BDNF. Science. 1990 Mar 23;247(4949 Pt 1):1446–1451. doi: 10.1126/science.247.4949.1446. [DOI] [PubMed] [Google Scholar]
- Matsuoka N., Maeda N., Ohkubo Y., Yamaguchi I. Differential effects of physostigmine and pilocarpine on the spatial memory deficits produced by two septo-hippocampal deafferentations in rats. Brain Res. 1991 Sep 20;559(2):233–240. doi: 10.1016/0006-8993(91)90007-i. [DOI] [PubMed] [Google Scholar]
- Monyer H., Sprengel R., Schoepfer R., Herb A., Higuchi M., Lomeli H., Burnashev N., Sakmann B., Seeburg P. H. Heteromeric NMDA receptors: molecular and functional distinction of subtypes. Science. 1992 May 22;256(5060):1217–1221. doi: 10.1126/science.256.5060.1217. [DOI] [PubMed] [Google Scholar]
- Nakanishi N., Shneider N. A., Axel R. A family of glutamate receptor genes: evidence for the formation of heteromultimeric receptors with distinct channel properties. Neuron. 1990 Nov;5(5):569–581. doi: 10.1016/0896-6273(90)90212-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi S. Molecular diversity of glutamate receptors and implications for brain function. Science. 1992 Oct 23;258(5082):597–603. doi: 10.1126/science.1329206. [DOI] [PubMed] [Google Scholar]
- Oppenheim R. W., Yin Q. W., Prevette D., Yan Q. Brain-derived neurotrophic factor rescues developing avian motoneurons from cell death. Nature. 1992 Dec 24;360(6406):755–757. doi: 10.1038/360755a0. [DOI] [PubMed] [Google Scholar]
- Phillips H. S., Hains J. M., Laramee G. R., Rosenthal A., Winslow J. W. Widespread expression of BDNF but not NT3 by target areas of basal forebrain cholinergic neurons. Science. 1990 Oct 12;250(4978):290–294. doi: 10.1126/science.1688328. [DOI] [PubMed] [Google Scholar]
- Represa A., Tremblay E., Ben-Ari Y. Kainate binding sites in the hippocampal mossy fibers: localization and plasticity. Neuroscience. 1987 Mar;20(3):739–748. doi: 10.1016/0306-4522(87)90237-5. [DOI] [PubMed] [Google Scholar]
- Rosenthal A., Goeddel D. V., Nguyen T., Lewis M., Shih A., Laramee G. R., Nikolics K., Winslow J. W. Primary structure and biological activity of a novel human neurotrophic factor. Neuron. 1990 May;4(5):767–773. doi: 10.1016/0896-6273(90)90203-r. [DOI] [PubMed] [Google Scholar]
- Sendtner M., Holtmann B., Kolbeck R., Thoenen H., Barde Y. A. Brain-derived neurotrophic factor prevents the death of motoneurons in newborn rats after nerve section. Nature. 1992 Dec 24;360(6406):757–759. doi: 10.1038/360757a0. [DOI] [PubMed] [Google Scholar]
- Sommer B., Burnashev N., Verdoorn T. A., Keinänen K., Sakmann B., Seeburg P. H. A glutamate receptor channel with high affinity for domoate and kainate. EMBO J. 1992 Apr;11(4):1651–1656. doi: 10.1002/j.1460-2075.1992.tb05211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmusk T., Palm K., Metsis M., Reintam T., Paalme V., Saarma M., Persson H. Multiple promoters direct tissue-specific expression of the rat BDNF gene. Neuron. 1993 Mar;10(3):475–489. doi: 10.1016/0896-6273(93)90335-o. [DOI] [PubMed] [Google Scholar]
- Werner P., Voigt M., Keinänen K., Wisden W., Seeburg P. H. Cloning of a putative high-affinity kainate receptor expressed predominantly in hippocampal CA3 cells. Nature. 1991 Jun 27;351(6329):742–744. doi: 10.1038/351742a0. [DOI] [PubMed] [Google Scholar]
- Wetmore C., Ernfors P., Persson H., Olson L. Localization of brain-derived neurotrophic factor mRNA to neurons in the brain by in situ hybridization. Exp Neurol. 1990 Aug;109(2):141–152. doi: 10.1016/0014-4886(90)90068-4. [DOI] [PubMed] [Google Scholar]
- Yan Q., Elliott J., Snider W. D. Brain-derived neurotrophic factor rescues spinal motor neurons from axotomy-induced cell death. Nature. 1992 Dec 24;360(6406):753–755. doi: 10.1038/360753a0. [DOI] [PubMed] [Google Scholar]
- Zafra F., Castrén E., Thoenen H., Lindholm D. Interplay between glutamate and gamma-aminobutyric acid transmitter systems in the physiological regulation of brain-derived neurotrophic factor and nerve growth factor synthesis in hippocampal neurons. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10037–10041. doi: 10.1073/pnas.88.22.10037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafra F., Hengerer B., Leibrock J., Thoenen H., Lindholm D. Activity dependent regulation of BDNF and NGF mRNAs in the rat hippocampus is mediated by non-NMDA glutamate receptors. EMBO J. 1990 Nov;9(11):3545–3550. doi: 10.1002/j.1460-2075.1990.tb07564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]